In the crystal of 7-iodo-4-oxo-4H-chromene-3-carbaldehyde, molecules are linked through stacking interactions and C—H⋯O hydrogen bonds. Halogen bonds between the formyl O and I atoms are also formed.
Keywords: crystal structure, π–π stacking, hydrogen bond, halogen bond
Abstract
In the title compound, C10H5IO3, an iodinated 3-formylchromone derivative, the non-H atoms are essentially coplanar (r.m.s. deviation = 0.0344 Å), with the largest deviation from the least-squares plane [0.101 (3) Å] being found for the formyl O atom. In the crystal, molecules are linked through stacking interactions [centroid–centroid distance between the benzene rings = 3.700 (3) Å] and C—H⋯O hydrogen bonds. Halogen bonds between the I atoms at 7-position and the formyl O atoms [I1⋯O3 = 3.056 (2) Å, C6—I1⋯O3 = 173.18 (8)° and I1⋯O3—C10 = 111.12 (18)°] are also formed along [110], resulting in sheets perpendicular to the c axis, constructed by C—H⋯O hydrogen bonds and I⋯O halogen bonds.
Chemical context
3-Formylchromone and its derivatives show versatile biological activities such as anti-inflammatory activity (Khan et al., 2010 ▸) and the inhibition of protein tyrosine phosphatase 1B (Shim et al., 2005 ▸), thymidine phosphorylase (Khan et al., 2009 ▸), carbonic anhydrase (Ekinci et al., 2012 ▸), and metallo-β-lactamase (Christopeit et al., 2016 ▸). Interestingly, 6,8-dichloro- and 6,8-dibromo-3-formylchromones possess potent urease inhibitory activity, whereas 6-fluoro-, 6-chloro- and 6-bromo-3-formylchromones exhibit no ability to inhibit urease (Kawase et al., 2007 ▸). Thus, the position of halogen atoms on the chromone ring should be associated with the urease inhibitory activity.
We have previously reported the crystal structures of 6,8-dichloro-4-oxochromene-3-carbaldehyde (6,8-dichloro-3-formylchromone; Ishikawa & Motohashi, 2013 ▸) and 6,8-dibromo-4-oxo-4H-chromene-3-carbaldehyde (6,8-dibromo-3-formylchromone; Ishikawa, 2014a ▸). In these crystals, halogen bonds are observed between the formyl oxygen atoms and the halogen atoms at the 8-position. Halogen bonding is defined as a net attractive interaction between an electrophilic region of a halogen atom in a molecule and a nucleophilic region of an atom in a molecule, and is characterized by a shorter contact between the two atoms. Halogen bonding has attracted much attention in medicinal chemistry, chemical biology, supramolecular chemistry and crystal engineering (Scholfield et al., 2013 ▸; Wilcken et al., 2013 ▸; Persch et al., 2015 ▸; Cavallo et al., 2016 ▸).
As part of an investigation of halogenated 3-formylchromones relevant to urease inhibitory activity and halogen bonding, I herein report the crystal structure of 7-iodo-4-oxo-4H-chromene-3-carbaldehyde (7-iodo-3-formylchromone). The main objective of this study is to reveal the interaction mode of the iodine substituent at the 7-position of the chromone ring in the solid state.
Structure commentary
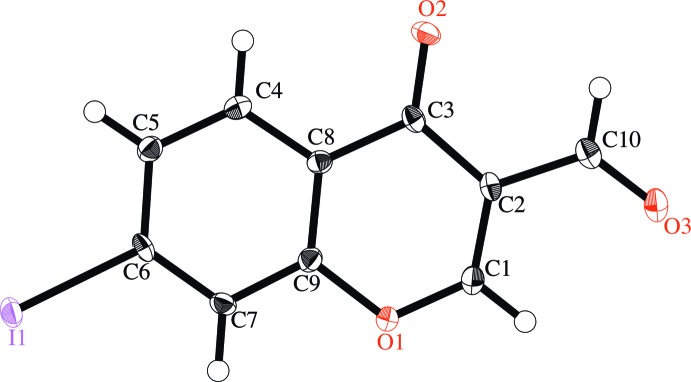
The mean deviation of the least-square planes for the non-hydrogen atoms is 0.0344 Å, and the largest deviation is 0.101 (3) Å for O3, indicating that these atoms are essentially coplanar (Fig. 1 ▸). All bond distances and angles are within their expected ranges.
Figure 1.
The molecular structure of the title compound, with displacement ellipsoids drawn at the 50% probability level. H atoms are shown as small spheres of arbitrary radius.
Supramolecular features
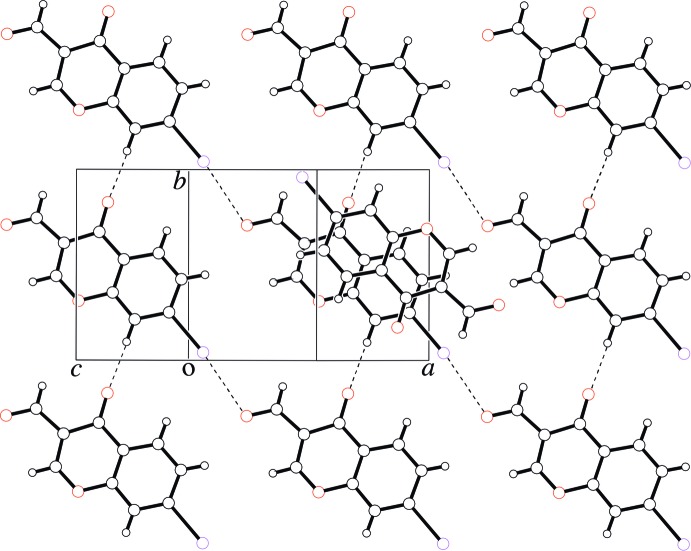
In the crystal, the molecules are linked through π–π stacking interactions between inversion-symmetry-equivalenti molecules [centroid–centroid distance between the benzene rings of the 4H-chromene units = 3.700 (3) Å; symmetry code: (i) −x, −y, −z], and through C—H⋯O hydrogen bonds (Table 1 ▸) that involve the C7/O2 atoms. In particular, significant shorter contacts are observed between the iodine atoms and the formyl oxygen atoms of translation-symmetry equivalentii molecules [I1⋯O3 = 3.056 (2) Å, C6—I1⋯O3 = 173.18 (8)°, I1⋯O3—C10 = 111.12 (18)°; symmetry code: (ii) x + 1, y − 1, z] along [1 1 0], resulting in sheets perpendicular to the c-axis, constructed by C—H⋯O hydrogen bonds and I⋯O halogen bonds (Fig. 2 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C7—H4⋯O2i | 0.95 | 2.35 | 3.208 (4) | 150 (1) |
Symmetry code: (i)  .
.
Figure 2.
A packing view of the title compound. C—H⋯O hydrogen bonds and I⋯O halogen bonds are represented as dashed lines.
Database survey
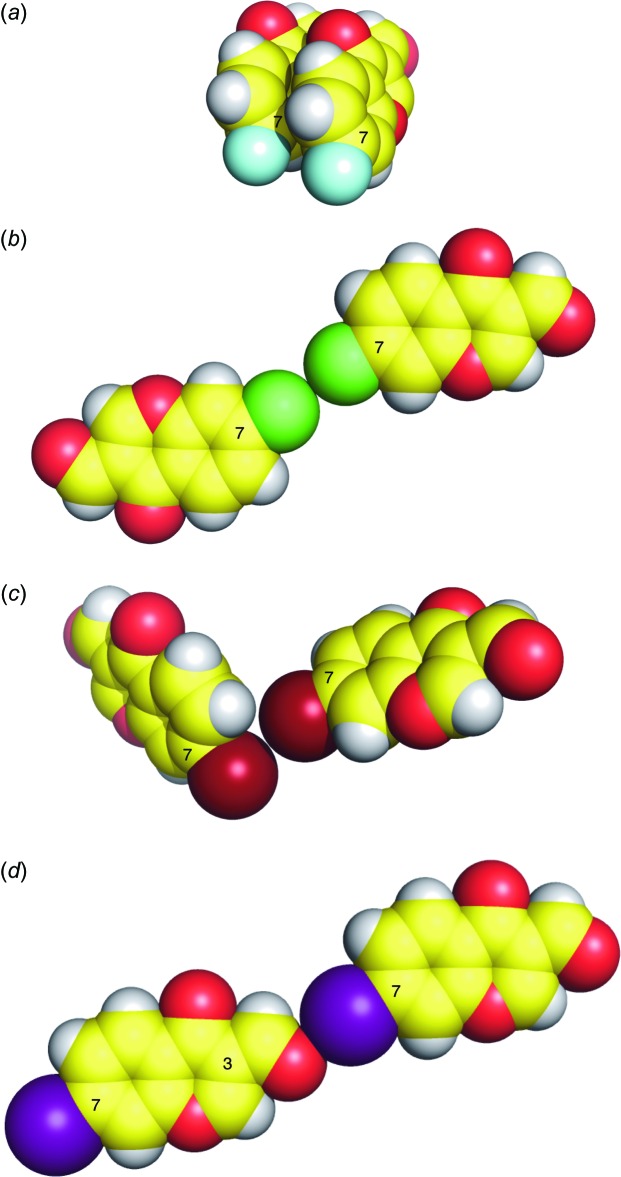
A search of WebCSD (Version 1.1.2, last update Oct 2016; Groom et al., 2014 ▸) for 7-halogeno-3-formylchromones gave the following three hits: 7-fluoro- (Asad et al., 2011 ▸), 7-chloro- (Ishikawa, 2014b ▸), and 7-bromo-3-formylchromone (Ishikawa, 2014c ▸). In 7-fluoro-3-formylchromone, no contact around the fluorine atom is seen (Fig. 3 ▸ a). In the crystals of 7-chloro- and 7-bromo-3-formylchromones, type I and type II halogen⋯halogen contacts are found, respectively (Fig. 3 ▸ b and 3c), and these halogen⋯halogen contacts are commonly found for Cl and Br atoms (Mukherjee et al., 2014 ▸). It should be noted that shorter contacts between oxygen atoms and halogen atoms are observed in 7-iodo-3-formylchromone (this work, Fig. 3 ▸ d), but not in 7-fluoro-, 7-chloro-, and 7-bromo-3-formylchromones. This is in agreement with an assumption that the iodine atom should have the largest σ-hole (Clark et al., 2007 ▸) among the halogen atoms in 7-halogeno-3-formylchromones. These findings should be helpful in understanding the interaction of halogenated 3-formylchromones with urease, and is thus valuable for rational drug design.
Figure 3.
Sphere models of the crystal structures of (a) 7-fluoro-4-oxo-4H-chromene-3-carbaldehyde (Asad et al., 2011 ▸), (b) 7-chloro-4-oxo-4H-chromene-3-carbaldehyde (Ishikawa, 2014b ▸), (c) 7-bromo-4-oxo-4H-chromene-3-carbaldehyde (Ishikawa, 2014c ▸) and (d) the title compound (this work).
Synthesis and crystallization
2′-Hydroxy-4′-iodoacetophenone was prepared from 3-acetoxyiodobenzene by a Fries rearrangement reaction. To a solution of 2′-hydroxy-4′-iodoacetophenone (4.4 mmol) in N,N-dimethylformamide (10 ml) was added dropwise POCl3 (13.2 mmol) at 273 K. After the mixture was stirred for 14 h at room temperature, water (100 ml) was added. The precipitates were collected, washed with water, and dried in vacuo at 333 K (yield 86%). 1H NMR (400 MHz, CDCl3): δ 7.84 (dd, 1H, J = 8.8 and 1.5 Hz), 7.96 (d, 1H, J = 1.5 Hz), 7.99 (d, 1H, J = 8.3 Hz), 8.49 (s, 1H), 10.36 (s, 1H). Single crystals suitable for X-ray diffraction were obtained by slow evaporation of a 1,2-dichloroethane solution of the title compound at room temperature.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The C-bound hydrogen atoms were placed in geometrical positions and refined using a riding model [C—H = 0.95 Å and U iso(H) = 1.2U eq(C)].
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C10H5IO3 |
| M r | 300.05 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 100 |
| a, b, c (Å) | 9.572 (4), 7.533 (4), 13.095 (9) |
| β (°) | 103.06 (4) |
| V (Å3) | 919.8 (9) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 3.46 |
| Crystal size (mm) | 0.25 × 0.18 × 0.15 |
| Data collection | |
| Diffractometer | Rigaku AFC7R |
| Absorption correction | ψ scan (North et al., 1968 ▸) |
| T min, T max | 0.528, 0.595 |
| No. of measured, independent and observed [F 2 > 2.0σ(F 2)] reflections | 2538, 2119, 1916 |
| R int | 0.017 |
| (sin θ/λ)max (Å−1) | 0.650 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.021, 0.053, 1.06 |
| No. of reflections | 2119 |
| No. of parameters | 128 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.56, −0.60 |
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989016016972/zl2682sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016016972/zl2682Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016016972/zl2682Isup3.cml
CCDC reference: 1511202
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
This work was supported by JSPS KAKENHI (Grant No. JP16K08199). I acknowledge University of Shizuoka for instrumental support.
supplementary crystallographic information
Crystal data
| C10H5IO3 | F(000) = 568.00 |
| Mr = 300.05 | Dx = 2.167 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71069 Å |
| a = 9.572 (4) Å | Cell parameters from 25 reflections |
| b = 7.533 (4) Å | θ = 15.1–17.1° |
| c = 13.095 (9) Å | µ = 3.46 mm−1 |
| β = 103.06 (4)° | T = 100 K |
| V = 919.8 (9) Å3 | Prismatic, yellow |
| Z = 4 | 0.25 × 0.18 × 0.15 mm |
Data collection
| Rigaku AFC7R diffractometer | Rint = 0.017 |
| ω–2θ scans | θmax = 27.5°, θmin = 3.0° |
| Absorption correction: ψ scan (North et al., 1968) | h = −12→12 |
| Tmin = 0.528, Tmax = 0.595 | k = −9→0 |
| 2538 measured reflections | l = −9→17 |
| 2119 independent reflections | 3 standard reflections every 150 reflections |
| 1916 reflections with F2 > 2.0σ(F2) | intensity decay: −0.3% |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.021 | H-atom parameters constrained |
| wR(F2) = 0.053 | w = 1/[σ2(Fo2) + (0.0244P)2 + 1.8229P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max = 0.001 |
| 2119 reflections | Δρmax = 0.56 e Å−3 |
| 128 parameters | Δρmin = −0.60 e Å−3 |
| 0 restraints | Extinction correction: SHELXL2014 (Sheldrick, 2015) |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0024 (4) |
| Secondary atom site location: difference Fourier map |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement was performed using all reflections. The weighted R-factor (wR) and goodness of fit (S) are based on F2. R-factor (gt) are based on F. The threshold expression of F2 > 2.0 σ(F2) is used only for calculating R-factor (gt). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| I1 | 0.24361 (2) | 0.04012 (2) | 0.39087 (2) | 0.01629 (8) | |
| O1 | −0.2715 (2) | 0.3092 (3) | 0.39728 (15) | 0.0152 (4) | |
| O2 | −0.1775 (2) | 0.8253 (3) | 0.33965 (16) | 0.0172 (4) | |
| O3 | −0.5743 (2) | 0.7067 (3) | 0.39144 (17) | 0.0209 (4) | |
| C1 | −0.3676 (3) | 0.4391 (4) | 0.3915 (2) | 0.0160 (5) | |
| H1 | −0.4603 | 0.4076 | 0.4004 | 0.019* | |
| C2 | −0.3435 (3) | 0.6125 (4) | 0.3740 (2) | 0.0135 (5) | |
| C3 | −0.2044 (3) | 0.6703 (4) | 0.3577 (2) | 0.0128 (5) | |
| C4 | 0.0421 (3) | 0.5595 (4) | 0.3565 (2) | 0.0143 (5) | |
| H2 | 0.0697 | 0.6769 | 0.3434 | 0.017* | |
| C5 | 0.1408 (3) | 0.4232 (4) | 0.3655 (2) | 0.0144 (5) | |
| H3 | 0.2365 | 0.4468 | 0.3604 | 0.017* | |
| C6 | 0.0979 (3) | 0.2499 (4) | 0.3822 (2) | 0.0133 (5) | |
| C7 | −0.0402 (3) | 0.2124 (4) | 0.3922 (2) | 0.0135 (5) | |
| H4 | −0.0685 | 0.0945 | 0.4039 | 0.016* | |
| C8 | −0.0982 (3) | 0.5267 (4) | 0.3665 (2) | 0.0123 (5) | |
| C9 | −0.1358 (3) | 0.3530 (4) | 0.3847 (2) | 0.0132 (5) | |
| C10 | −0.4587 (3) | 0.7427 (4) | 0.3717 (2) | 0.0162 (5) | |
| H5 | −0.4422 | 0.8619 | 0.3538 | 0.019* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| I1 | 0.01410 (10) | 0.01533 (11) | 0.01997 (11) | 0.00472 (7) | 0.00501 (7) | −0.00105 (7) |
| O1 | 0.0119 (9) | 0.0131 (9) | 0.0217 (10) | 0.0010 (7) | 0.0064 (8) | 0.0019 (8) |
| O2 | 0.0186 (10) | 0.0101 (9) | 0.0221 (10) | −0.0001 (8) | 0.0029 (8) | 0.0011 (8) |
| O3 | 0.0155 (10) | 0.0215 (11) | 0.0263 (11) | 0.0054 (8) | 0.0064 (8) | 0.0012 (9) |
| C1 | 0.0120 (12) | 0.0173 (14) | 0.0190 (13) | 0.0022 (10) | 0.0042 (10) | 0.0010 (11) |
| C2 | 0.0119 (12) | 0.0149 (13) | 0.0135 (12) | 0.0041 (10) | 0.0020 (10) | 0.0009 (11) |
| C3 | 0.0132 (12) | 0.0126 (12) | 0.0115 (12) | 0.0031 (10) | 0.0001 (9) | −0.0006 (10) |
| C4 | 0.0139 (12) | 0.0140 (13) | 0.0149 (12) | −0.0029 (10) | 0.0029 (10) | −0.0004 (10) |
| C5 | 0.0125 (12) | 0.0151 (14) | 0.0156 (13) | −0.0014 (10) | 0.0033 (10) | −0.0017 (10) |
| C6 | 0.0154 (12) | 0.0111 (12) | 0.0137 (12) | 0.0051 (10) | 0.0035 (10) | −0.0025 (10) |
| C7 | 0.0154 (12) | 0.0093 (12) | 0.0154 (12) | −0.0005 (10) | 0.0027 (10) | −0.0014 (10) |
| C8 | 0.0129 (12) | 0.0119 (12) | 0.0121 (12) | −0.0013 (10) | 0.0026 (9) | −0.0010 (10) |
| C9 | 0.0121 (12) | 0.0142 (13) | 0.0140 (12) | −0.0008 (10) | 0.0044 (10) | 0.0001 (10) |
| C10 | 0.0164 (13) | 0.0156 (13) | 0.0153 (13) | 0.0040 (11) | 0.0008 (10) | −0.0012 (11) |
Geometric parameters (Å, º)
| I1—C6 | 2.094 (3) | C4—C5 | 1.383 (4) |
| O1—C1 | 1.334 (3) | C4—C8 | 1.400 (4) |
| O1—C9 | 1.386 (3) | C4—H2 | 0.9500 |
| O2—C3 | 1.230 (3) | C5—C6 | 1.401 (4) |
| O3—C10 | 1.222 (4) | C5—H3 | 0.9500 |
| C1—C2 | 1.355 (4) | C6—C7 | 1.387 (4) |
| C1—H1 | 0.9500 | C7—C9 | 1.389 (4) |
| C2—C3 | 1.462 (4) | C7—H4 | 0.9500 |
| C2—C10 | 1.471 (4) | C8—C9 | 1.392 (4) |
| C3—C8 | 1.471 (4) | C10—H5 | 0.9500 |
| C1—O1—C9 | 118.2 (2) | C7—C6—C5 | 121.6 (2) |
| O1—C1—C2 | 125.1 (3) | C7—C6—I1 | 118.6 (2) |
| O1—C1—H1 | 117.4 | C5—C6—I1 | 119.8 (2) |
| C2—C1—H1 | 117.4 | C6—C7—C9 | 117.7 (3) |
| C1—C2—C3 | 120.5 (2) | C6—C7—H4 | 121.2 |
| C1—C2—C10 | 119.4 (3) | C9—C7—H4 | 121.2 |
| C3—C2—C10 | 120.1 (3) | C9—C8—C4 | 118.2 (2) |
| O2—C3—C2 | 123.2 (3) | C9—C8—C3 | 120.2 (2) |
| O2—C3—C8 | 122.8 (2) | C4—C8—C3 | 121.5 (3) |
| C2—C3—C8 | 113.9 (2) | O1—C9—C7 | 115.5 (2) |
| C5—C4—C8 | 120.8 (3) | O1—C9—C8 | 122.0 (2) |
| C5—C4—H2 | 119.6 | C7—C9—C8 | 122.5 (2) |
| C8—C4—H2 | 119.6 | O3—C10—C2 | 123.9 (3) |
| C4—C5—C6 | 119.1 (3) | O3—C10—H5 | 118.0 |
| C4—C5—H3 | 120.5 | C2—C10—H5 | 118.0 |
| C6—C5—H3 | 120.5 | ||
| C9—O1—C1—C2 | 0.1 (4) | O2—C3—C8—C9 | −178.5 (3) |
| O1—C1—C2—C3 | 1.1 (4) | C2—C3—C8—C9 | 2.4 (4) |
| O1—C1—C2—C10 | −178.8 (2) | O2—C3—C8—C4 | 1.2 (4) |
| C1—C2—C3—O2 | 178.6 (3) | C2—C3—C8—C4 | −177.9 (2) |
| C10—C2—C3—O2 | −1.5 (4) | C1—O1—C9—C7 | 179.6 (2) |
| C1—C2—C3—C8 | −2.3 (4) | C1—O1—C9—C8 | 0.0 (4) |
| C10—C2—C3—C8 | 177.6 (2) | C6—C7—C9—O1 | −178.8 (2) |
| C8—C4—C5—C6 | 1.6 (4) | C6—C7—C9—C8 | 0.7 (4) |
| C4—C5—C6—C7 | −1.5 (4) | C4—C8—C9—O1 | 179.0 (2) |
| C4—C5—C6—I1 | 177.9 (2) | C3—C8—C9—O1 | −1.4 (4) |
| C5—C6—C7—C9 | 0.3 (4) | C4—C8—C9—C7 | −0.6 (4) |
| I1—C6—C7—C9 | −179.1 (2) | C3—C8—C9—C7 | 179.1 (2) |
| C5—C4—C8—C9 | −0.6 (4) | C1—C2—C10—O3 | 4.9 (4) |
| C5—C4—C8—C3 | 179.7 (2) | C3—C2—C10—O3 | −175.0 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C7—H4···O2i | 0.95 | 2.35 | 3.208 (4) | 150 (1) |
Symmetry code: (i) x, y−1, z.
References
- Asad, M., Oo, C.-W., Osman, H., Hemamalini, M. & Fun, H.-K. (2011). Acta Cryst. E67, o766. [DOI] [PMC free article] [PubMed]
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., Giacovazzo, C., Mallamo, M., Mazzone, A., Polidori, G. & Spagna, R. (2012). J. Appl. Cryst. 45, 357–361.
- Cavallo, G., Metrangolo, P., Milani, R., Pilati, T., Priimagi, A., Resnati, G. & Terraneo, G. (2016). Chem. Rev. 116, 2478–2601. [DOI] [PMC free article] [PubMed]
- Christopeit, T., Albert, A. & Leiros, H. K. (2016). Bioorg. Med. Chem. 24, 2947–2953. [DOI] [PubMed]
- Clark, T., Hennemann, M., Murray, J. S. & Politzer, P. (2007). J. Mol. Model. 13, 291–296. [DOI] [PubMed]
- Ekinci, D., Al-Rashida, M., Abbas, G., Şentürk, M. & Supuran, C. T. (2012). J. Enzyme Inhib. Med. Chem. 27, 744–747. [DOI] [PubMed]
- Groom, C. R. & Allen, F. H. (2014). Angew. Chem. Int. Ed. 53, 662–671. [DOI] [PubMed]
- Ishikawa, Y. (2014a). Acta Cryst. E70, o439. [DOI] [PMC free article] [PubMed]
- Ishikawa, Y. (2014b). Acta Cryst. E70, o831. [DOI] [PMC free article] [PubMed]
- Ishikawa, Y. (2014c). Acta Cryst. E70, o996. [DOI] [PMC free article] [PubMed]
- Ishikawa, Y. & Motohashi, Y. (2013). Acta Cryst. E69, o1416. [DOI] [PMC free article] [PubMed]
- Kawase, M., Tanaka, T., Kan, H., Tani, S., Nakashima, H. & Sakagami, H. (2007). In Vivo, 21, 829–834. [PubMed]
- Khan, K. M., Ambreen, N., Hussain, S., Perveen, S. & Choudhary, M. I. (2009). Bioorg. Med. Chem. 17, 2983–2988. [DOI] [PubMed]
- Khan, K. M., Ambreen, N., Mughal, U. R., Jalil, S., Perveen, S. & Choudhary, M. I. (2010). Eur. J. Med. Chem. 45, 4058–4064. [DOI] [PubMed]
- Mukherjee, A. & Desiraju, G. R. (2014). IUCrJ, 1, 49–60. [DOI] [PMC free article] [PubMed]
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Persch, E., Dumele, O. & Diederich, F. (2015). Angew. Chem. Int. Ed. 54, 3290–3327. [DOI] [PubMed]
- Rigaku (1999). WinAFC Diffractometer Control Software. Rigaku Corporation, Tokyo, Japan.
- Rigaku (2015). CrystalStructure. Rigaku Corporation, Tokyo, Japan.
- Scholfield, M. R., Zanden, C. M., Carter, M. & Ho, P. S. (2013). Protein Sci. 22, 139–152. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Shim, Y. S., Kim, K. C., Lee, K. A., Shrestha, S., Lee, K. H., Kim, C. K. & Cho, H. (2005). Bioorg. Med. Chem. 13, 1325–1332. [DOI] [PubMed]
- Wilcken, R., Zimmermann, M. O., Lange, A., Joerger, A. C. & Boeckler, F. M. (2013). J. Med. Chem. 56, 1363–1388. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989016016972/zl2682sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016016972/zl2682Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016016972/zl2682Isup3.cml
CCDC reference: 1511202
Additional supporting information: crystallographic information; 3D view; checkCIF report