Abstract
Smooth muscle cells (SMC) and endothelial cells are the major cell types in blood vessels. The principal function of vascular SMC in the body is to regulate blood flow and pressure through contraction and relaxation. The endothelium performs a crucial role in maintaining vascular integrity by achieving whole-organ metabolic homeostasis via the production of factors associated with vasoconstriction or vasorelaxation. In this review, we have focused on the production of nitric oxide (NO), a vasorelaxation factor. The extent of NO production represents a key marker in vascular health. A decrease in NO is capable of inducing pathological conditions associated with endothelial dysfunction, such as obesity, diabetes, cardiovascular disease, and atherosclerosis. Recent studies have strongly implicated the involvement of G-protein-coupled receptor kinase 2 (GRK2) in the progression of cardiovascular disease. Vasculature which is affected by insulin resistance and type 2 diabetes expresses high levels of GRK2, which may induce endothelial dysfunction by reducing intracellular NO. GRK2 activation also induces changes in the subcellular localization of GRK2 itself and also of β-arrestin 2, a downstream protein. In this review, we describe the pathophysiological mechanisms of insulin resistance and diabetes, focusing on the signal transduction for NO production via GRK2 and β-arrestin 2, providing novel insights into the potential field of translational investigation in the treatment of diabetic complications.
Keywords: endothelial dysfunction, NO production, Akt/eNOS signaling pathway, GRK2, β-arrestin 2
Introduction
Diabetes mellitus continues to be a leading cause of hypertension and cardiovascular diseases in which vasodilation is impaired, as found in both diabetic humans and in animal models (1,2,3,4,5). The prevalence of hypertension is increased in type 2 diabetes, with diabetic hypertensive patients being markedly sensitive to blood pressure lowering as far as cardiac events are concerned (6,7,8). In fact, the main purpose of blood pressure-lowering therapy in diabetic patients is to protect against the progression of cardiovascular complications. The endothelium performs a crucial role in maintaining vascular integrity to facilitate whole-organ metabolic homeostasis by producing factors associated with vasoconstriction or vasorelaxation. In this review, we have focused on the production of nitric oxide (NO; a vasorelaxation factor). NO is a vital component in maintaining vascular health. A decrease in NO induces pathological conditions associated with endothelial dysfunction, such as obesity, diabetes, cardiovascular disease, and atherosclerosis. Presently, we think that revolutionary treatment facilitating not only tight glycemic control but also exhibiting a cardiovascular protective action can be achieved by reversing endothelial dysfunction.
We have focused on recent findings regarding the role of G-protein-coupled receptor kinase 2 (GRK2) in the regulation of the vascular condition found in diabetes. Over the last decade, GRK2 has been reported to be involved in GPCR-desensitization, -internalization, and -signalization through protein-protein interactions (9,10,11). Regarding the present issue of diabetic complications, we report the novel findings that GRK2 levels and activities correlate with endothelial dysfunction (12,13,14,15,16).
Here we review the current literature regarding the role of GRK2, with a focus on the more established therapeutic value of GRK2 targeting, and the emerging roles of GRK2 and β-arrestin 2 signaling in diabetic pathophysiology.
G-protein-coupled receptor kinase 2
GRKs possess a central catalytic region that is a serine/threonine kinase which facilitates specificity toward GPCRs (17, 18). This family of kinases is composed of seven members (GRK1 to GRK7) and is widely expressed, which is suggestive of their important role in the regulation of GPCR responsiveness (19, 20). GRKs are divided into three subfamilies based on differences in structure and regulation (21). GRK2 and GRK3, the most unique members among the GRK family, have an amino-terminal α-helix that stabilizes interaction with ligand-bound GPCRs, a regulator of the G protein signaling homology domain that binds to activated Gα, and a pleckstrin homology (PH) domain that mediates membrane localization via Gβγ and phospholipid interactions (22). Especially, GRK2 is a multidomain kinase that regulates GPCR signaling interacting with different proteins of the signaling cascade: although the catalytic domain phosphorylates the receptor (important for homologous desensitization), the PH domain interacts with dissociated Gβγ (important for kinase targeting of the membrane), and the amino-terminal α-helix interacts with activated Gα (important for Gα signaling regulation) (23,24,25,26,27). When activated, most GPCRs undergo phosphorylation, which is followed by receptor interaction with β-arrestins, and desensitization, and endocytosis (23). Other GRKs have different domains at their C termini that are similarly involved in membrane targeting (21, 28). Furthermore, they are grouped functionally into 3 classes: GRK1-like (GRK1 and GRK7), GRK2-like (GRK2 and GRK3), and GRK4-like (GRK4, GRK5 and GRK6). The GRK1-like class is found exclusively in the retina and modulates opsins. The GRK2-like form is widely expressed, although GRK2 is typically more abundant. GRK4 is found mostly in the testis and proximal tubules of the kidney. GRK5 and GRK6 are widely distributed among tissues (20). Therefore, most GPCRs in the body are regulated by 4 GRKs: GRK2, GRK3, GRK5, and GRK6. Traditionally, it has been believed that the function of GRKs is to protect cells against overstimulation. However, in certain situations (cardiovascular and inflammatory diseases), this process is impaired with progression of the disease. For example, as shown in Table 1, GRK2 levels increase under several conditions (12,13,14,15,16, 18, 29,30,31,32,33,34,35,36,37,38,39,40). Such a finding suggests that this kinase could be a potential diagnostic marker and/or therapeutic target for many conditions. In this review, we discuss the role of GRK2 in the presence of diabetes and endothelial dysfunction.
Table 1. Changes observed in GRK2 levels and functions in various diseases.
| Disease | Change in GRK2 levels | Condition/experimental model | Reference |
|---|---|---|---|
| Cardiac hypertrophy | Increase in cytosolic and membrane GRK activity attributed predominnantly to GRK2 protein upregulation | Pressure overload cardiac hypertrophy in the mouse was achieved following 7 days of transverse aortic constriction | 30 |
| Cardiac ischemia | GRK2 mRNA and activity in the particulate fraction significantly increased | Acute cardiac ischemia model based on stop-flow and low flow ischemia in the isolated perfused rat heart | 31 |
| Cardiomyopathy | Elevated left ventricular GRK2 mRNA and activity | Human patients with the ischemic and idiopathic dilated forms of cardiomyopathy | 32 |
| Cardiomyopathy | Myocardial GRK2 mRNA levels were induced in the failing hearts, as well as GRK2 protein | Rats subjected to ligation of the left coronary artery or sham-operated | 33 |
| Diabetes | Elevated GRK2 protein levels and activity in the aorta in the presence of diabetes | Spontaneously and experimental diabetic model mice | 12, 13, 14, 15, 16 |
| Heart failure | Biochemical abnormalities of heart failure and cardiac dysfunction were preceded by elevated GRK2 expression and activity | Spontaneously hypertensive heart failure (SHHF) rats that develop left ventricular hypertrophy and progress to heart failure | 34 |
| Heart failure | GRK2 activity and protein elevated only in the hypertrophic failing heart and not in hypertrophic but non-failing cardiac tissue | Rat model of heart infarction based on surgical occlusion of the coronary artery and tissue damage based on the existence of pulmonary edema | 35 |
| Hypertension | GRK2 activity increased in lymphocytes from hypertensive subjects, paralleled by an increase in GRK2 protein expression | Lymphocytes from younger hypertensive subjects as compared with older and younger normotensive subjects | 36 |
| Hypertension | GRK2 protein expression and GRK activity were elevated in hypertensive subjects | Black American participants with hypertension and cardiovascular injury | 18 |
| Insulin resistance and obesity | GRK2 protein levels were increased in muscle and adipose tissue in animal models of insulin resistance | Three different models of insulin resistance: tumor necrosis factor-α infusion, aging, and high-fat diet | 37 |
Involvement of GRK2 in G-protein-independent signaling
GRK2 and β-arrestin 2 are cytosolic proteins in their inactive state. Phosphorylation of the receptor by GRK2 recruits β-arrestin 2, leading to further receptor desensitization (41,42,43). β-Arrestin 2 binds to various regions of GPCR cytoplasmic tail domains and regulates endocytosis, frequently in response to GRK2-mediated receptor phosphorylation (44,45,46). Thus, the GRK-catalyzed phosphorylation and binding of β-arrestin 2 to the receptors are believed to be a common mechanism of GPCR desensitization (23, 26). GPCRs are key regulators of cell physiology and control processes ranging from glucose homeostasis to contractility of the heart (47,48,49). Thus, GRK2 plays an essential role in maintaining the homeostasis of cells and tissues by regulating GPCR signaling in normal states.
This classical understanding has been supported by numerous physiological studies. However, this simple paradigm has been altered by an increasing appreciation of the biochemical importance of both the negative regulation of G protein-dependent signaling, and G protein-independent signal transduction by GPCRs (10, 15, 50). We reported that G-protein-independent signaling requires β-arrestin 2, not GRK2, under normal conditions (15). Our study indicated that β-arrestin 2 may play a vital role in the mouse aorta. Although β-arrestin 2 was mainly localized diffusely in the cytosol in quiescent cells, we observed that its translocation from the cytosol to membrane fraction occurred in normal aorta preparations treated with insulin. Furthermore, we revealed that in diabetic states, GRK2 is activated and translocated to the membrane in spite of non-GPCR stimulation, and cytosolic β-arrestin 2 is not translocated to the membrane under insulin stimulation. This means that GRK2 antagonizes the action of β-arrestin 2. GRK2/β-arrestin 2-dependent signaling induces physiological responses that are different from G-protein-mediated responses (51,52,53). It is possible that the activation of β-arrestin 2 could be beneficial, whereas the activation of GRK2 could be harmful. So, it is valuable to limit the activation of GRK2 and recruit a specific β-arrestin 2, leading to the research and development of new pharmaceuticals.
GRK2 and insulin resistance
An emerging role of GRK2 involves its ability to modulate the response to insulin. GRK2 has been identified as serine/threonine kinases that participate, together with β-arrestin 2, in the regulation of multiple GPCRs. In contrast, insulin receptors are of the tyrosine kinase-type, not GPCRs, and insulin activates a signaling pathway involving the insulin receptor, insulin receptor substrate-1 (IRS-1), PI3-K, and Akt, and this leads to eNOS activation. The first suggestion that GRK2 is involved was based on the observation that insulin induces GRK2 upregulation (54), which in turn inhibits insulin signaling and glucose extraction (15, 38, 54, 55). This puts GRK2 at the center of the stage as a possible mechanism for insulin resistance. Interestingly, the higher protein expression and activity of GRK2, directly related to hypertension, insulin resistance, diabetes, or obesity, confirms previous evidence (20, 56). We and others have reported that treatment with GRK2 inhibitor or siRNA against GRK2 increased insulin signaling, while GRK2 overexpression led to insulin resistance (13, 15, 55, 57).
Previous reports indicated that GRK2 could act as an inhibitor of insulin action in cellular models. Insulin induces an increase of GRK2 levels and causes a GRK2- IRS1 association (54, 57). Other authors have also reported that IRS1 levels depend on GRK2 expression, and that increased GRK2 inhibit insulin-stimulated signaling in a kinase-activity independent manner, by mechanisms involving the formation of dynamic GRK2-IRS1 complexes (36). Overall, these data suggest that altered GRK2 levels could lead to the modulation of insulin signals through a GRK2-IRS1 association. Moreover, Luan et al. reported that insulin stimulated the formation of a new β-arrestin 2 signal complex, in which β-arrestin 2 acts as a scaffold to join Akt to the insulin receptor (58). The precise contribution of such mechanisms under different physiological conditions remains to be investigated. If the observed upregulation of GRK2 triggers insulin resistance, it is tempting to speculate that its inhibition would have positive effects. Confirmation of such evidence is based on our diabetic models (14). We have experimented with the use of a selective GRK2 inhibitor that prevents its activity. The GRK2 inhibitor corrected glucose and insulin levels in a glucose tolerance test when administered to diabetic models (ob/ob mice, a useful animal model of human type 2 diabetes, and also nicotinamide + streptozotocin-induced diabetic mice, a relatively new animal model of type 2 diabetes). A similar mechanism has been confirmed in mouse cardiomyocytes whereby Ser307 of IRS1 is a target for GRK2 phosphorylation, significantly increasing overall knowledge about the regulation of insulin signaling in terms of both physiology and pathology (59). In animal models of type 2 diabetes, insulin induces the translocation of GRK2 from the cytosol to plasma membrane, and the depletion of cellular GRK2 reduces IRS1 serine phosphorylation and increases IRS1 tyrosine phosphorylation, although the authors did not identify the kinase responsible for IRS1 phosphorylation (60). Interestingly, in spontaneously hypertensive rats, chronic treatment with a similar inhibitor of GRK2 kinase activity not only leads to an amelioration of the glucose homeostasis and IRS1 tyrosine phosphorylation, but also to reduction of the blood pressure levels (54). These findings are in agreement with other literature showing that GRK2 inhibition clearly delays the reduction in glucose uptake and protects insulin signaling in the heart, preserving the cardiac dimensions and function (59). These data support the novel hypothesis that part of the therapeutic effect of GRK2 inhibition under pathophysiological conditions such as insulin resistance, hypertension, or heart failure includes the correction of abnormal cardiac metabolism.
GRK2 and endothelial dysfunction
Individuals with type 2 diabetes mellitus are particularly prone to the detrimental effects of endothelial dysfunction, a key mechanism in the pathogenesis of atherosclerosis, and this explains the increased risk of cardiovascular events in type 2 diabetes (1, 2, 4, 5, 16, 61, 62). One of the most important functions of the endothelium is the production of nitric oxide (NO) in response to a variety of hormonal, mechanical, and chemical stimuli. A previous investigation (63) suggested that impaired NO production can result from endothelial dysfunction.
The mechanisms underlying endothelial dysfunction may be multifactorial. Previous evidence indicated a crucial for endothelial nitric oxide synthase (eNOS) in diabetes (63), whereas recent reports suggested that Akt signaling stimulates NO production via the Akt-dependent phosphorylation of eNOS at Ser1177 (64, 65, 66). We and others found that activation of the Akt/eNOS pathway by clonidine or insulin is impaired in the aorta in the presence of diabetes (15, 67,68,69,70), associated with endothelial dysfunction. Furthermore, GRK2 has been reported to be involved in impaired clonidine- or insulin-induced relaxation via Akt/eNOS signaling in the diabetic aorta (12,13,14,15,16). It has been clearly shown that the α2 adrenergic receptor is an excellent substrate for GRK2 (71, 72). Clonidine is classified as a selectively acting α2 adrenergic agonist. We revealed a link between α2 adrenergic receptor-stimulated GPCR signaling (specifically the βγ subunit) and Akt /eNOS activity/NO production, and that a defect in the upstream kinase, GRK2, is associated with impaired eNOS activity in diabetes (12). The insulin receptor is not a GPCR, and so it may not be linked to the βγ subunit. However, the insulin-stimulated relaxation is increased in the diabetic aorta treated with GRK2 inhibitor or GRK2 siRNA (15). Recently, Luan et al. reported that insulin stimulates the formation of a new β-arrestin 2 signal complex in which β-arrestin 2 acts as a scaffold for the translocation of Akt to the insulin receptor (58). Furthermore, we reported that the upregulation of GRK2 and a decrease in β-arrestin 2 inhibit the insulin-induced stimulation of Akt/eNOS signaling, and that GRK2 overactivation may result from an increase in PKC activity in preparations of aortas from diabetic mice with hyperinsulinemia (13, 15). Along with, the above reciprocal role of GRK2/β-arrestin 2, accumulating evidence indicates that GRK2 and β-arrestin 2 are both able to interact with Akt, as described below in detail.
GRK2 and β-arrestin 2 in diabetic vascular dysfunction
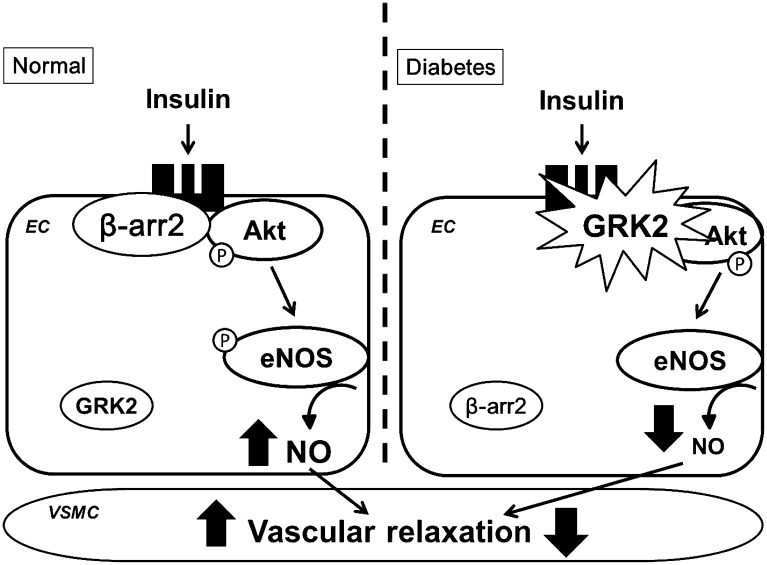
β-arrestin comprises two ubiquitously expressed isoforms, β-arrestin 1 and β-arrestin 2, both of which are abundantly expressed in the vasculature. Their binding to receptors sterically inhibits the receptor coupling to G-protein, leading to the inactivation of effectors such as second messenger-generating enzymes (73, 74). Besides this classical function, recently accumulating evidences have revealed novel functions of β-arrestins as signal transducers in various signaling pathways (51). For example, β-arrestins operate as adaptor proteins to recruit and activate Src under agonist-stimulated receptors (76). In addition, GPCR-mediated MAP kinase (MAPK) ERK1/2 phosphorylation results from a combination of G protein and β-arrestin signaling (76, 77), and β-arrestins were also shown to contribute to the activation of several Ras-family GTPases (78, 79). Regarding, the molecular function of β-arrestin 2, several laboratories (19, 26) have shown that this protein can act as a scaffold molecule that brings different signaling molecules such as Akt, Src, JNK, and ERK1/2 into the receptor complex. Recently, it was reported that insulin stimulates the formation of a new β-arrestin 2 signal complex in which β-arrestin 2 acts as a scaffold for the translocation of Akt and Src to the insulin receptor (58). Therefore, we addressed the associations among GRK2, β-arrestin 2, and insulin signaling (15). β-arrestin 2 siRNA significantly reduced insulin-induced vascular relaxation and NO production, suggesting that insulin signaling is mediated through β-arrestin 2 and that GRK2 may stimulate formational changes in β-arrestin 2, Akt, and the insulin receptor. This means that in the healthy vasculature, β-arrestin 2 binds to Akt under insulin stimulation, whereas, in the case of diabetes, insulin causes the translocation of GRK2 to the membrane, where it binds to Akt, preventing β-arrestin 2 binding to Akt because GRK2 remains bound. In other words, GRK2 and β-arrestin 2 compete for the insulin receptor upon insulin stimulation n (Fig. 1). Thus, β-arrestin 2-mediated insulin signaling may be of therapeutic benefit for patients with diabetic vascular dysfunction.
Fig. 1.
Subcellular localization and function of GRK2 and β-arrestin 2 in relation to NO production with insulin stimulation under normal and diabetic conditions. β-arrestin 2 may be beneficial by preventing the membrane translocation of GRK2 in the endothelium of the aorta under the normal condition, while GRK2 may be unfavorable by suppressing the positive effect of β-arrestin 2 in the aorta under the diabetic condition. See text for details. β-arr2: β-arrestin 2; eNOS: endothelial nitric oxide synthase; GRK2: G-protein-coupled receptor kinase-2; NO: nitric oxide.
GRK2 and sex differences in diabetes
Pre-menopausal women have lower rates of cardiovascular disease and cardiovascular morbidity and mortality than men of the same age. Diabetes is one of the major cardiovascular risk factors in both men and women, and there have been many studies focusing on women with both diabetes mellitus and cardiovascular disease (80,81,82). However, there have been fewer studies focusing on the detailed mechanisms. We previously showed that female diabetic mice have more favorably preserved vascular functions via the Akt pathway, as well as show greater NO production via augmented eNOS activity and expression (61, 83), suggesting that females have an inherent cardioprotective advantage. Additionally, we clarified the involvement of GRK2 (16). GRK2 localization in the diabetic aorta may differ between the sexes. Although GRK2 expression in the membrane and GRK2 activity were both increased in male diabetic aortas, GRK2 expression in the cytosol was increased and GRK2 activity was decreased in preparations of female diabetic aortas; that is, GRK2 was not abundantly translocated to the membrane. Based on the above, we speculate that in the female diabetic aorta, a mechanism exists that prevents the upregulation of GRK2 activity and translocation of GRK2 protein to the membrane. Although we remain unable to identify it, we expect to detect some endogenous substrate. We believe that we are one step closer to a full explanation of the pathogenesis of endothelial dysfunction in diabetes.
Concluding remarks and future directions
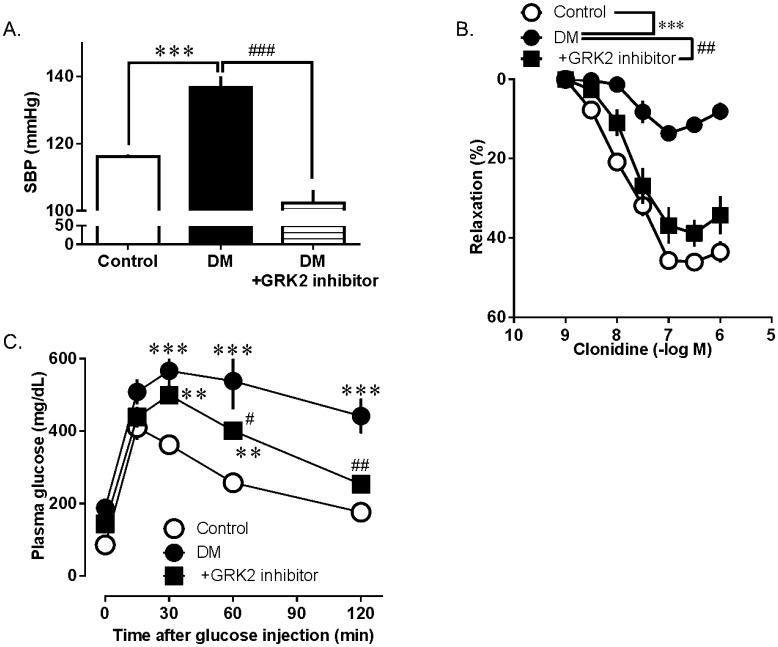
Presently, a large and ever-expanding body of pre-clinical evidence exists that strongly supports cardiac GRK2 inhibition as a therapeutic modality for heart failure. However, recent studies by us and others have begun to establish GRK2 targeting in extracardiac tissues, and more specifically in the vasculature, as another novel therapeutic possibility for diabetes and insulin resistance. Since inhibition of the activated GRK2 is able to normalize vascular insulin signaling and lower blood glucose, GRK2 inhibition is a novel strategy against the development of diabetes and diabetes-associated vascular complications. Adversely, it has been shown that the removal of GRK2 from specific tissues, such as the endothelium (84), increases the production of reactive oxygen species. Moreover, it was recently demonstrated that the endothelium-specific knockout of GRK2 is associated with impaired angiogenesis (85). Accordingly, GRK2 deficiency in endothelial cells in vitro increases inflammatory signaling and enhances leukocyte recruitment to activated endothelial cells (86), suggesting that GRK2 is a negative regulator of the vascular endothelial cell function. Either overexpression or deletion of GRK2 induces malfunctions, indicating that the proper level of GRK2 maintains the normal cell function. We have demonstrated that single-injection treatment of diabetic mice with a highly selective GRK2 inhibitor reduced the blood pressure, endothelial dysfunction, and glucose intolerance (Fig. 2) (14). This suggests that GRK2 has a potential to become a therapeutic target, and so in vivo studies are now required involving chronic administration.
Fig. 2.
GRK2 inhibitor improves hypertension (A), endothelial dysfunction (B), and glucose tolerance (C). (A) Measurement of the systolic blood pressure (SBP). SBP was measured 30 min after the injection of either the GRK2 inhibitor (200 µg/kg) or vehicle. (B) Clonidine-induced relaxation in the aortic rings from mice with diabetes (DM) and controls. At 60 min before the isolation of aortas, mice were treated with either the GRK2 inhibitor (200 µg/kg) or vehicle. (C) Plasma glucose immediately before and 15, 30, 60, 90, and 120 min after the ip. injection of glucose (2 g/kg) in mice that had received a single iv. injection of the GRK2 inhibitor or vehicle at 30-min. Values are means ± SE; n = 8. **P< 0.01, or ***P< 0.001 vs. controls or DM; #P< 0.05, ##P< 0.01 or ###P< 0.001 vs. DM or DM+GRK2 inhibitor [modified from ref.16].
In addition, the recently emerging and constantly expanding field of GPCR/ β-arrestin-dependent signaling also offers several exciting new opportunities regarding therapeutic intervention for diabetes and insulin resistance. Despite still being in its infancy, as far as the identification of specific physiological and pathophysiological effects is concerned, there is a potential in exploiting this area for therapeutic advancement. Admittedly, the picture is still unclear regarding the physiological actions of β-arrestin 2, particularly in relation to the vascular system. For example, total β-arrestin 2 expression in aortas remained unchanged between preparations from diabetic mice and controls, whereas the β-arrestin 2 level in the membrane fraction decreased significantly in diabetic aorta preparations upon insulin stimulation (15). Moreover, β-arrestin 2 siRNA significantly reduced insulin-induced vascular relaxation and NO production, and no effects of excess β-arrestin 2 were observed on these responses, suggesting that insulin signaling is mediated through β-arrestin 2, and that GRK2 may stimulate formational changes in β-arrestin 2, Akt, and the insulin receptor (15). Future studies will clarify the picture. In any case, targeting GRK2 and β-arrestin 2-dependent signal transduction represents exciting and promising possibilities for novel therapeutic approaches to diabetic vascular dysfunction, which urgently require new and innovative effective drugs and therapies.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Frankel DS, Meigs JB, Massaro JM, Wilson PW, O’Donnell CJ, D’Agostino RB, Tofler GH. Von Willebrand factor, type 2 diabetes mellitus, and risk of cardiovascular disease: the framingham offspring study. Circulation. 2008; 118(24): 2533–9. doi: 10.1161/CIRCULATIONAHA.108.792986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998; 31(5): 1047–60. doi: 10.1161/01.HYP.31.5.1047 [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998; 114(2): 344–51. doi: 10.1016/S0016-5085(98)70487-1 [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, King G, Lopes-Virella M, Reusch J, Ruderman N, Steiner G, Vlassara H. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation. 2002; 105(18): e138–43. doi: 10.1161/01.CIR.0000013954.65303.C5 [DOI] [PubMed] [Google Scholar]
- 5.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004; 286(5): H1597–602. doi: 10.1152/ajpheart.00026.2004 [DOI] [PubMed] [Google Scholar]
- 6.Alderman MH, Cohen H, Madhavan S. Diabetes and cardiovascular events in hypertensive patients. Hypertension. 1999; 33(5): 1130–4. doi: 10.1161/01.HYP.33.5.1130 [DOI] [PubMed] [Google Scholar]
- 7.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001; 37(4): 1053–9. doi: 10.1161/01.HYP.37.4.1053 [DOI] [PubMed] [Google Scholar]
- 8.Verdecchia P, Reboldi G, Angeli F, Borgioni C, Gattobigio R, Filippucci L, Norgiolini S, Bracco C, Porcellati C. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension. 2004; 43(5): 963–9. doi: 10.1161/01.HYP.0000125726.92964.ab [DOI] [PubMed] [Google Scholar]
- 9.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002; 3(9): 639–50. doi: 10.1038/nrm908 [DOI] [PubMed] [Google Scholar]
- 10.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005; 308(5721): 512–7. doi: 10.1126/science.1109237 [DOI] [PubMed] [Google Scholar]
- 11.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007; 129(3): 511–22. doi: 10.1016/j.cell.2007.02.046 [DOI] [PubMed] [Google Scholar]
- 12.Taguchi K, Kobayashi T, Takenouchi Y, Matsumoto T, Kamata K. Angiotensin II causes endothelial dysfunction via the GRK2/Akt/eNOS pathway in aortas from a murine type 2 diabetic model. Pharmacol Res. 2011; 64(5): 535–46. doi: 10.1016/j.phrs.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Taguchi K, Kobayashi T, Matsumoto T, Kamata K. Dysfunction of endothelium-dependent relaxation to insulin via PKC-mediated GRK2/Akt activation in aortas of ob/ob mice. Am J Physiol Heart Circ Physiol. 2011; 301(2): H571–83. doi: 10.1152/ajpheart.01189.2010 [DOI] [PubMed] [Google Scholar]
- 14.Taguchi K, Matsumoto T, Kamata K, Kobayashi T. Inhibitor of G protein-coupled receptor kinase 2 normalizes vascular endothelial function in type 2 diabetic mice by improving β-arrestin 2 translocation and ameliorating Akt/eNOS signal dysfunction. Endocrinology. 2012; 153(7): 2985–96. doi: 10.1210/en.2012-1101 [DOI] [PubMed] [Google Scholar]
- 15.Taguchi K, Matsumoto T, Kamata K, Kobayashi T. G protein-coupled receptor kinase 2, with β-arrestin 2, impairs insulin-induced Akt/endothelial nitric oxide synthase signaling in ob/ob mouse aorta. Diabetes. 2012; 61(8): 1978–85. doi: 10.2337/db11-1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taguchi K, Matsumoto T, Kamata K, Kobayashi T. Suppressed G-protein-coupled receptor kinase 2 activity protects female diabetic-mouse aorta against endothelial dysfunction. Acta Physiol (Oxf). 2013; 207(1): 142–55. doi: 10.1111/j.1748-1716.2012.02473.x [DOI] [PubMed] [Google Scholar]
- 17.Pao CS, Barker BL, Benovic JL. Role of the amino terminus of G protein-coupled receptor kinase 2 in receptor phosphorylation. Biochemistry. 2009; 48(30): 7325–33. doi: 10.1021/bi900408g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohn HI, Xi Y, Pesant S, Harris DM, Hyslop T, Falkner B, Eckhart AD. G protein-coupled receptor kinase 2 expression and activity are associated with blood pressure in black Americans. Hypertension. 2009; 54(1): 71–6. doi: 10.1161/HYPERTENSIONAHA.108.125955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007; 69: 511–34. doi: 10.1146/annurev.physiol.69.022405.154731 [DOI] [PubMed] [Google Scholar]
- 20.Harris DM, Cohn HI, Pesant S, Eckhart AD. GPCR signalling in hypertension: role of GRKs. Clin Sci (Lond). 2008; 115(3): 79–89. doi: 10.1042/CS20070442 [DOI] [PubMed] [Google Scholar]
- 21.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998; 67: 653–92. doi: 10.1146/annurev.biochem.67.1.653 [DOI] [PubMed] [Google Scholar]
- 22.Huang CC, Yoshino-Koh K, Tesmer JJ. A surface of the kinase domain critical for the allosteric activation of G protein-coupled receptor kinases. J Biol Chem. 2009; 284(25): 17206–15. doi: 10.1074/jbc.M809544200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallese M, Mariggiò S, D’Urbano E, Iacovelli L, De Blasi A. Selective regulation of Gq signaling by G protein-coupled receptor kinase 2: direct interaction of kinase N terminus with activated galphaq. Mol Pharmacol. 2000; 57(4): 826–31. [PubMed] [Google Scholar]
- 24.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002; 415(6868): 206–12. doi: 10.1038/415206a [DOI] [PubMed] [Google Scholar]
- 25.Hata JA, Koch WJ. Phosphorylation of G protein-coupled receptors: GPCR kinases in heart disease. Mol Interv. 2003; 3(5): 264–72. doi: 10.1124/mi.3.5.264 [DOI] [PubMed] [Google Scholar]
- 26.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006; 17(4): 159–65. doi: 10.1016/j.tem.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 27.Lymperopoulos A. GRK2 and β-arrestins in cardiovascular disease: Something old, something new. Am J Cardiovasc Dis. 2011; 1(2): 126–37. [PMC free article] [PubMed] [Google Scholar]
- 28.Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998; 38: 289–319. doi: 10.1146/annurev.pharmtox.38.1.289 [DOI] [PubMed] [Google Scholar]
- 29.Choi DJ, Koch WJ, Hunter JJ, Rockman HA. Mechanism of beta-adrenergic receptor desensitization in cardiac hypertrophy is increased beta-adrenergic receptor kinase. J Biol Chem. 1997; 272(27): 17223–9. doi: 10.1074/jbc.272.27.17223 [DOI] [PubMed] [Google Scholar]
- 30.Ungerer M, Kessebohm K, Kronsbein K, Lohse MJ, Richardt G. Activation of beta-adrenergic receptor kinase during myocardial ischemia. Circ Res. 1996; 79(3): 455–60. doi: 10.1161/01.RES.79.3.455 [DOI] [PubMed] [Google Scholar]
- 31.Ungerer M, Böhm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993; 87(2): 454–63. doi: 10.1161/01.CIR.87.2.454 [DOI] [PubMed] [Google Scholar]
- 32.Vinge LE, Øie E, Andersson Y, Grøgaard HK, Andersen G, Attramadal H. Myocardial distribution and regulation of GRK and beta-arrestin isoforms in congestive heart failure in rats. Am J Physiol Heart Circ Physiol. 2001; 281(6): H2490–9. [DOI] [PubMed] [Google Scholar]
- 33.Anderson KM, Eckhart AD, Willette RN, Koch WJ. The myocardial beta-adrenergic system in spontaneously hypertensive heart failure (SHHF) rats. Hypertension. 1999; 33(1 Pt 2): 402–7. doi: 10.1161/01.HYP.33.1.402 [DOI] [PubMed] [Google Scholar]
- 34.Theilade J, Strøm C, Christiansen T, Haunsø S, Sheikh SP. Differential G protein receptor kinase 2 expression in compensated hypertrophy and heart failure after myocardial infarction in the rat. Basic Res Cardiol. 2003; 98(2): 97–103. doi: 10.1007/s00395-003-0395-x [DOI] [PubMed] [Google Scholar]
- 35.Gros R, Benovic JL, Tan CM, Feldman RD. G-protein-coupled receptor kinase activity is increased in hypertension. J Clin Invest. 1997; 99(9): 2087–93. doi: 10.1172/JCI119381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Díez J, Murga C, Fernández-Veledo S, Mayor F, Jr , Lorenzo M. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010; 59(10): 2407–17. doi: 10.2337/db10-0771 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Penela P, Ribas C, Mayor F, Jr . Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003; 15(11): 973–81. doi: 10.1016/S0898-6568(03)00099-8 [DOI] [PubMed] [Google Scholar]
- 38.Ciccarelli M, Cipolletta E, Iaccarino G. GRK2 at the control shaft of cellular metabolism. Curr Pharm Des. 2012; 18(2): 121–7. doi: 10.2174/138161212799040493 [DOI] [PubMed] [Google Scholar]
- 39.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005; 26(17): 1752–8. doi: 10.1093/eurheartj/ehi429 [DOI] [PubMed] [Google Scholar]
- 40.Penela P, Murga C, Ribas C, Lafarga V, Mayor F, Jr. The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol. 2010; 160(4): 821–32. doi: 10.1111/j.1476-5381.2010.00727.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouvier M, Hausdorff WP, De Blasi A, O’Dowd BF, Kobilka BK, Caron MG, Lefkowitz RJ. Removal of phosphorylation sites from the beta 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature. 1988; 333(6171): 370–3. doi: 10.1038/333370a0 [DOI] [PubMed] [Google Scholar]
- 42.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990; 248(4962): 1547–50. doi: 10.1126/science.2163110 [DOI] [PubMed] [Google Scholar]
- 43.Watari K, Nakaya M, Kurose H. Multiple functions of G protein-coupled receptor kinases. J Mol Signal. 2014; 9(1): 1. doi: 10.1186/1750-2187-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol. 2008; 153(Suppl 1): S379–88. doi: 10.1038/sj.bjp.0707604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson SS, Ménard L, Barak LS, Koch WJ, Colapietro AM, Caron MG. Role of phosphorylation in agonist-promoted beta 2-adrenergic receptor sequestration. Rescue of a sequestration-defective mutant receptor by beta ARK1. J Biol Chem. 1995; 270(42): 24782–9. doi: 10.1074/jbc.270.42.24782 [DOI] [PubMed] [Google Scholar]
- 46.Ferguson SS, Downey WE, 3rd , Colapietro AM, Barak LS, Ménard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996; 271(5247): 363–6. doi: 10.1126/science.271.5247.363 [DOI] [PubMed] [Google Scholar]
- 47.Thal DM, Yeow RY, Schoenau C, Huber J, Tesmer JJ. Molecular mechanism of selectivity among G protein-coupled receptor kinase 2 inhibitors. Mol Pharmacol. 2011; 80(2): 294–303. doi: 10.1124/mol.111.071522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tachibana H, Naga Prasad SV, Lefkowitz RJ, Koch WJ, Rockman HA. Level of beta-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation. 2005; 111(5): 591–7. doi: 10.1161/01.CIR.0000142291.70954.DF [DOI] [PubMed] [Google Scholar]
- 49.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007; 13(3): 315–23. doi: 10.1038/nm1553 [DOI] [PubMed] [Google Scholar]
- 50.Lefkowitz RJ, Whalen EJ. β-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol. 2004; 16(2): 162–8. doi: 10.1016/j.ceb.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 51.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007; 69: 483–510. doi: 10.1146/annurev.physiol.69.022405.154749 [DOI] [PubMed] [Google Scholar]
- 52.Schmid CL, Bohn LM. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol Ther. 2009; 121(3): 285–93. doi: 10.1016/j.pharmthera.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010; 62(2): 305–30. doi: 10.1124/pr.109.002436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cipolletta E, Campanile A, Santulli G, Sanzari E, Leosco D, Campiglia P, Trimarco B, Iaccarino G. The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. Cardiovasc Res. 2009; 84(3): 407–15. doi: 10.1093/cvr/cvp252 [DOI] [PubMed] [Google Scholar]
- 55.Usui I, Imamura T, Satoh H, Huang J, Babendure JL, Hupfeld CJ, Olefsky JM. GRK2 is an endogenous protein inhibitor of the insulin signaling pathway for glucose transport stimulation. EMBO J. 2004; 23(14): 2821–9. doi: 10.1038/sj.emboj.7600297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorriento D, Ciccarelli M, Santulli G, Illario M, Trimarco B, Iaccarino G. Trafficking GRK2: Cellular and Metabolic consequences of GRK2 subcellular localization. Transl Med UniSa. 2014; 10: 3–7. [PMC free article] [PubMed] [Google Scholar]
- 57.Usui I, Imamura T, Babendure JL, Satoh H, Lu JC, Hupfeld CJ, Olefsky JM. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol Endocrinol. 2005; 19(11): 2760–8. doi: 10.1210/me.2004-0429 [DOI] [PubMed] [Google Scholar]
- 58.Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X, Li D, Jia W, Kang J, Pei G. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009; 457(7233): 1146–9. doi: 10.1038/nature07617 [DOI] [PubMed] [Google Scholar]
- 59.Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei Z, Peroutka RJ, Gold JI, Gumpert A, Chen M, Otis NJ, Dorn GW, 2nd , Trimarco B, Iaccarino G, Koch WJ. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation. 2011; 123(18): 1953–62. doi: 10.1161/CIRCULATIONAHA.110.988642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anis Y, Leshem O, Reuveni H, Wexler I, Ben Sasson R, Yahalom B, Laster M, Raz I, Ben Sasson S, Shafrir E, Ziv E. Antidiabetic effect of novel modulating peptides of G-protein-coupled kinase in experimental models of diabetes. Diabetologia. 2004; 47(7): 1232–44. doi: 10.1007/s00125-004-1444-1 [DOI] [PubMed] [Google Scholar]
- 61.Takenouchi Y, Kobayashi T, Taguchi K, Matsumoto T, Kamata K. Gender differences in endothelial function in aortas from type 2 diabetic model mice. J Pharmacol Sci. 2009; 111(1): 91–9. doi: 10.1254/jphs.09133FP [DOI] [PubMed] [Google Scholar]
- 62.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf). 2009; 196(2): 193–222. doi: 10.1111/j.1748-1716.2009.01964.x [DOI] [PubMed] [Google Scholar]
- 63.Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, Groszmann RJ. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999; 117(5): 1222–8. doi: 10.1016/S0016-5085(99)70408-7 [DOI] [PubMed] [Google Scholar]
- 64.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007; 42(2): 271–9. doi: 10.1016/j.yjmcc.2006.05.023 [DOI] [PubMed] [Google Scholar]
- 65.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999; 399(6736): 601–5. doi: 10.1038/21224 [DOI] [PubMed] [Google Scholar]
- 66.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999; 399(6736): 597–601. doi: 10.1038/21218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension. 2004; 44(6): 956–62. doi: 10.1161/01.HYP.0000147559.10261.a7 [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi T, Taguchi K, Nemoto S, Nogami T, Matsumoto T, Kamata K. Activation of the PDK-1/Akt/eNOS pathway involved in aortic endothelial function differs between hyperinsulinemic and insulin-deficient diabetic rats. Am J Physiol Heart Circ Physiol. 2009; 297(5): H1767–75. doi: 10.1152/ajpheart.00536.2009 [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi T, Taguchi K, Takenouchi Y, Matsumoto T, Kamata K. Insulin-induced impairment via peroxynitrite production of endothelium-dependent relaxation and sarco/endoplasmic reticulum Ca(2+)-ATPase function in aortas from diabetic rats. Free Radic Biol Med. 2007; 43(3): 431–43. doi: 10.1016/j.freeradbiomed.2007.04.019 [DOI] [PubMed] [Google Scholar]
- 70.Lee JH, Palaia T, Ragolia L. Impaired insulin-mediated vasorelaxation in diabetic Goto-Kakizaki rats is caused by impaired Akt phosphorylation. Am J Physiol Cell Physiol. 2009; 296(2): C327–38. doi: 10.1152/ajpcell.00254.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007; 13(3): 315–23. doi: 10.1038/nm1553 [DOI] [PubMed] [Google Scholar]
- 72.Pei G, Tiberi M, Caron MG, Lefkowitz RJ. An approach to the study of G-protein-coupled receptor kinases: an in vitro-purified membrane assay reveals differential receptor specificity and regulation by G β γ subunits. Proc Natl Acad Sci USA. 1994; 91(9): 3633–6. doi: 10.1073/pnas.91.9.3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. β-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J Biol Chem. 2009; 284(13): 8855–65. doi: 10.1074/jbc.M808463200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hall RA, Premont RT, Lefkowitz RJ. Heptahelical receptor signaling: beyond the G protein paradigm. J Cell Biol. 1999; 145(5): 927–32. doi: 10.1083/jcb.145.5.927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coureuil M, Lécuyer H, Scott MG, Boularan C, Enslen H, Soyer M, Mikaty G, Bourdoulous S, Nassif X, Marullo S. Meningococcus Hijacks a β2-adrenoceptor/β-Arrestin pathway to cross brain microvasculature endothelium. Cell. 2010; 143(7): 1149–60. doi: 10.1016/j.cell.2010.11.035 [DOI] [PubMed] [Google Scholar]
- 76.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA. 2001; 98(5): 2449–54. doi: 10.1073/pnas.041604898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeFea KA, Zalevsky J, Thoma MS, Déry O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000; 148(6): 1267–81. doi: 10.1083/jcb.148.6.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. β-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005; 280(9): 8041–50. doi: 10.1074/jbc.M412924200 [DOI] [PubMed] [Google Scholar]
- 79.Bhattacharya M, Anborgh PH, Babwah AV, Dale LB, Dobransky T, Benovic JL, Feldman RD, Verdi JM, Rylett RJ, Ferguson SS. Beta-arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat Cell Biol. 2002; 4(8): 547–55. [DOI] [PubMed] [Google Scholar]
- 80.Skafar DF, Xu R, Morales J, Ram J, Sowers JR. Clinical review 91: Female sex hormones and cardiovascular disease in women. J Clin Endocrinol Metab. 1997; 82(12): 3913–8. [DOI] [PubMed] [Google Scholar]
- 81.Sowers JR. Diabetes mellitus and cardiovascular disease in women. Arch Intern Med. 1998; 158(6): 617–21. doi: 10.1001/archinte.158.6.617 [DOI] [PubMed] [Google Scholar]
- 82.Kaseta JR, Skafar DF, Ram JL, Jacober SJ, Sowers JR. Cardiovascular disease in the diabetic woman. J Clin Endocrinol Metab. 1999; 84(6): 1835–8. doi: 10.1210/jcem.84.6.5735 [DOI] [PubMed] [Google Scholar]
- 83.Taguchi K, Matsumoto T, Kamata K, Kobayashi T. Akt/eNOS pathway activation in endothelium-dependent relaxation is preserved in aortas from female, but not from male, type 2 diabetic mice. Pharmacol Res. 2012; 65(1): 56–65. doi: 10.1016/j.phrs.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 84.Ciccarelli M, Sorriento D, Franco A, Fusco A, Del Giudice C, Annunziata R, Cipolletta E, Monti MG, Dorn GW, 2nd , Trimarco B, Iaccarino G. Endothelial G protein-coupled receptor kinase 2 regulates vascular homeostasis through the control of free radical oxygen species. Arterioscler Thromb Vasc Biol. 2013; 33(10): 2415–24. doi: 10.1161/ATVBAHA.113.302262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rivas V, Carmona R, Muñoz-Chápuli R, Mendiola M, Nogués L, Reglero C, Miguel-Martín M, García-Escudero R, Dorn GW, 2nd , Hardisson D, Mayor F, Jr , Penela P. Developmental and tumoral vascularization is regulated by G protein-coupled receptor kinase 2. J Clin Invest. 2013; 123(11): 4714–30. doi: 10.1172/JCI67333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stevenson NL, Martin-Martin B, Freeman J, Kriston-Vizi J, Ketteler R, Cutler DF. G protein-coupled receptor kinase 2 moderates recruitment of THP-1 cells to the endothelium by limiting histamine-invoked Weibel-Palade body exocytosis. J Thromb Haemost. 2014; 12(2): 261–72. doi: 10.1111/jth.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]