Abstract
The purpose of this study is to evaluate dose–volume histogram (DVH) predictors for the development of chronic gastrointestinal (GI) complications in patients with cervical cancer who have undergone postoperative concurrent chemotherapy and whole-pelvic radiation therapy (WPRT). The subjects were 135 patients who had undergone postoperative WPRT with concurrent nedaplatin-based chemotherapy between 2000 and 2014. Associations between selected DVH parameters and the incidence of chronic GI complications of G3 or higher were evaluated. Chronic GI complications of severity G3 occurred in 18 (13%) patients. Patients with GI complications had significantly greater V5–V45, mean dose and the generalized equivalent uniform dose (gEUD) of the small bowel loops, compared with those without GI complications. V30–V45, mean dose and gEUD of the bowel bag also showed significant differences between patients with and without GI complications. In contrast, no parameter for the large bowel loop was correlated with GI complications. Receiver operating characteristics curve analysis indicated that V30–V45 of the small bowel loops were better predictors than these respective parameters for the bowel bag. Next, patients were divided into four groups based on the median V15 and V40 of the small bowel loops. The group with both a high V15 and a high V40 showed a significantly higher probability of chronic GI complications. In conclusion, the small bowel loops are better predictors of chronic GI complications compared with the bowel bag, and a relatively high-dose volume (e.g. V40) of the small bowel loops is a useful predictor of chronic GI complications.
Keywords: cervical cancer, chronic complication, dose–volume histogram, gastrointestinal, postoperative, radiotherapy
INTRODUCTION
Postoperative whole-pelvic radiation therapy (WPRT) with or without chemotherapy is recommended in patients after radical hysterectomy for uterine cervical cancer with high- or intermediate-risk clinicopathological factors. However, patients who have received WPRT after radical hysterectomy may suffer chronic gastrointestinal (GI) complications. The small bowel is a critical organ in these complications, and we previously found that dose–volume histogram (DVH) parameters of the small bowel loops are predictive for chronic GI complications in conventional 2D radiation therapy (2DRT) or 3D conformal radiation therapy (3DCRT) given concurrently with nedaplatin [1].
Based on these results, since October 2010 we have used intensity-modulated radiation therapy (IMRT) as postoperative WPRT for uterine cervical cancer [2]. Since publication of our DVH findings, several other reports have shown a correlation between DVH parameters and chronic GI complications in patients with postoperative cervical cancer [3, 4]. However, several questions remain. First, it is unclear which of the small bowel loops or the bowel bag is a better predictor of chronic GI complications. Second, there is inconsistency with regard to the optimal parameters (high-dose or low-dose) for chronic GI complications. Therefore, we evaluated the optimal organs (small bowel loops or bowel bag) and optimal parameters (high-dose or low-dose) for prediction of chronic GI complications. We hypothesized that differences in optimal parameters may be partially due to variation in radiation therapy (RT) planning (because DVH parameters differ considerably among planning methods) and that clearer results may be obtained using data from a variety of DVH profiles, including IMRT. Therefore, we evaluated DVH predictors for the development of chronic GI complications in patients with cervical cancer who had undergone postoperative concurrent chemotherapy and WPRT with RT planning, including 2DRT, 3DCRT and IMRT.
MATERIALS AND METHODS
Patients
The study was performed as a retrospective chart review and was approved by our institutional review board. A total of 196 patients with clinical IB1–IIB uterine cervical cancer underwent open radical hysterectomy and postoperative RT at our institute between April 2000 (when we started to use concurrent nedaplatin-based chemotherapy) and June 2014. Postoperative RT is indicated when a pathological report includes one of the following high-risk prognostic factors: parametrial invasion, pelvic node metastasis, and a positive surgical margin; or one of the following intermediate-risk prognostic factors: deep stromal invasion, lymphovascular invasion, and a large tumor (>4 cm in diameter). Sixty-one patients were excluded from the study: 18 who received extended-field RT alone because of multiple lymph node metastases [5], 19 who underwent clinical trials of WPRT with concurrent carboplatin and paclitaxel [6], 13 who refused concurrent chemotherapy, 3 who received intracavitary brachytherapy with WPRT because of a close surgical margin, 4 who were lost to follow-up within six months, and 4 early patients who did not undergo treatment-planning computed tomography in the 2DRT era. The remaining 135 patients were analyzed in this study and had a minimum follow-up period of 6 months.
Radiation therapy and chemotherapy
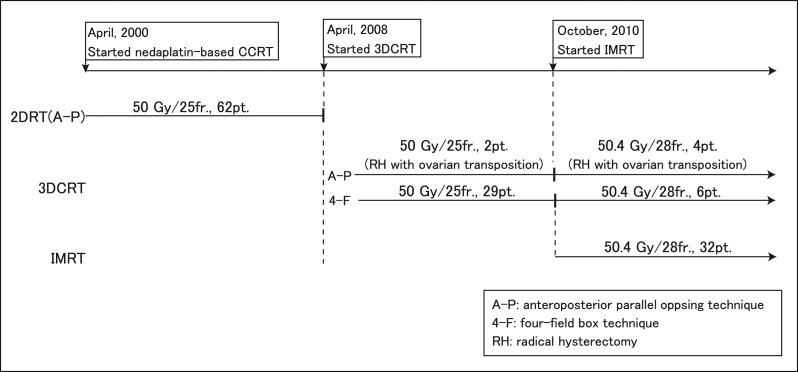
Changes in treatment planning for postoperative concurrent nedaplatin-based chemotherapy and WPRT at our institution are shown in Fig. 1. Briefly, WPRT was delivered with 3DCRT planning starting in April 2008 and with IMRT planning starting in October 2010. 2DRT planning was delivered with an anteroposterior parallel opposing technique and 3DCRT planning with a four-field box technique (0, 90, 180 and 270°), except for patients with ovarian transposition. The prescribed radiation doses were 50 Gy in 25 fractions before October 2010. After IMRT was started in October 2010, the prescribed radiation dose was 50.4 Gy in 28 fractions. The organs at risk that were contoured comprised the small bowel loops, large bowel loop and bowel bag. Details of RT and contouring of bowels have been described elsewhere [1, 2]. Nedaplatin (40 mg/m2) was given intravenously on a weekly basis for 5–6 weeks during the course of WPRT.
Fig. 1.
Changes in radiation treatment planning for postoperative nedaplatin-based CCRT at our institute.
Evaluation of gastrointestinal complications
Patients were followed up by gynecologic and radiation oncologists on an outpatient basis every 2 months in Year 1 after treatment, every 3 months in Year 2, every 4 months in Year 3, and every 6 months in Years 4–5. Follow-up after Year 5 was done on an individual basis. A chronic complication was defined as a GI event that occurred >3 months after RT was started. The severity of a chronic GI complication was classified according to Common Terminology Criteria for Adverse Events ver. 4.0. Toxicity data, including the grade of each GI complication, were collected retrospectively from hospitalization and follow-up records.
Statistical analysis
Associations between selected DVH parameters and the incidence of chronic GI complications of G3 or higher were evaluated. Relationships between clinical or DVH parameters and the incidence of chronic GI complications were analyzed by the Mann–Whitney U test for quantitative variables and the Fisher exact test for categorical variables. The mean DVH parameters for the small bowel loops, large bowel loop, and bowel bag of patients with and without G3 or higher GI complications were compared by the Mann–Whitney U test. The generalized equivalent uniform dose (gEUD), as proposed by Niemierko [7], represents the homogeneous dose distribution that results in the same probability of complications as that of an inhomogeneous dose distribution. The gEUD was calculated as follows:
where n is a parameter that describes the volumetric dependence of the dose–response relationship for each organ. When n = 1, the gEUD is equal to the mean dose, and a lower n value indicates stronger high-dose sensitivity. The value of n of 0.15 for the bowels was taken from Burmen et al. [8].
Receiver operating characteristics (ROC) curve analysis of each DVH parameter was performed to select the most relevant threshold for prediction of chronic GI complications of G3 or higher. The predictive value of a parameter was evaluated based on the area under the ROC curve (AUC). The actuarial incidence of GI complications was calculated with the Kaplan–Meier method, and differences between groups were compared by log-rank test. The predictive value of DVH parameters (V40 or gEUD of the small bowel loops) for GI complications was evaluated in a binary logistic regression model. Development of GI complications was used as the quantal endpoint. All statistical analyses were performed with R ver. 3.2.0 (The R Foundation for Statistical Computing, Vienna, Austria). A P value <0.05 or a 95% confidence interval not encompassing 1 were considered to be significant. All statistical tests were 2-sided.
RESULTS
The characteristics of the 135 patients are shown in Table 1. The median follow-up period from the start of RT was 55 months (range 6–145 months) for all patients, 74 months (range 8–145 months) for 2DRT, 59 months (range 7–80 months) for 3DCRT, and 31 months (range 6–51 months) for IMRT. The Eastern Cooperative Oncology Group performance status was 0–1 for all patients. Chronic GI complications of severity G0–2 and G3 occurred in 117 (87%) and 18 (13%) patients, respectively. There were no G4–5 complications. All G3 complications were enterocolitis, obstruction or both. The median time from the start of RT to onset of G3 chronic GI complications was 13 months (range 3–109 months), and 83% of G3 complications occurred within 31 months.
Table 1.
Patient and treatment characteristics
| No. (%) | |
|---|---|
| Age (years) | |
| Mean | 49 |
| SD | ±11 |
| BMI (kg/m2) | |
| Mean | 21.4 |
| SD | ±3.6 |
| Smoking | |
| None | 98 (73) |
| Yes | 37 (27) |
| Diabetes | |
| None | 130 (96) |
| Yes | 5 (4) |
| RT total dose (Gy) | |
| ≥50 | 128 (95) |
| <50 | 7 (5) |
| Total nedaplatin (mg) | |
| Mean | 278 |
| SD | ±57 |
| pT-stage | |
| T1 | 83 (61) |
| T2 | 52 (39) |
| pN-stage | |
| N0 | 98 (73) |
| N1 | 37 (27) |
SD = standard deviation, BMI = body mass index, RT = radiation therapy.
The incidence of G3 chronic GI complications was analyzed as a function of clinical factors. The results of univariate analysis are shown in Table 2. Low body mass index (≤21) was significantly associated with GI complications. Smoking habit also tended to be higher in patients with GI complications, but without significance.
Table 2.
Univariate analysis (Mann–Whitney U test and Fisher exact test) of development of Grade 3 chronic GI complications
| Variable | Grade 0–2 | Grade 3 | P value | Odds ratio |
|---|---|---|---|---|
| No. | No. | |||
| Age (years) | ||||
| ≤50 | 60 | 11 | 0.44 | 0.67 |
| >50 | 57 | 7 | ||
| Total nedaplatin (mg) | ||||
| ≤290 | 59 | 9 | 0.97 | 1.02 |
| >290 | 58 | 9 | ||
| pT-stage | ||||
| T1 | 73 | 10 | 0.58 | 1.33 |
| T2 | 44 | 8 | ||
| pN-stage | ||||
| N0 | 85 | 13 | 0.97 | 1.02 |
| N1 | 32 | 5 | ||
| Histology | ||||
| SCC | 79 | 12 | 0.94 | 1.04 |
| non-SCC | 38 | 6 | ||
| RT total dose (Gy) | ||||
| <50 | 6 | 1 | 0.94 | 0.92 |
| ≥50 | 111 | 17 | ||
| Smoking | ||||
| None | 88 | 10 | 0.08 | 2.42 |
| Yes | 29 | 8 | ||
| BMI | ||||
| ≤21 | 53 | 13 | 0.03 | 0.32 |
| >21 | 64 | 5 |
GI = gastrointestinal, SCC = squamous cell carcinoma, BMI = body mass index.
The mean DVH parameters of the small bowel loops, large bowel loop and bowel bag of patients with or without GI complications are shown in Table 3. Patients with GI complications had significantly greater V5–V45, mean dose and the generalized equivalent uniform dose (gEUD) of the small bowel loops, compared with those without GI complications. V30–V45, mean dose and gEUD of the bowel bag also showed significant differences between patients with and without GI complications. In contrast, no parameter for the large bowel loop was correlated with GI complications.
Table 3.
Comparison (Mann–Whitney U test) of mean DVH parameters of the small bowel loops, large bowel loop, and bowel bag in patients with and without chronic GI complications
| Overall | Grade 0–2 | Grade 3 | P value | |
|---|---|---|---|---|
| Small bowel loops | ||||
| Mean volume ± SE (ml) | ||||
| V5 | 385 ± 14 | 361 ± 14 | 542 ± 41 | <0.001 |
| V10 | 368 ± 14 | 344 ± 13 | 525 ± 39 | <0.001 |
| V15 | 357 ± 13 | 333 ± 13 | 514 ± 37 | <0.001 |
| V20 | 346 ± 13 | 321 ± 12 | 504 ± 36 | <0.001 |
| V30 | 304 ± 11 | 280 ± 11 | 464 ± 29 | <0.001 |
| V40 | 260 ± 11 | 234 ± 10 | 429 ± 28 | <0.001 |
| V45 | 242 ± 11 | 215 ± 12 | 413 ± 28 | <0.001 |
| Mean dose (Gy ± SE) | ||||
| 36.6 ± 0.5 | 35.9 ± 0.5 | 40.7 ± 1.3 | <0.01 | |
| gEUD (n = 0.15) | ||||
| 45.7 ± 0.3 | 45.4 ± 0.3 | 47.9 ± 0.5 | <0.001 | |
| Large bowel loop | ||||
| Mean volume ± SE (ml) | ||||
| V5 | 287 ± 12 | 286 ± 13 | 294 ± 36 | 0.96 |
| V10 | 265 ± 11 | 265 ± 12 | 270 ± 33 | 0.91 |
| V15 | 253 ± 11 | 253 ± 11 | 258 ± 31 | 0.96 |
| V20 | 240 ± 10 | 239 ± 11 | 248 ± 30 | 0.91 |
| V30 | 194 ± 8 | 193 ± 9 | 205 ± 22 | 0.58 |
| V40 | 161 ± 8 | 160 ± 9 | 166 ± 16 | 0.36 |
| V45 | 149 ± 8 | 148 ± 9 | 156 ± 15 | 0.28 |
| Mean dose (Gy ± SE) | ||||
| 29.8 ± 0.5 | 29.9 ± 0.5 | 29.0 ± 1.4 | 0.38 | |
| gEUD (n = 0.15) | ||||
| 43.7 ± 0.2 | 43.6 ± 0.3 | 44.1 ± 0.4 | 0.76 | |
| Bowel bag | ||||
| Mean volume ± SE (ml) | ||||
| V5 | 1320 ± 31 | 1305 ± 34 | 1416 ± 72 | 0.14 |
| V10 | 1254 ± 29 | 1238 ± 32 | 1356 ± 68 | 0.11 |
| V15 | 1215 ± 28 | 1199 ± 30 | 1322 ± 65 | 0.10 |
| V20 | 1175 ± 26 | 1157 ± 28 | 1292 ± 63 | 0.06 |
| V30 | 1031 ± 21 | 1009 ± 22 | 1177 ± 56 | 0.01 |
| V40 | 898 ± 23 | 871 ± 24 | 1070 ± 58 | <0.01 |
| V45 | 840 ± 25 | 811 ± 26 | 1027 ± 59 | <0.01 |
| Mean dose (Gy ± SE) | ||||
| 35.8 ± 0.3 | 35.5 ± 0.3 | 37.8 ± 1.0 | 0.04 | |
| gEUD (n = 0.15) | ||||
| 45.9 ± 0.2 | 45.7 ± 0.2 | 47.1 ± 0.3 | <0.01 | |
DVH = dose–volume histogram, GI = gastrointestinal, SE = standard error, gEUD = the generalized equivalent uniform dose.
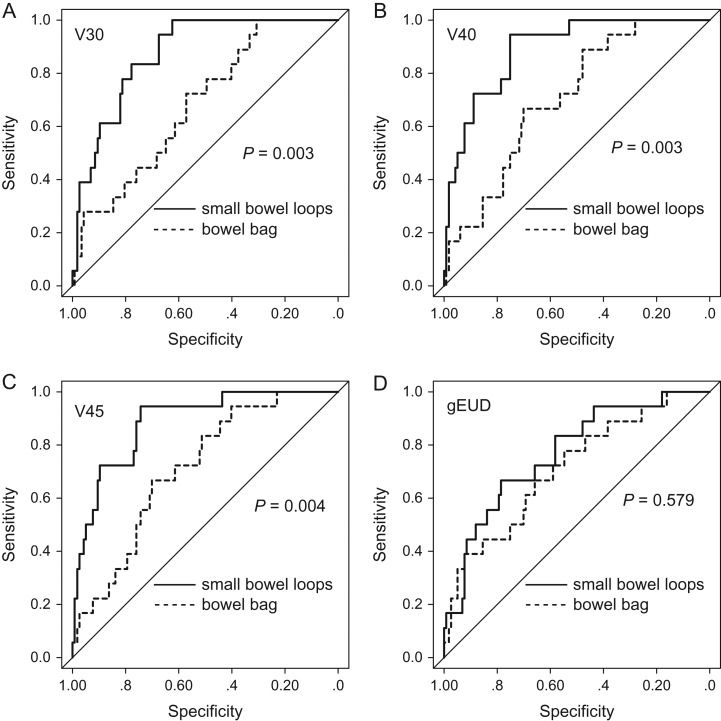
ROC curve analysis was performed to identify predictors of GI complications among DVH parameters for the small bowel loops and bowel bag. The results are shown in Table 4. AUCs for V5–V45 of the small bowel loops were >0.8, indicating that V5–V45 have good accuracy for prediction of GI complications. Among these parameters, V40 of the small bowel loops had the highest AUC (0.89). Comparisons of ROC curves between the small bowel loops and bowel bag for the respective parameters are shown in Fig. 2. This analysis indicated that V30, V40 and V45 of the small bowel loops were better predictors than these respective parameters for the bowel bag.
Table 4.
ROC curve analysis of DVH parameters in relation to Grade 3 chronic GI complications
| AUC | 95% CI | Optimal threshold | Chi–square | ||||
|---|---|---|---|---|---|---|---|
| Value | Sensitivity/specificity (%) | P value | Odds ratio | ||||
| Small bowel loops | V5 | 0.81 | 0.73–0.88 | 430 ml | 72.2/82.1 | <0.001 | 8.91 |
| V10 | 0.82 | 0.74–0.89 | 360 ml | 77.8/76.1 | <0.001 | 8.30 | |
| V15 | 0.82 | 0.75–0.89 | 395 ml | 72.2/82.1 | <0.001 | 12.21 | |
| V20 | 0.83 | 0.75–0.90 | 330 ml | 83.3/73.5 | <0.001 | 8.30 | |
| V30 | 0.87 | 0.81–0.93 | 300 ml | 83.3/77.8 | <0.001 | 31.51 | |
| V40 | 0.89 | 0.82–0.95 | 225 ml | 94.4/70.9 | <0.001 | 19.16 | |
| V45 | 0.88 | 0.82–0.94 | 235 ml | 88.9/74.4 | <0.001 | 27.20 | |
| Mean dose | 0.73 | 0.63–0.83 | 36.7 Gy | 61.1/72.6 | 0.02 | 3.36 | |
| gEUD (n = 0.15) | 0.75 | 0.65–0.84 | 46.0 Gy | 66.7/65.9 | <0.01 | 5.26 | |
| Bowel bag | V30 | 0.69 | 0.59–0.79 | 940 ml | 72.2/57.3 | 0.06 | 3.24 |
| V40 | 0.72 | 0.63–0.82 | 850 ml | 66.7/62.4 | <0.01 | 7.10 | |
| V45 | 0.71 | 0.62–0.81 | 800 ml | 72.2/60.7 | 0.01 | 4.59 | |
| Mean dose | 0.66 | 0.54–0.79 | 35.3 Gy | 61.1/69.2 | 0.16 | 2.15 | |
| gEUD (n = 0.15) | 0.71 | 0.61–0.82 | 45.2 Gy | 65.8/66.7 | 0.11 | 3.28 | |
ROC = receiver operating characteristics, DVH = dose–volume histogram, GI = gastrointestinal, AUC = area under the ROC curve, CI = confidence interval, gEUD = the generalized equivalent dose.
Fig. 2.
ROC curve analyses for the small bowel loops and bowel bag. (A) V30, (B) V40, (C) V45, (D) gEUD.
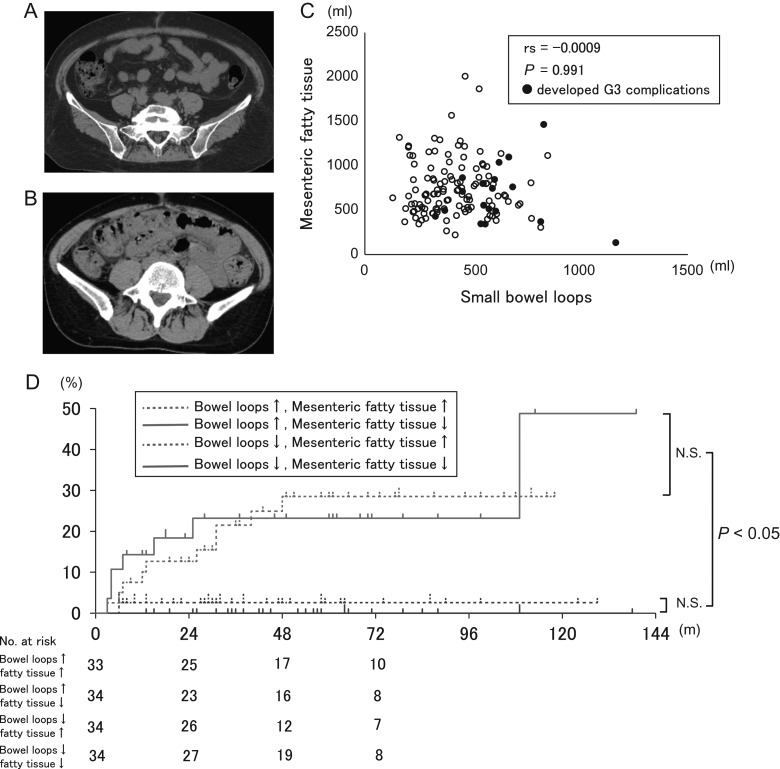
We next investigated why parameters of the small bowel loops have greater accuracy than those of the bowel bag. The bowel bag consists of the small bowel loops, large bowel loop and mesenteric fatty tissue (Fig. 3A, B). Mean DVH parameters for the small bowel loops strongly correlated with GI complications, but those for the large bowel loop did not do so (Table 3). The remaining volume of mesenteric fatty tissue may also affect GI complications, but there was no correlation between this volume and that of the small bowel loops (Fig. 3C). Also, when patients were divided into four groups by the median V40 of the small bowel loops (mV40SB; 239 ml) and the median volume of mesenteric fatty tissue, those with V40 > mV40SB had a higher rate of GI complications than those with V40 ≤ mV40SB regardless of the mesenteric fatty tissue volume (Fig. 3D). These data indicate that neither large bowel loop parameters nor the volume of mesenteric fatty tissue are correlated with GI complications.
Fig. 3.
Effect of mesenteric fatty tissue on chronic GI complications. (A) A case in which the bowel bag is rich in fatty tissue. (B) A case in which the bowel bag has little fatty tissue. (C) Relationship of mesenteric fatty tissue volume and small bowel loops volume. (D) Actuarial chronic GI complications for four groups based on threshold values of the median V40 of the small bowel loops and the volume of mesenteric fatty tissue.
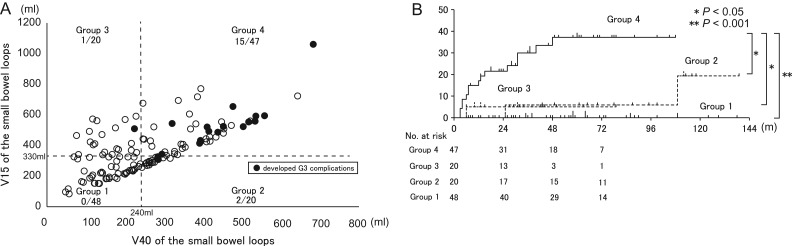
Our data suggest that several DVH parameters of the small bowel loops have high accuracy for prediction of chronic GI complications. Therefore, we evaluated the relationships between V15 (relatively low dose) and V40 (relatively high dose) of the small bowel loops and the correlation between GI complications and these parameters. Patients were divided into four groups based on the median V15 (mV15SB; 330 ml) and mV40SB: Group 1 (V15 ≤ mV15SB and V40 ≤ mV40SB), Group 2 (V15 ≤ mV15SB and V40 > mV40SB), Group 3 (V15 > mV15SB and V40 ≤ mV40SB, and Group 4 (V15 > mV15SB and V40 > mV40SB). The actuarial rates of GI complications for these groups are shown in Fig. 4A. The level of GI complications of Group 4 was significantly higher than that of Groups 1–3 (Fig. 4B).
Fig. 4.
(A) Relationship of V15 and V40 of the small bowel loops for each patient. Group 1 (≤median V15 and ≤median V40), Group 2 (≤median V15 and >median V40), Group 3 (>median V15 and ≤median V40), Group 4 (>median V15 and >median V40). (B) Actuarial chronic GI complications for the four groups based on threshold values of the median V15 and V40 of small bowel loops.
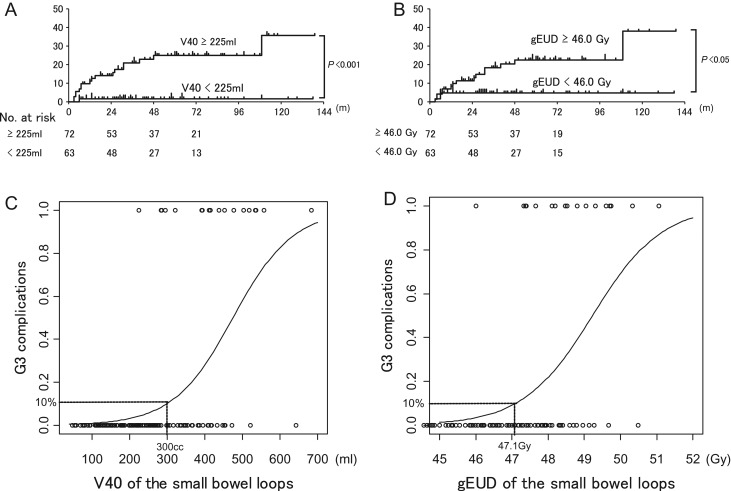
The 3-year cumulative incidence of G3 chronic GI complications was significantly higher in patients with V40 of the small bowel loops >225 ml compared with those with V40 ≤ 225 ml (P < 0.001) (Fig. 5A), and in patients with gEUD of the small bowel loops >46.0 Gy compared with those with gEUD ≤ 46.0 Gy (P < 0.05) (Fig. 5B). The logistic regression curve indicated that V40 of 300 ml or gEUD of 47.1 Gy resulted in a 10% probability of G3 chronic GI complications (Fig. 5C, D).
Fig. 5.
Kaplan–Meier estimates of the cumulative incidence of G3 chronic GI complications stratified by V40 of the small bowel loops (A) and gEUD (B). (A) Patients with V40 > 285 ml had higher rates of complications compared with those with V40 ≤ 285 ml. (B) Patients with gEUD > 46.0 Gy had higher rates of complications compared with those with gEUD ≤ 46.0 Gy. (C, D) Probability of chronic GI complications according to a binary logistic regression model for (C) V40 of the small bowel loops and (D) gEUD of the small bowel loops.
DISCUSSION
Several reports have shown that DVH parameters of the bowels are associated with chronic GI complications postoperatively in patients with cervical cancer [3, 4]. However, the best parameters for prediction of chronic GI complications are still poorly understood. Chopra et al. found that V15 of the small bowel loops or V15 of the large bowel loop is an independent predictor of chronic GI complications of G3 or higher [3]. Our data indicated that several parameters of the small bowel loops, including V15, have good accuracy for prediction of GI complications, whereas parameters for the large bowel loop did not correlate with radiation-induced GI complications. This discrepancy in the contribution of the large bowel loop to chronic GI complications between Chopra et al. and the current study may be due to treatment with or without brachytherapy, different surgery (in Chopra et al., 68% of cases were treated with simple hysterectomy), and the higher DVH parameters of the small bowel loops and large bowel loop in our study (Table 3). In fact, all of these parameters were much higher than those in Chopra et al., and the difference in DVH parameters might be due to differences in the physical characteristics of the patients in the two studies. There were many thin patients in our study: 66/135 patients (49%) had BMI ≤ 21. A further study is required to confirm the best predictors of chronic GI complications.
We have also shown that the small bowel loops may be better predictors of chronic GI complications compared with the bowel bag in 2DRT and 3DCRT [1]. In the current study, we examined which parameter correlated with development of chronic GI complications in 2DRT, 3DCRT and IMRT. V30–V45 of the bowel bag correlated with development of chronic GI complications, but ROC curve analysis clearly indicated that parameters for the small bowel loops are better predictors of these complications. The small bowel loops may be better predictors of chronic GI complications because the bowel bag includes mesenteric fatty tissue and the large bowel, which are not correlated with chronic GI complications, as well as the small bowel loops. Therefore, we recommend contouring of the small bowel loops for prediction of chronic GI complications, in addition to contouring of the bowel bag for evaluation of potential acute GI complications [9] or use of dose constraints [10]. However, the small bowel DVH parameters were estimated based on only one radiation treatment planning CT before RT, while daily variability in distension or movement of the small bowel during the treatment course may affect the dose–volume profile. Hysing et al. reported that isotropic margins of up to 30 mm are required to account for all intestinal motion in 90% of bladder cancer patients [11]. Therefore, further studies will be necessary to investigate the influence of intra- and interfraction motion of the small bowel loop on chronic GI complications.
We evaluated the relationship between V15 (relatively low dose) and V40 (relatively high dose) of the small bowel loops because several DVH parameters of the small bowel loops were predictive for chronic GI complications. Our data indicated that both V15 and V40 of the small bowel loops affected chronic GI complications. It should be noted that patients with a volume above the threshold volume did not show a greater frequency of chronic GI complications unless another volume exceeded the threshold. Previous clinical reports have suggested that IMRT is useful for decreasing chronic GI complications by reducing the relatively high-dose volume to the bowels [2, 12]. Also, due to the properties of IMRT, it is difficult to reduce the volume of a relatively low dose. Therefore, a relatively high-dose volume (e.g. V40) to the small bowel loops is an achievable goal for dose constraint that may reduce chronic GI complications. Additionally, we evaluated the gEUD of the small bowel loops because varied and inhomogeneous RT planning was included in the study. The 3-year cumulative incidence of G3 chronic GI complications was significantly higher in cases with gEUD > 46.0 Gy compared with those with gEUD ≤ 46.0 Gy. Therefore, a gEUD of the small bowel loops >46.0 Gy is also a useful predictor of chronic GI complications.
We used weekly nedaplatin as concurrent chemotherapy, whereas chemotherapy with 40 mg/m2 cisplatin weekly is now accepted as the standard first-line treatment, and we cannot exclude the possibility that the DVH predictors found in this study may be chemotherapy specific. However, nedaplatin (cis-diammine-glycoplatinum), a derivative of cisplatin, was developed by the Shionogi Pharmaceutical Company in Japan with the aim of reducing renal toxicity but maintaining efficacy, compared with cisplatin [13]. The G3 chronic GI complication rate of 13% in our study is similar to that observed with the use of other postoperative concurrent platinum-based chemotherapy and WPRT for cervical cancer [14, 15]. Therefore, we believe that our results are likely to be similar to those that would be obtained with cisplatin.
In conclusion, DVH parameters of the small bowel loops serve as predictors of chronic GI complications of severity G3 after postoperative concurrent chemotherapy and WPRT. Relatively high-dose volumes (e.g. V40) are useful predictors of chronic GI complications and are candidates for dose constraints in IMRT. We recommend that V40 of the small bowel loop should be <300 ml to avoid chronic GI complications in postoperative pelvic irradiation.
FUNDING
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 25861097.
CONFLICT OF INTEREST
All authors have indicated that they do not have any conflicts of interest in relation to this work.
REFERENCES
- 1.Isohashi F, Yoshioka Y, Mabuchi S, et al. . Dose–volume histogram predictors of chronic gastrointestinal complications after radical hysterectomy and postoperative concurrent nedaplatin-based chemoradiation therapy for early-stage cervical cancer. Int J Radiat Oncol Biol Phys 2013;85:728–34. [DOI] [PubMed] [Google Scholar]
- 2.Isohashi F, Mabuchi S, Yoshioka Y, et al. . Intensity-modulated radiation therapy versus three-dimensional conformal radiation therapy with concurrent nedaplatin-based chemotherapy after radical hysterectomy for uterine cervical cancer: comparison of outcomes, complications, and dose–volume histogram parameters. Radiat Oncol 2015;10:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chopra S, Dora T, Chinnachamy AN, et al. . Predictors of grade 3 or higher late bowel toxicity in patients undergoing pelvic radiation for cervical cancer: results from a prospective study. Int J Radiat Oncol Biol Phys 2014;88:630–5. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Zhu L, Zhang B, et al. . Dose–volume histogram predictors of chronic gastrointestinal complications after radical hysterectomy and postoperative intensity modulated radiotherapy for early-stage cervical cancer. BMC Cancer 2014;14:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mabuchi S, Okazawa M, Isohashi F, et al. . Postoperative whole pelvic radiotherapy plus concurrent chemotherapy versus extended-field irradiation for early-stage cervical cancer patients with multiple pelvic lymph node metastases. Gynecol Oncol 2011;120:94–100. [DOI] [PubMed] [Google Scholar]
- 6.Mabuchi S, Takahashi R, Isohashi F, et al. . A phase I study of concurrent weekly carboplatin and paclitaxel combined with intensity-modulated pelvic radiotherapy as an adjuvant treatment for early-stage cervical cancer patients with positive pelvic lymph nodes. Int J Gynecol Cancer 2013;23:1279–86. [DOI] [PubMed] [Google Scholar]
- 7.Niemierko A. A generalized concept of equivalent uniform dose (EUD). Med Phys 1999;26:1100 [Abstract]. [Google Scholar]
- 8.Burman C, Kutcher GJ, Emami B, et al. . Fitting of normal tissue tolerance data on an analytic function. Int J Radiat Oncol Biol Phys 1991;21:123–35. [DOI] [PubMed] [Google Scholar]
- 9.Kavanagh BD, Pan CC, Dawson LA, et al. . Radiation dose–volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 2010;76 Suppl 3:S101–7. [DOI] [PubMed] [Google Scholar]
- 10.Klopp AH, Moughan J, Portelance L, et al. . Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys 2013;86:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hysing LB, Kvinnsland Y, Lord H, et al. . Planning organ at risk volume margins for organ motion of the intestine. Radiother Oncol 2006;80:349–54. [DOI] [PubMed] [Google Scholar]
- 12.Chen MF, Tseng CJ, Tseng CC, et al. . Clinical outcome in posthysterectomy cervical cancer patients treated with concurrent Cisplatin and intensity-modulated pelvic radiotherapy: comparison with conventional radiotherapy. Int J Radiat Oncol Biol Phys 2007;67:1438–44. [DOI] [PubMed] [Google Scholar]
- 13.Mabuchi S, Kimura T.. Nedaplatin: a radiosensitizing agent for patients with cervical cancer. Chemother Res Pract 2011;2011:963159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsuhashi A, Uno T, Usui H, et al. . Postoperative concurrent daily low-dose cisplatin-based chemoradiation improves the prognosis of patients with pathologic T2b or N1 cervical cancer. Anticancer Res 2010;30:2341–6. [PubMed] [Google Scholar]
- 15.Heinzelmann F, Henke G, von Grafenstein M, et al. . Adjuvant radiochemotherapy in patients with locally advanced high-risk cervical cancer. Strahlenther Onkol 2012;188:568–75. [DOI] [PubMed] [Google Scholar]