Abstract
Objective
Test the effectiveness of a 6-week functional electrical stimulation (FES)-assisted rowing intervention to increase aerobic fitness and decrease shoulder pain in manual wheelchair users with spinal cord injury (SCI)
Methods
Ten adults with SCI (47 ± 12 years, 86 ± 19.7 kg, 175.5 ± 13.2 cm) 18 ± 14 years since injury, AIS classification A–C who had pain in one or both shoulders for >6 months took part in a pre-test, post-test experiment in our human performance laboratory. Participants took part in 30 minutes of FES-assisted rowing, 3 days/week × 6 weeks. Participants were evaluated for VO2 peak (FES-row and arm bike), distance rowed, arm power output, Wheelchair User Shoulder Pain Index (WUSPI), upper extremity isokinetic strength, scapular stabilization, participation (LIFE-H), quality of life (QOL-SCI), qualitative exit interview.
Results
Participants increased distance rowed by 257 ± 266 m and increased arm power output by 6.7 ± 7.9 W. An 8% increase in VO2 peak and 10.5 ± 4.4 point decrease in shoulder pain were observed (all P < 0.05). There were no changes in upper extremity strength, scapular stabilization, or survey-based measures of participation or quality of life. Qualitative interviewing indicated overall enjoyment of the intervention and improvement in perceived quality of life.
Conclusions
FES-assisted rowing is effective to increase aerobic fitness and decrease shoulder pain in manual wheelchair users with SCI. Further research is necessary to determine if rowing without FES can provide similar benefits, and to determine mechanisms driving improvements in shoulder pain, as no changes in measures of upper extremity strength or scapular stabilization were observed.
Keywords: FES-assisted exercise, Functional electrical stimulation, Rowing, Shoulder pain, Spinal cord injury
Introduction
Physical activity is an important component of health and quality of life. Adults with spinal cord injury (SCI) typically report low levels of physical activity and subsequently have poor aerobic fitness.1 In addition to poor aerobic fitness, shoulder pain is reported to occur in up to 83% of adults with SCI and may impact their ability to engage in routine physical activity.2 Low aerobic fitness levels and increased shoulder pain are significantly correlated with early death from cardiovascular disease, decreased quality of life and declining participation in daily activities within this population.2–4
Currently, upper-body cycling and wheelchair pushing are frequently prescribed to improve aerobic fitness in adults with SCI,5–9 and targeted shoulder strengthening exercises are used to improve shoulder pain.10–12 Despite the effectiveness of these interventions, several problems persist. First, exercise intensity during upper-body cycling is relatively low, because the activity is limited to small muscle groups above the level of injury; and it does not specifically address prevention or improvement of shoulder pain. Second, targeted shoulder strengthening exercises do not typically increase aerobic fitness to a significant extent, with the exception of severely deconditioned individuals. Adults with SCI frequently identify a lack of time as a barrier to exercise, indicating that exercise efficiency (accomplishing multiple goals with a minimal number of activities) is very important.13 Therefore, it would be beneficial to identify a mode of exercise that yields moderate to high exercise intensity while targeting the posterior shoulder musculature, which is shown to be weaker in adults with SCI.14 This could provide a feasible and efficient activity that can improve aerobic capacity and reduce strain on the shoulders with a reduced time commitment.
Previous investigators have shown that hybrid exercise, exercise using voluntary upper extremity motion in combination with electrically-stimulated paralyzed muscles, can induce an increased exercise intensity and more favorable aerobic response than upper extremity exercise alone.15,16 There is also evidence that resistance training programs targeting posterior shoulder muscle strengthening and anterior shoulder muscle stretching is are effective to reduce shoulder pain.10–12,17 Improvement in shoulder pain from programs targeting posterior muscle strength may be effective in SCI because they strengthen the posterior shoulder muscles which have been shown to be significantly weaker in manual wheelchair users with SCI.14,18 Posterior shoulder muscle strengthening can also improve scapular stability (lower ratio of upper to lower trapezius activity) which is impaired in individuals with subacromial impingement syndrome.14,19–22 FES-assisted rowing is a promising mode of exercise in this population because it (1) utilizes both upper and lower extremities, which increases aerobic demand (2) is better tolerated than other forms of hybrid exercise (lower rating of perceived exertion for the same level of oxygen consumption when FES-rowing compared to hybrid cycling)23 and (3) provides repeated activation of the posterior shoulder muscles that are not typically used during wheelchair propulsion and are often weak in adults with SCI.24
Previous investigations into the effectiveness of FES-assisted rowing have shown a significant improvement in upper extremity muscle strength, body composition and aerobic fitness.25–27 Most FES-assisted rowing machines require the participant to have enough electrically-stimulated lower extremity muscle strength and endurance to perform multiple resisted knee extensions prior to the initiation of FES-row training, i.e. sufficient force produced to move the weight of the rower seat plus the participant's body weight. In current FES-assisted rowing machine models, low levels of strength and endurance in the lower extremities can limit the duration and intensity of FES-rowing because participants must have enough stimulated knee extension strength to achieve and maintain knee extension when performing the upper-extremity pulling motion at the end of each stroke. Participants without sufficient force production must perform remedial FES-assisted strength training until they have enough strength to move the seat, their body mass, and overcome the resistance of the flywheel to perform FES-assisted rowing on current models, and the intensity may still be limited by their lower extremity strength and endurance.26,27 Additionally, the rowing stroke produced by current FES-assisted rowing machine models is not typical of the able-bodied rowing stroke, the participants do not achieve and maintain full knee extension throughout the arm pulling action because the legs collapse under the force of the arm pull. The FES-assisted rowing machine developed by our laboratory and utilized in this study is a promising mode of exercise for adults with SCI who have shoulder pain. The device attempts to provide an increased aerobic demand by using FES-assisted leg movement, targets posterior shoulder strength and scapular stability with a rowing motion that is not restricted by lower extremity strength, and does not require remedial FES-assisted strength training prior to the initiation of the activity.
Therefore, the purpose of this study was to examine changes in aerobic fitness, shoulder pain, shoulder strength and scapular stability in adults with SCI and shoulder pain after a six week intervention using our innovative FES-assisted rowing device and its subsequent impact on quality of life and participation in daily activities.
Methods
Study design and participants
Ten adults (2 female) with paraplegia (American Spinal Injury Association class A-C, T4–T12) completed the pre-post test study design. Mean age was 47.2 ± 18.3 years and mean time since injury was 18.2 ± 14.3 years. All participants used a manual wheelchair as their primary means of mobility and had pain in one or both shoulders. Eight of the ten participants had at least two positive clinical tests for subacromial impingement (Neer, Hawkins-Kennedy, or empty can) in their painful shoulder. Participants had no clinical signs of joint instability (sulcus sign) and/or labral tear (O'Brien's),28 and were able to raise both arms overhead. Participants were not included if they had shoulder surgery, fracture, subluxation or dislocation in the past year, and if they had a known cardiovascular or pulmonary disease or pressure ulcer greater than grade 2.
FES-Assisted rowing device
Rower
The Concept 2 Dynamic Indoor Rower was adapted to incorporate FES-assisted knee extension and flexion. Previous Concept 2 rower models that have been used in FES-assisted rowing applications have a static footplate and a dynamic seat moving in accordance with the rowing motion. The Concept 2 Dynamic model rower has a static seat and a dynamic footplate so that the body mass remains fixed in space while the footplate moves away from the body. In its original design, the footplate and pull handle provide opposing forces to allow both arm and leg contribution to drive the flywheel of the rower. In our pilot testing, we found that this coupling provides too much resistance for the leg extension movement and hinders FES-assisted rowing intensity for most people with SCI who are not currently engaged in NMES exercise of the lower extremities. In order to eliminate the need for remedial FES-assisted strength training prior to initiating the rowing activity, we disengaged the ergometer's flywheel from the driveline allowing the legs to minimally move against the force of gravity. In order to maintain some demand on the lower extremity beyond the force of gravity, elastic resistance bands were connected between the rower's seat at the footplate, allowing for progressively increasing leg extension resistance during training. This design removed the need for remedial strength training prior to the initiation of FES-row training, allowing for maximal upper-extremity force production during FES-assisted rowing, and still providing progressive resistance to the lower extremities.
Seating and support solutions
Additional items implemented for seated stability on the Concept 2 Dynamic rower included addition of leg supports and an adapted rowing seat. Elastic bands of variable resistance were attached between the seat and the footplate in order to progressively increase the resistance being applied during leg extension and to assist in knee flexion. Additionally, high-tension springs were connected from the seat back to the leg support. These springs acted in the same manner as the hip flexors during able-bodied rowing and assisted with returning the footplate to the start position which was challenging because the hip flexors are a major mover to return the footplate, but are not part of the electrically stimulated musculature. (Fig. 1A) In previous models, this is overcome by having the rowing machine on a downward slope to allow gravity to assist in knee flexion, along with the force created by the arms.
Figure 1.
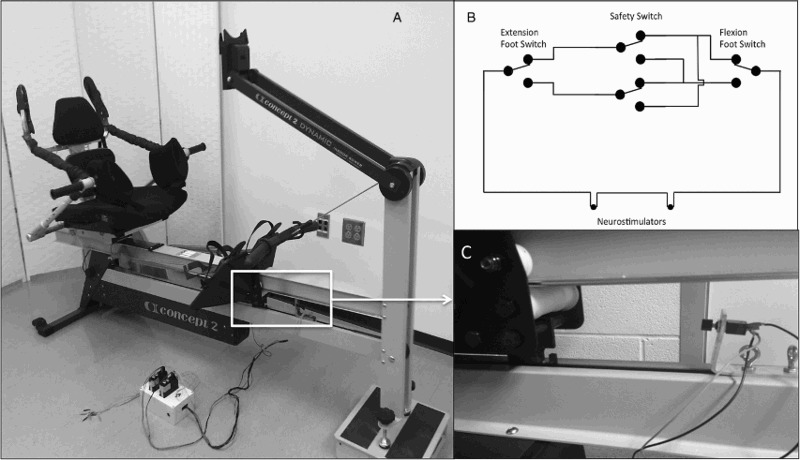
Design of functional electrical stimulation rowing machine.
Electrical stimulation
Electrical stimulation was delivered by two electrical stimulators (Medtronic, Respond II) through eight surface electrodes, two to each muscle group (quadriceps and hamstrings) and could be adjusted between 0 and 100 mA. In order to alternate the stimulation between the hamstrings and the quadriceps a circuit was created between two single-pole double-throw (SPDT) pushbutton switches and two 2.5 mm mono audio connectors for electrical stimulator control. The circuit allowed for the current to alternate between quadriceps and hamstrings as appropriate. A double-pole, double-throw (DPDT) toggle switch was located between the pushbutton switches, allowing for easier setup of the FES rower (Fig. 1B). To control the circuit, two SPDT switches were placed along the footplate track of the ergometer. When the legs and the footplate are at maximum flexion or extension, the footplate contacts a switch and the current alternates from one muscle group to the other, reversing the direction of leg movement. Two brackets were manufactured to position the pushbutton switches onto the Concept 2 Dynamic rower and were required to remain adjustable in order to accommodate individuals of different heights. An upper steel L-bracket mounted the switches and a lower aluminum bar, in combination with two eye bolts for each bracket which locked the switches in place along the footplate track. Non-identical brackets were created due to different sizing constraints on the front and rear of the rower (Fig. 1C).
Exercise program
Participants were familiarized with the FES-assisted rowing machine prior to the first training session in order to ensure participants did not have any negative responses to FES-assisted activity, e.g. skin irritation, autonomic dysreflexia, inability to tolerate stimulation. If the force of the electrically stimulated muscle contraction was not sufficient to extend and flex the legs, the footplate motion was assisted by the investigators. All participants were able to extend the legs with stimulation, 4 participants were able to return the footplate to its starting position without assistance from the investigator, participants who could not return the footplate to its starting position without assistance received assistance for this motion throughout the intervention. Participants trained with the FES-assisted rower three days per week for 6 weeks (18 total sessions). During each session participants were required to accumulate a total of 30 minutes of FES-assisted rowing. The majority of participants performed 10, 3-minute intervals with one minute rest in between intervals, in addition to warm-up and cool-down. During each session, participants transferred onto the ergometer and were secured with a lap belt and chest strap. The switches were adjusted along the track of the footplate to the point of full knee extension and maximal knee flexion. Participants rowed at a self-selected stroke rate. While rowing, participants were instructed to monitor their power output (W), which was displayed on the ergometer monitor. Prior to each session, participants were informed of their average power output from the previous session, and were instructed to attempt to match or exceed the power output for the previous day's exercise. Heart rate was monitored during activity using a heart rate monitor (Garmin Ltd, Olathe, KS, USA) synced to the ergometer's monitor display.
Pre- and post-testing protocols
Aerobic fitness
Peak oxygen consumption was assessed using a graded exercise test on an upper body ergometer (UBE) and during FES-row training sessions. For both tests, metabolic measures were taken using an open circuit spirometry metabolic cart (Parvo) using the manufacturer's software, which outputs 15-second VO2 averages. For the UBE protocol, participants were seated in their own daily-use wheelchair with wheels secured. The protocol consisted of a two minute warm-up at 60 revolutions per minute, followed by an incremental test in which the resistance was increased by 10 W every minute until the participant reached volitional fatigue, or 3 of 4 criteria for maximal oxygen consumption were met (>85% age predicted HR max, RPE ≥ 17, RER ≥ 1.1, plateau in oxygen consumption).29 Total testing time was 8 to 12 minutes and all participants achieved either RER ≥ 1.1, plateau in oxygen consumption or both. For the FES-row test, participants performed the last 2 intervals of their 3rd and 18th training sessions (3 minutes max FES row, 1 minute rest, 3 minutes max FES row) while expired gasses were collected, the highest 15-second average was selected as the peak VO2 for the FES-row condition.
Body composition
Body composition was analyzed pre- and post-training using dual-energy X-ray absorptiometry (DXA) (GE Lunar, GE Healthcare, Barrington, IL, USA). Total body bone mineral density (BMD) and body composition were measured using the internal software (GE Lunar Prodigy), along with changes in thigh lean mass, which was defined as a hexagon with points at lateral aspect of the greater trochanters and lateral aspects of the tibial plateaus. Weight was measured pre and post training using a standard wheelchair scale.
Upper body strength and scapular stabilization
Isokinetic strength and scapular stabilization (muscle activity of upper and lower trapezius) were collected using an isokinetic dynamometer (Biodex Medical Systems, Shirley, NY, USA) integrated with surface EMG (ADinstruments, Inc., Colorado Springs, CO, USA). The testing method was developed by the authors based upon previous investigators work in the area of subacromial impingement in able-bodied adults.20,22 Bipolar surface electrodes were placed over the muscle belly, in line with the fibers of the upper and lower trapezius. A reference electrode was placed over the clavicle. Signals were amplified with a differential amplifier (ADinstruments, Inc., Colorado Springs, CO, USA) with a high input impedance (>15 Ω at 100 Hz), a common mode rejection ratio of 85 dB at 60 Hz, and a bandwidth (–3 dB) of 20 to 2000 Hz. Root mean square (RMS)-processed signals were collected in real time with a sampling rate of 2000 Hz using a 16-bit A/D board (ADinstruments, Inc., Colorado Springs, CO, USA). In pilot testing our test/retest reliability was consistently greater than 0.9.
Participants were positioned on the dynamometer and resting level of electrical activity of the upper and lower trapezius was recorded. EMG signal quality was then verified and maximal electrical activity was determined by having the participant perform maximal voluntary isometric contractions (MVIC) against a stationary lever arm for each muscle group.
For the LT muscle, the attachment was positioned diagonally overhead in 160 degrees of forward flexion and 145 degrees of abduction. Participants were instructed to keep their elbow straight while applying a backward force on the attachment as if they were winding up to throw a ball. For the UT muscle, the attachment was positioned at 90 degrees of arm abduction and 5 degrees of horizontal flexion. Participants were instructed to keep their elbow straight while applying an upward force as if they were going to perform a jumping jack. Participants performed three 5sec maximum voluntary isometric muscle contractions against the attachment while receiving encouragement from the principle investigator. A 15 sec pause occurred between each contraction. EMG data collected during the MVICs were used to create a normalization reference for each participant. After signal filtering, the average RMS value for the middle 3 seconds of each contraction were averaged to determine the normalization value for each muscle group.
Isokinetic testing consisted of three protocols (Table 1): abduction (ABD) and adduction (ADD) in the frontal plane internal (IR) and external rotation (ER) in the scapular plane and closed chain flexion (push) and closed chain extension (pull) in the sagittal plane at waist level. We included the novel test of closed-chain flexion and extension for two reasons; (1) a multi-joint activity may better indicate function of the entire shoulder girdle than isolated single-joint movements, and (2) manual wheelchair use is a chest-dominant activity that would logically favor increased push versus pull strength, however this hypothesis has not been tested previously.
Table 1.
Upper extremity strength testing
| Test | Seat back | Arm position | Dynamometer position | Movement |
|---|---|---|---|---|
| Abduction/Adduction | 75° | Scapular Plane 0° elbow flexion | 10° tilt Aligned with posterior acromion | 30–150° abduction |
| Internal/External Rotation | 85° | 45° abduction 30° forward flexion 90° elbow flexion | 50° tilt Aligned with olecranon process | 10° IR to 75° ER |
| Push/Pull | 85° | 90° supination (thumb up) Hand below shoulder 0° abduction | 0° tilt Aligned with elbow when hand is in the lap | 0–30 cm push |
After setting the range of motion limits for each test, participants were familiarized with the motion and speed, and were instructed to stop testing if the motion was too painful. For all tests, participants were stabilized with a lap belt, footrest and belt across the contralateral shoulder. After familiarization repetitions, gravity correction was performed with the arm in a relaxed position. Participants then performed 5 maximal repetitions at 60 degrees/s with verbal encouragement from the PI and study staff. During the maximal isokinetic movements, EMG data from the two trapezius parts were collected synchronously with the arm movement.
Raw isokinetic dynamometer signals were analog/digital converted (16-bit resolution) at 2000 Hz. Signals were converted to force, position and velocity following standardized calibrations. Range of motion markers were automatically generated to define 60–120 degrees of ABD/ADD, 0–20 degrees of IR/ER, and 5–15 cm of push/pull.
EMG signal processing
Raw EMG signals were analog/digital converted (16-bit resolution) at 2000 Hz, and were low and high-pass filtered. A 35 ms moving average was used to smooth the signal. The average RMS values over 60–120 degrees of arm abduction were averaged across the five repetitions. The mean amplitude EMG signal, expressed as a percentage of MVIC was used to assess the activity of the two trapezius parts during arm abduction.
Shoulder pain
Shoulder pain was assessed using the Wheelchair User's Shoulder Pain Index (WUSPI). The WUSPI is a reliable, validated measure of shoulder pain specific to manual wheelchair users. The 15 item survey asks participants to indicate how much pain they have experienced doing each task in the past seven days. The score ranges from 0–150, lower scores indicated less pain and higher function.30
Participation and quality of life
Participation was assessed using the Assessment of Life Habits survey (LIFE-H). The LIFE-H is a validated measure specific to adults with physical disabilities.31 The survey consists of 77 items addressing participation with measures of how much difficulty the person has, and how much and what type of assistance is needed to perform the activity. The LIFE-H addresses the majority of relevant Activities and Participation constructs from the International Classification of Function, Disability and Health.32,33
Quality of life was assessed using the Quality of Life Index, spinal cord injury version (QOL-SCI). The QOL-SCI is a 37-item survey that asks participants to rate first how satisfied they are with an aspect of daily living, and then to rate how important that aspect is to them. The QOL-SCI has shown excellent internal consistency and concurrent validity.34–36
Qualitative feedback
At the conclusion of FES-row training and after post-testing, participants had the option to participate in a qualitative interview about their experience with the study. Open-ended questions were asked during an in-person interview while the PI took detailed notes. Participants provided feedback about how they felt the study affected their pain, health, quality of life and energy level. They were also asked to identify their most and least favorite parts of participating in the study and how they might utilize the FES-assisted rowing machine if it was available in a fitness center.
Data analysis
Data were first analyzed to determine if a difference existed between the pre- and post-test values (paired t-test, α = 0.05). If a difference existed, then linear regression was used to determine if other variables significantly predicted these changes. All analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, USA).
Results
Ten participants completed 18 training sessions within the 6-week time frame. Participant characteristics are presented in Table 2. Participants’ average heart rate (HR) during training was 86.1 ± 7.8% of max HR achieved during the graded exercise test and 76.7 ± 9.4% of age-predicted max HR, indicating participants achieved a level of vigorous intensity exercise.29
Table 2.
Participant characteristics
| Participant | Sex | Years since injury | Lesion level | ASIA score |
|---|---|---|---|---|
| 1 | M | 3 | T7 | C |
| 2 | F | 24 | T10 | A |
| 3 | M | 6 | T7 | B |
| 4 | F | 2 | T7 | B |
| 5 | M | 11 | T7 | A |
| 6 | M | 21 | T7 | A |
| 7 | M | 32 | T12 | A |
| 8 | M | 37 | T12 | C |
| 9 | M | 39 | T5 | A |
| 10 | M | 6.5 | T4 | C |
Participants significantly increased their distance rowed from 4054 ± 815 to 4312 ± 869 m (P = 0.014), and rowing power output from 36.1 ± 19.0 W to 42.8 ± 24.7 W (P = 0.026). Changes in aerobic fitness and shoulder pain are shown in Fig. 2. VO2 peak for the FES-Row condition increased by 8.1% (P = 0.034) and also increased by 7.1% in the UBE graded exercise test (P = 0.024). Shoulder pain in the participant identified more painful shoulder decreased by 47.8% (P = 0.002). There were no significant changes in body composition (P = 0.638), body weight (P = 0.18), thigh lean mass (P = 0.11), quality of life (P = 0.96), or participation (P = 0.74) (Table 3).
Figure 2.
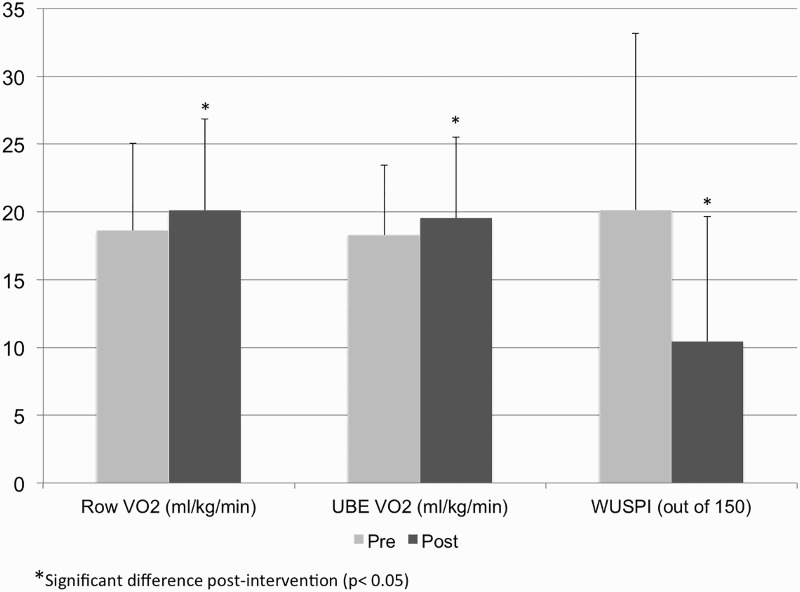
Changes in aerobic fitness and shoulder pain.
Table 3.
Changes in body composition, quality of life and participation
| QOL-SCI |
LIFE-H |
Body mass (Kg) |
% Body fat |
Thigh lean mass (Kg) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Mean | 11.1 | 12.2 | 7.8 | 7.7 | 85.1 | 85.1 | 36.9 | 36.7 | 11.9 | 11.6 |
| SD | 4.3 | 7.1 | 2.6 | 2.6 | 19.6 | 19.6 | 5.9 | 6.2 | 4.1 | 4.2 |
Table 4 shows the changes in upper body strength and muscle activation. There was a significant increase in dominant internal rotation strength from 37.3 ± 15.1 Nm to 41.6 ± 18.3 Nm, however there were no other significant changes in upper extremity strength or muscle activity (P > 0.05).
Table 4.
Upper extremity strength changes
| More painful pre |
More painful post |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| UT:LT | 1.6 | 0.7 | 1.4 | 0.5 |
| ABD (Nm) | 46.5 | 16.1 | 45.9 | 18.9 |
| ADD (Nm) | 45.1 | 27.3 | 44.3 | 21.8 |
| IR (Nm) | 38.2 | 16.3 | 38.2 | 18 |
| ER (Nm) | 28.2 | 8.7 | 28.0 | 9.0 |
| PUSH (Kg) | 55.7 | 22.2 | 55.9 | 25.4 |
| PULL (Kg) | 41.3 | 12.7 | 39.5 | 11.7 |
There was a significant correlation between change in power output and change in VO2, both for the FES-Row condition (r = 0.62, P = 0.049) and UBE graded exercise test (r = 0.58, P = 0.041).
Discussion
Our results demonstrate that a six-week rowing intervention with FES-assistance to the lower extremities can improve aerobic fitness and decrease shoulder pain in manual wheelchair users with SCI. FES-assisted rowing on our device improved oxygen consumption both during the rowing activity and on a UBE graded exercise test. These results are consistent with oxygen consumption values reported for hybrid training16,37 and for relative changes in peak VO2.26,27,38 The FES-row condition for oxygen consumption demonstrated an increased level of oxygen consumption while FES rowing than during arm ergometry. Participants were able to maintain training HR levels >80% of measured HR max, which is sufficient for improving aerobic fitness in adults with SCI.39
As expected, linear regression indicated that those participants who had greater improvements in power output had a significantly greater increase in VO2 peak. We found no significant change in body composition, body weight or thigh lean mass. When considering the length of our intervention (6 weeks), it is not that surprising that we did not see a change in body composition, though others have reported some favorable improvements in body composition measured via bioelectrical impedance analysis (decreased fat mass, increased lean mass).25 We did not observe any increase in thigh lean mass or whole-body bone mineral density. Lower extremity FES-assisted resistance training interventions have previously shown increases in thigh lean mass with short training interventions, suggesting that it may be necessary to perform isolated progressive resistance training if increases in thigh lean mass are desired.40–42
The FES-assisted rowing machine developed by our lab utilizes the Concept 2 Dynamic Indoor Rower, which allowed us to separate the movement of the legs from the force created by the arms. This allowed for participants who have very low electrically stimulated force output in their legs to begin rowing without the need for any remedial FES resistance training and because the force of the arms was mechanically uncoupled from the legs, participants were not limited in their arm power output. Although the resistance applied to the legs via elastic band tension was progressed throughout training, our device design may not have influenced the effects on body composition and thigh lean mass because the resistance applied to the legs was likely lower than the forces obtained by maintaining the arm-leg force couple or during electrically stimulated progressive resistance training. We also utilized two battery-powered stimulation units, which were limited to stimulation amplitudes of 100mA, which may have been insufficient to progress stimulated leg force sufficiently. Lower muscle strength and stimulation intensity may have also limited the potential for the FES component of the activity to increase oxygen consumption to the same level as that observed in other studies. Despite the lack of improvement in body composition and thigh lean mass, aerobic fitness improved and participants indicated that they generally enjoyed the activity, indicating that FES-assisted rowing with our design is an effective manner of improving aerobic fitness. Additionally, we observed that with the improved design, participants were able to row with form and technique that is typical of able-bodied rowing, i.e. full knee extension is maintained through the arm pull phase, which is not attained with current FES-assisted rower models. It should be noted, however, that the design may be further improved by providing mechanical assistance to return the footplate to the flexed knee position, as most participants could not accomplish this motion with the electrical stimulation and leg support, requiring study staff to provide manual assistance which would not be feasible as an independent form of exercise without remedial strengthening.
On average, our participants’ shoulder pain was reduced by 10 points on the WUSPI. The minimal detectable change in WUSPI scores is 5.5 points, suggesting that our intervention resulted in both clinical and statistical significance for the participants’ most painful shoulder.30 Arm-crank ergometry has previously been shown to be ineffective at reducing shoulder pain, as measured by the WUSPI (11.5 to 8.5 points).6 While two previous resistance training programs have shown significant decreases in WUSPI scores (–26.2 points17 and –36.3 points10), FES-assisted rowing may be a viable option for decreasing shoulder pain while also improving aerobic fitness without the need for separate aerobic and strength-training interventions. However, it should be noted that similar outcomes may be possible with upper body only rowing, for individuals without access to a customized, FES-assisted rowing device. Further studies should be conducted to determine if the addition of FES is worth the added expense and trainer effort.
The mechanism for the reduction in shoulder pain requires further study as our measures of upper extremity strength and muscle activation did not change significantly. In qualitative interviews, participants generally stated that their shoulder pain had decreased. Previous work in our lab demonstrated an increased UT:LT activation ratio indicating poor scapular stabilization in adults with impingement. We expected to see a decrease in this ratio if participants had a decrease in shoulder pain, however this was not observed. Participants also stated in qualitative interviews that they enjoyed having both their arms and legs involved in the activity and that they enjoyed having rowing as an alternative to arm crank or wheelchair pushing for aerobic exercise. Several participants who consistently monitor their exercise heart rate noticed that their heart rate was higher during the FES-assisted rowing than during their normal aerobic activities. Although we did not objectively measure this difference, this is in agreement with previous studies comparing arms-only versus hybrid activities.15,16
No significant changes in quality of life or participation as measured by the QOL-SCI and LIFE-H surveys were observed. Upon examination of our qualitative exit interviews, we believe that a ceiling effect may have occurred because participants were independent community-dwelling adults who scored at the very high-end for both surveys. Several survey items that decreased QOL and participation scores were determined by use of assistive devices such as a wheelchair, which was not expected to change with the intervention. Participants stated in their qualitative interviews that they felt their quality of life had improved. Three participants stated that they felt their transfers and wheelchair propulsion required less effort, which made daily activities easier. Participants consistently stated that they had more energy throughout the day, and one participant noted a significant improvement in sleep quality. Several participants noted that they had less fatigue when sitting unsupported and felt that their posture had improved.
Safety is always a concern when working with adults with SCI so we monitored several outcomes throughout the study. These included skin health where the electrodes were applied, prevention of pressure ulcers while using the FES-assisted rower, monitoring for autonomic dysreflexia and ensuring safe transfers onto and off of the ergometer. The investigators and participant monitored the skin under the electrodes after each session and there were no issues observed. Additionally, we did not have any blood pressure or transfer issues throughout the course of the study. Safety concerns have been raised about the use of FES associated with fracture in the lower extremities. All participants in our study were pre-screened and had typical bone mineral density for their age and were therefore at a very low risk of fracture from the force of electrically stimulated muscle contraction and no issues were encountered.
In the qualitative interviews, participants stated that they enjoyed the activity and would be very likely to use a similar device if it were available in a fitness center. Participants consistently stated that wearing the mask for the metabolic cart was their least favorite part of the study, while having a power goal every day was their favorite part of the study. Given the low injury risk level and potential to both increase aerobic fitness and decrease shoulder pain, it is reasonable to conclude that FES-assisted rowing without a mechanical arm-leg force couple is an acceptable exercise alternative for people with SCI, in particular those with shoulder pain.
Conclusion
The rowing intervention utilized in this study was effective at improving aerobic fitness and decreasing shoulder pain in this population. Additionally, training with the device was both safe and enjoyable for people with SCI. We did not observe a measurable increase in upper extremity muscle strength; therefore it is still warranted to recommend progressive resistance training in addition to aerobic training in this population. The design of our ergometer can be improved so that it is not necessary to have an external person to assist with set-up and movement of the footplate. This may be accomplished by incorporating a motor-driven footplate similar to the motor driven seat design of Kim et al.25
Disclaimer statements
Contributors SW: Conception, design of study, manuscript preparation; RR: manuscript preparation, data analysis; SP: Conception and design of study, data analysis, manuscript preparation; CSB: Conception and design of study, data analysis, manuscript preparation.
Funding The contents of this publication were developed under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research [NIDILRR grant number 90RE5009]. NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this (insert type of publication; e.g., book, report, film) do not necessarily represent the policy of NIDILRR, ACL, HHS, and you should not assume endorsement by the Federal Government.
Conflicts of interest All authors have been fully involved with the study and the manuscript preparation. None of the authors have declared a conflict of interest.
Ethics approval Ethical approval was granted by the University Institutional Review Board.
Acknowledgment
We would like to acknowledge the contributions of Dustin Dew, Dr. Alan Eberhardt and Whitney Neal.
References
- 1.Noreau L, Shephard RJ. Spinal cord injury, exercise and quality of life. Sports Med 1995;20(4):226–50. doi: 10.2165/00007256-199520040-00003 [DOI] [PubMed] [Google Scholar]
- 2.Subbarao J, Klopfstein J, Turpin R. Prevalence and impact of wrist and shoulder pain in patients with spinal cord injury. J Spinal Cord Med 1994;18(1):9–13. [DOI] [PubMed] [Google Scholar]
- 3.Kemp BJ, Bateham AL, Mulroy SJ, Thompson L, Adkins RH, Kahan JS. Effects of reduction in shoulder pain on quality of life and community activities among people living long-term with SCI paraplegia: A randomized control trial. J Spinal Cord Med 2011;34(3):278–84. doi: 10.1179/107902611X12972448729486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–77. [DOI] [PubMed] [Google Scholar]
- 5.Bougenot MP, Tordi N, Betik AC, Martin X, Le Foll D, Parratte B, et al. Effects of a wheelchair ergometer training programme on spinal cord-injured persons. Spinal Cord 2003;41(8):451–6. doi: 10.1038/sj.sc.3101475 [DOI] [PubMed] [Google Scholar]
- 6.Dyson-Hudson TA, Sisto SA, Bond Q, Emmons R, Kirshblum SC. Arm crank ergometry and shoulder pain in persons with spinal cord injury. Arch Phys Med Rehabil 2007;88(12):1727–9. doi: 10.1016/j.apmr.2007.07.043 [DOI] [PubMed] [Google Scholar]
- 7.El-Sayed MS, Younesian A. Lipid profiles are influenced by arm cranking exercise and training in individuals with spinal cord injury. Spinal Cord 2005;43(5):299–305. doi: 10.1038/sj.sc.3101698 [DOI] [PubMed] [Google Scholar]
- 8.Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, et al. Long-term exercise training in persons with spinal cord injury: Effects on strength, arm ergometry performance and psychological well-being. Spinal Cord 2003;41(1):34–43. doi: 10.1038/sj.sc.3101389 [DOI] [PubMed] [Google Scholar]
- 9.Valent L, Dallmeijer A, Houdijk H, Talsma E, van der Woude L. The effects of upper body exercise on the physical capacity of people with a spinal cord injury: A systematic review. Clin Rehabil 2007;21(4):315–30. doi: 10.1177/0269215507073385 [DOI] [PubMed] [Google Scholar]
- 10.Mulroy SJ, Thompson L, Kemp B, Hatchett PP, Newsam CJ, Lupold DG, et al. Strengthening and optimal movements for painful shoulders (stomps) in chronic spinal cord injury: A randomized controlled trial. Phys Ther 2011;91(3):305–24. doi: 10.2522/ptj.20100182 [DOI] [PubMed] [Google Scholar]
- 11.Curtis KA, Tyner TM, Zachary L, Lentell G, Brink D, Didyk T, et al. Effect of a standard exercise protocol on shoulder pain in long-term wheelchair users. Spinal Cord 1999;37(6):421–9. doi: 10.1038/sj.sc.3100860 [DOI] [PubMed] [Google Scholar]
- 12.Nawoczenski DA, Ritter-Soronen JM, Wilson CM, Howe BA, Ludewig PM. Clinical trial of exercise for shoulder pain in chronic spinal cord injury. Phys Ther 2006;86(12):1604–18. doi: 10.2522/ptj.20060001 [DOI] [PubMed] [Google Scholar]
- 13.Scelza WM, Kalpakjian CZ, Zemper ED, Tate DG. Perceived barriers to exercise in people with spinal cord injury. Am J Phys Med Rehabil 2005;84(8):576–83. doi: 10.1097/01.phm.0000171172.96290.67 [DOI] [PubMed] [Google Scholar]
- 14.Wilbanks SR, Bickel CS.. Scapular stabilization and muscle strength in manual wheelchair users with spinal cord injury and subacromial impingement. Top Spinal Cord Inj Rehabil 2016;22(1): 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verellen J, Vanlandewijck Y, Andrews B, Wheeler GD. Cardiorespiratory responses during arm ergometry, functional electrical stimulation cycling, and two hybrid exercise conditions in spinal cord injured. Disabil Rehabil Assist Technol 2007;2(2):127–32. doi: 10.1080/09638280600765712 [DOI] [PubMed] [Google Scholar]
- 16.Hooker S, Figoni S, Rodger M. Metabolic and hemodynamic responses to concurrent voluntary upper extremity crank and electrical stimulation to lower extremity cycle exercise in quadriplegics. J Rehab Res 1992;29(3):1–11. doi: 10.1682/JRRD.1992.07.0001 [DOI] [PubMed] [Google Scholar]
- 17.Nash MS, van de Ven I, van Elk N, Johnson BM. Effects of circuit resistance training on fitness attributes and upper-extremity pain in middle-aged men with paraplegia. Arch Phys Med Rehabil 2007;88(1):70–5. doi: 10.1016/j.apmr.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 18.Mulroy S, Gronley J, Newsam C, Perry J. Electromyograpic activity of shoulder muscles during wheelchair propulsion by paraplegic persons. Arch Phys Med Rehabil 1996;77(2):187–93. doi: 10.1016/S0003-9993(96)90166-5 [DOI] [PubMed] [Google Scholar]
- 19.Cools AM, Declercq GA, Cambier DC, Mahieu NN, Witvrouw EE. Trapezius activity and intramuscular balance during isokinetic exercise in overhead athletes with impingement symptoms. Scand J Med Sci Sports 2007;17(1):25–33. [DOI] [PubMed] [Google Scholar]
- 20.Cools A, Witvrouw E, Declerq G, Danneels L, Cambier D. Scapular muscle recruitment patterns: Trapezius muscle latency with and without impingement symptoms. Am J Sports Med 2003;31(4):542–9. [DOI] [PubMed] [Google Scholar]
- 21.De Mey K, Danneels L, Cagnie B, Cools AM. Scapular muscle rehabilitation exercises in overhead athletes with impingement symptoms: Effect of a 6-week training program on muscle recruitment and functional outcome. Am J Sports Med 2012;40(8):1906–15. doi: 10.1177/0363546512453297 [DOI] [PubMed] [Google Scholar]
- 22.Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther 2000;80(3):276–91. [PubMed] [Google Scholar]
- 23.Hettinga DM, Andrews BJ. The feasibility of functional electrical stimulation indoor rowing for high-energy training and sport. Neuromodulation 2007;10(3):291–7. doi: 10.1111/j.1525-1403.2007.00117.x [DOI] [PubMed] [Google Scholar]
- 24.Olenik L, Laskin J, Burnham R, Wheeler G, Steadward R. Efficacy of rowing, backward wheeling and isolated scapular retractor exercise as remedial strength activities for wheelchair users: Application of electromyography. Paraplegia 1995;33(3):148–52. doi: 10.1038/sc.1995.32 [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Park D, Lee B, Jeon J. A six-week motor-driven functional electronic stimulation rowing program improves muscle strength and body compositon in people with spinal cord injury: A pilot study. Spinal Cord 2014;52(8):621–4. doi: 10.1038/sc.2014.76 [DOI] [PubMed] [Google Scholar]
- 26.Wheeler GD, Andrews B, Lederer R, Davoodi R, Natho K, Weiss C et al. Functional electric stimulation-assisted rowing: Increasing cardiovascular fitness through functional electric stimulation rowing training in persons with spinal cord injury. Arch Phys Med Rehabil 2002;83(8):1093–9. doi: 10.1053/apmr.2002.33656 [DOI] [PubMed] [Google Scholar]
- 27.Jeon JY, Hettinga D, Steadward RD, Wheeler GD, Bell G, Harber V. Reduced plasma glucose and leptin after 12 weeks of functional electrical stimulation-rowing exercise training in spinal cord injury patients. Arch Phys Med Rehabil 2010;91(12):1957–9. doi: 10.1016/j.apmr.2010.08.024 [DOI] [PubMed] [Google Scholar]
- 28.Michener LA, Walsworth MK, Doukas WC, Murphy KP. Reliability and diagnostic accuracy of 5 physical examination tests and combination of tests for subacromial impingement. Arch Phys Med Rehabil 2009;90(11):1898–903. doi: 10.1016/j.apmr.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 29.American College of Sports Medicine ACSM's Resource Manual for Guidelines for Exercise Testing and Prescription. 6 edn Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 30.Curtis KA, Roach KE, Applegate EB, Amar T, Benbow CS, Genecco TD, et al. Reliability and validity of the wheelchair user's shoulder pain index (WUSPI). Paraplegia 1995;33(10):595–601. doi: 10.1038/sc.1995.126 [DOI] [PubMed] [Google Scholar]
- 31.Desrosiers J, Noreau L, Robichaud L, Fougeyrollas P, Rochette A, Viscogliosi C. Validity of the assessment of life habits in older adults. J Rehab Med 2004;36(4):177–82. doi: 10.1080/16501970410027485 [DOI] [PubMed] [Google Scholar]
- 32.Escorpizo R, Graf S, Marti A, Noreau L, Post MW, Stucki G, et al. Domain sets and measurement instruments on participation and environmental factors in spinal cord injury research. Am J Phys Med Rehabil 2011;90(11 Suppl 2):S66–78. doi: 10.1097/PHM.0b013e318230fbf9 [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization International classification of functioning, disability and health. World Health Organization; 2001. [Google Scholar]
- 34.Ferrans CE, Powers MJ. Quality of life index: Development and psychometric properties. ANS Adv Nurs Sci 1985;8(1):15–24. doi: 10.1097/00012272-198510000-00005 [DOI] [PubMed] [Google Scholar]
- 35.May LA, Warren S. Measuring quality of life of persons with spinal cord injury: Substantive and structural validation. Qual Life Res 2001;10(6):503–15. doi: 10.1023/A:1013027520429 [DOI] [PubMed] [Google Scholar]
- 36.May LA, Warren S. Measuring quality of life of persons with spinal cord injury: External and structural validity. Spinal Cord 2002;40(7):341–50. doi: 10.1038/sj.sc.3101311 [DOI] [PubMed] [Google Scholar]
- 37.Mutton D, Scremin A, Barstow T, Scott M, Kumkel C, Cagle T. Physiological responses during functional electrical stimulation lower extremity cycling and hybrid exercise in spinal cord injured subjects. Arch Phys Med Rehabil 1997;78(7):712–8. doi: 10.1016/S0003-9993(97)90078-2 [DOI] [PubMed] [Google Scholar]
- 38.Krauss JC, Robergs RA, Depaepe JL, Kopriva LM, Aisenbury JA, Anderson MA et al. Effects of electrical stimulation and upper body training after spinal cord injury. Med Sci Sports Exerc 1993;25(9):1054–61. doi: 10.1249/00005768-199309000-00014 [DOI] [PubMed] [Google Scholar]
- 39.Bizzarini E, Saccavini M, Lipanje F, Magrin P, Malisan C, Zampa A. Exercise prescription in subjects with spinal cord injuries. Arch Phys Med Rehabil 2005;86(6):1170–5. doi: 10.1016/j.apmr.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 40.Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: Case report. J Spinal Cord Med 2010;33(1):90–5. doi: 10.1080/10790268.2010.11689681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahoney ET, Bickel CS, Elder C, Black C, Slade JM, Apple D Jr, et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil 2005;86(7):1502–4. doi: 10.1016/j.apmr.2004.12.021 [DOI] [PubMed] [Google Scholar]
- 42.Skold C, Lonn L, Harms-Ringdahl K, Hultling C, Levi R, Nash M, et al. Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord-injured individuals. J Rehab Med 2002;34(1):25–32. doi: 10.1080/165019702317242677 [DOI] [PubMed] [Google Scholar]


