Abstract
To transfer the preplan for the head and neck brachytherapy to the clinical implantation procedure, a preplan-based 3D-printed individual template for needle insertion guidance had previously been designed and used. The accuracy of needle insertion using this kind template was assessed in vivo. In the study, 25 patients with head and neck tumors were implanted with 125I radioactive seeds under the guidance of the 3D-printed individual template. Patients were divided into four groups based on the site of needle insertion: the parotid and masseter region group (nine patients); the maxillary and paranasal region group (eight patients); the submandibular and upper neck area group (five patients); and the retromandibular region group (six patients). The distance and angular deviations between the preplanned and placed needles were compared, and the complications and time required for needle insertion were assessed. The mean entrance point distance deviation for all 619 needles was 1.18 ± 0.81 mm, varying from 0.857 ± 0.545 to 1.930 ± 0.843 mm at different sites. The mean angular deviation was 2.08 ± 1.07 degrees, varying from 1.85 ± 0.93 to 2.73 ± 1.18 degrees at different sites. All needles were manually inserted to their preplanned positions in a single attempt, and the mean time to insert one needle was 7.5 s. No anatomical complications related to inaccurately placed implants were observed. Using the 3D-printed individual template for the implantation of 125I radioactive seeds in the head and neck region can accurately transfer a CT-based preplan to the brachytherapy needle insertion procedure. Moreover, the addition of individual template guidance can reduce the time required for implantation and minimize the damage to normal tissues.
Keywords: 3D-printed, individual template, brachytherapy, head and neck
INTRODUCTION
Permanent radioactive seed implantation (low-dose-rate brachytherapy) as a kind of radiotherapy has been used to treat different malignant tumors, such as prostate cancer, breast cancer and recurrent head and neck cancer [1–3]. With this technique, radiotherapy is delivered directly to target areas by radioactive seeds implanted with needles (usually 18- or 17-gauge) [4]. Accurate seed placement, which is mainly determined by the trajectory of the needle in the tissue according to a CT-based preplan, is therefore vital for effective conformal radiotherapy and for limiting adverse side effects resulting from damage to adjacent normal tissues. In prostate brachytherapy, image-guidance and template-guidance as two important technical improvements are now classical techniques for implanting radioactive seeds [5–10]. However, use of these techniques in head and neck cancers has not been well studied.
Brachytherapy in the head and neck region presents two specific problems. First, given the proximity of many critical organs and tissues (e.g. bones, sinuses, eyes and major vessels), implantation is more difficult and dangerous in this region than in other regions of the body; thus, a good preplan is needed to decrease needle- and radiation-related damage to these unaffected tissues. Second, the issue of how to accurately transfer this virtual preplan to the implantation procedure, while also limiting seed placement error caused by patient and clinician, presents more unique difficulties. While several image-guidance techniques (such as ultrasound, CT, MRI and navigation) have been used in guiding needle insertion [11–16], it is still difficult to avoid sources of human error leading to seed misplacement, even at the hands of a skilled clinician. Furthermore, every image-guidance technology has its own limitations. While the Syed–Neblett template has been widely used as a guide for brachytherapy [7, 17], it may not be suitable for use in the head and neck region because it may lead to severe deviations due to the motion and complicated structures of the head and neck.
In a study previously published in Journal of Radiation Research, we described the design of a preplan-based 3D-printed individual template for needle insertion guidance that constrains the site and orientation of implantation needles and the transfer of this template to the clinical implantation procedure [18]. In this study, we improved upon this template design and evaluated the accuracy of this technique in vivo.
MATERIALS AND METHODS
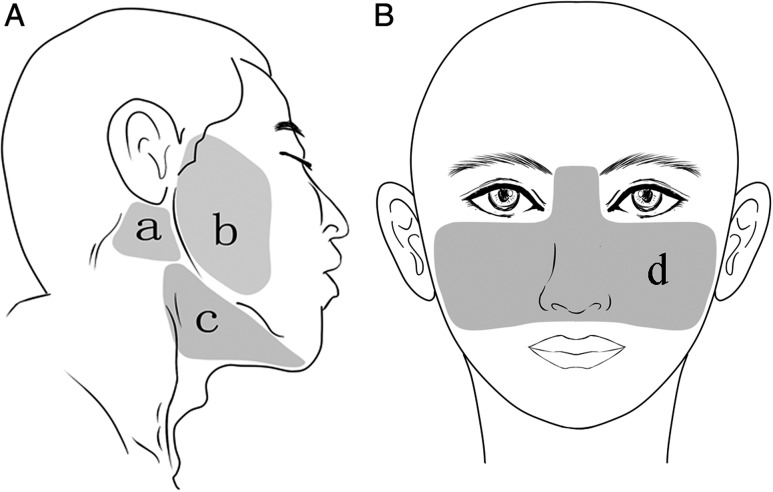
This study included 25 patients with recurrent and local advanced malignant head and neck tumors who received 3D-printed individual template–guided brachytherapy at the Peking University School and Hospital of Stomatology. The patients were divided into four groups, based on the site of needle insertion at the skin: the parotid and masseter region group (nine patients); the maxillary and paranasal region group (eight patients); the submandibular and upper neck area group (five patients); and the retromandibular region group (six patients) (Fig. 1A and B). The pathology of the patients included adenoid cystic carcinoma (10 cases), mucoepidermoid carcinoma (5 cases), squamous cell carcinoma (2 cases), myoepithelial carcinoma (2 cases), epithelial myoepithelial carcinoma (2 cases), non-specific adenocarcinoma (2 cases), acinar cell carcinoma (1 case) and oncocytic carcinoma (1 case). The planned dose (PD) was 80–120 Gy for recurrent tumors that had previously received radiotherapy and 120–160 Gy for tumors without prior radiotherapy. This study was approved by the Ethics Committee of the Peking University School and Hospital of Stomatology.
Fig. 1.
Regional distribution of needle insertion sites included in the study: (a) retromandibular region; (b) parotid and masseter region; (c) the submandibular and upper neck area; (d) maxillary and paranasal region.
Preplanning procedure
Each patient initially underwent a CT examination (Siemens AG, Munich, Germany, at 120 kV and 150 mA, with a slice thickness of 0.75 mm) for preplanning. To minimize patients’ movement during the examination and needle insertion, patients were immobilized in the same posture for both procedures, using an external fixator (vacuum pad). CT data was imported into a brachytherapy treatment planning system (BTPS; Beijing Astro Technology Ltd Co., Beijing, China), which was used to design a preplan to implant the 125I radioactive seeds (length 4.5 mm, diameter 0.8 mm; model 6711, China Institute of Atomic Energy). In the preplan, the needle entrance point, orientation of the needle pathway, depth of the needles and distribution of needles were determined. Correct delivery of the determined dose to the target area and minimal trauma to normal tissues were the primary points of focus in the preplan design.
Individual template design
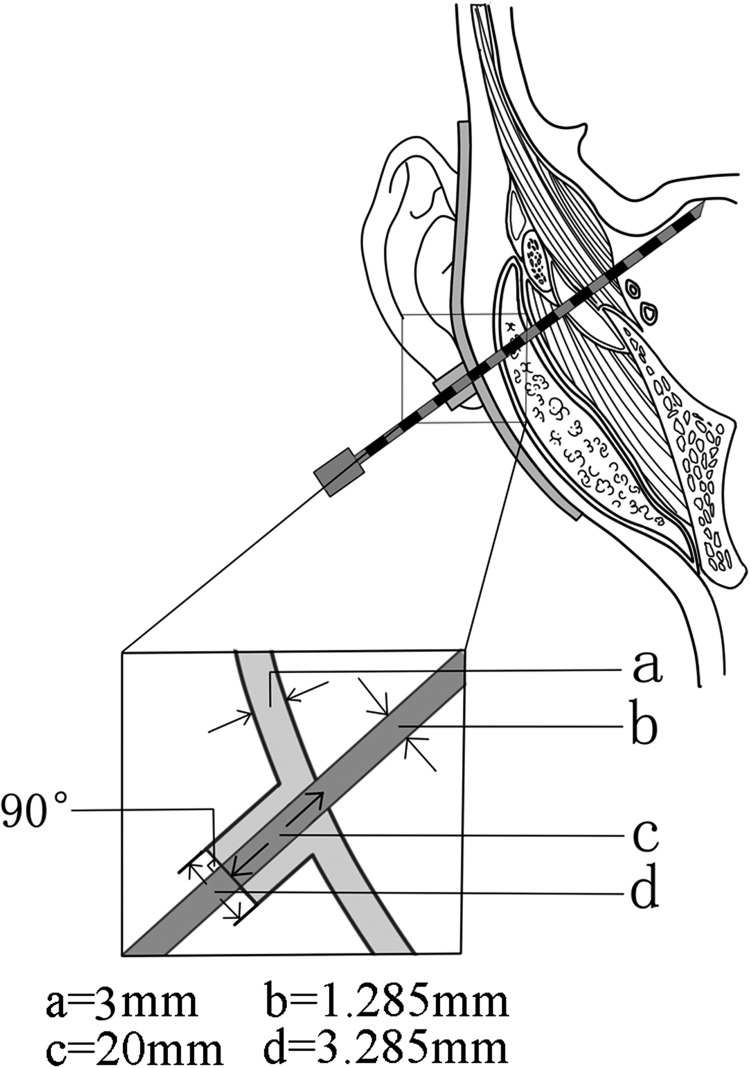
In the previously published study, we described the procedure of making the individual template [18]. In this study, the original template design process was used, albeit with a few key modifications. On the premise of ensuring the template strength, the thickness of the template was decreased to 0.3 cm to save printing materials and time. Cylinders, representing the needle pathways, with a longer fixed height of 2 cm were designed at the needle entrance points on the skin to constrain the site and direction of the needles. The cylinder (outside diameter 3.285 mm) was designed with a hole inside (inner diameter 1.285 mm), allowing for improved orientation of needle guidance during insertion (Fig. 2). The external surface of the cylinder was designed to be perpendicular to the needle path (Fig. 2). During implantation, the needle depth in the tissue was calculated from the needle scale (at the external surface of the cylinder) minus 2 cm for the cylinder (Fig. 2). Using this method, the individual template can also be used to help control and confirm needle depth. According to the design, the individual template was constructed from medial light-cured resin using the rapid forming machine Eden250 (Objet Company, Israel).
Fig. 2.
Schematic diagram of 3D-printed individual template shows that a needle avoiding the obstruction of the zygomatic arch and arriving in the skull base area: (a) thickness of the template; (b) inner diameter of the cylinder for needle guidance; (c) height of the guiding cylinder for needle guidance; (d) outside diameter of the cylinder for needle guidance.
Individual template–guided implantation
With the patient under general anesthesia, the individual template was placed at the preplanned site, and needles (18-gauge × 150 mm; MTP-1820-C, Hakko Co., Ltd, Japan) were manually inserted through the template to the target area (Fig. 3). After all of the needles had been inserted, an intermediate CT scan was acquired to confirm the sites of placed needles (Fig. 4). Under the strict direction of the preplan, 125I seeds were then placed into the target area through the needles.
Fig. 3.
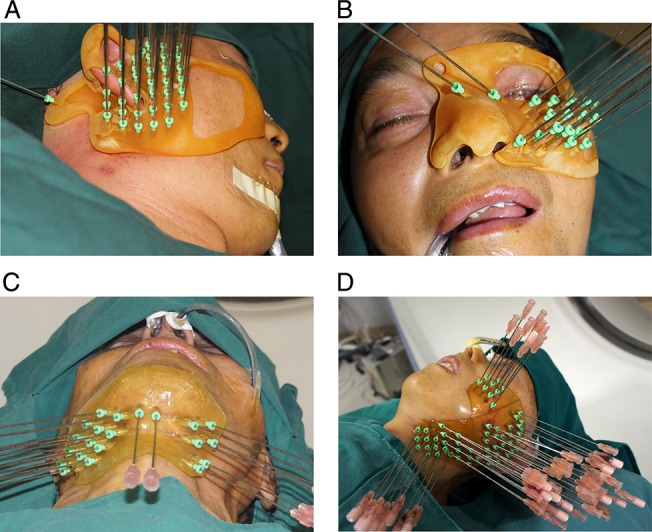
Needle implantation guided by 3D-printed individual template in different regions.
Fig. 4.
(A) Preplanned needle pathway and radioactive seeds; (B) intermediate CT scan acquired to confirm actual needle placement sites; (C) CT scan shows the implanted radioactive seeds.
Accuracy measurement
To evaluate the accuracy of needle positioning, the placed needle position determined by the intermediate CT dataset was compared with the preplanned needle position in the pre-implant CT dataset. The coordinates of the placed and preplanned needles were defined in reference to the same bony landmarks to maintain a uniform coordinate system for comparison. Specifically, deviation from the preplan in terms of entrance point site (distance deviation) and insertion angle (angular deviation) at the skin surface following needle placement were calculated by Mimics 10.01 software for Windows (Materialise, Belgium) and Geomagic software (3D Systems, USA) (Figs 5 and 6). In the calculate system, the needles were virtualized as straight lines according to the longitudinal centerline of needles to measure the distance and angular deviation. The amount of time required for needle insertion was also recorded.
Fig. 5.

(A and B) Distance deviations between the planned and the placed needles at the entrance point.
Fig. 6.
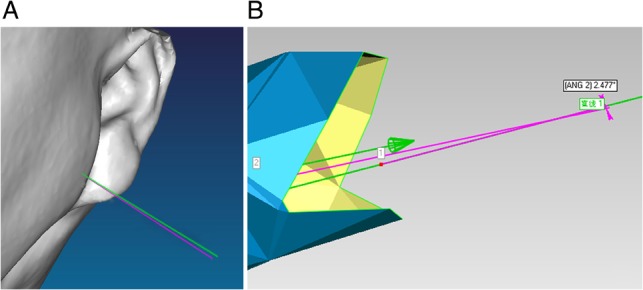
(A and B) Angular deviations between the planned and the placed needles at the entrance point. (The green line represents the preplanned needle; the red line represents the implanted needle).
Evaluation of postplan
A postplan, which was made by the BTPS after implantation of the seeds, was used to evaluate the D90 (dose delivered to 90% of the target volume), V100 (the percentage of the target volume receiving at least 100% of the planned dose) and V150 (the percentage of the target volume receiving at least 150% of the planned dose) in all patients.
Statistical analysis
All continuous numerical variables were presented as mean values ± standard deviations with ranges. Statistical analysis was carried out using SPSS 13.0 (SPSS Inc., Chicago, IL, USA) for Windows. The influence of the implant region on the distance and angular deviation of needles was assessed using a one-way ANOVA test. A two-sided P < 0.05 was considered significant.
RESULTS
A total of 619 needles were inserted, with an average needle depth of 40.20 ± 14.29 mm (range 10–80 mm). The average needle depth at each of the four distinct regions was as follows: parotid region, 30.63 ± 9.17 mm (range 10–50 mm); maxillary and paranasal regions, 43.65 ± 13.58 mm (range 10–80 mm); floor of the mouth, 46.80 ± 11.84 mm (range 20–65 mm); and the retromandibular region, 62.59 ± 10.06 mm (range 40–75 mm). All needles arrived at the preplanned position in one attempt manually, and the average time needed for insertion of a single needle was 7.5 s. No abnormal hemorrhages or anatomical complications related to inaccurately placed implants were observed.
A comparison of the distance and angular deviation results for different sites between the preplanned and placed needles is shown in Table 1. The total mean distance and angular deviations were 1.18 ± 0.81 mm and 2.08 ± 1.07 degrees, respectively. The distance deviation was significantly smaller (indicating more accurate placement) in the parotid and maxillary regions, and the angular deviation was significantly larger (indicating less accurate placement) in the submandibular and upper neck area, than in the other regions (P < 0.05).
Table 1.
Distance and angular deviation of needles at various sites
| Site | n | Distance deviation (mm) | Angular deviation (º) | ||||
|---|---|---|---|---|---|---|---|
| Range | Mean | SD | Range | Mean | SD | ||
| a | 29 | 0.307–3.724 | 1.811 | 0.846 | 0.5–5.56 | 2.52 | 1.36 |
| b | 239 | 0.053–2.569 | 0.857 | 0.545 | 0.1–4.3 | 1.85 | 0.93 |
| c | 136 | 0.459–4.035 | 1.930 | 0.843 | 0.3–5.98 | 2.73 | 1.18 |
| d | 215 | 0.175–2.692 | 0.982 | 0.678 | 0.2–4.4 | 1.87 | 0.90 |
a = retromandibular region, b = parotid and masseter region, c = submandibular and upper neck, d = maxillary and parasinus region, n = number of needles.
In the postplan for each patient after implantation of the seeds, the D90 was larger than the PD and ranged from 122 Gy to 198 Gy (mean 163.8 ± 22.6 Gy) . The V100 was larger than 95% and the V150 was less than 50% in all patients.
DISCUSSION
The use of templates is an important technical improvement in brachytherapy, evident by its promising use in prostate brachytherapy [19], that plays an important role in the guidance of needle insertion today [5–7, 10]. Templates are typically used to constrain the orientation of needles, to space needles evenly during insertion and to limit the influence of human error [7, 17]. Indeed, experiments using freehand techniques resulted in high failure rates due to poor dose distribution [19]. In this study, we introduced the design and use of a 3D-printed individual template to simultaneously guide the position and orientation of brachytherapy needles according to a CT-based preplan and to space the needles accordingly. The in vivo results presented are considered to be clinically acceptable, which is the basis of accurate radioactive seed placement.
Individual template advantages
As we have previously reported, the use of individual templates has certain advantages [18]. An individual template provides preplanned data in a convenient and easy-to-handle format without forfeiting the preoperatively achieved accuracy, which is the most important aspect of preplan to intra-operative realization [14, 20–22]. Moreover, 3D body surface information as well as needle position and angle are captured in the individual template, so there is no need to redefine each of the pathways during insertion; quick, accurate, single-attempt insertion allows for minimal invasiveness and operation time, reducing patient morbidity. By combining the use of the individual template with needle depth control, needles can be better aimed, and the distance from the needle tip to the target area can be more accurately ascertained (Fig. 2).
The pathway of the placed needle is determined by the entrance site and the angle of the needle. Because the radioactive seeds are delivered through the needles, placing the needles in the planned positions is the basis of ensuring the seeds are implanted at the planned sites. In this study, by using the 3D individual template to constrain the entrance site and angle of each needle delivering the 125I seeds, good dose distribution was achieved in each patient.
Because the diameter of the template holes can be adjusted, we can also use the template for more than just normal soft tissue needle insertion. For example, tumors in some sites are enveloped by bone (as in the paranasal sinus and skull base regions) and cannot be reached by normal insertion methods. In this study, the template was used to guide an air drill (bur diameter ~1.45 mm) through bone, based on the preplanned pathway, and then the brachytherapy needle was inserted through the drilled hole. Using this method, the implantation procedure can be simplified, and a more accurate seeding and dose distribution can be achieved. Moreover, the individual template can be used for other needle-based procedures, such as needle aspiration biopsy of deep tissues and deep abscess punctures.
Preplanning system
Use of a BTPS has been an important part of modern brachytherapy [23]. A precise preplan prior to implantation surgery is necessary in order to identify vital structures and to ensure a predictable dose distribution. In the BTPS, needle insertion pathways, the planned target volume (PTV) and doses can be planned on preoperative images (CT or MRI) prior to the needle insertion procedure. This feature gives oncologists enough time to optimize needle pathway entrance points and orientations, minimizing the risk of harming surrounding vital structures, and to plan optimal dose distribution fields in the target area [2]. Because of the many closely oriented vital structures in the head and neck region, preplanning is a beneficial approach for head and neck brachytherapy; in this study, needles were inserted using various orientations to avoid damage to vital structures (Fig. 3).
On the other hand, use of a BTPS also has some inherent disadvantages, such as alterations in patient position, set-up and organ volumes and shapes between designing the preplan and the implantation procedure [1, 24, 25]. In this study, these potential errors were minimized to some extent by external fixation of the patient.
Considerations for reducing errors
Several papers have written regarding the accuracy of needle insertion in brachytherapy. For in vitro tests of brachytherapy techniques, materials such as polystyrene, polyvinylchloride and agar are commonly used to simulate the soft tissues of the human body [1, 26]; however, these materials cannot accurately simulate the complicated structure of the head and neck region, and in vivo data of brachytherapy in this region is very limited. Other clinical studies have revealed that targeting error (needle misplacement) may be caused by imaging limitations, image misalignments, target uncertainty, human error, target movement due to tissue deformation, or needle deflection [1, 27–35]. Notably, McGill et al. reported that reducing the distance the needle travels between the template and the patient's body surface also reduces needle deflection within the tissue [4]. In consideration of the last point, the individual template used in this study was designed to lie on the surface of the head and neck to limit needle deflection. Additionally, to limit the influence of soft tissue deformation on the accuracy of needle insertion and to minimize set-up error during CT data recording and needle insertion, external fixation of the patients was used. Overall, the results showed that the template allowed for easy alignment and insertion of the brachytherapy needles with high targeting accuracy according to the preplan.
This study further demonstrates that 3D-printed individual templates can effectively constrain the positioning and direction of needles during brachytherapy and achieve effective and accurate transfer of a preplan to clinical application. Use of this template design method could also improve upon other needle-based diagnostic and therapeutic procedures (e.g. deep tissue biopsy). Future studies are planned combining real-time visualization and high-precision imaging techniques with individual templates to further increase the accuracy of needle insertion.
FUNDING
This work was supported by the New Technology and Therapy Fund of the Peking University School and Hospital of Stomatology [2010].
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGEMENTS
The authors thank Yi-Jiao Zhao (Peking University School and Hospital of Stomatology) and Yan-Sheng Li (School of Mechanical Engineering and Applied Electronics Technology, Beijing University of Technology) for valuable discussion. Part of this work was presented by Ming-Wei Huang (the first author) at the 2015 Annual Meeting of the American Brachytherapy Society.
REFERENCES
- 1.Krempien R, Hassfeld S, Kozak J, et al. . Frameless image guidance improves accuracy in three-dimensional interstitial brachytherapy needle placement. Int J Radiat Oncol Biol Phys 2004;60:1645–51. [DOI] [PubMed] [Google Scholar]
- 2.Pappas IP, Ryan P, Cossmann P, et al. . Improved targeting device and computer navigation for accurate placement of brachytherapy needles. Med Phys 2005;32:1796–801. [DOI] [PubMed] [Google Scholar]
- 3.Strassmann G, Heyd R, Cabillic-Engenhart R, et al. . Accuracy of 3-D needle navigation in interstitial brachytherapy in various body regions. Strahlenther Onkol 2002;178:644–7; discussion 648-9; author reply 650. [DOI] [PubMed] [Google Scholar]
- 4.McGill CS, Schwartz JA, Moore JZ, et al. . Precision grid and hand motion for accurate needle insertion in brachytherapy. Med Phys 2011;38:4749–59. [DOI] [PubMed] [Google Scholar]
- 5.Budrukkar AN, Shrivastava SK, Jalali R, et al. . Transperineal low-dose rate iridium-192 interstitial brachytherapy in cervical carcinoma stage IIB. Strahlenthe Onkol 2001;177:517–24. [DOI] [PubMed] [Google Scholar]
- 6.Corn BW, Lanciano RM, Rosenblum N, et al. . Improved treatment planning for the Syed–Neblett template using endorectal-coil magnetic resonance and intraoperative (laparotomy/laparoscopy) guidance: a new integrated technique for hysterectomized women with vaginal tumors. Gynecol Oncol 1995;56:255–61. [DOI] [PubMed] [Google Scholar]
- 7.Paley P, Koh W, Stelzer K, et al. . A new technique for performing Syed template interstitial implants for anterior vaginal tumors using an open retropubic approach. Gynecol Oncol 1999;73:121–5. [DOI] [PubMed] [Google Scholar]
- 8.Strassmann G, Kolotas C, Heyd R, et al. . Navigation system for interstitial brachytherapy. Radiother Oncol 2000;56:49–57. [DOI] [PubMed] [Google Scholar]
- 9.Strassmann G, Walter S, Kolotas C, et al. . Reconstruction and navigation system for intraoperative brachytherapy using the flab technique for colorectal tumor bed irradiation. Int J Radiat Oncol Biol Phys 2000;47:1323–9. [DOI] [PubMed] [Google Scholar]
- 10.Syed A, Feder B.. Technique of after-loading interstitial implants. Radiol Clin (Basel) 1977;46:458–75. [PubMed] [Google Scholar]
- 11.Auer T, Hensler E, Eichberger P, et al. . 3D navigation for interstitial stereotaxic brachytherapy. Strahlenther Onkol 1998;174:82–7. [DOI] [PubMed] [Google Scholar]
- 12.Dolezel M, Odrazka K, Zizka J, et al. . MRI-based Preplanning Using CT and MRI Data Fusion in Patients With Cervical Cancer Treated With 3D-based Brachytherapy: Feasibility and Accuracy Study. Int J Radiat Oncol Biol Phys 2012;84:146–52. [DOI] [PubMed] [Google Scholar]
- 13.Kolotas C, Birn G, Baltas D, et al. . CT guided interstitial high dose rate brachytherapy for recurrent malignant gliomas. Br J Radiol 1999;72:805–8. [DOI] [PubMed] [Google Scholar]
- 14.Krempien R, Hoppe H, Kahrs L, et al. . Projector-based augmented reality for intuitive intraoperative guidance in image-guided 3D interstitial brachytherapy. Int J Radiat Oncol Biol Phys 2008;70:944–52. [DOI] [PubMed] [Google Scholar]
- 15.Trejos AL, Lin AW, Pytel MP, et al. . Robot-assisted minimally invasive lung brachytherapy. Int J Med Robot 2007;3:41–51. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K, Shimizutani K, Tanaka E, et al. . Ultrasonographic monitoring of high dose rate interstitial implant using template technique for oral tongue cancer. Radiat Med 1999;17:337–41. [PubMed] [Google Scholar]
- 17.Nag S, Martínez-Monge R, Ellis R, et al. . The use of fluoroscopy to guide needle placement in interstitial gynecological brachytherapy. Int J Radiat Oncol Biol Phys 1998;40:415–20. [DOI] [PubMed] [Google Scholar]
- 18.Huang MW, Liu SM, Zheng L, et al. . A digital model individual template and CT-guided 125I seed implants for malignant tumors of the head and neck. J Radiat Res 2012;53:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath R, Roberts K, Ng M, et al. . Correlation of medical dosimetry quality indicators to the local tumor control in patients with prostate cancer treated with iodine-125 interstitial implants. Med Phys 1998;25:2293–307. [DOI] [PubMed] [Google Scholar]
- 20.Fitzpatrick JM, West JB.. The distribution of target registration error in rigid-body point-based registration. IEEE Trans Med Imaging 2001;20:917–27. [DOI] [PubMed] [Google Scholar]
- 21.Jolesz FA. Image-guided procedures and the operating room of the future. Radiology 1997;204:601–12. [DOI] [PubMed] [Google Scholar]
- 22.Peters TM. Image-guidance for surgical procedures. Phys Med Biol 2006;51:R505–40. [DOI] [PubMed] [Google Scholar]
- 23.Kirisits C, Siebert FA, Baltas D, et al. . Accuracy of volume and DVH parameters determined with different brachytherapy treatment planning systems. Radiother Oncol 2007;84:290–7. [DOI] [PubMed] [Google Scholar]
- 24.Nag S, Beyer D, Friedland J, et al. . American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys 1999;44:789–99. [DOI] [PubMed] [Google Scholar]
- 25.Zelefsky MJ, Yamada Y, Cohen G, et al. . Postimplantation dosimetric analysis of permanent transperineal prostate implantation: improved dose distributions with an intraoperative computer-optimized conformal planning technique. Int J Radiat Oncol Biol Phys 2000;48:601–8. [DOI] [PubMed] [Google Scholar]
- 26.McGill CS, Schwartz JA, Moore JZ, et al. . Effects of insertion speed and trocar stiffness on the accuracy of needle position for brachytherapy. Med Phys 2012;39:1811–7. [DOI] [PubMed] [Google Scholar]
- 27.Abolhassani N, Patel R, Moallem M.. Needle insertion into soft tissue: a survey. Med Eng Phys 2007;29:413–31. [DOI] [PubMed] [Google Scholar]
- 28.Carr JJ, Hemler PF, Halford PW, et al. . Stereotactic localization of breast lesions: how it works and methods to improve accuracy. Radiographics 2001;21:463–73. [DOI] [PubMed] [Google Scholar]
- 29.Hussain HK, Kingston JE, Domizio P, et al. . Imaging-guided core biopsy for the diagnosis of malignant tumors in pediatric patients. Am J Roentgenol 2001;176:43–7. [DOI] [PubMed] [Google Scholar]
- 30.Lüdemann L, Wybranski C, Seidensticker M, et al. . In vivo assessment of catheter positioning accuracy and prolonged irradiation time on liver tolerance dose after single-fraction 192Ir high-dose-rate brachytherapy. Radiat Oncol 2011;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narayana V, Roberson PL, Winfield RJ, et al. . Optimal placement of radioisotopes for permanent prostate implants. Radiology 1996;199:457–60. [DOI] [PubMed] [Google Scholar]
- 32.Podder T, Clark D, Sherman J, et al. . Vivo motion and force measurement of surgical needle intervention during prostate brachytherapy. Med Phys 2006;33:2915–22. [DOI] [PubMed] [Google Scholar]
- 33.Roberson PL, Narayana V, McShan DL, et al. . Source placement error for permanent implant of the prostate. Med Phys 1997;24:251–7. [DOI] [PubMed] [Google Scholar]
- 34.Taschereau R, Pouliot J, Roy J, et al. . Seed misplacement and stabilizing needles in transperineal permanent prostate implants. Radiother Oncol 2000;55:59–63. [DOI] [PubMed] [Google Scholar]
- 35.Wan G, Wei Z, Gardi L, et al. . Brachytherapy needle deflection evaluation and correction. Med Phys 2005;32:902–9. [DOI] [PubMed] [Google Scholar]