ABSTRACT
Booster influenza vaccination has been recommended for patients with chronic renal disease in order to enhance the immune response to the influenza vaccine; however, the efficacy of a booster influenza vaccination is a matter of controversy. Therefore, we made a meta-analysis to determine the efficacy in patients with hemodialysis (HD), peritoneal dialysis (PD) and renal transplant recipient (RT). The sero-protection rate was used as a serologic parameter to describe the immune response to the vaccine. Statistical analysis was performed to calculate the pooled rate difference (RD) and 95% confidence interval (CI). The pooled RD for the H1N1, H3N2 and B influenza vaccines was 0.02 (95% CI: −0.02–0.06), 0.05 (95% CI: −0.01–0.11), 0.04 (95% CI: −0.02–0.10), respectively. We concluded that a booster dose of the influenza vaccine did not effectively enhance immunogenicity. Therefore, a booster dose of vaccine is not recommended for patients with hemodialysis, peritoneal dialysis and renal transplant recipients.
KEYWORDS: hemodialysis, influenza vaccine, immunogenicity, meta-analysis, peritoneal dialysis, renal transplant recipient
Introduction
Influenza leads to a substantial increase in morbidity and mortality each year. Worldwide, influenza infection causes 3–5 million severe illnesses and 250,000–500,000 deaths annually.1 Patients with chronic renal disease have a higher mortality rate than the general population. Compared with the general population, the annual mortality rate for sepsis is 20-fold higher in transplant recipients and 100 to 300-fold higher in dialysis patients.2 Because chronic renal disease patients have compromised immunity, they are vulnerable to influenza infection.3,4 Moreover, influenza infection results in severe complications. Influenza infection is a significant threat to this population of patients. For the above reasons, these patients benefit from prevention of influenza infection more so than the general population.
In healthy people the influenza vaccination is safe and effective, but challenges exist when the influenza vaccine is administered to patients with chronic renal disease. Indeed, it has been reported that the influenza vaccine has weakened efficacy in patients with chronic renal disease.5 Under such circumstances, many strategies have been proposed to strengthen the immunogenicity of the influenza vaccine; a booster vaccination is one compelling example. Although this measure was implemented in 1987 by Versluis et al.,6 the anticipated effect has not been clearly established in these patients. The booster vaccination means increasing the influenza antigen supply to patients with chronic renal disease, which would accelerate depletion of the limited global influenza antigen supply.
The benefit of a booster vaccination has been a matter of controversy in patients with HD, PD and RT. Only a few studies have addressed this issue, and the reliability of the studies was limited because of the small number of patients. A meta-analysis is thus a useful tool to collate the scattered evidence.
In the current study we determined the efficacy of a booster influenza vaccination in patients with chronic renal disease, including patients undergoing hemodialysis, peritoneal dialysis, and kidney transplantation, and verified the clinical benefit of a booster influenza vaccination.
Results
Literature review
Three hundred 3 relevant records were retrieved from the China National Knowledge Infrastructure (CNKI), Pubmed, Cochrane Library, Embase, and ScienceDirect databases. Only nine full text articles were eligible after screening. All of the studies were cohort studies. The quality of all studies was >5 stars. The details of the process are shown in Figure 1.
Figure 1.
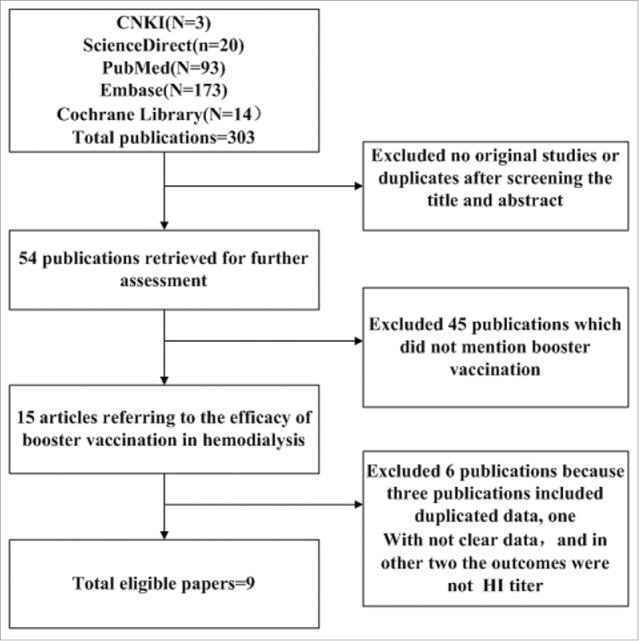
Flow chart of study selection.
Three records from Germany collected data during the same season and in the same location.7-9 We had doubts that the data came from one population. Similarly, 2 records from The Netherlands were based on data collection during the same season and in the same location.6,10 Under such conditions, we abstracted information from one study that had the most detailed data to avoid pooling the data from overlapping populations.
Description of included studies
The eligible publications included 2 from The Netherlands, 2 from Belgium, and one each from Italy, Korea, Spain, Germany, and France (Table 1). All of the papers were published between 1987 and 2013. Most of the patients who were recruited in the studies were elderly. The exact number of patients could not be derived from the original studies. In only one study was a comparison (first vs. booster vaccination) made between different groups of patients.11
Table 1.
Description of characteristics of the included studies.
| First Author | year of publication | study year | country | population | Age | F/M | Interval between transplantation and vaccination (years) | Years on dialysis | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Beyer | 1987 | 1985–1986 | Netherlands | HD | 57(17,76) | 55/53 | NA | 2(0.2,12) | S2;C2;O3;T7 |
| Vogtlander | 2004 | 1998–1999 | Netherlands | HD | 70 | 22/22 | NA | 2.7 | S4;C2;O2;T8 |
| Tanzi | 2007 | 2003–2004 | Italy | HD | 65.3 ± 13.5 | 23/35 | NA | NA | S0;C2;O3;T5 |
| Song | 2006 | 2003–2004 | Korea | HD | 50.0 ± 6.9 | 17/23 | NA | 2.2 ± 1.1 | S4;C1;O2;T7 |
| HD_C | 48.6 ± 6.7 | 25/20 | NA | 1.8 ± 0.8 | |||||
| Scharpe | 2009 | 2003–2004 | Belgium | HD | 67 ± 14 | 79/122 | NA | 3.83 ± 4.67 | S2;C2;O1;T5 |
| Scharpe | 2008 | 2003–2004 | Belgium | RT | 56 ± 13 | 60/105 | 6.3(3.1,10.4) | NA | S3;C2;O3;T8 |
| Quintana | 2013 | 2009–2010 | Spain | HD | 59.84 ± 15 | 24/34 | NA | 3.83 ± 4.70 | S2;C2;O3;T7 |
| PD | 55.29 ± 14.72 | 5/9 | NA | 2.08 ± 1.1 | |||||
| RT | 54.04 ± 12.04 | 26 % 26 | 6.88 ± 7.28 | NA | |||||
| Brakemeier | 2012 | 2009–2010 | Germany | RT | 52.2 ± 15 | NA | >=0.5 | NA | S1;C2;O3;T6 |
| Le Corre | 2012 | 2009–2010 | France | RT | 50.9(20,64) | 32/89 | 3.4 (0.5,31) | NA | S2;C2;O3;T7 |
Note. HD, hemodialysis; HD_C, control group who was hemodialysis patient receiving one dose of vaccine;
PD, peritoneal dialysis; RT, renal transplant; F/M, female/male; NA, not available;
NOS, the Newcastle–Ottawa Scale for cohort studies; SN, the number of stars for cohort selection section;
CN, the number of stars for cohort comparability section; ON, the number of stars for cohort outcome section;
TN, the total number of stars.
The vaccine used in all the studies was monovalent or trivalent, and administered via the intramuscular route (Table 2). The formation of influenza vaccine was subunit and split. MF-59 and AS03A adjuvant was applied in 3 studies.8,12,13 The interval between the first and booster vaccination ranged from 28–90 d. The interval between vaccination and serology was 21–30 d. The dosage of influenza vaccine in 8 eligible studies was 15 µg for each virus strain. In the other 2 studies, the dosage of H1N1 vaccine was 3.75 µg.
Table 2.
Information of vaccine used in the eligible studies and the immunogenicity.
| GMT |
|||||||
|---|---|---|---|---|---|---|---|
| First Author | vaccine formation | vaccine strains | dosage (ug) | interval between the first and the boost dose | one dose | booster dose | fold increase in GMT |
| Beyer | split trivalent vaccine | A/Chile/1/83(H1N1) | 15 | 30 days | NA | NA | NA |
| A/Pilippines/2/82(H3N2) | 15 | NA | NA | NA | |||
| B/USSR/100/83 | 15 | NA | NA | NA | |||
| Vogtlander | subunit trivalent vaccine | A/Beijing/262/95(H1N1) | 15 | 56 days | 46 | 53.4 | 0.16 |
| A/Sydney/5/97(H3N2) | 15 | 36.4 | 60.9 | 0.67 | |||
| B/Beijing/184/93 | 15 | 46 | 47.2 | 0.03 | |||
| Tanzi | subunit trivalent | A/NewCaledonia/20/99 (H1N1) | 15 | 30 days | 52 | 61.5 | 0.18 |
| MF59-adjuvanted vaccine | A/Panama/2007/99(H3N2) | 15 | 44 | 49 | 0.11 | ||
| B/HongKong/330/2001 | 15 | 26.3 | 27.9 | 0.06 | |||
| Song | split trivalent vaccine | A/New Caledonia/20/99 (H1N1) | 15 | 28 days | 21.54 | 24.28 | 0.13 |
| A/Moscow/10/99 (H3N2) | 15 | 37.13 | 64.09 | 0.73 | |||
| B/HongKong/330/2001 | 15 | 25.62 | 21.14 | 0.83 | |||
| Scharpe (2009) | split trivalent vaccine | A/New Caledonia/20/99 (H1N1) | 15 | 90 days | NA | NA | NA |
| A/Panama/2007/99 (H3N2) | 15 | NA | NA | NA | |||
| B/Shangdong/7/97 | 15 | NA | NA | NA | |||
| Scharpe (2008) | split trivalent vaccine | A/New Caledonia/20/99 (H1N1) | 15 | 90 days | NA | NA | NA |
| A/Panama/2007/99 (H3N2) | 15 | NA | NA | NA | |||
| B/Shangdong/7/97 | 15 | NA | NA | NA | |||
| Quintana | split monovalent | A%California%7% 2009(H1N1) | 3.75 | 21 days | 95.5 | 124.9 | 0.31(HD) |
| AS03A-adjuvanted vaccine | 344.5 | 350.2 | 0.01(PD) | ||||
| 31.8 | 46.5 | 0.46(RT) | |||||
| Brakemeier | split monovalent AS03A-adjuvanted vaccine | A%California%7%2009(H1N1) | 3.75 | 21 ± 3.5 days | 46.3 | 67.4 | 0.46 |
| Le Corre | split monovalent vaccine | A%California%7%2009(H1N1 | 15 | 21 days | 47.6 | 59 | 0.23 |
Note. GMT, Geometric Mean Titer; NA, not available.
Risk of bias assessment
Several aspects of the study might have caused bias. Booster vaccination might have a distinct influence on patients with different renal conditions. The interval between the first and booster vaccination was different in the eligible studies. Different schedules for booster vaccination may have diverse efficacy. Different formations of the vaccine could produce inconsistent immunogenicity. Age is also a significant factor which affects the immunogenicity of a vaccine. The age of the patients was heterogeneous in different groups. Years on dialysis might have an effect on the immune system. The patients with different years on dialysis might have various humoral responses to the influenza vaccine. Because of possible risk of bias from above sources, subgroup analysis for H1N1 vaccine was performed to determine them. Subgroup analysis showed they did not induce bias (data shown in Table 3).
Table 3.
Rate difference of Sero-protection (H1N1) by subgroup analysis.
| booster dose |
one dose |
||||||
|---|---|---|---|---|---|---|---|
| subgroup | numuber of group | events | tatol | events | tatol | rate difference (95%CI) | Test for subgroup difference (P value) |
| overall | 12 | 584 | 775 | 652 | 884 | 0.02 [−0.02, 0.06] | NA |
| Renal conditions | |||||||
| PD | 2 | 30 | 33 | 27 | 33 | 0.09 [−0.08, 0.26] | 0.73 |
| HD | 6 | 290 | 390 | 368 | 497 | 0.02 (−0.04, 0.07) | |
| RT | 4 | 264 | 352 | 257 | 354 | 0.02 [−0.04, 0.08] | |
| Interval between the first and booster vaccination | |||||||
| ≦30 | 9 | 314 | 464 | 302 | 474 | 0.04 [−0.02, 0.10] | 0.27 |
| >30 | 3 | 270 | 311 | 350 | 410 | 0.00 [−0.05, 0.05] | |
| Formation of vaccine | |||||||
| subunit | 9 | 485 | 628 | 553 | 729 | 0.02 [−0.02, 0.06] | 0.67 |
| split | 3 | 99 | 147 | 99 | 155 | 0.04 [−0.05, 0.14] | |
| Mean or median age | |||||||
| ≦50 | 4 | 148 | 227 | 149 | 234 | 0.02 [−0.06, 0.10] | 0.96 |
| >50 | 8 | 436 | 548 | 503 | 650 | 0.02 [−0.02, 0.07] | |
| Mean or median years on dialysis | |||||||
| ≦2.5 | 3 | 163 | 204 | 240 | 303 | 0.01 [−0.06, 0.08] | 0.66 |
| >2.5 | 4 | 143 | 205 | 142 | 213 | 0.03 [−0.05, 0.12] | |
The efficacy of the booster vaccination could not influence publication because negative and positive results have equal research value. After analysis, all the evidence from funnel plots and the Egger's test showed that there was no publication bias in the meta-analysis (data not shown).
Efficacy of booster vaccination in patients with HD, PD and RT
Eight hundred eighty-four patients in the current study had detailed information about the immunogenicity of the first dose of the influenza vaccine. For each virus strain of influenza vaccine, a serologic response of approximately 72% of the patients reached sero-protection (HI antibody titer >40). The sero-protection rates in the patients met the Committee for Proprietary Medical Products (CPMP) criteria.14
The rate difference (RD) for sero-protection (first vs. booster vaccination) was used to measure the efficacy of an additional dose of the influenza vaccine. The pooled results showed (Fig. 2–4) that the booster vaccination could not significantly increase the sero-protection rate in patients with HD, PD and RT. The pooled RD for the H1N1 vaccine was 0.02 (95% confidence interval (CI): −0.02–0.06). For the H3N2 vaccine, the pooled RD was 0.05 (95% CI: −0.01–0.11). For the B-type influenza vaccine, the pooled RD was 0.04 (95% CI: −0.02–0.10).
Figure 3.
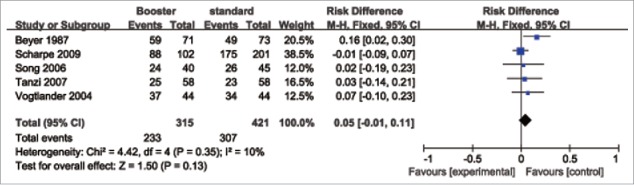
Forest plot of the efficacy of a booster (H3N2) influenza vaccine in patients with HD, PD and RT.
Figure 4.
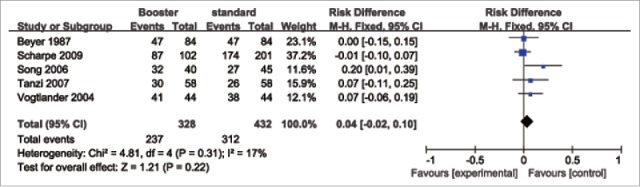
Forest plot of the efficacy of a booster (B-type) influenza vaccine in patients with HD, PD and RT.
Figure 2.
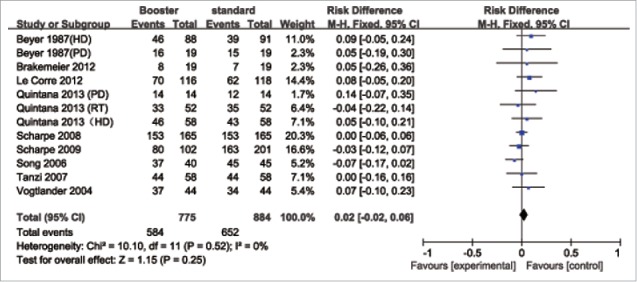
Forest plot of the efficacy of a booster (H1N1) influenza vaccine in patients with HD, PD and RT.
In order to better understand the efficacy of booster vaccination in dialysis patients and renal allograft recipients, fold-increase in geometric mean titer (GMT) from pre-booster to post-booster time point was also taken into consideration. The fold-increase in GMT was 0.24(95% CI:0.09–0.39) For the H1N1 vaccine, 0.50(95% CI:0.17–0.84) for the H3N2 vaccine, 0.06(95% CI:−0.07–0.31) for the B-type vaccine.
Discussion
Our findings showed that a single dose of influenza vaccine induces a sub-optimal immune response, and the effect reaches the CPMP criteria. Thus, a single dose of influenza vaccine is capable of protecting patients with HD, PD and RT from influenza infection. After a booster dose of influenza vaccine was administered to patients, the sero-protection rates increased; however, a booster vaccination did not yield a satisfactory immunopotentiating effect
An influenza vaccine is the most cost-effective way to prevent influenza infection. An influenza vaccination can also significantly decrease the number of severe complications and morality rate. It has been reported that influenza vaccine activity is 70%–90% in healthy populations >65 y of age.15 Our outcomes showed that influenza vaccine activity was slightly reduced in patients with HD, PD and RT. Therefore, the beneficial effect of influenza vaccine would also be realized in patients with HD, PD and RT.
Booster vaccination has long been recommended to patients with a compromised immune system to improve the level of protection of patients with chronic renal disease; however, our results showed that a booster dose of vaccine was not effective. This finding does not mean that there is no benefit of booster vaccination in other populations. As Gueller et al. reported,16 after hematopoietic stem cell transplantation, patients benefit from a booster influenza vaccination. The potentiating effect of a booster dose influenza vaccine was also reported in liver transplant recipients.17 Additional studies are needed to determine the efficacy of booster vaccination in other populations, such as the very elderly, HIV patients, and patients with autoimmune disease.
The sero-protection rate, mean fold increase in HI antibody titer, and sero-conversion rate are generally used to describe the antibody response to an influenza vaccine. We chose the sero-protection rate as the serologic parameter to measure the immunogenicity of the influenza vaccine because of the public health objectives. HI titer of 40 is generally accepted to represent a 50% protective titer for seasonal influenza A viruses in adult populations. The sero-conversion rate and mean fold increase in HI antibody are only related to the immunologic response and cannot assess the economic effectiveness in a population.
An annual influenza vaccine has been recommended, thus a portion of patients with HD, PD and RT had pre-vaccination antibody to the vaccine antigen. In this study we could not assess the influence of pre-vaccination because of lacking adequate information about the baseline HI titer. One may ponder the effect of the baseline HI titer on assessing the efficacy of a booster vaccination; however, the situation that a part of the patients had pre-vaccination was close to an actual real-life situation. One research by Agnieszka Mastalerz-Migas et al. may help us to eliminate above concern.18 They concluded that although influenza vaccination in previous seasons leads to higher baseline HI titer, it is of little influence on immunoresponse to current influenza immunization in the dialysis patients.
On the basis of our findings, we believe it is not prudent to recommend a booster influenza vaccination in patients with chronic renal disease. A booster dose of influenza vaccine would result in wasting the limited antibody supply. Our findings would reduce an excess dose of influenza vaccine being offered to patients. Although patients with chronic renal disease have impaired immune response to the influenza vaccine, one dose of influenza vaccine is protective. This phenomenon should attract us to further consider whether or not other efforts on improving the immunogenicity of influenza vaccine in patients are cost-effective.
The present meta-analysis had several limitations. Firstly, sero-protection rate did not completely demonstrate the actual effectiveness of booster vaccination. It is a great challenge to directly assess the actual effectiveness because lacking related studies. Sero-protection rate is an indirect parameter to show the actual effectiveness of vaccine, for at least 50% of vaccinees are protected when the HI titer is >40. Second, Subgroup analysis was performed only on the H1N1 vaccine. Without enough information about the other strain vaccine in the included studies, the present data was difficult to be divided into subgroups.
Conclusion
Although the influenza vaccine had impaired immunogenicity, one dose of influenza vaccine induced an adequate immune response in the patients. A booster dose of the influenza vaccine did not effectively enhance immunogenicity. Therefore, a booster dose of vaccine is not recommended for in patients with hemodialysis, peritoneal dialysis and renal transplant recipients.
Methods
Retrieval strategy and selection criteria
We searched PubMed, Embase, the Cochrane Library, the China National Knowledge Infrastructure, and Science Direct databases for articles published before January 2016. The key words used in the retrieval process were influenza vaccine, influenza vaccination, dialysis, hemodialysis, continuous ambulatory peritoneal dialysis, CAPD, peritoneal dialysis, renal failure, chronic renal failure, chronic kidney disease, chronic renal insufficiency, end stage renal disease, ESRD, and CKD. The articles were independently screened by 2 reviewers (YPL and XJX) in the sequence of title, abstract, and full text. Disagreements were resolved by discussion until consensus was achieved. An eligible study must have met the inclusion criteria, as follows: studies involving patients with chronic renal diseases who received an influenza vaccination; the study outcome was hemagglutination-inhibiting (HI) antibody against influenza virus; a comparison of the immunogenicity of the influenza vaccine was made between the standard and booster vaccination in the same or a different group of patients. The exclusion criteria were as follows: study sample size <5; not clear and original data; and duplicated data.
Data abstraction and quality assessment
We discussed which data we needed from each article and designed a questionnaire to survey the articles. The extracted information included the following: authors' names; publication year; study design; sample size; age of patients; years on dialysis; and vaccine type. The outcome that we abstracted from the articles was the sero-protection rate. The sero-protection rate is a percentage of vaccine recipients with a serum HI titer >1:40 after vaccination. A HI titer >1:40 can be viewed as protective in healthy adults.19 Study quality was assessed using the Newcastle–Ottawa Scale.20 The full NOS score was 9 stars which consist of 3 stars from section of cohort selection, 4 stars from section of comparability, and 3 stars from section of outcome. At least 2 researchers (XJX, YPL, and ZFL) independently finished extraction and quality assessment after reviewing each article. If there were discrepancies during the abstraction and assessment process between the reviewers, the discrepancies were resolved by discussion.
Data analysis
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement.21 We pooled the outcomes using the Mantel–Haenszel fixed or random model.22 Selection of the fixed- or random-effects model depended on the result of the Cochrane's Q test. Heterogeneity was significant when the p value of the Cochrane's Q test was >10%.23 When the heterogeneity was significant, the Mantel–Haenszel random model was used. If not, we preferred the Mantel–Haenszel fixed model. The I2 value indicated the degree of inconsistency among the studies, as follows 24: <25%, homogeneity; 25%–50%, moderate heterogeneity; 50%–75%, large heterogeneity; and >75%, extreme heterogeneity.25 Publication bias was assessed through visual inspection of funnel plot asymmetry. Asymmetry was also tested by Egger's linear regression analysis.26 Statistical analysis was performed using RevMan version 5.2 (provided by the Cochrane Collaboration) and STATA 13 (StataCorp, College Station, TX, USA).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was partly supported by grants from Natural Science Foundation of Guangdong Province, China (2015A030313516), and special fund for discipline construction of colleges and universities in Guangdong Province, China (2013KJCX0091).
References
- [1].Influenza (Seasonal) World Healther Organization (WHO), 2014 [Google Scholar]
- [2].Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney int 2000; 58:1758–64; PMID:11012910; http://dx.doi.org/ 10.1111/j.1523-1755.2000.00337.x [DOI] [PubMed] [Google Scholar]
- [3].Kim KW, Chung BH, Jeon EJ, Kim BM, Choi BS, Park CW, Kim YS, Cho SG, Cho ML, Yang CW. B cell-associated immune profiles in patients with end-stage renal disease (ESRD). Exp Mol Med 2012; 44:465–72; PMID:22617684; http://dx.doi.org/ 10.3858/emm.2012.44.8.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marcelli D, Marelli C, Richards N. Influenza A(H1N1)v pandemic in the dialysis population: first wave results from an international survey. Nephrol Dial Transplant 2009; 24:3566–72; PMID:19846392; http://dx.doi.org/ 10.1093/ndt/gfp557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Remschmidt C, Wichmann O, Harder T. Influenza vaccination in patients with end-stage renal disease: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness, and safety. BMC Med 2014; 12:244; PMID:25523432; http://dx.doi.org/ 10.1186/s12916-014-0244-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Versluis DJ, Beyer WE, Masurel N, Weimar W, Kramer P, Diderich PP. Value of booster immunisation with influenza vaccine in patients undergoing haemodialysis. Br Med J (Clin Res ed) 1987; 294:348; PMID:3101868; http://dx.doi.org/ 10.1136/bmj.294.6568.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brakemeier S, Schweiger B, Glander P, Diekmann F, Neumayer HH, Budde K. An adjuvanted influenza a h1n1 vaccine (pandemrix®) does not provide a protective immune response in the majority of renal transplant recipients. Transplantation 2010; 90:320; http://dx.doi.org/ 10.1097/00007890-201007272-00603 [DOI] [PubMed] [Google Scholar]
- [8].Brakemeier S, Schweiger B, Lachmann N, Glander P, Schonemann C, Diekmann F, Neumayer HH, Budde K. Immune response to an adjuvanted influenza A H1N1 vaccine (Pandemrix((R))) in renal transplant recipients. Nephrol Dial Transplant 2012; 27:423–8; PMID:21613386; http://dx.doi.org/ 10.1093/ndt/gfr278 [DOI] [PubMed] [Google Scholar]
- [9].Rambal V, Muller K, Dang-Heine C, Sattler A, Dziubianau M, Weist B, Luu SH, Stoyanova A, Nickel P, Thiel A, et al.. Differential influenza H1N1-specific humoral and cellular response kinetics in kidney transplant patients. Med Microbiol Immunol 2014; 203:35–45; PMID:24057515; http://dx.doi.org/ 10.1007/s00430-013-0312-3 [DOI] [PubMed] [Google Scholar]
- [10].Beyer WE, Versluis DJ, Kramer P, Diderich PP, Weimar W, Masurel N. Trivalent influenza vaccine in patients on haemodialysis: impaired seroresponse with differences for A-H3N2 and A-H1N1 vaccine components. Vaccine 1987; 5:43–8; PMID:3577356; http://dx.doi.org/ 10.1016/0264-410X(87)90008-9 [DOI] [PubMed] [Google Scholar]
- [11].Song JY, Cheong HJ, Ha SH, Kee SY, Jeong HW, Kim WJ. Active influenza immunization in hemodialysis patients: comparison between single-dose and booster vaccination. Am J Nephrol 2006; 26:206–11; PMID:16699258; http://dx.doi.org/ 10.1159/000093306 [DOI] [PubMed] [Google Scholar]
- [12].Tanzi E, Amendola A, Pariani E, Zappa A, Colzani D, Logias F, Perego A, Zanetti AR. Lack of effect of a booster dose of influenza vaccine in hemodialysis patients. J Med Virol 2007; 79:1176–9; PMID:17596830; http://dx.doi.org/ 10.1002/jmv.20936 [DOI] [PubMed] [Google Scholar]
- [13].Quintana LF, Serra N, De Molina-Llaurado P, Blasco M, Martinez M, Campos B, Bayas JM, Pumarola T, Campistol JM. Influence of renal replacement therapy on immune response after one and two doses of the A(H1N1) pdm09 vaccine. Influenza Other Respir Viruses 2013; 7:809–14; PMID:23078139; http://dx.doi.org/ 10.1111/irv.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Products ECfPM Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96). London: European Agency for the Evaluation of Medicinal Products, 1997 [Google Scholar]
- [15].Mastalerz-Migas A, Gwiazda E, Brydak LB. Effectiveness of influenza vaccine in patients on hemodialysis–a review. Med Sci Monit 2013; 19:1013–8; PMID:24241247; http://dx.doi.org/ 10.12659/MSM.889671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gueller S, Allwinn R, Mousset S, Martin H, Wieters I, Herrmann E, Serve H, Bickel M, Bug G. Enhanced immune response after a second dose of an AS03-adjuvanted H1N1 influenza A vaccine in patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17:1546–50; PMID:21324375; http://dx.doi.org/ 10.1016/j.bbmt.2011.02.004 [DOI] [PubMed] [Google Scholar]
- [17].Soesman NM, Rimmelzwaan GF, Nieuwkoop NJ, Beyer WE, Tilanus HW, Kemmeren MH, Metselaar HJ, de Man RA, Osterhaus AD. Efficacy of influenza vaccination in adult liver transplant recipients. J Med Virol 2000; 61:85–93; PMID:10745238; http://dx.doi.org/ 10.1002/(SICI)1096-9071(200005)61:1%3c85::AID-JMV14%3e3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- [18].Mastalerz-Migas A, Bujnowska-Fedak M, Brydak LB. Immune efficacy of first and repeat trivalent influenza vaccine in healthy subjects and hemodialysis patients. Adv Exp Med Biol 2015; 836:47–54; PMID:25248348; http://dx.doi.org/ 10.1007/5584_2014_36 [DOI] [PubMed] [Google Scholar]
- [19].de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol 2003; 115:63–73; PMID:15088777 [PubMed] [Google Scholar]
- [20].Newcastle-ottawa quality assessment scale cohort studies [Google Scholar]
- [21].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8:336–41; PMID:20171303; http://dx.doi.org/ 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- [22].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–48; PMID:13655060 [PubMed] [Google Scholar]
- [23].Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10:101–29; http://dx.doi.org/ 10.2307/3001666 [DOI] [Google Scholar]
- [24].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–58; PMID:12111919; http://dx.doi.org/ 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- [25].Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003; 327:557–60; PMID:12958120; http://dx.doi.org/ 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 1997; 315:629–34; PMID:9310563; http://dx.doi.org/ 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]


