ABSTRACT
Capsaicin (CAP) is the major pungent component of chili pepper and is being evaluated for use against numerous types of tumors. Although CAP is indicated to target multiple signaling pathways, exact mechanisms of how it disturb cancer cell metablism remain obscure. Recent studies revealed Sirtuin 1 (SIRT1) serves as a potential target of CAP in cancer cells, indicating a direct regulation of cancer cell histone acetylation by capsaicin. The present study evaluated the effect of CAP on gastric cancer (GC) cell lines to understand the mechanism of cell growth inhibition. The results showed that CAP could significantly suppress cell growth, while altering histone acetylation in GC cell lines. Further studies found that hMOF, a major histone acetyltranferase for H4K16, is central to CAP-induced epigenetic changes. Reduced hMOF activity was detected in GC tissues, which could be restored by CAP both in vivo and in vitro. These findings revealed an important role of hMOF-mediated histone acetylation in CAP-directed anti-cancer processes, and suggested CAP as a potential drug for use in gastric cancer prevention and therapy.
KEYWORDS: Anti-cancer, capsaicin, gastric cancer, H4K16 acetylation, MOF
Abbreviations
- GC
gastric cancer
- CAP
capsaicin
- HDAC
histone deacetylase
- HAT
histone acetyltransferases
- SIRT1
sirtuin 1
Introduction
Although the incidence of gastric cancer (GC) has decreased in recent years, it is still one of the most common cancer globally and the third-leading cause of the cancer deaths in year 2015.1 There are several risk factors for GC: Helicobacter pylori (H. pylori) and EBV infection, high-salt and low-vegetable diet, smoking, and chronic gastritis with intestinal metaplasia.2 According to Lauren's classification, approximately 95 % of GC are adenocarcinomas by histological phenotype as intestinal type, diffuse type and mixed type.3 Most GC patients are diagnosed at the advanced stage often accompanied with extensive invasion and lymphatic metastasis. Although different drugs are currently available for GC, the prognosis for the metastatic setting still remains poor.4 Unlike current pharmaceutical drugs that have single target and often result in relapse of cancer or drug resistance, natural compounds can target multiple signaling pathways that are deregulated in cancer cells.5 Published studies have shown the efficacy of natural compounds against different types of cancer, suggesting increasing intake of fruits and vegetables may serve as efficient and less toxic way for cancer prevention.6
Several recent studies have found that Capsaicin (CAP, 8-methyl-N-vanillyl-6-noneamide), a pungent alkaloid found in the plant genus Capsicum, inhibits cell proliferation and induces apoptosis in various GC cell lines, and it is widely accepted that CAP target multiple signaling pathways in GC cells, including ROS (reactive oxygen species) production, cell cycle arrest, influence of transcription factor expression, and change of growth/survival signal transduction pathways, such as NF-κB inactivation and EGFR/HER-2 pathway.7-11 More interestingly, it has also been suggested that CAP has carcinogenic and tumorigenic functions like a double-edged sword.12 Thus the complicated mechanisms involving in CAP's anti-cancer activity remain to be clarified.
Epigenetic mechanisms may be involved in many cellular processes by regulating gene expression and altering chromatin structure without altering gene sequences. Studies have indicated that many diseases, including cancer, is associated with abnormal epigenetic regulation.13 Epigenetic mechanisms controlling gene transcription are often involved in cell proliferation, differentiation, and survival and are casually linked with tumor development. Among all the epigenetic regulation pathways, histone acetylation is one of the first described epigenetic modifications related to carcinogenesis.14 Acetylation of the lysine residues on the N-terminal tails of histones H3 and H4 is generally associated with transcriptional activation.15
Recent studies revealed Sirtuin 1 (SIRT1), a deacetylase that regulates the deacetylation of both histone and non-histone proteins,16,17 serves as a potential target of CAP in cancer cells, indicating a direct regulation of cancer cell histone acetylation by CAP.18,19 However, whether or not CAP can affect epigenetic modifications in GC cells is still unknown. To address this issue, we use MGC-803 and SGC-7901 GC cells to explore the effects of CAP on histone modification. In this study, we present evidences for the first time that hMOF, a major histone acetyltranferase for H4K16, is central to the regulation of CAP-induced GC cell growth inhibition.
Results
HPLC-purified capsaicin showed inhibitory effect on cancer cell viability
In order to get purified capsaicin (CAP, Fig. 1A), we separated capsicum oleoresin. First, capsaicinoids including CAP and dihydrocapsaicin, were obtained by supercritical carbon dioxide extraction (Fig. 1B, upper panel). Next, semi-preparative HPLC was performed to yield a higher purity product of CAP (Fig. 1B, lower panel).
Figure 1.
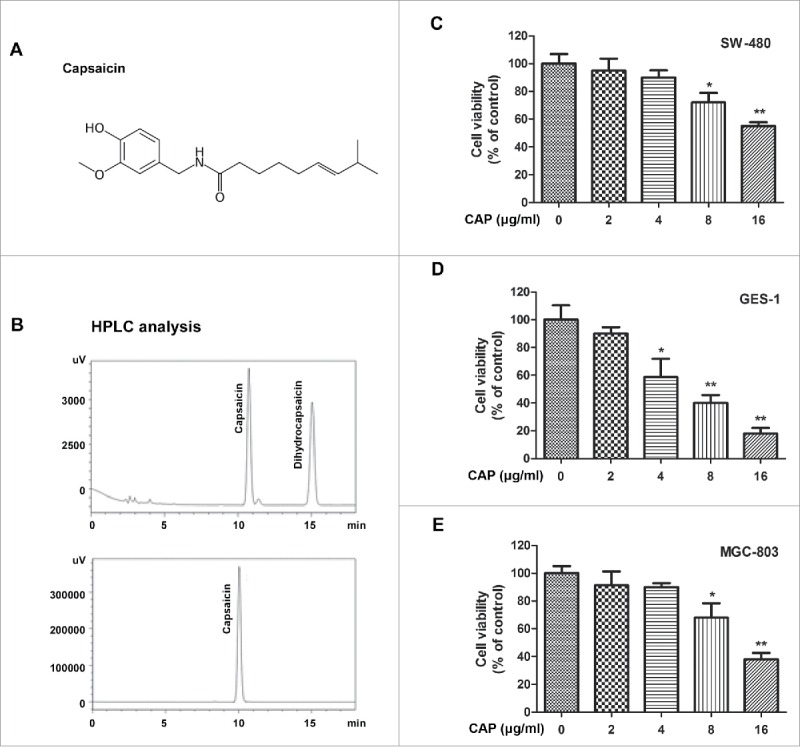
HPLC-purified CAP showed inhibitory effect on cancer cell viability. (A) Chemical formula for CAP. (B) HPLC analysis of CAP-containing products. Upper panel: capsaicinoids obtained by supercritical carbon dioxide extraction. Lower panel: Highly purified CAP product obtained by semi-preparative HPLC. (C-E) Cell viability of CAP-treated cancer cells. Cells were treated for 48 h with 0–16 μg/ml of CAP. Asterisk: Significant difference (*: p < 0.05, **: p < 0.01) compared to DMSO treatment.
To verify the cytotoxicity of CAP, we chose 3 different types of cell lines, colon cancer SW-480, gastric cancer MGC-803 and gastric mucosal GES-1 cells, treated with different amount of CAP for 48 hours, and measured cell viability through MTT assay. As expected, dose dependent cytotoxicity of CAP was detected in all the 3 cell lines examined (Fig. 1C-1E). Over forty percent of reduction rate was achieved by 16μg/ml of CAP treatment in 2 cancer cell lines (Fig. 1C and E). While on the other hand, noncancerous cells GES-1 presented intensive sensitivity to CAP treatment, 16 μg/ml of CAP eliminated 80 percent of all the living cells suggesting that CAP induced cytotoxicity effect was not specific for cancer cells only (Fig. 1D).
Capsaicin inhibited cell proliferation in gastric cancer cells
To further investigate the influence of capsaicin on cancer cell proliferation, culture image of CAP treated cells were recorded at different treating time point, and living cells stained by trypan blue were counted out to plot cell growth curves. As shown in Fig. 2A, CAP significantly inhibited proliferation of MGC-803 cells, the coverage of cells cultured in normal medium (∼50% after 24 hours and ∼80% after 48 hours) was reduced after adding different amount of CAP to the culture medium, highest inhibitory rate (∼50%) occurred among 8 μg/ml CAP treated cells. This inhibitory effect was further confirmed in cell growth curve that 96 hours of 10 μg/ml CAP treatment resulted in significant cell growth suppression in both MGC-803 (Fig. 2B) and HeLa cells (Fig. 2C).
Figure 2.
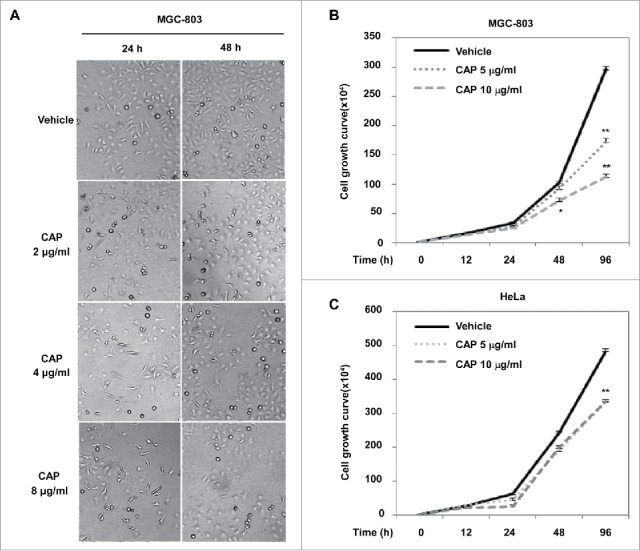
CAP inhibited cancer cell growth. (A) Photographs of MGC-803 cells grown in CAP-containing medium. (B and C): Cell growth curve of CAP-treated MGC-803 (B) and HeLa (C) cells. Vehicle: DMSO control treatment. Asterisk: Significant difference (*: p < 0.05, **: p < 0.01) compared to DMSO treatment.
In order to reveal the mechanism by which CAP suppress cell proliferation, we performed cell cycle analysis by FCM assay. Compared to DMSO-treated control group, CAP-treated groups had more cells accumulated in G1 phase (73.5% compared to 65.8% in SCG-7901 cells, Fig. 3A and C, and 66.8% compared to 52.1% in MGC-803 cells, Fig. 3B and D), indicating a CAP-induced G1 arrest may account for the inhibited cell proliferation.
Figure 3.
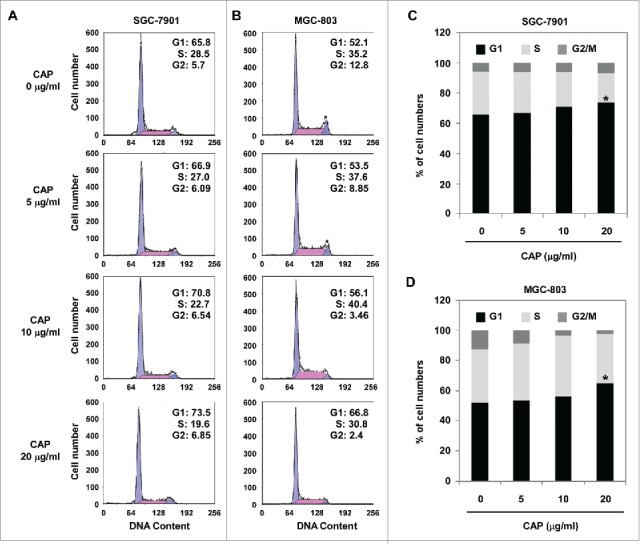
CAP induced cell cycle arrest in gastric cancer cells. SGC-7901 or MGC-803 cells were treated with 0–20 μg/ml of CAP for 48 h. (A and B) Histogram plot of cell cycle distribution. (C and D) Bar plot of cell cycle distribution. Asterisk: Significant difference (*: p < 0.05) compared to DMSO treatment.
Capsaicin affected epigenetic modifications in gastric cancer cells
Histone acetylation, driven by a set of histone modification enzymes that add or remove acetyl groups at specific lysine sites of the histone tails, is believed to be crucial for the control of gene expression during carcinogenesis. We have previously reported the reduction of hMOF in several kinds of cancer tissues, leading to down-regulated H4K16 acetylation. To further address whether the CAP-induced elimination of cancer cells depends on epigenetic histone modifications, we measured the levels of major histone modifications, including acetylation of H4K5, K8, K12 and K16, and methylation of H3K4. Results from western blot assay showed a significant enhancement of H4K16ac in MGC-803 cells treated with CAP (Fig. 4B, row 2), which was further confirmed in immunofluorescence assay (Fig. 4A, left column). Consistently, hMOF, as the responsible HATs for H4K16, was also strengthened by CAP treatment (Fig. 4B, row 1). Apart from the MSL complex, hMOF in human cells can form another complex, NSL complex, which is able to acetylate histone H4 not only at K16 but also at K5 and K8 sites.20 It was thus unsurprisingly to find H4K8ac level increased after CAP treatment (Fig. 4B, row 4). These altered acetyl modifications seem to rely on the acetyltransferase activity of hMOF since the major HDACs including HDAC1/2/4 and SIRT1 showed no obvious response to CAP (Fig. 4B, row 9–12). At last, an elevated H3K4me2 level was detected while monomethylation and trimethylation at the same site remained unchanged (Fig. 4B, row 6–8). This was also in line with our previous finding that hMOF-NSL complex crosstalk with MLL complex and interfere H3K4 methylation in HeLa cells.21
Figure 4.
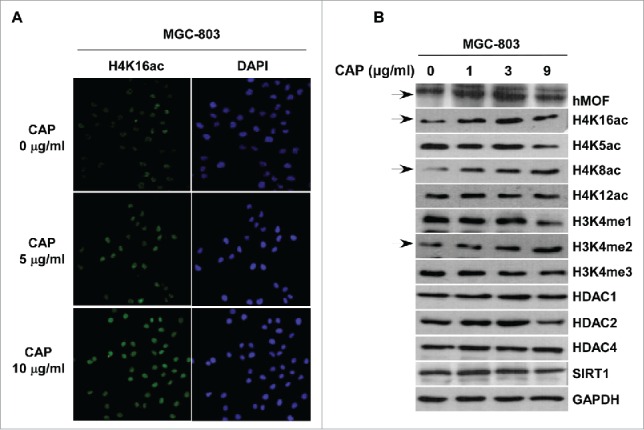
CAP affected hMOF-related epigenetic modifications in MGC-803 cells. MGC-803 cells were treated with 0–10 μg/ml of CAP for 24 h. (A) Immunofluorescent staining of hMOF (red signal, snapshot with the same exposure time). DAPI: nuclear indicator. (B) Western blot assay for cellular alterations of epigenetic modifications and related enzymes. Solid arrows indicate significant altered bands. GAPDH served as the loading control.
Capsaicin restored hMOF function in gastric cancer cells
Since hMOF and its mediated H4K16ac modification was altered by capsaicin treatment, we speculate that hMOF might be a major target of capsaicin in gastric cancer cells. We have previously reported a correlation of low expression of hMOF with clinicopathological features of various type of cancer including GC.22 To confirm the involvement of hMOF gene expression in the carcinogenesis of gastric cancer, we measured hMOF mRNA level using qPCR in 43 patients diagnosed with gastric cancer. Compared to matched normal tissues, the gene expression of hMOF was significantly decreased in gastric cancer tissues (p < 0.01, n = 43, Fig. 5B). As shown in Fig. 5A, hMOF gene expression was significant (> 2-fold decreased) downregulated in 86% (37/43) of patients, whereas only 2% (1/43) of patients showed significant (> 2-fold increased) upregulation of hMOF. In line with the tissue assay, hMOF gene expression was also depressed in gastric cancer cells (SGC-7901 and MGC-803) compared to normal gastric mucosal cells (GES-1), resulting in decreased level of H4K16ac (Fig. 5C). Moreover, the decreased H4K16ac could be rescued by hMOF restoration with extraneous hMOF-encoding plasmid, leading to a mild suppression of cancer cell viability (Fig. 5D). All these data suggested a reduced hMOF activity, which is important for cancer cell survival and might be reactivated by CAP treatment, prompting us to further investigate the underlying molecular mechanisms by which CAP triggered hMOF activation. Given the increased hMOF protein level we found in CAP treated MGC-803 cells (Fig. 4B, row 1), we thus designed a luciferase reporter assay to determine the direct regulation effect of CAP on hMOF gene expression. For this purpose, a luciferase reporter plasmid containing wild type promoter region of MYST1 gene, which encodes hMOF protein in human cells, was constructed (Fig. 6A, upper panel). In CAP treated MGC-803 cells, the luciferase activity regulated by Myst1 promoter region was significantly elevated in a dose dependent manner (Fig. 6A, lower panel), indicating a powerful activating effect of CAP on hMOF gene transcription through interacting with promoter region of MYST1 gene. In addition to its regulation on hMOF gene expression, CAP also presented capability to directly enhance acetyltransferase activity of hMOF. As shown in Fig. 6B, baculovirus expressed/purified hMOF protein catalyzed specific H4K16 acetylation in a well optimized in vitro HAT assay system with reconstituted histone octamer as substrate (Fig. 6B). However, when we fixed hMOF amount at a relative low level and added CAP to the system with increasing dosage, the H4K16ac became stronger along with the CAP addition (Fig. 6C).
Figure 5.
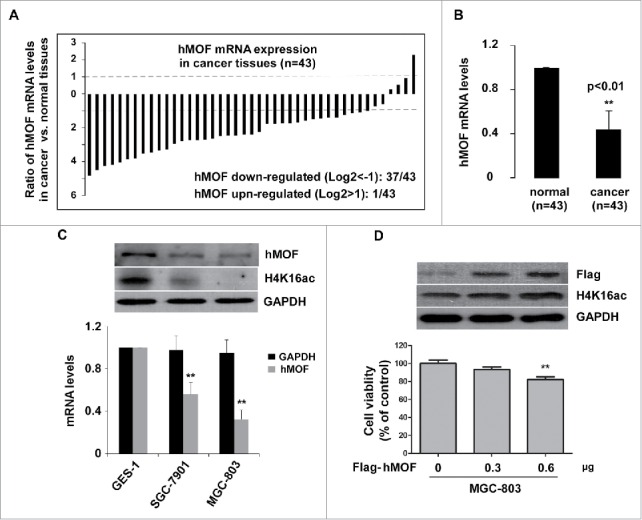
hMOF expression is down-regulated in gastric cancer tissues. (A) hMOF expression level in gastric cancer tissue compared to normal tissue of the same case (n = 43). mRNA level of hMOF was measured with Q-PCR method. Cases with more than 2 folds of alteration were counted. (B) Bar plot summarization of hMOF transcription level between gastric normal and cancer tissues. (C) Upper panel: whole cellular hMOF protein levels and H4K16ac levels in 3 gastric cell lines. Lower panel: Relative hMOF mRNA levels of gastric cancer cells (SGC-7901 and MGC-803) normalized to somatic gastric epithermal cells (GES-1). (D) Upper panel: restoration of H4K16ac in MGC-803 cells by exogenous hMOF (Flag-tagged). Lower panel: cell viability of hMOF-transfected MGC-803 cells. GAPDH served as loading control. Asterisk: Significant difference (**: p < 0.01) compared to normal tissues/noncancerous cells/control plasmid treatment.
Figure 6.
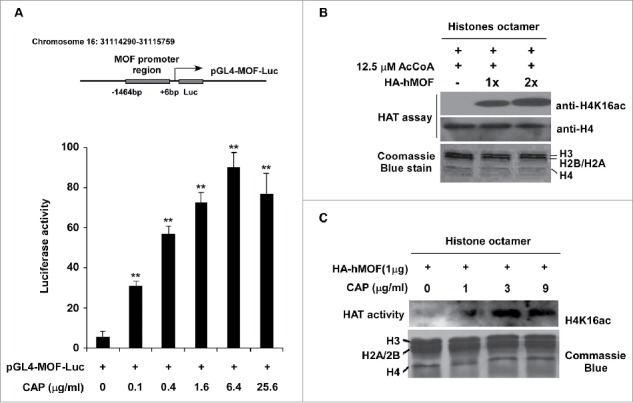
CAP restored hMOF function in MGC-803 cells. (A) Luciferase reporter assay of CAP-induced hMOF transactivation. Upper panel: schematic of the promoter region of MYST1 gene (−1464 to +6 bp) used for luciferase reporter assay. TSS: transcription start site. Lower panel: luciferase activity detected in CAP-treated MGC-803 cells. Twenty-four h after transfected with pGL4-MOF-Luc reporter plasmid, cells were treated with 0 to 25.6 μg/ml of CAP for 24 h before final measurement. (B) hMOF catalyzed H4K16 acetylization in vitro. Row 1 and 2 showed H4K16ac and H4 levels detected by specific antibodies. Row 3 showed the protein levels of major histones in the HAT assay system. (C) CAP stimulate the HAT activity of hMOF in vitro. 0–9 μg/ml of CAP was introduced into the HAT assay system to assess the stimulating effects of CAP on hMOF. Asterisk: Significant difference (**: p < 0.01) compared to DMSO treatment.
Discussion
As the major pungent chili pepper component, CAP is one of the most controversial phytochemicals, despite being well-studied. Even though widely consumed, CAP has a long and checkered history disputing whether its consumption or topical use is carcinogenic. Conflicting epidemiologic and basic research studies suggest that CAP could have a role in either preventing cancer or causing cancer.23 Hundreds of basic research studies show that CAP suppresses growth of numerous types of cancer cell, suggesting that it has chemopreventive activities.24-26 In contrast, epidemiologic studies seem to indicate that consumption of hot peppers, which contain variable levels of CAP, might be associated with an increased risk of cancer.27,28 Several recent studies with various cancer cell lines also suggest increased rate of carcinogenesis with the use of capsaicin.24,29,30 However, most of these studies have severe limitations and are mostly descriptive studies with speculative conclusions. Thus, a complete consensus as to whether the primary effect of CAP is cancer prevention or promotion has not yet been reached. It's been suggested that controversial results obtained from CAP-related toxicological studies may be attributed, to some extent, to the variability of CAP purification strategies.23 Many of the published toxicological researches on capsaicin relate to extracts of capsaicin derived from peppers, which are typically a mixture of capsaicin, norhydrocapsaicin, dihydrocapsaicin, homocapsaicin, homodihydrocapsaicin, and nonivamide. The actual percentage of capsaicin varies depending on the pepper source and method of extraction.31 Therefore, in this study, we adopted semi-preparative HPLC method to get highly purified CAP product (purity >99%, Fig. 1B) to avoid potentially toxic impurities. Although not clearly credited, CAP's anticancer effects and its molecular mechanism have still been extensively studied, indicating multiple signal pathways probably involved in CAP-related cancer inhibition. These potential CAP-regulated signal pathways or molecular targets include signal transducer and activator of transcription 3 (STAT3),32 nuclear factor-erythroid 2-related factor 2 (Nrf2),33 peroxisome proliferator-activated receptor-γ (PPAR-γ),34 epidermal growth factor receptor (EGFR),35 5′ adenosine monophosphate-activated protein kinase (AMPK),36 interleukin-6 (IL-6),37 and Cyclooxygenase (COX).38
The disruption of epigenetic changes, including DNA methylation, histone modifications and noncoding RNA-mediated silencing, has proved to underlies a wide variety of pathologies, including cancer.39 Thus, cancer is no doubt a multistep process derived from combinational crosstalk between genetic alterations and epigenetic influences through various environmental factors.40 Alterations in epigenetic processes, including chromatin modifications such as DNA methylation and histone acetylation, are common targets studied in cancer epigenomics.41 In this study, we first reported a positive regulatory effect of CAP on H4K16 acetylation through histone acetyltransferase hMOF in gastric cancer cells. Our results identified CAP as a dual-activator of hMOF, both promoting the transcription of MYST1 gene and stimulating the histone acetyltransferase activity of hMOF, leading to elevation of H4K16ac which occurred simultaneously with cancer cell cycle arrest and apoptosis. Moreover, noncancerous GES-1 cells, which possess much higher level of hMOF protein and H4K16ac than cancerous MGC-803 cells, presented more intensive sensitivity to CAP treatment, suggesting dependence of CAP-induced growth inhibition effect on the existence of high level of active MOF which works in a way of positive feed-back loop since CAP itself can reciprocally enhance MOF gene transcription and reactivate it's enzymatic activity. All these results indicate an important role of hMOF-mediated histone acetylation in CAP-directed anti-cancer processes. Nevertheless, in consideration of the fact that CAP acts on multiple targets relating to various signaling pathway, the actual interaction relationship between CAP and MOF may be more complicated than currently revealed.
As we all know, anti-cancer effect of most drugs fall into 2 major categories: non-genetic effects, such as DNA damage, metabolic inhibitory and tumor suppressor restore, and genetic effects that alter the gene expression pattern by epigenetic modifications thus switch the cancer cell to “scavengeable status.” Some spice-derived nutraceuticals have already been proved to induce epigenetic changes by regulating the activity of histone modification enzymes, these include curcumin, which has been identified as a strong inhibitor for DNA methyltransferase 1 (DNMT1) and p300/CREB-binding protein (CBP) in both in vitro and in vivo cancer models,42 and ursolic acid, which induces cell death in HL60 cells partially through increasing acetylation of histone H3 and inhibition of HDAC activity.43 It's also important to note that most intracellular targets of CAP reported are affected through gene expression regulation. Therefore, it's reasonable to speculate that MOF-mediated histone acetylation, as a major way that cell regulates its gene expression, may serve as a key signaling node altering downstream gene expression profile in response to CAP treatment. However, the dependency of CAP-induced anti-cancer activity on MOF and its related H4K16ac is not determined yet, and further studies are needed to clarify the relationship between CAP-triggered MOF activation and downstream cancer cell signaling pathways.
In summary, the results presented here suggest that CAP exerts its anti-cancer activity in gastric cancer cells partly by reactivating MOF and associated H4K16ac. These findings add a new member, MOF, to the targets of spice-derived nutraceuticals that influence epigenetic modifications, and suggest CAP as a potential drug for use in gastric cancer prevention and therapy.
Materials and methods
Antibodies
Anti-H4K16ac (H9164) and anti-M2 Flag antibodies were obtained from Sigma (USA). Anti-hMOF rabbit polyclonal antibody was from Bethyl Laboratories (A300-992A, USA). Anti-H3K4me1 (07–436), anti-H3K4me2 (07–030), anti-H3K4me3 (07–473), Anti-H4K5ac (07–327), anti-H4K8ac (07–328) and anti-H4K12ac (07–595) antibodies were purchased from Merck Millipore (Darmstadt, Germany). Anti-HDAC1 (10197-1-AP), anti-HDAC2 (12922-3-AP), anti-HDAC4 (17449-1-AP), and anti-SIRT1 (13161-1-AP) antibodies were from Proteintech Group (China, Wuhan). Anti-GAPDH rabbit polyclonal antibodies were raised against bacterially expressed proteins (Jilin University).
Purification of capsaicin
Capsicum oleoresin was provided by Jinta company (China). Capsaicinoids were extracted from capsicum oleoresin by supercritical carbon dioxide at 300 bar and 313 K as described.44 Capsaicin was purified by semi-preparative HPLC (MeOH-H2O, 65:35, v/v; tR 77.1 min) on Hitachi instrument (pump LC-2130, UV detector LC-2030) equipped with a YMC-Pack ODS-AM column (10 mm × 300 mm, 5 μm) with a flow rate 1.0 mL/min. After lyophilization the purified CAP was dissolved in DMSO for cell treatment.
Cell culture and transient transfection
Human gastric cancer cell lines SGC-7901 and MGC-803, human colon cancer SW-480 were obtained from Department of Gastrointestinal Surgery, the First Bethune Hospital of Jilin University. Human gastric mucosal cell line GES-1 was provided by the Cancer Hospital of Beijing University. Human cervical cancer cell line HeLa was obtained from ATCC (USA). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) with 5% glucose and 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. For transient transfection, MGC-803 cells were cultured in 6-well tissue culture plates (∼2 × 105 cells/well) and transfected with 0.3 and 0.6 µg of hMOF cDNAs using polyethylenimine (PEI). After 48 h of transfection, cells were harvested and lysed for protein gel blotting.
Tissue collection
104 tissue samples including 52 primary diagnosed gastric cancer and paired 52 normal tissues (> 5 cm away from the tumor area) from the same patients were collected. All patients underwent radical surgery between September 2008 and July 2013 at The First Bethune Hospital of Jilin University (Jilin, China) and did not receive any adjuvant therapy before the surgical operation. were collected from patients. Written informed consent was obtained from all participants, and the study was approved by the Institutional Ethics Board of School of Medicine, Jilin University.
Cell viability and growth assay
For viability assay, cells (2 × 103 cells/well) were seeded in 96-well plates and cultured overnight. Then, cells were treated with 0–16 μg/ml of capsaicin. After 48 h, cell proliferation and viability was determined by MTT assay. Upon termination of treatment, MTT was applied to each well (10 μl) at a final concentration of 0.5 mg/ml. After incubation for 2 h at 37°C, the supernatant was removed and 100 μl SDS was applied, and the MTT-formazan products were extracted. The absorbance was read at 570 nm using a 96-well microplate reader (BioTek, USA).
For growth assay, cells (1 × 105 cells/well) were seeded in 6-well plates and cultured overnight. Then, cells were treated with 0–10 μg/ml of capsaicin for up to 96 h. Cell images were captured at x200 magnification with an inverted microscope (Olympus, Tokyo, Japan) and cell growth curves were plotted by Trypan blue staining and cell counting (living cell number per well).
Cell cycle analysis
CAP-treated SGC-7901 and MGC-803 cells (1.0 × 106) were harvested and rinsed with PBS. The cell pellets were fixed in 70% ethanol at 4°C for 30 minutes. After washing twice with PBS, the cells were stained with 1.0 mL of PI solution (Dingguo Biotech, Beijing) containing 50 mg/L of PtdIns and 10 mg/L of RNase, followed by incubation on ice in dark condition for 30 minutes. The samples were then analyzed by FACS (Becton Dickinson, Franklin Lakes, NJ, USA).
Immunofluorescence staining
MGC-803 cells were cultured and grown to ∼60% confluence in 24-well plates containing a cover-slip (8D1007, Nest) on each well. Cells were washed by PBS buffer, and then fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature, permeabilized with 0.5% Tritonx-100 in PBS buffer for 5 min, followed by blocking with 1% bovine serum albumin in PBS for 1 h at 37°C. Sequentially, cells were washed for 5 min in PBST 3 times, and incubated with hMOF (1:500), H4K16ac (1:100) primary antibody at room temperature then stained with FITC-conjugated secondary antibody (1:300, Santa Cruz sc-2012). Cell nuclei were stained by Vectashield with DAPI (Vecter Laboraries, Inc., H-1200). Fluorescence images were observed with Olympus Bx40F Microscope (Olympus Corp.).
Western blotting
Whole-cell lysate from cultured cells was mixed with 4x SDS loading buffer (0.25 M Tris-HCl pH 6.8, 8% SDS, 30% glycerol, 0.02% Bromophenol Blue containing 10% BME), and boiled for 5 min at 95°C. 10–50 ug of denatured proteins were then separated by 12 or 18% SDS-PAGE and transferred to PVDF film. Specific proteins were detected using indicated antibodies. Signals were detected with a chemiluminescence scanner (ChemiScope 5300, Clinx Science Instruments Co., Ltd, China).
Reverse transcription and quantitative real-time PCR (qRT-PCR)
Total RNA from tissues (include tumor, or normal tissues) or cultured cells (include GES-1, SGC-7901 and MGC-803 cell lines) was isolated using TRIzol® LS Reagent (Invitrogen, Carlsbad, CA, USA). Total RNA (1 µg) from each sample was used as a template to produce cDNA with PrimeScript First-strand cDNA Synthesis kit (Takara). The resulting cDNA was analyzed by quantitative real-time PCR (qPCR) with an Eco Real-Time PCR System (Illumina, San Diego, CA, USA). All PCR reactions were finished as follows: initial denaturation step at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec and extension at 72°C for 30 sec. Primer sets used for PCR were as follows: hMOF, 5′-GGCTGGACGAGTGGGTAGACAA-3′ (forward) and 5′-TGGTGATCGCCTCATGCTCCTT-3′ (reverse), yielding a 227 bp product; GAPDH, 5′-GAGCCACATCGCTCAGACAC-3′ (forward) and 5′- CATGTAGTTGAGGTCAATGAAGG-3′ (reverse), yielding a 150 bp product;
Luciferase-reporter activity assay
Luciferase reporter was successfully constructed using molecular cloning technology. Target sequence for hMOF promoter region was obtained by PCR from genome DNA extract of HeLa cells. MGC-803 cells were seeded in 24-well plates for 24 h, after which they were transfected with 1 μg of Luciferase-reporter plasmids per well following CAP treatment. Luciferase activities were measured using the dual-luciferase-reporter gene assay kit (Promega) according to the manufacturer's instructions.
Histone acetyltransferase (HAT) assay
HAT assays were performed as described.45 Briefly, 40 μl reaction mixtures containing 50 mM Tris-HCl (pH8.0), 50 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, 5% (v/v) glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium butyrate, 12.5 μM cold acetyl coenzyme A (Sigma), 0.5 μg E.coli expressed and purified core histones, 0.5–1 μg baculovirus expressed and purified HA-hMOF were incubated at 30°C. After 60 min, the reaction was stopped by adding 4×SDS loading buffer (0.25 M Tris-HCl pH 6.8, 8% SDS, 30% Glycerol, 0.02% Bromophenol Blue containing 10% BME). Aliquots of reaction mixtures were subjected to 18% SDS-PAGE. Modified residues on histone H4 were detected by western blotting with acetylation-specific antibody, and the histone proteins were visualized by Coomassie R-250 blue staining.
Statistical analysis
Statistical analysis was achieved using GraphPad Prism 5 (La Jolla, CA, USA). Data are reported as the mean ± SEM. Statistically significant differences were determined by Mann-Whitney U test for gene expression between tumor and normal tissues, or by one-way ANOVA test for cell viability, growth and cell cycle assay. p < 0.05 was considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31571316), the Project of Jilin Province Science and Technology Development Program (20130206005YY and 20140413057GH) and the Open Foundation of Key Laboratory of the Ministry of Education in Yanbian University.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87-108; PMID:25651787; http://dx.doi.org/ 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K , Sasaki N, Schlemper RJ.. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345:784-9; PMID:11556297; http://dx.doi.org/ 10.1056/NEJMoa001999 [DOI] [PubMed] [Google Scholar]
- 3.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64:31-49; PMID:14320675 [DOI] [PubMed] [Google Scholar]
- 4.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE.. Chemotherapy for advanced gastric cancer. The Cochrane database of systematic reviews 2010:CD004064; PMID:20238327; http://dx.doi.org/ 10.1002/14651858.CD004064.pub3 [DOI] [PubMed] [Google Scholar]
- 5.Millimouno FM, Dong J, Yang L, Li J, Li X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev Res (Phila) 2014; 7:1081-107; PMID:25161295; http://dx.doi.org/ 10.1158/1940-6207.CAPR-14-0136 [DOI] [PubMed] [Google Scholar]
- 6.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal 2008; 10:475-510; PMID:18154485; http://dx.doi.org/ 10.1089/ars.2007.1740 [DOI] [PubMed] [Google Scholar]
- 7.Kim JD, Kim JM, Pyo JO, Kim SY, Kim BS, Yu R, Han IS. Capsaicin can alter the expression of tumor forming-related genes which might be followed by induction of apoptosis of a Korean stomach cancer cell line, SNU-1. Cancer Lett 1997; 120:235-41; PMID:9461043; http://dx.doi.org/ 10.1016/S0304-3835(97)00321-2 [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Kim JY, Lee SM, Jun CH, Cho SB, Park CH, Joo YE, Kim HS, Choi SK, Rew JS. Capsaicin induces apoptosis and modulates MAPK signaling in human gastric cancer cells. Mol Med Rep 2014; 9:499-502; PMID:24337453; http://dx.doi.org/ 10.3892/mmr.2013.1849 [DOI] [PubMed] [Google Scholar]
- 9.Meral O, Alpay M, Kismali G, Kosova F, Cakir DU, Pekcan M, Yigit S, Sel T. Capsaicin inhibits cell proliferation by cytochrome c release in gastric cancer cells. Tumour Biol 2014; 35:6485-92; PMID:24682934; http://dx.doi.org/ 10.1007/s13277-014-1864-6 [DOI] [PubMed] [Google Scholar]
- 10.Wang HM, Chuang SM, Su YC, Li YH, Chueh PJ. Down-regulation of tumor-associated NADH oxidase, tNOX (ENOX2), enhances capsaicin-induced inhibition of gastric cancer cell growth. Cell Biochem Biophys 2011; 61:355-66; PMID:21735133; http://dx.doi.org/ 10.1007/s12013-011-9218-0 [DOI] [PubMed] [Google Scholar]
- 11.Sarkar A, Bhattacharjee S, Mandal DP. Induction of Apoptosis by Eugenol and Capsaicin in Human Gastric Cancer AGS Cells–Elucidating the Role of p53. Asian Pac J Cancer Prev 2015; 16:6753-9; PMID:26434906; http://dx.doi.org/ 10.7314/APJCP.2015.16.15.6753 [DOI] [PubMed] [Google Scholar]
- 12.Surh YJ, Lee SS. Capsaicin in hot chili pepper: carcinogen, co-carcinogen or anticarcinogen? Food Chem Toxicol 1996; 34:313-6; PMID:8621114; http://dx.doi.org/ 10.1016/0278-6915(95)00108-5 [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis 2010; 31:27-36; PMID:19752007; http://dx.doi.org/ 10.1093/carcin/bgp220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archer SY, Hodin RA. Histone acetylation and cancer. Curr Opin Genet Dev 1999; 9:171-4; PMID:10322142; http://dx.doi.org/ 10.1016/S0959-437X(99)80026-4 [DOI] [PubMed] [Google Scholar]
- 15.Kurdistani SK, Grunstein M. Histone acetylation and deacetylation in yeast. Nat Rev Mol Cell Biol 2003; 4:276-84; PMID:12671650; http://dx.doi.org/ 10.1038/nrm1075 [DOI] [PubMed] [Google Scholar]
- 16.Stunkel W, Campbell RM. Sirtuin 1 (SIRT1): the misunderstood HDAC. J Biomol Screen 2011; 16:1153-69; PMID:22086720; http://dx.doi.org/ 10.1177/1087057111422103 [DOI] [PubMed] [Google Scholar]
- 17.Bosch-Presegue L, Vaquero A. Sirtuin-dependent epigenetic regulation in the maintenance of genome integrity. FEBS J 2015; 282:1745-67; PMID:25223884; http://dx.doi.org/ 10.1111/febs.13053 [DOI] [PubMed] [Google Scholar]
- 18.Pramanik KC, Fofaria NM, Gupta P, Srivastava SK. CBP-mediated FOXO-1 acetylation inhibits pancreatic tumor growth by targeting SirT. Mol Cancer Ther 2014; 13:687-98; PMID:24419059; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YH, Chen HY, Su LJ, Chueh PJ. Sirtuin 1 (SIRT1) deacetylase activity and NAD(+)/NADH ratio are imperative for capsaicin-mediated programmed cell death. J Agric Food Chem 2015; 63:7361-70; PMID:26255724; http://dx.doi.org/ 10.1021/acs.jafc.5b02876 [DOI] [PubMed] [Google Scholar]
- 20.Su J, Wang F, Cai Y, Jin J. The functional analysis of histone acetyltransferase MOF in tumorigenesis. Int J Mol Sci 2015; 17:99–116; PMID:26784169; http://dx.doi.org/24244196 10.3390/ijms17010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Su J, Wang F, Liu D, Ding J, Yang Y, Conaway JW, Conaway RC, Cao L, Wu D, et al.. Crosstalk between NSL histone acetyltransferase and MLL/SET complexes: NSL complex functions in promoting histone H3K4 di-methylation activity by MLL/SET complexes. PLoS Genet 2013; 9:e1003940; PMID:24244196; http://dx.doi.org/ 10.1371/journal.pgen.1003940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao L, Zhu L, Yang J, Su J, Ni J, Du Y, Liu D, Wang Y, Wang F, Jin J, et al.. Correlation of low expression of hMOF with clinicopathological features of colorectal carcinoma, gastric cancer and renal cell carcinoma. Int J Oncol 2014; 44:1207-14; PMID:24452485; http://dx.doi.org/ 10.3892/ijo.2014.2266 [DOI] [PubMed] [Google Scholar]
- 23.Bley K, Boorman G, Mohammad B, McKenzie D, Babbar S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol Pathol 2012; 40:847-73; PMID:22563012; http://dx.doi.org/ 10.1177/0192623312444471 [DOI] [PubMed] [Google Scholar]
- 24.Bode AM, Dong Z. The two faces of capsaicin. Cancer Res 2011; 71:2809-14; PMID:21487045; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-3756 [DOI] [PubMed] [Google Scholar]
- 25.Lin CH, Lu WC, Wang CW, Chan YC, Chen MK. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complement Altern Med 2013; 13:46; PMID:23433093; http://dx.doi.org/ 10.1186/1472-6882-13-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pramanik KC, Boreddy SR, Srivastava SK. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS One 2011; 6:e20151; PMID:21647434; http://dx.doi.org/ 10.1371/journal.pone.0020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serra I, Yamamoto M, Calvo A, Cavada G, Baez S, Endoh K, Watanabe H, Tajima K. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int J Cancer 2002; 102:407-11; PMID:12402311; http://dx.doi.org/ 10.1002/ijc.10716 [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Carrillo L, Hernandez Avila M, Dubrow R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. Am J Epidemiol 1994; 139:263-71; PMID:8116601 [DOI] [PubMed] [Google Scholar]
- 29.Liu NC, Hsieh PF, Hsieh MK, Zeng ZM, Cheng HL, Liao JW, Chueh PJ. Capsaicin-mediated tNOX (ENOX2) up-regulation enhances cell proliferation and migration in vitro and in vivo. J Agric Food Chem 2012; 60:2758-65; PMID:22353011; http://dx.doi.org/ 10.1021/jf204869w [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Li TZ, Xu GH, Luo BB, Chen YX, Zhang T. Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways. Neoplasma 2013; 60:364-72; PMID:23581408; http://dx.doi.org/ 10.4149/neo_2013_048 [DOI] [PubMed] [Google Scholar]
- 31.Johnson W. Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin. Int J Toxicol 2007; 26(Suppl 1):3-106; PMID:17365137; http://dx.doi.org/ 10.1080/10915810601163939 [DOI] [PubMed] [Google Scholar]
- 32.Bhutani M, Pathak AK, Nair AS, Kunnumakkara AB, Guha S, Sethi G, Aggarwal BB. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res 2007; 13:3024-32; PMID:17505005; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2575 [DOI] [PubMed] [Google Scholar]
- 33.Joung EJ, Li MH, Lee HG, Somparn N, Jung YS, Na HK, Kim SH, Cha YN, Surh YJ. Capsaicin induces heme oxygenase-1 expression in HepG2 cells via activation of PI3K-Nrf2 signaling: NAD(P)H:quinone oxidoreductase as a potential target. Antioxid Redox Signal 2007; 9:2087-98; PMID:17979524; http://dx.doi.org/ 10.1089/ars.2007.1827 [DOI] [PubMed] [Google Scholar]
- 34.Kim CS, Park WH, Park JY, Kang JH, Kim MO, Kawada T, Yoo H, Han IS, Yu R. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor gamma in HT-29 human colon cancer cells. J Med Food 2004; 7:267-73; PMID:15383218; http://dx.doi.org/ 10.1089/jmf.2004.7.267 [DOI] [PubMed] [Google Scholar]
- 35.Thoennissen NH, O'Kelly J, Lu D, Iwanski GB, La DT, Abbassi S, Leiter A, Karlan B, Mehta R, Koeffler HP. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010; 29:285-96; PMID:19855437; http://dx.doi.org/ 10.1038/onc.2009.335 [DOI] [PubMed] [Google Scholar]
- 36.Kim YM, Hwang JT, Kwak DW, Lee YK, Park OJ. Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann N Y Acad Sci 2007; 1095:496-503; PMID:17404062; http://dx.doi.org/ 10.1196/annals.1397.053 [DOI] [PubMed] [Google Scholar]
- 37.Kang JH, Kim CS, Han IS, Kawada T, Yu R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett 2007; 581:4389-96; PMID:17719033; http://dx.doi.org/ 10.1016/j.febslet.2007.07.082 [DOI] [PubMed] [Google Scholar]
- 38.Lee YS, Kwon EJ, Jin DQ, Park SH, Kang YS, Huh K, et al.. Redox status-dependent regulation of cyclooxygenases mediates the capsaicin-induced apoptosis in human neuroblastoma cells. J Environ Pathol Toxicol Oncol 2002; 21:113-20; PMID:12086397 [PubMed] [Google Scholar]
- 39.Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007; 128:683-92; PMID:17320506; http://dx.doi.org/ 10.1016/j.cell.2007.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther 2009; 8:1409-20; PMID:19509247; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0860 [DOI] [PubMed] [Google Scholar]
- 41.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet 2007; 8:286-98; PMID:17339880; http://dx.doi.org/ 10.1038/nrg2005 [DOI] [PubMed] [Google Scholar]
- 42.Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes & nutrition 2011; 6:93-108; PMID:21516481; http://dx.doi.org/ 10.1007/s12263-011-0222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen IH, Lu MC, Du YC, Yen MH, Wu CC, Chen YH, Hung CS, Chen SL, Chang FR, Wu YC. Cytotoxic triterpenoids from the stems of Microtropis japonica. J Nat Prod 2009; 72:1231-6; PMID:19534471; http://dx.doi.org/ 10.1021/np800694b [DOI] [PubMed] [Google Scholar]
- 44.Catchpole OJ, Grey JB, Perry NB, Burgess EJ, Redmond WA, Porter NG. Extraction of chili, black pepper, and ginger with near-critical CO2, propane, and dimethyl ether: analysis of the extracts by quantitative nuclear magnetic resonance. J Agric Food Chem 2003; 51:4853-60; PMID:12903935; http://dx.doi.org/ 10.1021/jf0301246 [DOI] [PubMed] [Google Scholar]
- 45.Cai Y, Jin J, Swanson SK, Cole MD, Choi SH, Florens L, Washburn MP, Conaway JW, Conaway RC. Subunit composition and substrate specificity of a MOF-containing histone acetyltransferase distinct from the male-specific lethal (MSL) complex. J Biol Chem 2010; 285:4268-72; PMID:20018852; http://dx.doi.org/ 10.1074/jbc.C109.087981 [DOI] [PMC free article] [PubMed] [Google Scholar]


