ABSTRACT
Tumor immunologic microenvironment is strongly involved in tumor progression and the presence of tumor infiltrating lymphocytes (TIL) with different phenotypes has been demonstrated to be of prognostic relevance in different malignancies. We investigated whether TIL infiltration of tumor tissues could also predict the outcome of prostate cancer patients. To this end, we carried out a retrospective analysis correlating the outcome of locally advanced prostate cancer patients undergone salvage radiotherapy upon relapse after radical surgery with the infiltration by different TIL populations. Twenty-two patients with resectable prostate cancer, with a mean age of 67 (+/−3.93) years, who received salvage radiotherapy with a mean of 69.66 (+/− 3.178) Gy in 8 weeks, between June 1999 and January 2009 and with a median follow up of 123 (+/− 55.82) months, were enrolled in this study. We evaluated, by immunohistochemistry, the intratumoral (t) and peripheral stroma (p) infiltration by CD45, CD3, CD4, CD8, CCR7, FoxP3 or PD-1-positive cells on tumor samples taken at the diagnosis (d) and relapse times (R). We correlated these variables with patients' biochemical progression free survival (bPFS), post-radiotherapy progression free survival (PFS), and overall survival (OS). Substantial changes in the rate of TIL subsets were found between the first and the second biopsy with progressive increase in CD4, CCR7, FoxP3, PD-1+ cells. Our analysis revealed that higher CD8p,R+ and lower PD-1R+ TIL scores correlated to a longer bPFS. Higher CD8p,R+ and CCR7t,R+ TIL scores and lower CD45p,R+ and FoxP3p,R+ TIL scores correlated to a prolonged PFS and OS. These results suggest that the immunological microenvironment of primary tumor is strictly correlated with patient outcome and provide the rationale for immunological treatment of prostate cancer.
KEYWORDS: Chemokyne Receptor 7, disease-free survival, FoxP3, overall survival, prostate cancer, PD-1, radiotherapy, prognosis, tumor infiltrating lymphocytes, T regulators lymphocytes
Introduction
Prostate cancer (PC) is the most common malignancy among men worldwide, associated to a high morbidity and mortality rate on the long term.1 At present, the best therapeutic option for the loco-regional disease is represented by radical prostatectomy (RP) and/or radiotherapy (RT) eventually followed by androgen blockade.2 RT is often delivered after RP, either in the adjuvant setting for PC patients at high risk for local relapse (LR), or consequently to LR occurring after wait-and-watch policy (salvage RT; SRT). The latter is frequently followed by biochemical relapse (BR) defined by PSA value above 0.2 ng/ml, which often precedes of almost 5 years the clinical onset and imaging evidence of recurrence. Adjuvant RT, usually delivered on a standardized elective volume, including the surgical bladder-urethral anastomosis, has been shown to be more effective than wait-and-watch option with SRT at the occurrence of local relapse in achieving a better therapeutic outcome in 3 different randomized trials.3-5 However, in common practice, SRT is often adopted at relapse but it is also employed in the absence of histological confirmation of recurrence for patients whose PSA value increases by 0.2 ng/ml without instrumental evidence of distant metastases and involvement of locoregional nodes. Although SRT allows to achieve a satisfactory long-term overall survival (OS) of 13.6 years, a second BR may occur in 50% of patients.6 Therefore, it is critical to identify patients undergoing SRT that necessitate additional treatments. Analysis of the efficiency of second or third line hormonal treatment for PC patients relapsed after hormone therapy, revealed the effectiveness of nonsteroidal antiandrogens as alternative therapies, particularly for second line responders, whose survival was significantly higher than nonresponders, indicating a potential predictive prognostic value for second line responsiveness and supporting the concept that second line responders, although androgen independent, are still hormonally sensitive.7,8 Taking into account that hormone manipulation and chemotherapy in this case may achieve only a transient therapeutic result, while reducing patients' quality of life, the research of new markers able to identify high-risk patients who really need these treatments is strongly required.
Immunological manipulation has proven to induce a severe immune response against several tumors, with encouraging results especially in combination with other drugs or therapeutic agents. This is the case of human recombinant IL-2 plus Lanreortide, whose effectiveness in the treatment of MTC was described in in vitro, in vivo and clinical studies by Vitale et al.9 Interestingly, the combination of low-dose IL-2 and Lanreotide was able to induce valuable responses in advanced and symptomatic MTC patients refractory to previous treatments.9 The same authors also demonstrated the synergistic antiproliferative activity of combined IFN-β and troglitazone treatment on pancreatic cancer cell lines10 and the antitumor activity of IFN-β in human neuroendocrine cancer cells, mainly counteracting the IGF-II autocrine/paracrine growth loop.11 The current development of cancer immunotherapy and the relative success of immune-check point inhibitors such as Ipilimumab (anti-CTLA-4) and Nivolumab/Pembrolizumab (anti-PD-1) in the treatment of different malignancies has prompted the design of multiple studies aimed to test this kind of treatment also in PC patients.1,12,13 At this aim, it has already been shown that PC cells can be recognized and killed by activated cytotoxic T lymphocyte (CTL) precursors sensitized to recognize PTHrP- or PSA-derived epitopes in vitro and in vivo.14,15 It has also been shown that several vaccine constructs derived from these antigens may be used to sensitize PBMCs derived from PC patients and are under investigation in clinical trials enrolling PC patients.14,15 On the other hand, there is poor knowledge concerning the immunological microenvironment associated with a better outcome in PC patients. The results of several studies have already shown that occurrence of autoimmunity, a systemic chronic inflammation profile, as well as the level of tumor infiltration by different lymphocyte subsets and macrophages, may indeed predict the outcome of patients with different disease including non small cell lung cancer (NSCLC), colorectal carcinoma (CRC), breast cancer, melanoma and other malignant diseases.16-25 In particular, the results of previous studies by our group showed that primary tumor infiltration by immune-regulatory T cells (CD25+FoxP3+, namely Tregs) and/or central memory T cells (CD8+CD45RA−CCR7+, namely Tcm) are associated to a prolonged PFS and OS in patients with metastatic colorectal carcinoma undergone frontline chemotherapy.26-28 On these bases, we investigated whether infiltration of the primary tumor by different lymphocyte subsets may predict the outcome of PC patients. In order to avoid possible interferences and biases related to frequent cancer co-morbidities, multiple medications, aging, and massive bone marrow infiltration associated with metastatic prostate cancer, we focused our retrospective study to a well-defined PC patient population, already treated with radical prostatectomy, with a good performance status, who received RT upon BR. We also took advantage by the fact that for this selected patient population we had availability of tumor tissues derived from biopsies performed at the time of diagnosis (d) and BR (r) (before RT). Therefore, we performed an immune-histochemical analysis on these tissues by scoring the rate of tumor infiltrating lymphocytes (TIL) positive for the expression of CD45, CD3, CD4, CD8, CCR7, FoxP3 or PD-1 inside the tumor (t) and in its periphery (p). Subsequently, these scores were statistically correlated with patients' biochemical progression free survival (bPFS, before RT), progression free survival (PFS) and overall survival (OS).
Patient and methods
Patient characteristics
This retrospective study includes 22 patients, among those referred for SRT for BR after prostatectomy form June 1999 to January 2009, whose minimal local relapse was confirmed by an ultrasound-guided biopsy. In these patients, local nodal involvement and systemic tumor progression were enrolled before RT. These patients had undergone the first diagnostic biopsy from 1985 to 2004, before prostatectomy, in our institution, and both the biopsy samples of each patient were available. Conversely, in the majority of these cases the prostatectomy was performed elsewhere. Therefore, any evaluation here reported is based on trans-perineal needle core-biopsy performed before prostatectomy (d) and after BR (r), just before the SRT was performed. Mean age at enrollment for BR was 67 (+/− 3.93) years while the other features of the patients enrolled in the study are reported in Table 1. All the patients received SRT in our center, with a mean dose of 69.66 (+/−3.178) Gy, delivered with a 6-15 MeV linear accelerator photon beams and 3-dimensional or intensity-modulation techniques, over 7/8 weeks.29 Pelvic MRI findings (available in any case) were also considered, while no hormonal manipulation was administered, unless further PC progression was diagnosed after SRT. All these patients were included in a follow-up program, consisting of periodical (q. Six months) physical examination (including rectal palpation), serum PSA dosage, blood count and chemistry, and imaging studies. bPFS represented the time between the 2 subsequential biopsies performed at diagnosis and at the BR time. Outcome analyses were based on standard progression-free survival (PFS) and overall survival (OS) since they completed SRT.
Table 1.
Characteristics of the patients series.
| Patients' Characteristics | Mean | Standard deviation | Frequency |
|---|---|---|---|
| Age | 67.00 years | 3.93 years | |
| Gleason Score | |||
| 6 | 6 (27%) | ||
| 7 | 14 (64%) | ||
| 8 | 2 (9%) | ||
| TNM | |||
| T2 | 13 (59%) | ||
| T3N0 | 6 (27%) | ||
| T3N1 | 3 (14%) | ||
| PSA (pre-irradiation) | 2.60 | 2.57 | |
| Radiation Therapy Dose | 6966 cGy | 455 cGy | |
| Follow up (months) | 123 months | 55.82 months |
TNM: TNM Classification of Malignant Tumors; T: primary tumor; N: regional lymph nodes; M: distant metastasis.
Pathology study
Tumor tissue samples were fixed in 10% buffered formalin and embedded in paraffin. From each block, 4 μm thick sections had been cut and stained with haematoxylin and eosin (H&E). All the slides were independently reviewed by 2 expert uropathologists (MTdV, and MRA). Tumor grading was established according to updated Gleason grading system.30
Immunohistochemistry
The most representative tumor blocks were selected on the basis of the morphological features and the Gleason score. Immunohistochemical stainings were performed on 4 ± 0.5 μm thick sections of each block employing the Ultravision Detection System Anti-poly-valent HRP (Ultra V Block) (LabVision, Fremont, CA, USA, Bio-Optica). All the procedures were carried out automatically by using the Bond-III machine. Slides were incubated with anti-human CD45 (ready to use-RTU, Novocastra, Firenze, Italy, CD3 (RTU, Menarini, Firenze, Italy), CD4 (RTU, Menarini, Firenze, Italy), CD8 (RTU, Dako, Milan, Italy), CCR7 (dilution: 1:50, RD Systems, Minneapolis USA), FoxP-3 (dilution: 1:50, Abcam, Cambridge, UK), PD-1 (dilution: 1:50, Dianova, Hamburg, Germany) antibodies, and the reaction revealed using DAB (Dako, Milan, Italy) as chromogen. Sections were weakly counterstained with Harris' haematoxylin and examined under a light microscope. Non-immune serum immunoglobulins were used as negative control. Adequate positive controls were added along the respective antibody. Immune staining was examined with a Zeiss Axioplan 2 microscope (Carl Zeiss Microscopy, Jena, Germany).
Staining assessment
The samples were independently evaluated and scored by 2 investigators (BJR and AB). Five different fields (at least 200 cells/field) were evaluated at ×400 magnification (i.e. high power field). In PC samples, protein expression level of all the antibodies was evaluated and recorded both in the tumor area (t) and in non-neoplastic peripheral area (p). The agreement between the 2 pathologists was about 90%. Cases with discrepancies were reviewed and discussed to reach 100% concordance. CD45, CD3, CD4, CD8, CCR7, FoxP3 and PD-1 positive TILs were scored as it follows: 0 = no positive lymphocytes; 1 = 1-10 positive lymphocytes; 2 = 11-20 positive lymphocytes; 3 = > 21 positive lymphocytes as reported elsewhere.26
Statistical analysis
We carried out a Wilcoxon signed Rank test to compare the differences among TIL scores between the 2 consecutive biopsies. Subsequently, the Pearson's test was used to correlate these scores to known PC prognostic factors such as disease stage, PSA value at BR and Gleason's score in biopsies performed at the time of diagnosis (d) and BR (r) (before RT). In order to perform a survival analysis we divided the different TIL scores into 2 subgroups with low (A) and high (B) infiltration rate, according to their respective median value. Kaplan Meier's method and Log-Rank test were used to evaluate bPFS, PFS and OS and correlate them with patients' associated variables. All analyses were performed using SPSS statistical package, version 17.0.
Results
Patients' characteristics
This study included 22 PC patients (Table 1) who presented a pre-irradiation median PSA value of 2.60 (+/− 2.62) ng/dl with an average age of 67 (+/− 3.93) years and a median follow up of 123 (+/− 55.82) months. Along the follow up, 8 patients experienced disease progression and 5 died.
Immune-infiltrate differences between tumor tissues at the diagnosis and relapse
Our immunohistochemical analysis first scored the rates of infiltration of TILs expressing CD45, CD3, CD4, CD8, CCR7, FoxP3 and PD-1 positive phenotype in tumor samples taken at the diagnosis and at the biochemical relapse. By comparing the 2 subsequent biopsies, we found a statistically significant progressive increase in the rate of TIL expressing CD45(t/p) (p < 0.001); CD3(t/p) (p < 0.002); CD4(t) (p < 0.001); CD8(t) (p = 0.013); CCR7(t) (p < 0.001), CCR7(p) (p = 0.038); FoxP3(t/p) (p < 0.025) and PD-1 (p = 0.034) (Fig. 1). No significant differences were observed for the other evaluated parameters.
Figure 1.
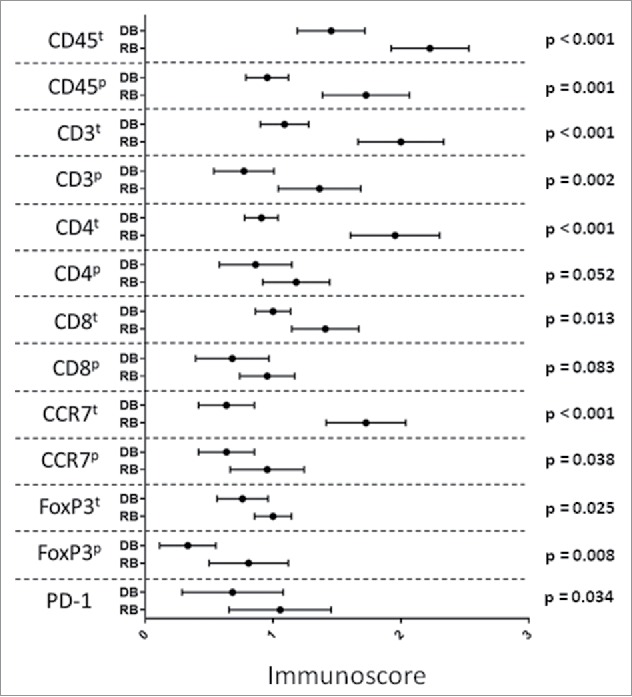
Change in infiltration rate of CD45(t/p); CD3(t/p); CD4(t); CD8(t); CCR7(t/p); FoxP3(t/p) and PD-1 positive cells in the tumor at the time of diagnosis and at relapse. Statistical analyses were performed using SPSS statistical package, version 17.0.
Tumor immune-infiltrate correlation with patients' outcome
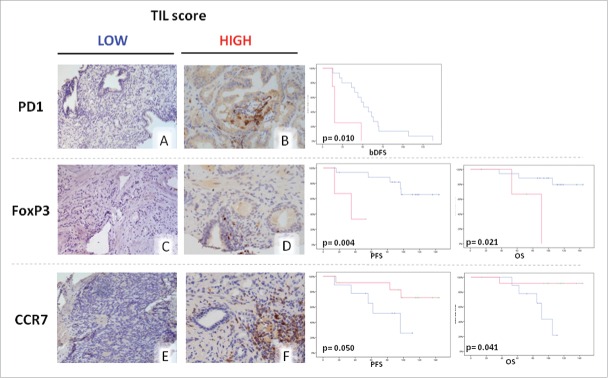
Kaplan Meyer's curves and log-rank test were performed to evaluate patients' bPFS, PFS and OS in the presence of a low (subgroup A) and high (subgroup B) tumor infiltration score by different lymphocyte subsets. Our analysis revealed a whole group median bPFS, PFS and OS of 47 months, 115 months and 140 months respectively, which were in line with those reported in the literature.3-5 On these bases, our patients may be considered a good representative series for our study. We found only PD-1 tumor infiltration score significantly associated with patients' bPFS. Specifically, a low PD-1 infiltration score in the first biopsy was significantly correlated with a longer bPFS [subgroup A vs. subgroup B: 56.067 +/−8.33 (95%CI 39.73-72.403) vs. Twenty-two.5 +/− 8.52 (95%CI5.78-39.21) months, P = 0.01] (Fig. 2). Tumor immune-infiltrate evaluated at the relapse, was then correlated with both PFS and OS. We found that a low FoxP3p infiltration score was predictive of longer PFS [subgroup A vs subgroup B: 119.37 +/−9.67 (95%CI 100.41-138.33) vs. 34.0 +/− 9.20 (95%CI 15.96-52.03) months, P = 0.004] and OS [subgroup A vs subgroup B: 129.44 +/− 7.86 (95%CI 114-144) vs. 78.33 +/−14.62 (95%CI 49-107) months, P = 0.021] (Fig. 2). Additionally, we also found that a high CCR7t tumor infiltration score was predictive of longer OS [subgroup A vs. subgroup B : 90.123 +/−6.25 (95%CI 76-103) vs 135.08 +/− 8.53 (95%CI 118-151) months, P = 0.010]. A high CCR7t tumor infiltration score also showed a trend to significance in predicting a longer PFS [subgroup A vs. subgroup B: 74.4 +/− 11.57 (95%CI 51.71-97.09) vs. 122.89 +/− 11.4 (95%CI 100.53-145.25) months, P = 0.057] (Fig. 2). In our series, the score of tumor infiltration by CD45+, CD3+ and CD4+ cells did not show any correlation with either PFS and OS of these patients (data not shown).
Figure 2.
Kaplan Meier curves related to survival analysis according to lymphocyte infiltration scores. Blue line: low infiltration score group (≤ 10 marker positive cells/ HPF). Red line: high infiltration score (> 10 marker positive cells/HPF). Representative immunohistochemical pictures for each different parameters are reported in A-F. Statistical analyses were performed using SPSS statistical package, version 17.0.
Tumor immune-infiltrate correlation with differentiation grade and PSA levels at relapse
By performing a Pearson correlation we evaluated the potential correlation of the immune-infiltrate with the most conventional prognostic parameters in PC patients represented by differentiation grade according to the Gleason's scale, disease stage and PSA at the BR (Table 2).
Table 2.
Tumor immune-infiltrate correlation with differentiation grade and PSA levels at relapse.
| Tumor infiltration scores | Prognostic factor | Correlation (p-value) |
|---|---|---|
| First biopsy | ||
| LCAt | Gleason Score | 0.017 |
| CD3t | Gleason Score | 0.011 |
| CD8t | Gleason Score | 0.027 |
| Recurrence Biopsy | ||
| CCRTt | Gleason Score | 0.029 |
(Pearson Correlation).
t: intratumoral
Tumor immune-infiltrate evaluated at the diagnosis was correlated with Gleason score in the diagnosis biopsies (d) and disease stage, whereas tumor immune-infiltrate evaluated at the BR was correlated with PSA level and Gleason score in the BR biopsies (r) (Table 2).
A lower TIL LCAt (p = 0.017), as well as a lower CD3t (p = 0.011) and a lower CD8t (p = 0.027) were significantly associated with an higher Gleason score at the diagnosis, whereas no TIL parameter was associated with disease stage.
A lower CCRTt (p = 0.029) resulted to be significantly associated with a lower Gleason score at the BR.
Discussion
Our analysis, comparing the immune-infiltration at the diagnosis and at the relapse, showed a significant increase in the rate of almost all the rates of TILs investigated, both intratumoral (t) and in the peripheral stroma (p). It's noteworthy to underline that the tumor immunologic microenvironment represents a dynamic process, affected in our analysis only by the surgery (i.e., tumor debulking), as the patients did not receive any hormonal manipulation.
The TILs differences, consequently, are an epiphenomenon of the mutations of the cancer cells gathered at the recurrence.
The results of this study also revealed that tumor infiltration by different lymphocyte subsets predicts the outcome of PC patients showing only local relapse after primary surgery and subsequently receiving SRT. This observation allowed us to establish a premise for a possible immunological therapy associated with RT for selected patients in this setting. Recently, in fact, several authors highlighted that a synergistic interaction between chemotherapy/radiotherapy and host immune response, is mandatory to achieve long-lasting responses.31 Indeed, both treatments could induce DNA double-strand breaks that, in turn, produce mutations and neo-antigens generation, promote immunological danger signals 32 and reduce tumor infiltrating immunosuppressive cell populations, such as inhibitory myeloid cells.23-25,33-40 All these events are necessary to trigger antigen-specific CTLs (CD3+CD8+), able to recognize and destroy tumor cells presenting altered and/or over-expressed molecular structures recognized as tumor-associated antigens.41,42 On these bases, several studies in PC patients have investigated, with contrasting results, the prognostic role of the basal inflammatory status and lymphocytes density in the primary tumor.43-45 However, all these studies are limited as they have been performed on elderly patients with bone metastases, who had undergone several years of hormone-manipulation and more or less severe prostate-cancer associated co-morbidities, all together potentially affecting the immune response. Conversely, our analysis has been performed on a homogeneous patients' population with minimal disease and a very long follow-up. This set of patients was considered suitable for immunological studies because of a low risk of bias related to the presence of co-morbidities commonly associated to metastatic disease. Anyway, a clear evidence exists that an efficient immune-surveillance may prevent and eventually antagonize tumor development and growth.12,13,33,34 Accordingly, in our study, we observed that increased infiltration of CCR7+ lymphocytes and reduced presence of FoxP3+ or PD-1+ cells was associated with a better patients' outcome. Specifically, CTLs expressing the homing chemokine receptor (CCR)-7 include at least 2 different populations of immune-effectors which, depending on CD45 isoform expression, can be distinguished in naïve (CD45/CD45Ra+) and central memory T cells (CD45/CD45Ra−, namely Tcms). Once engaged by its chemokine ligands (CCL)-19 and 21, produced by activated dendritic cells and other inflammatory cells,46-48 CCR7 regulates the homeostatic recirculation through body cavities and primes an intracellular process in the Tcms that drives their chemotactic homing to lymph nodes, tumor/infected tissues and target cells.49 Once achieved their target sites, these cells may differentiate in long-term memory cells (CD27+) or may lose CCR7 expression thus becoming highly efficient effector-memory CTLs.50 Thus, an increased number of CCR7+ T-cells in the tumor sites indicates a greater amount of freshly mobilized resource of immune-effectors. In a previous study, we already showed that a greater infiltration by CD8+CCR7+ T-cells in the primary tumors is predictive of better outcome in metastatic colorectal carcinoma patients 27,28 and similar results were also achieved by others in different malignancies.51-54 Conversely, Tregs (CD3+CD4+CD25+FoxP3+) are an immune-suppressive T lymphocyte subset which expresses the high affinity IL-2 receptor (CD25), the FoxP3 fork-head transcription factor controlling immunosuppressive gene expression and the inhibitory co-accessory molecule CTLA-4. This immune-suppressive lymphocyte subset represents a powerful inhibitory feedback to hyperactive CTLs, specifically aimed to avoid dangerous over-reactions or autoimmunity during acute viral infections, chronic inflammatory diseases and cancer.55-58 The prognostic role of tumor infiltrating Tregs concerns a controversial scenario in cancer patients. Accordingly, Treg over-expression has been considered detrimental for cancer patient survival, even though, we and others have described that a high Treg primary tumor infiltration score represents a good prognostic factor for colorectal cancer patients.18,26,52,59 Even though our results have a major limitation in the low number of patients enrolled, we showed for the first time, to the best of our knowledge, a potential inverse correlation between PD-1+ TIL score and prostate patient bDFS. PD-1 is an inhibitory immune checkpoint receptor which is expressed on activated antigen specific CTLs during the efferent phase of the immune response. Its binding to specific ligands (PD-1 ligands), expressed on inhibitory cell lineages and tumor cells, promotes reversible CTL anergy and, on the long term, induces antigen-specific CTL exhaustion.12,60-62 This event represents a major mechanism of tumor escape and acquired tumor cell resistance to the immune-effectors. More recently, the clinical development of mAbs to PD-1, such as Nivolumab and Pembrolizumab, has given a strong pulse to the design of cancer immunotherapy trials also outside the malignant melanoma area.63-65 On the wave of the first positive trial results, in terms of both response and survival, these mAbs have been approved for the treatment of NSCLC and are currently under active investigation for different malignancies.63-65 Since both mAbs act on a pre-existing immune-response, the positive results of these trials demonstrated: i) the presence of active and efficient immune-effectors also in patients with advanced malignant diseases; ii) that anergic CTLs may be rescued by anti-PD-1 agents; iii) that these effector cells may really counteract tumor development and consequently, prolong patient survival.
In conclusions, we observed that CTL and Tcm tumor infiltration scores represent a positive prognostic factor for locally advanced PC patients, while a high score of Tregs and PD-1+ TILs have a detrimental effect for patients' outcome. These data underline the critical role of the tumor immunologic microenvironment in conditioning both tumor development and survival and offer the rationale to design new immunotherapeutic strategies for PC patients, possibly associated with radiation treatment. In particular, our findings support the potential activity of immune-checkpoint inhibitor mAbs to PD-1, such as Nivolumab and Pembrolizumab, currently under investigation for the treatment of PC patients.66
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1. Ambrosio MR, Pirtoli L, Del Vecchio MT. Emerging targets for prostate adenocarcinoma therapy: How molecular biology may drive towards a more tailored approach. Current Drug Targets 2015; 17:266–75; PMID:26477458; http://dx.doi.org/10.2174/1389450116666151019102220 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer J Int Du Cancer 2010; 127:2893-917; PMID:21351269; http://dx.doi.org/ 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, et al.. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 2009; 181:956-62; PMID:19167731; http://dx.doi.org/ 10.1016/j.juro.2008.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven R, Maréchal JM, et al.. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012; 380:2018-27; PMID:23084481; http://dx.doi.org/ 10.1016/S0140-6736(12)61253-7 [DOI] [PubMed] [Google Scholar]
- 5.Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, Golz R, Störkel S, Willich N, Semjonow A, et al.. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol 2014; 66:243-50; PMID:24680359; http://dx.doi.org/ 10.1016/j.eururo.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 6.Ying J, Wang CJ, Yan J, Liauw SL, Straka C, Pistenmaa D, Xie XJ, Lotan Y, Roehrborn C, Kim DN. Long-term outcome of prostate cancer patients who exhibit biochemical failure despite salvage radiation therapy after radical prostatectomy. Am J Clin Oncol 2015; PMID:26165416; http://dx.doi.org/ 10.1097/COC.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 7.Kojima S, Suzuki H, Akakura K, Shimbo M, Ichikawa T, Ito H. Alternative antiandrogens to treat prostate cancer relapse after initial hormone therapy. J Urol 2004; 171:679-83; PMID:14713785; http://dx.doi.org/ 10.1097/01.ju.0000106190.32540.6c [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Okihara K, Miyake H, Fujisawa M, Miyoshi S, Matsumoto T, Fujii M, Takihana Y, Usui T, Matsuda T, et al.. Alternative nonsteroidal antiandrogen therapy for advanced prostate cancer that relapsed after initial maximum androgen blockade. J Urol 2008; 180:921-27; PMID:18635218; http://dx.doi.org/ 10.1016/j.juro.2008.05.045 [DOI] [PubMed] [Google Scholar]
- 9.Vitale G, Lupoli G, Guarrasi R, Colao A, Dicitore A, Gaudenzi G, Misso G, Castellano M, Addeo R, Facchini G, et al.. Interleukin-2 and lanreotide in the treatment of medullary thyroid cancer: in vitro and in vivo studies. J Clin Endocrinol Metab 2013; 98:E1567-74; PMID:23884781; http://dx.doi.org/ 10.1210/jc.2013-1443 [DOI] [PubMed] [Google Scholar]
- 10.Dicitore A, Caraglia M, Gaudenzi G, Manfredi G, Amato B, Mari D, Persani L, Arra C, Vitale G. Type I interferon-mediated pathway interacts with peroxisome proliferator activated receptor-γ (PPAR-γ): at the cross-road of pancreatic cancer cell proliferation. Biochim Et Biophys Acta 2014; 1845:42-52; PMID:24295567; http://dx.doi.org/ 10.1016/j.bbcan.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Vitale G, van Koetsveld PM, de Herder WW, van der Wansem K, Janssen JA, Colao A, Lombardi G, Lamberts SWJ, Hofland LJ. Effects of type I interferons on IGF-mediated autocrine/paracrine growth of human neuroendocrine tumor cells. American journal of physiology. Endocrinol Metab 2009; 296:E559-66; PMID:19141687; http://dx.doi.org/ 10.1152/ajpendo.90770.2008 [DOI] [PubMed] [Google Scholar]
- 12.Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: Evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol 2015; 68:267-79; PMID:25824720; http://dx.doi.org/ 10.1016/j.eururo.2015.02.032 [DOI] [PubMed] [Google Scholar]
- 13.Simons JW. Prostate cancer immunotherapy: beyond immunity to curability. Cancer Immunol Res 2014; 2:1034-43; PMID:25367978; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0174 [DOI] [PubMed] [Google Scholar]
- 14.Correale P, Walmsley K, Zaremba S, Zhu M, Schlom J, Tsang KY. Generation of human cytolytic T lymphocyte lines directed against prostate-specific antigen (PSA) employing a PSA oligoepitope peptide. J Immunol 1998; 161:3186-94; PMID:97433879048833 [PubMed] [Google Scholar]
- 15.Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang KY. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst 1997; 89:293-300; PMID:9048833; http://dx.doi.org/ 10.1093/jnci/89.4.293/content/89/4/293.abstract [DOI] [PubMed] [Google Scholar]
- 16.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation andtreatment effectiveness. Lancet Oncol 2014; 15:e493-503; PMID:25281468; http://dx.doi.org/ 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 17. Talebian Yazdi M, van Riet S, van Schadewijk A, Fiocco M, van Hall T, Taube C, Hiemstra PS, Van Der Burg SH. The positive prognostic effect of stromal CD8C tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget 2015; 7:3477–88; PMID:26658106; http://dx.doi.org/10.18632/oncotarget.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCoy MJ, Hemmings C, Miller TJ, Austin SJ, Bulsara MK, Zeps N, Nowak AK, Lake RA, Platell CF. Low stromal Foxp3(+) regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br J Cancer 2015; 113:1677-86; PMID:26645238; http://dx.doi.org/ 10.1038/bjc.2015.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daster S, Eppenberger-Castori S, Hirt C, Soysal SD, Delko T, Nebiker CA, Weixler B, Amicarella F, Iezzi G, Governa V, et al.. Absence of myeloperoxidase and CD8 positive cells in colorectal cancer infiltrates identifies patients with severe prognosis. Oncoimmunology 2015; 4:e1050574; PMID:26587320; http://dx.doi.org/ 10.1080/2162402X.2015.1050574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Harten-Gerritsen AS, Balvers MG, Witkamp RF, Kampman E, van Duijnhoven FJ. Vitamin D, inflammation, and colorectal cancer progression: A review of mechanistic studies and future directions for epidemiological studies. Cancer Epidemiol Biomarkers Prevention 2015; 24:1820-8; PMID:26396142; http://dx.doi.org/23760488 10.1158/1055-9965.EPI-15-0601 [DOI] [PubMed] [Google Scholar]
- 21.Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, Staropoli N, Pastina P, Marvaso G, Martellucci I, et al.. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Therapy 2013; 14:469-75; PMID:23760488; http://dx.doi.org/ 10.4161/cbt.24425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correale P, Tagliaferri P, Fioravanti A, Del Vecchio MT, Remondo C, Montagnani F, Rotundo MS, Ginanneschi C, Martellucci I, Francini E, et al.. Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 Trial). Clin Cancer Res 2008; 14:4192-9; PMID:18593999; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-5278 [DOI] [PubMed] [Google Scholar]
- 23.Correale P, Botta C, Martino E, Ulivieri C, Battaglia G, Carfagno T, Rossetti MG, Fioravanti A, Guidelli GM, Cheleschi S, et al.. Phase Ib study of poly-epitope peptide vaccination to thymidylate synthase (TSPP) and GOLFIG chemo-immunotherapy for treatment of metastatic colorectal cancer patients. Oncoimmunology 2015; 5(4):e1101205; PMID:27141384; http://dx.doi.org/ 10.1080/2162402X.2015.1101205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correale P, Botta C, Rotundo MS, Guglielmo A, Conca R, Licchetta A, Pastina P, Bestoso E, Ciliberto D, Cusi MG, et al.. Gemcitabine, oxaliplatin, levofolinate, 5-fluorouracil, granulocyte-macrophage colony-stimulating factor, and interleukin-2 (GOLFIG) versus FOLFOX chemotherapy in metastatic colorectal cancer patients: the GOLFIG-2 multicentric open-label randomized phase III trial. J Immunotherapy 2014; 37:26-35; PMID:24316553; http://dx.doi.org/ 10.1097/CJI.0000000000000004 [DOI] [PubMed] [Google Scholar]
- 25.Cusi MG, Botta C, Pastina P, Rossetti MG, Dreassi E, Guidelli GM, Fioravanti A, Martino EC, Gandolfo C, Pagliuchi M, et al.. Phase I trial of thymidylate synthase poly-epitope peptide (TSPP) vaccine in advanced cancer patients. Cancer Immunol Immunother 2015; 64:1159-73; PMID:26031574; http://dx.doi.org/ 10.1007/s00262-015-1711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L, et al.. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother 2010; 33:435-41; PMID:20386463; http://dx.doi.org/ 10.1097/CJI.0b013e3181d32f01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correale P, Rotundo MS, Botta C, Del Vecchio MT, Ginanneschi C, Licchetta A, Conca R, Apollinari S, De Luca F, Tassone P, et al.. Tumor infiltration by T lymphocytes expressing chemokine receptor 7 (CCR7) is predictive of favorable outcome in patients with advanced colorectal carcinoma. Clin Cancer Res 2012; 18:850-7; PMID:22142823; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-3186 [DOI] [PubMed] [Google Scholar]
- 28.Correale P, Rotundo MS, Botta C, Del Vecchio MT, Tassone P, Tagliaferri P. Tumor infiltration by chemokine receptor 7 (CCR7)(+) T-lymphocytes is a favorable prognostic factor in metastatic colorectal cancer. Oncoimmunology 2012; 1:531-2; PMID:22754775; http://dx.doi.org/ 10.4161/onci.19404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poortmans P, Bossi A, Vandeputte K, Bosset M, Miralbell R, Maingon P, Boehmer D, Budiharto T, Symon Z, van den Bergh AC, et al.. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC radiation oncology group. Radiother Oncol 2007; 84:121-7; PMID:17706307; http://dx.doi.org/ 10.1016/j.radonc.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 30.Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 2013; 111:753-60; PMID:23464824; http://dx.doi.org/ 10.1111/j.1464-410X.2012.11611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015; 28:690-714; PMID:26678337; http://dx.doi.org/ 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 2013; 73:2782-94; PMID:23418320; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frey B, Gaipl US. Radio-immunotherapy: the focused beam expands. Lancet Oncol 2015; 16:742-3; PMID:26095779; http://dx.doi.org/ 10.1016/S1470-2045(15)00055-8 [DOI] [PubMed] [Google Scholar]
- 34.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015; 16:e498-509; PMID:26433823; http://dx.doi.org/ 10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 35.La-Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune checkpoint inhibitors: New insights and current place in cancer therapy. Pharmacotherapy 2015; 35:963-76; PMID:26497482; http://dx.doi.org/ 10.1002/phar.1643 [DOI] [PubMed] [Google Scholar]
- 36.Deutsch E, Levy A, Chargari C. Radiation therapy and immunomodulation: Focus on experimental data. Cancer Radiotherapie 2015; 19:515-8.37; PMID:26293415; http://dx.doi.org/ 10.1016/j.canrad.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 37.Botta C, Gulla A, Correale P, Tagliaferri P, Tassone P. Myeloid-derived suppressor cells in multiple myeloma: pre-clinical research and translational opportunities. Frontiers Oncol 2014; 4:348; PMID:25538892; http://dx.doi.org/ 10.3389/fonc.2014.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correale P, Botta C, Cusi MG, Del Vecchio MT, De Santi MM, Gori Savellini G, Bestoso E, Apollinari S, Mannucci S, Marra M, et al.. Cetuximab +/− chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer J Int Du Cancer 2012; 130:1577-89; PMID:21618510; http://dx.doi.org/ 10.1002/ijc.26181 [DOI] [PubMed] [Google Scholar]
- 39.Botta C, Bestoso E, Apollinari S, Cusi MG, Pastina P, Abbruzzese A, Sperlongano P, Misso G, Caraglia M, Tassone P, et al.. Immune-modulating effects of the newest cetuximab-based chemoimmunotherapy regimen in advanced colorectal cancer patients. J Immunother 2012; 35:440-7; PMID:22576349; http://dx.doi.org/ 10.1097/CJI.0b013e31825943aa [DOI] [PubMed] [Google Scholar]
- 40.Harris TJ, Hipkiss EL, Borzillary S, Wada S, Grosso JF, Yen HR, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, et al.. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate 2008; 68:1319-29; PMID:18561247; http://dx.doi.org/ 10.1002/pros.20794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leng C, Li Y, Qin J, Ma J, Liu X, Cui Y, Sun H, Wang Z, Hua X, Yu Y, et al.. Relationship between expression of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8+ T cells. Oncology Reports 2016; 35:699-708; PMID:26718132; http://dx.doi.org/ 10.3892/or.2015.4435 [DOI] [PubMed] [Google Scholar]
- 42.Yu Z, Tan Z, Lee BK, Tang J, Wu X, Cheung KW, Lo NT, Man K, Liu L, Chen Z, et al.. Antigen spreading-induced CD8+T cells confer protection against the lethal challenge of wild-type malignant mesothelioma by eliminating myeloid-derived suppressor cells. Oncotarget 2015; 6:32426-38; PMID:26431275; http://dx.doi.org/ 10.18632/oncotarget.5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuksel OH, Urkmez A, Akan S, Yldirim C, Verit A. Predictive value of the Platelet-To-Lymphocyte ratio in diagnosis of prostate cancer. Asian Pacific J Cancer Prevention 2015; 16:6407-12; PMID:26434851; http://dx.doi.org/ 10.7314/APJCP.2015.16.15.6407 [DOI] [PubMed] [Google Scholar]
- 44.Ness N, Andersen S, Valkov A, Nordby Y, Donnem T, Al-Saad S, Busund LT, Bremnes RM, Richardsen E. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 2014; 74:1452-61; PMID:25111810; http://dx.doi.org/ 10.1002/pros.22862 [DOI] [PubMed] [Google Scholar]
- 45.Woo JR, Liss MA, Muldong MT, Palazzi K, Strasner A, Ammirante M, Varki N, Shabaik A, Howell S, Kane CJ, et al.. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Translational Med 2014; 12:30; PMID:24475900; http://dx.doi.org/ 10.1186/1479-5876-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99:23-33; PMID:10520991; http://dx.doi.org/ 10.1016/S0092-8674(00)80059-8 [DOI] [PubMed] [Google Scholar]
- 47.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol 2005; 42:799-809; PMID:15829268; http://dx.doi.org/ 10.1016/j.molimm.2004.06.040 [DOI] [PubMed] [Google Scholar]
- 48.Hopken UE, Winter S, Achtman AH, Kruger K, Lipp M. CCR7 regulates lymphocyte egress and recirculation through body cavities. J Leukocyte Biol 2010; 87:671-82; PMID:20028772; http://dx.doi.org/ 10.1189/jlb.0709505 [DOI] [PubMed] [Google Scholar]
- 49.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al.. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009; 27:5944-51; PMID:19858404; http://dx.doi.org/ 10.1200/JCO.2008.19.6147 [DOI] [PubMed] [Google Scholar]
- 50.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708-12; PMID:10537110; http://dx.doi.org/ 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 51.Schultze FC, Andag R, Alwahsh SM, Toncheva D, Maslyankov S, Yaramov N, von Ahsen N, Brandhorst G, Walson PD, Oellerich M, et al.. FoxP3 demethylation is increased in human colorectal cancer and rat cholangiocarcinoma tissue. Clin Biochem 2014; 47:201-5; PMID:24291052; http://dx.doi.org/ 10.1016/j.clinbiochem.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 52.Lopes LF, Guembarovski RL, Guembarovski AL, Kishima MO, Campos CZ, Oda JM, Ariza CB, de Oliveira KB, Borelli SD, Ehara Watanabe MA. FOXP3 transcription factor: a candidate marker for susceptibility and prognosis in triple negative breast cancer. Biomed Res Int 2014; 2014:341654; PMID:24877082; http://dx.doi.org/ 10.1155/2014/341654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ladoire S, Mignot G, Dalban C, Chevriaux A, Arnould L, Rebe C, Apetoh L, Boidot R, Penault-Llorca F, Fumoleau P, et al.. FOXP3 expression in cancer cells and anthracyclines efficacy in patients with primary breast cancer treated with adjuvant chemotherapy in the phase III UNICANCER-PACS 01 trial. Annals Oncol 2012; 23:2552-61; PMID:22431701; http://dx.doi.org/20087581 10.1093/annonc/mds028 [DOI] [PubMed] [Google Scholar]
- 54.Polcher M, Braun M, Friedrichs N, Rudlowski C, Bercht E, Fimmers R, Sauerwald A, Keyver-Paik MD, Kübler K, Büttner R, et al.. Foxp3(+) cell infiltration and granzyme B(+)/Foxp3(+) cell ratio are associated with outcome in neoadjuvant chemotherapy-treated ovarian carcinoma. Cancer Immunol Immunother 2010; 59:909-19; PMID:20087581; http://dx.doi.org/ 10.1007/s00262-010-0817-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu LF, Rudensky A. Molecular orchestration of differentiation and function of regulatory T cells. Genes Dev 2009; 23:1270-82; PMID:19487568; http://dx.doi.org/ 10.1101/gad.1791009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151-64; PMID: 763618418510923 [PubMed] [Google Scholar]
- 57.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133:775-87; PMID:18510923; http://dx.doi.org/ 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 58.Radstake TR, van der Voort R, ten Brummelhuis M, de Waal Malefijt M, Looman M, Figdor CG, et al.. Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis, and regulation by Fc gamma receptors. Annals Rheumatic Dis 2005; 64:359-67; PMID:15331393; http://dx.doi.org/ 10.1136/ard.2003.017566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Scientific Reports 2015; 5:15179; PMID:26462617; http://dx.doi.org/ 10.1038/srep15179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Slovin S. Biomarkers for immunotherapy in genitourinary malignancies. Urologic Oncol 2015; 34:205–13; PMID:25791754; http://dx.doi.org/10.1016/j.urolonc.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Massari F, Ciccarese C, Calio A, Munari E, Cima L, Porcaro AB, Novella G, Artibani W, Sava T, Eccher A, et al. . Magnitude of PD-1, PD-L1 and T Lymphocyte expression on tissue from Castration-Resistant prostate adenocarcinoma: An exploratory analysis. Targeted Oncol 2015; 11(3):345–51; PMID:26566945; http://dx.doi.org/10.1007/s11523-015-0396-3 [DOI] [PubMed] [Google Scholar]
- 62.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007; 8:239-45; PMID:17304234; http://dx.doi.org/ 10.1038/ni1443 [DOI] [PubMed] [Google Scholar]
- 63.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al.. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Eng J Med 2015; 373:1627-39; PMID:26412456; http://dx.doi.org/ 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al.. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Eng J Med 2015; 373:123-35; PMID:26028407; http://dx.doi.org/ 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al.. Nivolumab versus everolimus in advanced Renal-Cell Carcinoma. N Eng J Med 2015; 373:1803-13; PMID:26406148; http://dx.doi.org/ 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bracarda S, Altavilla A, Hamzaj A, Sisani M, Marrocolo F, Del Buono S, Danielli R. Immunologic checkpoints blockade in renal cell, prostate, and urothelial malignancies. Semin Oncol 2015; 42:495-505; PMID:25965369; http://dx.doi.org/ 10.1053/j.seminoncol.2015.02.004 [DOI] [PubMed] [Google Scholar]