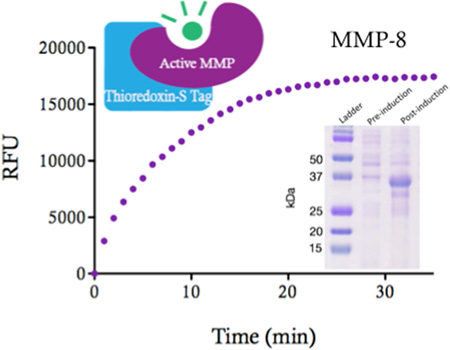
Abstract
Matrix metalloproteinases (MMPs) are crucial proteases in maintaining the health and integrity of many tissues, however their dysregulation often facilitates disease progression. In disease states these remodeling and repair functions support, for example, metastasis of cancer by both loosening the matrix around tumors to enable cellular invasion and by affecting proliferation and apoptosis, and they promote degradation of biological restorations by weakening the substrate to which the restoration is attached. As such, MMPs are important therapeutic targets. MMP-8 participates in cancer, arthritis, asthma and failure of dental fillings. MMP-8 differs from other MMPs in that it has an insertion that enlarges its active site. To elucidate the unique features of MMP-8 and develop selective inhibitors to this therapeutic target, a stable and active form of the enzyme is needed. MMP-8 has been difficult to express at high yield in a soluble, active form. Typically recombinant MMPs accumulate in inclusion bodies and complex methods are applied to refold and purify protein in acceptable yield. Presented here is a streamlined approach to produce in E. coli a soluble, active, stable MMP-8 fusion protein in high yield. This fusion shows much greater retention of activity when stored refrigerated without glycerol. A variant of this construct that contains the metal binding claMP Tag was also examined to demonstrate the ability to use this tag with a metalloprotein. SDS-PAGE, densitometry, mass spectrometry, circular dichroism spectroscopy and an activity assay were used to analyze the chemical integrity and function of the enzyme.
Keywords: MMP-8, Thioredoxin, soluble protein, metal abstraction peptide, claMP Tag
Graphical abstract
Introduction
Matrix metalloproteinases are a class of proteins responsible for degrading extracellular matrix (ECM) proteins to support tissue remodeling and repair.1,2 MMPs are of interest because of their complex biological roles and because of their participation in a variety of disease states, including cancer, metastasis and tumorigenesis, which is caused by the inappropriate up- or down-regulation of MMPs and/or their activity.3 Elevated activity leads to loosening of the supporting matrix, which facilitates dissemination of cancer cells to enable metastasis.4 Many MMP inhibitors have been created, but most have failed in clinical trials because of the complex set of contributions MMP activity exerts on disease progression, and as such, these enzymes remain the subject to much study.5,6
MMPs utilize a zinc-bound metal active site and are secreted as glycosylated zymogens, with a prodomain bound to the metal active site.2 Catalysis is activated by proteolytic cleavage of the prodomain, which exposes the active site. These enzymes are often difficult to produce recombinantly in a soluble form because of their complex composition, and generating them typically requires numerous steps, which include expression as proenzymes, refolding and use of several diverse purification approaches.7,8,9 MMPs also are difficult to produce because of their proteolytic function, which permits autoproteolysis to occur.10 Significant efforts have resulted in the production of a few MMPs in the amounts necessary to study their structure in atomic detail.11,12,13,14 Crystal structures of MMP-8 have been solved (PDB: 3DNG, 3DPE, 1ZVX)15,16 utilizing the complex protein production methods, and moreover, require the addition of an inhibitor to prevent autoproteolysis when concentrated.17 The ability to obtain high-resolution information about the protein enables design of selective inhibitors and provides the ability to characterize other ligand and protein-protein interactions that regulate enzyme activity.18,19,20
MMP-8 plays a role in cancer, arthritis, and asthma and promotes premature degradation of dental fillings by degrading the underlying collagen matrix within the dentin of demineralized tooth.21,22 While the fold of MMP-8 is related to other MMPs, it differs from them in the active site because it contains an insertion that creates a significantly larger binding pocket.23 The ability to study this enzyme and its unique active site in atomic detail in solution, without using an inhibitor would be extremely valuable for simplifying production and understanding how to design more potent and selective inhibitors of this particular MMP.24,25
Here, we present a method to express an active, stable form of matrix metalloproteinase-8 (MMP-8) in E. coli in sufficient amounts to enable structural evaluation. Several fusion constructs were generated that did not result in high yield of soluble, active, stable protein, but one construct met these criteria. This MMP-8 fusion includes two tags (thioredoxin and S Tag) to aid in folding and stability and a polyhistidine tag for affinity purification. Using this system, over 100 mg of MMP-8 can be expressed as a fusion protein in the soluble fraction of the cell lysate and purified quickly using immobilized metal affinity chromatography (IMAC) to recover appreciable amounts of catalytically active enzyme that retains full activity when stored refrigerated in simple buffer. Recognition sites for removal of the thioredoxin and thioredoxin-S Tag fusion partners were engineered into the construct. In addition, a spacer sequence also containing the metal-binding claMP Tag was inserted between the fusion partner and the enzyme. An additional aim of this study was to demonstrate compatibility of use of the metal abstraction peptide (MAP) technology inline with a metalloprotein.26 Genetic engineering of MAP into a plasmid creates, the claMP Tag, a linker-less carrier for many transition metals with utility in healthcare applications.27 The claMP Tag is a tripeptide consisting of the amino acid sequence Asn-Cys-Cys, which scavenges small transition metals from chelating agents.28 Because of the uniquely beneficial properties of the claMP Tag, which includes extraordinarily tight binding, resistance to metal release upon dilution at serum pH and specific release in acidic conditions as in endosomes, the claMP Tag is being investigated as a method for targeted delivery of metals for therapeutic and diagnostic applications by designing targeting proteins to contain the metal bound tripeptide.27 The claMP Tag has not been investigated previously in a system, such as matrix metalloproteinases, in which a structural and/or catalytic metalbinding site is present. Analysis of the activity and stability of these two fusion proteins and their cleaved products is presented herein.
Materials and Methods
Genetic Engineering
Human matrix metalloproteinase-8 (MMP-8) in the pCMV6-XL4 vector was obtained (OriGene Technologies, Inc. Cat # SC 127843) and the catalytic domain was amplified with designed primers (Integrated DNA Technologies). Two constructs of MMP-8 were prepared, one containing solely MMP-8 and one bearing an additional N-terminal claMP Tag (Asn-Cys-Cys).27,29 The primers contained a region matching the pET-32Xa/LIC vector (underlined), a portion matching the MMP-8 catalytic domain (italics), the claMP Tag (bold) and an inserted linker (bold and underlined) between the claMP Tag and MMP-8. 5’-GGT ATT GAG GGT CGC AAT CCA GGA AAC CCC AAG TG-3’ (forward primer no claMP Tag), 5’- TGC GGC TCT TCT GGC ATT GAG GGT CGC AAC CCC AAG TGG GAA-3’ (first forward primer for claMP-link insertion), 5’-GGT ATT GAG GGT CGC CCA GAT CTG GGT AAC TGC TGC GGC TCT TCT GGC-3’ (second forward primer for claMP-link insertion), and 5’-AGA GGA GAG TTA GAG CCT TAT CCA TAG ATG GCC TG-3’ (reverse primer for both constructs). The MMP-8 PCR reaction was purified using QIAquick PCR Purification Kit (Qiagen) and was inserted into the pET-32Xa/LIC vector by ligation independent cloning (protocol provided by Novagen). claMP-link-MMP-8 required two sequential PCR steps to incorporate the full-length linker. The PCR reaction was then purified using QIAquick PCR Purification Kit (Qiagen) and was inserted into the pET-32Xa/LIC vector by ligation independent cloning (protocol provided by Novagen). The inserted tag contained a BglII site so that the original Factor Xa recognition sequence could be removed by cleaving with this endonuclease. The new plasmid was cut with the restriction enzyme, BglII and was religated with T4 DNA ligase.
Using the standard heat shock procedure, the reactions were transformed into DH5α Escherichia coli (E. coli) cell strain. Luria broth (LB) agar plates with 100 µg/mL ampicillin were used to select transformed cells. The plates were incubated overnight at 37 °C. Individual colonies were grown in 5 mL LB with 100 µg/mL ampicillin overnight at 37 °C and 250 rpm. The starter cultures were spun d own at 1717 × g for 15 min and the supernatant was discarded. A miniprep kit (Quiagen) was used to purify the plasmid DNA. DNA sequences were confirmed by UC Berkeley DNA Sequencing Facility.
Four additional constructs bearing a variety of tags that may improve folding and/or solubility were generated and tested for expression in E. coli. These constructs did not lead to accumulation of soluble protein and are described in Supplemental Information.
Expression of MMP-8
Plasmids were transformed into BL21 E. coli cell strain using standard heat shock techniques. The cells were plated on LB agar plates with 100 µg/mL ampicillin and incubated overnight at 37 °C. Cultures were started using a single colony to inoculate 50 mL of LB with 100 µg/mL ampicillin and grown for 16 hrs at 37 °C, 250 rpm in an orbital shaker. Twenty milliliters of starter culture was transferred to 1 L of LB with 100 µg/mL ampicillin in a 3 L fernbach flask. The cells were grown at 37 °C, 250 rpm until the OD600 reached approximately 0.7. One milliliter of 1 M isopropyl β-D-1-thiogalactopyranoside (IPTG) was used to induce the cells, which were harvested after four hours by centrifugation at 1391 × g for 8 minutes. The cell pellets were stored at −80 °C until use.
Protein Purification
All solutions were made to an ionic strength of 150 mM by adjusting the concentration of NaCl. A one-liter pellet was resuspended in 25 mL lysis buffer (50 mM Tris-Cl, 20 mM imidazole, 59 mM NaCl, 10 µM N-Isobutyl-N-(4-methoxyphenylsulfonyl)glycyl hydroxamic acid (NNGH), pH 7.9) and three passes through a french press at 21,000 psi was used to lyse the cells. Lysates were centrifuged for 1 hour at 21,000 × g and 4 °C. The supernatant containing the protein was filtered through a 0.45 µm filter followed by a 0.2 µm filter and applied to a nickelated 5 mL Hi-Trap Chelating HP column (GE Lifesciences) equilibrated in lysis buffer. The column was washed with 50 mM Tris-Cl, 58 mM NaCl, 40 mM imidazole, 10 µM NNGH, pH 7.9 for 10 CV at a flow rate of 1.25 mL/min at 4 °C. The protein was eluted from the column using a linear gradient elution from 0 to 100% of 50 mM Tris, 37 mM NaCl, 500 mM imidazole, 10 µM NNGH, pH 7.9. The eluate was concentrated using Amicon Ultra 10 kDa MWCO (Millipore) concentrators to approximately 2 mL and injected on a HiLoad 26/600 Superdex 75 prep grade column (pack size 1 × 320 mL, GE Lifesciences #28-98930-34) equilibrated in 50 mM Tris-Cl, 60 mM NaCl, 10 µM NNGH, pH 7.9 to separate degradation fragments and eliminate the imidazole in the sample. The fractions were concentrated using an Amicon Ultra 10 kDa MWCO (Millipore) to approximately 2 mL. To cleave the fusion into thioredoxin and S Tag-MMP-8, 70 Units of Thrombin were added and the reaction was incubated at room temperature for 12 hours. The sample was injected on a Superdex 75 column (GE Lifesciences) to separate the fragments. The S tag was cleaved by adding 9.8 ng of Factor Xa and allowing the reaction to proceed at room temperature for 16 hrs and run over the Superdex 75 column (GE Lifesciences). The final product was concentrated to 1.5 mL and stored at 4 °C. All solutions used in these procedures contained 10 µM NNGH to stabilize the protein and protect against autoproteolysis, however, it was determined herein that this compound is not required for the fusion proteins.
claMP-link-MMP-8 abstracts nickel from the IMAC resin during purification, generating a translucent, rusty orange solution, typical of the Ni-claMP complex.27 As previously reported, UV-vis absorbance spectroscopy at 425 nm was used to assess metal occupancy of the claMP Tag in the fusion construct.27 The molar extinction coefficient for 425 nm is 350 M−1cm−1. Following purification, at 0.5 mM, the highest concentration of the fusion protein, the A425 indicates complete nickel insertion into the claMP Tag has been achieved. Factor Xa cleaves the nickel-bound claMP Tag from MMP-8.
Liquid Chromatography and Mass Spectrometry
Whole protein electrospray ionization mass spectra (ESI-MS) were acquired on a Qtof Premier (Waters/Micromass, Manchester UK) hybrid mass spectrometer operated in MS mode and acquiring data with the time of flight analyzer. The instrument was operated in V mode or at 9000 resolution. The source was optimized using lysozyme infused at the HPLC flow rate. The cone voltage was 45 V, the source ion guide with added gas flow (N2, 20 mL/min) and Ar was admitted to the collision cell. The cell was operated at 10 eV or maximum transmission without increasing water loss ions. Spectra were acquired covering the mass range 300 to 3000 amu and accumulating data for 4 seconds per cycle. Time to mass calibration was made with NaI cluster ions acquired under the same conditions. Spectral mass correction was made with the peptide leucine encephalin (YGGFL) dimer (1111.5463) observed in the lock mass spray channel. The average mass of proteins was determined from the charge state distribution with the "Transform" and/or MaxEnt I routines in Masslynx software.
Microbore HPLC/MS experiments were performed with a Waters Acquity H class chromatograph at 180 µL/min on a 1-mm ID C4 RP column eluting into the standard ESI source of the Qtof premier. The solvents were: A H2O, B 90% CH3CN, 10% isopropanol, both 0.08% formic acid. Separations were performed on a 1-mm ID × 50-mm long C4 reverse phase column (Zorbax C4, 300Å pore size, 3.5 µm particles packed by Micro-Tech) with a 1-mm × 2-cm dry-packed guard column (Upchurch Scientific model C.128, Oak Harbor, WA) filled with Zorbax C3 resin. The gradient was to ramp from 1% to 20% B in 1 min then to 50% B by 9 min, and finally to 75% B by 10 min.
Circular Dichroism Spectroscopy
Circular dichroism (CD) spectra were collected using a Chirascan spectropolarimeter (Applied Photophysics). The samples were analyzed at a concentration of 0.2 mg/mL in 10 mM sodium phosphate, pH 7.9 and were added to a quartz cuvette with a 0.1-cm path length (Starna Cells). Spectra were collected at 10 °C and analyzed using several wavelength ranges, beginning at 190, 195, 200, 205 and 210 nm through 260 nm. Background signal from the solution was subtracted. CDNN software (Applied Photophysics) was used for spectral deconvolution.
SDS PAGE Analysis
SDS-PAGE samples were obtained by mixing 30 µL of sample with 30 µL of reducing Laemmli buffer and heated at 90 °C for 10 min. Samples were stored at −20 °C until used. Gel samples were loaded onto standard 15% (v/v) resolving, 4% (v/v) stacking gels and run at 135 V for 2 hrs. A pre-stained molecular weight ladder (BioRad, #161-0374) was used for Coomassie staining and an unstained molecular weight ladder (BioRad, #161-0363) was used as a reference for gels stained with SYPRO Ruby Protein Gel Stain (Life Technologies, cat# S-12000).
Densitometry Analysis
Densitometry analysis was done on SYPRO Ruby-stained gels. A Typhoon TRIO Variable Mode Imager (Amersham Biosciences) was used to image the gels and ImageQuantTL Software was used to perform the quantitative analysis. Gels were stained with SYPRO Ruby Protein Gel Stain (Life Technologies, cat# S-12000) according to the manufacturer’s instructions.
Activity Assay
The MMP-8 Fluorometric Drug Discovery Kit, RED (Enzo Life Sciences cat# BML-AK305) was used to examine enzyme activity of the recombinant proteins and cleavage products. The kit components were thawed to room temperature and diluted in assay buffer according to the manufacture’s instructions. Briefly, assay buffer, enzyme (final concentration 1 µM), and either inhibitor (final concentration 5 µM) or an equivalent amount of buffer were pipetted into the wells of a 96-well plate and incubated at 37 °C for 1 hour. The fluorescent plate reader was set at Ex/Em=545/576 nm, with cut off at 570 nm. To begin the reaction, 10 µL of substrate equilibrated to 37 °C was added to obtain a final concentration of 1 µM. Buffer blanks and control solutions were analyzed to account for background signal and samples were analyzed in triplicate. Data were processed and fitting and error analysis was performed using Microsoft Excel and plots were made using GraphPad Prism. Error bars are representative of the standard deviation between replicates.
Results
Recombinant MMP-8 Expression and Purification
The catalytic domain of MMP-8 was cloned into a set of eight vectors containing a variety of tags to identify a construct that would produce soluble, active protein in high yield. The pET-32Xa/LIC vector containing N-terminal thioredoxin and S Tag successfully supported this objective. This fusion construct enables folding of MMP-8 to generate milligram quantities of the catalytically active enzyme while avoiding accumulation in inclusion bodies, which allows one-step affinity purification from the soluble fraction of the cell lysate.
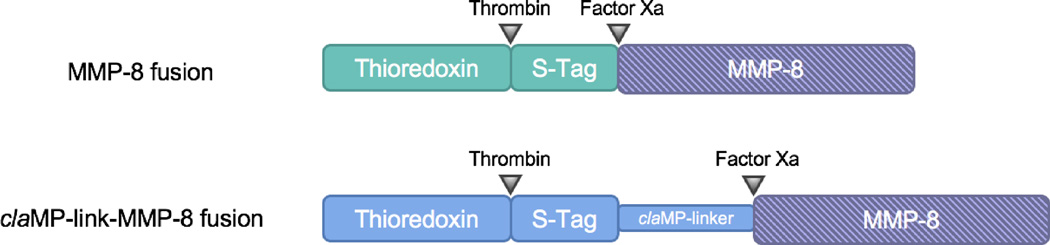
To test the robustness of the general construct, an additional variant of the MMP-8 fusion protein was created that alters the spacing between the stabilizing fusion partner and the enzyme. The claMP Tag developed by our lab was inserted N-terminal to MMP-8 with an additional flexible spacer sequence (Gly-Ser-Ser-Gly-Ile-Glu-Gly-Arg) (Figure 1). The claMP Tag is an extremely stable, inline module for targeted metal delivery.27,30 It consists of the amino acid sequence Asn-Cys-Cys and rapidly and quantitatively binds metal at basic pH,30,31 here during purification upon exposure to the Ni-IMAC resin. The recognition sequence of Factor Xa follows this claMP-link spacer in the claMP-link-MMP-8 construct to permit cleavage of the additional tag and release of the same MMP-8 product.
Figure 1.
Cartoon of the fusion constructs of matrix metalloproteinase-8 (MMP-8) containing fusion partners and cleavage sites.
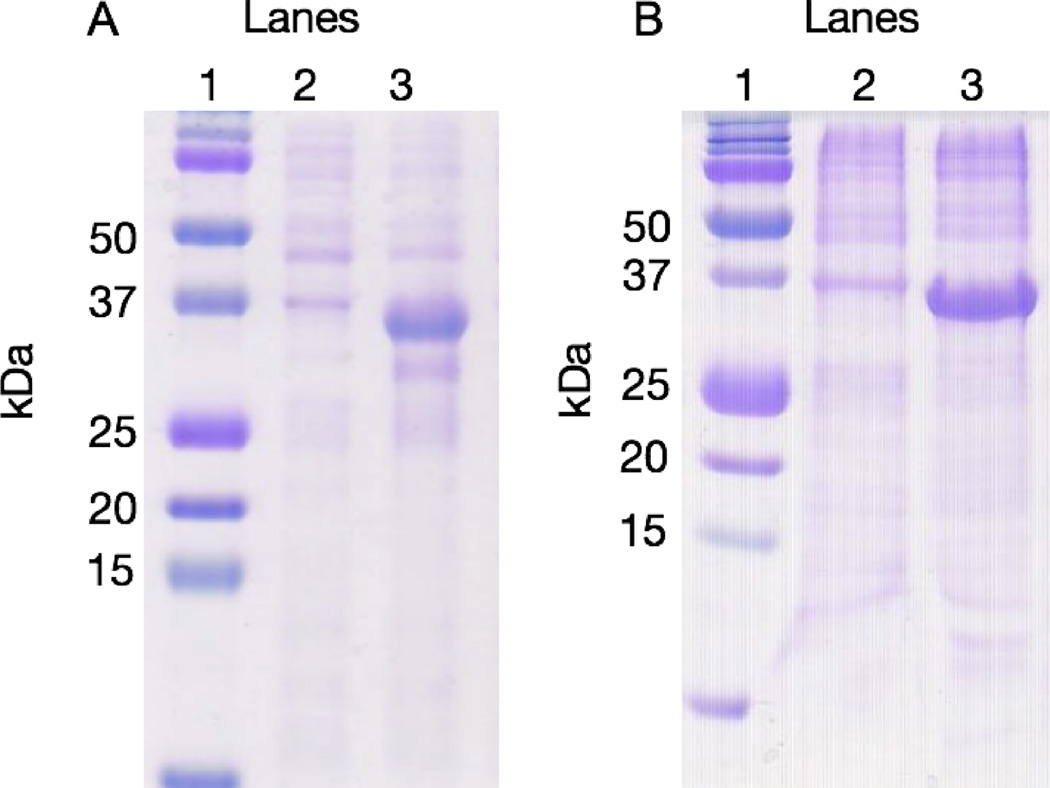
SDS-PAGE analysis of pre- and post-induction samples shows expression of the proteins in E. coli at the expected molecular weight of approximately 35 kDa (Figure 2).
Figure 2.
Coomassie stained SDS-PAGE showing expression of the two different MMP-8 constructs. (A) MMP-8 fusion cell lysate pre-induction (lane 2) and post-induction (lane 3). (B) claMP-link-MMP-8 fusion cell lysate pre-induction (lane 2) and post-induction (lane 3). In panels A and B, lane 1 is the molecular weight ladder (BioRad, #161-0374).
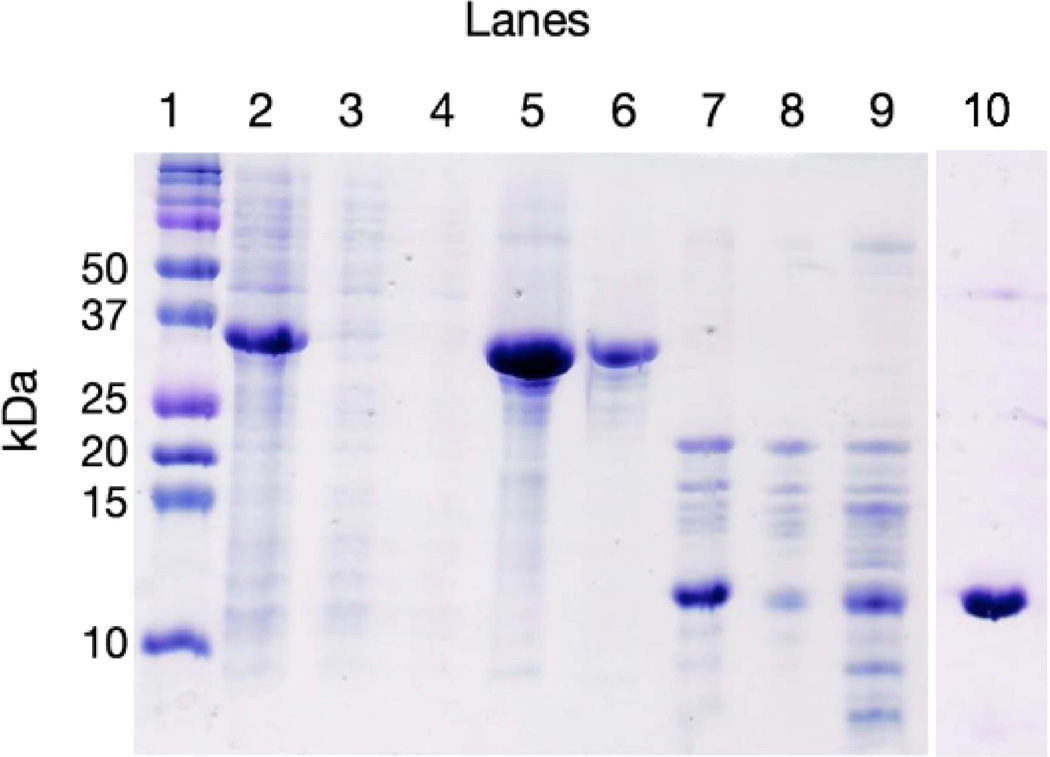
A representative SDS-PAGE analysis of the purification of MMP-8 fusion is shown in Figure 3. Following cell lysis and centrifugation to remove insoluble debris, the MMP-8 fusion protein remained present in the soluble fraction of the cell lysate, while it was not detectable in the pellet (Figure 3, lane 2). A Ni-IMAC column was used to affinity purify MMP-8 fusion from the cell lysate. The flow through during application of the cell lysate to the column (lane 3) and the 40 mM imidazole wash (lane 4) were collected to verify retention of protein on the column. A gradient elution of imidazole from 40 mM to 500 mM over 12 CV was used to elute the protein (lane 5). The gel displays molecular weight proteins below the expected 35-kDa MMP-8 fusion indicative of proteolytic degradation. To remove the lower molecular weight fragments SEC was used (lane 6) and mass spectrometry confirmed the presence of full-length fusion MMP-8 (Figure 4).
Figure 3.
Coomassie stained SDS-PAGE of the purification process of MMP-8. (Lane 1) molecular weight standards, (lane 2) soluble fraction of the cell lysate, (lane 3) the flow through of application of the cell lysis to the Ni-IMAC, (lane 4) the flow through of a 40 mM wash of the Ni-IMAC, (lane 5) the gradient elution peaks from 40 to 500 mM imidazole, (lane 6) SEC separation of degradants from the gradient elution step, (lane 7) after incubating with thrombin for 12 hours, (lane 8) separation of thrombin cleavage fragments by SEC, (lane 9) after incubating with factor Xa for 16 hours, (lane 10) thrombin-cleaved thioredoxin tag separated by SEC.
Figure 4.
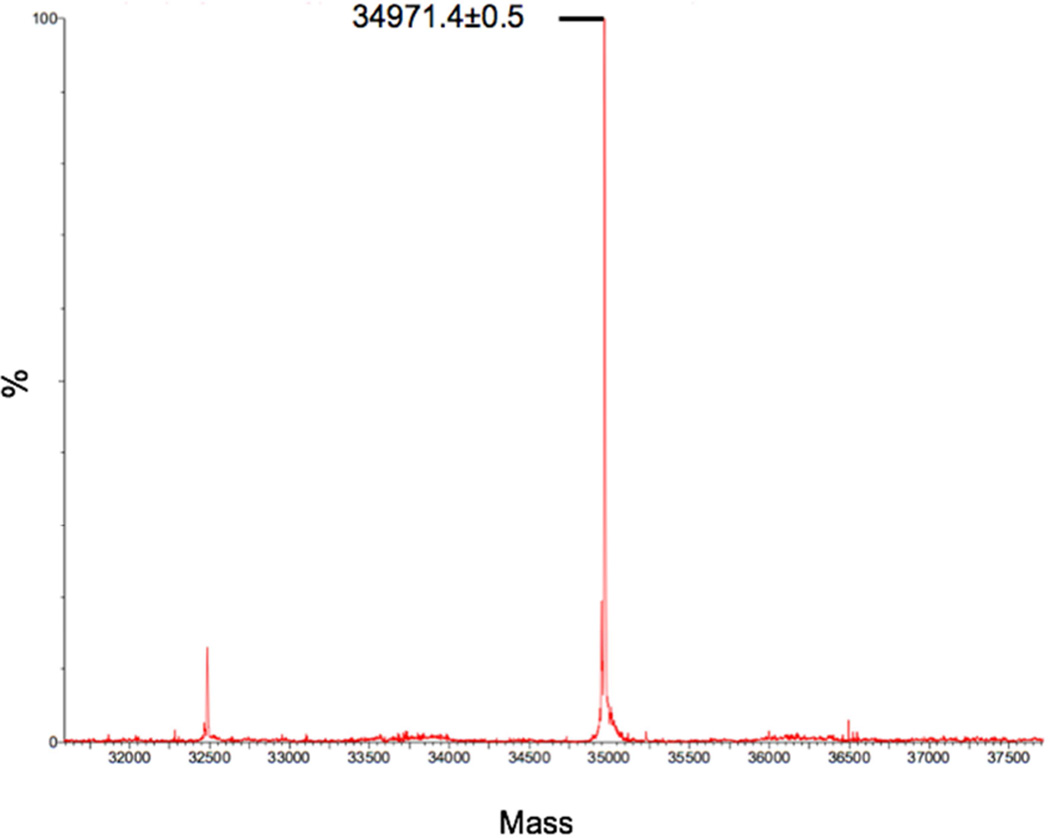
Mass spectrum of MMP-8 fusion (average theoretical mass of 34973.8 Da).
Thrombin and/or Factor Xa was/were added to the purified protein in an effort to remove the fusion tags and release MMP-8. Reaction with Factor Xa leads to incomplete cleavage but the uncut fusion protein is easily removed by SEC. Unfortunately productive cleavage generates two proteins (MMP-8 and thioredoxin-S Tag) of approximately 17–18 kDa, which cannot be separated by SEC and attempts to isolate MMP-8 from the fusion partner using IMAC after Factor Xa cleavage were unsuccessful. Consequently, a serial reaction with thrombin and Factor Xa was performed. Thrombin was added to cleave the thioredoxin tag (13 kDa), leaving S Tag-MMP-8 (21-kDa) (lane 7). While complete cleavage at the desired position is observed, additional fragments appear in the sample (lane 7). SEC effectively separates the cleaved thioredoxin tag (lane 10) from the MMP-8 fusion protein (lane 8) though a band appears on the gel at 13 kDa (lane 8), it is not thioredoxin; mass spectrometry shows that thioredoxin is not present in the MMP-8 fraction and this band instead corresponds to a fragment of MMP-8 (mass spectrum peak at 13875.8 ± 0.2 Da corresponds to MMP-8 fragmentation at residue 72 between Ala and His, with an average theoretical mass of 13876.1 Da) presumably due to autoproteolysis (data not shown). Multiple degradation fragments are present that would not separate using SEC. Because MMP-8 is prone to autoproteolysis, it seems likely that degradation proceeds once thioredoxin is cleaved. It is evident from the higher molecular weight bands in lane 8 and mass spectrometry data that Factor Xa fails to accomplish complete cleavage, resulting in a mixture of S Tag-MMP-8 and MMP-8 in the final product (lane 9). Attempts to isolate MMP-8 by SEC after this step result in extremely low recovery of protein, which remains a mixture of fragments.
Approximately 130 mg of protein was obtained from the cell lysate of a one-liter culture of MMP-8 fusion, determined by a Bradford assay after Ni-IMAC elution. 75 mg was injected onto the SEC and approximately 50% was recovered at the correct size (with fewer degradation fragments) (Figure 3, lane 6). The amount of MMP-8 obtained at each purification step was determined and is reported as the weight fraction of MMP-8 within the total fusion protein (Table 1). Although the results are not quantitative for cleaved MMP-8, the table summarizes the high-yield of protein produced in the form of the fusion construct and relative losses that accompany removal of the stabilizing fusion partner. The table shows that when using the thioredoxin-S Tag fusion construct large amounts, 20–40 mg of MMP-8 were obtained following affinity purification. The results show clearly that substantial loss of MMP-8 occurs when the fusion partner is cleaved. The percent yield of MMP-8 recovered after complete processing was calculated to be approximately 2.5% ±2.3% of the original amount present in the fusion constructs, but this value is clearly falsely elevated because MMP-8 cannot be obtained in a pure form and the sample contains numerous fragments. Because these species cannot be isolated and their individual activity assessed, accurate quantitation of native MMP-8 is not possible.
Table 1.
Relative MMP-8 equivalentsa recovered at each purification step
| Construct | Post Ni- IMAC (mg) |
Post SEC (mg) |
Post Thrombin Cleavage (mg) |
Post Factor Xa Cleavage (mg) |
|---|---|---|---|---|
| MMP-8 | 39.6±7.7 | 20.5±3.7 | 9.8±0.3 | 0.9±0.7b |
|
claMP-link- MMP-8 |
22.2±2.6 | ND | ND | 1.2±0.2b |
Mass equivalents of MMP-8 were calculated by taking the mass of MMP-8 as a fraction of the fusion protein, which initially was 75 mg.
This value reflects the total protein content of cleaved fragments present, not native MMP-8.
Mass spectrometric analysis
The correct protein size was initially determined using an SDS-PAGE analysis. To confirm the results from SDS-PAGE, a mass spectrometric analysis was completed of the fusion protein isolated by affinity chromatography. The spectrum shows a peak at 34971.4 ±0.5 Da, which corresponds to fusion MMP-8 with the N-terminal methionine removed (average theoretical mass = 34,973.8 Da) (Figure 4). The thrombin and Xa-cut mass spectrometry analysis displayed peaks of the expected masses but also many fragments of lower molecular weight, which presumably result from autoproteolysis (data not shown). Mass spectrometry data also was collected for the claMP Tagged fusion construct and it displayed the expected mass of 34893.13 ±0.44 Da for the fusion protein lacking the lead methionine residue as well (data not shown).
Circular Dichroism (CD) Analysis
To verify the correct fold for the fusion proteins is achieved, the secondary structure of MMP-8 fusion and claMP-link-MMP-8 fusion was analyzed using CD. Each protein was determined to be folded properly, containing the expected proportion of secondary structure that was predicted from the structures of the individual domains for thioredoxin and MMP-8. The spectra of MMP-8 fusion and claMP-link-MMP-8 fusion are very similar overall, but differ substantially below 200 nm. Differences in this region correspond to the tag itself. The calculated helical content decreases by 5% and random coil content increases by approximately 5% when this region of the spectrum is excluded from the calculation. This region is often omitted from CD analysis because of interference from buffers and other components, and the values obtained for secondary structure calculated using 205–260 nm produces comparable results for the two variants, indicating the claMP Tag does not affect the fold of MMP-8. The helical content of MMP-8 fusion and claMP-link-MMP-8 fusion was determined to be 19% and 18%, while the β-strand composition was 28% and 29% and random coil was 47% and 48%, respectively. This is consistent with previous CD analysis of MMPs, which have approximately 20% helical, 20% β-strand and 50% random coil structures.32 The two constructs examined have an N-terminal thioredoxin tag, and independently thioredoxin has approximately 35% helical and 18% β-strand character.33 An intervening linker sequence is also present between these two domains, which comprises approximately 10% of the fusion protein. This region may adopt a non-random coil structure and lead to the great increase in stability of the fusion system. Because the structure of this region is unknown, the experimentally determined value of the amalgamated fusion secondary structures matches that expected for thioredoxin fused to MMP-8.
Densitometry analysis of stability
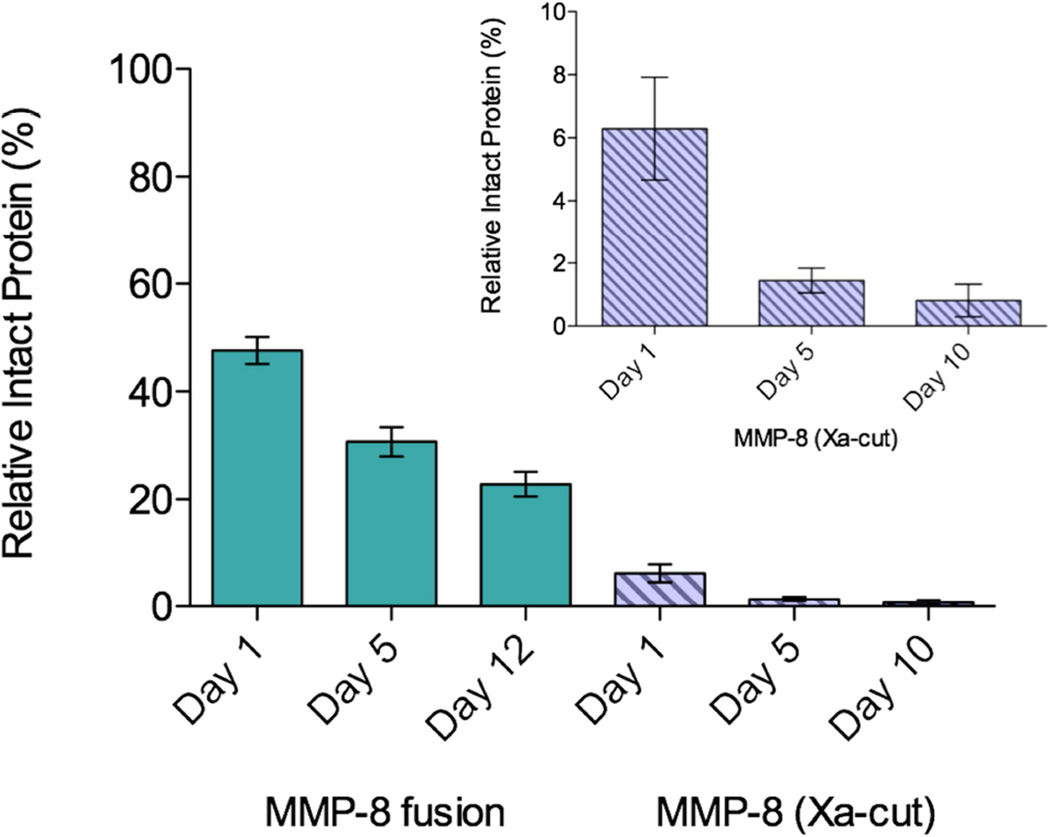
MMP-8 fusion and purified MMP-8 were stored at 4 °C in 50 mM Tris, 60 mM NaCl, 10 µM NNGH, pH 7.9 and sampled over several days to determine the stability of the purified enzymes. SDS-PAGE and densitometry analysis were performed to compare the amount of intact enzyme to the total protein content to determine the extent of degradation. The intact fusion protein represents approximately 50% of the total protein in the sample at day 1, and 25% of the full-length fusion protein remains after 12 days (Figure 5). As soon as MMP-8 is isolated from the fusion protein, less intact protein is present in the sample, with only 6% of the total protein in the sample being intact MMP-8. Within 10 days the amount of MMP-8 decreases to less than 1% intact (Figure 5, inset). Not only is the amount of intact fusion MMP-8 eight times greater than isolated MMP-8, but approximately 50% of fusion MMP-8 on day 1 remains intact after 12 days, while only 15% of MMP-8 on day 1 remains intact on day 10. These results clearly demonstrate that the fusion tags on MMP-8 have a stabilizing effect and autoproteolysis is decreased greatly in the fusion protein compared to MMP-8.
Figure 5.
Stability analysis of MMP-8 fusion protein and MMP-8 (Xa-cut) protein separated after Factor Xa cleavage. Values were determined using densitometry analysis. Inset is zoomed in bar graph of the isolated MMP-8 protein.
Activity assay
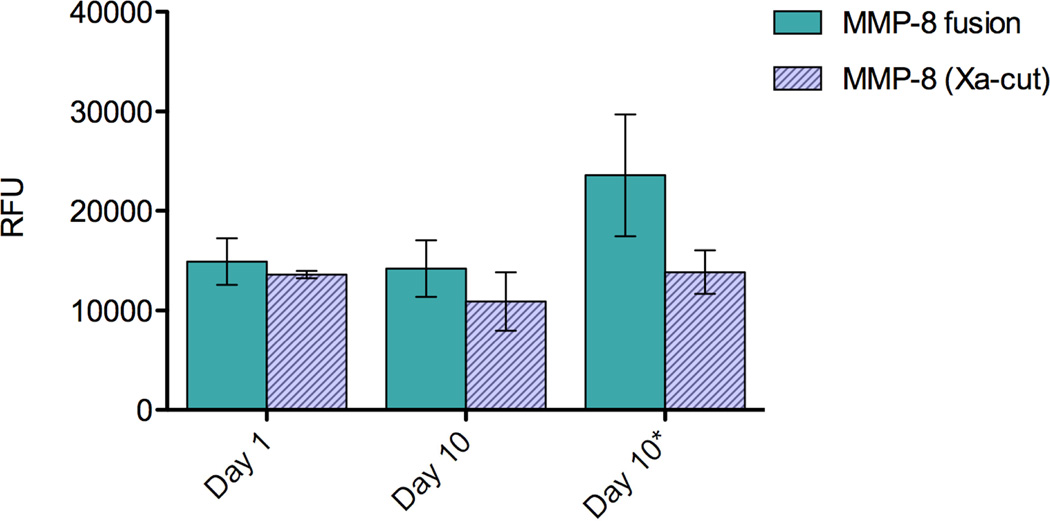
A fluorescent activity assay for MMP-8 was used to examine the activity of the purified fusion and isolated Xa-cut MMP-8 (Figure 6). The enzyme was examined 1 and 10 days after purification and was stored in 50 mM Tris, 60 mM NaCl pH 7.9 with and without(*) 10 µM NNGH, an inhibitor predicted to act as a protective stabilizer against proteolysis. Approximately 100-fold dilution of these protein samples into the assay conditions reduced the inhibitor concentration well below the IC50, eliminating its inhibitory effect. After 25 minutes of incubation with the substrate, MMP-8 fusion emits approximately 15,000 RFU on day 1 and day 10. Although degradation of the polypeptide chain was observed by SDS-PAGE, the MMP-8 fusion retains activity over 10 days both with and without NNGH indicating that addition of this compound has no effect. When the fusion tags are removed, MMP-8 appears to lose activity compared to MMP-8 fusion when stored without the inhibitor.
Figure 6.
Comparison of catalytic activity in relative fluorescence units (RFU) of the MMP-8 fusion protein and its cleaved products as analyzed using MMP-8 Fluorescent Drug Discovery Kit, RED (Enzo Life Sciences) at 25 minutes. Samples were analyzed immediately after purification (day 1) and on day 10. The proteins were stored with (day 10) or without (day 10*) a protective stabilizer at 4 °C prior to analysis. Solid bars represent the uncut MMP-8 fusion and hashed bars represent isolated MMP-8 following Factor Xa cleavage.
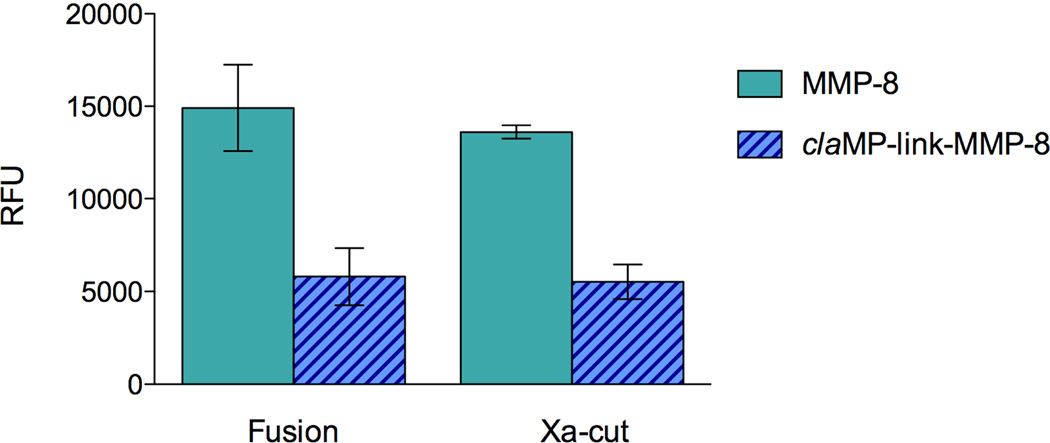
The activity of claMP-link-MMP-8 fusion and Xa-cut protein (MMP-8) were obtained to compare to the original MMP-8 fusion and Xa-cut MMP-8 protein lacking the claMP Tag (Figure 7). MMP-8 fusion was determined to have 3-times higher activity than claMP-link-MMP-8 fusion when analyzed directly after purification (day 1). Immediately after cleavage with Factor Xa, both MMP-8 and claMP-link-MMP-8 retain comparable activity to their original fusion construct. Although the claMP Tag and inserted linker decreases the activity of MMP-8 in both the fusion and Xa-cut constructs, the extent of diminution is minimal. A UV-Vis scan of claMP-link-MMP-8 fusion displays all the expected unique spectral features of the Ni-claMP complex (data not shown), including the non-overlapping feature at 425 nm to facilitate quantitation. Comparison of the A425 value with the total protein concentration, as determined by Bradford assay, demonstrated 1:1 binding of the claMP Tag with nickel. The Ni-claMP complex is extraordinarily stable and its formation in the fusion construct would preclude the tag from interacting with the zinc ion in MMP-8.
Figure 7.
Comparison of catalytic activity in relative fluorescence units (RFU) of the MMP-8 and claMP-link-MMP-8 fusion and Xa-cut proteins using MMP-8 Fluorescent Drug Discovery Kit, RED (Enzo Life Sciences) at 25 minutes. Samples were analyzed immediately after purification (day 1). Solid bars represent the MMP-8 construct and hashed bars represent the claMP-link-MMP-8 construct.
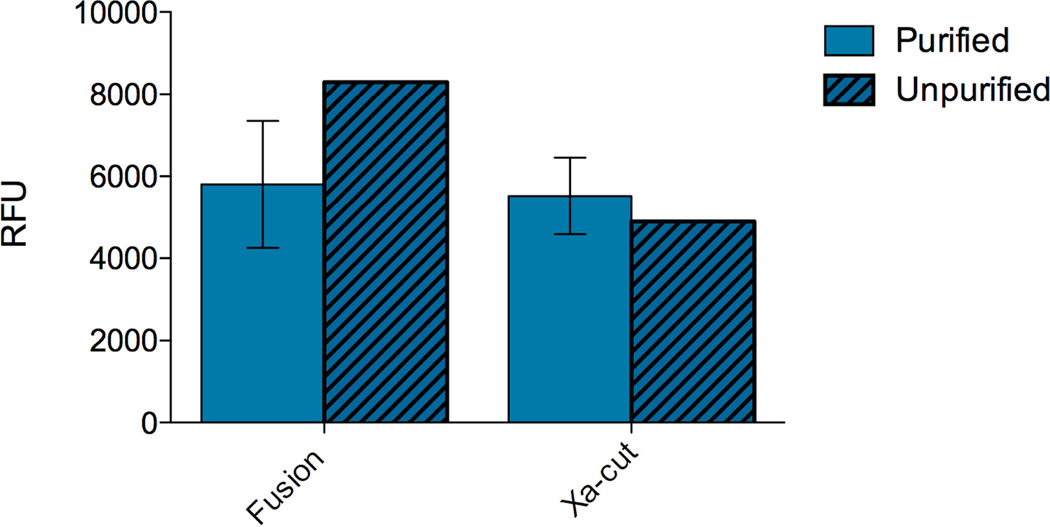
Because degradation of MMP-8 proceeds quickly once the fusion partner is cleaved, the impact on catalytic activity of leaving the fusion partner in the sample to avoid additional purification was examined. When the claMP-link-MMP-8 fusion protein was cleaved by Factor Xa, it was either separated from the released tag (purified) or was analyzed as a mixture of thioredoxin and MMP-8 Xa-cut protein (unpurified). Comparing the two methods of protein handling reveals that retention of the fusion partner has an insignificant effect on activity (Figure 8). Previous work has shown that the metal-free claMP module effectively inhibits MMP-8,29 and new data shows that loading claMP with nickel prevents binding to MMP-8 (manuscript in preparation). This data indicates that the claMP Tag may be placed inline with a metallo-enzyme and when it is loaded with another metal, such as nickel in this study, active enzyme is recovered.
Figure 8.
Comparison of catalytic activity in relative fluorescence units (RFU) of the claMP-link-MMP-8 fusion and Xa-cut proteins either being purified by SEC after cleavage by Factor Xa or not purified, using MMP-8 Fluorescent Drug Discovery Kit, RED (Enzo Life Sciences) at 25 minutes. Samples were analyzed immediately after purification (day 1). Solid bars represent the separated claMP-link-MMP-8 construct and hashed bars represent the mixture of cleaved claMP-link-MMP-8 fusion partner and MMP-8 protein.
Discussion
A fusion construct containing thioredoxin and S Tag N-terminal to MMP-8 resulted in the ability to accumulate large amounts of functional enzyme in the soluble fraction of E. coli. Subsequent purification using Ni-IMAC and a single SEC step enabled recovery of 20–40 mg of MMP-8 from 1 L of cell culture and the purified protein retained full activity at 10 days when stored refrigerated at high concentration (~0.5 mM) in a simple buffer solution. The catalytic domain of MMP-8 alone is typically stored in 50% glycerol at −80 °C at very low enzyme concentration to ensure activity remains, which is not optimal because the presence of glycerol can interfere with structural studies and this compound can be difficult to remove. SDS-PAGE shows that proteolysis of the fusion protein occurs over the course of several days but this is vastly improved compared to MMP-8 alone. Because the activity remains constant for at least 10 days, the functional core of the enzyme must remain intact and therefore provides useful material for numerous types of analysis, including structural studies where high concentration is required.
Removal of the fusion tags leads to proteolytic degradation and decreased activity. Inclusion of the inhibitor NNGH with MMP-8 following Factor Xa cleavage acts as a protective stabilizer and improves storage stability of the enzyme. Complete inhibition of MMP-8 is accomplished at 7 µM under the conditions of the catalytic assay (MMP-8 Fluorescent Drug Discovery Kit, RED (Enzo Life Sciences)), but at the much higher enzyme concentrations produced and examined for storage stability herein, 10 µM NNGH was insufficient to prevent cleavage of MMP-8 degradation (Figure 6).34 Because this concentration is near the limit of solubility of NNGH in water, higher concentrations could not be tested. Although NNGH did not provide adequate protection against autoproteolysis, the results indicate that identification of inhibitors with higher solubility and/or affinity for the enzyme should improve stability and could be used to preserve MMP-8 in the absence of the fusion partner.
The claMP Tag was added to the MMP-8 construct to examine the effect of an additional metal binding entity inline with a metal-containing enzyme. The claMP Tag binds metal extraordinarily tightly at basic pH and this fusion system becomes occupied with nickel during IMAC purification.30 The metal-free claMP Tag has been shown to inhibit MMP-8 activity and is being investigated as a potential regulator of the activity of MMP-8.29 claMP-link-MMP-8 was originally designed to contain a linker peptide sequence that would allow the claMP Tag to interact with the active site zinc to potentially inhibit the activity of the enzyme as a pro-moiety that is releasable by adjusting the pH to below neutral or by inserting another metal into the tag. Inclusion of the claMP Tag and linker did not interfere with folding of the enzyme but it did impair catalytic function by three fold. Investigation of the amount of metal bound to the claMP Tag determined complete occupancy of the claMP Tag with nickel. The rich UV-Vis spectrum of the Ni-claMP complex confirms the claMP Tag is not available to interact with zinc in MMP-8 and therefore cannot inhibit the enzyme in the fusion construct examined. Although demonstrating the reason for this modest decrease in activity is beyond the scope of the present study, it is likely that introducing 13 extra residues between the fusion tags and MMP-8 gives greater flexibility, allowing for better interaction between the enzyme and the stabilizing partner, thereby decreasing activity. Importantly, like the construct that lacks the claMP Tag, the activity remains unchanged with cleavage of the fusion partner (Figure 7).
Conclusion
Recombinant MMP-8 fusion was expressed and purified using a straightforward procedure to obtain active enzyme that is much more stable. The fusion protein can be stored refrigerated in common buffer without glycerol at high concentration and it retains full activity for longer than one week. This approach to preparation of MMP-8 is efficient and sufficiently simply to enable a wide variety of studies on MMP-8. The claMP Tag may be included in the construct and exogenous metal inserted during purification, demonstrating that the inline claMP Tag is compatible for use with metalloproteins.
Supplementary Material
Highlights.
Thioredoxin-S tag fusion produces soluble, active MMP-8
Over 100 mg of fusion protein produced per 1L culture
Two-step purification to obtain stable and active MMP-8
Inline metal-binding claMP Tag may be used with metalloproteins
Acknowledgments
Funding was provided by NIH/NIDCR grant R01-DE014392 and Collaborative Administrative Supplement R01-DE014392-03S1 and by NIH COBRE P20 RR017708. Takeru Higuchi Predoctoral Fellowship and the NIH Chemical Biology Training Grant (T32GM008545) provided support for M.L.M. We thank Dr. T. Williams, Dr. N. Galeva, and Mr. L. Seib for technical assistance with ESI-MS, Ms. H. Shinogle and Microscopy and Analytical Imaging Laboratory for assistance with and use of the Typhoon Imager and ImageQuant TL software, Dr. B. Taktak Karaca for assistance with the Cytation 3 plate reader, Ms. A. Cooper for protein expression testing, Drs. D. Volkin, C.R. Middaugh, O. Kumru and H. Khasa for use of equipment and assistance with CD data collection, and Drs. C. Berrie and J. Tucker for discussion of the manuscript.
JSL is co-owner of Echogen Inc., a limited liability company that has licensed the patent-protected metal abstraction peptide/claMP Tag technology from University of Kansas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All other authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
Participated in research design: McNiff, Dixit, Gao, Laurence
Conducted experiments: McNiff, Dixit, Haynes, Gao
Performed data analysis: McNiff, Dixit, Haynes, Gao, Laurence
Wrote or contributed to the writing of the manuscript: McNiff, Laurence
References
- 1.Lu P, Takai K, Weaver VM, Werb Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harbor perspectives in biology. 2011;3(12) doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular research. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez-Fernández A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL, Jones JL, Span PN. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer research. 2008;68(8):2755–2763. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- 4.Kessenbrock K, Plaks V, Werb Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadler-Olsen E, Winberg J-O, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013;34(4):2041–2051. doi: 10.1007/s13277-013-0842-8. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Current Opinion in Cell Biology. 1998;10(5):602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 7.Windsor LJ, Steele DL. Expression of Recombinant Matrix Metalloproteinases in Escherichia coli #. T Matrix Metalloproteinase Protocols. 2009;622:67–81. doi: 10.1007/978-1-60327-299-5_4. [DOI] [PubMed] [Google Scholar]
- 8.Oneda H, Inouye K. Refolding and Recovery of Recombinant Human Matrix Metalloproteinase 7 (Matrilysin) from Inclusion Bodies Expressed by Escherichia coli. Journal of Biochemistry. 1999;126(5):905–911. doi: 10.1093/oxfordjournals.jbchem.a022533. [DOI] [PubMed] [Google Scholar]
- 9.Ye QZ, Johnson LL, Hupe DJ, Baragi V. Purification and characterization of the human stromelysin catalytic domain expressed in Escherichia coli. Biochemistry. 1992;31(45):11231–11235. doi: 10.1021/bi00160a038. [DOI] [PubMed] [Google Scholar]
- 10.Hyun Min K, Joo-Hyon K, In Kwan H, Seo-Jin L, Tae-Han K, Ki-Hyeong R, Seung-Taek L. Refolding of the Catalytic and Hinge Domains of Human MT1-MMP Expressed in Escherichia coli and Its Characterization. Molecules & Cells. 2002;13(1):118. [PubMed] [Google Scholar]
- 11.Vaisar T, Kassim SY, Gomez IG, Green PS, Hargarten S, Gough PJ, Parks WC, Wilson CL, Raines EW, Heinecke JW. MMP-9 Sheds the β(2) Integrin Subunit (CD18) from Macrophages. Molecular & Cellular Proteomics : MCP. 2009;8(5):1044–1060. doi: 10.1074/mcp.M800449-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmier MO, Fulcher YG, Bhaskaran R, Duong VQ, Fields GB, Van Doren SR. NMR and Bioinformatics Discovery of Exosites That Tune Metalloelastase Specificity for Solubilized Elastin and Collagen Triple Helices. Journal of Biological Chemistry. 2010;285(40):30918–30930. doi: 10.1074/jbc.M110.136903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Doren SR. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biology. 2015;44–46:224–231. doi: 10.1016/j.matbio.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandstetter H, Grams F, Glitz D, Lang A, Huber R, Bode W, Krell H-W, Engh RA. The 1.8-Å Crystal Structure of a Matrix Metalloproteinase 8-Barbiturate Inhibitor Complex Reveals a Previously Unobserved Mechanism for Collagenase Substrate Recognition. Journal of Biological Chemistry. 2001;276(20):17405–17412. doi: 10.1074/jbc.M007475200. [DOI] [PubMed] [Google Scholar]
- 15.Gavuzzo E, Pochetti G, Mazza F, Gallina C, Gorini B, D'Alessio S, Pieper M, Tschesche H, Tucker PA. Two Crystal Structures of Human Neutrophil Collagenase, One Complexed with a Primed- and the Other with an Unprimed-Side Inhibitor: Implications for Drug Design†. Journal of Medicinal Chemistry. 2000;43(18):3377–3385. doi: 10.1021/jm9909589. [DOI] [PubMed] [Google Scholar]
- 16.Pochetti G, Gavuzzo E, Campestre C, Agamennone M, Tortorella P, Consalvi V, Gallina C, Hiller O, Tschesche H, Tucker PA, Mazza F. Structural Insight into the Stereoselective Inhibition of MMP-8 by Enantiomeric Sulfonamide Phosphonates. Journal of Medicinal Chemistry. 2006;49(3):923–931. doi: 10.1021/jm050787+. [DOI] [PubMed] [Google Scholar]
- 17.Pochetti G, Montanari R, Gege C, Chevrier C, Taveras AG, Mazza F. Extra Binding Region Induced by Non-Zinc Chelating Inhibitors into the S1′ Subsite of Matrix Metalloproteinase 8 (MMP-8)†. Journal of Medicinal Chemistry. 2009;52(4):1040–1049. doi: 10.1021/jm801166j. [DOI] [PubMed] [Google Scholar]
- 18.Spicer TP, Jiang J, Taylor AB, Choi JY, Hart PJ, Roush WR, Fields GB, Hodder PS, Minond D. Characterization of Selective Exosite-Binding Inhibitors of Matrix Metalloproteinase 13 That Prevent Articular Cartilage Degradation in Vitro. Journal of Medicinal Chemistry. 2014;57(22):9598–9611. doi: 10.1021/jm501284e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moy FJ, Chanda PK, Cosmi S, Pisano MR, Urbano C, Wilhelm J, Powers R. High-resolution solution structure of the inhibitor-free catalytic fragment of human fibroblast collagenase determined by multidimensional NMR. Biochemistry. 1998;37(6):1495–1504. doi: 10.1021/bi972181w. [DOI] [PubMed] [Google Scholar]
- 20.Ikejiri M, Bernardo MM, Bonfil RD, Toth M, Chang M, Fridman R, Mobashery S. Potent Mechanism-based Inhibitors for Matrix Metalloproteinases. Journal of Biological Chemistry. 2005;280(40):33992–34002. doi: 10.1074/jbc.M504303200. [DOI] [PubMed] [Google Scholar]
- 21.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Archives of Oral Biology. 2007;52(2):121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Current Opinion in Cell Biology. 2001;13(5):534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 23.Dorman G, Cseh S, Hajdu I, Barna L, Konya D, Kupai K, Kovacs L, Ferdinandy P. Matrix Metalloproteinase Inhibitors: A Critical Appraisal of Design Principles and Proposed Therapeutic Utility. Drugs. 2010;70(8):949–964. doi: 10.2165/11318390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the Activities of Matrix Metalloproteinases 2, 8, and 9 by Chlorhexidine. Clinical and Diagnostic Laboratory Immunology. 1999;6(3):437–439. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13(12):904–927. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
- 26.Laurence JAS, Vartia AA, Krause ME. Metal abstraction peptide (MAP) tag and associated methods. Google Patents. 2012 [Google Scholar]
- 27.Mills BJ, Mu Q, Krause ME, Laurence JS. claMP Tag: A Versatile Inline Metal-Binding Platform Based on the Metal Abstraction Peptide. Bioconjugate Chemistry. 2014;25(6):1103–1111. doi: 10.1021/bc500115h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krause ME, Glass AM, Jackson TA, Laurence JS. MAP ping the Chiral Inversion and Structural Transformation of a Metal-Tripeptide Complex Having Ni-Superoxide Dismutase Activity. Inorganic Chemistry. 2011;50(6):2479–2487. doi: 10.1021/ic102295s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixit N, Settle JK, Ye Q, Berrie CL, Spencer P, Laurence JS. Grafting MAP peptide to dental polymer inhibits MMP-8 activity. Journal of Biomedical Materials Research Part B. Applied Biomaterials. 2015;103(2):324–331. doi: 10.1002/jbm.b.33205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills BJ, Laurence JS. Stability Analysis of an Inline Peptide-based Conjugate for Metal Delivery: Nickel(II)–claMP Tag Epidermal Growth Factor as a Model System. Journal of Pharmaceutical Sciences. 2015;104(2):416–423. doi: 10.1002/jps.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krause ME, Glass AM, Jackson TA, Laurence JS. Novel tripeptide model of nickel superoxide dismutase. Inorganic chemistry. 2009;49(2):362–364. doi: 10.1021/ic901828m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, Makaroff K, Paz N, Aitha M, Crowder MW, Tierney DL. Metal Ion Dependence of the Matrix Metalloproteinase-1 Mechanism. Biochemistry. 2015;54(23):3631–3639. doi: 10.1021/acs.biochem.5b00379. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo K, Sakurada Y, Yonehara R, Kataoka M, Gekko K. Secondary-Structure Analysis of Denatured Proteins by Vacuum-Ultraviolet Circular Dichroism Spectroscopy. Biophysical Journal. 2007;92(11):4088–4096. doi: 10.1529/biophysj.106.103515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee E-J, Moon P-G, Baek M-C, Kim H-S. Comparison of the Effects of Matrix Metalloproteinase Inhibitors on TNF-α Release from Activated Microglia and TNF-α Converting Enzyme Activity. Biomolecules & Therapeutics. 2014;22(5):414–419. doi: 10.4062/biomolther.2014.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.