Abstract
The calculated risk of cancer in humans due to radiation exposure is based primarily on long-term follow-up studies, e.g. the life-span study (LSS) on atomic bomb (A-bomb) survivors in Hiroshima and Nagasaki. Since A-bomb radiation consists of a mixture of γ-rays and neutrons, it is essential that the relative biological effectiveness (RBE) of neutrons is adequately evaluated if a study is to serve as a reference for cancer risk. However, the relatively small neutron component hampered the direct estimation of RBE in LSS data. To circumvent this problem, several strategies have been attempted, including dose-independent constant RBE, dose-dependent variable RBE, and dependence on the degrees of dominance of intermingled γ-rays. By surveying the available literature, we tested the chromosomal RBE of neutrons as the biological endpoint for its equivalence to the microdosimetric quantities obtained using a tissue-equivalent proportional counter (TEPC) in various neutron fields. The radiation weighting factor, or quality factor, Qn, of neutrons as expressed in terms of the energy dependence of the maximum RBE, RBEm, was consistent with that predicted by the TEPC data, indicating that the chromosomally measured RBE was independent of the magnitude of coexisting γ-rays. The obtained neutron RBE, which varied with neutron dose, was confirmed to be the most adequate RBE system in terms of agreement with the cancer incidence in A-bomb survivors, using chromosome aberrations as surrogate markers. With this RBE system, the cancer risk in A-bomb survivors as expressed in unit dose of reference radiation is equally compatible with Hiroshima and Nagasaki cities, and may be potentially applicable in other cases of human radiation exposure.
Keywords: neutron RBE, mixed-radiation field, microdosimetry, chromosomal effectiveness, atomic bomb survivor, cancer risk
INTRODUCTION
The year 2015 marked the seventieth anniversary since the atomic bomb (A-bomb) explosions over the cities of Hiroshima and Nagasaki in August 1945. As unique cases of human exposure to radiation on a large scale, the health effects of the A-bomb radiation events have attracted worldwide attention. A long-term follow-up of A-bomb survivors in the life-span study (LSS), initiated in 1950 and continued by the US Atomic Bomb Causality Commission (ABCC) and its successor, the USA–Japan binational Radiation Effects Research Foundation (RERF), has provided fundamental information on cancer and non-cancer disease risk in humans following exposure to ionizing radiation [1–3]. The dose–effect relationship obtained from the LSS formed the scientific basis of the radiation protection standard. Despite uncertainties at low-doses (<0.2 Sv) the dose–risk relationship has been pragmatically dealt with in line with an unproven assumption of the ‘linear non-threshold (LNT)’ model [4]. In addition, there is also an upsurge of interest regarding more prospective use of the obtained knowledge as a generalized measure of risk assessment in various scenarios of human radiation exposure, including radiation accidents, medical exposures, and radiation in the environment and workplace. To date, health effects in A-bomb survivors have been correlated with direct radiation exposure, i.e. prompt and delayed γ-rays and neutrons, of which the dosimetry system has been improved by continued revisions up to the present dosimetry system 2002, or ‘DS02’ [5]. For the dose–effect relationships in A-bomb survivors to be applied beyond the quality of radiation as a generalized measure of risk assessment at a Gy or Gy-equivalent basis of reference radiation, the neutron component should be adequately weighted by its relative biological effectiveness (RBE).
To date, neutrons in A-bomb radiation in Hiroshima and Nagasaki have been conventionally weighted by a constant value of RBE = 10. However, this has been repeatedly questioned in light of common radiobiological knowledge. Indeed, an extensive study recently performed on chromosomal effectiveness of fission neutrons with varying energy spectra indicated that the dose-dependent variable RBE was most suitable for explaining the variability of dose–respons for somatic chromosome aberrations and cancer risk in A-bomb survivors [6]. However, this assertion has been criticized by Cullings et al. [7], who claimed that the experimentally derived RBE might be implausible for determining cancer rates in the LSS, and that in the γ–neutron mixed radiation field, the neutron RBE might be highly suppressed at low levels compared with that estimated for neutrons alone in the experimental system. The discrepancy in the neutron weighting system with reference to A-bomb survivors might have a strong impact on the risk evaluation following human exposure to neutrons, since neutron exposure often occurs in association with a mixture of γ-rays of either extrinsic or intrinsic origin. Here, we show that the unique neutron RBE described in Cullings et al. [7] in A-bomb survivors is due largely to an a priori linear setting of dose–response curves for biological endpoints; this can otherwise be better explained by the dose-dependent variable RBE without including biological endpoints in random dosimetry errors. The present weighting system best provides the commonality for Hiroshima and Nagasaki, despite large difference in their γ–neutron ratios, and may provide a common platform for radiobiology, microdosimetry, risk evaluation and setting of radiological protection.
DATA ASSESSMENT AND RESULTS OF ANALYSES
Uncertainties in the ‘survivor dose estimate’ in determining neutron RBE
The cancer incidence (or mortality) in A-bomb survivors has been correlated with organ dose of radiation, in which prompt and delayed source-term γ and neutron data are first converted to the free-in-air (FIA) kerma at the survivor location by considering the transporting factors of the air, then to the shielded kerm by shielding transmission factors, then to the organ dose by considering body transmission factors specific to the particular organ together with neutron-activated γ-rays [5, 7, 8]. In addition, RERF recently introduced further modifications of doses, in the so-called ‘survivor dose estimate’. Modifications include the truncation of unreasonably high doses to 4 Gy, and a dose adjustment made on the basis of random dosimetry errors, which include systematic and random dosimetry errors and imprecise knowledge of survivor location and shielding [9, 10]; these are incorporated as coefficients of variation over an assumed linear dose–response relationship [11–14]. Also considered in the estimation of error magnitude were uncertainties in the various biological endpoints, such as chromosome aberrations and epilation in survivors [15–17].
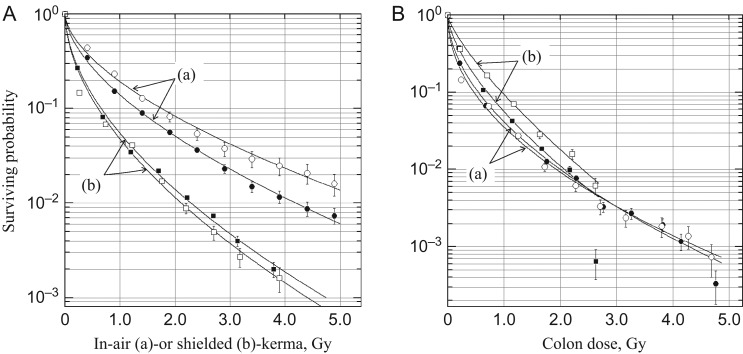
Fig. 1 shows survivor distribution expressed in terms of relative survivor density against dose, S(D), which is fitted to a Weibull hazard function.
| (1) |
where D is dose in Gy, δ is a location parameter, η is a scale parameter and κ is a shape parameter. The mean dose, Ď, and median dose, , are given by
| (2) |
in which Γ(…) is a gamma function. Since the location parameters are generally very small, δ ≈ 0, at κ = 1, survival is randomly distributed along with the exponential function of dose, and 37% survival probability is given by R(η) = 1 − S(η). The survivor distribution is not the real probability of surviving, but is a surrogate index in which a relative number of survivors receive at least the indicated doses. Fig. 1A(a) is replotted from Table II of Pierce et al. [11] and shows survivor distribution, where the cumulative number of survivors is plotted against the DS86 ‘In-Air’ tissue kerma. The In-Air tissue kerma is a tissue-equivalent kerma in air at a survivor's location after adjustment for external shielding. The Weibull parameters are ηH = 31.810 ± 12.828 and κH = 0.592 ± 0.121 for Hiroshima (H), ηN = 42.778 ± 15.289 and κN = 0.592 ± 0.134 for Nagasaki (N), δ = 0.1 Gy for both cities. City-specific differences appear in the scale parameters. The monotonically decreasing densities were the same as those of Pierce et al. [11]. By setting x as the true dose, z as the estimated dose, and ε as the response variable, they determined the adjustment factor, p(z) = [z − E(x|z)]/z, that satisfied the variate response E(ε|x) at several levels of coefficient of variation. The variate E(ε|x) was linear for x and was tested for various biological endpoints. The adjustment factor was thus dose-dependent and differed between the two cities. Currently, a 35% level of error in the coefficient of variation at each dose level has been adopted by RERF, by which shielded kerma and organ doses have been adjusted [1–3, 5]. The dose adjustment factors are dose- and city-dependent, varying from 1.0 at 0.38 Gy to 0.82 at 4 Gy in Hiroshima and from 1.0 at 0.52 Gy to 0.86 at 4 Gy in Nagasaki. The use of these adjustments results in a 10–15% increase in cancer risk [1, 5]. However, it should be noted that when survivor probability is plotted against the DS86 shielded kerma, the Weibull probability densities of the two cities become very similar (ηH = 16.893 ± 2.999, ηN = 13.156 ± 6.036, κH = 0.584 ± 0.062, κN = 0.550 ± 0.114) and the city difference disappears (Fig. 1A(b)). The shielded kerma is a dose that the survivor could receive after transmission of all shielding structures. Since the LSS cohort was not selected on the basis of dose, the results indicate that the city difference is derived largely from the difference in the external shielding, including city-specific geographical differences, rather than from the difference in systematic dosimetry errors. When the shielding calculation is properly applied, the dose adjustment by city-specific ‘random dosimetry errors’ may not be justified.
Fig. 1.
Relative number of survivors in life-span study (LSS) cohort for unmodified and unweighted dose. (A) DS86 dose system. (a) DS86 ‘In-Air’ tissue kerma. The original data were taken from Table II of Pierce et al. [11]. (b) DS86 shielded kerma. The distribution of survivors was calculated from the RERF ‘DS02can’ database. (B) DS02 colon dose. The distribution of survivors was calculated from the RERF ‘lssinc07’ database. (a) Surviving probability plotted against unmodified colon dose (coluDS02w1). (b) Surviving probability plotted against modified colon dose (colaDS02w1), in which unreasonably high are truncated to 4 Gy and the doses were adjusted by dose- and city-specific adjustment factors. Solid symbols = Hiroshima, open symbols = Nagasaki.
Further to the above, to clarify the problem of dose adjustment, the distribution of survivors was studied for the DS02 dosimetry system using the RERF ‘lssinc07’ database. The survivor distribution was similarly compiled, except for the effects of dose modifications. The survivor distributions are presented in Table 1, and their fit to the Weibull distribution is shown in Fig. 1B. As was the case using DS86 shielded kerma, the city difference was not seen, or commonality between the two cities was greatly improved, when an unadjusted (pre-adjustment) and unweighted (RBE = 1 for neutrons) DS02 colon dose, coluDS02w1, was used (Fig. 1B(a)). In contrast, when the survivor distributions were studied after dose modifications (dose adjustment and truncation) colaDS02w1, the dose–responses clearly dissociated between Hiroshima and Nagasaki (Fig. 1B(b)). The difference was again in their scale parameters (Table 1). As seen at the last dose points (>2.5 Gy) in Fig. 1B(b), the effects of truncation alone are more evident in Hiroshima survivors, reflecting the dominance of truncated doses in this dose category in Hiroshima.
Table 1.
Distribution of survivors according to DS02 colon dose and parameters of the best fit to the Weibull distribution
| Dose systema | (a) DS02 colon dose (unmodified) | (b) DS02 colon dose (modified) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| City | Hiroshima | Nagasaki | Hiroshima | Nagasaki | |||||
| Dose category | Mean | Survivor | Mean | Survivor | Mean | Survivor | Mean | Survivor | |
| <0.1 | 0.017 | 41 144 | 0.009 | 22 190 | 0.032 | 21105 | 0.026 | 6684 | |
| 0.1–0.5 | 0.227 | 9383 | 0.246 | 2079 | 0.226 | 9383 | 0.246 | 2079 | |
| 0.5–1.0 | 0.688 | 2176 | 0.738 | 996 | 0.661 | 2200 | 0.722 | 996 | |
| 1.0–1.5 | 1.256 | 737 | 1.260 | 430 | 1.180 | 807 | 1.195 | 446 | |
| 1.5–2.0 | 1.771 | 272 | 1.729 | 120 | 1.701 | 299 | 1.664 | 132 | |
| 2.0–2.5 | 2.302 | 241 | 2.279 | 74 | 2.204 | 316 | 2.324 | 101 | |
| 2.5–3.0b | 2.778 | 31 | 2.715 | 24 | 2.661 | 22 | 2.649 | 65 | |
| 3.0–3.5 | 2.273 | 42 | 3.173 | 14 | |||||
| 3.5–4.0 | 3.838 | 41 | 3.813 | 12 | |||||
| 4.0–4.5 | 4.162 | 46 | 4.285 | 17 | |||||
| 4.5–5.0 | 4.784 | 18 | 4.694 | 19 | |||||
| Weibull parameters | μ | 10.446 ± 4.424 | 8.090 ± 4.343 | 19.314 ± 3.838 | 32.199 ± 4.571 | ||||
| κ | 0.519 ± 0.199 | 0.482 ± 0.291 | 0.643 ± 0.110 | 0.761 ± 0.077 | |||||
| Ď | 0.212 | 0.182 | 0.299 | 0.404 | |||||
| 0.068 | 0.046 | 0.141 | 0.224 | ||||||
aDS02 colon doses with neutrons unweighted (RBE = 1): (a) unmodified doses, in which doses are neither truncated nor adjusted. (b) modified doses, in which unreasonably high doses are truncated to 4 Gy and doses are adjusted according to the dose- and city-specific adjustment factors. The number of survivors was compiled from the RERF ‘lssinc07’ database. The surviving probability in Fig. 1 was calculated assuming that the survivors at a given dose category could also tolerate the preceding doses, i.e. Si = ∑Ni/T, where T is total number of survivors and Ni is the number of survivors at the ith dose category.
b0.25 ≤ Gy for modified dose system.
The continued efforts of the USA–Japan working group on the assessment of the A-bomb radiation dosimetry for Hiroshima and Nagasaki will have greatly reduced systematic bias and perhaps random errors as well. Therefore, the magnitude of the allowable level of dosimetry error could be based on a flawed assumption concerning neutron RBE and the dose–response model of the biological endpoints considered. The importance of neutron RBE in determining dosimetry errors has been pointed out previously by Jablon [9] and Gilbert [10], and this still needs to be investigated.
Radioactive fallout shadow and its influence on the dose–response model
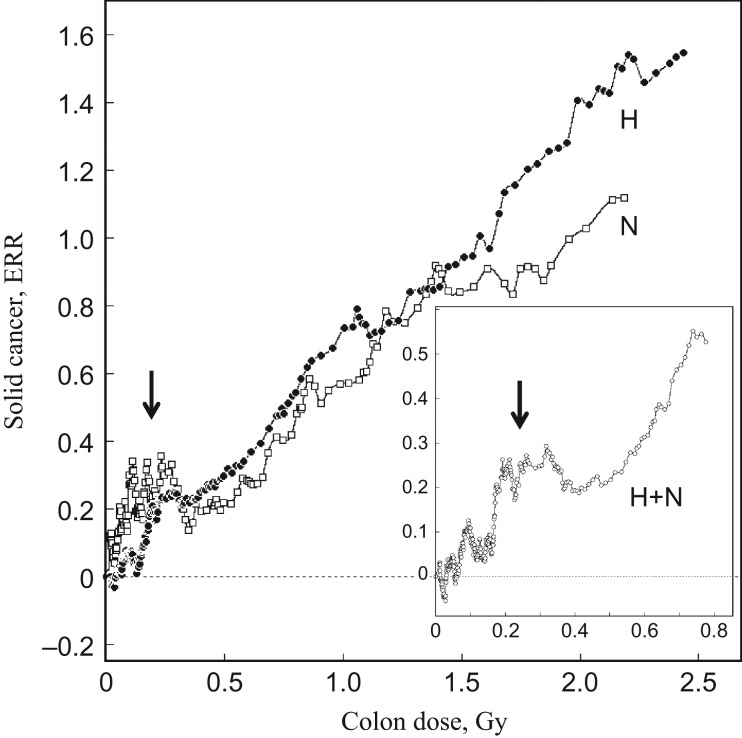
In the mixed radiation field, the a priori setting of the dose–response model for biological endpoints strongly influences the allowance for the kinetics and magnitude of neutron RBE as well. While there is no city-specific difference in the survivor distribution profile as a function of shielded kerma or organ doses (Fig. 1B(a)), differences do exist between the two cities in the dose–response patterns of the biological endpoints, such as chromosome aberrations and cancer risk. This can be seen clearly in Fig. 2, which shows a model-free visualization of excess relative risk (ERR) of solid cancer against the unadjusted colon dose in Gy, in which the neutrons are weighted by RBE = 1, coluDS02w1. ERR was calculated from the RERF ‘lssinc07’ database, as described [6]. The ‘lssinc07’ database contains cancer incidence data for 111 952 persons followed up during the period encompassing 1958–1998, in which information on the occurrence of cancer is cross-tabulated in a total of 26 806 data cells containing information on city, sex, person years at risk, age at the time of bombing, attained age, DS02 dose, etc. Persons exposed to doses <0.05 Gy and those who were not in the city at the time of bombing (NIC) were treated as controls. Instead of following a dose category strategy, the dose–response profiles were visualized by moving window averaging, whereby, to avoid systematic distortion of the distribution of relative risk around the mean, a window of 1500 cells was consecutively moved with a step size of 50, here referred to as MW = w1500s50. The mean dose within the window was expressed as the person-year weighted dose.
Fig. 2.
Model-free visualization of excessive relative risk (ERR) of solid cancer by moving window averaging for unadjusted and unmodified DS02 colon dose, coluDS02w1. Insert shows the ERR at low doses in the two cities combined cohort. Solid circles = Hiroshima (H), open squares = Nagasaki (N), open circles = two cities combined (H + N). The abnormal elevation of ERR at low doses is shown by arrow.
It is evident from Fig. 2 that, in spite of the comparability of dose distribution between the two cities (Fig. 1B(a)), the cancer rate clearly differs between the two cities, implying that the late effects are not simply a function of the shielded kerma or organ dose, or coluDS02w1. It should also be noted that abnormal elevations of the cancer rate are apparent at low doses, below 0.2 or 0.3 Gy, in a manner that is more pronounced in Nagasaki. In order to adjust for the cohort size of the two cities, the calculation was made in a combined cohort. As seen in the insert of Fig. 2, the abnormal elevation at low doses was again evident. The origin of this abnormal elevation will be discussed later. It is highly likely that, in addition to the dose-dependent downward shift of doses by adjustment factors, this abnormal elevation at low doses adds a factor to the global dose–response curve in favor of dose linearity.
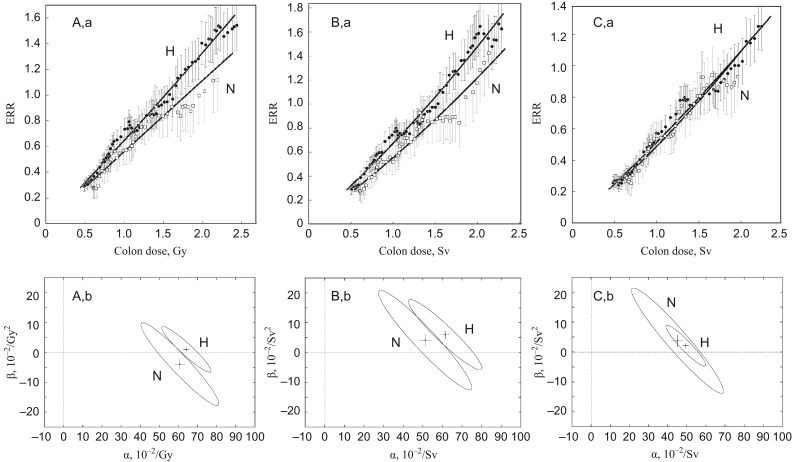
In order to avoid the influence of this abnormality at low doses, ERR dose responses were calculated for doses in which a lower cut-off point of 0.3 Gy (or Sv) and an upper cut-off point of 3 Gy (or Sv) of the doses of individual survivors were used (Fig. 3). The dose-dependent RBE of neutrons used in Fig. 3C is the same as that reported previously [6], i.e.
| (3) |
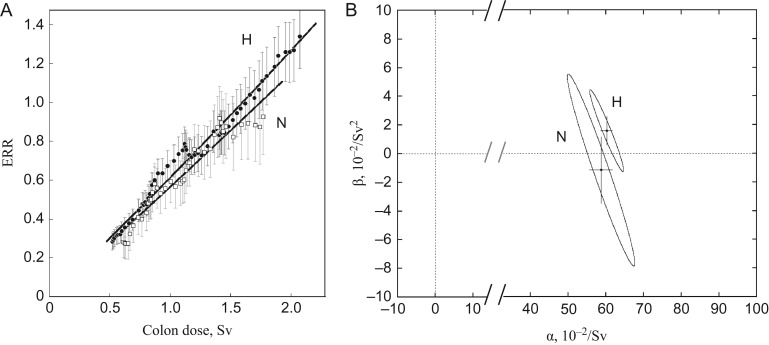
where the ratio of the iso-effective doses of neutrons (Dn) and 60Co γ-rays as reference radiation (Dγ) is expressed by the linear–quadratic model, y = C + αD + βD2. The parameters used are αγ = 1.310 × 10−2/Gy, αn = 1.127/Gy, βγ = βn = 5.355 × 10−2/Gy2 and Rm = αn/αγ for maximum RBE (RBEm). The quotient θ = β/α is the curvature parameter, a large value for which implies a strongly curved dose–response relationship. It is θγ = 4.08 for γ-rays and θn = 0.05 for neutrons. Because of the difference in curvature, the ratio of two doses, Dγ and Dn, yielding the same effect, i.e. RBE, is variable depending on dose. The dose–response relationship of ERR was fitted to a linear–quadratic model, y = αD + βD2, by the maximum likelihood method. As seen in Fig. 3, it is apparent that the choice of neutron weighting system is a critical determinant for giving an equivalence of cancer risk between the two cities. In contrast to the survivor distribution presented in Fig. 1B(a), the city difference in cancer risk was already apparent when RBE = 1 was used for neutrons (Fig. 3A), and could not be eliminated by the conventional use of a constant RBE = 10 together with or without the dose adjustment (Fig. 3B). The use of other values for constant RBE did not improve the city equivalence (data not shown). The dose–response relationships of the two cities were highly matched only when neutrons were weighted by a dose-dependent variable RBE (Fig. 3C). The dose–response relationship of ERR for all solid cancers for a combined cohort of the two cities is:
| (4) |
where D is the DS02 unadjusted colon dose in Sv in which neutrons are weighted by a dose-dependent variable RBE (Eq. 3). The city difference in the dose–response relatioship was tested by comparing the joint confidence regions of the α- and β-terms as calculated by:
| (5) |
where S(α,β) is the residual sum of squares, S(α*,β*) is the variance. C(v1;1 − q) is the percentile of χ2 distribution at the 100 × (1 − q)% level (two-sided test) for the number of degrees of freedom, v1 = p and v2 = n − p, where p is the number of parameters and n is the number of data points. As shown in Fig. 3 (lower panel), half-sized 95% confidence ellipses (q = 0.05) of the two cities fully overlap only when neutrons are weighted by a dose-dependent variable RBE (Fig. 3C and b). This indicates that the dose responses of cancer risk in the two cities are comparable and mutually compatible and are potentially applicable to other cases of human radiation exposure as a reference dose–response function. These results thereby reject the use of a constant RBE and dose adjustment by random dosimetry errors. The a priori setting of a linear dose–response relationship could be one reason that renders a large variability in biological endpoints and the neutron RBE inflexible. Indeed, uncertainties in dose–response relationships of chromosome aberrations have been solved by introducing the dose-dependent variable RBE of neutrons [6, 18]. Severe epilation could also be a possible outcome (our unpublished results). In this dose range, the dose–response kinetics of solid cancers and leukemia are also comparable beyond the city difference only when the neutrons are weighted by a dose-dependent RBE [19].
Fig. 3.
Dose–responses of excessive relative risk (ERR) of solid cancers in Hiroshima and Nagasaki (upper panel) and the testing of their statistical significance between two cities by 95% joint confidence regions (lower panel). (A) Unadjusted colon dose with neutrons weighted by RBE = 1 (coluDS02w1). (B) Adjusted colon dose with neutrons weighted by RBE = 10 (colaDS02w10). (C) Unadjusted colon dose with neutrons weighted by dose-dependent variable RBE (coluDS02wgn). Vertical and horizontal lines in the confidence ellipse show the SEs of the mean. For dose-dependent variable RBE, see Eq. (3). H = Hiroshima, N = Nagasaki.
In the context of the abnormal elevation at low doses, here we focused only on solid cancers, because while leukemia/lymphoma did not follow such an abnormal response in Hiroshima, the low-dose response pattern of leukemia/lymphoma in Nagasaki was not clear, possibly due to the inclusion of endemic adult T-cell leukemia/lymphoma, which was assumed to be refractory to the radiation carcinogenesis [19]. The origin of the abnormal elevation of the cancer rate at low doses is not clear. A subset (~20%) of the LSS cohort, called the adult health study (AHS) cohort, was reported to have received more frequent X-ray examinations during biennial medical examinations at ABCC/RERF [20–22]. Cumulative doses to the colon and stomach up to the year 1970 have been estimated to be ~90 mGy and ~500 mGy, respectively. However, the excess medical exposure may not be a factor, since exclusion of the AHS cohort did not eliminate the abnormal elevation of ERR at low doses (data not shown). According to the nuclear explosion scenario, human exposure to ionizing radiations resulting from A-bomb explosion is diverse, and includes prompt and delayed radiation (γ-rays and neutrons), fallout of radioactive fission products and vaporized radioactive fuel materials, and radiation from neutron-activated secondary radionuclides. The pathways are also multiple, consisting of direct acute or chronic external and internal exposure by inhalation or digestion (Ohtaki M, personal communication). However, the evaluation of radiation doses other than direct radiation suffers from the scarcity of systematic information necessary for the calculation of individual survivor doses, while not excluding other possible sources of exposure. Furthermore, uncertainties may also be due to disturbances in predicted levels of radioactive pollution/contamination due to rainfall 20–30 min after the bombing (black rain) and later by the large typhoon on the 17 September. The typhoon was one of the three largest typhoons ever to hit Japan, making landfall first on the island of Kyushu in a region ~160 km south of Nagasaki and then directly hitting Hiroshima. Nevertheless, aided by modern technologies, the radioactive fallout has been estimated from the radiation levels detected in some soil samples taken soon after the bombing: black rain streaks on walls, black rain deposits in roof guttering, and in undisturbed soil samples taken over a wide area [23]. The low-dose abnormality could be a reflection of radioactive fallout in general or of neutron-activated radionuclides. Cancer risk of early entrance into the bombed area is inconclusive; for instance, Kato et al. [24] reported no significant increase in the LSS cohorts of Hiroshima and Nagasaki, whereas in the different setting of the study cohort in Hiroshima, Matsuura et al. [25] suggested an elevation of cancer mortality, particularly in those entering on the day of the bombing. It is noteworthy, however, that the low-dose abnormality is more pronounced in survivors from the Nagasaki bombing, particularly with regards to cancers of the lung, liver and gallbladder [19]. It is thus tempting to correlate such cancer rates with the inhalation and/or ingestion of fuel materials, particularly of 235U/238U and 239Pu (isotope enrichment being 80% for 235U and 94.5% for 239Pu), in addition to other transmuted radionuclides. These fuel materials are easily vaporized by fission thermal energy (boiling temperature is 3930°C for 235U/238U and 3235°C for 239Pu). The internally incorporated 235U/238U and 239Pu continue to emit alpha-particles of high biological effectiveness. The air pollution resulting from these radionuclides is suggested by their inclusion in the fallout and/or black rain [26–32]. The biological half-life of 235U/238U is 15–100 days and that of 239Pu is ~200 years [33]. Uncertainties about the origin of the abnormal elevation of the cancer rate at low doses should be rigorously discussed before determining the direct A-bomb radiation as a sole concern in the cancer risk dose response and its modeling.
Assessment of neutron RBE by microdosimetric measurements
Cullings et al. [7] concluded that the RBE of neutrons in a γ–neutron mixed field such as that in A-bomb radiation differed from that of a so-called ‘pure neutron field’ experimental system, or that calculated independently from the intermingled γ-rays. Experimental systems using fission neutrons are usually a mixture of differing levels of γ-rays. Yet, at least at biologically relevant moderate doses, nil or negligible levels of interaction of high– and low–linear energy transfer (LET) radiations have been reported for various biological endpoints in mixed radiation fields [34–41]; however, others have reported synergistic-like effects [42, 43].
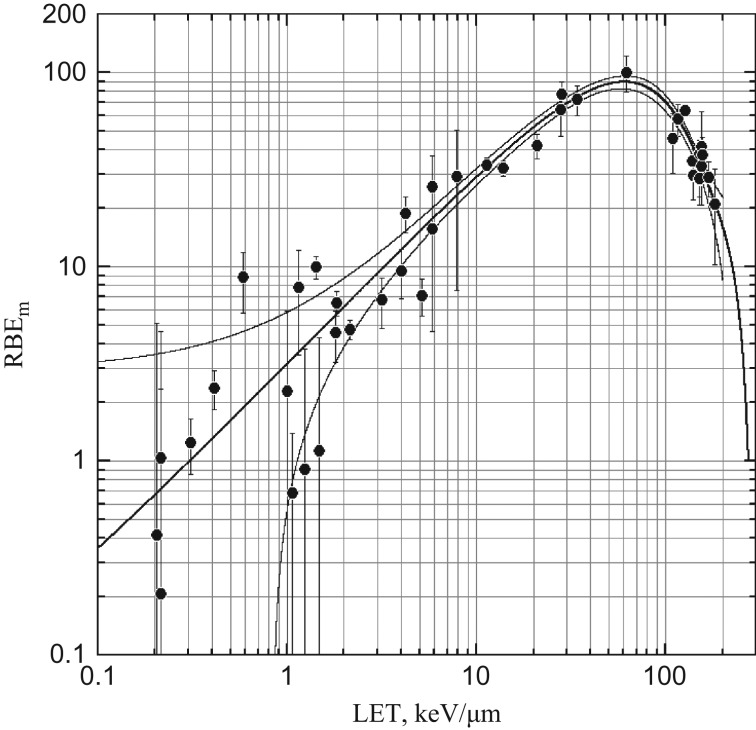
This independency was further confirmed here by comparing the microdosimetric profile of neutrons and neutron-induced chromosome aberrations in human peripheral blood lymphocytes. Fig. 4 shows the LET (L) dependence of RBEm of dicentric chromosomes induced in human G0 lymphocytes irradiated in vitro. This quantity, referred to here as r(L), corresponds to the quality factor, Q(L), as defined in ICRP Publication 60 [44], but is restricted here to chromosome aberrations by external beams of monoenergetic light charged particles, i.e. electrons, protons and alpha-particles. The data were taken from literature reports and compiled in Fig. 3 of Sasaki [45], in which the LET dependence of RBEm was expressed as a function of binary interactions of DNA double-strand breaks (DSB). However, for simplicity it was expressed here as a polynomial function of unrestricted track-average LET, i.e. r(L) = ∑aiLi, in which i = 0, 1, 2, 3, , , , k, and k was the degree of polynomial as determined by the Akaike information criterion (AIC) maximum likelihood method. Parameters of the best fit are a0 = (4.053 ± 2.947) × 10−2, a1 = 3.194 ± 0.011, a3 = −(1.682 ± 0.074) × 10−4, a4 = (3.247 ± 0.043) × 10−6, a5 = −(1.655 ± 0.011) × 10−8, a6 = (3.731 ± 0.010) × 10−11 and a7 = −(3.187 ± 0.0003) × 10−17.
Fig. 4.
LET dependence of maximum RBEm of dicentric chromosomes induced in human lymphocytes irradiated in vitro with various charged particles. The LET dependence was fitted to a polynomial function, r(L) = ∑αiLi. Curves show regression line (thick line) and 95% confidence intervals (thin lines). Vertical lines show 95% confidence limits.
The LET–RBEm relationship, r(L), was applied to the track-average LET of recoil protons estimated from the microdosimetric spectrum of various neutron fields, and the resulting RBEm distributions were integrated to estimate the average RBEm, or Cn(E). The Cn(E) is analogous to the more generalized quantity, mean quality factor of neutrons, Qn(E), i.e. the mass element-based radiation weighting factor of neutrons. Fig. 5 shows the outline of the estimation of Qn of the neutron field. The microdosimetry data measured by TEPC with the sensitive cavity of diameter d = 1 or d = 2 μm were selected from the literature, including accelerator-produced monoenergetic and quasimonoenergetic neutrons and fission neutrons [46–66]. The microdosimetric quantities are defined as:
| (6) |
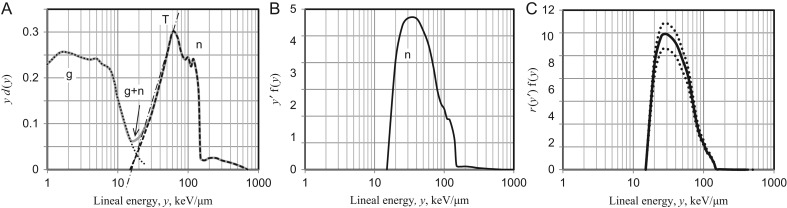
where Etr is the initial energy of recoil protons, and l is the mean chord length of the sensitive cavity [67]. The neutron fields are usually contaminated with varying amounts of γ-rays. To subtract the γ component, the γ–neutron boundary was determined by the principle of triangular chord length distribution [68, 69] (Fig. 5A). After subtracting the γ component, the dose distribution, yd(y), was converted to the frequency distribution, yf(y), and then normalized to unity. The lineal energy, y, is analogous to the track-average LET. However, for the low-energy recoil protons, the LET tends to be underestimated, with the track length being shorter than the mean chord length of the sensitive cavity. The correction was made for measured y that produced recoil protons with initial energy insufficient to traverse the cavity diameter. The energy dependence of the continuously slowing down approximation (CSDA) range, R, and track-average LET of recoil protons, y’ = T/R, were calculated according to the Proton Table of ICRU Report 49 for methane-based TE-gas (ρ = 1.0641 × 10−3) and ICRU muscle (ρ = 1.04) as a tissue substitute [70]. Accordingly, the adjusted LET, y’, implies y’ > y for R < l and y’ = y for R ≥ l. The break point was y = 70 keV/μm for the sensitive cavity of d = 1 μm and y = 80 keV/μm for d = 2 μm.
Fig. 5.
Microdosimetric estimation of RBEm of neutrons in mixed γ–neutron field (example). (A) TEPC reading of lineal energy spectrum (solid line) and determination of γ (dotted line) and neutron (dashed line) components. Slashed line (T) shows a tangent to the assumed triangular spectrum. (B) Distribution of lineal energy after adjustment by CSDA range of recoil protons and their LET for ICRU muscle. (C) Distribution of RBEm. Dotted lines show lower and upper bounds based on 95% confidence intervals of RBEm-LET curve, r(L), in Fig. 4.
Since the energy dependence of LET of TE-gas was slightly higher than that of ICRU muscle, mainly due to the difference in their electronic collisional stopping power and hence in their CSDA range, the LET values for TE-gas were converted to those for ICRU muscle according to their proportionality (Fig. 5B). Referring to the LET dependence of RBEm, r(L), the RBEm distribution against the TEPC reading of y was obtained (Fig. 5C). The average RBEm of a given neutron field that corresponded to a chromosome version of mean quality factor (Qn) was calculated by:
| (7) |
The chord length modification, y-to-y’ conversion, mentioned above was not applied in the following instances: (a) high energy spectra (above the proton tail, which is y = 115 keV/μm and y = 225 keV/μm for the sensitive cavity with d = 1 μm and d = 2 μm, respectively) that correspond to alpha recoils by 17O(n,α)14C reaction and recoil C-, N- and O-ions; (b) neutron fields with a mean energy of E > 1.5 MeV, in which the low y counts cannot be distinguished from those due to high-energy (fast) protons with low LET. In these neutrons the y’ = y rule may tend to underestimate the RBE; (c) thermal and epithermal neutrons, where the reactions are mostly capture reactions such as 1H(n,γ)d, 14N(n,p)14C and 17O(n,α)14C. Their kerma contribution in ICRU muscle is 35.94% for protons (0.59 MeV), 37.24% for γ (2.25 MeV) and 25.94% for 14C heavy ions (0.40 MeV). The contribution of other components is <1%. High energy γ-rays may not contribute to the RBE. Heavy ions may be disregarded because of their extremely high LET, which is so high that it likely kills cells. Therefore, protons are major species relating to the RBEm of the thermal and epithermal neutrons. The 0.59 MeV protons have a CSDA range of 11.15 μm and a track-average LET of 52.94 keV/μm. As such, the y’ = y rule will be properly applied for the whole spectrum of the thermal and epithermal neutrons.
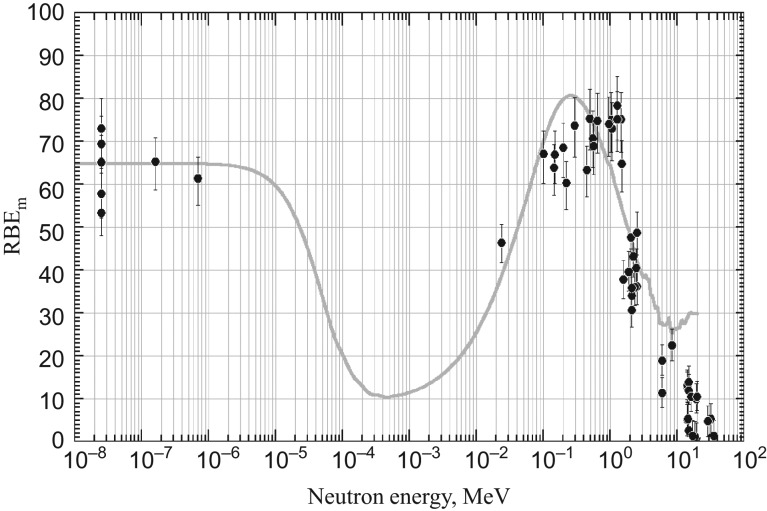
The source-term information for neutron fields and their calculated energy-averaged RBEm, i.e. Qn values, are presented in an attached table (supplementary data). The table also includes a simple arithmetic mean of lineal energy, . Although such a rounded quantity may be informative in dealing with cell inactivation, it cannot be applied to such endpoints as mutations, including chromosome aberrations, that could be registered only in living cells. The cells are highly abortive at LET beyond the proton edge. Fig. 6 shows the average RBEm, i.e. chromosomally determined mean quality factor, Qn, plotted against neutron energy. Except for their magnitude, the energy dependency of Qn was highly comparable with that of Schuhmacher and Siebert [71] calculated by MCNP Monte Carlo neutron transport code applied to the ICRU tissue substitute. Their calculation was carried out based on the unspecified radiation quality factor, Q(L), of ICRP Publication 60 [44], whereas the r(L) presented here is specific to chromosome aberrations. The values follow similar LET dependence, but differ in their magnitude, each giving a maximum value of Q(L)max ≈ 30 and r(L)max ≈ 90 according to their response functions. When the Qn value was rescaled according to the r(L)max/Q(L)max ratio, the energy dependencies were highly comparable with each other (Fig. 6). The Qn was broadly compatible with the operational radiation protection quantities of ICRP [4], e.g. the effective quality factor, , and the radiation weighting factor, wR, at the high-energy range, giving a maximum at ~1 MeV. However, and wR dissociate from Qn, being less sensitive to neutron energy at low energies (<100 MeV). This is because and wR are specifically defined quantities expressed as the ambient dose equivalent at 10 mm depth in a tissue-equivalent ICRU sphere of 30 cm diameter, where secondary photons from the (n,γ) capture reaction largely contribute to the dose and hence moderate the contribution of the high-LET charged particles from (n,el) elastic recoil and (n,p) and (n,α) capture reactions. In the ICRU tissue substitute, the probability of (n,γ) reaction is highest (σ = 0.037 barn) for thermal neutrons (0.025 eV) and decreases with an increase in neutron energy to σ ≈ 5.3 × 10−6 barn at 100 MeV.
Fig. 6.
Average RBEm, i.e. mean quality factor of neutron, Qn, calculated from microdosimetry spectra of various neutron fields plotted against neutron energy. Vertical lines represent the 95% confidence intervals derived from curve fitting of r(L). The thin curve represents the mean quality factor of neutron, Qn, of Schuhmacher and Siebert [71] after rescaling by the r(L)max/Q(L)max ratio of the radiation quality factor.
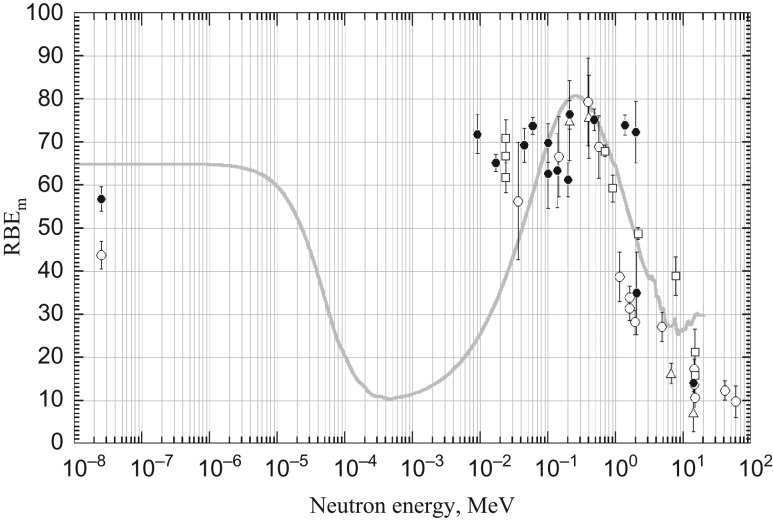
Fig. 7 shows the RBEm of dicentrics observed in human G0 lymphocytes irradiated in vitro with neutrons. The dose–response relationships were taken from the literature, and RBEm was calculated as the ratio of the initial slope of the dose–response relationship to the neutron component and that of 60Co γ-rays as the reference radiation, i.e. RBEm = αn/αγ. The initial slope of the γ-ray response was determined from pooled data for laboratories with large-scale experiments, i.e. RBC Kyoto [7, 18, 45, 72–74], GSF Munchen [36, 37, 39, 75–81] and NRPB Harwell [82–87], but was determined individually for solitary head-to-head experiments in other laboratories [88–90]. The relationship between chromosomally determined RBEm and the neutron energy in the experimental system was highly comparable with those estimated from the microdosimetric spectra of TEPC counting (Fig. 6) and those estimated from the MCNP neutron transport code [71]. The latter two calculations are based purely on neutrons. However, in the chromosomal evaluation, the neutrons are contaminated with varying amounts of γ-rays. The equivalence among the three estimates strongly indicates that, at least at biologically relevant low or moderate doses, the interaction between the γ and neutron components may be negligibly small, if it exists at all.
Fig. 7.
RBEm of dicentrics observed in human G0 lymphocytes irradiated with neutrons in vitro. Data are taken from the literature: RBC Kyoto (solid circles), GSF Munchen (open circles), NRPB Harwell (open squares) and other laboratories (open triangles). The vertical lines represent the 95% confidence interval based on the SE of the initial slope for neutrons. For explanation of thin curve, see legend to Fig. 6.
Neutron RBE in the mixed-radiation field
Considerably large RBE values at low doses have been also suggested in A-bomb survivors, but with very large uncertainties due to a small neutron component fraction, particularly in the DS86 and DS02 dosimetry systems [91–98]. Cullings et al. [7] raised the point that neutron RBE values determined independently from the coexisting γ-rays might lead to an erroneous risk evaluation because the neutron RBE could be strongly suppressed in the mixed-radiation field. They assumed that the effects in the mixed field, E(Dγ,Dn), were equivalent to the effects of γ-ray dose, Dγ, plus an increment of γ-equivalent dose, Δ, due to neutrons, E(Dγ + Δ, 0), i.e.:
| (8) |
The RBE in the mixed field is defined by R(Dγ,Dn) = Δ/Dn. The RBE is dependent both on γ-rays and neutrons (Equation A2 of Cullings et al. [7]). The formula is similar to that previously proposed by Rossi and Zaider [91] for neutron RBE in the mixed field.
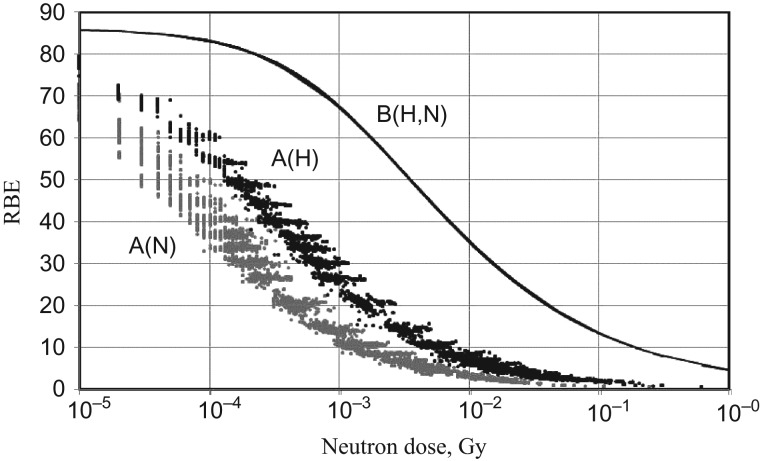
Fig. 8 shows the neutron RBE in the LSS cohorts of Hiroshima and Nagasaki. The neutron RBEs in the mixed field were calculated by the R(Dn,Dγ) formulated by Cullings et al. [7], using the same dose–response parameters presented for Eq. (3) (as was also the case for Cullings et al. [7]). The R(Dn,Dγ) values are scattered and lower in Nagasaki (Fig. 8 A(N)) than in Hiroshima (Fig. 8 A(H)), reflecting varying neutron/γ-ray ratios in individual survivors and their lower ratio in Nagasaki. Fig. 8 also includes the neutron RBEs for individual survivors calculated independently from the γ-rays (Fig. 8 B(H,N)). They are simply dependent on the neutron dose, irrespective of the coexisting γ-rays and were the same for the two cities. In any case, the neutron RBE varies depending on dose, irrespective of whether the calculation is based on the γ-dependence or -independence model, although the magnitude is much smaller for the γ-dependence model.
Fig. 8.
RBE of neutrons in LSS cohorts from Hiroshima (H) and Nagasaki (N) expressed as a function of neutron dose (Gy) for the unadjusted DS02 colon dose, coluDS02w1. (A) RBE of neutrons in terms of γ-dependence model, R(Dγ,Dn) of Cullings et al. [7]. (B) RBE in terms of γ-independence model. Calculations were made using the ‘lssinc07’ database.
The RBE in the γ-dependence model, R(Dγ,Dn), was applied to the dose–response relationship of the ERR of solid cancer in Hiroshima and Nagasaki (Fig. 9). As seen in the figure, equivalence of the two cities was not improved, and even yielded much poorer matching than when a constant RBE = 10 was used. The difference in the dose–response relationship between the two cities was statistically significant, as shown by the non-overlap of the half-sized 95% ellipses (Fig. 9B). Thus, the RBE value for the γ-independence model was more appropriate in eliminating the city difference (Fig. 3C). The reason for the discrepancy remains to be elucidated. It should be noted, however, that in the dependence model, the RBE is defined as an increment of effective dose, ∆, that satisfies RBE = ∆/Dn. This differs from the canonical definition of the equi-effective dose ratio. Since in this case the dimension of ∆ is not dose but ‘effectiveness’, the interactions, Dγ∆ and ∆2, are theoretically unrealistic.
Fig. 9.
Dose–responses for ERR of solid cancer in Hiroshima (H) and Nagasaki (N) and test for difference by 95% joint confidence intervals (CIs). Doses are unadjusted colon dose, coluDS02wmix, in which neutrons were weighted by R(Dγ,Dn) for the γ-dependence model. (A) Dose–response relationships. The vertical lines represent the 80% CIs. (B) The 95% joint CIs are based on the χ2 distribution and shown in half size. The vertical and horizontal lines within the ellipse represent the SEs of the mean.
Alternatively, by setting the RBE as an equi-effective dose ratio ω = Dγ/Dn, the yield in the mixed field can be expressed by
| (9) |
where the neutron RBE of an individual survivor is given by ω = [−1 + {1 + 4θγ(Rm + θnDn)Dn}1/2]/2θγDn. The RBE thus obtained in the LSS cohort turned out to be independent of the magnitude of the intermingled γ-ray dose, identical to that for the γ-independence model, e.g. RBE given in Eq. (3), and (moreover) identical between the two cities as well (Fig. 8 B(H,N)).
DISCUSSION AND CONCLUSION
On the basis of the microdosimetric analysis presented here, we could not find any evidence for deviation of the neutron RBE in the γ–neutron mixed radiation field. The neutron RBE was independent of the magnitude of the coexisting γ-rays. In the microdosimetric aspect, a possible interaction between the two components could be a function of ζ(DγDn), which represents the mutual interaction between the primary DNA damage generated by neutrons and photons. However, its magnitude may be negligibly small at low doses of low-energy neutrons because the energy deposition of recoil protons is highly localized. In contrast, that of γ-associated Compton and photoelectrons is sparsely distributed over the cell nucleus. The difference in cancer risk between Hiroshima and Nagasaki can be readily explained by differences in the neutron component amount, which completely disappear only when the neutrons are weighted by a dose-dependent variable RBE. This indicates that even a small amount of neutrons cannot be disregarded in the risk evaluation.
Interestingly, a chromosome aberration–based RBE provided the most appropriate weighting factor for neutrons for cancer rates in survivors. Obviously, chromosome aberrations themselves may not be the sole and integral events leading to the development of cancer. Yet, the parallelism strongly suggests that they share the same underlying molecular mechanisms, such as pathway choice for the repair of DNA double-strand breaks [19]. The processes leading to cancer are complex and may include a variety of confounding factors. Therefore, the curvature of the dose–response relationship itself does not guarantee the underlying causal mechanisms. The equivalence of cancer rates between the two cities may verify the adequacy of radiation weighting by chromosomally derived RBE and suggests that chromosome structural aberrations (rearrangements) could serve as surrogate biomarkers for radiological cancers. In spite of the proposed modulation of neutron RBE in a mixed-radiation field, chromosomal RBE as assessed by a chromosomal version of quality factor of neutron, Qn, against neutron energy was confirmed to be independent of the intermingled incident γ-rays; this is consistent with predictions based on microdosimetric measurements and the Monte Carlo simulation of energy transport of neutrons alone. However, it should be noted that the radiation quality factor, r(L), and derived quality factor of neutrons, Qn, thus chromosomally obtained were much larger than those currently adopted for radiological protection [44, 99]. For radiological protection purposes, ICRP uses a maximum radiation quality factor, Q(L), of the rounded number of ~20, with its possible peak value of ~30 in its recommended response function [4, 44]. This value is a rounded number obtained from experimental data compiled by Sinclair [100], the ICRU Report No. 40 [101] and NCRP Report No. 104 [102]. In conveying a possible variability of biological effects, the Q(L) is not specified for a particular biological endpoint. Endpoints include a range of biological events relevant to radiological protection in general, such as chromosome aberrations, mutations, malignant transformation in cultured mammalian cells, tumor induction and life shortening in experimental animals. It was thus apparent that the current Q(L) and its derived quality factor, Qn, effective quality factor, and radiation weighting factor, wR, of neutrons are seriously underestimated for the calculation of cancer risk, possibly by a factor of ~3.
All those lines of evidence presented here indicate that the RBE of fission neutrons is variable depending only on neutron dose, not on the magnitude of the intermingled γ-rays in the mixed field. The cancer risk in A-bomb survivors can best be explained by introducing such a dose-dependent variable RBE system, and thus provides a generalized measure for risk assessment in other cases of human radiation exposures.
Supplementary Material
ACKNOWLEDGEMENTS
This report makes use of publicly available databases, ‘DS02can’, ‘lssins07’ and ‘lssinc07ahs’, released from RERF (http://www.rerf.or.jp). The conclusions in this report are those of the authors and do not necessarily reflect the scientific judgment of the RERF or its funding agencies.
SUPPLEMENTARY DATA
Supplementary data on the neutron field and its mean quality factor, Qn, calculated from TEPC-measured microdosimetric spectra are available at the Journal of Radiation Research online.
CONFLICT OF INTEREST
The authors report that there are no conflicts of interest. The authors alone are responsible for the content and writing of this report.
REFERENCES
- 1.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007;168:1–64. [DOI] [PubMed] [Google Scholar]
- 2.Richardson D, Sugiyama H, Nishi N, et al. Ionizing radiation and leukemia mortality among Japanese atomic bomb survivors, 1950–2000. Radiat Res 2009;172:368–82. [DOI] [PubMed] [Google Scholar]
- 3.Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res 2012;177:229–43. [DOI] [PubMed] [Google Scholar]
- 4.ICRP (International Commission on Radiological Protection) The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP 2007;37:1–332. [DOI] [PubMed] [Google Scholar]
- 5.Cullings HM, Fijita S, Funamoto S.. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiat Res 2006;166:219–54. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki MS, Nomura T, Ejima Y, et al. Experimental derivation of relative biological effectiveness of A-bomb neutrons in Hiroshima and Nagasaki and implications for risk assessment. Radiat Res 2008;170:101–17. [DOI] [PubMed] [Google Scholar]
- 7.Cullings HM, Pierce DA, Kellerer AM.. Accounting for neutron exposure in the Japanese atomic bomb survivors. Radiat Res 2014;182:587–98. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich WF, Cullings HM.. Use of the individual data of the A-bomb survivors for biologically based cancer models. Radiat Environ Biophys 2010;49:39–46. [DOI] [PubMed] [Google Scholar]
- 9.Jablon S. Atomic bomb radiation dose estimation at ABCC. RERF Technical Report. 23–71 1971;1–41.
- 10.Gilbert ES. Some effects of random dose measurement errors on analysis of atomic bomb survivor data. Radiat Res 1984;98:591–605. [PubMed] [Google Scholar]
- 11.Pierce DA, Stram DO, Vaeth M.. Allowing for random errors in radiation dose estimates for the atomic bomb survivor data. Radiat Res 1990;123:275–84. [PubMed] [Google Scholar]
- 12.Pierce DA, Preston DL, Stram DO, et al. Allowing for dose estimation errors for the A-bomb survivor data. J Radiat Res 1991;32 Suppl:108–21. [DOI] [PubMed] [Google Scholar]
- 13.Pierce DA, Stram DO, Vaeth M, et al. The errors-in-variables problem: considerations provided by radiation dose–response analyses of the A-bomb survivor data. J Am Stat Assoc 1992;87:351–9. [Google Scholar]
- 14.Pierce DA, Vaeth M, Cologne JB.. Allowance for radiation dose estimation errors in atomic bomb survivor studies: a revision. Radiat Res 2008;170:118–26. [DOI] [PubMed] [Google Scholar]
- 15.Sposto R, Stram DO, Awa AA.. An investigation of random errors in the DS86 dosimetry using data on chromosome aberrations and severe epilation. Technical Report No. 7-90. Radiation Effects Research Foundation, Hiroshima, 1990. [PubMed] [Google Scholar]
- 16.Stram DO, Sposto R.. Recent uses of biological data for the evaluation of A-bomb radiation dosimetry. J Radiat Res 1991;32 Suppl:122–35. [DOI] [PubMed] [Google Scholar]
- 17.Stram DO, Sposto R, Preston D, et al. Stable chromosome aberrations among A-bomb survivors: an update. Radiat Res 1993;136:29–36. [PubMed] [Google Scholar]
- 18.Sasaki MS, Endo S, Ejima Y, et al. Effective dose of A-bomb radiation in Hiroshima and Nagasaki as assessed by chromosomal effectiveness of spectrum energy photons and neutrons. Radiat Environ Biophys 2006;45:79–91. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki MS, Tachibana A, Takeda S.. Cancer risk at low doses of ionizing radiation: artificial neural networks inference from atomic bomb survivors. J Radiat Res 2014;55:391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto O, Antoku S, Russell WJ, et al. Medical x-ray exposure doses as contaminants of atomic bomb doses. Health Phys 1988;54:257–69. [DOI] [PubMed] [Google Scholar]
- 21.Kazuo K, Antoku S, Sawada S, et al. Organ doses received by atomic bomb survivors during radiological examinations at the Radiation Effects Research Foundation. Br J Radiol 1991;64:720–7. [DOI] [PubMed] [Google Scholar]
- 22.Kato K, Sawada S.. Medical X-ray doses’ contributions to the ionizing radiation exposures of atomic-bomb survivors. J Radiat Res 1991;32 Suppl:136–53. [DOI] [PubMed] [Google Scholar]
- 23.Aoyama M, Oochi Y (eds). Revisit THE Hiroshima A-bomb with a Database. Hiroshima: Hiroshima City, 2011. [Google Scholar]
- 24.Kato H, Brown CC, Hoel DG, et al. Studies of the mortality of A-bomb survivors. Report 7. Mortality, 19501978: Part 2. Mortality from causes other than cancer and mortality in early entrants. Radiat Res 1982;91:243–64. [PubMed] [Google Scholar]
- 25.Matsuura M, Hayakawa N, Shimokata H.. Survival analyses of atomic bomb survivors in Hiroshima Prefecture, Japan, 1968–1982. Cancer mortality risk among early entrants. Hiroshima J Med Sci 1995;44:29–38. [PubMed] [Google Scholar]
- 26.Sakanoue M, Tsuji T.. Plutonium content of soil at Nagasaki. Nature 1971;234:92–3. [Google Scholar]
- 27.Takada J, Hoshi M, Sawada S, et al. Uranium isotopes in Hiroshima ‘black rain’ soil. J Radiat Res 1983;24:229–36. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto M, Komura K, Sakanoue M, et al. Pu isotopes, 241Am and 137Cs in soils from the atomic bombed area in Nagasaki and Hiroshima. J Radiat Res 1985;26:211–23. [DOI] [PubMed] [Google Scholar]
- 29.Kudo A, Mahara Y, Santry DC, et al. Geographical distribution of fractionated local fallout from the Nagasaki A-bomb. J Environ Radioact 1991;14:305–16. [Google Scholar]
- 30.Fujikawa Y, Shizuma K, Endo S, et al. Anomalous 235U/238U ratios and metal elements detected in the black rain from the Hiroshima A-bomb. Health Phys 2003;84:155–62. [DOI] [PubMed] [Google Scholar]
- 31.Saito-Kokubu Y, Yasuda K, Magura M, et al.. Distribution of plutonium isotopes and 137Cs found in the surface soils of Nagasaki, Japan. J Geosci (Osaka City Univ) 2007;50:7–13. [Google Scholar]
- 32.Sakaguchi A, Kawai K, Steier P, et al. Feasibility of using 236U to reconstruct close-in fallout deposition from the Hiroshima atomic bomb. Sci Total Environ 2010;408:5392–8. [DOI] [PubMed] [Google Scholar]
- 33.Eisenbud M. Environmental Radioactivity. New York: Academic Press, 1973. [Google Scholar]
- 34.Barendsen GW, Beusker TL, Vergroesen AJ, et al. Effects of different radiations on human cells in tissue culture. II. Biological experiments. Radiat Res 1960;13:841–9. [PubMed] [Google Scholar]
- 35.Booz J, Fidorra J.. Microdosimetric investigations on collimated fast neutron beams for radiation therapy: II. The problem of radiation quality and RBE. Phys Med Biol 1981;26:43–56. [DOI] [PubMed] [Google Scholar]
- 36.Bauchinger M, Kühn H, Dresp J, et al. Dose–effect relationship for 14.5 MeV (d+T) neutron-induced chromosome aberrations in human lymphocytes irradiated in a man phantom. Int J Radiat Biol Relat Stud Phys Chem Med 1983;43:571–8. [DOI] [PubMed] [Google Scholar]
- 37.Schmid E, Schraube H, Bauchinger M.. Chromosome aberration frequencies in human lymphocytes irradiated in a phantom by a mixed beam of fission neutrons and γ-rays. Int J Radiat Biol 1998;73:263–7. [DOI] [PubMed] [Google Scholar]
- 38.Ballarini F, Biaggi M, Edwards A, et al. Estimating mixed field effects: an application supporting the lack of a non-linear component for chromosome aberration induction by neutrons. Radiat Prot Dosimetry 2003;103:19–28. [DOI] [PubMed] [Google Scholar]
- 39.Schmid E, Wagner FM, Romm H, et al. Dose–response relationship of dicentric chromosomes in human lymphocytes obtained for the fission neutron therapy facility MEDAPP at the research reactor FRM II. Radiat Environ Biophys 2009;48:67–75. [DOI] [PubMed] [Google Scholar]
- 40.Phoenix B, Green S, Hill MA, et al. Do the various radiations present in BNCT act synergistically? Cell survival experiments in mixed alpha-particle and gamma-ray fields. Appl Radiat Isot 2009;67:S318–20. [DOI] [PubMed] [Google Scholar]
- 41.Wojcik A, Obe G, Lisowska H, et al. Chromosomal aberrations in peripheral blood lymphocytes exposed to a mixed beam of low energy neutrons and gamma radiation. J Radiol Prot 2012;32:261–75. [DOI] [PubMed] [Google Scholar]
- 42.Mason AJ, Giusti V, Green S, et al. Interaction between the biological effects of high- and low-LET radiation dose components in a mixed exposure. Int J Radiat Biol 2011;87:1162–72. [DOI] [PubMed] [Google Scholar]
- 43.Staaf E, Deperas-Kaminska M, Brehwens K, et al. Complex aberrations in lymphocytes exposed to mixed beams of 241Am alpha particles and X-rays. Mutat Res 2013;756:95–100. [DOI] [PubMed] [Google Scholar]
- 44.ICRP (International Commission on Radiological Protection) 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP, 1991;21(1–3). [PubMed] [Google Scholar]
- 45.Sasaki MS. Advances in the biophysical and molecular bases of radiation cytogenetics. Int J Radiat Biol 2009;85:26–47. [DOI] [PubMed] [Google Scholar]
- 46.Rodgers RC, Gross W.. Microdosimetry of monoenergetic neutrons. In: Booz J, Ebert HG, Eickel R, et al. (eds). Proceedings of the 4th Symposium on Microdosimetry, 1974. Commission of the European Communities, Luxembourg (cited in ICRU Report 36, 1983) p. 1027 (Report No. EUR 5122).
- 47.Amols HI, Dicello JF, Awschalom M, et al. Physical characterization of neutron beams produced by protons and deuterons of various energies bombarding beryllium and lithium targets of several thicknesses. Med Phys 1977;4:486–93. [DOI] [PubMed] [Google Scholar]
- 48.Stinchcomb TG, Kuchnir FT, Skaggs LS.. Comparison of the microdosimetric event-size method and the twin-chamber method of separating dose into neutron and gamma components. Phys Med Biol 1980;25:51–64. [DOI] [PubMed] [Google Scholar]
- 49.Fidorra J, Booz J.. Microdosimetric investigations on collimated fast-neutron beams for radiation therapy: I. Measurements of microdosimetric spectra and particle dose fractions in a water phantom for fast neutrons from 14 MeV deuterons on beryllium. Phys Med Biol 1981;26:27–41. [DOI] [PubMed] [Google Scholar]
- 50.Stafford PM, Horton JL, Almond PR.. A microdosimetric characterization of a cyclotron-produced therapeutic neutron beam. Med Phys 1987;14:1015–9. [DOI] [PubMed] [Google Scholar]
- 51.Kliauga P, Horton J, Stafford P.. Microdosimetry of a 42 MeV therapy neutron beam. Int J Radiat Oncol Biol Phys 1989;16:845–8. [DOI] [PubMed] [Google Scholar]
- 52.Tubiana M, Dutreix J, Wambersie A.. Neutrons and other heavy particles In: Tubiana M, Dutreix JD, Wambersie A (eds). Introduction to Radiobiology. London: Taylor and Francis, 1990, 273–312. [Google Scholar]
- 53.Wuu CS, Amols HI, Kliauga P, et al. Microdosimetry for boron neutron capture therapy. Radiat Res 1992;130:355–9. [PubMed] [Google Scholar]
- 54.Waker AJ. Principles of experimental microdosimetry. Radiat Prot Dosimetry 1995;61:297–308. [Google Scholar]
- 55.Srdoč D, Marino SA.. Microdosimetry of monoenergetic neutrons. Radiat Res 1996;146:466–74. [PubMed] [Google Scholar]
- 56.Endo S, Hoshi M, Takada J, et al. Neutron generator (HIRRAC) and dosimetry study. J Radiat Res 1999;40 Suppl:14–20. [DOI] [PubMed] [Google Scholar]
- 57.Onizuka Y, Endo S, Ishikawa M, et al. Microdosimetry of epithermal neutron field at the Kyoto University reactor. Radiat Prot Dosimetry 2002;99:383–5. [DOI] [PubMed] [Google Scholar]
- 58.Ménard S, Chau Q, Lahaye T.. Active neutron dosimeters based on microdosimetric principles: research studies. Radiat Prot Dosimetry 2002;99:375–6. [DOI] [PubMed] [Google Scholar]
- 59.Martín G, Gerdung S, Raabe M, et al. A practical design of a cylindrical tissue-equivalent proportional counter for microdosimetry. Radiat Prot Dosimetry 2002;99:379–80. [DOI] [PubMed] [Google Scholar]
- 60.Endo S, Onizuka Y, Ishikawa M, et al. Microdosimetry of neutron field for boron neutron capture therapy at Kyoto University reactor. Radiat Prot Dosimetry 2004;110:641–4. [DOI] [PubMed] [Google Scholar]
- 61.Burmeister J, Yudelev M, Kota C, et al. Boron neutron capture enhancement of fast neutron radiotherapy utilizing a moderated fast neutron beam. Med Phys 2005;32:666–72. [DOI] [PubMed] [Google Scholar]
- 62.Moro D, Colautti P, Lollo M, et al. BNCT dosimetry performed with a mini twin tissue-equivalent proportional counter (TEPC). Appl Radiat Isot 2009;67:S171–4. [DOI] [PubMed] [Google Scholar]
- 63.Endo S, Tanaka K, Fujikawa K, et al. Microdosimetry on a mini-reactor UTR-KINKI for educational uses and biological researches. J Radiat Res 2009;50:83–7. [DOI] [PubMed] [Google Scholar]
- 64.Agosteo S, Colautti P, Fanton I, et al. Study of a solid state microdosimeter on a monolithic silicon telescope: irradiations with low-energy neutrons and direct comparison with a cylindrical TEPC. Radiat Prot Dosimetry 2011;143:432–5. [DOI] [PubMed] [Google Scholar]
- 65.Colauti P, Moro D, Chiriotti S, et al. Microdosimetric measurements in the thermal neutron irradiation facility of LENA reactor. Appl Radiat Isot 2014;88:147–52. [DOI] [PubMed] [Google Scholar]
- 66.Zhang W, Wang Z, Liu Y, et al. TEPC performance for a reference standard. Radiat Prot Dosimetry 2014;158:246–50. [DOI] [PubMed] [Google Scholar]
- 67.ICRU Microdosimetry. ICRU Report 36. International Commission on Radiation Units and Measurements, Bethesda, Maryland, USA, 1983.
- 68.Rossi HH, Rosenzweig W.. A device for the measurement of dose as a function of specific ionization. Radiology 1955;64:404–11. [DOI] [PubMed] [Google Scholar]
- 69.Glass WA, Braby LA.. A wall-less detector for measuring energy deposition spectra. Radiat Res 1969;39:230–40. [PubMed] [Google Scholar]
- 70.ICRU Stopping powers and ranges for protons and alpha particles. ICRU Report 49. International Commission on Radiation Units and Measurements, Bethesda, Maryland, USA, 1993.
- 71.Schuhmacher H, Siebert BR.. Quality factors and ambient dose equivalent for neutrons based on the new ICRP recommendations. Radiat Prot Dosimetry 1992;40:85–9. [Google Scholar]
- 72.Sasaki MS. Radiation-induced chromosome aberrations in human lymphocytes: possible biological dosimeter in man. In: Sugahara T, Hug O (eds). Biological Aspects of Radiation Protection, Tokyo: Igaku Shoin ltd; Berlin: Springer, 1971, 81–91.
- 73.Sasaki MS, Saigusa S, Kimura I, et al. Biological effectiveness of fission neutrons: energy dependency and its implication for the risk assessment. In: Japan Atomic Energy Research Institute (ed). Proceedings of the International Conference on Radiation Effects and Protection, Mito, 1992 pp. 33–5.
- 74.Sasaki MS, Takatsuji T, Ejima Y.. The F value cannot be ruled out as a chromosomal fingerprint of radiation quality. Radiat Res 1998;150:253–8. [PubMed] [Google Scholar]
- 75.Schmid E, Schlegel D, Guldbakke S, et al. RBE of nearly monoenergetic neutrons at energies of 36 keV–14.6 MeV for induction of dicentrics in human lymphocytes. Radiat Environ Biophys 2003;42:87–94. [DOI] [PubMed] [Google Scholar]
- 76.Schmid E, Regulla D, Guldbakke S, et al. Relative biological effectiveness of 144 keV neutrons in producing dicentric chromosomes in human lymphocytes compared with 60Co γ-rays under head-to-head conditions. Radiat Res 2002;157:453–60. [DOI] [PubMed] [Google Scholar]
- 77.Schmid E, Regulla D, Guldbakke S, et al. The effectiveness of monoenergetic neutrons at 565 keV in producing dicentric chromosomes in human lymphocytes at low doses. Radiat Res 2000;154:307–12. [DOI] [PubMed] [Google Scholar]
- 78.Bauchinger M, Koester L, Schmid E, et al. Chromosome aberrations in human lymphocytes induced by fission neutrons. Int J Radiat Biol Relat Stud Phys Chem Med 1984;45:449–57. [DOI] [PubMed] [Google Scholar]
- 79.Bauchinger M, Schmid E, Rimpl G, et al. Chromosome aberrations induced in human lymphocytes after irradiation with 15.0 MeV neutrons in vitro. I. Dose–response relation and RBE. Mutat Res 1975;27:103–9. [DOI] [PubMed] [Google Scholar]
- 80.Nolte R, Mühlbradt K-H, Meulders JP, et al. RBE of quasi-monoenergetic 60 MeV neutron radiation for induction of dicentric chromosomes in human lymphocytes. Radiat Environ Biophys 2005;44:201–9. [DOI] [PubMed] [Google Scholar]
- 81.Schmid E, Wagner FM, Canella L, et al. RBE of thermal neutrons for induction of chromosome aberrations in human lymphocytes. Radiat Environ Biophys 2013;52:113–21. [DOI] [PubMed] [Google Scholar]
- 82.Lloyd DC, Edwards AA, Prosser JS, et al. In vitro induction of chromosomal aberrations in human lymphocytes, with and without boron 10, by radiations connected in boron neutron capture therapy. Br J Radiol 1988;61:1136–41. [DOI] [PubMed] [Google Scholar]
- 83.Edwards AA, Lloyd DC, Prosser JS.. The induction of chromosome aberrations in human lymphocytes by 24 keV neutrons. Radiat Prot Dosimetry 1990;31:265–8. [Google Scholar]
- 84.Aghamohammadi SZ, Goodhead DT, Savage JR.. Production of chromosome aberrations, micronuclei, and sister-chromatid exchanges by 24-keV epithermal neutrons in human G0 lymphocytes. Mutat Res 1989;211:225–30. [DOI] [PubMed] [Google Scholar]
- 85.Lloyd DC, Purrott RJ, Dolphin GW, et al. Chromosome aberrations induced in human lymphocytes by neutron irradiation. Int J Radiat Biol Relat Stud Phys Chem Med 1976;29:169–82. [DOI] [PubMed] [Google Scholar]
- 86.Lloyd DC, Purrott RJ, Reeder EJ, et al. Chromosome aberrations induced in human lymphocytes by radiation from 252Cf. Int J Radiat Biol Relat Stud Phys Chem Med 1978;34:177–86. [DOI] [PubMed] [Google Scholar]
- 87.Lloyd DC, Edwards AA, Prosser JS, et al. Chromosome aberrations induced in human lymphocytes by D-T neutrons. Radiat Res 1984;98:561–73. [PubMed] [Google Scholar]
- 88.Dobson RL, Straume T, Carrano AV, et al. Biological effectiveness of neutrons from Hiroshima bomb replica: results of a collaborative cytogenetic study. Radiat Res 1991;128:143–9. [PubMed] [Google Scholar]
- 89.Vulpis N, Tognacci L, Scarpa G.. Chromosome aberrations as a dosimetric technique for fission neutrons over the dose-range 0.2–50 rad. Int J Radiat Biol Relat Stud Phys Chem Med 1978;33:301–6. [DOI] [PubMed] [Google Scholar]
- 90.Fabry L, Leonard A, Wambersie A.. Induction of chromosome aberrations in G0 human lymphocytes by low doses of ionizing radiations of different quality. Radiat Res 1985;103:122–34. [PubMed] [Google Scholar]
- 91.Rossi HH, Zaider M.. Contribution of neutrons to the biological effects in Hiroshima. Health Phys 1990;58:645–7. [PubMed] [Google Scholar]
- 92.Zaider M. Evidence of a neutron RBE of 70 (±50) for solid-tumor induction at Hiroshima and Nagasaki and its implication for assessing the effective neutron quality factor. Health Phys 1991;61:631–6. [DOI] [PubMed] [Google Scholar]
- 93.Rossi HH, Zaider M.. Comment on the contribution of neutrons to the biological effect at Hiroshima. Radiat Res 1996;146:590–3. [PubMed] [Google Scholar]
- 94.Little MP. Estimates of neutron relative biological effectiveness derived from the Japanese atomic bomb survivors. Int J Radiat Biol 1997;72:715–26. [DOI] [PubMed] [Google Scholar]
- 95.Kellerer AM, Nekolla E.. Neutron versus γ-ray risk estimates. Inferences from the cancer incidence and mortality data in Hiroshima. Radiat Environ Biophys 1997;36:73–83. [DOI] [PubMed] [Google Scholar]
- 96.Rühm W, Walsh L, Chomentowski M.. Choice of model and uncertainties of the gamma-ray and neutron dosimetry in relation to the chromosome aberrations data in Hiroshima and Nagasaki. Radiat Environ Biophys 2003;42:119–28. [DOI] [PubMed] [Google Scholar]
- 97.Kellerer AM, Rühm W, Walsh L.. Indication of the neutron effect contribution in the solid cancer data of the A-bomb survivors. Health Phys 2006;90:554–64. [DOI] [PubMed] [Google Scholar]
- 98.Walsh L. Neutron relative biological effectiveness for solid cancer incidence in the Japanese A-bomb survivors: an analysis considering the degree of independent effects from γ-ray and neutron absorbed doses with hierarchical partitioning. Radiat Environ Biophys 2013;52:29–36. [DOI] [PubMed] [Google Scholar]
- 99.ICRP (International Commission on Radiological Protection) Relative Biological Effectiveness (RBE), Quality Factor (Q) and Radiation Weighting Factor (wR). ICRP Publication 92. Ann ICRP 2003;33(4). [DOI] [PubMed] [Google Scholar]
- 100.Sinclair WK. Experimental RBE values of high-LET radiation at low doses and the implications for quality factor assignment. Radiat Prot Dosimetry 1985;13:319–26. [Google Scholar]
- 101.ICRU The Quality Factor in Radiation Protection. ICRU Report No. 40. International Commission on Radiation Units and Measurements, Bethesda, Maryland, USA, 1986.
- 102.NCRP The relative biological effectiveness of radiations of different quality. NCRP Report No. 104. National Council on Radiation Protection and Measurements, Bethesda, Maryland, USA, 1990.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.