ABSTRACT
The PcrV cap structure of the type III secretory apparatus of Pseudomonas aeruginosa is a vaccine target. Human immunoglobulin G (IgG) molecules extracted from sera containing high or low anti-PcrV titers were tested for their effects against P. aeruginosa pneumonia in a mouse model. Among 198 volunteers, we selected the top 10 high anti-PcrV titer sera and the bottom 10 low anti-PcrV titer sera and extracted the IgG fraction from each serum sample. First, we examined the effects of the IgG against virulent P. aeruginosa. A lethal dose of P. aeruginosa premixed with saline, low titer human IgG, high titer human IgG, or rabbit-derived polyclonal anti-PcrV IgG was intratracheally administered into the lungs of mice, and their survival and lung inflammation were evaluated for 24 h. The high anti-PcrV titer human IgG had a prophylactic effect. Next, the prophylactic effects of intravenous administration of extracted and pooled high or low anti-PcrV titer human IgG were examined. Here, prophylactic intravenous administration of pooled high anti-PcrV titer human IgG, which showed binding capacity to P. aeruginosa PcrV, was more effective than the administration of its low titer pooled equivalent, and the measured physiological and inflammatory parameters correlated with the anti-PcrV titer levels. This result indirectly implies that high anti-PcrV titers in blood can help to protect against virulent P. aeruginosa infections. In addition, the IgG fractions from such high titer sera have potential to be a source of specific intravenous immunoglobulin products for passive vaccination against virulent P. aeruginosa infections.
KEYWORDS: anti-PcrV titer, bacterial pneumonia, intravenous immunoglobulin, PcrV, Pseudomonas aeruginosa, type III secretion system
Introduction
Infections in immunocompromised people caused by multi-drug resistant pathogenic bacteria have become an increasing problem worldwide.1,2 Pseudomonas aeruginosa is a major opportunistic pathogen capable of causing acute and fatal infections, such as ventilator-associated pneumonia, bacteremia, and sepsis in critically ill individuals.3-5 P. aeruginosa clinical isolates are often resistant to most β-lactams and fluoroquinolones and, sometimes, resistant to aminoglycosides, such as gentamicin and amikacin, thus categorizing them as multi-drug resistant P. aeruginosa (MDRP).4,6-9 Limitations in the number of effective antimicrobial agents for treating MDRP infections leads to the high mortality rates associated with the acute lung injury induced by this bacterium.5
While seeking new prophylactic or therapeutic strategies that do not rely on conventional antimicrobial agents, we have investigated the use of an immunotherapy approach that targets the P. aeruginosa type III secretion system.10 The type III secretion system is the major virulence mechanism in P. aeruginosa responsible for acute lung injury, bacteremia, and sepsis.11 In the type III secretion system of P. aeruginosa, PcrV, a cap structure on the tip of the type III secretory apparatus, is a key molecule through which cytotoxic P. aeruginosa delivers lethal cytotoxic toxins into its target eukaryotic cells.12 We have previously reported that the blockade of PcrV by specific antibodies can inhibit translocation of type III secretory toxins.10,13,14 Active immunization with recombinant PcrV protects animals from lethal P. aeruginosa infections 14-16, and anti-PcrV antibodies also protect infected animals from acute lung injury, bacteremia, and sepsis.14,17-22 Based on these experimental results, an engineered human anti–PcrV antibody was tested in patients in Phase II trials 23-25, but no therapies based on it have been adapted for clinical use as yet.
We recently reported that a commercially available immunoglobulin solution possesses anti-PcrV titers and intravenous administration of this solution protects mice from infection with cytotoxic P. aeruginosa and several clinical isolates of this bacterium.26 Additionally, the efficacy of the immunoglobulin solution against a P. aeruginosa clinical isolate was confirmed in leukopenic mice.27 The above results imply that a certain subset of the blood donor population has efficaciously high anti-PcrV titers in their sera. Thus, we performed an epidemiological study in which serum anti-PcrV titers were measured in 198 volunteers.28 As a result, in 21 participants (10.6%), the anti-PcrV titers exceeded an approximate 3-fold rise (>12 nM) compared with the median value.28 However, we were uncertain whether sera containing high anti-PcrV titers would be efficacious against virulent P. aeruginosa infections. For the effective blockade of type III secretion-associated virulence, for example, a monoclonal antibody needs to bind a specific blocking epitope region on the PcrV molecule.20 This means that a high serum titer against PcrV may not always correlate with an effective blocking capability against type III secretion. Therefore, in this study, we extracted IgG fractions from human sera that possessed high or low anti-PcrV titers in our epidemiological survey. The protective capacities of the extracted IgGs against type III secretion-associated virulence were tested in a mouse model of P. aeruginosa pneumonia, and the results suggest that high titer human sera possess blocking capacities against P. aeruginosa. We found that the immunoglobulin product from human blood containing high anti-PcrV titers has potential to act as a therapeutic agent against P. aeruginosa infections.
Results
In vivo testing of pre-mixed human IgG
Among the sera collected from 198 participants in the epidemiological study28, the top and the bottom 10 sera in terms of their anti-PcrV titer levels were selected for further small-scale purification of the IgG fractions by affinity column chromatography (Table 1). The top 10 high anti-PcrV titer sera contained anti-PcrV titers ranging from 113.81 to 17.50 nM (mean ± SD = 39.3 ± 29.9 nM), and the bottom 10 low anti-PcrV titer sera contained anti-PcrV titers ranging from 1.93 to 1.01 nM (mean ± SD = 1.55 ± 0.27 nM). The difference in the anti-PcrV titers between the high and low titer sera was 25.4 times the mean values.
Table 1.
Anti-PcrV titers of the sera selected for IgG fraction purification.
| Group | No. | Age and sex | Anti-PcrV titer (6F5 equivalent, nM) |
|---|---|---|---|
| High anti-PcrV titer | 1 | 71 F | 113.81 |
| 2 | 47 F | 64.60 | |
| 3 | 63 M | 42.96 | |
| 4 | 73 M | 36.61 | |
| 5 | 29 F | 30.23 | |
| 6 | 61 F | 27.68 | |
| 7 | 47 M | 23.99 | |
| 8 | 37 M | 17.96 | |
| 9 | 76 F | 17.61 | |
| 10 | 83 F | 17.50 | |
| Mean ± SD | 58.7 ± 17.9 | 39.3 ± 29.9 | |
| Low anti-PcrV titer | 1 | 71 F | 1.93 |
| 2 | 47 F | 1.91 | |
| 3 | 63 M | 1.66 | |
| 4 | 73 M | 1.65 | |
| 5 | 29 F | 1.61 | |
| 6 | 61 F | 1.54 | |
| 7 | 47 M | 1.50 | |
| 8 | 37 M | 1.34 | |
| 9 | 76 F | 1.34 | |
| 10 | 83 F | 1.01 | |
| Mean ± SD | 51.2+20.7 | 1.55 ± 0.27 | |
| Total | Mean ± SD | 55.0 ± 19.3 | 20.4 ± 28.3 |
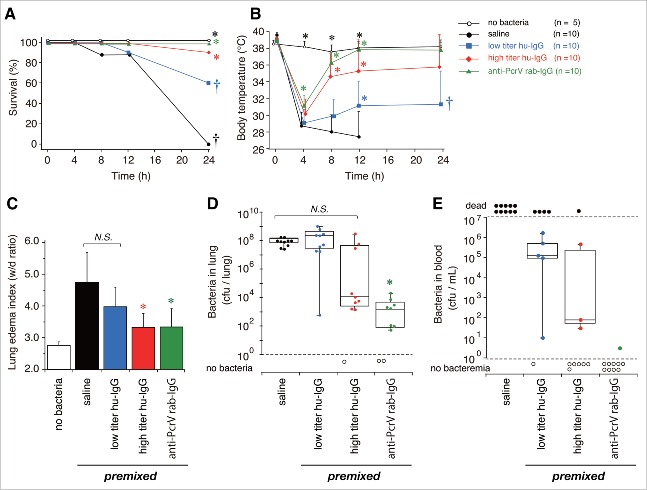
We first evaluated the effect of each purified human IgG derived from individual volunteers on bacterial solutions in vivo (Fig. 1). From our previous experience of screening for effective antibody clones or IgG extracted from hyper-immune serum that can block type III secretion-associated lung injury, we found that the pre-mixed setting produced the clearest direct effects with which to evaluate the efficacy of antibodies in blocking acute lung injury in infected animals.14,20,23 In other words, if no protective effect was observed in the pre-mixed setting, administration of the antibodies via different routes (i.e., intravenous and intramuscular) would be unlikely to generate any protective effects. The effect of 10 human IgGs displaying high anti-PcrV titers (high titer hu-IgG) were compared with that of 10 human IgGs displaying low anti-PcrV titers (low titer hu-IgG), with saline used as a negative control and rabbit-derived anti-PcrV polyclonal IgG (anti-PcrV rab-IgG) used as a positive control. IgG (10 μg) was mixed directly with solutions each containing the same lethal dose of P. aeruginosa, and these solutions (60 µL/mouse) were instilled intratracheally into the mouse lungs. This IgG dose was selected on the basis of the results from our previous animal studies, where we reported on the dose-dependent effect of commercially available intravenous immunoglobulin solution.26 The survival rates and body temperatures of the mice were monitored for 24 h after infection (Fig. 1A and B). As a non-infected control, 5 mice each received intratracheal instillation of saline with no bacteria, after which they were monitored for 24 h. Mice in the infected control group each received bacteria pre-mixed with anti-PcrV rab-IgG (10 µg) or saline. All mice that received bacteria pre-mixed with saline became severely hypothermic within 4 h, became more hypothermic after 12 h, and all of them died within 24 h of bacterial administration (†, p < 0.05, vs. premixed anti-PcrV rab-IgG). In contrast, all mice that received bacteria pre-mixed with 10 µg of anti-PcrV rab-IgG showed mild hypothermia after 4 h, recovered, and then survived for 24 h (*, p < 0.05, vs. premixed saline). Ninety percent of mice that received bacteria pre-mixed with high titer hu-IgG recovered from hypothermia at 4 h post-administration (*, p < 0.05, vs. premixed saline at each time point). In contrast, only 60% of mice that received bacteria pre-mixed with low titer hu-IgG recovered from hypothermia at 4 h post-administration and survived for 24 h (*, p < 0.05, vs. premixed saline at each time point; †, p < 0.05, vs. premixed anti-PcrV rab-IgG).
Figure 1.
Survival, body temperature, lung edema and bacteriological data in the premixed setting. Five mice that received intratracheal instillation of saline with no bacteria acted as the control mice. Saline, low titer hu-IgG, high titer hu-IgG, or anti-PcrV rab-IgG was premixed with a lethal dose (1.5 × 106 CFU/mouse) of Pseudomonas aeruginosa PA103 just before tracheal instillation. A. Survival was monitored for 24 h after infection. B. Mouse body temperature for 24 h after bacterial instillation. C. Lung edema index (wet to dry weight ratio, W/D ratio) 24 h after bacterial instillation. D. The number of bacteria remaining in infected mouse lungs 24 h after bacterial instillation. E. The number of bacteria in the blood of surviving mice 24 h after bacterial instillation. Data on body temperature are presented as the mean + SD. *, p < 0.05, vs. the saline-premixed group; †, p < 0.05, vs. the 10-µg anti-PcrV rab-IgG premixed group.
Lung edema in the 5 mouse groups was evaluated 24 h after the bacterial instillation (Fig. 1C). Severe lung edema with wet-to-dry (W/D) weight ratios above 4.5 was observed at 24 h post-bacterial instillation in mice that received saline. The low titer hu-IgG premixture did not significantly decrease the edema level. In contrast, premixture of 10 µg of high titer hu-IgG or anti-PcrV rab-IgG in bacterial solution significantly decreased the edema levels with a W/D weight ratio below 4.0 (*, p < 0.05, vs. premixed saline).
The number of bacteria in the lungs of all the mice and the bacteremia in those of the 24 h survivors was calculated (Fig. 1D and E). First, about 108 colony forming units (CFUs; 8.5 × 107 and 1.4 × 108 median values, respectively) were detected in the lungs of mice treated with the premixed saline or the premixed low titer hu-IgG bacterial solution. Fewer bacteria (2.6 × 103, median value) were detected in the mice treated with the premixed high titer hu-IgG bacterial solution, although there was no statistically significant difference in comparison with the saline-premixed control. A significantly decreased number of bacteria (2.4 × 102 CFU, median value) was detected in the mice that received the premixed anti-PcrV rab-IgG (*, p < 0.05, vs. premixed saline). Survivors in the group that received premixed low titer hu-IgG had bacteremia levels of 105 CFU/mL (1.1 × 105 CFU, median value). Over 60% of the survivors in the group that received high titer hu-IgG or anti-PcrV rab-IgG showed no bacteremia (no statistical analysis on the bacteremia levels was performed because all mice in the saline-premixed group died, while 90% of the mice in the anti-PcrV rab-IgG premixed group showed no bacteremia).
The levels of inflammatory cytokines and myeloperoxidase (MPO) activities in the lung homogenates of the mice and the cytokine levels in the blood of the 24-h survivors were measured 24 h after bacterial instillation (Figs. 2 and 3). Three mice that received intratracheal instillation of saline (60 µL) without bacteria were treated as the non-infected control group. No increases in cytokine levels and MPO activity were detected in the non-infected control mice, and no cytokines were measured in blood samples from all the mice that received the saline premixture because all of these mice died within 24 h of the bacterial instillation. Significant increases in IL-1β, IL-6, TNF-α, and MPO levels were detected in the lungs of mice that received the premixed saline and bacteria (‡, p < 0.05, vs. the no bacteria control); these levels decreased slightly in the lungs of mice that received low titer hu-IgG (*, p < 0.05, vs. saline premixed with TNF-α). Significantly lower levels of IL-1β, IL-6, TNF-α, and MPO were detected in the lungs of mice treated with premixtures of high titer hu-IgG or anti-PcrV rab-IgG (*, p < 0.05, vs. saline premixture).
Figure 2.
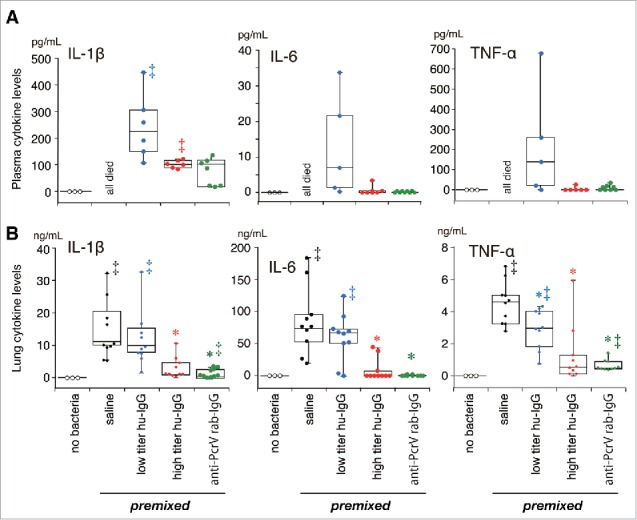
Cytokine concentrations in plasma (A) and lung homogenates (B) in the premixed setting. Saline, low titer hu-IgG, high titer hu-IgG, or anti-PcrV rab-IgG was premixed with a lethal dose (1.5 × 106 CFU/mouse) of Pseudomonas aeruginosa PA103 just before tracheal instillation. Blood from the surviving mice and the lungs from all mice were collected 24 h post-instillation. Data are presented as median, first (boxes), and second quartiles (bars) with each value indicated by circles; *, p < 0.05 compared with the saline-premixed group; ‡, p < 0.05 compared with the no bacteria control group.
Figure 3.
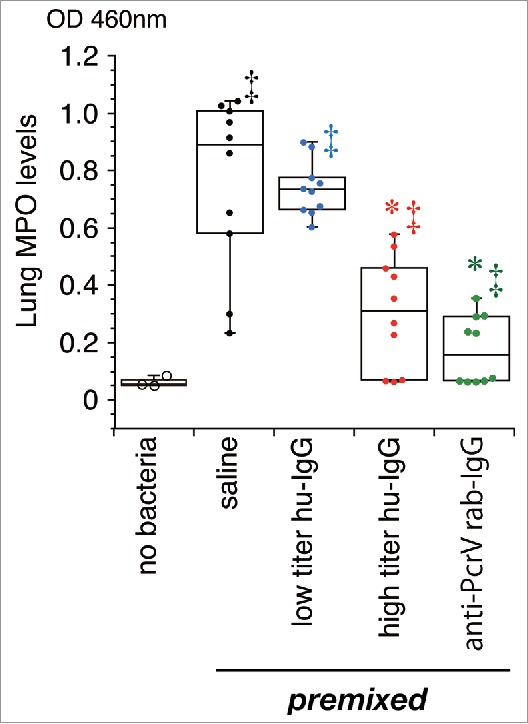
Myeloperoxidase activity in lung homogenates. Saline, low titer hu-IgG, high titer hu-IgG, or anti-PcrV rab-IgG was administered to the mice and their lungs were removed 24 h post-instillation. MPO: myeloperoxidase activity; Dots represent animals. Data are presented as median, first (boxes), and second quartiles (bars) with each value indicated by circles; *, p < 0.05 compared with the saline-premixed group; ‡, p < 0.05 compared with the no bacteria control group.
In a separate series of experiments, we evaluated lung histology in the mice at 24 h post-intratracheal instillation of a lethal dose of P. aeruginosa (Fig. 4). Edematous morphological changes with inflammatory cell recruitment in the alveolar space were observed in the lungs of mice treated with saline or low titer hu-IgG. The edematous morphological changes were not seen in the lungs of mice treated with premixtures of either high titer hu-IgG or anti-PcrV rab-IgG.
Figure 4.
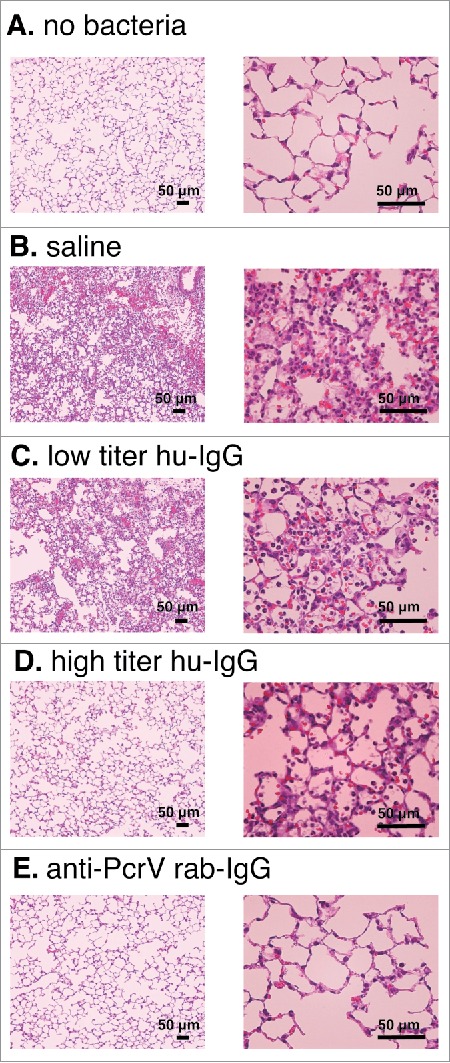
Lung histology 24 h after bacterial instillation. Saline, low titer hu-IgG, high titer hu-IgG, or anti-PcrV rab-IgG was administered to the mice and their lungs were removed 24 h post-instillation. A. saline solution alone (no bacteria). B. P. aeruginosa PA103 was pre-mixed with saline. C. P. aeruginosa PA103 was pre-mixed with low titer hu-IgG. D. P. aeruginosa PA103 was pre-mixed with high titer hu-IgG. E. P. aeruginosa PA103 was pre-mixed with anti-PcrV rab-IgG. Mouse lungs were fixed 10 h after bacterial instillation. Hematoxylin-eosin staining was conducted after 10% formaldehyde fixation and paraffin embedding. Magnification, 100× and 400×.
Pre-treatment with intravenous human IgG (pre-iv)
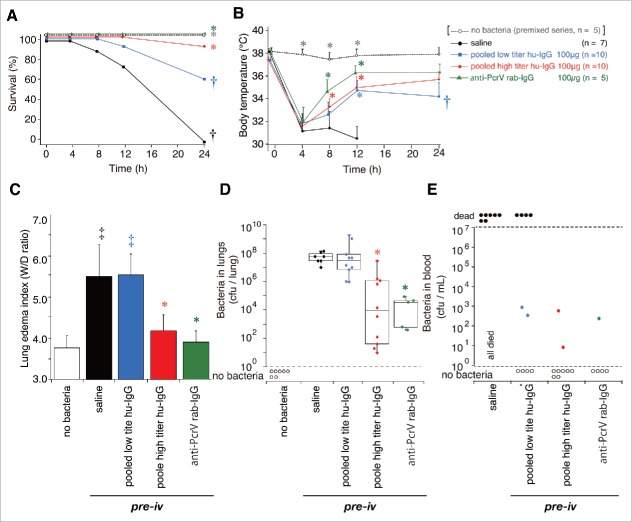
Here, we evaluated the prophylactic effect of serum-derived human IgG on bacterial infection (Fig. 5). In this series of experiments, because of the dose-limitation per mouse, we equally combined 10 purified low titer hu-IgGs (pooled low titer hu-IgG), and 10 high titer hu-IgGs (pooled high titer hu-IgG), and tested them among the 5 following groups: no bacteria, saline alone, pooled low titer hu-IgG (100 µg), pooled high titer hu-IgG (100 µg), and the anti-PcrV rab-IgG (100 µg). The IgGs (100 μg) were intravenously administered 1 h prior to tracheal instillation of a lethal dose of P. aeruginosa. Next, mouse survival and body temperature were monitored for 24 h post-infection (Fig. 5A and B). Mice in the infected control groups were intravenously administered saline (100 µL) or 100 µg of anti-PcrV rab-IgG (100 µL) 1 h prior to bacterial instillation. All mice that were intravenously administered saline 1 h prior to bacterial infection became severely hypothermic at 4 and 12 h post-administration, and died within 16 h. In contrast, all mice intravenously administered 100 µg anti-PcrV rab-IgG recovered from temporal hypothermia at 4 h, and survived for 24 h. Sixty percent of the mice that received pooled low titer hu-IgG survived for 24 h (†, p < 0.05, vs. anti-PcrV rab-IgG-pretreatment; N.S. vs. saline pretreatment), and the survivors were still hypothermic at 24 h post-administration (†, p < 0.05, vs. anti-PcrV rab-IgG pretreatment). Although the pooled high titer hu-IgG-treated mice were hypothermic after 4 h, 90% of these mice survived for 24 h (*, p < 0.05 vs. saline pretreatment).
Figure 5.
Survival, body temperature, lung edema and bacteriological data in the pre-iv setting. Saline, pooled low titer hu-IgG, pooled high titer hu-IgG, or anti-PcrV rab-IgG was administered 1 h prior to tracheal instillation of a lethal dose (1.5 × 106 CFU/mouse) of P. aeruginosa PA103. A. Survival was monitored for 24 h after infection. B. Mouse body temperature over the 24 h post bacterial instillation period. C. Lung edema index (wet to dry weight ratio, W/D ratio) 24 h after bacterial instillation. D. The number of bacteria remaining in the infected mouse lungs 24 h after bacterial instillation. E. The number of bacteria in the blood of the surviving mice 24 h after bacterial instillation. The data for the 5 non-infected control mice are from the premixed series in Fig. 1. Data for body temperature are presented as the mean + SD. *, p< 0.05, vs. the saline pre-iv group; †, p < 0.05, vs. the 100-µg anti-PcrV rab-IgG pre-iv group; ‡, p < 0.05 vs. the no bacteria control group.
Severe lung edema with W/D weight ratios above 5.0 was observed at 24 h post-bacterial instillation in mice that received saline as well as in mice that received pooled low titer hu-IgG (Fig. 5C). Administration of pooled high titer hu-IgG or anti-PcrV rab-IgG significantly decreased the edema levels in each group with a W/D weight ratio below 4.0 (*, p < 0.05 vs. saline pretreatment).
The number of bacteria in the lungs of all the mice and the bacteremia levels in the lungs of the 24-h survivors were calculated (Fig. 5D and E). First, >107 CFUs (6.0 × 107 and 3.3 × 107 CFU median values) were detected in the lungs of mice treated with saline or the pooled low titer hu-IgG, respectively. Lower numbers of bacteria (9.2 × 103, median value) were detected in the mice treated with the pooled high titer hu-IgG (*, p < 0.05, vs. saline pretreatment). A significantly decreased number of bacteria (2.5 × 104 CFU, median value) was detected in the mice that received anti-PcrV rab-IgG (*, p < 0.05, vs. saline pretreatment). Bacteremia was not detected in most of the survivors pretreated with any of the IgG types.
We measured inflammatory cytokines and MPO activity levels in the lung homogenates of all the mice, and cytokine levels in the blood samples from the 24-h survivors, 24 h after bacterial instillation (Figs. 6 and 7). Three mice that received pretreatment with intravenous saline 1 hour prior to intratracheal instillation of saline (60 µL) without bacteria were treated as the negative control group. No increases in cytokine and MPO activity levels were detected in the non-infected control mice, and no cytokines in the blood were measured in all the mice that received the saline premixture because all of them died within 24 h of the bacterial instillation. Significant increases in IL-1β, IL-6, TNF-α, and MPO levels were detected in the lungs of mice that received intravenous saline before bacterial instillation (‡, p < 0.05, vs. the no bacteria control); these levels did not decrease in the lungs of mice that received pooled low titer hu-IgG. However, lower levels of IL-1β, IL-6, TNF-α, and MPO were detected in the lungs of mice treated with pooled high titer hu-IgG or anti-PcrV rab-IgG (*, p < 0.05, vs. saline pretreatment).
Figure 6.
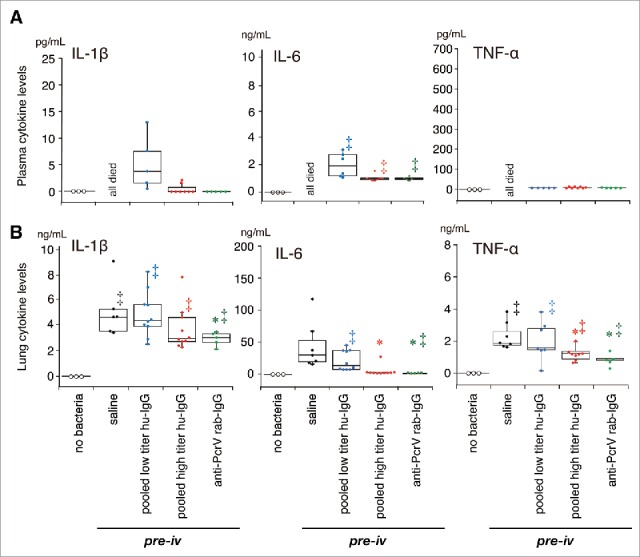
Cytokine concentrations in plasma and lung homogenates in the premixed setting. Saline, pooled low titer hu-IgG, pooled high titer hu-IgG, or anti-PcrV rab-IgG was intravenously administered 1 h prior to tracheal instillation of a lethal dose (1.5 × 106 CFU/mouse) of P. aeruginosa PA103. The blood from the surviving mice and lungs from all the mice were collected 24 h post-instillation. Data are presented as median, first (boxes), and second quartiles (bars) with each value indicated by circles; *, p < 0.05 compared with the saline pre-iv group; ‡, p < 0.05 compared with the no bacteria control group.
Figure 7.
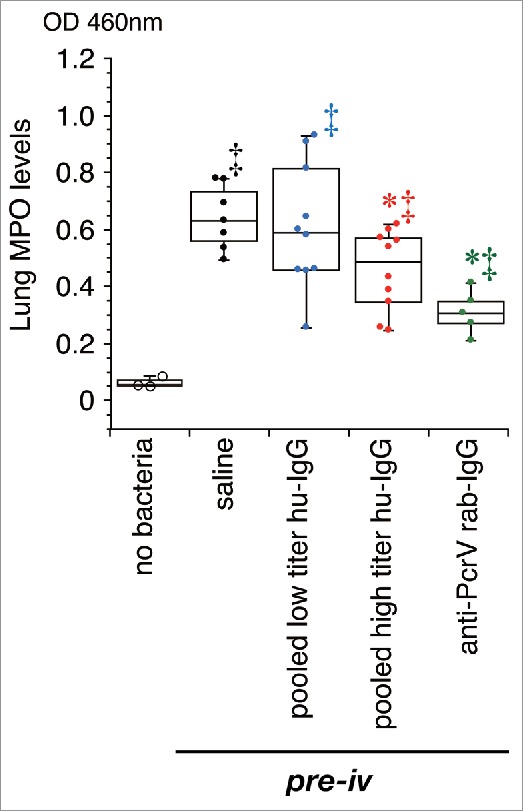
Myeloperoxidase activity in lung homogenates. Saline, pooled low titer hu-IgG, pooled high titer hu-IgG, or anti-PcrV rab-IgG was intravenously administered 1 h prior to tracheal instillation of a lethal dose (1.5 × 106 CFU/mouse) of P. aeruginosa PA103. Mouse lungs were collected 24 h post-instillation. MPO: myeloperoxidase activity; Dots represent animals. Data are presented as median, first (boxes), and second quartiles (bars) with each value indicated by circles; *, p < 0.05 compared with the saline pre-iv group; ‡, p < 0.05 compared with the no bacteria control group.
In a separate series of experiments, we evaluated the lung histology of the mice at 24 h post-intratracheal instillation of a lethal dose of P. aeruginosa (Fig. 8). Inflammatory cell recruitment and alveolar bleeding were observed in the lungs of mice pretreated with saline or pooled low titer hu-IgG. Contrastingly, no morphological changes were seen in the lungs of mice pretreated with pooled high titer hu-IgG or anti-PcrV rab-IgG.
Figure 8.
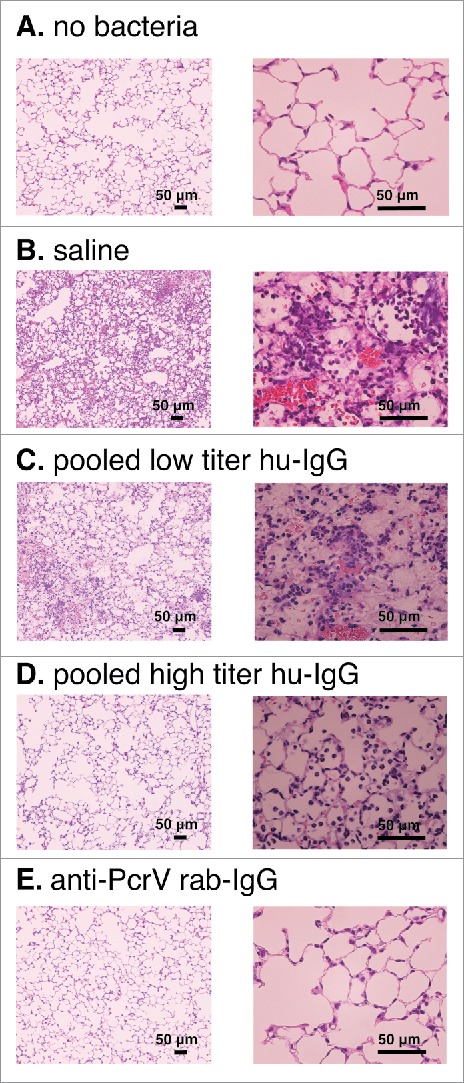
Lung histology 24 h after bacterial instillation. Saline, pooled low titer hu-IgG, pooled high titer hu-IgG, or anti-PcrV rab-IgG was intravenously administered 1 h prior to tracheal instillation of a lethal dose (1.5 × 106 CFU/mouse) of P. aeruginosa PA103. A. no bacterial control (tracheal instillation of saline solution alone). B. intravenously pretreated with saline. C. intravenously pretreated with pooled low titer hu-IgG. D. intravenously pretreated with pooled high titer hu-IgG. E. intravenously pretreated with anti-PcrV rab-IgG. Mouse lungs were fixed 24 h after bacterial instillation. Hematoxylin-eosin staining was conducted after 10% formaldehyde fixation and paraffin embedding. Magnification, 100× and 400×.
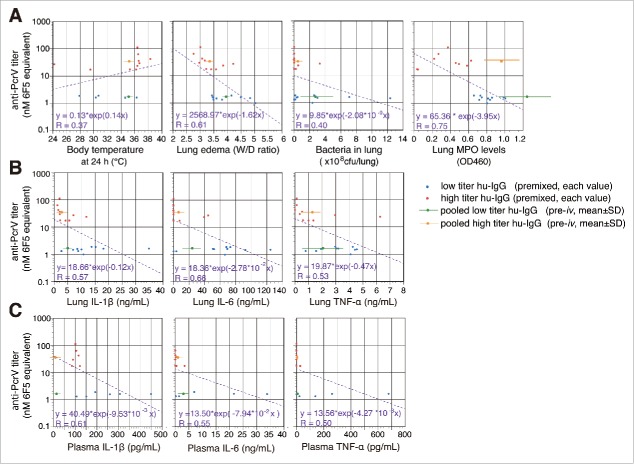
Next, we analyzed the xy-correlation between individual values (x) of the measured parameters in the premixed condition and the anti-PcrV titers (y) for each IgG (Fig. 9). The data (mean ± SD) for the pre-iv series were also plotted on the same figures. The linear regression between the MPO levels in the lungs and the anti-PcrV titers gave the highest correlation coefficient of 0.754 for the parameters measured. The next highest correlation coefficients between the anti-PcrV titers and lung IL-6, lung edema, and plasma IL-1β were all greater than 0.6.
Figure 9.
Correlation and linear regression analyses (in hemi-logarithmic plots) between anti-PcrV titers and various biological values of premixed series. A. The xy correlations between body temperature, lung edema, bacteria in lungs and lung myeloperoxidase levels (x values), and anti-PcrV titers (nM 6F5 equivalent, y values) at 24 h post-infection. B. The xy correlations between various lung cytokine levels (x values) and anti-PcrV titers (y values). C. The xy correlations between plasma cytokine levels (x values) and anti-PcrV titers (y values). Red circles are the values from premixed high titer hu-IgG, and the blue circles are values from premixed low titer hu-IgG. An orange circle with a bar denotes the mean ± SD values from the pooled high titer hu-IgG pre-iv series, and a green circle with a bar denotes the mean ± SD values from the pooled low titer hu-IgG pre-iv series.
Immunoblot analysis of human IgG binding to P. aeruginosa surface proteins
Finally, we analyzed the binding of high and low titer anti-PcrV IgGs to P. aeruginosa surface proteins including PcrV. P. aeruginosa surface proteins were eluted from a bacterial pellet using the osmotic shock method described in Materials and Methods. The eluted proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Recombinant PcrV (306 amino acids, M.W. 33.8 kDa) derived from Escherichia coli was used as a reference. In a silver-stained SDS-PAGE, several intensely stained protein bands, including one corresponding to P. aeruginosa PcrV (294 amino acids, M.W. 32.4 kDa), were identified (Fig. 10A). Next, the electrophoresed proteins were blotted onto a polyvinylidene difluoride blotting membrane and immunostained with human anti-PcrV monoclonal antibody 6F5, pooled low titer hu-IgG, or pooled high titer hu-IgG as the primary antibodies (Fig. 10B and C). Monoclonal anti-PcrV 6F5 recognized both recombinant and P. aeruginosa PcrV among the eluted surface proteins. Pooled low titer IgG detected both E. coli-derived recombinant PcrV and P. aeruginosa PcrV with low intensity, as well as a high-intensity protein of ∼70kDa. Notably, the pooled high titer IgG detected recombinant P. aeruginosa PcrV and non-recombinant P. aeruginosa PcrV the most intensely. The pooled high titer IgG also detected several more smaller- and larger-sized proteins in comparison with the pooled low titer IgG.
Figure 10.
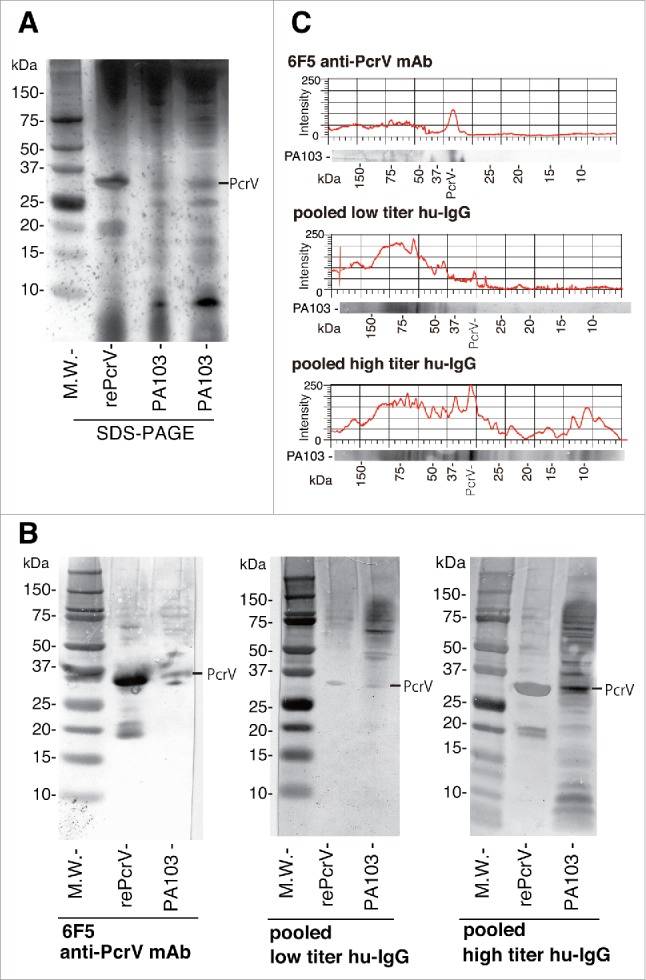
SDS-PAGE and immunoblot analyses. The binding capacity of pooled human IgG against P. aeruginosa PA103 surface proteins was analyzed using osmotic shock elution methodology, SDS-PAGE, and immunoblotting. A. P. aeruginosa surface proteins derived from osmotic shock treatment of the bacteria and recombinant PcrV were separated by SDS-PAGE (4–12% Bis-Tris gel), and the gel was silver-stained. B. After SDS-PAGE, the surface proteins of P. aeruginosa PA103 were electroblotted onto a polyvinylidene difluoride blotting membrane and then incubated with human monoclonal anti-PcrV IgG 6F5, pooled low titer human IgG, or pooled high titer human IgG. A secondary anti-human IgG molecule conjugated with horseradish peroxidase was applied to the blot, after which 3,3′-diaminobenzidine was used to visualize the immunostained protein bands. C. The intensities of the surface protein bands immunostained with human monoclonal anti-PcrV IgG 6F5, pooled low titer human IgG, or pooled high titer human IgG were digitalized in 256 gradations of a gray scale and displayed in xy-plot graphs by using the surface plot function of the image processing software, ImageJ. rePcrV: recombinant PcrV. M.W.: Molecular weight marker.
Discussion
When we recently analyzed the anti-PcrV titers in the sera of the 198 study participants, they ranged between 1.01 nM, and 113.81 nM (median 4.09 nM).28 High anti-PcrV titers beyond an approximate 3-fold rise (exceeding 12 nM) in comparison with the median value were observed in 21 patients (10.6%), and a significantly higher anti-PcrV titer was observed in the 70 y and older group compared with the other age groups.28 In the current study, we selected sera samples containing the top and bottom 10 anti-PcrV titers and extracted the IgG fractions from them.
As a result, the human IgGs from the high anti-PcrV titer sera had significantly higher potency than those from the low anti-PcrV titer sera in terms of their ability to neutralize the type III secretion-associated virulence of P. aeruginosa in the premixed conditions used for our mouse model. Anti-PcrV titers of premixed IgGs were inversely correlated with lung edema indices, lung IL-1β, IL-6 and MPO levels. In addition, intravenous administration of the pooled high titer hu-IgG was more prophylactic than administration of low titer hu-IgG. In our immunoblot analyses where the interactions of pooled low and high hu-IgG to P. aeruginosa surface proteins were tested, the pooled high titer hu-IgG showed a high binding capacity to P. aeruginosa among the various P. aeruginosa surface proteins, while the pooled low titer hu-IgG weakly recognized P. aeruginosa PcrV. In addition, the pooled high titer hu-IgG had the capacity to bind more non-PcrV P. aeruginosa surface proteins than pooled low titer hu-IgG. These results imply that high titer anti-PcrV serum from individuals is protective against virulent P. aeruginosa, predominantly by PcrV blockade, and that the antibody fraction from such high titer sera can target other surface proteins in this bacterium, thereby enhancing its prophylactic potency, as we have reported previously.26 Therefore, in addition to the inhibition of type III secretion-associated virulence by PcrV blockade, human IgG that contains specificities to other P. aeruginosa surface antigens might have contributed to the biological effects observed in our animal models.
Regardless of active or passive immunization, high titer anti-PcrV sera had prophylactic properties against virulent P. aeruginosa infections, and the IgG fraction derived from such high titer sera is a potential source of immunoglobulin products for treatment of P. aeruginosa pneumonia. While intravenous immunoglobulin (IVIG) has been used against infectious diseases with the expectation of opsonization, immune bacteriolysis and neutralization of toxins and viruses, our results suggest that in antibody-dependent cellular cytotoxicity the role of specific antibody fractions against pathogens in IVIG products should be more focused.26 Recent meta-analysis and systematic reviews have highlighted the requirement for more evidence from large, well-conducted randomized controlled trials.29-31 However, because IVIG products are derived from donor blood, product-by-product (or lot-by-lot) differences in the specific antibody titers in the IVIG should always be taken into account when interpreting the data from clinical trials on IVIG therapy performed in various countries.
Recent worldwide propagation of multi-drug resistant bacteria has enhanced the strong anticipation for the development of anti-bacterial antibody therapies for various Gram-negative bacterial nosocomial infections. However, although many trials of antibacterial monoclonal antibodies have been conducted for bacterial infections, no antibody-based products, except Obiltoxaximab for Bacillus anthracis 32, have proceeded to clinical application.33-35 Even in the arena of common infectious diseases, Palivizumab (Synagis® MedImmune, LLC. Gaithersburg, Maryland, USA), which is marketed for the prevention of respiratory syncytial virus in high-risk babies, is almost the only recombinant antibody in clinical use,36,37 although many anti-cancer monoclonal antibodies are in full clinical use.38 We have investigated the development of a monoclonal antibody targeting the P. aeruginosa virulence factor type III secretion system. Our target is PcrV, which forms the tip of the type III secretion apparatus responsible for the delivery of type III secretory toxins (i.e., ExoS, ExoT, ExoU, and ExoY) into target cells.12 Based on the preclinical data for the mAb166 murine anti-PcrV monoclonal antibody in various animal models,19,20,22,39 a humaneered anti-PcrV monoclonal antibody (KB001-A, KaloBios Pharmaceuticals) has been developed23 and tested for treating P. aeruginosa pneumonia in patients with cystic fibrosis and in mechanically ventilated patients in Phase II studies in the United States24 and France.25 Although KB001-A was generally safe and well-tolerated in these trials, the candidate molecule failed to demonstrate an increase in the time to needing antibiotics for worsening respiratory tract signs and symptoms, therefore showing that KB001-A did not reduce the risk of pulmonary disease exacerbation. Consequently, further development of KB001-A has halted before conducting the larger efficacy studies that were expected in the near future. As mentioned by others,40 most of the failed antibacterial monoclonal antibodies target a single bacterial antigen, while anthrax monoclonal antibodies are a cocktail of anti-toxin and anti-capsule monoclonal antibodies. To obtain anti-infective monoclonal mixtures, the need to target not only different epitopes on the same antigen, but different antigens also seems highly likely.
When we examine the immunoglobulin products from blood donors, several old and new products have been marketed for infectious diseases. Tetanobulin, a blood product for treatment of tetanus caused by Clostridium tetani, is still the only commercially available immunoglobulin product. Anthrax immune globulin (Anthrasil®, Emergent BioSolutions, Gaithersburg, MD, USA), which was granted approval by the US. Food and Drug Administration for treating inhalation anthrax in conjunction with antibiotics in 2015, is a human immune globulin that is used in combination with antibiotics to treat anthrax.41-43 Human hepatitis B immunoglobulin products (Nabi-HB®, Biotest Pharmaceuticals Co, Boca Raton, FL, USA; HepaGam B® Emergent BioSolutions, Gaithersburg, MD, USA; HyperHEP B®, Grifols Therapeutics Inc. Research Triangle Park, NC, USA) provide passive immunization for individuals exposed to the hepatitis B virus as evidenced by a reduction in the attack rate of hepatitis B following their use.37,44,45 These products, which contain antibodies to the hepatitis B surface antigen (anti-HBs), are prepared from plasma donated by individuals with high anti-HBs titers. Varicella-Zoster immunoglobulin (VariZIG®, Emergent BioSolutions), which is purified human IgG, contains antibodies to varicella zoster virus and provides passive immunization for non-immune individuals exposed to varicella zoster virus, thereby reducing the severity of varicella infections.46,47 VariZIG® is specifically indicated for post-exposure prophylaxis to varicella virus in high-risk individuals, such as immunocompromised children and adults, and the newborns of mothers with varicella shortly before or after delivery. Vaccinia immunoglobulin (VIGIV®, vaccinia immune globulin intravenous, Emergent BioSolutions) is the purified gamma globulin fraction from human plasma that contains antibodies to vaccinia virus via boosting with the vaccinia vaccine prior to plasma donation.48,49
As we have discussed above, when considering immunotherapies for infectious diseases, immunoglobulin products derived from blood donors are currently more active and look more promising than therapeutic strategies that rely on recombinant monoclonal antibody technologies. The results of our study raise the possibility that if we can determine the specific antigen titers against the P. aeruginosa PcrV antigen in serum derived from blood donors, immunoglobulin products derived from high anti-PcrV titer sera have potential to become potent prophylactic and therapeutic tools against lethal P. aeruginosa infections. Development of an immunoglobulin product against virulent P. aeruginosa may circumvent the struggle there has been to develop recombinant monoclonal antibody strategies against this bacterium.
Conclusion
Human IgGs extracted from human sera containing high anti-PcrV titers were tested for their prophylactic effects against P. aeruginosa pneumonia in a mouse model. Each IgG fraction extracted from high titer serum improved the acute lung injury and survival rate of mice infected with virulent P. aeruginosa. Prophylactic administration of pooled IgG extracted from high titer sera, which showed binding capacity to P. aeruginosa PcrV, was more effective than prophylactic administration of its low titer pooled equivalent. The results imply that the protective capacity of human IgG is conferred by inhibiting type III secretion-associated virulence through PcrV blockade, as well as via specificities to other P. aeruginosa surface antigens, which might have contributed to the adjunctive biological effect. These results raise the possibility of high anti-PcrV blood titers helping to protect people against virulent P. aeruginosa infections, and that IgG fractions from these high titer sera could be a source of specific IVIG products that can specifically target P. aeruginosa.
Materials and methods
This study was approved by the Kyoto Prefectural University of Medicine Ethics Committee, Japan.
Immunoglobulin
From April 2012 to March 2013, 198 adult patients who had anesthesia for scheduled surgeries in the operating theater of the Kyoto Prefectural University of Medicine under the management of the Department of Anesthesiology, Kyoto Prefectural University of Medicine, participated as volunteers in this study. Written informed consent for blood sample collection for serum titer measurement and purification of immunoglobulin was obtained from each study participant. Human serum-derived IgG molecules were prepared by affinity column chromatography using a protein A sepharose column (HiTrap Protein A HP, GE healthcare Life Sciences, #17-0402-01), according to the manufacturer's instructions. Affinity column chromatography based on a starting volume of 1 mL of serum produced 3 mg of IgG. The eluted IgG was dialyzed against phosphate-buffered saline for 72 h. Aliquots were stored at −80°C. The anti-PcrV polyclonal IgG fraction was prepared from a rabbit vaccinated (Kitayama Labes Co. Ltd., Ina, Japan) with recombinant PcrV.
P. aeruginosa and culture conditions
The cytotoxic P. aeruginosa PA103 strain was used in this study. PA103 came originally from a patient in Australia in the late 1960s, and is a cytotoxic strain with the exoS−, exoT+, exoU+, and exoY+ type III secretion system genotype, but has a negative ExoY secretion phenotype by virtue of a premature stop codon mutation.50-52 PA103, stored as individual bacterial stocks at −80°C, was prepared for the experiments as described previously.53,54 Briefly, bacterial cultures were grown at 33°C for 13 h in a shaking incubator, and then centrifuged at 8,500 × g for 5 min. The bacterial pellet was washed 3 times in lactated Ringer's solution and then diluted to the appropriate number of CFUs per milliliter, as determined by spectrophotometry (MTP-880Lab, Corona Electric Co., Hitachinaka, Japan). The number of bacteria was confirmed by determining the CFU of the diluted aliquots on sheep blood agar plates.
Anti-PcrV titer measurement
Quantification of the anti-PcrV titers was performed using an enzyme-linked immunosorbent assay (ELISA).28 Micro-well plates (Nunc C96 Maxisorp, Thermo Fisher Scientific Inc., #430341) were coated for 2 h at 4°C with recombinant PcrV suspended in coating buffer (1.5 μg/mL in coating solution, 0.05M NaHCO3, pH 9.6). Plates were washed twice with phosphate-buffered saline (PBS) with 0.05% Tween-20 (Sigma-Aldrich Co., P9416), and then blocked with 200 μL of 1% bovine serum albumin/PBS overnight at 4°C. Samples (serial dilution: 32–1024×) were applied to the plates (100 μL/well) and incubated overnight at 4°C. Peroxidase-labeled anti-rabbit IgG (Sigma-Aldrich Co., A6154) or peroxidase-labeled anti-human IgG (Sigma-Aldrich Co., A8667) were applied at 1:60,000 for 1 h at 37°C. Then, after 6 washes, the plates were incubated with 2,2′-azino-bis (3ethylbenzthiazoline-6-sulfonic acid) (ABTS, Sigma-Aldrich Co., A3219) at room temperature for 30 min. After the addition of 0.5 M H2SO4 (100 μL/well) to the plates, the optical density (OD) at 450 nm was measured by a microplate reader (MTP-880Lab), and the dilution indices for ODs > 0.15 were considered positive. In the ELISA assays, the human anti-PcrV monoclonal antibody 6F5 (Shionogi & Co., Ltd., Japan) was used as a standard to quantify the anti-PcrV titers as concentrations of monoclonal antibody 6F5 equivalents.
Protocols used for the animal studies
Male ICR mice (5 weeks old; body weight, 25 g) certified pathogen-free, were purchased from Shimizu Laboratory Supplies, Co. Ltd. (Kyoto, Japan). Mice were housed in cages with filter tops under pathogen-free conditions. The protocols for all animal experiments were approved by the Animal Research Committee of Kyoto Prefectural University of Medicine, Kyoto, Japan, prior to starting the experiments.
Mouse survival studies and lung edema index, bacteriology, cytokines, myeloperoxidase (MPO) activities, and lung histology
Intratracheal infections were induced using an endotracheal needle under short-term volatile anesthesia.53-55 The mice were briefly anesthetized with inhaled sevoflurane (Sevofrane®, Maruishi Pharmaceutical Co., Osaka, Japan) and were placed supine, at an angle of −30 degrees. The bacterial solution (60 μL) was instilled slowly into the left lobe of the lungs through a gavage needle (modified animal feeding needle, 24-gauge, Popper & Sons, Inc., #20068-606) inserted into the trachea via the oropharynx. The proper insertion of the needle in the trachea was confirmed by palpation of the tip of the blunt needle through the skin and the observation of movement of the solution inside the syringe with respiratory efforts. Once awake, the mice were returned to their cages, monitored regularly, and allowed access to food and water. Rectal temperature measurements were recorded and mouse survival was monitored for 24 h. After 24 h, the surviving mice were euthanized after the collection of arterial blood, and the lungs were harvested from the mice for physiological, microbiological, and biomolecular assays. The lung edema index was obtained by measuring the W/D weight ratios of the mouse lungs at the 24 h time point, as described previously.53,54 The concentrations of interleukin-1 β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the lung homogenates and in the plasma were quantified using an ELISA kit (BD OptEIA ELISA Sets, BD Biosciences, #559603, #555240, and #555268) according to the manufacturer's instructions. The MPO activities in the lung homogenates were measured using a previously described biochemical method.56 For the bacteriological assays, the lung homogenates and the collected blood were diluted and plated on sheep blood agar plates for quantitative assessment of the bacterial counts. For fixation, mouse lungs were perfused with 10% buffered formalin phosphate and then embedded in paraffin. Mounted sections that had been stained with hematoxylin and eosin were observed by light microscopy. In the pre-mixed series, anti-PcrV rab-IgG, human IgG, or saline was mixed with the P. aeruginosa solution (1.5 × 106 CFU, 60 μL) just before tracheal instillation into each animal's lungs. In the pre-iv series, saline, pooled human IgG, or anti-PcrV rab-IgG was intravenously administered to each mouse one h before the infection.
Immunoblot analysis
For the immunoblot analysis, a pellet of P. aeruginosa PA103 from a culture maintained in trypticase soy broth overnight was resuspended in 100 µL of osmotic shock solution #1 (20 mM Tris-HCl pH 8, 2.5 mM EDTA, 20% sucrose), and then incubated on ice for 10 min. The solution was centrifuged for 1 min at 4°C and the supernatant was decanted. Cell pellets were resuspended in 100 µL of osmotic shock solution #2 (20 mM Tris-HCl pH 8, 2.5 mM EDTA), incubated on ice for 10 min, and centrifuged for 10 min at 4°C. The supernatants (shock fluid, 10 μL aliquots for each sample) were mixed with sample buffer and then subjected to SDS-PAGE (4–12% NuPAGE Bis-Tris Gel, Thermo Fisher Scientific, Waltham, Massachusetts, USA) followed by silver-staining (Silver Stain Plus, Bio-Rad Laboratories, Hercules, CA, USA) of the gels. Alternatively, following SDS-PAGE, the proteins were blotted onto polyvinylidene difluoride blotting membranes (iBlot Gel Transfer Stacks PVDF, Mini, Novex) using an electroblotter (iBlot Gel Transfer Device, Invitrogen). Membranes were immunostained with pooled hu-IgG (10 µg/mL) or human anti-PcrV monoclonal antibody 6F5 (16 ng/mL, Shionogi & Co., Ltd.) as the primary antibodies, and anti-human IgG conjugated with horseradish peroxidase (A8667, Sigma-Aldrich Co.) as the secondary antibody; 3,3′-diaminobenzidine (SLBM4338V, Sigma-Aldrich Co.) was used as the developer. The stained membranes were analyzed with the image processing software, ImageJ.57
Statistical analysis
The Mantel-Cox log rank test was used to assess mouse survival with Prism4 (GraphPad Software, La Jolla, CA, USA). Differences in the body temperatures and the W/D weight ratio between the treatment groups were compared by an unpaired t test, and the Mann–Whitney U test was used for the bacteriology comparisons (Instat3, GraphPad Software, La Jolla, CA, USA). A p-value of < 0.05 was considered statistically significant. The curve fittings between the anti-PcrV titers and the individual biological values were performed using DeltaGraph 7 (Red Rock Software Inc., Salt Lake City, UT, USA).
Abbreviations
- Anti-PcrV rab-IgG
rabbit-derived polyclonal anti-PcrV IgG
- CFUs
colony forming units
- E. coli
Escherichia coli
- high titer hu-IgG
human IgG with high anti-PcrV titer
- low titer hu-IgG
human IgG with low anti-PcrV titer
- IVIG
intravenous immunoglobulin
- MDRP
multidrug resistant Pseudomonas aeruginosa
- MPO
myeloperoxidase
- OD
optical density
- PBS
phosphate-buffered saline
- P. aeruginosa
Pseudomonas aeruginosa
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- W/D weight ratios
wet to dry weight ratios
Disclosure of potential conflicts of interest
With regard to the content of the manuscript, Teiji Sawa received a patent fee from the Regent of the University of California, USA.
Author contributions
TS designed, coordinated and supervised the research. PL, ET, AT developed the economic model and carried out the economic analysis. HY, MK collected clinical samples and analyzed epidemiological data. MK, HK, MS, SH, YN carried out the animal experiments and biological assays. KM assisted the serum titer measurement. TS and MK wrote the manuscript. All authors revised the manuscript and contributed to improving the paper; all authors read and approved the final manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (KAKENHI No. 24390403, No. 26670791, and No. 15H05008) and from The Ministry of Education, Culture, Sports, Science and Technology, Japan to Teiji Sawa.
References
- [1].Bassetti M, Merelli M, Temperoni C, Astilean A. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob 2013; 12:22; PMID:23984642; http://dx.doi.org/ 10.1186/1476-0711-12-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Viale P, Giannella M, Tedeschi S, Lewis R. Treatment of MDR-Gram negative infections in the 21st century: a never ending threat for clinicians. Curr Opin Pharmacol 2015; 24:30-7; PMID:26210268; http://dx.doi.org/ 10.1016/j.coph.2015.07.001 [DOI] [PubMed] [Google Scholar]
- [3].Rello J, Lisboa T, Koulenti D. Respiratory infections in patients undergoing mechanical ventilation. Lancet Respir Med 2014; 2:764-74; PMID:25151022; http://dx.doi.org/ 10.1016/S2213-2600(14)70171-7 [DOI] [PubMed] [Google Scholar]
- [4].Pena C, Gomez-Zorrilla S, Suarez C, Dominguez MA, Tubau F, Arch O, Oliver A, Pujol M, Ariza J. Extensively drug-resistant Pseudomonas aeruginosa: risk of bloodstream infection in hospitalized patients. Eur J Clin Microbiol Infect Dis 2012; 31:2791-7; PMID:22552893; http://dx.doi.org/ 10.1007/s10096-012-1629-3 [DOI] [PubMed] [Google Scholar]
- [5].Tumbarello M, De Pascale G, Trecarichi EM, Spanu T, Antonicelli F, Maviglia R, Pennisi MA, Bello G, Antonelli M. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med 2013; 39:682-92; PMID:23370828; http://dx.doi.org/ 10.1007/s00134-013-2828-9 [DOI] [PubMed] [Google Scholar]
- [6].Vettoretti L, Floret N, Hocquet D, Dehecq B, Plesiat P, Talon D, Bertrand X. Emergence of extensive-drug-resistant Pseudomonas aeruginosa in a French university hospital. Eur J Clin Microbiol Infect Dis 2009; 28:1217-22; PMID:19504273; http://dx.doi.org/ 10.1007/s10096-009-0767-8 [DOI] [PubMed] [Google Scholar]
- [7].Park YS, Lee H, Chin BS, Han SH, Hong SG, Hong SK, Kim HY, Uh Y, Shin HB, Choo EJ, et al.. Acquisition of extensive drug-resistant Pseudomonas aeruginosa among hospitalized patients: risk factors and resistance mechanisms to carbapenems. J Hosp Infect 2011; 79:54-8; PMID:21764173; http://dx.doi.org/ 10.1016/j.jhin.2011.05.014 [DOI] [PubMed] [Google Scholar]
- [8].Liew YX, Tan TT, Lee W, Ng JL, Chia DQ, Wong GC, Kwa AL. Risk factors for extreme-drug resistant Pseudomonas aeruginosa infections in patients with hematologic malignancies. Am J Infect Control 2013; 41:140-4; PMID:22795726; http://dx.doi.org/ 10.1016/j.ajic.2012.02.025 [DOI] [PubMed] [Google Scholar]
- [9].Sawa T, Shimizu M, Moriyama K, Wiener-Kronish JP. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit Care 2014; 18:668; PMID:25672496; http://dx.doi.org/ 10.1186/s13054-014-0668-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sawa T, Ito E, Nguyen VH, Haight M. Anti-PcrV antibody strategies against virulent Pseudomonas aeruginosa. Hum Vaccin Immunother 2014; 10:2843-52; PMID:25483637; http://dx.doi.org/ 10.4161/21645515.2014.971641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sawa T. The molecular mechanism of acute lung injury caused by Pseudomonas aeruginosa: from bacterial pathogenesis to host response. J Intensive Care 2014; 2:10; PMID:25520826; http://dx.doi.org/ 10.1186/2052-0492-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sawa T, Katoh H, Yasumoto H. V-antigen homologs in pathogenic gram-negative bacteria. Microbiol Immunol 2014; 58:267-85; PMID:24641673; http://dx.doi.org/ 10.1111/1348-0421.12147 [DOI] [PubMed] [Google Scholar]
- [13].Sawa T, Wiener-Kronish JP. A therapeutic strategy against the shared virulence mechanism utilized by both Yersinia pestis and Pseudomonas aeruginosa. Anesthesiol Clin North America 2004; 22:591-606, viii-ix; PMID:15325721; http://dx.doi.org/ 10.1016/j.atc.2004.05.002 [DOI] [PubMed] [Google Scholar]
- [14].Sawa T, Yahr TL, Ohara M, Kurahashi K, Gropper MA, Wiener-Kronish JP, Frank DW. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med 1999; 5:392-8; PMID:10202927; http://dx.doi.org/ 10.1038/7391 [DOI] [PubMed] [Google Scholar]
- [15].Moriyama K, Wiener-Kronish JP, Sawa T. Protective effects of affinity-purified antibody and truncated vaccines against Pseudomonas aeruginosa V-antigen in neutropenic mice. Microbiol Immunol 2009; 53:587-94; PMID:19903258; http://dx.doi.org/ 10.1111/j.1348-0421.2009.00165.x [DOI] [PubMed] [Google Scholar]
- [16].Holder IA, Neely AN, Frank DW. PcrV immunization enhances survival of burned Pseudomonas aeruginosa-infected mice. Infect Immun 2001; 69:5908-10; PMID:11500471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Imamura Y, Yanagihara K, Fukuda Y, Kaneko Y, Seki M, Izumikawa K, Miyazaki Y, Hirakata Y, Sawa T, Wiener-Kronish JP, et al.. Effect of anti-PcrV antibody in a murine chronic airway Pseudomonas aeruginosa infection model. Eur Respir J 2007; 29:965-8; PMID:17301098; http://dx.doi.org/ 10.1183/09031936.00147406 [DOI] [PubMed] [Google Scholar]
- [18].Neely AN, Holder IA, Wiener-Kronish JP, Sawa T. Passive anti-PcrV treatment protects burned mice against Pseudomonas aeruginosa challenge. Burns 2005; 31:153-8; PMID:15683685; http://dx.doi.org/ 10.1016/j.burns.2004.09.002 [DOI] [PubMed] [Google Scholar]
- [19].Faure K, Fujimoto J, Shimabukuro DW, Ajayi T, Shime N, Moriyama K, Spack EG, Wiener-Kronish JP, Sawa T. Effects of monoclonal anti-PcrV antibody on Pseudomonas aeruginosa-induced acute lung injury in a rat model. J Immune Based Ther Vaccines 2003; 1:2; PMID:12089663; http://dx.doi.org/ 10.1186/1476-8518-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Frank DW, Vallis A, Wiener-Kronish JP, Roy-Burman A, Spack EG, Mullaney BP, Megdoud M, Marks JD, Fritz R, Sawa T. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J Infect Dis 2002; 186:64-73; PMID:12943554; http://dx.doi.org/ 10.1086/341069 [DOI] [PubMed] [Google Scholar]
- [21].Shime N, Sawa T, Fujimoto J, Faure K, Allmond LR, Karaca T, Swanson BL, Spack EG, Wiener-Kronish JP. Therapeutic administration of anti-PcrV F(ab')2 in sepsis associated with Pseudomonas aeruginosa. J Immunol 2001; 167:5880-6; PMID:11698464; http://dx.doi.org/ 10.1086/341069 [DOI] [PubMed] [Google Scholar]
- [22].Song Y, Baer M, Srinivasan R, Lima J, Yarranton G, Bebbington C, Lynch SV. PcrV antibody-antibiotic combination improves survival in Pseudomonas aeruginosa-infected mice. Eur J Clin Microbiol Infect Dis 2012; 31:1837-45; PMID:22187351; http://dx.doi.org/ 10.1007/s10096-011-1509-2 [DOI] [PubMed] [Google Scholar]
- [23].Baer M, Sawa T, Flynn P, Luehrsen K, Martinez D, Wiener-Kronish JP, Yarranton G, Bebbington C. An engineered human antibody fab fragment specific for Pseudomonas aeruginosa PcrV antigen has potent antibacterial activity. Infect Immun 2009; 77:1083-90; PMID:19103766; http://dx.doi.org/ 10.1128/IAI.00815-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Milla CE, Chmiel JF, Accurso FJ, VanDevanter DR, Konstan MW, Yarranton G, Geller DE, KB Study Group . Anti-PcrV antibody in cystic fibrosis: a novel approach targeting Pseudomonas aeruginosa airway infection. Pediatr Pulmonol 2014; 49:650-8; PMID:24019259; http://dx.doi.org/ 10.1002/ppul.22890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Francois B, Luyt CE, Dugard A, Wolff M, Diehl JL, Jaber S, Forel JM, Garot D, Kipnis E, Mebazaa A, et al.. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trial. Crit Care Med 2012; 40:2320-6; PMID:22622405; http://dx.doi.org/ 10.1097/CCM.0b013e31825334f6 [DOI] [PubMed] [Google Scholar]
- [26].Katoh H, Yasumoto H, Shimizu M, Hamaoka S, Kinoshita M, Akiyama K, Sawa T. IV immunoglobulin for acute lung injury and bacteremia in Pseudomonas aeruginosa pneumonia. Crit Care Med 2016; 44:e12-24; PMID:26317571; http://dx.doi.org/ 10.1097/CCM.0000000000001271 [DOI] [PubMed] [Google Scholar]
- [27].Shimizu M, Katoh H, Hamaoka S, Kinoshita M, Akiyama K, Naito Y, Sawa T. Protective effects of intravenous immunoglobulin and antimicrobial agents on acute pneumonia in leukopenic mice. J Infect Chemother 2016; 22:240-7; PMID:26867796; http://dx.doi.org/ 10.1016/j.jiac.2016.01.006 [DOI] [PubMed] [Google Scholar]
- [28].Yasumoto H, Katoh H, Kinoshita M, Shimizu M, Hamaoka S, Akiyama K, Naito Y, Sawa T. Epidemiological analysis of serum anti-Pseudomonas aeruginosa PcrV titers in adults. Microbiol Immunol 2016; 60:114-20; PMID:26696420; http://dx.doi.org/ 10.1111/1348-0421.12353 [DOI] [PubMed] [Google Scholar]
- [29].Turgeon AF, Hutton B, Fergusson DA, McIntyre L, Tinmouth AA, Cameron DW, Hebert PC. Meta-analysis: intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med 2007; 146:193-203; PMID:17283351 [DOI] [PubMed] [Google Scholar]
- [30].Laupland KB, Kirkpatrick AW, Delaney A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med 2007; 35:2686-92; PMID:18074465; http://dx.doi.org/ 10.1111/1348-0421.12353 [DOI] [PubMed] [Google Scholar]
- [31].Pildal J, Gotzsche PC. Polyclonal immunoglobulin for treatment of bacterial sepsis: a systematic review. Clin Infect Dis 2004; 39:38-46; PMID:15206051; http://dx.doi.org/ 10.1086/421089 [DOI] [PubMed] [Google Scholar]
- [32].Greig SL. Obiltoxaximab: First Global Approval. Drugs 2016; PMID:15206051; http://dx.doi.org/ 10.1007/s40265-016-0577-0 [DOI] [PubMed] [Google Scholar]
- [33].Reichert JM. Antibodies to watch in 2016. MAbs 2016; 8:197-204; PMID:26651519; http://dx.doi.org/ 10.1080/19420862.2015.1125583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reichert JM. Antibodies to watch in 2015. MAbs 2015; 7:1-8; PMID:25484055; http://dx.doi.org/ 10.4161/19420862.2015.988944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Reichert JM. Antibodies to watch in 2014. MAbs 2014; 6:5-14; PMID:24284914; http://dx.doi.org/ 10.4161/mabs.27333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sandritter T. Palivizumab for respiratory syncytial virus prophylaxis. J Pediatr Health Care 1999; 13:191-5; quiz 6-7; PMID:10690084; http://dx.doi.org/ 10.1016/S0891-5245(99)90039-1 [DOI] [PubMed] [Google Scholar]
- [37].Meissner HC, Welliver RC, Chartrand SA, Law BJ, Weisman LE, Dorkin HL, Rodriguez WJ. Immunoprophylaxis with palivizumab, a humanized respiratory syncytial virus monoclonal antibody, for prevention of respiratory syncytial virus infection in high risk infants: a consensus opinion. Pediatr Infect Dis J 1999; 18:223-31; PMID:19504273; http://dx.doi.org/ 10.1007/s10096-009-0767-8 [DOI] [PubMed] [Google Scholar]
- [38].Modjtahedi H, Ali S, Essapen S. Therapeutic application of monoclonal antibodies in cancer: advances and challenges. Br Med Bull 2012; 104:41-59; PMID:23118261; http://dx.doi.org/ 10.1093/bmb/lds032 [DOI] [PubMed] [Google Scholar]
- [39].Wang Q, Li H, Zhou J, Zhong M, Zhu D, Feng N, Liu F, Bai C, Song Y. PcrV antibody protects multi-drug resistant Pseudomonas aeruginosa induced acute lung injury. Respir Physiol Neurobiol 2014; 193:21-8; PMID:24418353; http://dx.doi.org/ 10.1016/j.resp.2014.01.001 [DOI] [PubMed] [Google Scholar]
- [40].Oleksiewicz MB, Nagy G, Nagy E. Anti-bacterial monoclonal antibodies: back to the future? Arch Biochem Biophys 2012; 526:124-31; PMID:22705202; http://dx.doi.org/ 10.1016/j.abb.2012.06.001 [DOI] [PubMed] [Google Scholar]
- [41].Kammanadiminti S, Patnaikuni RK, Comer J, Meister G, Sinclair C, Kodihalli S. Combination therapy with antibiotics and anthrax immune globulin intravenous (AIGIV) is potentially more effective than antibiotics alone in rabbit model of inhalational anthrax. PLoS One 2014; 9:e106393; PMID:25226075; http://dx.doi.org/ 10.1371/journal.pone.0106393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mytle N, Hopkins RJ, Malkevich NV, Basu S, Meister GT, Sanford DC, Comer JE, Van Zandt KE, Al-Ibrahim M, Kramer WG, et al.. Evaluation of intravenous anthrax immune globulin for treatment of inhalation anthrax. Antimicrob Agents Chemother 2013; 57:5684-92; PMID:23979731; http://dx.doi.org/ 10.1128/AAC.00458-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Malkevich NV, Basu S, Rudge TL Jr., Clement KH, Chakrabarti AC, Aimes RT, Nabors GS, Skiadopoulos MH, Ionin B. Effect of anthrax immune globulin on response to BioThrax (anthrax vaccine adsorbed) in New Zealand white rabbits. Antimicrob Agents Chemother 2013; 57:5693-6; PMID:23979740; http://dx.doi.org/ 10.1128/AAC.00460-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hoofnagle JH, Seeff LB, Bales ZB, Wright EC, Zimmerman HJ. Passive-active immunity from hepatitis B immune globulin. Reanalysis of a Veterans Administration cooperative study of needle-stick hepatitis. The Veterans Administration Cooperative Study Group. Ann Intern Med 1979; 91:813-8; PMID:517881 [DOI] [PubMed] [Google Scholar]
- [45].Seeff LB, Wright EC, Zimmerman HJ, Alter HJ, Dietz AA, Felsher BF, Finkelstein JD, Garcia-Pont P, Gerin JL, Greenlee HB, et al.. Type B hepatitis after needle-stick exposure: prevention with hepatitis B immune globulin. Final report of the Veterans Administration Cooperative Study. Ann Intern Med 1978; 88:285-93; PMID:343678 [DOI] [PubMed] [Google Scholar]
- [46].Centers for Disease C, Prevention . Updated recommendations for use of VariZIG–United States, 2013. MMWR Morb Mortal Wkly Rep 2013; 62:574-6; PMID:23863705 [PMC free article] [PubMed] [Google Scholar]
- [47].Centers for Disease C, Prevention . FDA approval of an extended period for administering VariZIG for postexposure prophylaxis of varicella. MMWR Morb Mortal Wkly Rep 2012; 61:212; PMID:22456121 [PubMed] [Google Scholar]
- [48].Shearer JD, Siemann L, Gerkovich M, House RV. Biological activity of an intravenous preparation of human vaccinia immune globulin in mouse models of vaccinia virus infection. Antimicrob Agents Chemother 2005; 49:2634-41; PMID:15980330; http://dx.doi.org/ 10.1128/AAC.49.7.2634-2641.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hopkins RJ, Kramer WG, Blackwelder WC, Ashtekar M, Hague L, Winker-La Roche SD, Berezuk G, Smith D, Leese PT. Safety and pharmacokinetic evaluation of intravenous vaccinia immune globulin in healthy volunteers. Clin Infect Dis 2004; 39:759-66; PMID:15472804; http://dx.doi.org/ 10.1086/422998 [DOI] [PubMed] [Google Scholar]
- [50].Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun 1997; 65:579-86; PMID:9009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, Wu C, Mende-Mueller L, Frank DW. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol 1997; 25:547-57; PMID:9302017 [DOI] [PubMed] [Google Scholar]
- [52].Liu PV. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis 1966; 116:481-9; PMID:4959184 [DOI] [PubMed] [Google Scholar]
- [53].Sawa T, Ohara M, Kurahashi K, Twining SS, Frank DW, Doroques DB, Long T, Gropper MA, Wiener-Kronish JP. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect Immun 1998; 66:3242-9; PMID:9632591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sawa T, Corry DB, Gropper MA, Ohara M, Kurahashi K, Wiener-Kronish JP. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol 1997; 159:2858-66; PMID:9300709 [PubMed] [Google Scholar]
- [55].Sawa T, Kurahashi K, Ohara M, Gropper MA, Doshi V, Larrick JW, Wiener-Kronish JP. Evaluation of antimicrobial and lipopolysaccharide-neutralizing effects of a synthetic CAP18 fragment against Pseudomonas aeruginosa in a mouse model. Antimicrob Agents Chemother 1998; 42:3269-75; PMID:9835525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].theLabRats Protocol for Assay of Myeloperoxidase in Frozen Lung Tissue in the LabRat.com. 2005. http://www.thelabrat.com/protocols/myeloperoxidaseassay.shtml [Google Scholar]
- [57].Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis, Nature methods 2012; 9:671-675; PMID:22930834 [DOI] [PMC free article] [PubMed] [Google Scholar]