ABSTRACT
The tumor suppressor Retinoblastoma (Rb) protein is highly phosphorylated in cancer cells largely due to the overexpression of cyclins or the loss of expression of cyclin dependent kinase inhibitors (cdki). Hyperphosphorylation of Rb promotes proliferation, and plays a role in the regulation of apoptosis. Recently, inhibition of cyclin dependent activity toward Rb has been identified as a strategy that has shown clinical efficacy. We utilized a method to induce phosphatase activity toward Rb in cells by shRNA silencing of PNUTS (Phosphatase Nuclear Targeting Subunit) that regulates PP1-mediated dephosphorylation of Rb. In this study, the effect of Rb dephosphorylation on the epithelial to mesenchymal transition (EMT) was determined. The EMT transition is observed in cancer cells that have acquired invasive characteristics. In breast cancer cells grown in 3D Matrigel cultures, MCF7 cells undergo apoptosis in response to Rb dephosphorylation, whereas MDA-MB-231 and Hs578T cells exhibit a reduction in the EMT. Cells devoid of phosphorylated Rb (nontransformed MCF10A and Rb-null MDA-MB-468) lacked any response to PNUTS depletion, showing the effect is Rb-dependent. In addition, these studies showed that Rb dephosphorylation in 3D Matrigel cultures of highly invasive HT1080 cells led to the inhibition of the EMT. Furthermore we observed association between dephosphorylated Rb with ZEB1, a zinc-finger E-box-binding transcription factor that regulates expression of E- and N-cadherins. Finally Rb dephosphorylation led to inhibition of ZEB1 transcriptional activity, this data supports the notion that Rb dephosphorylation modulates the EMT. These studies suggest targeting Rb phosphorylation in mesenchymal cancer cells may decrease invasiveness.
KEYWORDS: Apoptosis, EMT, MCF7, phosphatase, Retinoblastoma, TNBC, ZEB1
Introduction
The Retinoblastoma (Rb) tumor suppressor protein is inactivated by phosphorylation on several amino acid sites in many human cancers.1 Cyclin dependent kinases (cdks) are primarily responsible for phosphorylation of Rb that occurs in cells. It is well established that alterations in the Rb pathway (consisting of cdk4/6, D-type cyclins, the cdk inhibitor p16ink4a, and Rb itself) that lead to excessive phosphorylation of Rb have been observed in most cancer types.2,3 Because mutations that lead to increased cdk activity toward Rb are more common than mutations to the Rb gene itself, inhibitors of cdks that target Rb phosphorylation are in clinical development.4 Thus Rb phosphorylation is a clear target of cancer treatment and understanding the regulation of the Rb tumor suppressor by phosphorylation is of paramount significance in cancer.
The mechanism by which hyperphosphorylation of Rb promotes proliferation has been extensively studied. Unphosphorylated Rb suppresses proliferation by interaction with and inhibition of the E2F transcription factor that stimulates expression of genes required for S phase.5 Phosphorylation of Rb by cdks at the G1/S phase transition in response to proliferative signals leads to the release of the E2F that stimulates cell cycle progression.6 The cell cycle regulatory function of Rb is restored in late M phase when Protein Phosphatase 1 (PP1) dephosphorylates Rb.7,8
In addition to its role in cell proliferation, recent evidence suggests that phosphorylated Rb may protect cells from cell death. Rb dephosphorylation has been widely observed during apoptosis.9-12 In fact, the specific induction of Rb-directed phosphatase activity has been shown to be required for apoptosis to occur.13,14 It is likely that specific phosphorylation sites of Rb regulate apoptosis. For example, phosphorylation of specific sites such as Threonine-821 of Rb blocks the induction of apoptosis induced by UV radiation or the cdk inhibitor, Roscovitine.15 In addition, phosphorylation of Serine-807 of Rb promotes interaction with Bax, and inhibits apoptosis.16 Thus these studies suggest that Rb phosphorylation regulates cell death.
Cellular transition from the expression of epithelial to mesenchymal characteristics (EMT) is a process that occurs in embryonic development. In tumorigenesis, the EMT is associated with the development of invasive and metastatic capacity.17 A role for Rb in the regulation of the EMT has recently been proposed.18 In breast cancer cells, it was demonstrated that Rb depletion promotes a mesenchymal-like morphology with an invasive phenotype.19 Furthermore, experiments using mouse embryonic fibroblasts (MEFS) that lack Rb (and related family members p107 and p130) demonstrated that Rb loss promotes the EMT.20,21
Our previous studies have focused on the regulation of Rb phosphorylation by the phosphatase, PP1.13,23 Recently the idea has surfaced that phosphatases are rational targets in cancer.24 We have developed an alternate approach that targets Rb hyperphosphorylation in cancer cells. Specificity toward substrates is imparted onto PP1 by many different interacting proteins.25,26 In proliferating cells, PP1 is associated with a regulatory protein called PNUTS (Phosphatase Nuclear Targeting Subunit).27,28 Our previous experiments have shown that PNUTS inhibits PP1 activity toward Rb phosphorylation sites.29,30 Recently it was demonstrated that PNUTS blocks Rb binding to PP1, and therefore it was concluded that the PNUTS:PP1 complex is a cancer drug target.31 PNUTS dissociation from PP1, which permits Rb dephosphorylation, occurs due to changes in PKA mediated phosphorylation of PNUTS.32 We have found that siRNA mediated knockdown of PNUTS in breast, ovarian and colon cancer cell types leads to an increase in PP1 phosphatase activity toward Rb, Rb dephosphorylation on several sites, and a dramatic induction of apoptosis.13
In the current study our aim was to investigate the effect of Rb dephosphorylation in breast cancer cells grown in 3-dimensional (3D) structures reminiscent of in vivo tumor structure. Previous studies utilizing traditional 2-dimensional (2D) cell culture has provided a starting point for the understanding of pathways involved in carcinogenesis. However, more physiologically relevant data can be obtained using cells grown in 3D cell culture. In 3D epithelial culture, cells organize into structures that resemble the in vivo tissue.33,34 Nonmalignant breast epithelial cells grown on lrECM (laminin rich extracellular matrix) will form hollow spherical monolayers termed “acini” that resemble physiological structures while mammary tumor cells proliferate into disorganized masses.35 In this study, utilizing shRNA mediated knockdown of PNUTS to dephosphorylate Rb in breast cancer cells, we found the response was dependent on breast cancer cell type, and the results revealed a new role for Rb phosphorylation in the control of cancer cell EMT. In addition, we demonstrate that unphosphorylated Rb forms a complex with the transcription factor ZEB1, an important regulator of the EMT.
Results
Based upon our previous studies that showed targeting Rb phosphorylation in breast, colon and ovarian cancer cells led to an increase in apoptosis,13,23 in this study we sought to determine the effect of Rb dephosphorylation in breast cancer cells grown in 3D spheroids which more closely recapitulate the physiological structure of tumors. We utilized well-established methods of 3D Matrigel culture34,35 followed by immunofluorescence to show that phosphorylation of Rb is readily detected in 3D spheroids of MCF7 cancer cells but is absent in non-transformed MCF10A breast epithelial cells (Fig. 1A), similar to the situation in vivo. In order to activate phosphatase toward Rb in cells grown in 3D tumor spheroids in Matrigel, we generated cell lines expressing doxycyline-inducible lentiviral shRNA. For each cell type, viral transduction of both PNUTS shRNA and nontargeting shRNA was achieved. Experiments were performed in the presence or absence of Doxycyline (100ng/ml) stimulation, on cells infected with virus expressing either nontargeting (NT) or PNUTS shRNA. In MCF7 breast cancer cells, which are classified as luminal, we found PNUTS depletion caused a marked drop in cell number, likely due to an increase in apoptosis which was detected by TUNEL assays and by immunoblotting with antibodies to cleaved PARP (Fig. 1B and 1C). In addition, immunoblotting revealed that expression of PNUTS is reduced using this method and that Rb is dephosphorylated (loss of upper, slowly migrating band). In all experiments in this study, doxycycline addition to cells expressing nontargeting shRNA had no effect (data not shown). This induction of apoptosis is comparable to our previous observations using MCF7 cells grown in 2D cell culture.13
Figure 1.
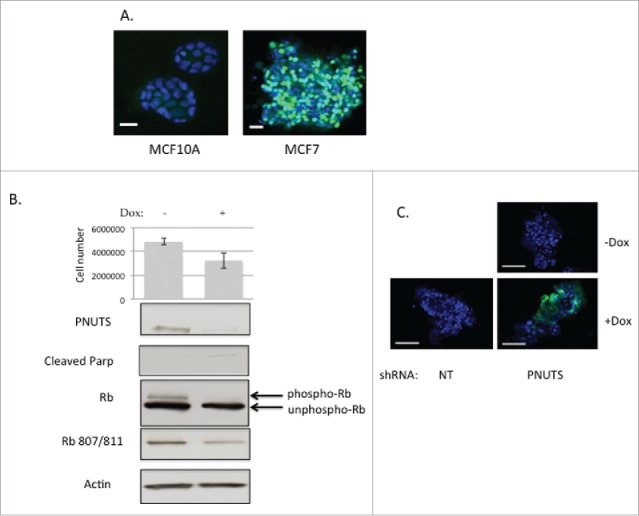
(A) Non-transformed MCF10A breast epithelial cells (left) and MCF7 breast cancer cells (right) were grown in Matrigel using published methods.34 After 10–12 d in culture, spheroids were analyzed using phosphospecific antibodies to Rb (Ser807/811) according to immunofluorescence protocols (Cell Signaling Technology). Green fluorescence shows phosphorylated Rb. Scale bar, 500 µm. (B) MCF7 cells expressing Dox-inducible PNUTS shRNA were grown in Matrigel cultures for approximately 12 days, +/−Dox stimulation. Cell number experiments were performed by the CellTiter-Glo 3D assay (Promega) with n = 12. Error bars represent standard deviation of the mean and data shown is representative of 5 independent experiments. Cellular protein was obtained from Matrigel cultures by Cell Recovery Solution (Corning). Phospho-Rb and unphospho-Rb are separated by differential migration on SDS-PAGE and are indicated by arrows. Cleaved Parp and Rb phosphorylated at S807/811 is shown. Equal protein loading is shown by Actin immunoblotting. (C) TUNEL assays (Roche) were performed using MCF7 cells grown in Matrigel 3D cell culture expressing NT (nontargeting) or PNUTS shRNA. Dox stimulation is indicated. Data shown is representative of 3 independent experiments.
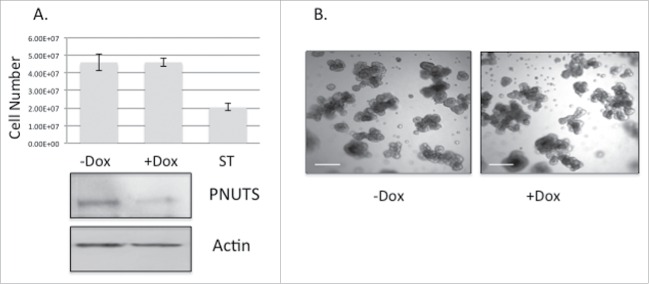
Experiments were then carried out using non-transformed MCF10A breast cancer cells grown in 3D cell culture. Because these cells do not contain hyperphosphorylated Rb like that found in cancer cells (Fig. 1A), the prediction is that PNUTS knockdown would have little effect. As shown in Fig. 2A, cell number is unaffected in MCF10A cells by targeting Rb by PNUTS depletion. Examination of the cell morphology of MCF10A cells grown in 3D culture shows that PNUTS depletion has little effect (Fig. 2B). These results suggest that the effect of PNUTS knockdown in 3D culture of breast epithelial cells is dependent on the presence of phosphorylated Rb. In another set of control experiments, we utilized the Rb-null breast cancer cell type MDA-MB-468. This cell line is considered invasive.37 In Fig. 3A we show that it is deficient in Rb phosphorylation and Rb expression. As revealed for MCF10A cells, this cell type devoid of phosphorylated Rb is unaffected by reduced expression of PNUTS (Fig. 3B, 3C).
Figure 2.
(A) MCF10A cells expressing Dox-inducible PNUTS shRNA were grown in 3D cultures for 12 days, +/− Dox, followed by immunoblotting for PNUTS and Actin. Cell number was determined by the CellTiter-Glo 3D assay (Promega). ST (1mM Staurosporine, 24 hr) was used as a positive control for cell death. Error bars represent standard deviation of the mean of n = 10 and the data shown is representative of 3 independent experiments. (B) Images of MCF10A cells grown in Matrigel 3D cell culture +/− Dox stimulation show cell morphology. Scale bar, 200µm.
Figure 3.
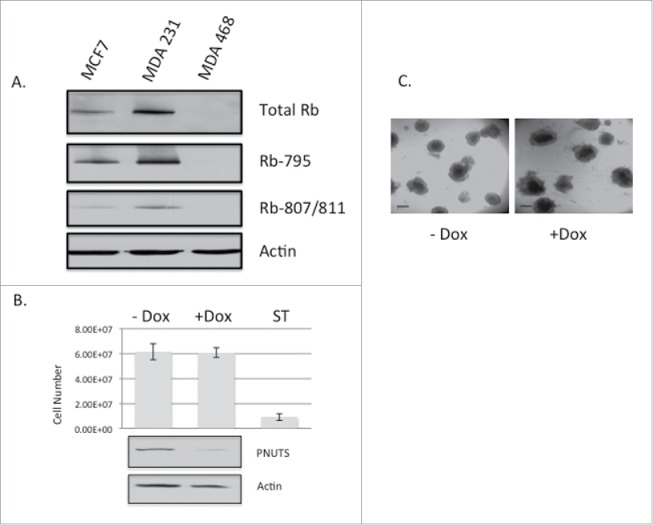
(A) Immunoblotting with Rb antibodies was performed on lysates from MCF7, MDA-MB-231, and MDA-MB-468 cell lines. Phosphospecific antibodies to Rb on S795 and S807/811 are indicated. Actin immunoblotting is shown as a loading control. (B) MDA-MB-468 cells expressing Dox-inducible PNUTS shRNA were grown in 3D cultures for 12 days, +/− Dox, followed by immunoblotting for PNUTS and Actin. Cell number was determined by the CellTiter-Glo 3D assay (Promega). ST (1mM Staurosporine, 24 hr) was used as a positive control for cell death. Error bars represent standard deviation of the mean of n = 10 and the data shown is representative of 3 independent experiments. (C) Images of MDA-MB-468 cells grown in Matrigel 3D cell culture +/− Dox stimulation show cell morphology. Scale bar, 200µm.
Finally in the invasive, Rb-positive breast cancer cell line MDA-MB-231 grown in 3D cell culture, PNUTS knockdown had an interesting effect. Although there was no decrease in cell number resulting from activation of Rb phosphatase as found in MCF7 cells, (data not shown) we observed a marked change in morphology in the cells from a stellate invasive morphology to a more rounded, less invasive appearance (Fig. 4A). In addition, immunoblotting showed dephosphorylation of Rb in response to PNUTS depletion, and changes in the expression of molecules involved in the epithelial-mesenchymal transition (EMT). In response to Rb dephosphorylation, cells exhibited reduced expression of the mesenchymal marker N-cadherin, with a concomitant increase the expression of the epithelial cell marker E-cadherin. Such a change in expression from mesenchymal to epithelial markers is associated with a decrease in invasive properties.38 We performed additional experiments using invasive Hs578T breast cancer cells grown in 3D cell culture. As we described for MDA-MB-231 cells, we observed no change in cell number due to PNUTS depletion in the Hs578T cells, however we did observe a change in morphology from an invasive to less invasive appearance (Fig. 4B). In addition, using these cells in 2D culture, immunoblotting revealed a decrease in expression of the mesenchymal marker N-cadherin in response to PNUTS depletion (Fig. 4C).
Figure 4.
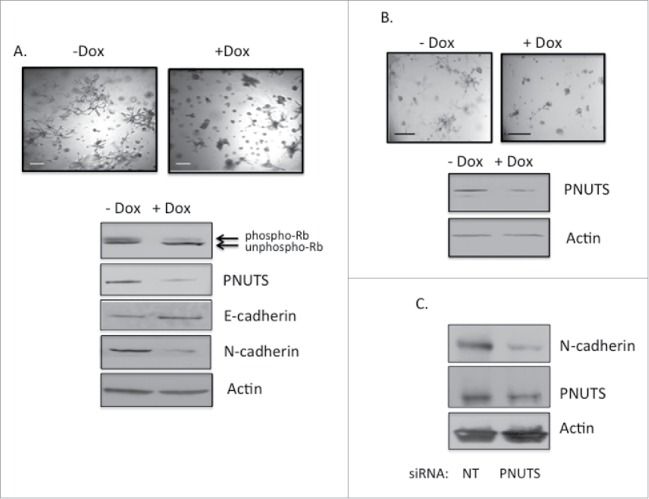
(A) MDA-MB-231 cells expressing Dox-inducible PNUTS shRNA were grown in 3D cultures for 12 days, +/− Dox, followed by immunoblotting for PNUTS, Actin, E- and N-cadherin. Phospho-Rb and unphospho-Rb are separated by differential migration on SDS-PAGE and are indicated by arrows. Images are shown of cells +/− Dox stimulation. Scale bar, 200µm. (B) Hs578T cells were grown and treated as in A. (C) Hs578T cells grown in 2D cell culture were subjected to PNUTS depletion13 and immunoblotting was performed. The data shown is representative of 2 independent experiments.
To determine whether targeting Rb phosphorylation by PNUTS depletion may effect the EMT of other cancer cell types, we utilized highly invasive HT1080 fibrosarcoma cells in subsequent experiments.39 These cells contain highly phosphorylated Rb (Fig. 5A) and in 3D cell culture exhibit an invasive appearance (Fig. 5B). PNUTS depletion was performed in cells grown in 3D cell culture using doxycycline-inducible shRNA as described for the breast cancer cell types above. Cell number was unaffected by PNUTS depletion in HT1080 cells (data not shown). However, dephosphorylation of Rb in these cells reduced the invasive appearance of these cells (Fig. 5B). Finally, a decrease in N-cadherin expression is observed in HT1080 cells grown in 2D cell culture in response to PNUTS depletion (Fig. 5C).
Figure 5.
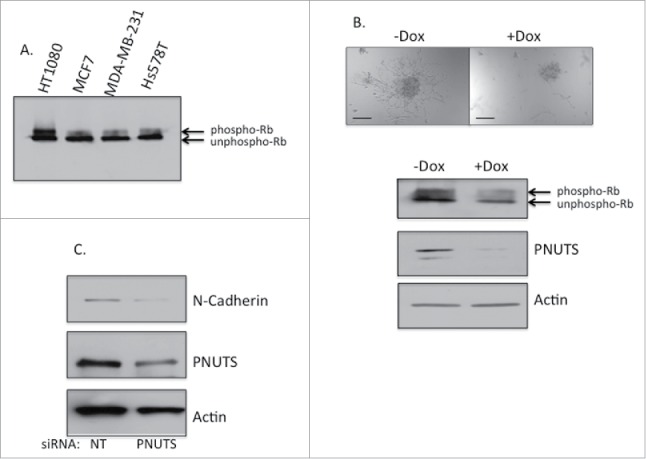
(A) Rb phosphorylation was assessed by immunoblotting in HT1080, MCF7, MDA-MB-231 and Hs578T cells. Phospho-Rb and unphospho-Rb are separated by differential migration on SDS-PAGE. (B) HT1080 cells expressing Dox-inducible PNUTS shRNA were grown in 3D cultures for 12 days, +/− Dox, followed by immunoblotting for PNUTS, Actin, and phospho-Rb and unphospho-Rb (indicated by arrows). Images are shown of cells +/− Dox stimulation. Scale bar, 200µm. (C) HT1080 cells grown in 2D cell culture were subjected to PNUTS depletion and immunoblotting was performed. The data shown is representative of 2 independent experiments.
The phosphorylation state of Rb regulates its association with several cellular proteins including the transcription factor E2F.5 Because targeting Rb phosphorylation by PNUTS depletion in the current study led to an increase in E-cadherin expression and a decrease in N-cadherin expression in the mesenchymal cell types, we sought to determine whether transcription factors that regulate the EMT could associate with Rb. First using mesenchymal MDA-MB-231 cells, we performed pull-down assays with GST-Rb fusion proteins and found that ZEB1, a zinc-finger E-box-binding transcription factor that regulates E- and N-cadherin expression, could bind to Rb (Fig. 6A). To detect endogenous complex formation between Rb and ZEB1 we utilized co-immunoprecipitation. We detected a complex between ZEB1 and dephosphorylated Rb in MDA-MB-231 cells subjected to PNUTS depletion (Fig. 6B). This result demonstrates that unphosphorylated Rb binds to ZEB1 and may regulate ZEB1 transcriptional activity. To explore this possibility we utilized reporter assays to measure transcriptional regulation of E-cadherin, which is repressed by ZEB1.40 Results of these experiments show an increase in E-cadherin promoter activity, due to the inhibition of ZEB1 in cells that contain dephosphorylated Rb (Fig. 7). In summary these data are consistent with a model in which dephosphorylation of Rb in response to PNUTS depletion leads to complex formation between Rb and ZEB1, inhibition of ZEB1 transcriptional repressor activity of E-cadherin, and an increase in E-cadherin expression that inhibits the EMT transition. Hyperphosphorylation of Rb widely observed in cancer cells would likely promote Rb-ZEB1 dissociation, and lead to stimulation of the EMT and invasion.
Figure 6.
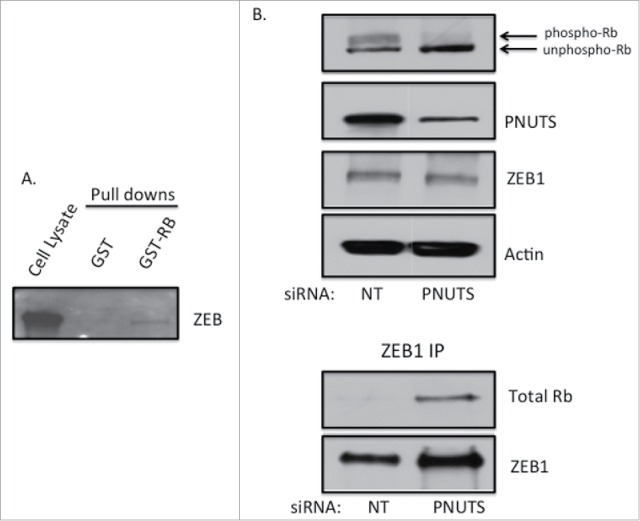
(A) Pull down assays were performed with GST alone or GST fused to full length Rb (GST-RB) as described previously.48 MDA-MB-231 cell lysate (500µg) was incubated with GST or GST-RB for 2 hours and associated proteins were collected using GST-sepharose. Immunoblotting with ZEB1 antibodies is shown. Data shown is representative of 3 separate experiments. (B) MDA-MB-231 cells were grown in 2D cell culture and subjected to PNUTS depletion. Co-immunoprecipitation was performed using ZEB1 antibodies using cells subjected to NT (nontargeting) siRNA or PNUTS siRNA followed by immunoblotting with antibodies to PNUTS, ZEB1 and Actin. Phospho-Rb and unphospho-Rb are separated by differential migration on SDS-PAGE and are indicated. Data shown is representative of 2 independent experiments.
Figure 7.
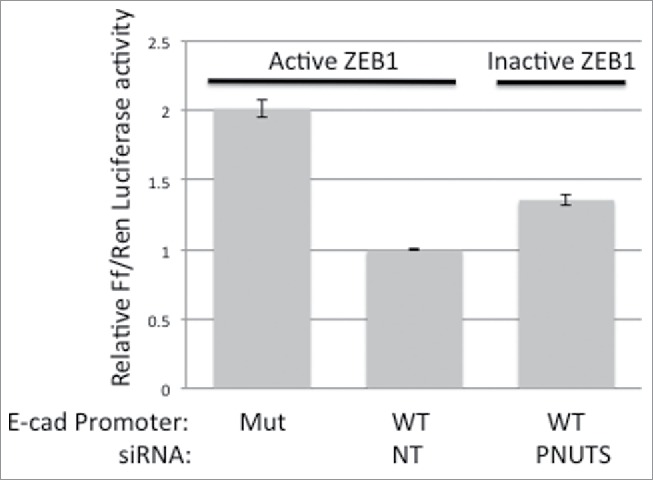
Reporter assays were performed using either WT or mutant E-cadherin promoter driven firefly luciferase expression constructs. proE-cad178-Luc (WT) contains the minimal region of the promoter required to exhibit activity and proE-cad178-Luc-mEbox (MUT) contains point mutations that abrogate promoter activity.40 MDA-MB-231 cells were transfected with firefly luciferase constructs and Renilla luciferase as an internal vector control. Two days later cells were subjected to PNUTS depletion using PNUTS or NT (nontargeting) siRNA. Relative firefly/Renilla Luciferase activity was measured using the Dual Luciferase assay (Promega). Activity at the WT cadherin promoter in cells transfected with nontargeting siRNA was normalized to one. Experiment shown is representative of 5 independent experiments with n = 4. Error bars represent standard deviation of the mean.
Discussion
Use of the 3D cell culture model may be predicted to yield relevant information regarding the true physiology of invasive tumors. This is the first study directly addressing the effect of Rb dephosphorylation on cancer cells grown in 3D cell culture. The data presented here suggests that targeting Rb phosphorylation in malignant cancer is a rational strategy to block invasiveness. The epithelial-mesenchymal transition (EMT) occurs when epithelial cells gain the ability to spread from the original tumor tissue via the expression of the mesenchymal genetic program. Cancer cells engage the EMT to promote invasiveness and metastasis.41 An important role for the tumor suppressor protein, Rb, in regulating the EMT in breast cancer was previously revealed using Rb and Zeb depletion experiments.18,19 In fact, it was shown that Rb and ZEB1 cooperate to promote tumor progression and invasion in the transformation of mouse embryonic fibroblasts by Ras.20,21 These previous studies utilized Rb knockout models to elucidate a role for Rb in the regulation of invasion. In the current study we focus on modulating the phosphorylation state of Rb to examine the effect of Rb activation in cancer cells. We compared the effect of Rb activation by driving its dephosphorylation, in breast cancer cells that are classified as epithelial (MCF7) or mesenchymal (MDA-MB-231 and Hs578T). Previous studies showed that depletion of the PP1 regulator PNUTS was a reliable method to target Rb phosphorylation in cells. PNUTS depletion increased PP1 activity toward Rb, Rb dephosphorylation and apoptosis in breast, colon and ovarian cancer cells grown in 2D culture.13,23 Here we utilized Matrigel 3D culture of cells to more closely recapitulate the physiology of a breast cancer tumor. In epithelial MCF7 cells, we found Rb dephosphorylation in cells grown in 3D caused the induction of apoptosis that was comparable to that observed in cells grown in 2D culture. However, using mesenchymal cell types (MDA-MB-231 and Hs578T) we found no effect on cell number due to Rb dephosphorylation, but a striking change in cellular morphology that occurred concomitant with changes in the expression of regulators of the EMT. These cell types are also classified as triple-negative (TNBC) due to the lack of expression of ER, PR, or HER-2/Neu amplification and represent the disease found in 10–15% of patients.42 These breast cancers are difficult to treat with agents that inhibit proliferation, our results here suggest that targeting invasiveness may be a clinically relevant approach in TNBC.
To demonstrate that the effect of PNUTS depletion on breast cancer cells grown in 3D cell culture was dependent upon modulation of the phosphorylation state of Rb, we utilized non-transformed breast epithelial cells (MCF10A) that have minimal phosphorylation of Rb in addition to Rb-negative MDA-MB-468 breast cancer cells (classified as TNBC). In both of these control experiments, PNUTS depletion had no effect on cell number or on the appearance of the cells. We found a similar dependence on Rb phosphorylation in our previous studies utilizing 2D cell cultures. In Rb-negative Saos-2 cells and nontransformed MCF10A breast epithelial and CCD-18Co colon epithelial cells PNUTS depletion had no effect on cell number and did not cause apoptosis.13 Another study showed that cell death induced by PNUTS depletion involved the PTEN tumor suppressor.43 However, as shown here and in our previous studies the ability of PNUTS depletion to effect cell number and/or induce cell death was shown to be dependent on Rb expression/phosphorylation. In addition, when comparing the 3 TNBC cell lines utilized in this study, in those that contain phosphorylated Rb (MDA-MB-231 and Hs578T) the EMT of the cells was affected, however in the Rb-negative MDA-MB-468 cells the EMT was unaffected.
To determine whether the effect of Rb dephosphorylation on the EMT may be operative in other types of invasive cancer cells, we utilized HT1080 fibrosarcoma cells that are classified as mesenchymal.39 Interestingly, we found these cells contain highly phosphorylated Rb which corroborates the finding that highly phosphorylated Rb is expressed in invasive projections of malignant melanoma.22 In these cells, we found that PNUTS depletion causes Rb dephosphorylation, a decrease in the mesenchymal marker N-cadherin, and a dramatic loss of invasive projections in cells grown in 3D cell culture.
Rb phosphorylation regulates its ability to bind with many cellular proteins.44 The most well studied example is that of Rb association with the E2F family of transcription factors that regulates proliferation at the G1-S transition.1 Dephosphorylated Rb (in early G1 phase) associates with and inhibits E2F S phase promoting gene expression. In late G1 phase, Rb phosphorylation promotes the release of E2F to allow transcription of genes required for progression into S phase. In this study, using PNUTS depletion to induce Rb dephosphorylation led to changes in the expression of the cadherins. Specifically, Rb dephosphorylation caused a decrease in expression of the mesenchymal marker N-cadherin and an increase in the expression of the epithelial marker E-cadherin, therefore we investigated the possibility that EMT transcription factors involved in regulating E- and N-cadherin expression, (Snail, Twist or ZEB1), may mediate the effect of Rb dephosphorylation on invasiveness in these cells.38 To do this we performed Rb binding assays and found that bacterially expressed (unphosphorylated) GST-Rb specifically bound to ZEB1 in cell lysates obtained from MDA-MB-231 cells. Furthermore by co-immunoprecipitation we confirmed endogenous complex formation between dephosphorylated Rb and ZEB1 in cells in which PNUTS was depleted. To our knowledge, these data are the first to demonstrate in vivo association of Rb with the transcription factor ZEB1. In response to Rb dephosphorylation, Zeb expression remained constant, only association of with Rb is facilitated. To determine whether Rb dephosphorylation could effect transcription of genes that regulate the EMT, we utilized reporter assays. These experiments support the concept that dephosphorylation of Rb in cancer cells causes association between Rb and ZEB1, leading to inhibition of ZEB1 transcriptional activity.
Recently, targeting Rb phosphorylation with the use of cdk inhibitors in the clinical setting has intensified.45 The cdk4 inhibitor Palpociclib has shown efficacy in breast cancer treatment via its effect on Rb phosphorylation.46,47 Our experiments target Rb phosphorylation not by inhibiting kinase activity toward Rb, but by activating phosphatase activity toward Rb. Activation of PP1 activity toward Rb by PNUTS depletion affects cells in a different way than cdk inhibition. For example, preclinical investigation of Palpociclib on 47 breast cancer cell lines grown in 2D cell culture showed a cytostatic effect on proliferation due to Rb dephosphorylation, but no induction of apoptosis.47 Interestingly, our experiments show that treatment of MDA-MB-231 cells grown in 3D culture with Palpociclib did not affect cell number or invasion (data not shown). In contrast, in several types of cancer cells, PNUTS depletion causes apoptosis, in only those cells that contain phosphorylated Rb.13 One plausible explanation to reconcile these findings is that cdk inhibition and PP1 activation target different Rb phosphorylation sites. Dephosphorylation of a subset of Rb phosphorylation sites may likely be required to trigger proliferation arrest versus apoptosis. Furthermore, the current study reveals an additional role of Rb phosphorylation in the regulation of cancer cell invasion. It is likely that dephosphorylation of specific sites of Rb regulate association with ZEB1 and subsequently the EMT. Elucidation of the exact Rb phosphorylation sites involved in the processes of proliferation, apoptosis and the EMT must wait further investigation.
Materials and methods
Cell culture
All cell lines utilized were obtained from ATCC and utilized within 4 months of receipt. Cell culture materials were obtained from Invitrogen unless otherwise indicated. MCF7, MDA-MB-231, MDA-MB-468 and HT1080 cells were grown in Dulbecco's modified Eagle's media (DMEM), supplemented with 10% fetal bovine serum (FBS), 100 U/ml Penicillin, 100 μg/ml Streptomycin (Pen/Strep) and 2mM glutamine. Hs578T cells were grown in DMEM media with 10% FBS, 0.01mg/ml Insulin and Pen/Strep. MCF10A cells were maintained in DMEM/F12, 5%HS, 20ng/ml EGF, 0.5µg/ml Hydrocortisone, 100ng/ml cholera toxin, 10µg/ml Insulin and Pen/Strep. Cells were routinely maintained at 37°C in a humidified, 5% CO2-containing atmosphere and were split 2–3 times weekly to maintain subconfluent cultures. Matrigel 3D cultures were generated according to published methods.34,36 Lentiviral Doxycycline-inducible shRNAs (Dharmacon) included the PNUTS target sequence: GAGTCGATGCACTTACTTG or nontargeting control sequences. Inducible vectors were transduced into each cell type and stable cell populations were generated by puromycin selection. Appropriate doxycycline concentration for shRNA expression was determined for each cell type and 100ng/ml was routinely used, given to cultures at 3 day intervals for 10–12 d. For all cell types experiments were conducted with cells stably transfected with nontargeting control sequences +/− Dox, which caused no effect on the cells. To guarantee rigor in these experiments, MCF7 cells expressing lentiviral PNUTS shRNA were generated twice, in newly purchased cells from ATCC each time. The results from replicate experiments were identical, conducted 6 months apart.
TUNEL, cell proliferation, reporter assays
For TUNEL assays, 3D cell cultures were grown for 12 d in Matrigel and treated +/−Dox. On day 12, cells were fixed with 4% PFA and washed 3x with PBS. Cells were permeabilized with 1% Triton-X solution and washed 3x with 1X PBS. TUNEL assay was conducted as instructed in the Roche In Situ Cell Death Detection, Fluorescein Kit protocol and incubated at 37° C for 1 hour in the dark. This was followed by 3 washes with 1X PBS and staining overnight with Cell Signaling Technology Prolong Gold Antifade with DAPI. Confocal microscopy imaging was performed the following day. Cell proliferation was measured in Matrigel 3D cultures using Cell Titer Glo-3D assay (Promega). Reporter assays were performed using MDA-MB-231 cells transfected using Fugene (Promega) according to the manufacturer's directions. All transfections were performed using 10 μg of total DNA/60 mm dish. The proE-cad178-Luc and proE-cad178-Luc-mEbox plasmids were gifts from Kumiko UiTei (Addgene plasmids 42081 and 42082).
Microscopy
Immunofluorescence was performed according to protocols from Cell Signaling Technology using antibodies to phospho-Rb 807/811. Microscopy was performed utilizing a Zeiss LSM 700 confocal microscope.
PNUTS depletion, immunoblotting, pull down assay, and immunoprecipitation
PNUTS knockdown in 2D cultures and immunoblotting was performed as described.13 Pull down assays were performed using GST or GST fused to full length Rb as described.48 In this study, we utilized the following primary antibodies: Rb (IF8, Santa Cruz Biotech) Phospho-Rb 807/811, Phospho-Rb 795, cleaved PARP, N-cadherin, E-cadherin, and ZEB1 (Cell signaling Technology), PNUTS (BD Bioscience) βActin (Sigma). Immunoblotting of lysates from cells grown in Matrigel was performed using Cell Recovery Solution (Corning) according to the manufacturer's directions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Aaron Steiner for assistance with confocal microscopy.
Funding
This work was supported by the National Cancer Institute of the NIH Award Number R15CA182723 awarded to NAK.
References
- 1.Burkhart DL, Sage J. Cellular mechanism of tumor suppression by the retinoblastoma gene. Nat Rev Cancer 2008; 8:671-82; PMID:18650841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittnacht S. The retinoblastoma protein- from bench to bedside. Eur J Cell Biol 2005; 84:97-107; PMID:15819393; http://dx.doi.org/ 10.1016/j.ejcb.2004.12.012 [DOI] [PubMed] [Google Scholar]
- 3.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell 2002; 2:103-12; PMID:12204530; http://dx.doi.org/ 10.1016/S1535-6108(02)00102-2 [DOI] [PubMed] [Google Scholar]
- 4.Stone A, Sutherland RL, Musgrove EA. Inhibitors of cell cycle kinases: recent advances and future prospects as cancer therapeutics. Crit Rev Oncog 2012; 17:175-98; PMID:22471707 [DOI] [PubMed] [Google Scholar]
- 5.Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev 2000; 14:2393-409; PMID:11018009; http://dx.doi.org/ 10.1101/gad.813200 [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ. Cancer Cell cycles. Science 1996; 274:1672-7; PMID:8939849; http://dx.doi.org/ 10.1126/science.274.5293.1672 [DOI] [PubMed] [Google Scholar]
- 7.Berndt N. Roles and regulation of serine/threonine-specific protein phosphatases in the cell cycle. Prog Cell Cycle Res 2003; 5:497-510; PMID:14593745 [PubMed] [Google Scholar]
- 8.Nelson DA, Krucher NA, Ludlow JW. High molecular weight protein phosphatase type 1 dephosphorylates the Retinoblastoma protein. J Biol Chem 1997; 272:4528-35; PMID:9020179; http://dx.doi.org/ 10.1074/jbc.272.7.4528 [DOI] [PubMed] [Google Scholar]
- 9.Jeon HS, Dracheva T, Tang SH, Meerzaman D, Fukuoka J, Shakoori A, Shilo K, Travis WD, Jen J. SMAD6 contributes to patient survival in non-small cell lung cancer and its knockdown reestablishes TGF-β homeostasis in lung cancer cells. Cancer Res 2008; 68:9686-92; PMID:19047146; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knudsen KE, Booth D, Naderi S, Sever-Chroneos Z, Fribourg AF, Hunton IC, Feramisco JR, Wang JYJ, Knudsen ES. Rb-dependent S-phase response to DNA damage. Mol Cell Biol 2000; 20:7751-63; PMID:11003670; http://dx.doi.org/ 10.1128/MCB.20.20.7751-7763.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payton M, Chung G, Yakowec P, Wong A, Powers D, Xiong L, Zhang N, Leal J, Bush TL, Santora V, et al.. Discovery and Evaluation of Dual cdk1 and cdk2 inhibitors. Cancer Res 2006; 66:4299-308; PMID:16618755; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-2507 [DOI] [PubMed] [Google Scholar]
- 12.Plastaras JP, Kim SH, Liu YY, Dicker DT, Dorsey JF, McDonough J, Cerniglia G, Rajendran RR, Gupta A, et al.. Cell Cycle dependent and schedule dependent antitumor effects of sorafenib combined with radiation. Cancer Res 2007; 67:9443-54; PMID:17909054; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1473 [DOI] [PubMed] [Google Scholar]
- 13.De Leon G, Sherry TC, Krucher NA. Reduced expression of PNUTS leads to activation of Rb-phosphatase and caspase-mediated apoptosis. Cancer Bio Ther 2008; 17:833-9; http://dx.doi.org/ 10.4161/cbt.7.6.5839 [DOI] [PubMed] [Google Scholar]
- 14.Dou QP, An B, Will PL. Induction of a retinoblastoma phosphatase by anticancer drugs accompanies p53-independent G1 arrest and apoptosis. Proc Natl Acad Sci U S A 1995; 92:9019-23; PMID:7568064; http://dx.doi.org/ 10.1073/pnas.92.20.9019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentine B, Antonucci L, Hunce R, Edwards J, Marallano V, Krucher NA. Dephosphorylation of threonine-821 of the retinoblastoma tumor suppressor protein (Rb) is required for apoptosis induced by UV and Cdk inhibition. Cell Cycle 2012; 11:3324-30.; PMID:22895174; http://dx.doi.org/ 10.4161/cc.21693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonucci LA, Egger JV, Krucher NA. Phosphorylation of the Retinoblastoma protein (Rb) on serine-807 is required for association with Bax. Cell Cycle 2014; 13:3611-7; PMID:25483096; http://dx.doi.org/ 10.4161/15384101.2014.964093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiery JP. Epithelial-mesenchymal tansitions in tumor progression. Nature Rev Cancer 2002; 2:442-54; http://dx.doi.org/ 10.1038/nrc822 [DOI] [PubMed] [Google Scholar]
- 18.Arima Y, Inoue Y, Shibata T, Hayashi H, Nagano O, Saya H, Taya Y. Rb depletion results in deregulation of e-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-to-mesenchymal transition. Cancer Res 2008; 68:5104-12; PMID:18593909; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5680 [DOI] [PubMed] [Google Scholar]
- 19.Arima Y, Hayashi H, Sasaki M, Hosonaga M, Goto TM, Chiyoda T, Kuninaka S, Shibata T, Ohata H, Nakagama H, et al.. Induction of ZEB Proteins by Inactivation of RB Protein Is Key Determinant of Mesenchymal Phenotype of Breast Cancer. J Biol Chem 2012; 287:7896-906; PMID:22262832; http://dx.doi.org/ 10.1074/jbc.M111.313759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Sanchez-Tillo E, Lu X, Huang L, Clem B, Telang S, Jenson AB, Cuatrecasas M, Chesney J, Postigo A, et al.. Sequential inductions of the ZEB1 transcription factor caused by mutation of Rb and then Ras proteins are required for tumor initiation and progression. J Biol Chem 2013; 288:11572-80; http://dx.doi.org/ 10.1074/jbc.M112.434951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Sanchez-Tillo E, Lu X, Huang L, Clem B, Telang S, Jenson AB, Cuatrecasas M, Chesney J, Postigo A, et al.. The ZEB1 transcription factor acts in a negative feedback loop with miR200 downstream of Ras and Rb1 to regulate Bmi1 expression. J Boil Chem 2014; 289:4116-25; http://dx.doi.org/ 10.1074/jbc.M113.533505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roesch A, Becker B, Meyer S, Hafner C, Wild PJ, Landthaler M, Vogt T. Overexpression and hyperphosphorylation of retinoblastoma protein in the progression of malignant melanoma. Mod Pathol 2005; 18:565-72; PMID:15502804; http://dx.doi.org/ 10.1038/modpathol.3800324 [DOI] [PubMed] [Google Scholar]
- 23.De Leon G, Cavino M, D'Angelo M, Krucher NA. PNUTS knockdown potentiates the apoptotic effect of Roscovitine in breast and colon cancer cells. Int J Oncol 2010; 36:1269-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoekstra E, Peppelenosch MP, Fuhler GM. Meeting report Europhosphatase 2015:Phosphatases as drug targets in cancer. Cancer Res 2016; 76:193-96; PMID:27309387; http://dx.doi.org/ 10.1158/0008-5472.CAN-15-2091 [DOI] [PubMed] [Google Scholar]
- 25.Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci 2010; 33:113-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolupaeva V, Janssens V. PP1 and PP2A phosphatases- cooperating partners in modulating retinoblastoma protein activation. FEBS J 2013; 280:627-43; PMID:22299668; http://dx.doi.org/ 10.1111/j.1742-4658.2012.08511.x [DOI] [PubMed] [Google Scholar]
- 27.Allen PB, Kwon YG, Nairn AC, Greengard P. Isolation and Characterization of PNUTs, a putative Protein Phosphatase 1 Nuclear Targeting Subunit. J Biol Chem 1998; 273:4089-95; PMID:9461602; http://dx.doi.org/ 10.1074/jbc.273.7.4089 [DOI] [PubMed] [Google Scholar]
- 28.Kreivi JP, Trinkle-Mulcahy L, Lyon CE, Morrice NA, Cohen P, Lamond AI. Purification and characterisation of p99, a nuclear modulator of protein phosphatase activity. FEBS Lett 1997; 420:57-62; PMID:9450550 [DOI] [PubMed] [Google Scholar]
- 29.Krucher NA, Rubin E, Tedesco VC, Roberts MH, Sherry TC, De Leon G. Dephosphorylation of Rb (Thr-821) in response to cell stress. Exp Cell Res 2006; 312:2757-63; PMID:16764854; http://dx.doi.org/ 10.1016/j.yexcr.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 30.Udho E, Tedesco VC, Zygmunt A, Krucher NA. PNUTS (Phosphatase Nuclear Targeting Subunit) inhibits retinoblastoma-directed PP1 activity. Biochem Biophys Res Comm 2002; 297:463-7; http://dx.doi.org/ 10.1016/S0006-291X(02)02236-2 [DOI] [PubMed] [Google Scholar]
- 31.Choy MS, Hieke M, Kumar GS, Lewis GR, Gonzalez-DeWhitt KR, Kessler RP, Stein BJ, Hessenberger M, Nairn AC, Peti W, et al.. Understanding the antagonism of retinoblastoma protein dephosphorylation by PNUTS provides insights into the PP1 regulatory code. Proc Natl Acad Sci U S A 2014; 111:4097-102; PMID:24591642; http://dx.doi.org/ 10.1073/pnas.1317395111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YM, Watanable T, Allen PB, Kim YM, Lee SJ, Greengard P, Nairn AC, Kwon YG. PNUTS, a protein phosphatase 1 (PP1) nuclear targeting subunit. Characterization of its PP1 and RNA-binding domains and regulation by phosphorylation. J Biol Chem 2003; 278:13819-28; PMID:12574161; http://dx.doi.org/ 10.1074/jbc.M209621200 [DOI] [PubMed] [Google Scholar]
- 33.Debnath J, Brugge JS. Modeling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer 2005; 5:675-88; PMID:16148884 [DOI] [PubMed] [Google Scholar]
- 34.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003; 30:256-68; PMID:12798140; http://dx.doi.org/ 10.1016/S1046-2023(03)00032-X [DOI] [PubMed] [Google Scholar]
- 35.Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin Cancer Biol 1995; 6:175-84; PMID:7495986; http://dx.doi.org/ 10.1006/scbi.1995.0021 [DOI] [PubMed] [Google Scholar]
- 36.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 2007; 4:359-65; PMID:17396127; http://dx.doi.org/ 10.1038/nmeth1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon LA, Mulligan KT, Maxwell-Jones H, Adams M, Walker RA, Jones JL. Breast cell invasive potential relates to the myoepithelial phenotype. Int J Cancer 2003; 106:8-16. [DOI] [PubMed] [Google Scholar]
- 38.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Bio 2014; 15:178-96; http://dx.doi.org/ 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benedict WF, Weissman BE, Mark C, Stanbridge EJ. Tumorigenicity of Human HT1080 Fibrosarcoma x Normal Fibroblast Hybrids: Chromosome Dosage Dependency. Cancer Res 1984; 44:3471-9; PMID:6744274 [PubMed] [Google Scholar]
- 40.Mazda M, Nishi K, Naito Y, Ui-Tei K. E-cadherin is transcriptionally activated via suppression of ZEB1 transcriptional repressor by small RNA-mediated gene silencing. PLoS One 2011; 6:e28688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Weinberg RA. Epithelial-Mesenchymal Transition: at the crossroads of development and tumor metasatasis. Dev Cell 2008; 14:818-29; PMID:18539112; http://dx.doi.org/ 10.1016/j.devcel.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 42.Chavez KJ, Garimmella SV, Lipkowitz S. Triple Negative Breast Cancer Cell Lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis 2010; 32:35-48; PMID:21778573; http://dx.doi.org/ 10.3233/BD-2010-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kavela S, Shinde SR, Ratheesh R, Viswakalyan K, Bashyam MD, Gowrishankar S, Vamsy M, Pattnaik S, Rao S, Sastry RA, et al.. PNUTS functions as a proto-oncogene by sequestering PTEN. Cancer Res 2013; 73:205-14; PMID:23117887; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giacinti C, Giordano A. Rb and cell cycle progression. Oncogene 2006; 25:5220-7; PMID:16936740; http://dx.doi.org/ 10.1038/sj.onc.1209615 [DOI] [PubMed] [Google Scholar]
- 45.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinase in cancer therapy. Nat Rev Drug Discovery 2015; 14:130-46; PMID:25633797; http://dx.doi.org/ 10.1038/nrd4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et al.. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole vs. letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16:25-35; PMID:25524798; http://dx.doi.org/ 10.1016/S1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 47.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desail AJ, Ginther C, Atefi M, Chen I, Fowst C, et al.. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11:25-35; http://dx.doi.org/ 10.1186/bcr2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krucher NA, Zygmunt A, Mazloum N, Tamrakar S, Ludlow JW, Lee MYWT. Interaction of the retinoblastoma protein (pRb) with the catalytic subunit of DNA polymerase delta (p125). Oncogene 2000; 19:5464-70; PMID:11114723; http://dx.doi.org/ 10.1038/sj.onc.1203930 [DOI] [PubMed] [Google Scholar]