Abstract
The aim of this study was to determine whether metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are associated with outcomes in Stage I lung cancer patients treated with stereotactic body radiation therapy (SBRT). Thirty-eight patients underwent [18F] fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) within 60 days before SBRT at our institution between January 2001 and December 2011. The maximum standardized uptake value (SUVmax), MTV2, MTV4, MTV6, TLG40%, TLG50% and TLG60% were calculated. Prognostic factors for overall survival (OS) and local control (LC) were analyzed using Cox's proportional hazards model, and survival curves were calculated using the Kaplan–Meier method. Receiver operating characteristics (ROC) curves of PET parameters for OS and LC were calculated. The median follow-up period for survivors was 37.7 months. Three-year OS and LC rates were 56.4% and 70.5%, respectively, and 5-year OS and LC rates were 36.8% and 70.5%, respectively. In univariate analyses, tumor diameter (P = 0.019), single dose ≥10 Gy (P = 0.017), MTV2 (P = 0.030) and MTV4 (P = 0.048) were significant predictors for OS. Tumor diameter (P < 0.001), single dose ≥10 Gy (P = 0.007), SUVmax (P = 0.035), MTV2 (P < 0.001), MTV4 (P = 0.003), MTV6 (P = 0.017), TLG40% (P < 0.001), TLG50% (P = 0.001) and TLG60% (P = 0.003) were significant predictors for LC. SUVmax was not a significant predictor for OS. We made the ROC curves at PET parameters, and the largest area under the curve value for OS was MTV2 and for LC was TLG40%. Tumor diameter, single dose ≥10 Gy, MTV2 and MTV4 are prognostic factors for OS and LC rates and MTV2 is a better prognostic factor for OS than other PET parameters.
Keywords: stereotactic body radiation therapy, non–small cell lung cancer, NSCLC, metabolic tumor volume, total lesion glycolysis, prognostic factor
INTRODUCTION
Surgery has been the standard treatment for early-stage non–small cell lung cancer (NSCLC). In recent times, patients who are medically inoperable because of comorbidities or because they have rejected surgery are being treated with stereotactic body radiation therapy (SBRT). Good local control (LC) rates have been achieved with SBRT for inoperable patients [1]. A good overall survival (OS) rate has also been achieved with SBRT for operable patients with Stage I NSCLC [2].
Positron emission tomography–computed tomography using 18F-fluorodeoxyglucose (FDG-PET/CT) is used to search for lymph node metastases and distant organ metastases, and the use of FDG-PET/CT has improved the rate of detection of these metastases in patients with NSCLC [3]. The SUVmax is mainly used as a measure of malignancy, but it is controversial whether SUVmax is a prognostic factor for Stage I NSCLC treated with SBRT [4]. Therefore, another parameter in FDG-PET/CT as a prognostic factor is required. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) have been reported as possible prognostic factors [5–7].
In this study, we investigated whether pretreatment MTV and TLG were associated with LC rates and OS in patients with Stage I NSCLC treated with SBRT.
MATERIALS AND METHODS
Patients
We retrospectively reviewed a clinical database of patients with Stage I primary lung cancer treated by SBRT in our institution between January 2001 and December 2011. A total of 80 patients with 82 lesions received FDG-PET/CT before SBRT, and data for 47 of those patients with 48 lesions were available to measure metabolic parameters. Standardized uptake value data before December 2004, except for the SUVmax written in the report, had already been deleted. We excluded patients for whom more than 60 days had passed from FDG-PET/CT to the first day of SBRT. Finally, 38 patients were included in this study.
The patients’ characteristics and irradiation dose characteristics are summarized in Table 1. The median age of the patients was 77 years (range, 48–88 years). Twenty-six lesions were pathologically proven, but 12 lesions were not proven. The median tumor diameter was 2.3 cm (range, 0.9–4.2 cm). All lesions were of peripheral type.
Table 1.
Patients’ characteristics and irradiation dose characteristics
| Number of lesions (%) | ||
|---|---|---|
| Gender | Female | 8 (21%) |
| Male | 30 (79%) | |
| Age (years) | <69 | 3 (8%) |
| 70–79 | 19 (50%) | |
| 80–89 | 16 (42%) | |
| Performance status | 0–1 | 32 (84%) |
| 2–3 | 6 (16%) | |
| Histology | SCC | 9 (24%) |
| Adeno | 15 (39%) | |
| NSCLC-NOS | 2 (5%) | |
| Undiagnosed | 12 (32%) | |
| Operability | Operable | 17 (45%) |
| Inoperable | 21 (55%) | |
| Tumor diameter (cm) | Median 2.3 (0.9–4.2) | |
| T stage | T1a | 11 (29%) |
| T1b | 15 (39%) | |
| T2a | 12 (32%) | |
| Tumor location | Upper lobe | 24 (63%) |
| Middle or lower lobe | 14 (37%) | |
| GGN type | Solid GGN | 36 (95%) |
| Part solid - pure GGN | 2 (5%) | |
| BED10 | ≥100 | 17 (45%) |
| <100 | 21 (55%) | |
| Single dose | ≥10 Gy | 21 (55%) |
| <10 Gy | 17 (45%) | |
SCC = squamous cell carcinoma, Adeno = adenocarcinoma, NSCLC-NOS = non–small cell lung cancer not otherwise specified, GGN = ground glass nodule, BED10 = biological effective dose calculated using α/β = 10.
SBRT procedure
The SBRT technique used in our institution has been reported several times [8–9]. The treatment procedure is summarized in Table 2. We observed respiratory-driven tumor motion on a simulator (Ximatron, Varian Medical Systems). When respiratory motion was large, we used an abdominal pressure belt.
Table 2.
Treatment procedure
| Immobilization: | Vacuum cushions (Vac-loc, Med-tek) with or without an abdominal pressure belt |
| Computed tomography: | Slow-rotation CT scanning (slice thickness, 2.5 mm; 4 s/slice) |
| ITV definition: | Slow-rotation CT and lung tumor motion on the simulator (Varian Ximatron) |
| SBRT planning | Varian CADPlan or Eclipse |
| Prescription: | |
| Isocenter prescription | 60 Gy/15 fr, 60 Gy/8 fr and 48 Gy/4 fr |
| D95 prescription | 50 Gy/8 fr and 40 Gy/4 fr |
| Algorithm: | PBC with Modified Batho Power Law correction or AAA |
| Irradiation machine: | Varian Clinac 23EX |
| Beam arrangement: | Multistatic beams, typically four non-coplanar and three coplanar static beams using 6-MVX beams |
ITV = internal target volume, SBRT = stereotactic body radiation therapy, D95 = 95% of the PTV.
The gross tumor volume (GTV) was defined as the visible extent of the tumor on a CT image with a pulmonary window. The clinical target volume (CTV) was extended for 0–5 mm from the GTV. The internal target volume (ITV) was determined from slow-rotation CT images and from respiratory-driven tumor motion on the simulator. The planning target volume (PTV) was extended for 5 mm from the ITV. The SBRT plan was created with a 3D radiotherapy planning system (CAD Plan/Eclipse, Varian Medical Systems). SBRT was delivered with a linear accelerator (Clinac 23EX, Varian Medical Systems) using 6-MV X-ray beams with five to seven non-coplanar multistatic ports and/or multidynamic arcs.
Before June 2009, the dose calculation algorithm was based on the pencil beam method with heterogeneity correction (modified Batho power law). Sixty Gy in 15 fractions, 60 Gy in 8 fractions, or 48 Gy in 4 fractions was prescribed to the isocenter. After June 2009, the dose calculation algorithm was changed to the analytical anisotropic algorithm. Fifty Gy in 8 fractions or 40 Gy in 4 fractions was delivered to cover 95% of the PTV (D95).
This study was approved by the Ethical Committee of our institution, and written informed consent was obtained from all patients.
Follow-up after SBRT
Patients consulted a radiation oncologist 4–6 weeks after treatment. Around the same time, CT scanning was performed. Thereafter, the patients had follow-up examinations every 3–6 months for 2 years following treatment, then every 6 months.
FDG-PET/CT methods
The median time from FDG-PET/CT to the first day of SBRT was 30 days (range, 3–58 days). After a 4-h fast, patients were injected with 3.7 MBq FDG/kg. After 60 min, a whole body scan was performed using a PET/CT scanner (Biograph Duo LSO or Biograh 40 Truepoint; Siemens Medical Solution, Erlangen Germany).
Metabolic parameters
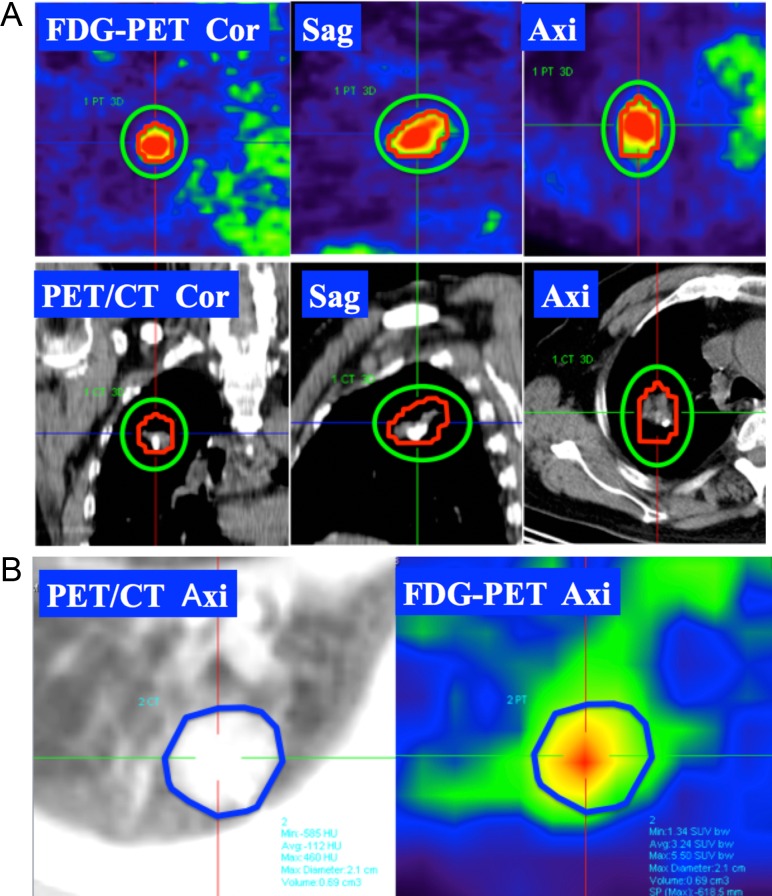
One radiologist analyzed pretreatment FDG-PET images for 38 patients and measured SUVmax, MTV2, MTV4, MTV6, TLG40%, TLG50% and TLG60%. MTVX was defined as the volume for which the SUV was over or equal to X [5]. The volume of interest (VOI) sufficiently enclosed the SUV area, and the volume greater or equal to SUV = X was automatically measured (Fig. 1A). TLGX% was defined as MTVX% × SUVmean [6]. MTVX% was defined as the volume over or equal to X% of SUVmax. For example, when SUVmax was 8, MTV40% was the volume over or equal to the SUV of 3.2. When there was confusion regarding the MTVX% area and lung background, we used the tumor volume as MTVX%. We contoured an outline of the tumor on the slice showing the maximum tumor dimension at the pulmonary window of PET/CT, and we defined MTVmean as the average of SUV in the outline (Fig. 1B).
Fig. 1.
Measurement of metabolic tumor volume (MTV) and mean maximum standardized uptake value (SUVmean). (A) We made the volume of interest (VOI) to sufficiently surround the fluorodeoxyglucose (FDG) uptake area (green line). The volume of over or equal to the FDG value X was automatically measured (red line). (B) SUVmean was defined as the average of the SUV in the outline of the tumor at the level of the greatest tumor dimensions at the pulmonary window of a positron emission tomography – computed tomography (PET/CT) axial image.
Statistical analysis
Statistical analysis was performed by using JMP® pro v.11.0.0 (SAS Institute). OS and LC rates were calculated from the first day of SBRT to the day of an event. Prognostic factors for OS and LC rates, including age, gender, tumor diameter, biological effective dose (BED10), single dose, SUVmax, MTV2, MTV4, MTV6, TLG40%, TLG50% and TLG60%, were investigated by Cox's proportional hazards model for univariate analyses. Multivariate analysis was not performed because of the small number of patients. BED10 was calculated using the following formula: BED10 = nd (1 + d/α/β), where n is the number of fractions, d is a single dose, and α/β is 10 Gy. OS and LC rates were calculated using the Kaplan–Meier method. We made receiver operating characteristics curves (ROC curves) for PET parameters for OS and LC rates. Regarding OS, the 3-year OS rate was 56.4%, and the median follow-up period in patients who survived was ~36 months. Therefore, we set the cut-off value to 36 months, and we considered survival for >36 months to be ‘true’ and survival for <36 months to be ‘false’. Absence of local recurrence was ‘true’ and presence of local recurrence was ‘false’ for LC. We compared them with the volumes of area under the curve (AUC) by using χ2 test. P < 0.05 was defined as significant in all tests. Toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events ver. 4.0.
RESULTS
Treatment outcomes
The median follow-up periods were 30 months (range, 5.6–82 months) in all patients and 37.7 months (range, 17.4–82 months) in patients who survived.
The 3-year OS rate was 56.4% [95% confidence interval (CI): 39.4–72.1] and the 5-year OS rate was 36.8% (95% CI: 18.3–60.2). Nine patients died of NSCLC, and nine patients died of other causes. Local recurrence was seen in seven patients, and nine patients had distant organ metastases. The 3-year LC rate was 70.5% (95% CI: 46.9–86.6) and the 5-year LC rate was 70.5% (95% CI: 46.9–86.6).
Grade 2 or 3 radiation pneumonitis occurred in seven patients, and Grade 5 radiation pneumonitis occurred in one patient.
FDG-PET/CT parameters
The median SUVmax was 6.32 (range: 0.65–23.6). The median values of MTV2, MTV4 and MTV6 were 5.75 cm3 (range: 0–82.1 cm3), 2.03 cm3 (range: 0–31.5 cm3) and 0.04 cm3 (range: 0–21.7 cm3), respectively. The median values of TLG40%, TLG50% and TLG60% were 12.7 (range: 0.016–205), 8.2 (range: 0.016–111) and 5.17 (range: 0.016–78.7), respectively.
Univariate analyses
In univariate analyses, tumor diameter [P = 0.019, hazard ratio (HR): 1.99, 95% CI: 1.12–3.58], single dose ≥10 Gy (≥10 Gy vs <10 Gy; P = 0.017, HR: 0.35, 95% CI: 0.11–0.81), MTV2 (P = 0.030, HR: 1.03, 95% CI: 1.00–1.05) and MTV4 (P = 0.047, HR: 1.06, 95% CI: 1.00–1.11) were significant predictors of OS, and tumor diameter (P < 0.001, HR: 11.1, 95% CI: 2.98–69.2), single dose ≥10 Gy (P = 0.007, HR: 0.10, 95% CI: 0.01–0.56), SUVmax (P = 0.035, HR: 1.18, 95% CI: 1.01–1.37), MTV2 (P < 0.001, HR: 1.10, 95% CI: 1.04–1.18), MTV4 (P = 0.003, HR: 1.15, 95% CI: 1.05–1.26), MTV6 (P = 0.017, HR: 1.17, 95% CI: 1.04–1.32), TLG40% (P < 0.001, HR: 1.03, 95% CI: 1.02–1.06), TLG50% (P = 0.001, HR: 1.04, 95% CI: 1.02–1.05) and TLG60% (P = 0.003, HR: 1.06, 95% CI: 1.02–1.10) were significant predictors of LC. SUVmax was not a significant predictor of OS (Table 3).
Table 3.
Univariate analyses for OS and LC
| Variables | UVA for OS | UVA for LC | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.07 (0.99–1.17) | 0.072 | 1.01 (0.92–1.14) | 0.789 |
| Gender | 2.66 (0.75–16.8) | 0.141 | 0.86 (0.18–6.03) | 0.859 |
| Tumor diameter (cm) | 1.99 (1.12–3.58) | 0.019* | 11.1 (2.98–69.2) | <0.001* |
| BED10 ≥100 or not | 0.78 (0.29–2.03) | 0.606 | 0.48 (0.07–2.25) | 0.365 |
| Single dose ≥10 Gy or not | 0.35 (0.11–0.81) | 0.017* | 0.10 (0.01–0.56) | 0.007* |
| SUVmax | 1.07 (0.99–1.14) | 0.086 | 1.18 (1.01–1.37) | 0.035* |
| MTV2 | 1.03 (1.00–1.05) | 0.030* | 1.10 (1.04–1.18) | <0.001* |
| MTV4 | 1.06 (1.00–1.11) | 0.047* | 1.15 (1.05–1.26) | 0.003* |
| MTV6 | 1.07 (0.97–1.15) | 0.155 | 1.17 (1.04–1.32) | 0.017* |
| TLG40% | 1.01 (0.99–1.02) | 0.056 | 1.03 (1.02–1.06) | <0.001* |
| TLG50% | 1.02 (0.99–1.03) | 0.057 | 1.04 (1.02–1.08) | 0.001* |
| TLG60% | 1.02 (0.99–1.04) | 0.070 | 1.06 (1.02–1.10) | 0.003* |
UVA = univariate analyses, OS = overall survival, LC = local control, HR = hazard ratio, CI = confidence interval, BED10 = biological effective dose calculated using α/β = 10.
*Including conventional radiation therapy.
ROC curves
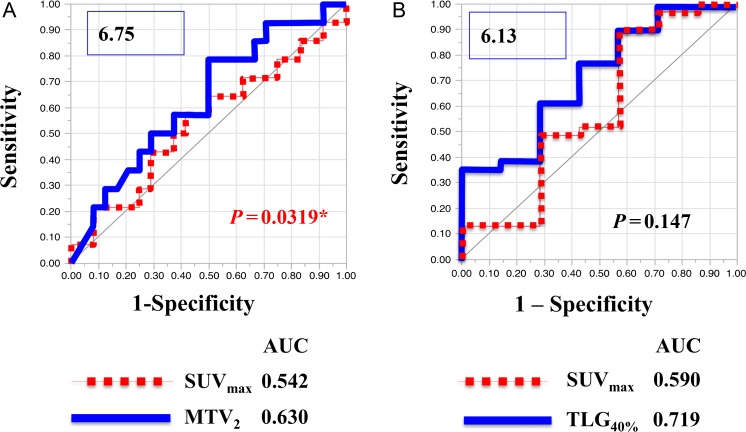
The AUC values of SUVmax, MTV2, MTV4, MTV6, TLG40%, TLG50% and TLG60% for survival were 0.542, 0.630, 0.601, 0.564, 0.625, 0.619 and 0.616, respectively. The largest AUC for survival was MTV2; the AUC of MTV2 was significantly different from the AUC of SUVmax (P = 0.0319, Fig. 2A). Of the patients who were followed up for <36 months, three patients (~7.9% of all patients) were lost to follow-up. On the other hand, AUC values of SUVmax, MTV2, MTV4, MTV6, TLG40%, TLG50% and TLG60% for LC were 0.590, 0.645, 0.668, 0.608, 0.719, 0.701 and 0.687, respectively. The largest AUC for LC was TLG40%; the AUC for LC of TLG40% was not significantly different from the AUC for SUVmax (P = 0.147, Fig. 2B).
Fig. 2.
Receiver operating characteristics curves (ROC curves) for survival and local recurrence. (A) The ROC curve for MTV2 for survival indicated that the cut-off value was 6.75. The AUC for MTV2 was 0.630 and was compared with the AUC for SUVmax, using the X2 test. (B) The ROC curve for TLG40% for local control indicated that the cut-off value was 6.13. The AUC for TLG40% was 0.719 and was compared with the AUC for SUVmax by using the X2 test.
DISCUSSION
SBRT is a safe and effective treatment method for inoperable patients with early-stage NSCLC [1]. The effectiveness of SBRT has also been shown in operable patients, and SBRT has become an option for treatment of patients with early-stage NSCLC. If we can predict that SBRT will be effective for a patient before treatment, we can strongly recommend SBRT. It will be possible to give patients tailor-made medicine if a predictive factor is established. We previously reported some possible prognostic factors, including BED10, metastatic tumor, broad attachment to the pleura, and minimum PTV dose [8–10]. The results of the present study suggest that MTV and TLG also have potential as prognostic factors for LC and OS after SBRT.
Several studies have shown results of SBRT for early-stage lung cancer. LC rates were 86.0–98.2% and 3-year OS rates were 42.7–83% in past studies [1,2,11,12]. In our study, the LC rate was 81.6% (31/38) and the 3-year OS rate was 56.4%. The 3-year OS rate in our study is consistent with the results of most past studies, but is worse than the result reported by Nagata et al. [11]. The LC rate in our study was relatively poor compared with the results of past studies. The possible reason for the worse LC rate might be that half of the patients were treated using a BED10 of <100 due to bad performance status and bad pulmonary function. Despite the fact that the median age of patients enrolled in our study was higher than the median ages of patients in other studies, the 3-year OS rate in our study was comparable with the rates in other studies.
In this study, BED10 was not a significant predictor of OS and LC rates in Cox's proportional hazards model. On the other hand, a single dose ≥10 Gy was a significant predictor of OS and LC rates. Our institution has recently been prescribing 40 Gy in four fractions to cover 95% of the PTV (D95) using the analytical anisotropic algorithm. A BED10 is calculated to be 80 when a single dose of 10 Gy is used. However, it is difficult to compare a BED10 of isocenter prescription with that of D95 prescription. In this study, 40 Gy in four fractions using D95 prescription was considered to be a BED10 of <100. This might be a reason why BED10 was not a significant predictor in this study.
SUVmax was not a significant predictor of OS in univariate analyses in our study. It is controversial whether SUVmax is useful as a prognostic factor. Hamamoto et al., Takeda et al. and Nair et al. reported that SUVmax had potential as a prognostic factor, but Hoopse et al. and Burdick et al. reported that SUVmax did not have potential as a prognostic factor [13–17] (Table 4). There are several pitfalls to be aware of concerning the value of SUVmax. For example, when the tumor is small, it is known that the value of SUVmax is underestimated (a volume effect), and when the tumor is located in a large physiological movement region, the value of SUVmax is underestimated (due to motion blur). Stage I lung cancer tumors are small and have respiratory movement, and it is therefore possible that the value of SUVmas can be underestimated. Therefore, it is not clear whether SUVmax is a prognostic factor in patients with Stage I NSCLC treated with SBRT.
Table 4.
Usefulness of pre-SBRT FDG-PET/CT
| Authors | N | TNM | Median follow-up period (months) | Possibility of SUVmax as a prognostic factor | |
|---|---|---|---|---|---|
| Hoopse et al. [13] | 32 | T1–2N0M0 | 42.5 |
|
|
| Hamamoto et al. [14] | 26 | T1–2N0M0 | 21 | LCR | Yes |
| Takeda et al. [15] | 97 | T1–4N0M0 | 18 | LCR | Yes |
| Burdick et al. [16] | 72 | T1–2N0M0 | 16.7 |
|
|
| Nair et al. [17] | 163* | T1–2N0M0 | 16 |
|
|
*Including SBRT and conventional radiation therapy.
N = number of patients, SBRT = stereotactic body radiation therapy, FDG-PET/CT = fluorodeoxyglucose positron emission tomography / computer tomography, SUVmax = maximum standardized uptake value, LCR = local control rate, OS = overall survival, LRFS = local recurrence-free survival, DMFS = distant metastasis-free survival.
The results of univariate analyses indicated that MTV2 and MTV4 were prognostic factors for OS, but that SUVmax was not. The AUC value of MTV2 was significantly larger than that of SUVmax. However, SUVmax was not a significant prognostic factor in univariate analysis, and the largest AUC value for OS was for MTV2. We think MTV2 is a better prognostic factor than SUVmax for OS. Regarding the LC rate, SUVmax, MTV2, MTV4, MTV6, TLG40%, TLG50% and TLG60% were prognostic factors in univariate analyses. There was no significant difference between the AUC value of TLG40% and that of SUVmax. However, the AUC value of TLG40% was larger than that of SUVmax, and it is therefore possible that TLG40% is a better prognostic factor than SUVmax for LC. In this study, local recurrence was observed in only seven patients. In the future, we will analyze data for a larger number of patients. The results of our study suggest that MTV2 was a prognostic factor for OS, and a significantly better factor than SUVmax. However, when the value of X goes down, it is possible that MTVx and TLGx correlate strongly with tumor volume. The reason that PET parameters of small X are better prognostic factors may be simply that they reflect tumor volume.
In order to calculate TLGx, SUVmean must be calculated. The definition of SUVmean is very difficult. In this study, we defined the region of interest for SUVmean on the slice showing the maximum tumor dimension at the pulmonary window of PET/CT. However, when the tumor is located at the lower lobe of the lung, there is sometimes deviation in PET and CT fusion images. Therefore, it is questionable whether it is the true SUV average of the tumor. In the future, it will be necessary to define SUVmean, and we propose to repeat this study using 4D PET, with the addition of respiratory motion.
MTVx and TLGx are parameters reflecting both tumor volume and FDG accumulation. In this study, MTV2 was a significant factor for OS in Cox's hazards model analysis, and MTV2 was shown by AUC comparison to be a significantly better prognostic factor than SUVmax for OS. FDG-PET/CT is very useful for detecting distant metastasis and lymph node metastasis, but it is inadequate for diagnosing and predicting prognosis from SUVmax only. It is necessary to examine other parameters in the future. Our results suggested that MTV2 is a better prognostic factor for OS of patients with Stage I lung cancer treated with SBRT. In order to provide patients with tailor-made medicine, further study of prognostic factors is necessary.
CONCLUSION
In conclusion, MTV2 on pretreatment FDG-PET/CT is a significant predictor of OS. MTV2 is a better prognostic factor than other FDG-PET/CT parameters, including SUVmax for OS. By using MTV2 as well as other predictors, personalized medicine might become a reality, but further study is needed.
ACKNOWLEDGEMENTS
We are grateful to our ex-colleagues for help with collection of data. We particularly thank Drs Keisuke Fujimoto, Toru Sakayauchi, Kakutaro Narazaki, Yumi Sato and Yuko Shirata for their support.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest associated with this manuscript.
REFERENCES
- 1.Timmerman R, Paulus R, Galvin J, et al.. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onishi H, Shirato H, Nagata Y, et al.. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2 Suppl 3:S94–100. [DOI] [PubMed] [Google Scholar]
- 3.Pieterman RM, van Putten JW, Meuzelaar, et al.. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 2000;343:254–61. [DOI] [PubMed] [Google Scholar]
- 4.Jingu K, Yamamoto T, Kaneta T, et al.. Prognostic probability of FDG-PET before stereotactic ablative radiotherapy for primary lung cancer—review of the literature. J Radiol Radiat Ther 2014;2:1041. [Google Scholar]
- 5.Abelson JA, Murphy JD, Trakul N, et al.. Metabolic imaging metrics correlate with survival in early stage lung cancer treated with stereotactic ablative radiotherapy. Lung Cancer 2012;78:219–24. [DOI] [PubMed] [Google Scholar]
- 6.Shadi A, Pedram H, Elkan FH, et al.. Baseline total lesion glycolysis measured with 18F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging 2013;3:272–281. [PMC free article] [PubMed] [Google Scholar]
- 7.Satoh Y, Onishi H, Nambu A, et al.. Volume-based parameters measured by using FDG PET/CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: prognostic value. Radiology 2014;270:275–81. [DOI] [PubMed] [Google Scholar]
- 8.Shirata Y, Jingu K, Koto M, et al.. Prognostic factors for local control of stage I non-small cell lung cancer in stereotactic radiotherapy: a retrospective analysis. Radiat Oncol 2012;7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto T, Jingu K, Shirata Y, et al.. Outcomes after stereotactic body radiotherapy for lung tumors, with emphasis on comparison of primary lung cancer and metastatic lung tumors. BMC Cancer 2014;14:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto T, Kadoya N, Shirata Y, et al.. Impact of tumor attachment to pleura measured by pretreatment CT image on outcome of stage I NSCLC treated with stereotactic body radiotherapy. Radiat Oncol 2015;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata Y, Takayama K, Matsuo Y, et al.. Clinical outcomes of a phase 2/3 study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005. ;63:1427–31. [DOI] [PubMed] [Google Scholar]
- 12.Fakiris AJ, McGarry RC, Yiannoutsos M, et al.. Stereotactic body radiation therapy for early-stage non–small-cell lung carcinoma: four-year results of a prospective Phase II study. Int J Radiat Oncol Biol Phys 2009;75:677–82. [DOI] [PubMed] [Google Scholar]
- 13.Hoopse DJ, Tann M, Fletcher JW, et al.. FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer 2007;56:229–34. [DOI] [PubMed] [Google Scholar]
- 14.Hamamoto Y, Sugawara Y, Inoue T, et al.. Relationship between pretreatment FDG uptake and local control after stereotactic body radiotherapy in stage I non-small-cell lung cancer: the preliminary result. Jpn J Clin Oncol 2011;41:543–7. [DOI] [PubMed] [Google Scholar]
- 15.Takeda A, Yokosuka N, Ohashi T, et al.. The maximum standardized uptake value (SUVmax) on FDG-PET is a strong predictor of local recurrence for localized non-small-cell lung cancer after stereotactic body radiotherapy (SBRT). Radiother Oncol 2011;101:291–7. [DOI] [PubMed] [Google Scholar]
- 16.Burdick MJ, Stephans KL, Reddy CA, et al.. Maximum standardized uptake value from staging FDG-PET/CT does not predict treatment outcome for early-stage non-small-cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1033–9. [DOI] [PubMed] [Google Scholar]
- 17.Nair VJ, MacRae R, Sirisegaram, et al.. Pretreatment [18F]-fluoro-2-deoxy-glucose positron emission tomography maximum standardized uptake value as predictor of distant metastasis in early-stage non–small cell lung cancer treated with definitive radiation therapy: rethinking the role of positron emission tomography in personalizing treatment based on risk status. Int J Radiat Oncol Biol Phys 2014;88:312–8. [DOI] [PubMed] [Google Scholar]