Abstract
Background
Multichannel electrogastrography (M-EGG) can be used to evaluate gastrointestinal motility. The myoelectric activity of the remnant stomach after surgery has not been measured by M-EGG. This study examined whether myoelectric activity varied with surgical technique and compared vagus nerve-preserving distal gastrectomy (VP-DG) with standard distal gastrectomy without vagus nerve preservation (DG). Furthermore, we examined the relationship between the M-EGG findings and patients' postoperative symptoms.
Methods
Twenty-six patients who underwent VP-DG, 20 who underwent DG, and 12 healthy volunteers as controls were examined with M-EGG. The Gastrointestinal Symptom Rating Scale (GSRS) was used to assess postoperative symptoms.
Results
Longer periods of normal gastric function (normogastria, 2.0–4.0 cycle min–1) were detected in channel 1 in the VP-DG group than in the DG group in either the fasted or fed state (P<0.05). The percentage of slow wave coupling (%SWC) in the fed state correlated negatively with GSRS scores (reflux, r=–0.59, P=0.02; abdominal pain, r=–0.51, P=0.04, indigestion, r=–0.59, P=0.02 and total score, r=–0.75, P=0.02).
Conclusions
Slow waves can be recorded non-invasively using M-EGG in the remnant stomach following gastrectomy. The VP-DG group showed better preserved gastric myoelectric activity than the DG group, and the %SWC showed a significant negative correlation with scores of GSRS (reflux, abdominal pain, indigestion and total score) in the VP-DG group.
Keywords: multichannel electrogastrography, vagus nerve-preserving distal gastrectomy, %normal, percentage of slow wave coupling, gastrointestinal symptom rating scale
Introduction
Many techniques allow gastrointestinal motility to be observed and measured. Electrogastrography (EGG) records the electrical activity associated with stomach peristalsis, and it has recently attracted attention as a new means of evaluating gastric motility. The first EGG was recorded in 1922 by Walter Alvarez (1) and utilized cutaneous electrodes applied to the epigastric region. Since then, single-channel EGG has become a widely used technique in clinical and research practice. Recently, multichannel EGG (M-EGG) has been developed, a technique that allows data to be recorded on 4 channels simultaneously.
Although vagus nerve preserving distal gastrectomy (VP-DG) has become a common operative method for early gastric cancer, there have been few scientific evaluations of remnant stomach function following this procedure. The aims of this study were to compare M-EGG recordings taken from patients who had undergone VP-DG, those who had undergone standard distal gastrectomy (DG) and explore the relationship between symptoms and M-EGG findings.
Recently many studies have used the Gastrointestinal Symptom Rating Scale (GSRS) (2) to evaluate postoperative symptoms following gastrectomy (3,4,5,6,7). The GSRS asks questions specific to gastrointestinal symptoms, and the specificity and sensitivity of the scale have been validated. We used the GSRS to evaluate symptoms in post-gastrectomy patients, and explored the relationship between symptoms and M-EGG findings.
Patients and Methods
Twelve healthy volunteers without abdominal complaints and not taking anticholinergic drugs and/or gastroprokinetic agents were examined as controls (healthy volunteers group). Twenty six patients who underwent vagus-nerve preserving distal gastrectomy (VP-DG group) and 20 patients who underwent standard distal gastrectomy (DG group) were enrolled. Informed consent had been obtained from all participants beforehand. The study was approved by the Kawasaki Medical School Ethics Committee. The participants' demographic details are summarized in Table 1. The gender distribution, mean age, body mass index in the three groups did not differ significantly.
Table 1. Clinical background of participants.
| Cases | Sex (male/female) | Age (years) | Body mass index (kg/m2) | |
|---|---|---|---|---|
| Healthy volunteers | 12 | 8/4 | 56.1 ± 12.9 | 20.6 ± 2.4 |
| VP-DG | 26 | 20/6 | 64.6 ± 12.8 | 21.9 ± 2.8 |
| DG | 20 | 13/7 | 62.7 ± 13.8 | 21.6 ± 2.5 |
VP-DG: Vagus-nerve preserving distal gastrectomy. DG: Standard distal gastrectomy. ± values are mean ± S.D. values.
Surgical procedures
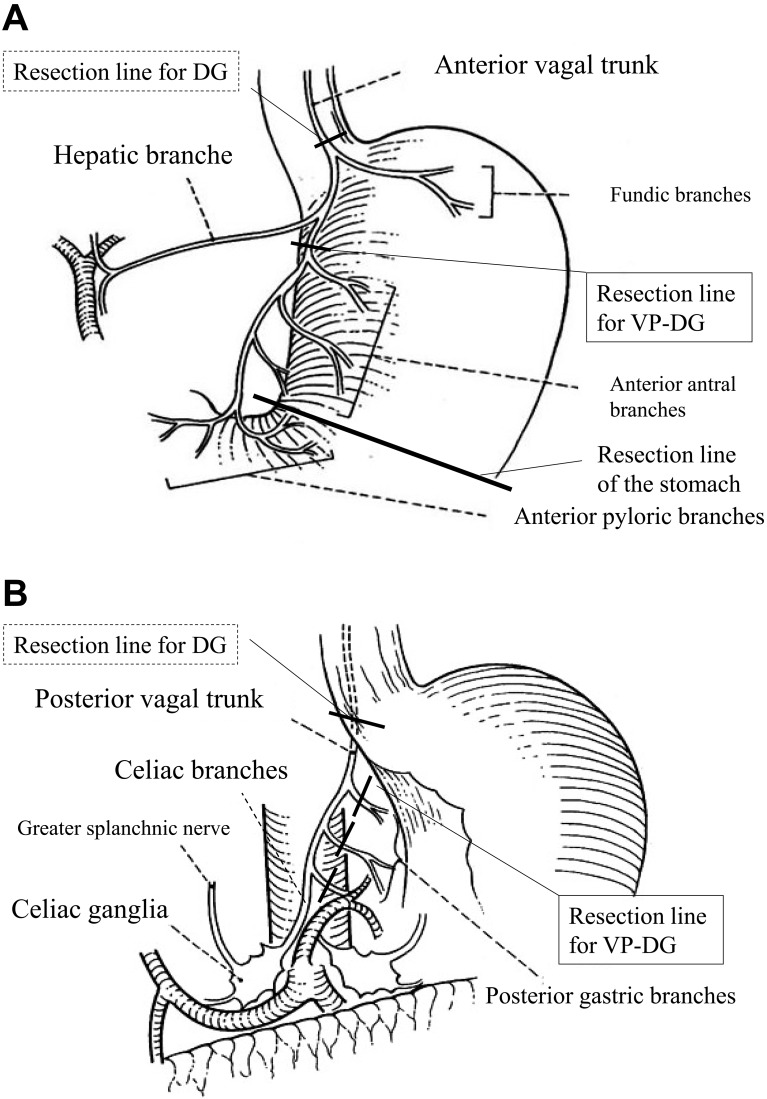
In both the VP-DG and DG groups, the anastomosis was made by Billroth's operation I by using the layer-to-layer hand-sewn method. The VP-DG group patients had early gastric cancer with D1+α lymph node dissection. In the VP-DG group, the hepatic branches of the anterior vagal trunk and the celiac branches of the posterior vagal trunk were preserved, while the gastric branches of both trunks were cut in their distal portions (Fig. 1A, 1B). In the DG group, all patients had advanced gastric cancer and underwent dissection of D2 lymph nodes and the anterior and posterior vagal trunks were cut at the proximal end. All cases underwent lymph node dissection, and met the curative potential criteria of "Resection A" as defined by the Japanese Classification of Gastric Carcinoma, 14th Edition (8).
Fig. 1.
Resection lines for standard distal gastrectomy without vagus nerve preservation (DG) and vagus nerve-preserving distal gastrectomy (VP-DG) procedures. (A) The anterior vagal trunk was resected in the DG group, while the hepatic branches and fundic branches were preserved in the VP-DG group (From Kazuya Yamaguchi Syuzyutu 2007; 61: 958, translated into English with permission). (B) The posterior vagal trunk was resected in the DG group, while the celiac branches were preserved in the VP-DG group (From Kazuya Yamaguchi Syuzyutu 2007; 61: 958, translated into English with permission).
M-EGG
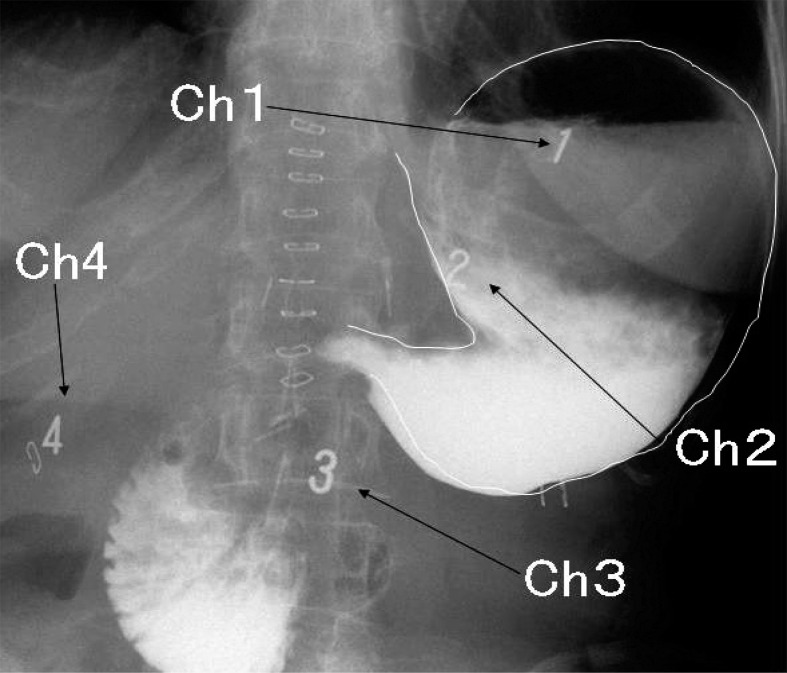
M-EGG was conducted two weeks after surgery. After overnight fasting, M-EEG was recorded in the early morning using four electrodes (Medtronic, Minneapolis, MN). The skin was cleaned with water and alcohol before applying the electrodes. The ground electrode (G) was placed on the left costal margin, horizontal to the midpoint of the line between the xyphoid process and the umbilicus. The reference electrode was placed on the xiphoid process. The Channel 3 electrode (Ch3) was positioned between the xiphoid process and umbilicus, and the Channel 4 electrode (Ch4) was placed 4 cm to the right of Ch3, the Channel 2 electrode (Ch2) and the Channel 1 electrode (Ch1) were placed with an interval of 4–6 cm on a line leading from Ch3 at a 45° angle towards the left costal margin. The grounding electrode was put on the left costal margin on a horizontal line stretching from Ch3 as standard for M-EGG. The electrodes of postoperative patients were placed in the same way. Gastric contrast radiography of DG patients with the electrodes in situ is shown in Fig. 2. Ch1 and Ch2 were placed above the fundus and upper gastric corpus, respectively. However, Ch3 or Ch4 electrodes were distant from the remnant stomach (Fig. 2) and only signals from Ch1 and Ch2 could be recorded. The recording data from Ch3 and Ch4 were not analyzed in this study. In distal gastrectomy the remnant stomach is anchored by the gastrophrenic and gastrosplenic ligaments. Because these ligaments are not resected in distal gastrectomy, they maintain the position of the gastric fundus in postoperative patients as in normal individuals. Therefore, it was decided that no X-ray examination was necessary to confirm or adjust electrode position. Fasting M-EEG was recorded for 20 min in the supine position whilst resting. Then each participant ate two commercially purchased rice balls with Japanese green tea (250 ml) before recording the fed M-EGG for 20 min. The position and conditions during fed M-EGG were identical to those during fasting M-EGG. The nutritional value of one rice ball was 180 kcal, with 4 g protein, 0.5 g fat, 40 g carbohydrates, 1 g salt, 25 mg cholesterol, 1 g fiber, and 0.6 mg vitamins.
Fig. 2.
The positions of electrodes in relation to the remnant stomach after DG are shown on a typical abdominal radiograph in a patient who underwent gastrectomy. Placement of Ch1 and Ch2 over the fundus of the remnant stomach and the upper gastric corpus is shown.
1) M-EGG conditions and environment
Each electrode was connected to a PolyGraf (Medtronic, Minneapolis, MN) via an EGG 4-channel extension cable. Signals were analyzed using a Medtronic Polygram Net EGG 3111224 system. A 1.8 cycle min–1 (cpm) high-pass filter and a 15 cpm low- pass filter were used.
2) Analysis of M-EGG
Bradygastria, normogastria, and tachygastria were defined as cycle frequency ranges of 0.5–2.0 cpm, 2.0–4.0 cpm, and 4.0–9.0 cpm, respectively. Frequencies outside these ranges were considered as arrhythmia. The relative times of each during the recording period are reported as %normal, %bradygastria, %tachygastria, and the %arrhythmia respectively. Spectrum analysis in each frequency range was performed, and signal data acquired every 256 s were filtered and processed by fast Fourier transformation to obtain power spectra. Then, the dominant frequency and dominant power were obtained from the power spectra in order to calculate the percentage of time during which the wave of a similar frequency was transmitted from one to the other electrodes (%slow wave coupling: %SWC). Slow wave coupling was defined as the percentage of time during which the peak power frequency difference was <0.2 cpm (9).
Evaluation of postoperative symptoms
After postoperative M-EGG, postoperative gastrointestinal symptoms were assessed using the Japanese version of the GSRS in 15 patients in the DG group and in 12 patients in the VP-DG group. The GSRS consists of 15 items that assess digestive tract symptoms on an interview-based rating scale. The 15 items on the GSRS evaluate the five domains: reflux, abdominal pain, ingestion, diarrhea, and constipation. The GSRS data are presented as syndrome scores and a total score as the mean of specific scores.
Statistical analysis
The results of data analysis are expressed as the mean ± S.D. Statistical significance was set at P<0.05 and determined using the chi-squared test for independence, and Tukey-Kramer's test for multiple comparisons (Post-hoc test). The correlation between M-EGG findings and reported satisfaction was determined using Pearson's correlation coefficient (r).
Results
The clinical factors at the time of the M-EGG recording, except the intraoperative blood loss, did not differ between the two patients groups (Table 2).
Table 2. Clinical factors at the M-EGG recording in the VP-DG and DG groups.
| VP-DG | DG | P value | ||
|---|---|---|---|---|
| Operation times (min) | 208 ± 34.6 | 215 ± 30.0 | 0.48 | |
| Intraoperative blood loss (ml) | 96 ± 102.7 | 263 ± 200 | 0.001 | |
| WBC (/μl) | 5,616 ± 1,111 | 5,342 ± 1,263 | 0.47 | |
| Hb (g/dl) | 12.7 ± 1.12 | 11.7 ± 1.32 | 0.99 | |
| Alb (g/dl) | 3.08 ± 0.38 | 3.69 ± 0.57 | 0.48 | |
| CRP (mg/dl) | 1.59 ± 1.09 | 0.75 ± 1.07 | 0.98 | |
| Body weight† | 94.0 ± 2.51 | 93.2 ± 3.62 | 0.19 | |
| GSRS scores | ||||
| Reflux score | 1.67 ± 0.80 | 1.50 ± 0.65 | 0.55 | |
| Abdominal pain score | 1.37 ± 0.95 | 1.39 ± 0.53 | 0.86 | |
| Ingestion score | 1.81 ± 0.91 | 1.79 ± 0.59 | 0.95 | |
| Diarrhea score | 1.52 ± 1.15 | 1.50 ± 0.97 | 0.96 | |
| Constipation score | 2.04 ± 1.22 | 2.13 ± 0.96 | 0.83 | |
| Total score | 1.62 ± 0.43 | 1.66 ± 0.59 | 0.82 | |
± values are mean ± S.D. values. *P<0.05. WBC: white blood cell, Hb: hemoglobin, Alb: albumin, CRP: C-reactive protein. †The amount is expressed as %, when the preoperative levels are adjusted to 100%. GSRS: Gastrointestinal Symptom Rating Scale.
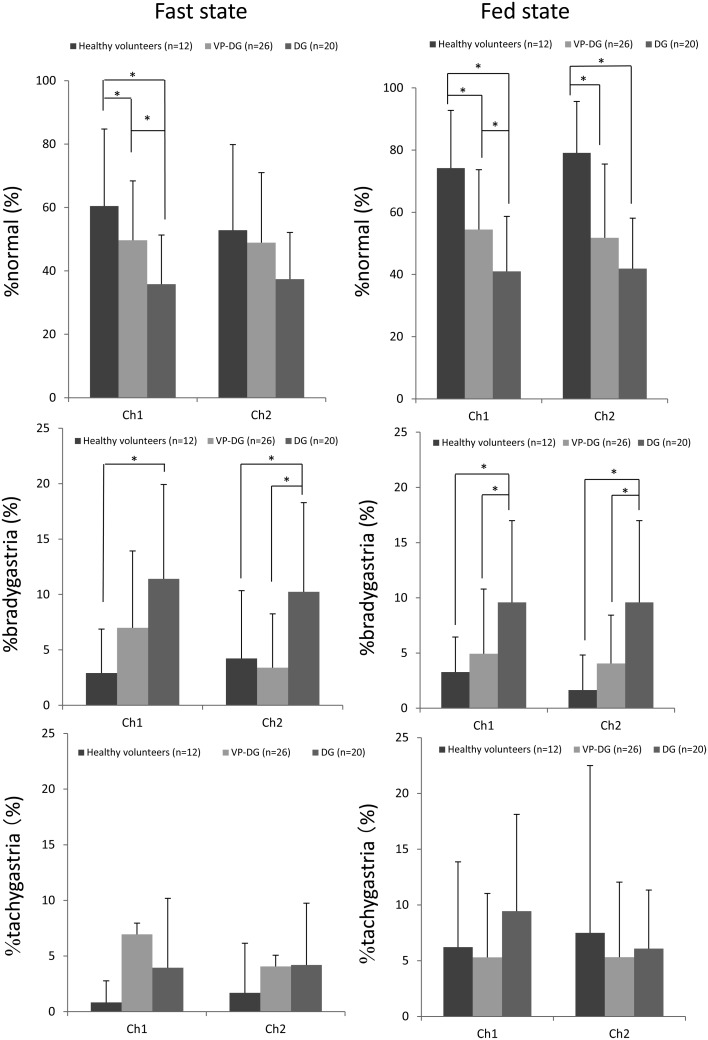
The comparison of the gastric myoelectric activity between the healthy volunteers, and both the VP-DG and DG groups are shown in Table 3. The %normal, % bradygastria and %tachgastria values recorded from Ch1 and Ch2 in each participant group are shown in Fig. 3.
Table 3. The comparison of the gastric myoelectric activity between the healthy volunteers, the VP-DG and DG groups.
| Ch1 | Ch2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Healthy
volunteers (n=12) |
VP-DG (n=26) |
DG (n=20) |
Healthy
volunteers (n=12) |
VP-DG (n=26) |
DG (n=20) |
|||
| %N | Fasting | 60.5 ± 24.3*** | 49.6 ± 18.7** | 35.8 ± 15.5 | 52.8 ± 27.0 | 48.9 ± 22.0 | 37.4 ± 14.7 | |
| Fed | 74.2 ± 18.6*, *** | 54.5 ± 19.2** | 41.0 ± 17.7 | 79.1 ± 16.5*, *** | 51.8 ± 23.8 | 41.9 ± 16.2 | ||
| %B | Fasting | 2.91 ± 3.96***, † | 6.49 ± 6.93 | 11.4 ± 8.50† | 4.23 ± 6.10*** | 4.40 ± 4.85** | 10.2 ± 8.04 | |
| Fed | 3.27 ± 5.91*** | 4.94 ± 5.85** | 9.52 ± 7.40 | 1.65 ± 3.17*** | 4.05 ± 4.37** | 9.58 ± 7.40 | ||
| %T | Fasting | 0.83 ± 1.94† | 6.95 ± 14.1 | 3.96 ± 6.23† | 1.69 ± 4.46 | 4.06 ± 3.23 | 4.20 ± 5.54 | |
| Fed | 6.21 ± 7.65 | 5.29 ± 5.73 | 9.44 ± 8.67 | 7.50 ± 15.0 | 5.31 ± 6.73 | 6.04 ± 8.55 | ||
| %A | Fasting | 35.0 ± 23.2 | 39.1 ± 17.3 | 48.8 ± 13.5 | 33.7 ± 18.9 | 45.5 ± 21.3 | 48.2 ± 14.8 | |
| Fed | 16.3 ± 11.8 | 35.3 ± 15.9 | 40.4 ± 15.1 | 15.5 ± 13.3 | 38.9 ± 18.9 | 42.4 ± 14.5 | ||
± values are mean ± S.D. values. VP-DG: Vagus-nerve preserving distal gastrectomy, DG: Standard distal gastrectomy. %N: %normal, %B: %bradygastria, %T: %tachygastria, %A: %arrhythmia. *P<0.05 Healthy volunteers vs. VP-DG, **P<0.05 VP-DG vs. DG, ***P<0.05 Healthy volunteers vs. DG, †P<0.05 fast vs. fed.
Fig. 3.
The gastric myoelectric activity between the healthy volunteers, VP-DG and DG groups. %normal and %bradygastria had significant difference between in the VP-DG and DG groups.
%normal from Ch1 in the healthy volunteers group was significantly higher than that in the DG groups (P<0.01) with a significant difference between the two patient groups (P<0.05) in the fasting state. In the fed state, %normal from Ch1 in the healthy volunteers group was significantly higher than that in the VP-DG (P<0.01) and DG groups (P<0.01), respectively, with a significant difference between the two patient groups (P<0.05) in the fasting state. %normal from Ch2 in the fasting state did not differ in all groups. %normal from Ch2 in the healthy volunteers group was higher than that in the VP-DG (P<0.01) and DG groups (P<0.01), without a significant difference between the two patient groups in the fed state. %normal from Ch1 and Ch2 had no significant difference between the fasting and fed state.
%bradygastria from Ch1 and Ch2 in the fasting state in the DG group was lower than that in the healthy volunteers (P<0.01). %bradygastria from Ch2 in the fasting state had significant difference between the two patient groups. In the fed state, %bradygastria from Ch1 and Ch2 in the DG groups was lower than that in the healthy volunteers group (P<0.01) with a significant difference between the two patient groups (Ch1: P<0.05, Ch2: P<0.05).%bradygastria from Ch1 had significant difference between the fasting and fed state in healthy volunteers (P<0.05) and DG group (P<0.05).
%tachygastria and %arrhythmia from Ch1 and Ch2 in the fasting and fed states had no significant difference between the three groups. %tachygastria from Ch1 had significant difference between in the fasting and fed state in healthy volunteers group (P<0.05) and DG group (P<0.05).
%SWC from Ch1-2 in the healthy volunteers group were significantly lower than that in the two patient groups (healthy volunteers group vs. VP-DG group: P<0.01, normal group vs. DG group: P<0.01) without a significant difference between the two patient groups in the fasting and fed states (Fasting state; healthy volunteers group: 61.6 ± 21.3%, VP-DG group: 38.6 ± 20.2, DG group: 43.5 ± 19.1, Fed state; healthy volunteers group: 76.2 ± 21.4%, VP-DG group: 43.9 ± 21.7, DG group: 49.8 ± 23.4). %SWC in the fed state is significantly higher than that in the fasting state in normal group (P<0.05). The two patients groups had no significant difference between in the fasting and fed state.
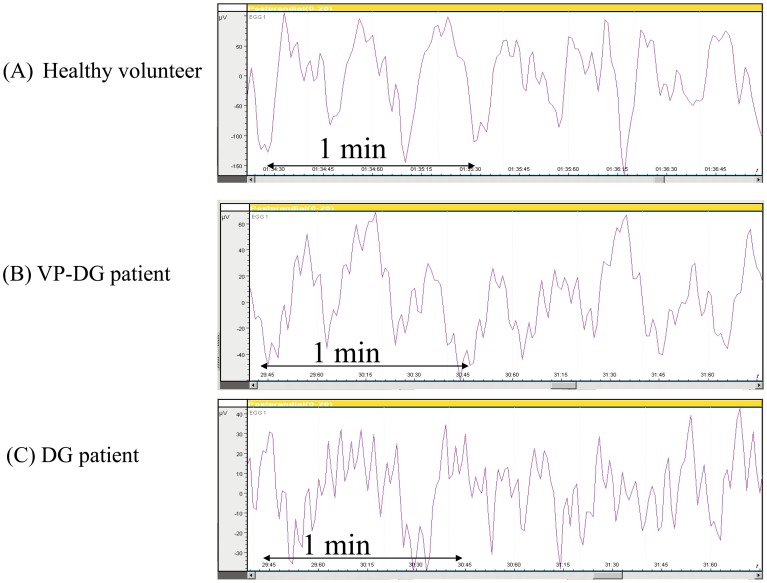
Typical gastric electrical recordings in the healthy volunteers, VP-DG and DG patient are shown in Fig.s 4A, 4B and 4C, respectively. The slow wave with a frequency of approximately 3 cpm was observed in the healthy volunteers group (Fig. 4A), indicating normal gastric electrical activity. A similar 3-cpm waveform was also seen in the VP-DG group (Fig. 4B), while the irregular slow wave were seen in the DG group (Fig. 4C).
Fig. 4.
Typical gastric electrical recordings in the healthy volunteer, VP-DG and DG patient from Ch1. A-C: M-EGG slow waves recorded from Ch1 of (A) Healthy volunteer (B) VP-DG patient and (C) DG patient.
The any of GSRS scores had no significant difference between the VP-DG and DG group (Table 2).
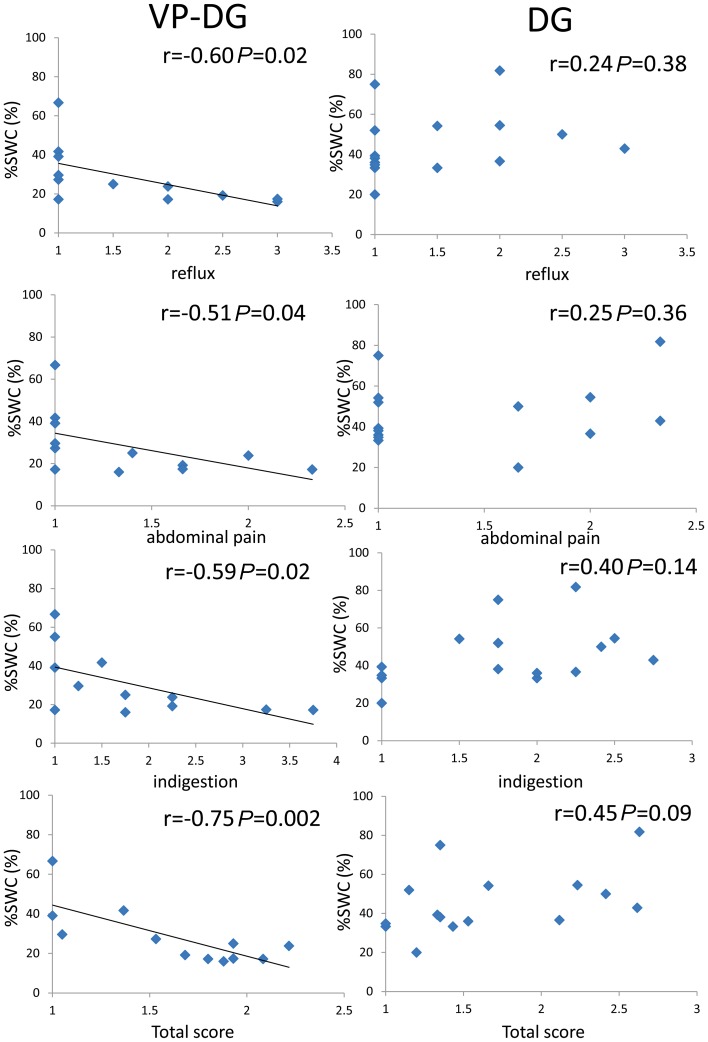
M-EGG parameters (%normal, %bradygastria, %tachygastria, %arrhythmia and %SWC) for the fasting and fed state did not correlate with any of the GSRS scores in the DG group. In the VP-DG group, there was no correlation between M-EGG parameters (%normal %bradygastria, %tachygastria, and %arrhythmia) in the fasting and fed state and GSRS scores. There was no correlation between %SWC in the fasting state and GSRS subscale scores, while the %SWC in the fed state correlated negatively with the GSRS scores (reflux: r=–0.60, P=0.02; abdominal pain: r=–0.51, P=0.04; indigestion: r=–0.59, P=0.04; total score: r=–0.75, P=0.002) (Fig. 5). %SWC in the fed state did not correlate with other GSRS subscale scores: diarrhea and constipation in VP-DG group. In VP-DG group and fasting state, %normal from Ch1 correlated negatively with diarrhea scale (r=–0.63, P=0.01) (Table 4).
Fig. 5.
Correlation between percentage of slow wave coupling of the M-EGG and GSRS scores (reflux, abdominal pain, indigestion and total score) in both patient groups in the fed state. The percentage of slow wave coupling (%SWC) in the fed state correlated negatively with GSRS scores (reflux, r=–0.59, P=0.02; abdominal pain, r=–0.51, P=0.04, indigestion, r=–0.59, P=0.02 and total score, r=–0.75, P=0.02).
Table 4. Correlation between parameters of the M-EGG and GSRS scores in the VP-DG group.
| Reflux | Abdominal pains | Indigestion | Diarrhea | Constipation | Total score | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |||||||||
| %N | Fasting | 1 ch | 0.27 | 0.39 | 0.12 | 0.64 | 0.08 | 0.60 | –0.63 | 0.01* | 0.35 | 0.87 | –0.10 | 0.36 | ||||||
| 2 ch | 0.29 | 0.36 | 0.42 | 0.92 | 0.32 | 0.84 | –0.20 | 0.26 | 0.13 | 0.66 | 0.24 | 0.77 | ||||||||
| Fed | 1 ch | –0.04 | 0.89 | –0.06 | 0.42 | –0.04 | 0.45 | 0.45 | 0.07 | 0.01 | 0.51 | 0.40 | 0.09 | |||||||
| 2 ch | –0.19 | 0.54 | –0.05 | 0.04 | –0.03 | 0.46 | –0.43 | 0.08 | –0.02 | 0.47 | –0.39 | 0.10 | ||||||||
| %B | Fasting | 1 ch | –0.06 | 0.86 | –0.05 | 0.43 | –0.12 | 0.33 | –0.14 | 0.32 | 0.14 | 0.67 | –0.03 | 0.46 | ||||||
| 2 ch | 0.07 | 0.82 | 0.05 | 0.56 | –0.03 | 0.46 | 0.20 | 0.74 | 0.05 | 0.56 | 0.24 | 0.78 | ||||||||
| Fed | 1 ch | 0.42 | 0.17 | 0.39 | 0.89 | 0.52 | 0.95 | 0.03 | 0.54 | –0.41 | 0.09 | 0.13 | 0.66 | |||||||
| 2 ch | –0.37 | 0.23 | –0.19 | 0.28 | –0.31 | 0.15 | 0.31 | 0.84 | –0.08 | 0.39 | –0.09 | 0.36 | ||||||||
| %T | Fasting | 1 ch | –0.09 | 0.36 | –0.29 | 0.19 | –0.08 | 0.90 | –0.37 | 0.12 | 0.01 | 0.51 | –0.52 | 0.08 | ||||||
| 2 ch | 0.63 | 0.98 | 0.12 | 0.65 | 0.38 | 0.89 | –0.07 | 0.42 | 0.12 | 0.64 | 0.10 | 0.62 | ||||||||
| Fed | 1 ch | –0.30 | 0.17 | –0.25 | 0.22 | –0.30 | 0.17 | 0.43 | 0.52 | 0.13 | 0.65 | 0.14 | 0.67 | |||||||
| 2 ch | –0.13 | 0.34 | 0.01 | 0.51 | 0.003 | 0.49 | –0.12 | 0.34 | 0.19 | 0.72 | 0.10 | 0.63 | ||||||||
| %A | Fasting | 1 ch | –2.02 | 0.26 | –0.03 | 0.45 | –0.002 | 0.50 | 0.68 | 0.99 | –0.36 | 0.12 | 0.20 | 0.79 | ||||||
| 2 ch | –0.45 | 0.07 | –0.52 | 0.06 | –0.41 | 0.90 | 0.15 | 0.68 | –0.09 | 0.27 | –0.38 | 0.11 | ||||||||
| Fed | 1 ch | 0.09 | 0.60 | 0.01 | 0.62 | 0.66 | 0.42 | 0.37 | 0.88 | 0.006 | 0.50 | 0.39 | 0.87 | |||||||
| 2 ch | 0.28 | 0.82 | 0.08 | 0.60 | 0.08 | 0.40 | 0.42 | 0.91 | –0.01 | 0.48 | 0.39 | 0.89 | ||||||||
| %SWC | Fasting | –0.15 | 0.32 | –0.19 | 0.27 | –0.20 | 0.73 | 0.04 | 0.55 | –0.01 | 0.48 | 0.13 | 0.66 | |||||||
| Fed | –0.60 | 0.02* | –0.51 | 0.04* | –0.59 | 0.04* | –0.29 | 0.17 | 0.57 | 0.97 | –0.75 | 0.002* | ||||||||
r: correlation coefficient, P: P value, *P<0.05, M-EGG: multichannel electrogastrography, %N: %normal, %b: %bradygastria, %t: %tachygastria, %A: %arrhythmia, VP-DG: Vagus nerve-preserving distal gastrectomy.
Discussion
Preservation of the vagus nerve is expected to improve the quality of life of patients after gastrectomy by decreasing post gastrectomy symptoms such as diarrhea. However, evidence to support this supposition is scant, not least because few objective methods of evaluation exist. M-EGG was specifically developed to evaluate gastric motility. This approach has enabled us to acquire substantial quantities of novel data on gastric motility. Therefore, we used the same technique to evaluate vagus nerve-preserving gastrectomy, and determine whether objective methods of recording gastric motility correlated with postoperative symptoms by means of a questionnaire-based survey.
Imai and colleagues (10) conducted EGG in healthy volunteers after administration of intravenous atropine sulfate, and found that normal gastric motility was almost completely abolished with clearly diminished waveform amplitudes in all subjects. As a result of this study it can be concluded that nearly all components of the EGG trace can be attributed to vagal activity. Geldof and colleagues (11) reported highly selective vagotomy is associated with abnormalities in myoelectivity. However, the effect of vagus nerve preservation on the gastric electrical activity after gastrectomy has not been reported until now. We found that the relative time of normogastria (%normal) was lower in the DG group than in the VP-DG group, in which the fundic branches were likely to have been preserved. This indicates that preservation of the vagus nerve influences the gastric electrical activity of the remnant stomach after gastrectomy. The fundic branches are several fine branches innervating the cardiac region and fundus of the stomach, and arise on the proximal side at the bifurcation points of the hepatic branch from the anterior vagal trunk and the celiac branch from the posterior vagal trunk. Loeweneck and colleagues (12) classified the branches of the anterior vagal trunk into four types and confirmed the fundic branches in each. Furthermore, Schemann reported that neurons in the gastric myenteric plexus receive multiple vagal inputs (13).
The vagus nerve plays an important role in gastric electrical activity. Relaxation of the fundus is believed to facilitate retention of gastric contents. Gastric receptive relaxation occurs when the fundus relaxes in response to stimulation during the passage of food (14). Abrahamsson showed that stimulation of vagus nerves induces relaxation of the fundus (15), and a barostat study by Le Blanc-Louvry and colleagues found that a 200-kcal liquid meal (200 ml) brought on gastric relaxation in 12 of 16 VP-DG patients (16).
We suggest that the remnant stomach with preserved vagal innervation might maintain the motility of the remnant stomach as well as receptive relaxation. Further examination of gastric myoelectrical activity regarding relaxation of the proximal stomach is needed.
As shown in this study, we found that under fed conditions, the %bradygastria recorded in Ch1 and Ch2 were higher in the DG group than in the VP-DG group. Furthermore, %bradygastria from Ch2 in the fasting state was higher in the DG group than in the VP-DG group. This is likely to have been caused by the loss of vagal efferent stimulation of the gastric plexuses due to the vagus nerve dissection in the DG group.
%SWC is a unique parameter of M-EGG, and is used in the evaluation of diseases accompanied by abnormal gastric motility. %SWC is significantly lower in patients with functional dyspepsia and systemic sclerosis compared with healthy individuals (17). Our previous study comparing %normal and %SWC with parameters obtained from a 13C-acetate breath test revealed that the %normal measured in Ch1 of the M-EGG correlated with elimination half-life (T1/2) and lagtime (Tlag). The %SWC as determined by M-EGG from all channels correlated with T1/2, Tlag, and the gastric emptying coefficient, suggesting that %normal and %SWC values indicate gastric emptying through gastric movement and gastric emptying through coordinated movement of the stomach, respectively (18). We did not find a significant difference in %SWC values between the VP-DG and DG groups. In the VP-DG group, the fundic branches were preserved in the remnant stomach (the gastric fundus) where Ch1 was placed, while the gastric branches were resected (the upper corpus of the stomach) where Ch2 was placed. This means that efferent vagal nerve fibers remained capable of stimulating the gastric myenteric plexus in the fundus, but not in the upper corpus of the stomach, and thus propagation of gastric electrical activity was abnormal, resulting in decreased %SWC values. While, in the DG group, the vagus nerves that branch into the fundus and the upper corpus of the stomach were generally cut as part of lymph node dissection. Thus the gastric myenteric plexus in both parts of the stomach did not receive efferent vagal inputs, and thus propagation of gastric electrical activity was abnormal, resulting in decreased %SWC values.
Imai and colleagues examined EGG recorded after total or subtotal gastrectomy and found that 3 cpm power peaks were absent after total gastrectomy, suggesting that EGG measures gastric electrical activity (19). On the other hand, two thirds of patients who had undergone subtotal gastrectomy exhibited waveforms similar to those observed in healthy individuals, indicating that the region serving as the gastric pacemaker was not removed during gastrectomy in these patients. Schaap and colleagues reported that EGG signals contained a component at approximately 3 cpm in 22 of 33 DG patients (20). Homma et al. (21) examined EGG recorded after subtotal gastrectomy, and reported that the postoperative to preoperative power ratio for the 3 cpm was significantly reduced following subtotal gastrectomy in the post prandial state. In the present study, a 3 cpm component was apparent in the EGG signals in the VP-DG group (Fig. 4B), which suggest that M-EGG is a reliable noninvasive means of measuring remnant stomach function.
Fluoroscopy often fails to detect peristalsis of the remnant stomach. The stomach can be divided into two regions depending on motility pattern: the proximal region that shows receptive relaxation and tonic contraction, and the distal region that shows mainly peristalsis (22). Cannon and colleagues reported that EGG waveforms were independent of gastric motility, as showed by fluoroscopy, which mainly represents the function of the proximal region of the stomach (22, 23). It appears that M-EGG is capable of detecting preserved function in the remnant stomach that cannot be assessed by gastric excretion tests such as fluoroscopy.
Tsuji et al. (24) compared the quality of life of VP-DG and DG patients, and found that body weight recovery was significantly better in the VP-DG group. In addition, the reduction in the visceral fat area was significantly greater in the DG group than in the VP-DG group (25). These findings indicate that vagus nerve preservation benefits patients by helping them achieve a better postoperative quality of life, but its underlying mechanisms are still unclear. If the remnant stomach has preserved function, patients might experience fewer symptoms and have better food intake, which will result in a better quality of life. We believe that our findings of preserved electrical activity in the remnant stomach of the VP-DG group presents a new perspective and scientific evidence for the benefits of vagus nerve preservation.
There are some studies that have examined the potential links between EGG findings and digestive symptoms (26,27,28,29,30,31,32,33). In systemic sclerosis patients, gastric dysrhythmias were associated with certain gastrointestinal symptoms (26). Significant correlation was found between the symptom score and the percentage arrhythmia in patients in the fed state or in patients after either bone marrow or stem cell transport (27). In patients with unresectable cancer, the severity of symptoms was significantly higher in patients with abnormal EGG results (28). On the other hand, some investigators reported no correlation was observed between EGG parameters and symptoms (34,35,36,37). As different methods have been applied in various investigations, there is only limited comparability. We found significant negative correlations between %SWC and GSRS subscale scores (reflux, abdominal pain, and indigestion) in the VP-DG group, although %SWC in the VP-DG group was not significantly different from that in the DG group. Since pain-related behavior after administration of acids is suppressed in animals that have undergone vagus nerve resection, the afferent fiber of the vagus nerve is believed to play an important role in the experience of abdominal symptoms (38). The afferent fiber of the vagus nerve was preserved in the VP-DG group, and therefore, abdominal symptoms were linked to the M-EGG findings. On the other hand, in the DG group, because the afferent fibers of the vagus nerve were cut, abdominal symptoms were independent of M-EGG. In this study, scores for the three GSRS subscales likely to represent upper abdominal symptoms were recorded: reflux (heartburn and regurgitation); abdominal pain (abdominal pain, hunger pains, and nausea); and indigestion (borborygmus, abdominal distension, eructation, and increased flatus). We found significant correlation between %SWC in the fed state in the VP-DG group. This correlation indicated that the M-EGG recording particularly reflected the degree of upper abdominal symptoms.
To our knowledge, this study is the first to demonstrate the correlation between M-EGG findings and gastrointestinal symptoms after gastrectomy. In the VP-DG group, it was surmised that gastric electrical activity was preserved because the fundic branches of the vagus nerve were preserved, and this may explain why gastrointestinal symptom scores correlate with gastric electrical activity (%SWC). In the DG group, gastrointestinal symptom scores did not correlate with gastric electrical activity (%SWC), probably because vagus nerve resection abolished efferent vagal inputs to the gastric myenteric plexus, thereby disturbing gastric electrical activity. In this study, we found a negative correlation between %SWC in VP-DG group and fed state but not in the fasting state. Because of the significant increase of %SWC after feeding in healthy volunteers group, we suggest that this negative correlation was affected by vagal stimulation following feeding. These results thus indicated that M-EGG was useful for evaluating postoperative function of the remnant stomach in VP-DG patients with preserved gastric electrical activity, on the basis of its correlation with gastrointestinal complaints. However, M-EGG was not useful for evaluating DG patients since disturbed gastric electrical activity did not correlate with complaints.
In conclusion, VP-DG patients showed better preserved gastric myoelectric activity than DG patients, and %SWC showed a significant negative correlation with scores of GSRS (stomach acid reflux, abdominal pain, indigestion, total score) in the VP-DG group. These data reveal the functional superiority in the myoelectric activity of VP-DG compared with DG using objective data obtained using M-EGG.
Award
A summary of this study was presented at the 53th Annual meeting of the Japan Society of Smooth Muscle Research held in 2011 Tokyo, Japan, and this study received the Best Presentation award.
Funding and Disclosures
This study was supported by a grant from the KAWASAKI Foundation for Medical Science & Medical Welfare.
Author Contributions
Haruaki Murakami, Hideo Matsumoto, Hisako Kubota, and Masaharu Higashida performed the research, Masafumi Nakamura and Toshihiro Hirai designed the research study, Haruaki Murakami and Hideo Matsumoto analyzed the data, and Haruaki Murakami wrote the paper.
References
- 1.Alvarez WC. The electrogastrogram and what it shows. JAMA. 1922; 78: 1116–9. doi: 10.1001/jama.1922.02640680020008 [DOI] [Google Scholar]
- 2.Svedlund J, Sjödin I, Dotevall G. GSRS-a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988; 33(2): 129–34. doi: 10.1007/BF01535722 [DOI] [PubMed] [Google Scholar]
- 3.Namikawa T, Kitagawa H, Okabayashi T, Sugimoto T, Kobayashi M, Hanazaki K. Double tract reconstruction after distal gastrectomy for gastric cancer is effective in reducing reflux esophagitis and remnant gastritis with duodenal passage preservation. Langenbecks Arch Surg. 2011; 396(6): 769–76. doi: 10.1007/s00423-011-0777-8 [DOI] [PubMed] [Google Scholar]
- 4.Hayami M, Seshimo A, Miyake K, Shimizu S, Kameoka S. Effects of emptying function of remaining stomach on QOL in postgastrectomy patients. World J Surg. 2012; 36(2): 373–8. doi: 10.1007/s00268-011-1379-x [DOI] [PubMed] [Google Scholar]
- 5.Kharbutli B, Velanovich V. Gastrointestinal symptomatic outcomes of laparoscopic and open gastrectomy. World J Gastrointest Surg. 2009; 1(1): 56–8. doi: 10.4240/wjgs.v1.i1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kono K, Iizuka H, Sekikawa T, Sugai H, Takahashi A, Fujii H, Matsumoto Y. Improved quality of life with jejunal pouch reconstruction after total gastrectomy. Am J Surg. 2003; 185(2): 150–4. doi: 10.1016/S0002-9610(02)01211-4 [DOI] [PubMed] [Google Scholar]
- 7.Namikawa T, Kitagawa H, Okabayashi T, Sugimoto T, Kobayashi M, Hanazaki K. Roux-en-Y reconstruction is superior to billroth I reconstruction in reducing reflux esophagitis after distal gastrectomy: special relationship with the angle of his. World J Surg. 2010; 34(5): 1022–7. doi: 10.1007/s00268-010-0452-1 [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma. 14th ed. Tokyo: Kanehara; 2010. [DOI] [PubMed] [Google Scholar]
- 9.Simonian HP, Panganamamula K, Parkman HP, Xu X, Chen JZ, Lindberg G, Xu H, Shao C, Ke MY, Lykke M, Hansen P, Barner B, Buhl H. Multichannel EGG in Normal Subjects: A Multicenter study. Dig Dis Sci. 2004; 49: 594–601. doi: 10.1023/B:DDAS.0000026304.83214.50 [DOI] [PubMed] [Google Scholar]
- 10.Imai K, Kitakoji H, Chihara E, Sakita M. Effects of atropine sulfate and neostigmine on gastric electrical activity in human subjects--electrogastrographic study. Hepatogastroenterology. 2008; 55(81): 294–7. [PubMed] [Google Scholar]
- 11.Geldof H, van der Schee EJ, van Blankenstein M, Smout AJ, Akkermans LM. Effects of highly selective vagotomy on gastric myoelectrical activity. An electrogastrographic study. Dig Dis Sci. 1990; 35(8): 969–75. doi: 10.1007/BF01537245 [DOI] [PubMed] [Google Scholar]
- 12.Loeweneck H, Lüdinghausen M, Mempel W. Vagus und cholinergishes System am Margen des Menschen. Munich. Med. Wochenschr. 1967; 109: 1754–62. [PubMed] [Google Scholar]
- 13.Schemann M, Grundy D. Am J Physiol. 1992; 263(5 Pt 1): G709–18. [DOI] [PubMed] [Google Scholar]
- 14.Cannon WB, Lieb CW. The receptive relaxation of the stomach. Am J Physiol. 1911; 29: 267–73. [Google Scholar]
- 15.Abrahamsson H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol Scand Suppl. 1973; 390: 1–38. [PubMed] [Google Scholar]
- 16.Le Blanc-Louvry I, Savoye G, Maillot C, Denis P, Ducrotté P. An impaired accommodation of the proximal stomach to a meal is associated with symptoms after distal gastrectomy. Am J Gastroenterol. 2003; 98(12): 2642–7. doi: 10.1016/j.amjgastroenterol.2003.08.026 [DOI] [PubMed] [Google Scholar]
- 17.Lin X, Chen JZ. Abnormal gastric slow waves in patients with functional dyspepsia assessed by multichannel electrogastrography. Am J Physiol Gastrointest Liver Physiol. 2001; 280(6): G1370–5. [DOI] [PubMed] [Google Scholar]
- 18.Haruaki M, Hideo M, Toshihiro H. Examination on the gastric motility and the parameter (%normal, %SWC (Percentage slow wave coupling)) of multichannel electrogastrography. J Smooth Muscle Res. 2010; 46(5): 249–58. 21187673 [Google Scholar]
- 19.Imai K, Sakita M. Pre- and postoperative electrogastrography in patients with gastric cancer. Hepatogastroenterology. 2005; 52(62): 639–44. [PubMed] [Google Scholar]
- 20.Schaap HM, Smout AJ, Akkermans LM. Myoelectrical activity of the Billroth II gastric remnant. Gut. 1990; 31(9): 984–8. doi: 10.1136/gut.31.9.984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homma S, Shimakage N, Yagi M, Hasegawa J, Sato K, Matsuo H, Tamiya Y, Tanaka O, Muto T, Hatakeyama K. Electrogastrography prior to and following total gastrectomy, subtotal gastrectomy, and gastric tube formation. Dig Dis Sci. 1995; 40(4): 893–900. doi: 10.1007/BF02064997 [DOI] [PubMed] [Google Scholar]
- 22.Cannon WB, Lieb CW. The receptive relaxation of the stomach. Am J Physiol. 1911; 29: 267–73. [Google Scholar]
- 23.Cannon WB. The movements of the stomach studied by the roentren rays. Am J Physiol. 1898; 1: 359–82. [Google Scholar]
- 24.Tsuji H, Ando S, Sakakibara K. The Clinical Evaluation of Vagus Nerve Preserving Gastric Operation with D2 Lymph Node Dissection for Early and Advanced Gastric Cancer. Nihon Shokaki Geka Gakkai Zasshi. 2003; 36(2): 78–84. doi: 10.5833/jjgs.36.78 [DOI] [Google Scholar]
- 25.Miyato H, Kitayama J, Hidemura A. Vagus nerve preservation selectively restores visceral fat volume in patients with early gastric cancer who underwent gastrectomy. J Surg Res. 2012; 173(1): 60–7. doi: 10.1016/j.jss.2010.08.040 [DOI] [PubMed] [Google Scholar]
- 26.McNearney TA, Sallam HS, Hunnicutt SE, Doshi D, Wollaston DE, Mayes MD, Chen JD. Gastric slow waves, gastrointestinal symptoms and peptides in systemic sclerosis patients. Neurogastroenterol Motil. 2009; 21(12): 1269–e120. doi: 10.1111/j.1365-2982.2009.01350.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Mandanas RA, Lin X, Chen JD. Impaired gastric slow wave rhythmicity in patients after bone marrow or stem cell transplant. Dig Dis Sci. 2002; 47(8): 1746–51. doi: 10.1023/A:1016484226110 [DOI] [PubMed] [Google Scholar]
- 28.Chasen M, Bhargava R. Gastrointestinal symptoms, electrogastrography, inflammatory markers, and PG-SGA in patients with advanced cancer. Support Care Cancer. 2012; 20(6): 1283–90. doi: 10.1007/s00520-011-1215-8 [DOI] [PubMed] [Google Scholar]
- 29.DiBaise JK, Brand RE, Lyden E, Tarantolo SR, Quigley EM. Gastric myoelectrical activity and its relationship to the development of nausea and vomiting after intensive chemotherapy and autologous stem cell transplantation. Am J Gastroenterol. 2001; 96(10): 2873–81. doi: 10.1111/j.1572-0241.2001.04241.x [DOI] [PubMed] [Google Scholar]
- 30.Riezzo G, Clemente C, Leo S, Russo F. The role of electrogastrography and gastrointestinal hormones in chemotherapy-related dyspeptic symptoms. J Gastroenterol. 2005; 40(12): 1107–15. doi: 10.1007/s00535-005-1708-7 [DOI] [PubMed] [Google Scholar]
- 31.Leung MW, Wong BP, Chao NS, Chung KW, Kwok WK, Liu KK. Electrogastrography in the management of pediatric functional dyspepsia and motility disorder. J Pediatr Surg. 2006; 41(12): 2069–72. doi: 10.1016/j.jpedsurg.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 32.Christensen CJ, Johnson WD, Abell TL. Patients with cyclic vomiting pattern and diabetic gastropathy have more migraines, abnormal electrogastrograms, and gastric emptying. Scand J Gastroenterol. 2008; 43(9): 1076–81. doi: 10.1080/00365520802085411 [DOI] [PubMed] [Google Scholar]
- 33.Chen CL, Hu CT, Lin HH, Yi CH. Clinical utility of electrogastrography and the water load test in patients with upper gastrointestinal symptoms. J Smooth Muscle Res. 2006; 42(5): 149–57. doi: 10.1540/jsmr.42.149 [DOI] [PubMed] [Google Scholar]
- 34.van der Voort IR, Osmanoglou E, Seybold M, Heymann-Mönnikes I, Tebbe J, Wiedenmann B, Klapp BF, Mönnikes H. Electrogastrography as a diagnostic tool for delayed gastric emptying in functional dyspepsia and irritable bowel syndrome. Neurogastroenterol Motil. 2003; 15(5): 467–73. doi: 10.1046/j.1365-2982.2003.00433.x [DOI] [PubMed] [Google Scholar]
- 35.Lu CL, Chen CY, Chang FY, Kang LJ, Lee SD, Wu HC, Kuo TS. Impaired postprandial gastric myoelectrical activity in Chinese patients with nonulcer dyspepsia. Dig Dis Sci. 2001; 46(2): 242–9. doi: 10.1023/A:1005684328217 [DOI] [PubMed] [Google Scholar]
- 36.Hoogerwerf WA, Pasricha PJ, Kalloo AN, Schuster MM. Pain: the overlooked symptom in gastroparesis. Am J Gastroenterol. 1999; 94(4): 1029–33. doi: 10.1111/j.1572-0241.1999.01008.x [DOI] [PubMed] [Google Scholar]
- 37.Adachi H, Kamiya T, Hirako M, Misu N, Kobayashi Y, Shikano M, Matsuhisa E, Kataoka H, Sasaki M, Ohara H, Nakao H, Orito E, Joh T. Improvement of gastric motility by hemodialysis in patients with chronic renal failure. J Smooth Muscle Res. 2007; 43(5): 179–89. doi: 10.1540/jsmr.43.179 [DOI] [PubMed] [Google Scholar]
- 38.Lamb K, Kang YM, Gebhart GF, Bielefeldt K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003; 125(5): 1410–8. doi: 10.1016/j.gastro.2003.07.010 [DOI] [PubMed] [Google Scholar]