Abstract
This article reviews our current knowledge about cell-derived extracellular vesicles (EVs), including microparticles and exosomes, and their emergence as mediators of a new important mechanism of cell-to-cell communication. Particular emphasis has been given to the increasing involvement of EVs in the field of radiation-induced vascular injury. Although EVs have been considered for a long time as cell “dust”, they in fact precisely reflect the physiological state of the cells. The role of microparticles and exosomes in mediating vascular dysfunction suggests that they may represent novel pathways in short- or long-distance paracrine intercellular signaling in vascular environment. In this article, the mechanisms involved in the biogenesis of microparticles and exosomes, their composition and participation in the pathogenesis of vascular dysfunction are discussed. Furthermore, this article highlights the concept of EVs as potent vectors of biological information and protagonists of an intercellular communication network. Special emphasis is made on EV-mediated microRNA transfer and on the principal consequences of such signal exchange on vascular injury and radiation-induced non-targeted effect. The recent progress in elucidating the biology of EVs has provided new insights for the field of radiation, advancing their use as diagnostic biomarkers or in therapeutic interventions.
INTRODUCTION
Radiation injury of blood vessels was originally identified more than a century ago and continues to be a clinical problem today despite dramatic advances in the field of radiation oncology. Radiation induces endothelial cell (EC) dysfunction, which is characterized by increased permeability, detachment from the underlying basement membrane and apoptosis (1, 2). EC dysfunction and apoptosis contribute to postirradiation inflammation and fibrosis. Within vessels, radiation induces a prothrombotic state, which is characterized by platelet aggregation, microthrombus formation and increased adhesion of inflammatory cells to ECs with subsequent diapedesis into the perivascular space (3). Structurally, irradiation of the vasculature causes the dose-dependent destruction of blood vessels, which affects the tissue microvasculature in particular (4).
The endothelium serves a critical role as a barrier and is the primary sensor of physical and chemical changes in the bloodstream. Endothelial dysfunction is an all-encompassing term for a shift from a normal, healthy endothelium to a stressed/damaged endothelium with a pro-vasoconstriction, pro-coagulation and pro-inflammatory phenotype (5, 6). Thus, the recognition of endothelial dysfunction can lead to earlier therapeutic intervention and potentially, reduced vascular damage. More importantly, evaluating circulating biomarkers may reveal mechanisms of endothelial pathology, as well as provide insights on endothelial functional status, while remaining minimally invasive. This article examines extracellular vesicles (EVs) as biomarkers of endothelial dysfunction and discusses their role in vascular homeostasis.
Extracellular vesicles constitute a heterogeneous group of cell-derived vesicles that are enclosed by a lipid bilayer containing various proteins and receptors, which envelopes a diverse array of proteins, nucleic acids, chemicals and structural molecules derived from the cell of origin, the nature of which depends on the cellular source, state and environmental conditions (7–12). Nonetheless, three main EV subpopulations have been consistently identified and are classified according to their size and biogenesis (Table 1) (13–15). The best studied of these are exosomes (sometimes called nanovesicles), which range in size from 30 to 100 nm. Exosomes are intraluminal vesicles generated by reverse budding of multivesicular bodies (MVBs) within cells before their secretion upon fusion of MVBs with plasma membrane (16). A second EV subpopulation consists of microparticles (MPs) or microvesicles (also known as shed vesicles or ectosomes), which range in size from 0.1 to 1 µm. MPs are directly shed from the plasma membrane of cells, arising from regions enriched in lipid rafts and expose phosphatidylserine (PS) in the outer leaflet of their membrane (13). A third EV subpopulation, is constituted by apoptotic bodies (ABs), which are larger vesicles (1–2 µm) released from apoptotic cells that are rapidly engulfed by phagocytic cells (9, 14). ABs are characterized by a permeable membrane, PS exposure and the presence of fragmented nuclear DNA.
TABLE 1.
Classification of Extracellular Vesicles
| Vesicle type | Origin | Size | Markers (enriched) | Content |
|---|---|---|---|---|
| Exosomes | Intraluminal budding of MVBs; fusion of MVBs with plasma membrane |
30–100 nm | Tetraspanins (CD63, CD9, CD81), LAMP1, ESCRT components, ALIX, TSG101, MFGE8 |
mRNA, miRNA, cytoplasmic and membrane proteins |
| Microparticles | Outward blebbing of cell membrane |
0.1–1 µm | PS, surface proteins from cell of origin (integrins, selectins, receptors) |
mRNA, miRNA, cytoplasmic and membrane proteins |
| Apoptic bodies | Blebs released from poptotic cells |
>1 µm | Elevated PS, permeable membrane (PI positive) |
DNA, nuclear fragments, cell organelles, proteins, mRNA, miRNA |
Notes. MVBs = multivesicular bodies. LAMP1 = lysosomal-associated membrane protein 1; ESCRT = endosomal sorting complex required for transport; ALIX = apoptosis-linked gene 2-interacting protein X; TSG101 = tumor susceptibility gene 101; PS = phosphatidylserine; PI = propidium iodide.
All three classes of subcellular vesicles are formed under conditions of endothelial damage, however, the relationship between exosomes and apoptotic bodies and endothelial dysfunction is unclear. EVs have been reported to be part of the disease mechanism in several conditions, such as inflammation and thrombosis, that are reported to be highly involved in the pathogenesis of vascular dysfunction. Finally, given their significant presence in most if not all bodily fluids, which makes them easily and noninvasively accessible, EVs have been investigated as potential biomarkers for many diseases (17).
MICROPARTICLES
Microparticle Characterization
The general consensus is that most cell types, including circulating cells and cells present in the vessel wall, are capable of vesiculating and releasing membrane-shed MPs in the extracellular media in response to cell activation or apoptosis. MPs originating from different cell types can be detected in the plasma of healthy subjects, resulting from the active balance between MP generation and clearance. MPs are anuclear fragment of cellular membrane that shed from stressed or damaged cells. With a typical diameter of 0.1–1.0 µm, MPs contain surface proteins and cytoplasmic material of the parent cells. MPs are distinguishable from other subcellular vesicles on the basis of size, mechanism of formation and content (18). MPs are typically identified in plasma samples by flow cytometry on the basis of size, the externalization of PS and the presence of specific surface antigens. The labeling of surface antigens allows for identification of the cellular origin of MPs, however, the precise criteria for identification on the basis of surface antigens have yet to be established. In plasma samples, MPs of endothelial (identified by the surface presence of CD144, CD62E or CD31), platelet (CD41a, CD42b, CD62P), leukocyte (CD45, CD4, CD8, CD14) and erythrocyte (CD235a) origin are present (19–21).
Given that MPs are released under conditions of cell stress/damage, it is not surprising that plasma levels of MPs are increased in a wide range of cardiovascular diseases (15, 22), cancer (23), lung injury (24), renal failure (25, 26) and decompression sickness (27). There is strong evidence to suggest that MPs of endothelial, platelet and leukocyte origin may be reflective of endothelial dysfunction and may in fact contribute to endothelial dysfunction (13, 28, 29). The current knowledge about the mechanisms of endothelial vesiculation has been derived mainly from experiments in isolated or cultured ECs, in which their capacity to generate MPs after activation by a variety of stimuli was documented. The data clearly show that endothelial MP shedding can occur independent of endothelial apoptosis (30).
Microparticle Stimuli
In addition to the broad structural changes governing MP formation, several signals have been identified that may stimulate or inhibit MP formation. Pro-inflammatory molecules are potent stimuli of microparticle formation from ECs. In this regard, tumor necrosis factor (TNF)-α (31, 32), lipopolysaccharide (LPS, in the presence of fatty acids) (33), interleukin-1α (34) and C-reactive protein (35, 36) all promote MP release from ECs. Similarly, the pro-coagulant factors thrombin (37) and plasminogen activator inhibitor-1 (32, 38) also increase endothelial MP formation. Uremic toxins such as p-cresol (39), p-cresyl sulfate (40), indoxyl sulfate (39) and homocysteine (41) are also associated with increased MP formation from ECs. Other stimuli implicated in endothelial MP formation include high glucose (42), angiotensin II (21), camptothecin (43), growth factor deprivation (44) and reactive oxygen species (ROS) (21, 45). Shear stress has also been associated with endothelial MP formation in vivo, although direct effects on isolated ECs have not been reported (46). Conversely, statin treatment (47) and NO (35) suppress MP production from endothelial cells.
Microparticle Generation
As MP formation begins with the outward budding of the plasma membrane, it is perhaps not surprising that cytoskeletal reorganization is a critical component of MP formation. In this regard, actin filament dynamics appear crucial for MP formation from multiple cell types. Different studies have implicated Rho kinase, an upstream regulator of myosin light-chain kinase and cytoskeletal dynamics, in the formation of MPs from ECs, a process which may involve caspase 2 (21, 29, 30). Finally, transglutaminase 2, an enzyme that catalyzes protein cross-linking and governs cytoskeletal reorganization, has recently been implicated in MP release from vascular smooth muscle cells (SMCs) (48). Thus, although the precise machinery necessary for MP formation is not fully understood and indeed may differ among various cell populations, cytoskeletal reorganization appears to represent a critical step in MP formation.
A second event implicated in MP formation is the externalization of PS. PS is an aminophospholipid that is found preferentially (if not exclusively) on the inner leaflet of the plasma membrane of healthy cells (49). The asymmetric distribution of PS is regulated by three distinct enzymes: flippases, floppases and scramblases. Flippases promote the translocation of PS and phosphatidylethanolamine against their electrochemical gradient towards the inner membrane in an ATP-dependent manner and are constitutively expressed. Floppases, which include members of the ATP-binding cassette (ABC) transporter family, catalyze the transport of PS to the outer membrane in an ATP-dependent fashion. Finally, scramblases are ATP independent and facilitate movement of PS between both membrane leaflets and include TMEM16F (transmembrane protein 16F) (49, 50). The majority of studies examining MPs report some degree of outer membrane PS exposure, and emerging evidence suggests that this exposure is a key mediator of the formation of MPs. The strongest evidence supporting this can be found in individuals with Scott syndrome, a condition characterized by an impaired ability to externalize PS and impaired coagulation that may result from defects in TMEM16F or the floppase ABCA1 (50–53). Individuals with Scott syndrome exhibit reduced MP shedding from platelets (54). Although some reports suggest that PS is not externalized in certain MP populations (an observation based on a lack of detectable annexin V binding), it is unclear whether these populations truly lack externalized PS or the level of externalization is simply below the limits of detection (55).
Recently, lipid-rich microdomains known as detergent-insoluble glycolipid-enriched complexes or lipid rafts and caveolae, have also been implicated in the formation of endothelial, monocyte and platelet MPs. Biro et al. (56) first observed a high cholesterol content in platelet MPs relative to the plasma membrane, suggesting an enrichment in lipid rafts. Additionally, perturbation of lipid-rich domains is associated with alterations in MP formation. In this regard, Liu et al. (57) have shown that cholesterol enrichment in monocytes/macrophages is associated with enhanced MP formation. Similarly, it has been observed that disruption of lipid-rich domains, with methyl-β-cyclodextrin or nystatin, impaired MP formation in ECs (21). Moreover, several proteins that localize to lipid rafts/caveolae have been identified in MPs, including CD39, flotillin-2, endothelial NO synthase and caveolin-1 (21). Of note, transport mechanisms not involving protein participation, but requiring large, local deformations of the plasma membrane (e.g., endocytosis, exocytosis and membrane fusion events) are also determined by changes in the composition of lipids.
More importantly, ionizing radiation has been shown to alter the concentration or chemical nature of plasma membrane lipids, which impacts plasma membrane function. In addition, radiation affects the functions mediated by transmembrane proteins by altering their expression or by changing the interaction(s) that normally takes place between membrane lipids and proteins, which can dramatically alter the way in which cells associate with each other within tissues and organs. Altered gap junctional associations, receptor/ligand cell-to-cell communication and ion transport all contribute to loss of tissue homeostasis, carcinogenesis and reduced cell viability (58, 59). Radiation-induced damage to the plasma membrane results from lipid peroxidation. Lipid peroxidation is initiated by a hydroxyl radical that initiates a self-perpetuating reaction through formation of lipid radicals, resulting in the oxidative deterioration of polyunsaturated lipid molecules. Membrane lipid peroxidation results in increased membrane permeability to small molecules and ions (58).
Recently, it has become evident that lipid rafts also play an important part in signal transduction processes (60). Many studies support the model in which rafts merge into larger membrane domains on hydrolysis of sphingomyelin (SM) to ceramide (CER) after exposure to various stimuli. Indeed, CER molecules dramatically change the biophysical properties of plasma membrane, which results in spontaneous self association of rafts to larger domains called platforms. Many receptors or stimuli are able to induce the formation of CER-enriched membrane domains or activation of sphingomyelinase(s) (acidic A-SMase and/or neutral N-SMase), or require the expression of SMase for transmission of the specific biological effect (60).
Biological Effects
MPs interact with recipient cells, which may be local or considerably distant from the originating cell, through a process entailing ligand/receptor signaling at the recipient cell surface and/or the fusion of vesicle and cell plasma membranes. Mechanisms involving integrin interaction have also been reported for MPs derived from other cell types (ECs, SMCs and leukocytes). Recently, Burger et al. (21) showed that direct interaction of heparin-binding EGF-like growth factor/positive MPs with the endothelial EGF receptor causes pro-oxidative and pro-inflammatory responses in ECs in vitro. Lipids, in particular externalized PS, are a key determinant of the interaction of membrane vesicles with target cells, although the PS-moiety of endothelial MPs can interact with endothelial PS receptor in an annexin I-dependent manner to prevent endothelial apoptosis (61). The biological effects of MPs have been extensively reviewed elsewhere (22, 62, 63). Discussed below are some aspects of MP biology related to vascular dysfunction.
Coagulation
In human diseases, the contribution of MPs originating from ECs to the circulating pool of tissue factor (TF)-positive MPs has already been documented in sickle cell anemia (64) and sepsis (65). Moreover, these MPs induce TF expression of monocytes after binding and thus, participate in the amplification of pro-coagulant cellular responses (66). Endothelial MPs (EMPs) harboring PS and TF-dependent pro-coagulant activity are released from cultured ECs, in response to a variety of stimuli including cytokines, complement and LPS (67–69). This is consistent with the endothelial expression of TF detected in animal models of endotoxemia and also in sickle cell mice (70, 71).
The discovery of fibrinolytic activity harbored by MPs further adds to their contribution in the regulation of hemostatic balance. This fibrinolytic activity is related to the evidence of molecular equipment that identifies MPs as an efficient support for plasmin generation. The plasmin generation capacity of MPs was more extensively studied in EMPs. This property has been initially demonstrated in a model of MPs generated from human microvascular ECs (HMECs) stimulated with TNF-α, in which the urokinase plasminogen activator (uPA/uPAR) system was identified as the major activator (72). Finally, tissue (t)-PA was also characterized as the activator that was detectable on the surface of MPs from human primary micro- and macrovascular ECs and was then further confirmed in MP samples from patients with thrombotic thrombocytopenia purpura and systemic lupus erythematosus (73).
Apart from their well-known pro-coagulant activities, platelet-, leukocyte-, endothelial- and cancer-derived MPs harbor a variety of anticoagulant factors, reflecting the pro-and anti-coagulant properties of their parent cells. This equilibrium reflects, at least in part, the coagulable state and could be useful in identifying thrombotic risk (74). Another regulatory mechanism by which EMPs and monocyte-derived MPs are thought to counteract thrombin generation is the exposure of the anticoagulant receptors thrombomodulin and the endothelial cell protein C receptor (75, 76). Platelet-derived MPs have also been found to accelerate factor Va inactivation by activated protein C (77).
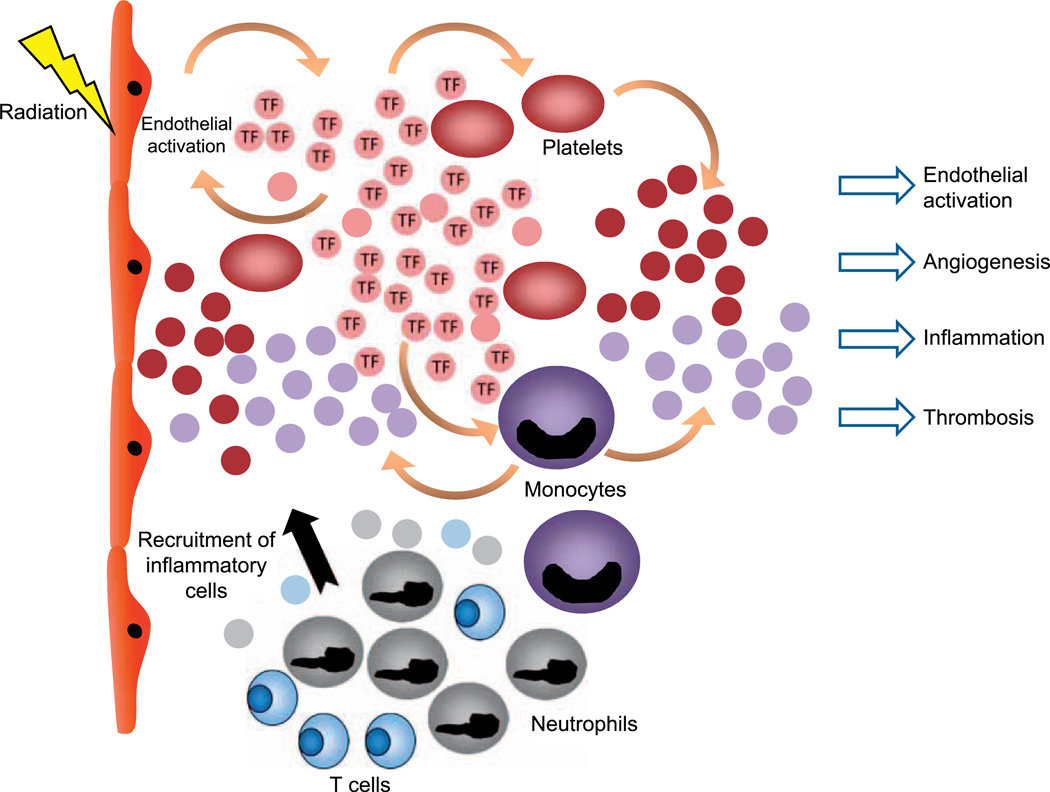
Ionizing radiation is associated with an increased risk of thrombotic events, with delayed re-endothelialization, fibrin deposition and platelet recruitment as potential causes of postirradiation thrombosis (78). In our laboratory, we investigated a cohort of 217 patients from the Epinal accident (France), where patients with prostate adenocarcinoma were overexposed to radiation during radiotherapy (79). We demonstrated that MPs isolated from peripheral blood samples could be used as potential biomarkers in association to the severity grade of the complications in this patient population. Furthermore, this study identified the cellular origin of MPs present in chronic radiation enteritis patients and demonstrated that the higher circulating MP level observed in severe-grade patients was due to an increase of MPs derived from platelets, monocytes and ECs (unpublished data). These results are in accordance with an elevation of these subpopulations in acute coronary syndrome, hypertension or atherosclerosis and in all thrombotic diseases occurring in both venous and arterial beds (46, 80–83). Our in vitro studies confirmed that radiation triggered a dose-dependent release of MPs by platelets. Moreover, it has been shown that radiation induced TF expression and an increase in the procoagulability of MPs derived from human peripheral blood mononuclear cells (78). These findings may explain a possible mechanism by which radiation enhances blood thrombogenicity (Fig. 1).
FIG. 1.
Schematic representation of the microparticle secretion after radiation-induced endothelial activation. MPs could be considered as signalosomes for several core biological processes. For example, microparticles may activate immune responses. In blood circulation, extracellular vesicles participate in the coagulation cascade by providing a surface for the assembly of clotting factors. Microparticles also take part in stem cell maintenance and plasticity, and they appear to have an essential role in the repair of injured tissue owing to their neoangiogenic, anti-apoptotic and cell proliferation-stimulating characteristics. Given their involvement in disease progression, extracellular vesicles can be considered as targets for therapeutic intervention as well as useful disease biomarkers.
Inflammation
There is now emerging evidence from in vitro and in vivo studies that MPs may play a role in inflammatory conditions, since they display a variety of proinflammatory activities. MPs from ECs, platelets and leukocytes can promote adhesion and rolling of leukocytes, contain pro-inflammatory cytokines and trigger the release of MPs from several cell types in vitro (84, 85). In addition, oxidized phospholipids from endothelial MPs released by oxidative stress may cause monocyte adherence to ECs and neutrophil activation (85). A recent study demonstrated also that MPs, isolated from septic shock patients, injected into rats induced the expression of inducible NO synthase, nuclear factor kappa B (NFκB) and cyclooxygenase-2 in the lungs and hearts of these animals (86).
Inflammation is one of the major consequences of radiation injury and adhesion molecules are known to play a key role in cellular traffic through vascular endothelium when leukocytes migrate from blood into tissues (87). In our laboratory, we observed endothelial activation in a P-selectin-dependent manner related to incubation of MPs isolated from patients with prostate adenocarcinoma who were overexposed to radiation during their radiotherapy (unpublished data). Similarly, our results have shown vascular cell adhesion molecule-1-dependent endothelial activation. Our in vitro studies confirmed that radiation triggered a dose-dependent release of MPs by monocytes and ECs. CD31+ and CD41+ MPs are considered to be valuable surrogate markers for reflecting the extent of EC dysfunction, and CD14+ MPs could be considered as proinflammatory molecules that enhance vascular inflammation (88, 89).
Angiogenesis
Different studies demonstrated that MPs play a part in development, angiogenesis, wound healing and, more generally, tissue remodeling, in the form of positive or negative gradients of information delivered to neighboring cells (15, 90). It has been shown that platelet MPs from healthy individuals promote proliferation, migration and tube formation in cultured ECs. The latter effects of MPs are mediated by their lipid components, probably sphingosine 1-phosphate. The ability of platelet MPs to induce angiogenesis is related to the activation of extracellular signal-regulated kinase and phosphoinositide 3-kinase pathways (91). Also, MPs of endothelial origin can elicit angiogenesis, but the mechanisms by which they mediate their effects are different from those reported for platelet MPs. Indeed, metalloproteinases harbored by endothelial MPs regulate the focalized proteolytic activity essential for invasion during neovascular structure formation (92). Although these effects have been described in in vitro systems, one would expect that this effect of EMPs may contribute to neovascularization in in vivo situations (93). Promotion of angiogenic processes by EMPs may have both beneficial and deleterious effects. EMPs could be endogenous survival signals responsible for vascular repair in ischemic tissues. However, promotion of angiogenic response may also have deleterious effects in cancer spreading, proliferative diabetic retinopathy (94) or atherosclerotic plaque destabilization by promoting intraplaque neovascularization (95). These examples suggest that involvement of EMPs in vascular homeostasis is more complex than initially thought and demonstrate that further studies are needed to examine the mechanisms involved (68). However, the effects of radiation on the angiogenic process need to be more fully investigated.
EXOSOMES
Exosome Biogenesis and Characterization
Exosomes are a population of vesicles of endocytic origin that are formed as intraluminal vesicles (ILVs) by budding into early endosomes and MVBs. Cargo sorting into exosomes involves the endosomal sorting complex required for transport (ESCRT) and other associated proteins such as apoptosis-linked gene 2-interacting protein X (ALIX) and tumor susceptibility gene 101 (TSG101). Recently, several ESCRT-independent mechanisms were also described in ILV formation and exosome biogenesis, involving lipids, tetraspanins or heat shock proteins (7, 96). Of note, mammalian cells depleted for key ESCRT components still form MVBs (97). Therefore, in some cells exosome production requires the lipid CER and nSMase (98). Exosomes are secreted after the fusion of MVBs with the cell membrane, which depends on several small Rab GTPases (99). Therefore, exosomes differ from MPs or apoptotic bodies that are released from the cell as a result of a direct budding process of the plasma membrane. The ultrastructure of exosomes obtained by transmission electron microscopy appears to be cup shaped, which is likely due to the collapse of these circular molecules as a result of processing and fixation. Indeed, quickly frozen exosomes analyzed by cryo-electron microscopy have a perfectly round shape (7). Typically, exosomes have a diameter of 30–100 nm and a density of 1.13–1.19 g/ml and are isolated through sucrose cushion or density gradient by ultracentrifugation at 100,000g (100).
Exosomes contain cytoplasmic proteins, certain lipid raft-interacting proteins and RNAs, however, owing to their highly regulated biogenesis, exosomes typically accommodate some additional defined components. These include endosome-associated proteins, such as: flotillin; ALIX; TSG101; the tetraspanins CD63, CD9 and CD81; and the lipids sphingomyelin, ceramides and cholesterol (7). Of interest, there have been numerous reported on the RNA contents of EVs isolated from cell cultures or body fluids (101–103). Although most studies focused on the presence of microRNAs (miRNAs), recent deep sequencing analyses revealed the presence of other small noncoding RNA species, many of which were found to be enriched in EVs relative to cellular RNA, suggesting a selective incorporation of vesicular RNA molecules (101, 103, 104). Once secreted from the cell, exosomes can deliver their cargo to adjacent or distant cells, including macrophages, ECs and tumor cells, thereby modifying the target cell’s gene expression, signaling and overall function. In most cases, this leads to EV uptake through endocytosis. During this process, EV membrane constituents can be delivered to the recipient cell membrane and EV cargo can enter the recipient cell cytoplasm or nucleus, thereby contributing additional signaling molecules and pathways and potentially resulting in a diverse range of functional consequences in the recipient cell (9, 105, 106).
EV-Mediated MicroRNA Transfer and Vascular Homeostasis
EVs transfer functional miRNA into recipient vascular cells
Valadi et al. first demonstrated the transfer of miRNA through EVs between mast cells in culture (101). EV-mediated miRNA transfer has since been demonstrated in many cellular settings, including the cardiovascular system, where vascular (ECs, SMCs, pericytes), cardiac (cardiomyocytes, fibroblasts) and stem/progenitor cells [mesenchymal stromal cells (MSCs), cardiac progenitor cells, endothelial progenitor cells (EPCs), circulating CD34+ cells] were shown to interact with each other by exchanging biological material through EV production/uptake (107). Transfer of functionally active miRNA has been validated by elegant in vitro studies showing the uptake of EVs released from macrophages or platelets by ECs in culture (Table 2). Laffont et al. demonstrated that miR-223-containing MPs released by platelets were internalized by ECs, where miR-223 regulated the expression of endogenous target genes (108). Squadrito et al. showed that macrophage-derived exosomes transferred a number of miRNAs in ECs, of which miR-142-3p was shown to regulate reporter gene expression (109). EV-mediated transfer of functionally active miRNA was also shown to exert a functional impact on recipient cells and vascular homeostasis. Expression of the miR-143/145 cluster was shown to be induced in ECs in response to shear stress, through Kruppel-like factor-2 transcription factor upregulation. These miRNAs were transferred through EVs to SMCs, where they regulated the expression of several target genes (110). The miR143/ 145 cluster is predominantly expressed in SMCs, where it plays an important role in the regulation of differentiation and proliferation (111). Accordingly, miR-143/145−/− mice display a thinner arterial medial layer and a decreased blood pressure, and they develop spontaneous neointimal lesions in the femoral arteries (112). Injection of EVs containing miR-143/145 in Apoe−/− mice on a high-fat diet resulted in the reduction of plaque size, thus conferring an atheroprotective function to these miRNAs (110). Interestingly, it was recently shown that miR-143/145 could be transferred in the opposite direction as well, through the formation of tunneling nanotubes (membrane protrusions), highlighting the importance of these miRNAs in cell-to-cell communication among SMCs and ECs for the regulation of EC function and vascular homeostasis (113).
TABLE 2.
Overview of the miRNAs Transferred bv Extracellular Vesicles Discussed in This Article
| Producing cells | Species | Vesicle type (as indicated in original publication) |
Purification method |
|---|---|---|---|
| Platelets | Human | Microparticles | Centrifugation 20,000g, 90 min |
| Primary and immortalized BM-derived macrophages |
Mouse | Exosomes | Precipitation (Exoquick, primary Mφ) and ultra 134,000g, 70 min (immortalized Mφ) |
| KLF2-transduced HUVECs; mouse lung ECs |
Human; mouse | Extracellular vesicles | Centrifugation 20,500g, 60 min |
| Apoptotic HUVECs | Human | Apoptotic bodies | Centrifugation 16,000g, 20 min |
| Apoptotic HCAECs | Human | Microparticles | Centrifugation 20,000g, 40 min |
| CD34+ PBMCs | Human | Extracellular vesicles (MVs and exosomes) |
Centrifugation 16,000g, 60 min (MVs) + ultra 120,000g, 60 min (exosomes) |
| CD34+ PBMCs | Human | Exosomes | Ultra 100,000g, 60 min (sucrose gradient) |
| THP-1 monocytes | Human | MVs (exosomes) | Ultra 110,000g, 120 min |
| HMEC-1 | Human | Exosomes | Ultra 100,000g, 60 min |
| Pericytes (SVPs) | Human | ND (CM) | Ultrafiltration (50× concentrate) |
| MDA-MB-231 breast cancer cells | Human | Exosomes | Ultra 110,000g, 110 min |
| 4T1 breast cancer cells | Mouse | Exosomes | Ultra 110,000g, 70 min |
| Hypoxic K562 leukemia cells | Human | Exosomes | Precipitation (Exoquick) |
| Hypoxic multiple myeloma cells | Human | Exosomes | Precipitation (Exoquick) |
| Irradiated MRC-5 fetal lung fibroblasts | Human | Exosomes | Precipitation (Exoquick) |
| Transferred miRNA |
Recipient cells | Target gene inhibition |
Biological effect | Reference |
|---|---|---|---|---|
| miR-223 | HUVECs | FBXW7, EFNA1 | n.d. | (108) |
| miR-142-3p | Immortalized mouse EC- like cells |
Reporter activity | n.d. | (109) |
| miR-143, miR-145 | Human aortic SMCs |
ELK, KLF4, PHACTR4, SSH2, MMP3 |
Atheroprotective in vivo | (110) |
| miR-126 | HUVECs | RGS16 | Atheroprotective in vivo | (114) |
| miR-126 | HCAECs in vitro and murine ECs in vivo |
SPRED1 | EC migration and proliferation in vitro; re-endofhelialization in vivo |
(115) |
| miR-126 | HAECs | n.d. | Pro-angiogenic in vitro | (119) |
| miR-126 | HUVECs | n.d. | Pro-angiogenic in vitro and in vivo | (120) |
| miR-150 | HMEC-1 | MYB | Increased cell migration in vitro | (123) |
| miR-214 | HMEC-1 | ATM | Reduced EC senescence in vitro; pro- angiogenic in vitro and ex vivo |
(124) |
| miR-132 | HUVECs | pl20RASGAP/RASAl | Pro-angiogenic in vitro | (125) |
| miR-105 | HMVECs | TJP1/Z0-1 | Increased cell migration and vascular permeability in vitro and in vivo |
(126) |
| miR-210 | HUVECs | Reporter activity | Pro-angiogenic in vitro | (127) |
| miR-210 | HUVECs | EFNA3 | Pro-angiogenic in vitro | (131) |
| miR-210, miR-135b | HUVECs | FIH-1 | Pro-angiogenic in vitro and ex vivo | (132) |
| miR-21 | Unirradiated MRC-5 cells | BCL-2 | RIBE | (148, 149) |
Notes. HUVECs = human umbilical vein endothelial cells (ECs); n.d. = not done; BM = bone marrow; ultra = ultracentrifugation; SMCs = smooth muscle cells; HCAECs = human carotid artery ECs; PBMCs = peripheral blood mononuclear cells; HAECs = human aortic ECs; HMEC/ HMVECs = human microvascular ECs; SVPs = saphenous vein-derived pericyte progenitors; CM = conditioned media; RIBE = radiation-induced bystander effect.
Angiogenic function of EV-derived miR-126
Zernecke et al. demonstrated that human umbilical vein EC (HUVEC)-derived ABs were generated during atherosclerosis, and that these ABs were enriched for miR-126 (114). AB uptake by recipient ECs led to the miR-126-dependent induction of CXCL12 expression, through inhibition of miR-126 target gene RGS16. It was finally shown that treatment with ABs released from apoptotic HUVECs, or isolated from atherosclerotic plaques from patients undergoing endarterectomy of carotid arteries, reduced plaque size in Apoe−/−mice on a high-fat diet, through the CXCL12 -dependent recruitment of progenitor cells for endothelial repair. This effect was abrogated when using ABs obtained from miR-126−/− mouse ECs compared to miR-126+/+ ECs, suggesting an atheroprotective role for miR-126 (114). Jansen et al. obtained similar results showing that EVs from apoptotic human coronary artery ECs promoted vascular EC repair by transferring mir-126 to ECs in vitro and in vivo, using a reendothelialization model after electric endothelial denudation of the common carotid artery in mice (115). This effect was mediated, at least in part, by inhibition of the miR-126 target gene SPRED1, resulting in enhancement of vascular endothelial growth factor (VEGF) signaling (116, 117). It is interesting to note that the two aforementioned studies demonstrated a similar atheroprotective role for miR-126-containing EVs released from apoptotic ECs, yet Zernecke et al. attributed this function to endothelial ABs, while Jansen et al. worked with endothelial MPs. Although these two classes of EVs are produced through distinct mechanisms and display different sizes (12, 118), they both expose PS at their surface, which was used for vesicle characterization in these two studies, and the procedures used for their isolation were similar, including removal of cells and cell debris at 800–l,500g for 10 min, followed by pelleting of EVs at 16,000–20,000g for 20–40 min. It is therefore tempting to speculate that these two studies indeed tested the same EV population, which was likely a mixture of both types of vesicles (118). Admittedly, this observation does not preclude the atheroprotective effect of apoptotic EC-derived EVs, though it remains unclear whether it is associated solely with ABs, MPs or both.
It has been well established that endothelial miR-126 governs vascular integrity and angiogenesis by regulating the responses of ECs to VEGF and fibroblast growth factor (FGF). Targeted deletion of miR-126 in mice and its knockdown in zebrafish causes vascular leakage during embryonic development and defective neovascularization after myocardial infarction, due to defects in the proliferation and migration of ECs and angiogenesis (116, 117). It is therefore not surprising that EV-mediated transfer of miR-126 to ECs exerts similar pro-angiogenic effects. Thus, it was found that bone marrow-derived CD34+ peripheral blood mononuclear cells secrete EVs with angiogenic activity both in vitro and in vivo. These EVs were enriched for miR-126, and treatment with anti-miR-126 abrogated the pro-angiogenic effect of CD34+ cell-derived EVs. Reduced miR-126 expression in high-glucose-treated or in type 2 diabetic patient-derived CD34+ cells resulted in altered pro-angiogenic capacity, although it could be rescued by miR-126 mimics (119, 120). Of note, EVs released by ECs grown in high-glucose conditions also showed reduced levels of miR-126, and reduced regenerative capacity in vitro and in vivo (115). These observations suggest a role for reduced circulating EVs miR-126 expression in the vascular impairment associated with diabetes mellitus, corroborating miRNA profiling studies showing reduced plasma miR-126 levels in type 2 diabetic patients (121). Interestingly, miR-126 was also reported to be transferred in a vesicle-independent manner from ECs to SMCs in a coculture system in vitro, where it regulated the expression of target genes FOXO3, BCL2 and IRS1, leading to increased proliferation of SMCs (122). This effect was abolished when applying laminar shear stress to ECs. The antiatherogenic effect of shear stress, mediated by inhibition of miR-126 secretion, was confirmed in vivo using a carotid artery ligation model in which the neointimal lesion formation seen in wild-type animals was attenuated in miR-126−/− mice. These studies suggest that miR-126 may exert pro- or anti-atherogenic functions, depending on its mode of extracellular transfer and recipient cell type.
Other angiogenesis-related miRNAs
Other EV-transferred miRNAs were shown to play a role in promoting and regulating angiogenesis (Table 2). The human monocytic cell line THP-1 was shown to transfer miR-150 to recipient ECs via EVs, where it decreased expression of its target gene MYB, resulting in enhanced cell migration. Interestingly, elevated levels of miR-150 were also found in EVs from plasma of patients with severe atherosclerosis, similarly resulting in decreased c-Myb protein level in recipient ECs and enhanced cell migration (123). Exosomes released by the human microvascular endothelial cell line HMEC-1 in culture were shown to stimulate angiogenesis through the transfer of miR-214 in recipient HMEC-1 cells, resulting in reduced expression of target gene ATM, leading to repression of cell senescence (124). HMEC-1-derived exosomes with reduced miR-214 levels had reduced capacity to stimulate HMEC-1 cell migration and angiogenesis in vitro and ex vivo (Matrigel® plug assay). In addition, miR-132 was shown to be produced and secreted by saphenous vein-derived pericyte progenitors (SVPs), especially in response to hypoxic culture conditions (125). Exposure to SVP conditioned media (CM) led to increased miR-132 levels in HUVECs, accompanied by inhibition of its target gene p120RASGAP. Notably, the pro-angiogenic capacity of SVP-CM was blunted upon transfection with anti-miR-132 in SVPs, leading to reduced HUVEC proliferation and tube formation. However, the method of transportation between SVPs and recipient ECs was not investigated in this study. Interestingly, miR-132 inhibition attenuated the effect of SVP transplantation on promoting reparative neovascularization in a mouse model of myocardial infarction (125).
Angiogenic potential of miRNAs from cancer-derived EVs
Exosome-mediated miRNA transfer from cancer cells to the niche was recently reported to promote metastasis through vascular remodeling. Zhou et al. showed that miR-105 is overexpressed in metastatic breast cancer MDA-MB-231 cells and their exosomes, compared to primary tumor cells (126). Exosome-mediated transfer of miR-105 to HMVECs induced EC migration and led to the inhibition of the target gene TJP1/ZO-1 (tight junction protein 1/zonula occludens-1). MDA-MB-231-derived exosomes induced vascular destruction and increased vascular permeability in vitro and in vivo, thereby promoting metastasis in the lung and brain. This effect was suppressed through inhibition of miR-105, which restored ZO-1 expression in vascular ECs and the barrier function of endothelial niche cells. Similarly, Kosaka et al. found that exosomes derived from metastatic breast cancer 4T1 cells enhanced tube formation and migration of HUVECs in vitro (127). This effect was partially mediated by exosomal transfer of miR-210 from cancer cells to HUVECs, where it regulated reporter gene expression. Expression of miR-210 is regulated by hypoxia-inducible factor (HIF)-1, and has been reported to induce tube formation and migration of HUVECs in response to hypoxia in vitro (128, 129). miR-210 aberrant expression has been reported in a number of pathological conditions where hypoxia is a major component, including tumors, myocardial infarction and cutaneous ischemic wound healing (130). Similar to breast cancer cells, it was shown that exosomes released from leukemia K562 cells grown in hypoxic conditions mediated the transfer of miR-210 in HUVECs, where it regulated expression of the target gene EFNA3 and enhanced tube formation (131). Using an in vitro chronic hypoxia model of multiple myeloma, the same group demonstrated that exosomes derived from hypoxic cells increased tube formation of HUVECs (132). In addition to miR-210, these exosomes were enriched with miR-135b, which was transferred into HUVECs via exosomes to mediate their pro-angiogenic potential in vitro and ex vivo (plug assay). In HUVECs, exosome-transferred miR-135b regulated expression of the factor-inhibiting HIF-1 (FIH-1) target gene, resulting in increased HIF-1 transcriptional activity.
MicroRNAs, EVs and Radiation-Induced Nontargeted Effects
It is now widely accepted that radiation causes damage not only to directly exposed cells, but also to unirradiated cells in contact with, or at a distance from directly irradiated cells. These nontargeted effects, which include low-dose hypersensitivity, genomic instability, adaptive response, radiation-induced bystander effect (RIBE) and distant (abscopal) effect, are the consequences of intercellular communication between irradiated and unirradiated cells. RIBE refers to the biological alterations that occur in bystander cells and tissues, including DNA damage, gene expression alteration and apoptosis. Such communication is known to occur through cell gap junctions (133) or molecular signals released in the extracellular milieu by irradiated cells. Some factors responsible for mediating RIBE have been identified, including soluble cytokines such as transforming growth factor (TGF)-β, nitric oxide, and ROS (134, 135). Radiation-induced abscopal effects occur at a distance from the irradiated site and have been documented in preclinical and clinical studies as systemic effects of local radiotherapy for the treatment of different tumor types. However, while some studies have shown a systemic tumor-enhancing effect, others have shown that local irradiation inhibits distant tumor growth (136). In vivo studies using animal models investigated the molecular mechanisms involved in the effect of radiation on distant, unirradiated tumor growth, and reported a role for p53 signaling and the immune system in mediating abscopal antitumor activity (137, 138). Since EVs can travel to distant sites because of their release in the blood circulation (139), the role of EVs and their cargo, in particular their miRNA content, as mediators of RIBE has been actively investigated recently. In fact, several studies have demonstrated RIBE on unirradiated cells in coculture with irradiated cells or upon exposure to CM harvested from irradiated cells, with involvement of miRNA expression variations. Using a transwell system, Chaudhry and Omaruddin showed that human lymphoblast TK6 cells underwent wide miRNA expression changes upon coculture with 2 Gy X-ray-irradiated cells (140). Using human non-small cell lung cancer (NSCLC) cell line H1299, the Yang team demonstrated increased ROS generation and p53 binding protein (53BP)-1 foci in cells exposed to CM harvested from 5 Gy X-ray-irradiated H1299 cells (141). These effects were TGF-β1 dependent and involved increased miR-21 expression in bystander cells. Of note, inhibition of miR-21 in bystander cells abrogated the biological effect induced by irradiated cells CM. Using a coculture system, the same group showed that human HaCaT keratinocytes irradiated with 0.56 Gy α particles induced micronuclei formation, elevated ROS levels and increased 53BP1 foci in unirradiated human embryonic dermal WS1 fibroblasts (142, 143). These effects were mediated by activation of the TGF-β1-SMAD2 pathway in irradiated HaCaT cells, and led to increased miR-21 level in bystander WS1 cells, with possible involvement of SOD2. Interestingly, HaCaT cells that received up to 10 Gy X-ray irradiation failed to elicit RIBE in WS1 cells in this system, nor did they show activation of SMAD2, suggesting that RIBE is likely dependent on radiation quality, as well as the cell type under study. In another study, TGF-β1 in CM from 4 Gy X-ray-irradiated HeLa cells was also shown to account for the RIBE observed on recipient unirradiated HeLa cells, with increased 53BP1 foci and increased frequency of micronuclei (144). This effect was attenuated by induction of miR-663 in bystander cells, where it directly inhibited TGF-β1 in a feedback regulation mode.
It was recently shown that EVs (exosomes) could mediate short- and long-term RIBE in human MCF7 breast epithelial cancer cells exposed to CM from 2 Gy X-ray-irradiated cells, involving some RNA component as well (145, 146). Along this line, Jella et al. showed that exosomes released from HaCaT cells gamma-irradiated with 0.05–0.5 Gy (60Co) induced increased cell death and ROS production in unirradiated cells (147). The Wang group demonstrated that CM from 2 Gy X-ray-irradiated human fetal lung MRC-5 fibroblasts mediated RIBE in unirradiated cells, with increased frequency of micronuclei and increased number of 53BP1 foci, as well as reduced clonogenic survival (148). Irradiated CM was enriched in miR-21, which was transferred to recipient bystander cells where it repressed its target gene BCL2 at the RNA and protein levels. Interestingly, transfection of miR-21 mimics into MRC-5 cells induced similar effects, suggesting a role for miR-21 in mediating RIBE. In a follow-up study, the same group demonstrated that exosomes isolated from irradiated CM could induce bystander effects by mediating miR-21 transfer from irradiated to bystander cells (149). Inhibition of miR-21 before irradiation blunted these effects, suggesting that exosome-mediated miR-21 transfer plays an important role in RIBE. Overall, these studies highlight the prevalent role of the TGF-β1 pathway and miR-21 for induction of RIBE.
Interestingly, miR-21 has been involved in vascular remodeling, where it was shown to be upregulated in SMCs after vascular injury. Notably, miR-21 regulated rat aortic SMC proliferation and apoptosis in vitro and in injured rat carotid arteries (150). miR-21 is also highly expressed in vascular ECs and is upregulated in a hypoxic environment (tumor, ischemic limb), leading to expression of HIF1 and its target gene VEGF, thereby promoting angiogenesis. Furthermore, miR-21 and HIF-1 are involved in a hypoxia-induced positive feedback loop, in which HIF-1α drives miR-21 expression and miR-21 indirectly stabilizes and upregulates HIF-1α via inhibition of target gene PTEN (148, 151). Nonetheless, miR-21 has also been reported to inhibit angiogenesis by targeting RHOB in ECs and HMGA2 in bone marrow-derived EPCs, leading to decreased cell migration and tube formation in vitro, and reduced angiogenesis in vivo in murine models of choroidal neovascularization and hind limb ischemia, respectively (152, 153). The effect of increased exosomal miR-21 released by irradiated cells on the vascular compartment remains to be explored, however.
Exosomes in Regenerative Medicine
Adult MSCs are multipotent stem cells that are routinely isolated from bone marrow or adipose tissue and expanded ex vivo. They have been extensively studied in the context of regenerative medicine, and they are currently evaluated in hundreds of clinical trials for a wide range of diseases, including cardiovascular diseases, graft-versus-host disease, cartilage and bone repair (154). Although the therapeutic capacity of MSCs was originally attributed to their differentiation potential into different reparative or supportive cell types, numerous studies have shown that the therapeutic benefit of MSCs essentially relied on their paracrine activity (14). Indeed, injection of MSC-CM could recapitulate MSCs beneficial effects in animal models of myocardium ischemia/reperfusion (155, 156) and cutaneous wound healing (157, 158). The Lim laboratory demonstrated that exosomes were the main contributor to the beneficial effect of MSCs paracrine activity for the treatment of myocardial injury (159). Since then, several studies have demonstrated the therapeutic efficacy of exosomes derived from different cell types (MSCs, ECs, adipose-derived progenitor cells) in multiple pathological animal models, including kidney (160), heart (159) and lung (161) ischemia.
In addition, conditioned media (162) or EVs (163) from human cord blood-derived EPCs were shown to promote angiogenesis in vitro and in vivo. Similarly, the Losordo group demonstrated that exosomes derived from mobilized human CD34+ cells recapitulated the angiogenic activity of CD34+ cells, both in vitro and in vivo using Matrigel plug and corneal assays (120). Recently, a comprehensive proteomic analysis revealed that MSCs exposed to hypoxic conditions released exosomes with a robust profile of key angiogenic proteins, including PDGF, FGF, EGF and NFκB signaling pathway (164). Hypoxia preconditioned MSC-derived exosomes induced tube formation in HUVECs, which was completely abrogated upon treatment with an NFκB inhibitor. Our group is currently investigating the beneficial effect of MSC-derived exosomes for the treatment of radiological cutaneous injury. Preliminary results revealed a stimulation of angiogenesis and an improvement of skin quality after local exosome administration, raising new hopes in regenerative medicine for the treatment of radiation injuries (unpublished data).
A number of clinical trials aiming at using exosomes as therapeutic tools or vehicle for drug delivery have been published (165). For example, exosomes purified from dendritic cells pulsed with antigenic peptides were used as anticancer vaccines for two phase I clinical trials against melanoma and NSCLC (166, 167), and more recently for a phase II clinical trial against NSCLC (168). Furthermore, the immunosuppressive potential of exosomes was demonstrated by Kordelas et al., who reported on the case of a patient with graft-vs.-host disease after allogeneic stem cell transplant for the treatment of acute lymphoblastic leukemia (169). The patient received allogeneic MSC-derived exosomes, resulting in attenuation of the symptoms and disease stabilization.
Altogether, these preclinical and clinical studies highlight the beneficial potential for exosome-based therapy with regard to angiogenesis and immunosuppression, and pave the way to the development of clinically compliant preparations of stem cell-derived exosomes for the treatment of radiological injuries.
CONCLUSION
An increasing number of studies highlight the contribution of EVs in signal transmission in a cell-to-cell communication process in different pathologies, including cardiovascular diseases and cancer. However, the extent of their contributions to patho/physiological processes and the mechanisms involved remain uncertain. An understanding of the molecular mechanisms governing EV formation, release and clearance, as well as those involved in cell-to-cell communication, will enable to us to envision new therapeutic strategies for limiting disease progression or favoring tissue repair. Over the past decade, most efforts have been focused on elucidating the involvement of exosomes in radiation-induced nontargeted effects and the role played by miRNAs in these processes. Thus far, there is a lack of data on MPs in the field of radiation, which may be the result of current technical concerns and limited standardization. Nonetheless, a complete vision of the role of EVs, integrating MPs and exosomes in regulating vascular homeostasis in the irradiation field is necessary. In addition, one should also be aware that exosomes reflect only a partial picture of the vascular status and should not exclude the simultaneous contribution of the MPs derived from the cells of interest (Fig. 1).
An overview of the literature clearly indicates that research on EVs is still in its infancy and suffers from limitations. Despite the recent advances, the terms “exosomes” and “extracellular vesicles” or “microvesicles” have been used interchangeably in many published studies due to the current limited understanding of extracellular vesicle biogenesis, inconsistencies in extracellular vesicle purification protocols and a lack of thorough vesicle characterization. There remains an urgent need for clarification on a number of technical points, such as the EV nomenclature and the standardized protocols for their preparation. However, the complexity of EV heterogeneity and functions has to be taken into account. One of the most urgent challenges is to establish methods to separately characterize each kind of EV to precisely define their individual cargoes and functions, including their miRNA content. The first step in addressing this challenge is determining how to define and measure EVs in a reproducible manner. The characterization of MPs and other types of vesicles remains difficult because of their small size and heterogeneity in terms of phospholipid content and antigenic composition. In addition, there is no consensus on the best markers for defining “rare” EV subpopulations such as endothelium-, leukocyte-, and cancer-derived MPs, which appear to be the most relevant in terms of predictive value. While important work to standardize the pre-analytical and analytical variables has been achieved on behalf of the International Society on Thrombosis and Haemostasis Standardization Subcommittee in vascular biology for the MPs (170, 171), efforts in this area continue. Similarly, the International Society for Extracellular Vesicles provides researchers with a minimal set of biochemical, biophysical and functional standards that should be used to attribute any specific biological cargo or functions to EVs (12, 172, 173).
Another major field of optimization and standardization resides in the thorough analysis of EV RNA content. It is clear that the different EV subpopulations, exosomes, MPs, ABs, display distinct total RNA and miRNA profiles (118, 173). In addition, discrepancies within a subgroup, i.e., exosomal RNA profiles, have been observed among studies, which could be partly explained by differences in the cellular source as well as methodological parameters (175). Several comparative studies highlighted the strong impact of the choice of both EV isolation method (176, 177) and RNA purification protocol (175, 178, 179), on downstream RNA profiling. In particular, comparison of RNA isolation protocols using phenol-based methods, column-based techniques or combined phenol/column-based approaches, revealed substantial variations regarding small RNA recovery and miRNA expression analysis in exosomes from cell culture and biofluids (175, 178, 179). To our knowledge, no consensus has been established so far, and the main recommendation remains to carefully choose the RNA isolation method according to the nature and amount of starting material, as well as the downstream experiments.
In this review we present information on the diverse implications of EV signaling in health and disease, how EVs may be utilized as diagnostic disease biomarkers and therapeutics and how their inherent properties potentially provide benefits over existing clinical interventions. Although the complexity of EVs naturally broadens their functional impact, this also makes investigations of their activity difficult. While the overall effect on a cell after EV uptake results from the combination of the particular EV-transferred components, including relevant angiomiRs, it is also determined by the cell of origin and its status at the time of secretion. In addition, it is also important to remember that different EVs can have synergistic or opposing effects on the recipient cell. Mechanistically, EVs participate in clearance of substances, information exchange and epigenetic modulation, and may even be responsible for the spreading of pathological proteins. This complex, multifunctional activity is explained by the highly heterogeneous content of EVs. All these regulatory functions have been delineated mostly using in vitro systems, although their in vivo relevance remains to be fully demonstrated. In the fields of radiation biology and oncology, a better understanding of EV biology together with standardized methods for EV quantification, isolation, storage and molecular characterization will greatly enhance the future promise for EV-based diagnostic and therapeutic applications.
REFERENCES
- 1.Heckmann M, Douwes K, Peter R, Degitz K. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp Cell Res. 1998;238:148–154. doi: 10.1006/excr.1997.3826. [DOI] [PubMed] [Google Scholar]
- 2.Langley RE, Bump EA, Quartuccio SG, Medeiros D, Braunhut SJ. Radiation-induced apoptosis in microvascular endothelial cells. Br J Cancer. 1997;75:666–672. doi: 10.1038/bjc.1997.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13:3047–3055. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker DG, Krochak RJ. The response of the microvascular system to radiation: a review. Cancer Invest. 1989;7:287–294. doi: 10.3109/07357908909039849. [DOI] [PubMed] [Google Scholar]
- 5.van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flammer AJ, Luscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- 7.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–718. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 9.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 10.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cellderived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 12.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci (Lond) 2013;124:423–441. doi: 10.1042/CS20120309. [DOI] [PubMed] [Google Scholar]
- 14.EL Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 15.Loyer X, Vion AC, Tedgui A, Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res. 2014;114:345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- 16.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200:367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126:1139–1143. doi: 10.1172/JCI87316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21–29. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- 19.Jy W, Horstman LL, Jimenez JJ, Ahn YS, Biro E, Nieuwland R, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–1851. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix R, Robert S, Poncelet P, Dignat-George F. Overcoming limitations of microparticle measurement by flow cytometry. Semin Thromb Hemost. 2010;36:807–818. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- 21.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/ Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31:1898–1907. doi: 10.1161/ATVBAHA.110.222703. [DOI] [PubMed] [Google Scholar]
- 22.Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009;335:143–151. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 23.Zahra S, Anderson JA, Stirling D, Ludlam CA. Microparticles, malignancy and thrombosis. Br J Haematol. 2011;152:688–700. doi: 10.1111/j.1365-2141.2010.08452.x. [DOI] [PubMed] [Google Scholar]
- 24.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L364–L381. doi: 10.1152/ajplung.00354.2011. [DOI] [PubMed] [Google Scholar]
- 25.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 26.Rabelink TJ, de Boer HC, van Zonneveld AJ. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat Rev Nephrol. 2010;6:404–414. doi: 10.1038/nrneph.2010.65. [DOI] [PubMed] [Google Scholar]
- 27.Thom SR, Yang M, Bhopale VM, Huang S, Milovanova TN. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J Appl Physiol (1985) 2011;110:340–351. doi: 10.1152/japplphysiol.00811.2010. [DOI] [PubMed] [Google Scholar]
- 28.Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J Am Soc Hypertens. 2012;6:85–99. doi: 10.1016/j.jash.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Burger D, Kwart DG, Montezano AC, Read NC, Kennedy CR, Thompson CS, et al. Microparticles induce cell cycle arrest through redox-sensitive processes in endothelial cells: implications in vascular senescence. J Am Heart Assoc. 2012;1:e001842. doi: 10.1161/JAHA.112.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–1876. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 31.Brown MD, Feairheller DL, Thakkar S, Veerabhadrappa P, Park JY. Racial differences in tumor necrosis factor-alpha-induced endothelial microparticles and interleukin-6 production. Vasc Health Risk Manag. 2011;7:541–550. doi: 10.2147/VHRM.S22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson DB, Sander T, Kaul S, Wakim BT, Halligan B, Twigger S, et al. Comparative proteomic analysis of PAI-1 and TNF-alpha-derived endothelial microparticles. Proteomics. 2008;8:2430–2446. doi: 10.1002/pmic.200701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Turco S, Basta G, Lazzerini G, Evangelista M, Rainaldi G, Tanganelli P, et al. Parallel decrease of tissue factor surface exposure and increase of tissue factor microparticle release by the n-3 fatty acid docosahexaenoate in endothelial cells. Thromb Haemost. 2007;98:210–219. [PubMed] [Google Scholar]
- 34.Abid Hussein MN, Boing AN, Sturk A, Hau CM, Nieuwland R. Inhibition of microparticle release triggers endothelial cell apoptosis and detachment. Thromb Haemost. 2007;98:1096–1107. doi: 10.1160/th05-04-0231. [DOI] [PubMed] [Google Scholar]
- 35.Devaraj S, Kumaresan PR, Jialal I. C-reactive protein induces release of both endothelial microparticles and circulating endothelial cells in vitro and in vivo: further evidence of endothelial dysfunction. Clin Chem. 2011;57:1757–1761. doi: 10.1373/clinchem.2011.169839. [DOI] [PubMed] [Google Scholar]
- 36.Wang JM, Wang Y, Huang JY, Yang Z, Chen L, Wang LC, et al. C-Reactive protein-induced endothelial microparticle generation in HUVECs is related to BH4-dependent NO formation. J Vasc Res. 2007;44:241–248. doi: 10.1159/000100558. [DOI] [PubMed] [Google Scholar]
- 37.Simoncini S, Njock MS, Robert S, Camoin-Jau L, Sampol J, Harle JR, et al. TRAIL/Apo2L mediates the release of procoagulant endothelial microparticles induced by thrombin in vitro: a potential mechanism linking inflammation and coagulation. Circ Res. 2009;104:943–951. doi: 10.1161/CIRCRESAHA.108.183285. [DOI] [PubMed] [Google Scholar]
- 38.Brodsky SV, Malinowski K, Golightly M, Jesty J, Goligorsky MS. Plasminogen activator inhibitor-1 promotes formation of endothelial microparticles with procoagulant potential. Circulation. 2002;106:2372–2378. doi: 10.1161/01.cir.0000033972.90653.af. [DOI] [PubMed] [Google Scholar]
- 39.Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, et al. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006;4:566–573. doi: 10.1111/j.1538-7836.2005.01780.x. [DOI] [PubMed] [Google Scholar]
- 40.Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, et al. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis. 2009;54:891–901. doi: 10.1053/j.ajkd.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Sekula M, Janawa G, Stankiewicz E, Stepien E. Endothelial microparticle formation in moderate concentrations of homocysteine and methionine in vitro. Cell Mol Biol Lett. 2011;16:69–78. doi: 10.2478/s11658-010-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terrisse AD, Puech N, Allart S, Gourdy P, Xuereb JM, Payrastre B, et al. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J Thromb Haemost. 2010;8:2810–2819. doi: 10.1111/j.1538-7836.2010.04088.x. [DOI] [PubMed] [Google Scholar]
- 43.Simak J, Holada K, Vostal JG. Release of annexin V-binding membrane microparticles from cultured human umbilical vein endothelial cells after treatment with camptothecin. BMC Cell Biol. 2002;3:11. doi: 10.1186/1471-2121-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–180. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 45.Szotowski B, Antoniak S, Goldin-Lang P, Tran QV, Pels K, Rosenthal P, et al. Antioxidative treatment inhibits the release of thrombogenic tissue factor from irradiation- and cytokine-induced endothelial cells. Cardiovasc Res. 2007;73:806–812. doi: 10.1016/j.cardiores.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Boulanger CM, Amabile N, Guerin AP, Pannier B, Leroyer AS, Mallat CN, et al. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension. 2007;49:902–908. doi: 10.1161/01.HYP.0000259667.22309.df. [DOI] [PubMed] [Google Scholar]
- 47.Tramontano AF, O’Leary J, Black AD, Muniyappa R, Cutaia MV, El-Sherif N. Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho-kinase pathway. Biochem Biophys Res Commun. 2004;320:34–38. doi: 10.1016/j.bbrc.2004.05.127. [DOI] [PubMed] [Google Scholar]
- 48.van den Akker J, van Weert A, Afink G, Bakker EN, van der Pol E, Boing AN, et al. Transglutaminase 2 is secreted from smooth muscle cells by transamidation-dependent microparticle formation. Amino Acids. 2012;42:961–973. doi: 10.1007/s00726-011-1010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bevers EM, Comfurius P, Dekkers DW, Zwaal RF. Lipid translocation across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1999;1439:317–330. doi: 10.1016/s1388-1981(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 51.Toti F, Satta N, Fressinaud E, Meyer D, Freyssinet JM. Scott syndrome, characterized by impaired transmembrane migration of procoagulant phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood. 1996;87:1409–1415. [PubMed] [Google Scholar]
- 52.Castoldi E, Collins PW, Williamson PL, Bevers EM. Compound heterozygosity for 2 novel TMEM16F mutations in a patient with Scott syndrome. Blood. 2011;117:4399–4400. doi: 10.1182/blood-2011-01-332502. [DOI] [PubMed] [Google Scholar]
- 53.Albrecht C, McVey JH, Elliott JI, Sardini A, Kasza I, Mumford AD, et al. A novel missense mutation in ABCA1 results in altered protein trafficking and reduced phosphatidylserine translocation in a patient with Scott syndrome. Blood. 2005;106:542–549. doi: 10.1182/blood-2004-05-2056. [DOI] [PubMed] [Google Scholar]
- 54.Dachary-Prigent J, Pasquet JM, Fressinaud E, Toti F, Freyssinet JM, Nurden AT. Aminophospholipid exposure, microvesiculation and abnormal protein tyrosine phosphorylation in the platelets of a patient with Scott syndrome: a study using physiologic agonists and local anaesthetics. Br J Haematol. 1997;99:959–967. doi: 10.1046/j.1365-2141.1997.5003302.x. [DOI] [PubMed] [Google Scholar]
- 55.Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044–1052. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 56.Biro E, Akkerman JW, Hoek FJ, Gorter G, Pronk LM, Sturk A, et al. The phospholipid composition and cholesterol content of platelet-derived microparticles: a comparison with platelet membrane fractions. J Thromb Haemost. 2005;3:2754–2763. doi: 10.1111/j.1538-7836.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 57.Liu ML, Reilly MP, Casasanto P, McKenzie SE, Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler Thromb Vasc Biol. 2007;27:430–435. doi: 10.1161/01.ATV.0000254674.47693.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albanese J, Dainiak N. Modulation of intercellular communication mediated at the cell surface and on extracellular, plasma membrane-derived vesicles by ionizing radiation. Exp Hematol. 2003;31:455–464. doi: 10.1016/s0301-472x(03)00050-x. [DOI] [PubMed] [Google Scholar]
- 59.Benderitter M, Vincent-Genod L, Berroud A, Voisin P. Simultaneous analysis of radio-induced membrane alteration and cell viability by flow cytometry. Cytometry. 2000;39:151–157. doi: 10.1002/(sici)1097-0320(20000201)39:2<151::aid-cyto8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 60.Bionda C, Hadchity E, Alphonse G, Chapet O, Rousson R, Rodriguez-Lafrasse C, et al. Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free Radic Biol Med. 2007;43:681–694. doi: 10.1016/j.freeradbiomed.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 61.Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, Becher MU, et al. Endothelial microparticle uptake in target cells is annexin I/ phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1925–1935. doi: 10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- 62.Meziani F, Tesse A, Andriantsitohaina R. Microparticles are vectors of paradoxical information in vascular cells including the endothelium: role in health and diseases. Pharmacol Rep. 2008;60:75–84. [PubMed] [Google Scholar]
- 63.Shantsila E, Kamphuisen PW, Lip GY. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost. 2010;8:2358–2368. doi: 10.1111/j.1538-7836.2010.04007.x. [DOI] [PubMed] [Google Scholar]
- 64.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 65.Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–4553. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 66.Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, Dignat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99:3962–3970. doi: 10.1182/blood.v99.11.3962. [DOI] [PubMed] [Google Scholar]
- 67.Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 69.Parry GC, Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15:612–621. doi: 10.1161/01.atv.15.5.612. [DOI] [PubMed] [Google Scholar]
- 70.Lupu C, Westmuckett AD, Peer G, Ivanciu L, Zhu H, Taylor FB, Jr, et al. Tissue factor-dependent coagulation is preferentially upregulated within arterial branching areas in a baboon model of Escherichia coli sepsis. Am J Pathol. 2005;167:1161–1172. doi: 10.1016/S0002-9440(10)61204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solovey A, Kollander R, Shet A, Milbauer LC, Choong S, Panoskaltsis-Mortari A, et al. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104:840–846. doi: 10.1182/blood-2003-10-3719. [DOI] [PubMed] [Google Scholar]
- 72.Lacroix R, Sabatier F, Mialhe A, Basire A, Pannell R, Borghi H, et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood. 2007;110:2432–2439. doi: 10.1182/blood-2007-02-069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lacroix R, Plawinski L, Robert S, Doeuvre L, Sabatier F, Martinez de Lizarrondo S, et al. Leukocyte- and endothelial-derived microparticles: a circulating source for fibrinolysis. Haematologica. 2012;97:1864–1872. doi: 10.3324/haematol.2012.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aharon A, Brenner B. Microparticles, thrombosis and cancer. Best Pract Res Clin Haematol. 2009;22:61–69. doi: 10.1016/j.beha.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Perez-Casal M, Downey C, Cutillas-Moreno B, Zuzel M, Fukudome K, Toh CH. Microparticle-associated endothelial protein C receptor and the induction of cytoprotective and antiinflammatory effects. Haematologica. 2009;94:387–394. doi: 10.3324/haematol.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satta N, Freyssinet JM, Toti F. The significance of human monocyte thrombomodulin during membrane vesiculation and after stimulation by lipopolysaccharide. Br J Haematol. 1997;96:534–542. doi: 10.1046/j.1365-2141.1997.d01-2059.x. [DOI] [PubMed] [Google Scholar]
- 77.Tans G, Rosing J, Thomassen MC, Heeb MJ, Zwaal RF, Griffin JH. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood. 1991;77:2641–2648. [PubMed] [Google Scholar]
- 78.Goldin-Lang P, Niebergall F, Antoniak S, Szotowski B, Rosenthal P, Pels K, et al. Ionizing radiation induces upregulation of cellular procoagulability and tissue factor expression in human peripheral blood mononuclear cells. Thromb Res. 2007;120:857–864. doi: 10.1016/j.thromres.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 79.Peiffert D, Simon JM, Eschwege F. [Epinal radiotherapy accident: passed, present, future] Cancer Radiother. 2007;11:309–312. doi: 10.1016/j.canrad.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123:8–23. doi: 10.1016/j.thromres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Matsumoto N, Nomura S, Kamihata H, Kimura Y, Iwasaka T. Increased level of oxidized LDL-dependent monocyte-derived microparticles in acute coronary syndrome. Thromb Haemost. 2004;91:146–154. doi: 10.1160/TH03-04-0247. [DOI] [PubMed] [Google Scholar]
- 82.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–217. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 83.Nomura S, Tandon NN, Nakamura T, Cone J, Fukuhara S, Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158:277–287. doi: 10.1016/s0021-9150(01)00433-6. [DOI] [PubMed] [Google Scholar]
- 84.Forlow SB, McEver RP, Nollert MU. Leukocyte-leukocyte interactions mediated by platelet microparticles under flow. Blood. 2000;95:1317–1323. [PubMed] [Google Scholar]
- 85.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, et al. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 86.Mastronardi ML, Mostefai HA, Meziani F, Martinez MC, Asfar P, Andriantsitohaina R. Circulating microparticles from septic shock patients exert differential tissue expression of enzymes related to inflammation and oxidative stress. Crit Care Med. 2011;39:1739–1748. doi: 10.1097/CCM.0b013e3182190b4b. [DOI] [PubMed] [Google Scholar]
- 87.Gaugler MH, Squiban C, van der Meeren A, Bertho JM, Vandamme M, Mouthon MA. Late and persistent up-regulation of intercellular adhesion molecule-1 (ICAM-1) expression by ionizing radiation in human endothelial cells in vitro. Int J Radiat Biol. 1997;72:201–209. doi: 10.1080/095530097143428. [DOI] [PubMed] [Google Scholar]
- 88.Hoyer FF, Giesen MK, Nunes Franca C, Lutjohann D, Nickenig G, Werner N. Monocytic microparticles promote atherogenesis by modulating inflammatory cells in mice. J Cell Mol Med. 2012;16:2777–2788. doi: 10.1111/j.1582-4934.2012.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Helbing T, Olivier C, Bode C, Moser M, Diehl P. Role of microparticles in endothelial dysfunction and arterial hypertension. World J Cardiol. 2014;6:1135–1139. doi: 10.4330/wjc.v6.i11.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freyssinet JM. Cellular microparticles: what are they bad or good for? J Thromb Haemost. 2003;1:1655–1662. doi: 10.1046/j.1538-7836.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 91.Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 92.Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leroyer AS, Ebrahimian TG, Cochain C, Recalde A, Blanc-Brude O, Mees B, et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119:2808–2817. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- 94.Chahed S, Leroyer AS, Benzerroug M, Gaucher D, Georgescu A, Picaud S, et al. Increased vitreous shedding of microparticles in proliferative diabetic retinopathy stimulates endothelial proliferation. Diabetes. 2010;59:694–701. doi: 10.2337/db08-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leroyer AS, Rautou PE, Silvestre JS, Castier Y, Leseche G, Devue C, et al. CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J Am Coll Cardiol. 2008;52:1302–1311. doi: 10.1016/j.jacc.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 96.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 97.Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 98.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 99.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 1–13. [DOI] [PubMed] [Google Scholar]
- 100.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;3:22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 101.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 102.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nolte-’t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, t Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol. 2011;2011:842849. doi: 10.1155/2011/842849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Emanueli C, Shearn Al, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24–30. doi: 10.1016/j.vph.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laffont B, Corduan A, Ple H, Duchez AC, Cloutier N, Boilard E, et al. Activated platelets can deliver mRNA regulatory Ago2•microRNA complexes to endothelial cells via microparticles. Blood. 2013;122:253–261. doi: 10.1182/blood-2013-03-492801. [DOI] [PubMed] [Google Scholar]
- 109.Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, et al. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014;8:1432–1446. doi: 10.1016/j.celrep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 110.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 111.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Climent M, Quintavalle M, Miragoli M, Chen J, Condorelli G, Elia L. TGFp triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ Res. 2015;116:1753–1764. doi: 10.1161/CIRCRESAHA.116.305178. [DOI] [PubMed] [Google Scholar]
- 114.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 115.Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, et al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 116.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mocharla P, Briand S, Giannotti G, Dorries C, Jakob P, Paneni F, et al. AngiomiR-126 expression and secretion from circulating CD34(+) and CD14(+) PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood. 2013;121:226–236. doi: 10.1182/blood-2012-01-407106. [DOI] [PubMed] [Google Scholar]
- 120.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 122.Zhou J, Li YS, Nguyen P, Wang KC, Weiss A, Kuo YC, et al. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ Res. 2013;113:40–51. doi: 10.1161/CIRCRESAHA.113.280883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 124.van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. doi: 10.1182/blood-2013-02-478925. S1–15. [DOI] [PubMed] [Google Scholar]
- 125.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, et al. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- 129.Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation. 2012;19:215–223. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288:34343–34351. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shao C, Folkard M, Prise KM. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene. 2008;27:434–440. doi: 10.1038/sj.onc.1210653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Temme J, Bauer G. Low-dose gamma irradiation enhances superoxide anion production by nonirradiated cells through TGF-beta1-dependent bystander signaling. Radiat Res. 2013;179:422–432. doi: 10.1667/RR3161.2. [DOI] [PubMed] [Google Scholar]
- 136.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Camphausen K, Moses MA, Menard C, Sproull M, Beecken WD, Folkman J, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63:1990–1993. [PubMed] [Google Scholar]
- 138.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 139.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chaudhry MA, Omaruddin RA. Differential regulation of microRNA expression in irradiated and bystander cells. Mol Biol (Mosk) 2012;46:634–643. [PubMed] [Google Scholar]
- 141.Jiang Y, Chen X, Tian W, Yin X, Wang J, Yang H. The role of TGF-beta1-miR-21-ROS pathway in bystander responses induced by irradiated non-small-cell lung cancer cells. Br J Cancer. 2014;111:772–780. doi: 10.1038/bjc.2014.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tian W, Yin X, Wang L, Wang J, Zhu W, Cao J, et al. The key role of miR-21 -regulated SOD2 in the medium-mediated bystander responses in human fibroblasts induced by alpha-irradiated keratinocytes. Mutat Res. 2015;780:77–85. doi: 10.1016/j.mrfmmm.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 143.Yin X, Tian W, Wang L, Wang J, Zhang S, Cao J, et al. Radiation quality-dependence of bystander effect in unirradiated fibroblasts is associated with TGF-β1-Smad2 pathway and miR-21 in irradiated keratinocytes. Sci Rep. 2015;5:11373. doi: 10.1038/srep11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu W, Xu S, Yao B, Hong M, Wu X, Pei H, et al. MiR-663 inhibits radiation-induced bystander effects by targeting TGFB1 in a feedback mode. RNA Biol. 2014;11:1189–1198. doi: 10.4161/rna.34345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Al-Mayah AH, Irons SL, Pink RC, Carter DR, Kadhim MA. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat Res. 2012;177:539–545. doi: 10.1667/rr2868.1. [DOI] [PubMed] [Google Scholar]
- 146.Al-Mayah A, Bright S, Chapman K, Irons S, Luo P, Carter D, et al. The non-targeted effects of radiation are perpetuated by exosomes. Mutat Res. 2015;772:38–45. doi: 10.1016/j.mrfmmm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 147.Jella KK, Rani S, O’Driscoll L, McClean B, Byrne HJ, Lyng FM. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat Res. 2014;181:138–145. doi: 10.1667/RR13337.1. [DOI] [PubMed] [Google Scholar]
- 148.Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X, et al. MiR-21 is involved in radiation-induced bystander effects. RNA Biol. 2014;11:1161–1170. doi: 10.4161/rna.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, et al. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015;12:1355–1363. doi: 10.1080/15476286.2015.1100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 151.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez ML, et al. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One. 2011;6:e16979. doi: 10.1371/journal.pone.0016979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu S, Deng S, Ma Q, Zhang T, Jia C, Zhuo D, et al. MicroRNA-10A* and MicroRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ Res. 2013;112:152–164. doi: 10.1161/CIRCRESAHA.112.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.ClinicalTrials.gov. U.S. National Library of Medicine. Bethesda: National Institutes of Health; https://clinicaltrials.gov. [Google Scholar]
- 155.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 156.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–264. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- 157.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mishra PJ, Banerjee D. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells. 2012;4:35–43. doi: 10.4252/wjsc.v4.i5.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/ reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 160.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim JY, Song SH, Kim KL, Ko JJ, Im JE, Yie SW, et al. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010;19:1635–1644. doi: 10.3727/096368910X516637. [DOI] [PubMed] [Google Scholar]
- 163.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 164.Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappab signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ohno S, Drummen GP, Kuroda M. Focus on extracellular vesicles: development of extracellular vesicle-based therapeutic systems. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Viaud S, Ploix S, Lapierre V, Thery C, Commere PH, Tramalloni D, et al. Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade: a critical role of interferon-gamma. J Immunother. 2011;34:65–75. doi: 10.1097/CJI.0b013e3181fe535b. [DOI] [PubMed] [Google Scholar]
- 169.Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 170.Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat-George F. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and SSC Haemostasis Collaborative workshop. J Thromb Haemost. 2013 doi: 10.1111/jth.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-George F. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and SSC Haemostasis Collaborative workshop. J Thromb Haemost. 2010;8:2571–2574. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- 172.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013:2. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lunavat TR, Cheng L, Kim DK, Bhadury J, Jang SC, Lasser C, et al. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells-evidence of unique microRNA cargos. RNA Biol. 2015;12:810–823. doi: 10.1080/15476286.2015.1056975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eldh M, Lotvall J, Malmhall C, Ekstrom K. Importance of RNA isolation methods for analysis of exosomal RNA: evaluation of different methods. Mol Immunol. 2012;50:278–286. doi: 10.1016/j.molimm.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 176.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 177.Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Crossland RE, Norden J, Bibby LA, Davis J, Dickinson AM. Evaluation of optimal extracellular vesicle small RNA isolation and qRT-PCR normalisation for serum and urine. J Immunol Methods. 2016;429:39–49. doi: 10.1016/j.jim.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 179.El-Khoury V, Pierson S, Kaoma T, Bernardin F, Berchem G. Assessing cellular and circulating miRNA recovery: the impact of the RNA isolation method and the quantity of input material. Sci Rep. 2016;6:19529. doi: 10.1038/srep19529. [DOI] [PMC free article] [PubMed] [Google Scholar]