Abstract
The gene-environment interactions that underlie development and progression of psychiatric illness are poorly understood. Despite a century of progress, genetic approaches have failed to identify new treatment modalities, perhaps because of the heterogeneity of the disorders and lack of understanding of mechanisms. Recent exploration into epigenetic mechanisms in health and disease has uncovered changes in DNA methylation and chromatin structure that may contribute to psychiatric disorders. Epigenetic changes suggest a variety of new therapeutic options due to their reversible chemistry. However, distinguishing causal links between epigenetic changes and disease from changes consequent to life experience has remained problematic. Here we define epigenetics and explore aspects of epigenetics relevant to causes and mechanisms of psychiatric disease, and speculate on future directions.
Keywords: epigenetics, DNA methylation, chromatin, neuropsychiatric disorders
Introduction
In 1942, Conrad Waddington introduced the word epigenetics, fusing “epi”, meaning “above”, with “genetic” to name the contributions of noninherited factors to embryonic development (Waddington, 1952). Waddington was so enamored of his eponym that he used it to name his laboratory at the University of Edinburgh and titled his first book “The Epigenetics of Birds” (Van Speybroeck, 2002). “Epigenetics” was slow to spread within the scientific community partly because Waddington’s definition—“all those events which lead to the unfolding of the genetic program for development”—was broad, and to many, confusing. Discovery of the molecular basis of inheritance and the altered patterning of control of gene expression during cell differentiation allowed the definition to be refined (for review, see Holliday, 2006). The definition of Wu and Morris (2001), “The study of changes in gene function that are mitotically and/or meiotically heritable and that do not entail a change in DNA sequence,” is most commonly used to describe this phenomenon. Here we argue that the defining aspect of epigenetic change is not whether DNA is modified (Liyanage et al., 2014) but the ready reversibility of epigenetic change. This change contrasts directly with nucleotide substitutions, site-directed recombination at T-cell and immunoglobulin genes, insertion of foreign DNA sequences from viruses or transposons, and other genetic events that are for all intents and purposes permanent and may irretrievably modify the genetic code. Taking these data into account, we define epigenetics as the study of readily reversible mitotically and/or meiotically heritable changes. Figure 1 illustrates the distinction between epigenetic and genetic events.
Figure 1.
Epigenetic and genetic alterations of DNA. Epigenetic changes, including methylation and hydroxymethylation of cytosines and other nucleotides, chromatin condensation and opening, and the shortening and lengthening of telomeres, are reversible and thus provide a capacity to rapidly adapt to changes in the environment (lightning bolt). Genetic changes, including DNA substitutions, insertion/deletions (not shown), recombination, and viral integration/transposition, are primarily irreversible. For example, it is rare that a second point mutation exactly reverses a mutation or that a second recombination event occurs at precisely the same location as a previous recombination event. The magnitude of reversibility is shown by the length of the blue arrow.
In this review, we focus on knowledge of epigenetics and the brain, including how epigenetic dysregulation can contribute to disordered behavior. We illustrate how epigenetic states of critical genomic loci contribute directly to the development of neuropsychiatric disorders. DNA methylation and chromatin structure changes at the molecular level restrict the range of gene function at these critical loci and in combination with genetic events can signal the onset of brain disorders.
Crosstalk between Genetic and Epigenetic Mechanisms
Genetic variation is not independent of epigenetic variation but can predict it. The likelihood of DNA mutation and repair is strongly influenced by the epigenetic state of DNA. Methylcytosines are vulnerable to mutation to thymine by deamination to uracil (Duncan and Miller, 1980). Also, transcribed regions of DNA in an epigenetically open state are both more vulnerable to mutation and amenable to transcription-coupled repair (Sweder and Hanawalt, 1993). When a spontaneous nucleotide lesion or DNA damage initially occurs, it is usually on a single strand of DNA and at that point it is readily repaired with high accuracy by a variety of DNA repair processes; in cells with normally functioning DNA repair systems, such nucleotide lesions are almost always correctly repaired. Because a nucleotide lesion on single-stranded DNA is reversible, by the definition we use here, single-strand lesions could be labeled as epigenetic events. However, if the lesion is not repaired and is copied on the second strand of DNA, or if it is incorrectly repaired and copied onto both strands, it becomes an irreversible, and therefore a genetic, event. Genetic variants can alter DNA methylation and also directly change gene expression, as has been detected on a genome-wide basis (Gibbs et al., 2010).
Chemical Modification of DNA Expands Its Information Content
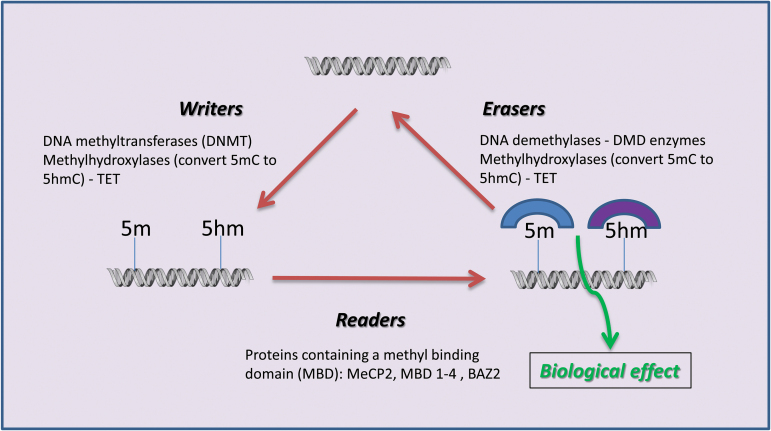
In a process distinct from nucleotide mutations, mammalian DNA is chemically modified via methylation, hydroxymethylation, and formyl- and carboxy-modifications of cytosines predominantly located in cytosine guanine dinucleotides (CpGs) and by N-methylation of adenine (Fu and He, 2012). Other nucleotides are also modified more rarely, but their functions are not well understood. Chemical modifications of cellular RNA, including ribosomal, transfer, long-noncoding, small nuclear, and messenger RNA—for example the N(6)-methyladenosine in mRNA—also mark a new epigenetic layer of chemical information and complexity (Roundtree and He, 2016). No doubt many other important modifications of DNA and RNA remain undiscovered. Cells have a dedicated enzymatic machinery to epigenetically modify nucleic acids and proteins and another set of machinery to reverse it (Goll and Bestor, 2005). As shown in Figure 2, 3 sets of enzymes, which can be classified as “writers” (which add DNA or protein marks), “readers” (which generate a biological effect based on the type of DNA or protein mark), and “erasers” (which erase the marks left by the writers), are needed for the reversible modification of DNA and protein.
Figure 2.
DNA epigenetic modifications and their editors. The 2 best-known DNA modifications are methylation (5m) and hydroxymethylation (5hm). Other nucleotides can be modified, but DNA methylation preferentially occurs on cytosine nucleotides adjacent to guanine nucleotides, a modification catalyzed by DNA methyltransferases (DNMTs). DNA methylation generally silences transcription whereas hydroxymethylation generally activates transcription, although exceptions are now widely known, and seem to be related to the genomic feature (e.g., promoter, intragenic region, 3’ UTR) in which the epigenetic modification is located.
Location, Location, Location
Transcription can be exquisitely sensitive to local levels of DNA methylation (Razin and Cedar, 1984). Cytosine methylation in promoters, enhancers, and transcription start sites usually silences gene expression (transcription), and hypomethylation of the same cytosine bases is generally associated with increased transcription (Wolffe and Matzke, 1999). The opposite trend is observed for genic (i.e., exonic and intronic) cytosine methylation. In these regions, DNA methylation is associated with increased transcription. Regions outside genes, particularly repetitive DNA sequences such as LINE (long interspersed nuclear elements) and SINE (short interspersed nuclear elements) are hypermethylated and transcriptionally silenced to prevent potentially disruptive genomic transposition (Yoder et al., 1997). Both allele-specific gene expression, such as imprinting, and allele-skewed gene expression, such as differential allele expression, are mediated by epigenetic mechanisms (Barton et al., 1984). The effects of other DNA modifications, including cytosine hydroxymethylation, are less well characterized but may functionally erase the effects of cytosine methylation as well as exert their own independent effects. Factors regulating access of the DNA methylation machinery and consequent chemical modification of DNA to highly selected regions of the genome are not well understand.
Chromatin
Another way to expand genome information content is to alter the way that the DNA strand, which would be 3 m long if stretched out, is packaged as a DNA/protein complex: chromatin. Packaging of DNA enhances or reduces accessibility for transcription and other processes, including DNA damage and repair. The 3-dimensional topology of chromatin, including long-range interactions within the same chromosomeand across different chromosomes, is modified via a complex network of histone chemical modifications, including phosphorylation, methylation, acetylation, ADP-ribosylation, and many others. Combinations of chemical modifications of histones that alter their ability to influence gene expression states are known as the histone code (Jenuwein and Allis, 2001). These covalent modifications, centered on amino acid residues in the tail portions of histones, alter histone-DNA interactions and chromatin structure and ultimately affect gene expression. Details of the combinatorial codes of modified histones and their biological effects are still being worked out, but some basic principles of the histone code have emerged. The predominant target in the histone octamer is histone H3, although other subunits are also chemically modified (for more detail, see Figure 3). Phosphorylation of serine residues 10 and 28 on histone H3 is a marker for chromosome condensation and gene silencing (Wei et al., 1998). On the other hand, the combination of phosphorylation of serine10 and acetylation of lysine14 on histone H3 is well correlated with active gene transcription (Strahl and Allis, 2000). Analogous to epigenetic modifications of DNA, the amino acid modifications of histone H3 can also be enzymatically reversed, with a subsequent return to the original gene expression state.
Figure 3.
The histone code and its modifiers. The basic functional unit of chromatin is the nucleosome, which is composed of 147bp of DNA wrapped tightly around an octamer of histone proteins (H2A, H2B, H3, and H4). Histone tails project from nucleosomes and are subject to posttranslational modifications, including methylation (Me), acetylation (Ac), phosphorylation (P), phosphoacetylation (p-Ac), ubiquitination (Ub), and ADP-ribosylation (ADP-R), in different combinations. Local combinations of differentially modified histone proteins form histone codes. Histone codes enhance or inhibit transcription by recruiting enzymes that catalyze the opening or condensing of chromatin, thus making the DNA more or less accessible to transcription factors and additional regulatory factors that modify transcription. The histone code is edited by an ensemble of enzymatic writers, erasers, and readers. Writers add covalent modifications. Erasers catalyze removal of modifications. Readers recognize and bind specific motifs.
Epigenetics and Development
Epigenetic changes control the unfolding of the developmental program in which pluripotent cells differentiate to cells with specialized functions (Reik et al., 2001). Cell differentiation requires expression and suppression of subsets of genes from among the 25,000 or more genes that are inherited and requires nuanced expression, including alternative transcription start sites, and variations in level of RNA expression and translation, including by regulatory RNAs. Via epigenetic modification, a hemoglobin gene cannot become a sodium channel gene but can be expressed in an erythrocyte and at the correct developmental time-point and level. Conversely, the sodium channel gene is correctly expressed in the neuron. A dramatic example of epigenetic variation is the random inactivation of 1 of the 2 X chromosomes in females, condensing the DNA and chromatin of the inactive chromosome into a Barr body and shutting down the expression of all but a few genes on that chromosome (Cooper, 1971).
Inherited Disorders of DNA Methylation Machinery
Rett syndrome (RTT) is a neurological disorder that features behavioral deficits, seizures, lack of verbal skills, and severe motor coordination predominantly in female patients (Rett, 1966). RTT was formerly classified as a developmental disorder by the DSM-IV but was removed after discovery that RTT is caused by genetic mutations in the X-linked MECP2 gene. MECP2 is a transcriptional repressor that binds specifically to methylated cytosine (Amir et al., 1999). Whole genome surveys by high throughput chromatin immunoprecipitation sequencing experiments of MECP2 binding sites have identified hundreds of loci, some of which are involved in neurodevelopmental signaling pathways (Yasui et al., 2013). In fibroblasts derived from patients with RTT and cortical neurons from mouse models of RTT, MeCp2 deletion leads to aberrant cell migration and differentiation. Importantly, these effects are reversed by tubastatin, an HDAC6-specific inhibitor, indicating that epigenetic therapy is a potential option for treating RTT patients (Xu et al., 2014).
Other diseases affecting brain development and function are also caused by genetic defects in the epigenetic machinery. Some of these are rare, including ICF syndrome (immunodeficiency, centromere instability, and facial anomalies), which is caused by autosomal recessive mutations in the DNA-methyltransferase-3b gene (Xu et al., 1999). More common variants in the machinery of DNA methylation that may be relevant to behavior are found in methylene tetrahydrofolate reductase (Rozen, 1996). The reader is referred to this recent review for more detail (Bjornsson, 2015).
Stress and Early-Life Trauma
Early-life stress is a powerful risk factor for several psychiatric disorders (for a recent review, see Klengel et al., 2014). Numerous studies linking maternal behavior and stress-induced depressive-like behaviors in animals have reported changes in DNA methylation of the glucocorticoid receptor (GR) gene (NR3C1) (Weaver et al., 2004), specifically, hypermethylation of regulatory areas of NR3C1 coupled to a decrease in GR transcription. The reduced GR levels induced by DNA hypermethylation then impair negative feedback of the HPA axis such that stress responses are poorly modulated (Weaver, 2014). Other key genes in the stress axis, including genes encoding neuropeptides, and the GR chaperone FKBP5 are also epigenetically programmed by early adversity. In mice, increased secretion of corticosterone following early maternal separation is accompanied by changes in expression and DNA methylation of the neuropeptide Avp promoter. Mice stressed by social defeat exhibited lower DNA methylation in regulatory elements of Crh and altered transcription of this gene, while resilient mice showed fewer changes. Finally, differential methylation of Fkbp5 has been implicated in stress response in a mouse model.
Many of the genes identified in animal studies also show epigenetic abnormalities in human postmortem brain samples or peripheral blood cells from patients with stress disorders. For example, changes in promoter DNA methylation of the NR3C1 gene in hippocampal tissues obtained from suicide victims show a correlation with exposure to early adversity. Importantly, the DNA methylation changes tracked with total GR expression. Exposure to childhood abuse in humans has been shown to correlate with lower allele-specific FKBP5 methylation associated with disinhibited GR-induced transcription of FKBP5 (Klengel et al., 2013). Recent evidence points to a potentially transgenerational transmissible effect of stress on an epigenetic level, as holocaust exposure had an effect on FKBP5 methylation that was observed in exposed parents as well as in their offspring (Yehuda et al., 2015).
Depression
The World Health Organization forecasts that depression will be the leading cause of disease burden by 2030 (Whiteford et al., 2013). However, the absence of a convincing animal model of depression, coupled with inconclusive genetic studies in humans, has hampered efforts to identify candidate depression genes (Lolak et al., 2014). New approaches have utilized DNA methylation patterns of individual CpGs in a manner similar to the use of single nucleotide polymorphisms (SNPs) in genome wide association studies (GWAS) studies (Cortijo et al., 2014). These epigenome-wide association studies (EWAS) have the advantage that they are unbiased and hypothesis free and can survey DNA methylation patterns in virtually any tissue.
EWAS studies of postmortem frontal cortex samples and saliva from depressed patients found methylation differences in 7 genes including 3 genes that were also previously discovered in genetic studies (Dempster et al., 2014). Another EWAS study found hypermethylation at the zinc finger and BTB domain containing 20 genes that is associated with hippocampus integrity, plays a role in neurogenesis and neurodevelopment, and is a candidate gene for depression (Davies et al., 2014). It is unclear whether these DNA methylation changes play a causal role in the development of depression by mediating the expression of these genes or may be useful as markers for diagnosis and categorization of disease state(s).
Given the crosstalk that occurs between chromatin and DNA modifications by virtue of their important role in homeostasis of cell functions and physiology, it comes as no surprise that recent studies have unearthed a relationship between histone modifications and depression. For example, studies of postmortem prefrontal cortex found that antidepressant use was associated with reduced methylation of lysine H3K27 at the BDNF promoter I, correlating with decreased serum BDNF levels (Duclot and Kabbaj, 2015).
Given the key role of DNA modifications, and crosstalk with histone modifications, in regulation of memory and other neurological processes (see below), more comprehensive EWAS studies encompassing a greater spectrum of CpG dinucleotides, or even better, unbiased surveys of genomic DNA methylation by whole genome sequencing will be vital to better understand the role of such processes in depression.
Learning, Memory, and Degenerative Disorders
Learning and formation of new memories require the structural and functional remodeling of neuronal synapses in response to specific patterns of neuronal activity (Martin et al., 2000). In turn, the neuronal signaling pathways trigger nuclear epigenetic activity, ultimately resulting in long-term alterations of gene expression (Dash et al., 2007). Experiments using the global histone deacetylase (HDAC) inhibitors trichostatin A and sodium butyrate (Vecsey et al., 2007), as well as genetic disruption of histone acetyltransferase genes (Alarcon et al., 2004), demonstrate clear effects on long-term memory and directly implicate histone acetylation as an important component of memory formation. Roles for Hdac2 (Morris et al., 2013), Hdac3 (McQuown et al., 2011), and HDAC Sirt1 (Michan et al., 2010) in synaptic plasticity, learning, long-term potentiation, and both long- and short-term memory have been identified. Also, studies of animals with genetic manipulations of the G9a (Gupta-Agarwal et al., 2012) and Mll2/Kmt2b genes (Kerimoglu et al., 2013) have firmly defined a role for histone methylation in both learning and memory. A role for HDAC3 has also been identified for addictions, where inhibition of this enzyme enhanced behavior performance in fear memory formation and the extinction of drug- seeking behavior (Malvaez et al., 2013).
DNA methylation has also been associated with memory formation and maintenance. Pharmacological inhibition and genetic knock-out studies of DNA methyltransferases (DNMTs) resulted in memory suppression and impaired memory consolidation, synaptic plasticity, learning, and memory (Lipsky, 2013). Studies of Tet1 knock-out mice demonstrate a key role for DNA demethylation in abnormal memory extinction, but not memory formation (Rudenko et al., 2013). DNA methylation and histone modifications cross-talk extensively during memory maintenance (Miller et al., 2008). The administration of a DNMT inhibitor, which prevented the reinstatement of old memories before extinction, affected not only DNA methylation but also reduced H3 and H4 deacetylation. Similarly, HDAC inhibitors could prevent the reinstatement of past memories when administered before extinction by increasing histone acetylation and reducing DNMT1 expression through the suppression of the ERK kinase signaling pathway.
Drugs of Abuse
Molecular neuroadaptation to addictive substances shares aspects with learning and memory. For both, pairing of cues with outcomes, and in contexts, leads to long-lasting synaptic changes. Neuroadaptaive changes associated with addictive behaviors have been identified in several circuits, including the mesolimbic dopaminergic system, which itself includes the ventral tegmental area, nucleus accumbens (NAc) (Tuesta and Zhang, 2014), hippocampus, amygdala, and medial prefrontal cortex. Although approximately 30% to 70% of vulnerability to different addictive agents is heritable (Goldman et al., 2005), the remainder is due to environmental factors such as psychological stress and social interactions (Renthal and Nestler, 2008). This observation suggests a major role for epigenetic mechanisms due to their environmental sensitivity and long-lasting effects.
Histone modifications (acetylation, phosphorylation, and methylation) have been shown to alter effects of both stimulant and depressive drugs in in vivo models. For example, acute exposure to cocaine, which is known to rapidly induce the immediate early genes c-fos and fosb in the NAc, increases histone H4 acetylation in their promoters. Chronic exposure to cocaine induced Bdnf and Cdk5 H3 promoter acetylation and transcription upregulation. Furthermore, inhibition of HDAC family members using sodium butyrate and trichostatin A or the knock-out of specific histone deacetylases (e.g., Hdac5) potentiates cocaine’s effects while overexpression of Hdac4 attenuates them. HDACs also help mediate neuroadaptations to other drugs of abuse, including opioids and nicotine. In selected alcohol-preferring animal models, inhibition of Hdac2 using siRNA or trichostatin A in the amygdala reduced voluntary alcohol intake was found to normalize deficits in Bdnf-Arc signaling (a pathway associated with anxiety behaviors in alcohol withdrawal) and dendritic spine density in the amygdala upon withdrawal from chronic ethanol exposure (Sakharkar et al., 2014). HDAC activity has also been found to be mechanistically important in other drug models: enhancement of morphine-induced locomotor sensitization and conditioned place preference (Sanchis-Segura et al., 2009) and regulation of nicotine preference (Pastor et al., 2011). Effects of epigenetic modifications in addiction are not unidirectional or simple. Histone methylation can activate or repress gene transcription depending on the specific lysine residue modified and as a result can have both negative and positive effects on drug-associated behaviors. Methylation of H3K9 in the NAc inhibits behavioral responses to cocaine and morphine (Maze et al., 2010), but methylation of H3K4 in the NAc enhances methamphetamine-induced conditioned place preference (Aguilar- Valles et al., 2014).
Along with histone modifications, though less studied, DNA methylation has been implicated in regulation of drug responses. In animal models, a role for DNMTs in response to cocaine was observed. Inhibition of Dnmt3a in the NAc via a pharmacological inhibitor or gene knock-down increased behavioral responses to cocaine and overexpression of Dnmt3a; supplementation with the methyl donor S-adenosylmethionine had the opposite effect. A methylation reader, Methyl-CpG-binding protein-2 (MeCP2), and a key modulator of neural activity-regulated gene expression was found to inhibit methamphetamine reward behavior using specific NAc knock-down and knock-in models (Godino et al., 2015). In contrast, and again showing context specificity of epigenetic effects, chronic cocaine increased MeCP2 expression in dorsal striatum, where local knock-down of the protein inhibited cocaine self-administration (Im et al., 2010).
Candidate gene epigenetic studies in blood and saliva have identified differential methylation associated with drug exposure in a multitude of genes, for example, ALDH1A2 in alcohol dependence (Harlaar et al., 2014), MAOA and MAOB in smoking (Launay et al., 2009), prodynorphin in alcoholism, and OPRM1 in opioid addiction (Andersen et al., 2015). Interestingly, a study conducted in human postmortem brain examined 3 SNPs in the prodynorphin gene that were previously reported to be associated with alcoholism. These SNPs were found to overlap with CpGs, which showed significant hypermethylation in alcoholic subjects carrying the protective genotype vs controls (Taqi et al., 2011). Thus a differential epigenetic signal (i.e., DNA methylation) has been shown to provide an additional level of heterogeneity to the known variation in the genetic code that is associated with alcohol addiction. These results emphasize the important role of DNA methylation, in combination with genetic polymorphisms and histone modifications, to regulate brain responses to drugs of abuse.
Schizophrenia and Bipolar Disorder
Studies of DNA methylation in schizophrenia (SCZ) and bipolar disorder (BD) illustrate progress in relating epigenetic changes to severe psychiatric disorders, but also the complexities of such studies in patients who will have experienced many other exposures with the potential to cause epigenetic changes (Kuratomi et al., 2008). Genome-wide methylome studies found altered DNA methylation of numerous genes implicated in SCZ and BD (Dempster et al., 2011). Aberrant methylation of numerous CpGs was found in genomic DNA from blood of twins with SCZ and BD compared with discordant twins. These differences included hypomethylation of the ST6GALNAC1 promoter, as had also been observed in patients with psychosis (Dempster et al., 2011). While the latter study found DNA hypermethylation of COMT in SCZ (Nohesara et al., 2011), another study provided evidence that methylation of the COMT promoter is affected both by the COMT Val158Met polymorphism and physical activity (Lott et al., 2013). A recent comprehensive EWAS found >4500 differentially methylated CpGs at 3000 genes (Wockner et al., 2014), while another study reported that elevated blood homocysteine levels in SCZ patients are associated with altered methylation at >1000 CpG sites, again implicating many genes (Xu et al., 2015). Another EWAS identified aberrant methylation of genes involved in neuronal differentiation, dopaminergic function, hypoxia, and infection (Castellani et al., 2015), many of which overlap with SNPs in putative SCZ risk loci (Hannon et al., 2016).
Researchers have long sought to tie variations in chromatin structure to SCZ. An increase in arginine-rich histones, reversible by a D2 agonist, was reported in the neutrophils of SCZ patients some 4 decades ago (Issidorides et al., 1975). Transcriptome studies have uncovered abnormal expression of many histone-related genes in SCZ patients. For example, dysregulation of HDAC3 in the temporal cortex and increased expression of HDAC1 have been reported in the frontal cortex of SCZ patients. Expression of HDAC1, HDAC3, and HDAC4 inversely correlates with expression of GAD67 (GAD1), a gene implicated in SCZ pathogenesis. For a recent comprehensive review of all chromatin changes associates with SCZ development, please see (Nestler et al., 2015).
Maternal Effects
Fetal alcohol spectrum disorders (FASD) are caused by maternal alcohol consumption, which alters the developmental trajectory of the fetus via epigenetic pathways. FASD may include facial abnormalities, low birth weight, microcephaly, poor coordination, low intelligence, microophthalmia, and deficits in hearing and vision (Chokroborty-Hoque et al., 2014). Recent studies show that alcohol exposure specifically during early development induces major alterations in gene promoter methylation and histone modification and deregulates noncoding RNAs that are functionally consequential (Kleiber et al., 2014). In an FASD cohort from South Africa, CpG methylation changes in imprinting control regions (e.g., lower KvDMR1 and PEG3 DMR) were found in genomic DNA isolated from peripheral blood (Masemola et al., 2015). Given the wide-ranging effects of alcohol on cellular physiology, many more studies are needed to identify all of the epigenetically regulated developmental pathways that are altered by fetal exposure to alcohol.
Transgenerational Effects
There is some evidence that different types of environmental stimuli can alter the epigenome of the whole brain or related neural circuits, contributing to long-lasting behavioral phenotypes that may be transmitted from parent to offspring via transgenerational mechanisms. For instance, food deprivation in first generation (F0) mice led to decreased serum glucose levels in both male and female offspring (F1), whereas the high-fat diet in male rats (F0) selectively resulted in pancreatic beta-cell dysfunction in female offspring (Anderson et al., 2006). This implies that parental exposures can transgenerationally impact the metabolic function of offspring. Another study examined the offspring of males fed with a low-protein diet. In their offspring, the expression of multiple genes related to lipid and cholesterol metabolism was higher, possibly because of increased methylation of a key lipid regulator gene (Carone et al., 2010). While maternal influence on health of offspring has been long recognized and can be attributed to intrauterine environment, these recent findings suggest that physiologically impactful epigenetic changes can be transmitted through the male germ line. In line with the abovementioned results, malnutrition in F0 pregnancies led to in utero undernourishment of F1 animals, subsequently altering the sperm DNA methylome of F1 adult males (Ng et al., 2010). Collectively, environmental stimuli can impact the sperm methylome even before maturation of the individual and may be due to the epigenetic changes in spermatogonium cells. Accordingly, the spermatogonium acts as a candidate target to prevent such transgenerational inheritance of certain diseased phenotypes. However, the involvement of DNA methylation changes in transgenerational inheritance by sperm has recently been called into question. An exhaustive analysis of cytosine methylation patterns in sperm obtained from mice consuming 1 of 3 diets by whole genome methylation mapping found that “epivariation,” either stochastic or due to unknown demographic or environmental factors, was a far stronger contributor to the sperm methylome than was the diet consumed (Shea et al., 2015).
Recently, Dias BG and Ressler KJ (2014) linked odorant conditioning and DNA hypomethylation of an olfactory receptor gene in mice. This odor sensitivity was shown to be transmitted for 2 generations, but it is unclear how olfactory stimulation could drive molecular events in the sperm. This highlights a general problem for proponents of transgenerational epigenetic behavioral transmission: how can a learned association be epigenetically encoded in the germline of the parent and then transmitted and decoded in the brain of the child?
Conclusion and Future Directions
Epigenetic change is a relatively new frontier of temporally dynamic, reversible, molecular change that can be measured genome wide, revealing mechanisms of genomic control as well as consequences of environmental exposures. Epigenetic influences on gene regulation help mediate response and adaptation to the environment, accounting for part of the liability to psychiatric diseases. Epigenetic mechanisms mediate gene by environment interactions in which certain combinations of inherited variation and exposures are particularly dangerous. They may even account for some nongenetic transgenerational transmission of liability, as is still controversial. Several frequently prescribed medications, including valproate and opioids, and psychotropic drugs that are susceptible to abuse, strongly influence epigenetic mechanisms and their effects are partially mediated thereby. Increasingly, epigenetic effects will be considered, and measured, in the diagnosis of mental disorders and in their treatment, where epigenetic actions and effects of drugs will need to be considered. Although speculative at the moment and challenged by the question of how to target specific genes, pharmacological modification of epigenetic processes holds promise. Compared with diseases such as immunodeficiency, centromere instability, and facial anomalies, RTT, and cancer, where epigenetic changes are widespread and of a large magnitude, it is likely that small changes in specific regions, perhaps at a single histone octamer or single CpG dinucleotide, would be targeted in psychiatric diseases and with effort taken to minimize off-target effects. A deeper understanding of the molecular factors, such as accessory proteins that open, close, and remodel chromatin in a locus-specific manner, will be needed before gene-specific drugs, with few off-target effects, are developed. Equally important is a better understanding of signaling pathways that translate behavioral interventions into molecular events that may reverse negative epigenetic changes associated with disease.
Most epigenetic association studies have utilized peripheral tissue samples such as blood, buccal tissue, or saliva samples. There is evidence for a modest correlation between methylation patterns in blood cells and the brain prefrontal cortex in rodent and rhesus monkey models (Davies et al., 2012) as well as between peripheral methylation and related brain metabolites in human positron emission tomography studies (Shumay et al., 2012). However, the majority of studies in animal model systems or postmortem tissues where blood and brain comparisons have been made show clear gene- and sometimes even CpG-specific methylation patterns, probably reflecting their tissue specificity, making the discovery of mechanistically informative disease-specific DNA methylation changes difficult. New algorithms, aided by the Human Epigenome Project, have identified tissue-specific methylation patterns, and these can be used to “normalize” samples with mixed cellular backgrounds. The development of induced pluripotent stem cells and reliable protocols to differentiate neuronal precursors into diverse neuronal, glial, and astrocytic lineages might seem to open the door to “patient”-specific epigenotyping; however, these cells are themselves derived from some peripheral tissue, such as skin or blood, and have been dedifferentiated. Thus, induced pluripotent stem cells will be more valuable for understanding effects of genotype, and in vitro exposures, and the epigenotype of the brain will remain somewhat a mystery.
Epigenetic changes may predict response to therapy and may be useful as biomarkers to diagnose disease and monitor disease progresssion. Recent data suggest epigenetic patterns such as serotonin transporter methylation status may predict antidepressant pharmacotherapy response (Domschke et al., 2014). Intriguingly, normalization of hypomethylation of the serotonin transporter and MAOA genes correlates with successful psychotherapeutic intervention in anxiety disorders (Roberts et al., 2014; Ziegler et al., 2016). Lithium increases H3K4me3 and acetylation of H3 and H4 at a specific gene promoter region in mouse brain and postmortem brains of patients with BD. The well-known atypical antipsychotic drug, clozapine, could also increase H3K4me in the murine brain. Thus, epigenetic plasticity may constitute a key mechanism of therapeutic interventions in mental disorders, and epigenetic changes may be biomarkers for lasting therapeutic effects, contributing to better monitoring and possibly even prediction of treatment success in a personalized medicine approach.
Although this review focuses on extensively studied epigenetic mechanisms such as chromatin remodeling and DNA methylation, several other mechanisms have been identified that can modify gene expression and cell function, and that can be both cause and consequence of psychiatric disease. For example; At chromosome ends, telomere length is maintained by telomerase, an enzyme that adds copies of a DNA repeat at open telomere ends. Telomere shortening, which ordinarily occurs with aging, is accelerated by stress and may be a biomarker of stress and, at extreme levels, may impair cell function. Extranuclear regulatory RNAs, some packaged in microvesicles known as exosomes, are capable of epigenetically modifying gene expression, possibly even trans-generationally as recently reported (Bale, 2015). Epigenetics continues to expand in new directions, revealing new layers of genomic, cellular, and neural function.
Statement of Interest
None.
Acknowledgments
The present work was supported by intramural funding from the National Institutes of Health, National Institute for Alcohol Abuse and Alcoholism, the German Research Foundation (DFG, SFB-TRR-58, C02 to K.D.), and the German Ministry of Research and Education (BMBF, 01EE1402A, PROTECT-AD, P5 to K.D.). We apologize to those whose work has not been cited due to space constraints.
References
- Aguilar-Valles A, Vaissiere T, Griggs EM, Mikaelsson MA, Takacs IF, Young EJ, Rumbaugh G, Miller CA. (2014. ) Methamphetamine-associated memory is regulated by a writer and an eraser of permissive histone methylation. Biol Psychiatry 76:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. (2004. ) Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42:947–959. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. (1999. ) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23:185–188. [DOI] [PubMed] [Google Scholar]
- Andersen AM, Dogan MV, Beach SR, Philibert RA. (2015. ) Current and future prospects for epigenetic biomarkers of substance use disorders. Genes (Basel) 6:991–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LM, Riffle L, Wilson R, Travlos GS, Lubomirski MS, Alvord WG. (2006. ) Preconceptional fasting of fathers alters serum glucose in offspring of mice. Nutrition 22:327–331. [DOI] [PubMed] [Google Scholar]
- Bale TL. (2015. ) Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci 16:332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton SC, Surani MA, Norris ML. (1984. ) Role of paternal and maternal genomes in mouse development. Nature 311:374–376. [DOI] [PubMed] [Google Scholar]
- Bjornsson HT. (2015. ) The Mendelian disorders of the epigenetic machinery. Genome Res 25:1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, Meissner A, Weng Z, Hofmann HA, Friedman N, Rando OJ. (2010. ) Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143:1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani CA, Laufer BI, Melka MG, Diehl EJ, O’Reilly RL, Singh SM. (2015. ) DNA methylation differences in monozygotic twin pairs discordant for schizophrenia identifies psychosis related genes and networks. BMC Med Genomics 8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokroborty-Hoque A, Alberry B, Singh SM. (2014. ) Exploring the complexity of intellectual disability in fetal alcohol spectrum disorders. Front Pediatr 2:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DW. (1971. ) Directed genetic change model for X chromosome inactivation in eutherian mammals. Nature 230:292–294. [DOI] [PubMed] [Google Scholar]
- Cortijo S, Wardenaar R, Colome-Tatche M, Gilly A, Etcheverry M, Labadie K, Caillieux E, Hospital F, Aury JM, Wincker P, Roudier F, Jansen RC, Colot V, Johannes F. (2014. ) Mapping the epigenetic basis of complex traits. Science 343:1145–1148. [DOI] [PubMed] [Google Scholar]
- Dash PK, Moore AN, Kobori N, Runyan JD. (2007. ) Molecular activity underlying working memory. Learn Mem 14:554–563. [DOI] [PubMed] [Google Scholar]
- Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, Coarfa C, Harris RA, Milosavljevic A, Troakes C, Al-Sarraj S, Dobson R, Schalkwyk LC, Mill J. (2012. ) Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol 13:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MN, Krause L, Bell JT, Gao F, Ward KJ, Wu H, Lu H, Liu Y, Tsai PC, Collier DA, Murphy T, Dempster E, Mill J, Consortium UKBE , Battle A, Mostafavi S, Zhu X, Henders A, Byrne E, Wray NR, Martin NG, Spector TD, Wang J. (2014. ) Hypermethylation in the ZBTB20 gene is associated with major depressive disorder. Genome Biol 15:R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster EL, Pidsley R, Schalkwyk LC, Owens S, Georgiades A, Kane F, Kalidindi S, Picchioni M, Kravariti E, Toulopoulou T, Murray RM, Mill J. (2011. ) Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum Mol Genet 20:4786–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster EL, Wong CC, Lester KJ, Burrage J, Gregory AM, Mill J, Eley TC. (2014. ) Genome-wide methylomic analysis of monozygotic twins discordant for adolescent depression. Biol Psychiatry 76:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ. (2014) Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 17 (1):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Tidow N, Schwarte K, Deckert J, Lesch KP, Arolt V, Zwanzger P, Baune BT. (2014. ) Serotonin transporter gene hypomethylation predicts impaired antidepressant treatment response. Int J Neuropsychopharmacol 17:1167–1176. [DOI] [PubMed] [Google Scholar]
- Duclot F, Kabbaj M. (2015. ) Epigenetic mechanisms underlying the role of brain-derived neurotrophic factor in depression and response to antidepressants. J Exp Biol 218:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan BK, Miller JH. (1980. ) Mutagenic deamination of cytosine residues in DNA. Nature 287:560–561. [DOI] [PubMed] [Google Scholar]
- Fu Y, He C. (2012. ) Nucleic acid modifications with epigenetic significance. Curr Opin Chem Biol 16:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JR, van der Brug MP, Hernandez DG, Traynor BJ, Nalls MA, Lai SL, Arepalli S, Dillman A, Rafferty IP, Troncoso J, Johnson R, Zielke HR, Ferrucci L, Longo DL, Cookson MR, Singleton AB. (2010. ) Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genet 6:e1000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godino A, Jayanthi S, Cadet JL. (2015. ) Epigenetic landscape of amphetamine and methamphetamine addiction in rodents. Epigenetics 10:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. (2005) The genetics of addictions: uncovering the genes. Nat Rev Genet 6 (7):521–532. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. (2005. ) Eukaryotic cytosine methyltransferases. Annu Rev Biochem 74:481–514. [DOI] [PubMed] [Google Scholar]
- Gupta-Agarwal S, Franklin AV, Deramus T, Wheelock M, Davis RL, McMahon LL, Lubin FD. (2012. ) G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation. J Neurosci 32:5440–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon E, Spiers H, Viana J, Pidsley R, Burrage J, Murphy TM, Troakes C, Turecki G, O’Donovan MC, Schalkwyk LC, Bray NJ, Mill J. (2016. ) Methylation QTLs in the developing brain and their enrichment in schizophrenia risk loci. Nat Neurosci 19:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlaar N, Bryan AD, Thayer RE, Karoly HC, Oien N, Hutchison KE. (2014. ) Methylation of a CpG site near the ALDH1A2 gene is associated with loss of control over drinking and related phenotypes. Alcohol Clin Exp Res 38:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. (2006. ) Epigenetics: a historical overview. Epigenetics 1:76–80. [DOI] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. (2010. ) MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issidorides MR, Stefanis CN, Varsou E, Katsorchis T. (1975. ) Altered chromatin ultrastructure in neutrophils of schizophrenics. Nature 258:612–614. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. (2001. ) Translating the histone code. Science 293:1074–1080. [DOI] [PubMed] [Google Scholar]
- Kerimoglu C, Agis-Balboa RC, Kranz A, Stilling R, Bahari-Javan S, Benito-Garagorri E, Halder R, Burkhardt S, Stewart AF, Fischer A. (2013. ) Histone-methyltransferase MLL2 (KMT2B) is required for memory formation in mice. J Neurosci 33:3452–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber ML, Diehl EJ, Laufer BI, Mantha K, Chokroborty-Hoque A, Alberry B, Singh SM. (2014. ) Long-term genomic and epigenomic dysregulation as a consequence of prenatal alcohol exposure: a model for fetal alcohol spectrum disorders. Front Genet 5:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. (2013. ) Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci 16:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Pape J, Binder EB, Mehta D. (2014. ) The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology 80:115–132. [DOI] [PubMed] [Google Scholar]
- Kuratomi G, Iwamoto K, Bundo M, Kusumi I, Kato N, Iwata N, Ozaki N, Kato T. (2008. ) Aberrant DNA methylation associated with bipolar disorder identified from discordant monozygotic twins. Mol Psychiatry 13:429–441. [DOI] [PubMed] [Google Scholar]
- Launay JM, Del Pino M, Chironi G, Callebert J, Peoc’h K, Megnien JL, Mallet J, Simon A, Rendu F. (2009. ) Smoking induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS One 4:e7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky RH. (2013. ) Epigenetic mechanisms regulating learning and long-term memory. Int J Dev Neurosci 31:353–358. [DOI] [PubMed] [Google Scholar]
- Liyanage VR, Jarmasz JS, Murugeshan N, Del Bigio MR, Rastegar M, Davie JR. (2014. ) DNA modifications: function and applications in normal and disease States. Biology (Basel) 3:670–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolak S, Suwannarat P, Lipsky RH. (2014. ) Epigenetics of depression. Prog Mol Biol Transl Sci 128:103–137. [DOI] [PubMed] [Google Scholar]
- Lott SA, Burghardt PR, Burghardt KJ, Bly MJ, Grove TB, Ellingrod VL. (2013. ) The influence of metabolic syndrome, physical activity and genotype on catechol-O-methyl transferase promoter-region methylation in schizophrenia. Pharmacogenomics J 13:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, Rusche JR, Wood MA. (2013. ) HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A 110:2647–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. (2000. ) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711. [DOI] [PubMed] [Google Scholar]
- Masemola ML, van der Merwe L, Lombard Z, Viljoen D, Ramsay M. (2015. ) Reduced DNA methylation at the PEG3 DMR and KvDMR1 loci in children exposed to alcohol in utero: a South African Fetal Alcohol Syndrome cohort study. Front Genet 6:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE, III, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ. (2010. ) Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 327:213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, Wood MA. (2011. ) HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 31:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. (2010. ) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30:9695–9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. (2008. ) DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem 89:599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Mahgoub M, Na ES, Pranav H, Monteggia LM. (2013. ) Loss of histone deacetylase 2 improves working memory and accelerates extinction learning. J Neurosci 33:6401–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Pena CJ, Kundakovic M, Mitchell A, Akbarian S. (2015) Epigenetic basis of mental illness. Neuroscientist. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. (2010. ) Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 467:963–966. [DOI] [PubMed] [Google Scholar]
- Nohesara S, Ghadirivasfi M, Mostafavi S, Eskandari MR, Ahmadkhaniha H, Thiagalingam S, Abdolmaleky HM. (2011. ) DNA hypomethylation of MB-COMT promoter in the DNA derived from saliva in schizophrenia and bipolar disorder. J Psychiatr Res 45:1432–1438. [DOI] [PubMed] [Google Scholar]
- Pastor V, Host L, Zwiller J, Bernabeu R. (2011. ) Histone deacetylase inhibition decreases preference without affecting aversion for nicotine. J Neurochem 116:636–645. [DOI] [PubMed] [Google Scholar]
- Razin A, Cedar H. (1984. ) DNA methylation in eukaryotic cells. Int Rev Cytol 92:159–185. [DOI] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. (2001. ) Epigenetic reprogramming in mammalian development. Science 293:1089–1093. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. (2008. ) Epigenetic mechanisms in drug addiction. Trends Mol Med 14:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rett A. (1966. ) [On a unusual brain atrophy syndrome in hyperammonemia in childhood]. Wien Med Wochenschr 116:723–726. [PubMed] [Google Scholar]
- Roberts S, Lester KJ, Hudson JL, Rapee RM, Creswell C, Cooper PJ, Thirlwall KJ, Coleman JR, Breen G, Wong CC, Eley TC. (2014. ) Serotonin transporter [corrected] methylation and response to cognitive behaviour therapy in children with anxiety disorders. Transl Psychiatry 4:e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, He C. (2016. ) RNA epigenetics-chemical messages for posttranscriptional gene regulation. Curr Opin Chem Biol 30:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen R. (1996. ) Molecular genetics of methylenetetrahydrofolate reductase deficiency. J Inherit Metab Dis 19:589–594. [DOI] [PubMed] [Google Scholar]
- Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, Faull KF, Jaenisch R, Tsai LH. (2013. ) Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron 79:1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, Pandey SC. (2014. ) Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol 17:1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Jancic D, Jimenez-Minchan M, Barco A. (2009. ) Inhibition of cAMP responsive element binding protein in striatal neurons enhances approach and avoidance responses toward morphine--and morphine withdrawal-related cues. Front Behav Neurosci 3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea JM, Serra RW, Carone BR, Shulha HP, Kucukural A, Ziller MJ, Vallaster MP, Gu H, Tapper AR, Gardner PD, Meissner A, Garber M, Rando OJ. (2015. ) Genetic and epigenetic variation, but not diet, shape the sperm methylome. Dev Cell 35:750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumay E, Logan J, Volkow ND, Fowler JS. (2012) Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics 7:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. (2000. ) The language of covalent histone modifications. Nature 403:41–45. [DOI] [PubMed] [Google Scholar]
- Sweder KS, Hanawalt PC. (1993. ) Transcription-coupled DNA repair. Science 262:439–440. [DOI] [PubMed] [Google Scholar]
- Taqi MM, Bazov I, Watanabe H, Sheedy D, Harper C, Alkass K, Druid H, Wentzel P, Nyberg F, Yakovleva T, Bakalkin G. (2011. ) Prodynorphin CpG-SNPs associated with alcohol dependence: elevated methylation in the brain of human alcoholics. Addict Biol 16:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Zhang Y. (2014. ) Mechanisms of epigenetic memory and addiction. EMBO J 33:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Speybroeck L. (2002. ) From epigenesis to epigenetics: the case of C. H. Waddington. Ann N Y Acad Sci 981:61–81. [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. (2007. ) Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27:6128–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. (1952) The epigenetics of birds. Cambridge: Cambridge University Press. [Google Scholar]
- Weaver IC. (2014. ) Integrating early life experience, gene expression, brain development, and emergent phenotypes: unraveling the thread of nature via nurture. Adv Genet 86:277–307. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. (2004. ) Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. (1998. ) Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci U S A 95:7480–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. (2013. ) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382:1575–1586. [DOI] [PubMed] [Google Scholar]
- Wockner LF, Noble EP, Lawford BR, Young RM, Morris CP, Whitehall VL, Voisey J. (2014. ) Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients. Transl Psychiatry 4:e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe AP, Matzke MA. (1999. ) Epigenetics: regulation through repression. Science 286:481–486. [DOI] [PubMed] [Google Scholar]
- Wu C, Morris JR. (2001. ) Genes, genetics, and epigenetics: a correspondence. Science 293:1103–1105. [DOI] [PubMed] [Google Scholar]
- Xu GL, Bestor TH, Bourc’his D, Hsieh CL, Tommerup N, Bugge M, Hulten M, Qu X, Russo JJ, Viegas-Pequignot E. (1999. ) Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature 402:187–191. [DOI] [PubMed] [Google Scholar]
- Xu J, He G, Zhu J, Zhou X, St Clair D, Wang T, Xiang Y, Zhao Q, Xing Q, Liu Y, Wang L, Li Q, He L, Zhao X. (2015. ) Prenatal nutritional deficiency reprogrammed postnatal gene expression in mammal brains: implications for schizophrenia. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Kozikowski AP, Pozzo-Miller L. (2014. ) A selective histone deacetylase-6 inhibitor improves BDNF trafficking in hippocampal neurons from Mecp2 knockout mice: implications for Rett syndrome. Front Cell Neurosci 8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui DH, Xu H, Dunaway KW, Lasalle JM, Jin LW, Maezawa I. (2013. ) MeCP2 modulates gene expression pathways in astrocytes. Mol Autism 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, Binder EB. (2015. ) Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol Psychiatry. Epub ahead of print. doi: 10.1016/j.biopsych.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Yoder JA, Walsh CP, Bestor TH. (1997. ) Cytosine methylation and the ecology of intragenomic parasites. Trends Genet 13:335–340. [DOI] [PubMed] [Google Scholar]
- Ziegler C, Richter J, Mahr M, Gajewska A, Schiele MA, Gehrmann A, Schmidt B, Lesch KP, Lang T, Helbig-Lang S, Pauli P, Kircher T, Reif A, Rief W, Vossbeck-Elsebusch AN, Arolt V, Wittchen HU, Hamm AO, Deckert J, Domschke K. (2016. ) MAOA gene hypomethylation in panic disorder-reversibility of an epigenetic risk pattern by psychotherapy. Transl Psychiatry 6:e773. [DOI] [PMC free article] [PubMed] [Google Scholar]