Abstract
Functional studies have shown that orchidectomy increases the effects of phenylephrine on rat portal veins, but that it is completely prevented in the presence of both ETA and ETB receptor antagonists. Although it suggests the involvement of endothelin-1 (ET-1), the local production of this vasoactive peptide has not been directly quantified in portal veins. Therefore, the aim of the present study was to verify if orchidectomy increases the local expression of ET-1 as well as ETA and ETB receptors in the rat portal vein. Indeed, the genic expression of ET-1, ETA and ETB receptors in rat portal veins taken from control (CONT), orchidectomized (ORX) and ORX plus testosterone-replacement therapy (ORX + T) animals were determined by Real Time RT-PCR. The results showed that orchidectomy induced a significant increment in genic expression of ET-1and ETB receptors in the rat portal veins, which was completely reversed by testosterone replacement treatment. In conclusion, the results suggest that orchidectomy increases the production of ET-1 in the rat portal vein and that, at least partially, it may be related to the previously reported elevation of responses to phenylephrine.
Keywords: endothelin-1, ETB receptor, orchidectomy, portal vein, testosterone
Introduction
The cardiovascular system is considered to be an important target of androgenic hormones (1). In this regard, the cardiovascular repercussions of long-term testosterone deprivation must be better understood, because it is involved in some clinical situations commonly observed in men such as hypogonadism (2, 3), age-related plasma testosterone reduction (4, 5) as well as in patients undergoing orchidectomy for the treatment of hormone-sensitive neoplasias (6).
Long-term variations of the plasma testosterone level may modify the gene expression of peptides that regulate vascular tone. However, the repercussions of plasma testosterone level variation upon the expression of ET-1 as well as of the endothelin receptors, subtype A (ETA receptor) or B (ETB receptor), are still misunderstood. Some authors report that testosterone treatment increases plasma ET-1 (7) or big-ET-1 level (8) in humans. Moreover, it was demonstrated that testosterone increased the percentage of human cultured endothelial cells that secrete ET-1 as well as the ET-1 mRNA expression on these cells (9). Furthermore, treatment with testosterone increased the contractile effects of ET-1 in segments of the thoracic aorta taken from hypercholesterolemic rabbits (10). On the other hand, there was a significant negative correlation between plasma ET-1 level and plasma testosterone level in patients with hypogonadism before and after testosterone replacement therapy (11).
In a previous study we observed that orchidectomy increased the effects of phenylephrine on rat portal veins, but that this was completely reversed by testosterone replacement. This orchidectomy-induced increase of response was not observed in the presence of either BQ-123 or BQ-788, selective antagonists of ETA and ETB receptors respectively (12). Based on these data, we can infer that orchidectomy increases the expression of endothelin-1 and/or ETA and ETB receptors in the rat portal vein, thereby resulting in elevation of portal vein responses to phenylephrine. This proposal, however, requires more direct evidence that local expression of ET-1 is indeed increased due to orchidectomy. Therefore, the aim of the present study was to verify if orchidectomy increases the genic expression of ET-1 as well as ETA and ETB receptors in the rat portal vein.
Methods
Animals
Male Wistar rats weighting 350–450 g were housed in plastic cages (50 × 40 × 20 cm), 3 animals per cage, with food and water ad libitum. During the experiment, the animals were maintained under a 12 h light-dark cycle beginning at 7:00 h, at room temperature (25 °C). Rats were used in accordance with instructions of the Guide for the Care and Use of Laboratory Animals, National Academy of Sciences (1996). This study was also approved by the Research Ethics Committee of the School of Medicine at Marília (protocol No.268/09).
Orchidectomy protocol
Animals were divided into three groups: control (CONT), orchidectomized (ORX) and ORX plus testosterone-replacement therapy (ORX + T). The orchidectomy as well as the testosterone replacement therapy was performed in accordance with the protocol described (12). Briefly, under anesthesia with 2,2,2-tribromoethanol (Acros Organics, Geel, Antwerp, Belgium; 250 mg/kg, i.p.), both testes of the ORX animals were surgically removed through a longitudinal incision made in the scrotum. Control animals were subjected to the same surgical procedures but their testes were not removed. Animals were subjected to orchidectomy on postnatal Day 90, when the plasma testosterone level appears to be more stable (13).
Testosterone replacement therapy protocol
Testosterone replacement was performed in ORX + T animals using testosterone propionate (10 mg/kg, i.m.; Sigma, St Louis, MO, USA) that was administered every 5 days for 3 weeks. Control and ORX animals were injected i.m. with vehicle (maize oil) instead. The replacement was started 23 days after orchidectomy, when the atrophy of the testosterone-dependent sexual accessory organs had already occurred (14).
Testosterone-dependent sexual accessory organs weight
The effectiveness of the orchidectomy was verified by the atrophy (reduction of wet weight) of the seminal vesicles and prostate gland, referred to as testosterone-dependent sexual accessory organs (14). In the same way, testosterone replacement was considered effective if it was able to revert the atrophy of the testosterone-dependent sexual accessory organs. At the day of experiment, the animals were anesthetized with 2,2,2-tribromoethanol (250 mg/kg, i.p.) and exsanguinated. Next, seminal vesicles and prostate gland were carefully removed and cleared of all connective tissue. The wet weight (in g) of both the isolated seminal vesicles and the prostate gland were reported as a proportion of total body weight (in kg).
Plasma testosterone level measurement
The effectiveness of the orchidectomy was confirmed by the decrease in plasma testosterone levels, while testosterone replacement was considered effective if it was able to reverse the reduction of the plasma testosterone levels induced by orchidectomy. At the day of experiment, the animals were anesthetized with 2,2,2-tribromoethanol (250 mg/kg, i.p.) and the blood samples were collected by puncture of the vena cava immediately before the sacrifice of animals. Samples were centrifuged (700 g) for 20 min at 2 °C. Supernatants were collected and stored at –20 °C until measurement of plasma testosterone level by chemiluminescence (15).
RNA extraction and cDNA synthesis
At the day of experiment, the animals were anesthetized with 2,2,2-tribromoethanol (250 mg/kg, i.p.) and exsanguinated. Next, portal vein rings (2–4 mm) were carefully removed, cleared of all connective tissue and stored at –80 °C. Total RNA was extracted from frozen portal vein samples using TRIZOL (Life Technologies, Gaithersburg, MD, USA), following the manufacturer's instructions. Total RNA was quantified using Spectrophotometer NanoDrop - 2000 (NANODROP, USA). The concentrations were adjusted and the samples were stored at –80 °C until use. cDNA synthesis was carried out using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems™, USA), following the protocol provided by the manufacturer. All cDNA was quantified using Spectrophotometer NanoDrop - 2000 (NANODROP, USA). The concentrations were adjusted and stored at –80 °C.
Gene Expression Analysis
All gene expression was measured using qRT-PCR on the Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems™, USA), according to the cycling conditions recommended by Applied Biosystems. We used Endothelin-1(ET-1) – Assay ID: Rn00561129_m1*, Endothelin receptor type A – Assay ID: Rn00561137_m1*, Endothelin receptor type B – Assay ID: Rn00569139_m1* and GAPDH – Assay ID: Rn99999916_s1.
The threshold values were uniformly set for all assays. All reactions were performed in duplicate. Replicates with standard deviations (SD) higher than 0.5 for the cycle threshold (CT) value were repeated or excluded from the analysis.
An amplification curve was created for each group, and the CT values obtained for all genes (ET-1, ETA, ETB and GAPDH). We used the comparative CT method (ΔΔCT method), where we first calculated ΔCT = CT target – CT endogenous controls in order to normalize the target gene to the endogenous controls. It is important to highlight that Relative Quantification (RQ) of ET-1, ETA, ETB genes were calculated using the control group as a reference and using the 2-ΔΔCT formula, which provides the percentage change, or how much one gene is more expressed in one group relative to another. All CT values were obtained using 7500 software 2.0 and these data were exported to Microsoft Excel (Microsoft, USA) to calculate 2-ΔCT and RQ.
Statistical analysis
Data are presented as mean ± standard error of the mean (S.E.M.). The one-way Analysis of Variance (ANOVA) followed by Bonferroni as the post-test was used for comparison between the groups. P values of less than 0.05 were considered to be statistically significant.
Results
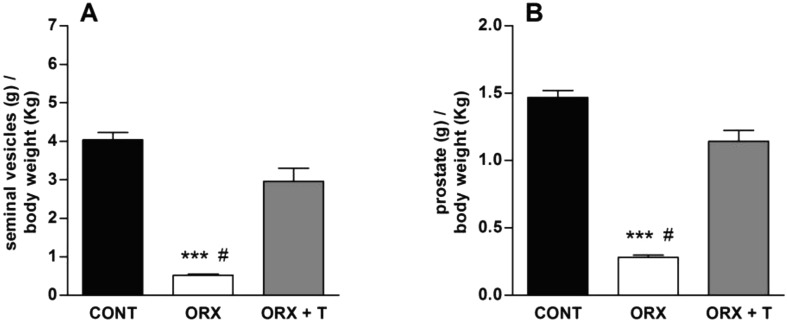
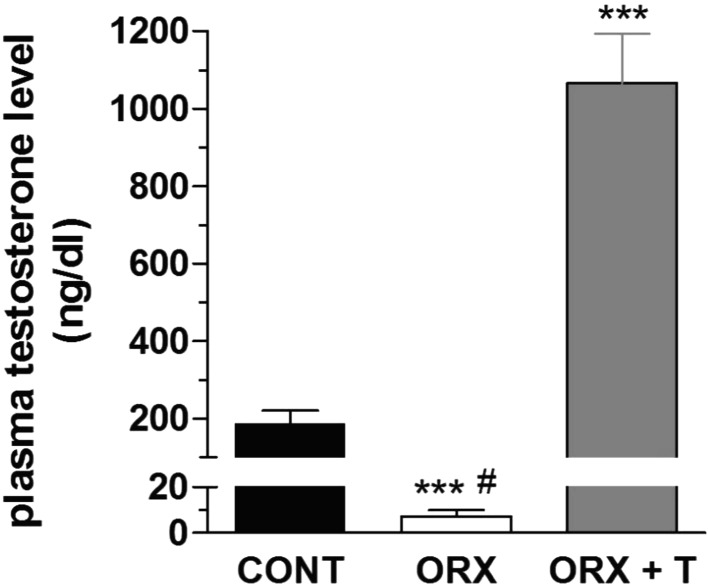
Orchidectomy significantly reduced the wet weight of the seminal vesicles (Fig. 1A) and the prostate gland (Fig. 1B), whereas the testosterone replacement treatment completely reversed this orchidectomy-induced atrophy. In parallel, orchidectomy significantly reduced plasma testosterone level in ORX animals and, moreover, the hormone replacement treatment not only restored but even promoted supraphysiological levels of this hormone in the plasma of ORX + T animals (Fig. 2).
Fig. 1.
Weight of seminal vesicles (A) and prostate gland (B) taken from control (CONT), orchidectomized (ORX) and ORX + testosterone-treated (ORX + T) animals. Data are the mean ± S.E.M. (n=10–12). ***P<0.001 related to the CONT; #P<0.001 related to the ORX + T (One-way ANOVA, followed by Bonferroni post-test).
Fig. 2.
Plasma testosterone levels (ng/dl) taken from control (CONT), orchidectomized (ORX) and ORX + testosterone-treated (ORX + T) animals. Data are the mean ± S.E.M. (n=10–12). ***P<0.001 related to the CONT; #P<0.001 related to the ORX + T (One-way ANOVA, followed by Bonferroni post-test).
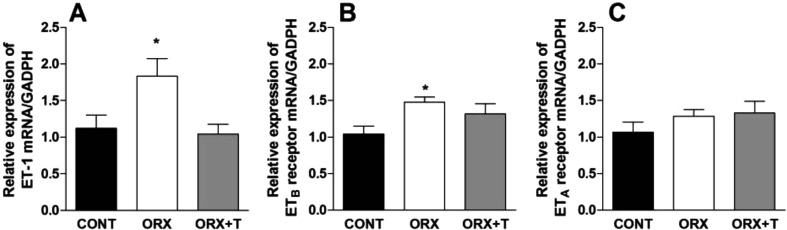
Moreover, orchidectomy elevated significantly the genic expression of ET-1 (Fig. 3A) and ETB receptors (Fig. 3B) in rat portal veins, and the testosterone replacement treatment completely reversed the orchidectomy-induced elevation of genic expression of both ET-1 and ETB (Fig. 3A and 3B). On the other hand, orchidectomy failed to increase the genic expression of ETA receptors in rat portal veins (Fig. 3C).
Fig. 3.
Gene expression of endothelin-1 (ET-1) (A) as well as endothelin receptor subtypes ETB (B) and ETA (C), related to the GAPDH gene, detected in rat portal veins taken from control (CONT), orchidectomized (ORX) and ORX + testosterone-treated (ORX + T) animals. Data are the mean ± S.E.M. (n=6). *P<0.05 related to CONT (one way ANOVA followed by Bonferroni's post-test).
Discussion
The present study was conducted to verify if the genic expression of ET-1 as well as ETA and ETB receptors are modified by orchidectomy and whether such modifications may be reversed by testosterone replacement treatment. In this regard, our primary concern was to know if the procedure used for orchidectomy really promoted a drastic reduction of the plasma testosterone level, and whether the hormonal replacement protocol effectively restored these plasma levels at the time of tissue collection for the Real time RT-PCR experiments. In this regard, it was found that orchidectomy significantly reduced the wet weight of the seminal vesicles and prostate gland whereas the testosterone replacement treatment completely reversed this orchidectomy-induced atrophy. In addition, orchidectomy significantly reduced plasma testosterone level in ORX animals and, moreover, the hormone replacement treatment not only restored but even promoted supraphysiological levels of this hormone in the plasma of ORX + T animals. This indicates that either the orchidectomy procedure or testosterone replacement protocol used in the present investigation were effective (16).
It is noteworthy that the choice of this hormone replacement protocol, despite the production of supraphysiological levels of testosterone, occurred because it is the only way to reverse atrophy in the testosterone-dependent organs induced by orchidectomy. Thus, as it completely reverses the atrophy of testosterone-dependent organs, this protocol may also reverse any changes in the production of ET-1 induced by orchiectomy in the portal veins of these animals.
These results, together with the quantification of the plasma testosterone level, reinforce the effectiveness of our experimental model, thereby supporting the results obtained by real time RT-PCR that show orchidectomy-induced increment of the ET-1 genic expression in the rat portal vein. It strongly suggests that the drastic reductions of plasma testosterone level in orchidectomized animals stimulate the production of ET-1 in this venous bed. This increment of the ET-1 genic expression was reversed by testosterone treatment, confirming the pivotal role that this hormone plays in this phenomenon. Therefore, the findings of the present study support the previously proposed hypothesis that orchidectomy promotes an increment of the expression of ET-1 on the portal vein, thereby increasing the α1-adrenoceptor-mediated contractile effects of phenylephrine. This hypothesis is also supported by previous studies which suggest that locally produced ET-1 potentiates the vascular effects of α1-adrenoceptor agonists (17, 18).
The reported increment of the phenylephrine effects induced by orchidectomy on the rat portal vein was not observed in the presence of ETA and ETB receptor inhibitors (12). In the analyzed rat portal veins, the genic expression of ETB receptors was not only increased by orchidectomy but also restored by testosterone replacement treatment. On the other hand, neither orchidectomy nor testosterone replacement treatment in orchidectomized animals modified the local expression of ETA receptors. However, this orchidectomy-induced increment of the ETB genic expression was not followed by modification of the rat portal vein responses to exogenous ET-1 (12). Perhaps this indicates a simultaneous action on ETB receptors overexpressed in both endothelium, where they promote the release of vasodilating substances (19), and also in vascular smooth muscle.
The present study shows that orchidectomy increases both ET-1 and ETB receptor mRNA expression in rat portal veins, but it did not specify the vascular wall layer where this increment occurs. Possibly the increment of ET-1 mRNA expression occurs in the endothelium since it is the main source of ET-1 (9). Moreover, the presence of ETB receptors has also been described in the endothelium (19). To solve this doubt, it would be necessary to verify the ET-1 and ETB mRNA expression in rat portal veins without endothelium. However, the endothelial removal was not possible in these veins before the mRNA extraction. Actually, this extraction process must be fast to avoid mRNA degradation. Thus, it would not be possible to remove the endothelium as well as to verify the absence of endothelial cells in these veins before the mRNA extraction was performed.
The increase of genic expression of ET-1 as well ETB receptors strongly indicates that these molecules are synthesized in greater amounts in the rat portal vein. However it is necessary to emphasize that the real-time PCR does not quantify protein synthesis. Real-time PCR was chosen in in this study because the portal vein of rats is small and the use of direct methods of quantification of peptides is difficult. Nevertheless, since the presented data are congruent with those previously obtained in functional experiments (12), this limitation does not invalidate the hypothesis that orchidectomy increases the synthesis of ET-1 and ETB receptors in rat portal vein.
This study expands the discussion about the effect of testosterone upon the vessels. Actually, it is not in agreement with some previous reports which have shown that testosterone elevates plasma levels of either ET-1 (7) or Big-ET-1 (8) in humans. It also contradicts data obtained from cultured human endothelial cells which demonstrated that testosterone increased the percentage of cells that secrete ET-1 as well as the ET-1 mRNA expression on those cells (9).
On the other hand, the effects of orchidectomy on rat portal vein were similar to those previously observed in other testosterone-sensitive organs. In the rat prostate gland, orchidectomy increased the expression of ET-1 and ETB receptors in both the dorsolateral and ventral regions, whereas it reduces the expression of ETA receptors in the dorsolateral region only (20). In dogs, orchidectomy increased the ET-1 binding in the prostate gland while it reduced the ET-1 binding in the brain (21). Moreover, the orchidectomy-induced increment of ET-1 as well as both ETA and ETB receptor expression in the rat seminal vesicle (22). In the rat epididymis, the expression of ET-1 was also up-regulated by orchidectomy (23). Together, the presented data indicate that testosterone may exert suppressive effects upon the expression of both ET-1 and ETB receptors in these testosterone-sensitive organs.
As we have shown, the rat portal veins are sensitive to drastic modifications of plasma testosterone level. However, this venous bed has a distinct response in relation to other blood vessels regarding the changes in ET-1 expression consequent to modification in plasma testosterone levels. It is noteworthy that the portal vein constitutes a crucial bed for the drainage of blood from the splanchnic region, the largest blood reservoir in the body (24). Therefore, this implies that special attention must be given to the splanchnic hemodynamics when marked reductions of plasma testosterone levels occur.
In conclusion, the present study suggests that the drastic reduction of plasma testosterone level induced by orchidectomy increases the genic expression of ET-1 and ETB receptors in the rat portal vein. Supported by previous functional evidences, the presented data strongly suggests that orchidectomy augments the production of both ET-1 and ETB receptors on the rat portal vein and that this, may at least partially explain the already described increased responses of the rat portal vein to phenylephrine.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (process No.09/08012–2). We thank Mr. Alisson Douglas Ventura Neves for technical assistance.
References
- 1.Lopes RAM, Neves KB, Carneiro FS, Tostes RC. Testosterone and vascular function in aging. Front Physiol. 2012; 3: 89. doi: 10.3389/fphys.2012.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernini G, Versari D, Moretti A, Virdis A, Ghiadoni L, Bardini M, Taurino C, Canale D, Taddei S, Salvetti A. Vascular reactivity in congenital hypogonadal men before and after testosterone replacement therapy. J Clin Endocrinol Metab. 2006; 91(5): 1691–7. doi: 10.1210/jc.2005-1398 [DOI] [PubMed] [Google Scholar]
- 3.Sader MA, Griffiths KA, Skilton MR, Wishart SM, Handelsman DJ, Celermajer DS. Physiological testosterone replacement and arterial endothelial function in men. Clin Endocrinol (Oxf). 2003; 59(1): 62–7. doi: 10.1046/j.1365-2265.2003.01796.x [DOI] [PubMed] [Google Scholar]
- 4.Baum NH, Crespi CA. Testosterone replacement in elderly men. Geriatrics. 2007; 62(9): 15–8. [PubMed] [Google Scholar]
- 5.Seidman SN. Androgens and the aging male. Psychopharmacol Bull. 2007; 40(4): 205–18. [PubMed] [Google Scholar]
- 6.Hynes PG, Kelly K. Prostate cancer stem cells: The case for model systems. J Carcinog. 2012; 11: 6. doi: 10.4103/1477-3163.93701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheugt FW, Gooren LJ. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993; 118(6): 429–32. doi: 10.7326/0003-4819-118-6-199303150-00006 [DOI] [PubMed] [Google Scholar]
- 8.van Kesteren PJ, Kooistra T, Lansink M, van Kamp GJ, Asscheman H, Gooren LJ, Emeis JJ, Vischer UM, Stehouwer CD. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb Haemost. 1998; 79(5): 1029–33. [PubMed] [Google Scholar]
- 9.Pearson LJ, Yandle TG, Nicholls MG, Evans JJ. Regulation of endothelin-1 release from human endothelial cells by sex steroids and angiotensin-II. Peptides. 2008; 29(6): 1057–61. doi: 10.1016/j.peptides.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 10.Ammar EM, Said SA, Hassan MS. Enhanced vasoconstriction and reduced vasorelaxation induced by testosterone and nandrolone in hypercholesterolemic rabbits. Pharmacol Res. 2004; 50(3): 253–9. doi: 10.1016/j.phrs.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 11.Kumanov P, Tomova A, Kirilov G. Testosterone replacement therapy in male hypogonadism is not associated with increase of endothelin-1 levels. Int J Androl. 2007; 30(1): 41–7. doi: 10.1111/j.1365-2605.2006.00706.x [DOI] [PubMed] [Google Scholar]
- 12.de Souza Rossignoli P, Pereira OCM, Chies AB. Orchidectomy enhances the effects of phenylephrine in rat isolated portal vein. Clin Exp Pharmacol Physiol. 2010; 37(3): 368–74. doi: 10.1111/j.1440-1681.2009.05313.x [DOI] [PubMed] [Google Scholar]
- 13.White RM, Rivera CO, Davison CA. Nitric oxide-dependent and -independent mechanisms account for gender differences in vasodilation to acetylcholine. J Pharmacol Exp Ther. 2000; 292(1): 375–80. [PubMed] [Google Scholar]
- 14.Valle RM, Abreu LC, Picarelli ZP, Valle JR. Influence of castration upon trophism and reactivity of rat seminal vesicles. Braz J Med Biol Res. 1982; 15(1): 49–53. [PubMed] [Google Scholar]
- 15.Yilmaz B, Canpolat S, Sandal S, Akpolat N, Kutlu S, Ilhan N, Kelestimur H. Paint thinner exposure inhibits testosterone synthesis and secretion in a reversible manner in the rat. Reprod Toxicol. 2006; 22(4): 791–6. doi: 10.1016/j.reprotox.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 16.Matsuda K, Ruff A, Morinelli TA, Mathur RS, Halushka PV. Testosterone increases thromboxane A2 receptor density and responsiveness in rat aortas and platelets. Am J Physiol. 1994; 267(3 Pt 2): H887–93. [DOI] [PubMed] [Google Scholar]
- 17.Henrion D, Laher I. Potentiation of norepinephrine-induced contractions by endothelin-1 in the rabbit aorta. Hypertension. 1993; 22(1): 78–83. doi: 10.1161/01.HYP.22.1.78 [DOI] [PubMed] [Google Scholar]
- 18.Thorin E, Cernacek P, Dupuis J. Endothelin-1 regulates tone of isolated small arteries in the rat: effect of hyperendothelinemia. Hypertension. 1998; 31(4): 1035–41. doi: 10.1161/01.HYP.31.4.1035 [DOI] [PubMed] [Google Scholar]
- 19.Mazzuca MQ, Khalil RA. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem Pharmacol. 2012; 84(2): 147–62. doi: 10.1016/j.bcp.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi W, Afiatpour P, Jr Foster HE, Ikeda K, Wada Y, Weiss RM, Latifpour J. The effect of castration on endothelins, their receptors and endothelin converting enzyme in rat prostate. Naunyn Schmiedebergs Arch Pharmacol. 2002; 366(2): 166–76. doi: 10.1007/s00210-002-0575-5 [DOI] [PubMed] [Google Scholar]
- 21.Padley RJ, Dixon DB, Wu-Wong JR. Effect of castration on endothelin receptors. Clin Sci (Lond). 2002; 103(Suppl 48): 442S–5S. doi: 10.1042/CS103S442S [DOI] [PubMed] [Google Scholar]
- 22.Takahashi W, Yono M, Wada Y, Ikeda K, Weiss RM, Latifpour J. Regulatory effect of castration on endothelins, their receptors and endothelin-converting enzyme in rat seminal vesicle. BJU Int. 2003; 92(7): 803–9. doi: 10.1046/j.1464-410X.2003.04466.x [DOI] [PubMed] [Google Scholar]
- 23.Hamzeh M, Robaire B. Identification of early response genes and pathway activated by androgens in the initial segment and caput regions of the regressed rat epididymis. Endocrinology. 2010; 151(9): 4504–14. doi: 10.1210/en.2010-0023 [DOI] [PubMed] [Google Scholar]
- 24.Kjekshus H, Risoe C, Scholz T, Smiseth OA. Methods for assessing hepatic distending pressure and changes in hepatic capacitance in pigs. Am J Physiol Heart Circ Physiol. 2000; 279(4): H1796–803. [DOI] [PubMed] [Google Scholar]