A tetrahedral CuP2S2 coordination geometry is found for the CuI ion in the title compound. The dithiocabamate ligand forms symmetric Cu—S bonds. In the crystal, supramolecular dimers of complex molecules are connected via eight-membered {⋯H—O⋯H—O}2 synthons. In addition, the chloroform molecule participates in Cl⋯π(arene) and S⋯Cl interactions.
Keywords: crystal structure, copper, dithiocarbamate, hydrogen bonding, Hirshfeld surface analysis, NMR
Abstract
The title compound, [Cu(C5H5NO2S2)(C18H15P)2]·CHCl3, features a tetrahedrally coordinated CuI atom within a P2S2 donor set defined by two phosphane P atoms and by two S atoms derived from a symmetrically coordinating dithiocarbamate ligand. Both intra- and intermolecular hydroxy-O—H⋯O(hydroxy) hydrogen bonding is observed: the former closes an eight-membered {⋯HOC2NC2O} ring, whereas the latter connects centrosymmetrically related molecules into dimeric aggregates via eight-membered {⋯H—O⋯H—O}2 synthons. The complex molecules are arranged to form channels along the c axis in which reside the chloroform molecules, being connected by Cl⋯π(arene) and short S⋯Cl [3.3488 (9) Å] interactions. The intermolecular interactions have been investigated further by Hirshfeld surface analysis, which shows the conventional hydrogen bonding to be very localized with the main contributors to the surface, at nearly 60%, being H⋯H contacts. Solution NMR studies indicate that whilst the same basic molecular structure is retained in solution, the triphenylphosphane ligands are highly labile, exchanging rapidly with free Ph3P at room temperature.
Chemical context
The motivation to prepare bis(phosphane)copper(I) dithiocarbamates of general formula (R
3P)2Cu(S2CNR′R′′) (R, R′, R′′ = alkyl, aryl) largely stems from the versatile biological properties exhibited by these types of compounds (Skrott & Cvek, 2012 ▸; Biersack et al., 2012 ▸) and metal dithiocarbamates in general, as summarized in a recent review (Hogarth, 2012 ▸). At present, research continues to develop promising anti-microbial agents in light of the growing prevalence of bacterial infections and threats associated with drug-resistant bacteria (Verma & Singh, 2015 ▸; Onwudiwe et al., 2016 ▸). In our recent efforts to develop anti-microbial agents, phosphanegold(I) dithiocarbamates, R
3PAu[S2CN(iPr)CH2CH2OH], were prepared and these compounds demonstrated prominent and distinctive anti-microbial activity against a broad range of Gram-positive and Gram-negative bacteria, dependent on the type of P-bound substituent employed (Sim et al., 2014 ▸). A distinct structure–activity relationship was noted in that when R = Et, the compound was potent against a broad range of Gram-positive and Gram-negative bacteria, whereas the R = Ph and Cy compounds showed specific activity against Gram-positive bacteria. Even greater, broad-range activity is apparent in triethylphosphanegold(I) dialkyldithiocarbamates (Chen et al., 2016 ▸). The above prompted an exploration of the anti-bacterial activity of related copper(I) and silver(I) derivatives, as these metals are known to possess noteworthy potential as anti-microbial agents (Losasso et al., 2014 ▸). Thus, a series of phosphanecopper(I) and silver(I) compounds of general formula (Ph3P)2
M[S2CN(R)CH2CH2OH] for M = Cu and Ag, and R = Me, iPr and CH2CH2OH, were prepared and evaluated for their anti-microbial activities (Jamaludin et al., 2016 ▸). While none of the studied compounds exhibited activity against Gram-negative bacteria, they were found to be selectively potent against Gram-positive bacteria. Following new syntheses to evaluate further the potential of this class of compounds, crystals became available for the title complex, (Ph3P)2Cu[S2CN(CH2CH2OH)2] (I), as its 1:1 chloroform solvate. Herein, the crystal and molecular structures of (I)·CHCl3 are described along with an analysis of its Hirshfeld surface. Finally, some non-standard, e.g. variable temperature, NMR measurements are presented in order to gain insight into the solution structure.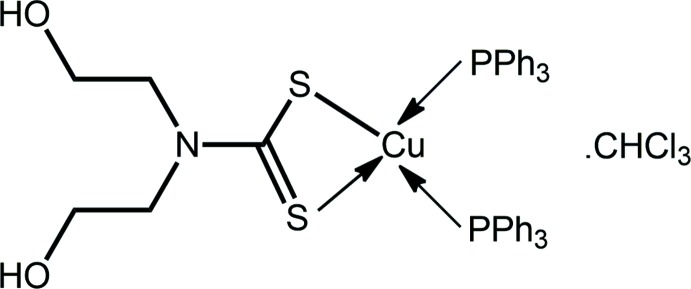
Structural commentary
The molecular structure of the complex in (I)·CHCl3 is shown in Fig. 1 ▸ and selected geometric parameters are collected in Table 1 ▸. The copper atom is bound by two dithiocarbamate-S atoms and two phosphane-P atoms. The dithiocarbamate ligand is coordinating in a symmetric mode with Δ(Cu—S) = 0.042 Å, being the difference between the Cu—Slong and Cu—Sshort bond lengths. This near equivalence in Cu—S bond lengths is reflected in the experimental equivalence of the associated C1—S1, S2 bond lengths. A small disparity, i.e. 0.02 Å, is noted in the Cu—P bond lengths. The resulting P2S2 donor set defines an approximate tetrahedral geometry. A measure of tetrahedral vs square-planar geometry is the value of τ4 (Yang et al., 2007 ▸) with values of 1.0 and 0.0° corresponding to ideal tetrahedral and square planar geometries, respectively. In the case of the complex in (I)·CHCl3, the value computes to 0.80. Distortions from the ideal tetrahedral geometry are clearly related to the acute angle subtended by the dithiocarbamate ligand and the wide angle subtended by the bulky triphenylphosphane ligands, Table 1 ▸.
Figure 1.
The molecular structure of the complex in (I)·CHCl3, showing the atom-labelling scheme and displacement ellipsoids at the 70% probability level. The solvent CHCl3 molecule is omitted.
Table 1. Geometric data (Å, °) for (I) in (I)·CHCl3 and (I) in its 1:1 Ph3P co-crystal.
| Parameter | (I) in (I)·CHCl3 | (I) in (I)·PPh3 a |
|---|---|---|
| Cu—S1 | 2.3791 (6) | 2.3948 (12) |
| Cu—S2 | 2.4213 (5) | 2.4288 (12) |
| Cu—P1 | 2.2602 (6) | 2.2849 (12) |
| Cu—P2 | 2.2380 (5) | 2.2594 (12) |
| C1—S1 | 1.714 (2) | 1.709 (4) |
| C1—S2 | 1.717 (2) | 1.702 (4) |
| S1—Cu—S2 | 75.264 (18) | 74.76 (4) |
| S1—Cu—P1 | 110.96 (2) | 109.85 (5) |
| S1—Cu—P2 | 109.81 (2) | 112.35 (4) |
| S2—Cu—P1 | 103.74 (2) | 102.50 (4) |
| S2—Cu—P2 | 123.17 (2) | 122.04 (5) |
| P1—Cu—P2 | 123.65 (2) | 124.52 (4) |
Note: (a) Jian et al. (2000 ▸).
The structure of (I) has also been determined in its 1:1 co-crystal with PPh3 (Jian et al., 2000 ▸), hereafter (I)·Ph3P, and key geometric parameters for this structure are also included in Table 1 ▸. Interestingly, within pairs of comparable bond lengths, those in (I)·PPh3 are systematically longer. However, the value of Δ(Cu—S) is slightly less at 0.034 Å. The value of τ4 is identical at 0.80. An overlay diagram for (I) in each of (I)·CHCl3 and (I)·PPh3 is shown in Fig. 2 ▸ which confirms the very similar conformations adopted for (I) in both structures.
Figure 2.
Overlay diagram of (I)·CHCl3 (red image) and (I)·Ph3P (blue). The molecules have been overlapped so the chelate rings are coincident. The CHCl3 and Ph3P molecules have been omitted.
Supramolecular features
Geometric parameters describing the salient intermolecular interactions in the crystal of (I)·CHCl3 are collated in Table 2 ▸. There are two types of hydroxy-O—H⋯O(hydroxy) hydrogen bonding in the molecular packing, one intramolecular and the other intermolecular. The former has hydroxy-O2—H as the donor and the hydroxy-O1 as the acceptor, and closes an eight-membered {⋯HOC2NC2O} ring. The key feature of the molecular packing is the presence of hydroxy-O—H⋯O(hydroxy) hydrogen bonding which connects centrosymmetriclly-related molecules into dimeric aggregates via eight-membered {⋯H—O⋯H—O}2 synthons, encompassing the intramolecular hydroxy-O—H⋯O(hydroxy) hydrogen bonds, Fig. 3 ▸ a. The only other identifiable directional interactions within standard distance criteria (Spek, 2009 ▸) involve the chloroform molecule. Thus, a chloroform-Cl3⋯π(arene) interaction is noted, Table 2 ▸. In addition, there is evidence for a close S1⋯Cl3 contact, i.e. involving the same chlorine atom as in the just mentioned Cl⋯π(arene) interaction. The separation of 3.3488 (9) Å is about 0.2 Å less than the sum of their van der Waals radii (Spek, 2009 ▸). In a very recent and exhaustive review of halogen bonding (Cavallo et al., 2016 ▸), it was mentioned that sulfur is well known to function as an acceptor in R—X⋯S synthons. The interactions involving the chloroform molecule are highlighted in Fig. 3 ▸ b. Globally, molecules of the copper(I) complex pack to define channels parallel to the c axis in which reside the solvent molecules, Fig. 3 ▸ c. Given the presence of Ph3P ligands in (I)·CHCl3, evidence was sought for phenyl-embraces (Dance & Scudder, 1995 ▸). While none was apparent for the P1-phosphane, centrosymmetrically related P2-phosphane ligands approach each other in this manner to generate a sixfold phenyl-embrace. The closest interactions between the phosphane residues in this embrace is a pair of edge-to-face-phenyl—H⋯π(arene) interactions, i.e. C63—H63⋯π(C51–C56)i = 3.25 Å with an angle at H62 of 133°; symmetry operation (i): 1 − x, 1 − y, 1 − z.
Table 2. Hydrogen-bond geometry (Å, °).
Cg1 is the ring centroid of (C51–C56).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2O⋯O1 | 0.84 | 1.95 | 2.710 (3) | 150 |
| O1—H1O⋯O2i | 0.86 | 1.97 | 2.697 (3) | 142 |
| C6—Cl3⋯Cg1 | 1.77 (1) | 3.81 (1) | 3.798 (3) | 76 (1) |
Symmetry code: (i)  .
.
Figure 3.
Molecular packing in (I)·CHCl3: (a) supramolecular dimer sustained by hydroxy-O—H⋯O(hydroxy) hydrogen bonding shown as orange dashed lines, (b) a view of the interactions between the complex and solvent molecules with the Cl⋯π(arene) and Cl⋯S interactions shown as purple and blue dashed lines, respectively, and (c) a view of the unit-cell contents in projection down the c axis, with chloroform molecules occupying one channel highlighted in space-filling mode.
Hirshfeld surface analysis
The protocols for the Hirshfeld surface analysis were as described recently (Yeo et al., 2016 ▸). In the present study, analyses were conducted on the following three species: (I) in (I)·CHCl3, (I)·CHCl3 and CHCl3 alone. Hirshfeld surface analysis provides visualization on the existence of any intermolecular interactions within close proximity in a crystal structure, for which contact distances shorter than the sum of the respective van der Waals radii appear red while at distances equal or longer than this would be white and blue in appearance, respectively. Figs. 4 ▸ a and b show Hirshfeld surfaces mapped over d norm for (I) and CHCl3, respectively. The former image exhibits intense red spots on the surface near the hydroxyethyl substituents which are correlated with the strong O—H⋯O hydrogen bonding. Apart from these dominant interactions, several other red spots attributed to the close contacts between the complex and chloroform molecules, i.e. C⋯H/H⋯C, S⋯Cl/Cl⋯S and H⋯Cl/Cl⋯H, are evident in Fig. 4 ▸ a and b.
Figure 4.
Comparison of the Hirshfeld surfaces of (a) molecule (I) in (I)·CHCl3 and (b) CHCl3 in (I)·CHCl3, highlighting intermolecular interactions formed with the other component of the structure. The Hirshfeld surfaces were mapped over d norm within the range −0.572 to 1.457 Å.
The combination of d i and d e distances resulted in two-dimensional cuttlefish- and chicken wing-like fingerprint plots for (I), (I)·CHCl3 and CHCl3, Fig. 5 ▸ a, which may be decomposed into several essential close contacts as shown in Fig. 5 ▸ b–d. In general, complex (I) and its chloroform solvate exhibit almost identical profiles except that the pincer form of (I) in its decomposed fingerprint plot delineated into C⋯H/H⋯C contacts shows two different tips at d e + d i ∼ 2.5 Å and ∼2.7 Å in contrast to the pincer form of (I)·CHCl3 with a pair of symmetrical tips at d e + d i ∼ 2.7 Å when the solvate is considered as a single entity. The close contact distance (d e + d i ∼ 2.5 Å), which is shorter than the sum of van der Waals radii of 2.9 Å (Spek, 2009 ▸), is also reflected in the lancet blade-like fingerprint plot of the solvent molecule corresponding to the Cl—H⋯C(π) interaction. The H⋯Cl/Cl⋯H contact, on the other hand, contributes to the half-pincer form in the decomposed fingerprint plot of (I) and develops into the full pincer form in (I)·CHCl3, both with d e + d i ∼ 2.9 Å that is very close to the sum of van der Waals radii (2.95 Å). As expected, O⋯H/H⋯O contacts constitute the strongest among all interactions with d e + d i ∼ 1.9 Å (cf. the sum of van der Waals radii of 2.75 Å) in the forceps form of both decomposed fingerprint plots of (I) and (I)·CHCl3, Fig. 5 ▸ d. Based on the asymmetric fingerprint patterns of the C⋯H/H⋯C and Cl⋯H/H⋯Cl contacts, Fig. 5 ▸ b and c, and the symmetric pattern of the O⋯H/H⋯O contacts, Fig. 5 ▸ d, it may be concluded that two complex molecules are very closely associated, as shown in Fig. 3 ▸ a, and these are flanked by two CHCl3 molecules, highlighted in Fig. 3 ▸ b.
Figure 5.
Comparison between (I) in (I)·CHCl3, (I)·CHCl3 and CHCl3 of the (a) full two-dimensional fingerprint plots, and the plots delineated into (b) C⋯H/H⋯C, (c) Cl⋯H/H⋯Cl and (d) O⋯H/H⋯O contacts.
The quantification on the distribution of each of the contacts to the Hirshfeld surface reveals that H⋯H, C⋯H/H⋯C and H⋯Cl/Cl⋯H are the three main components for (I) in (I)·CHCl3, with the corresponding contributions of ca 59.4, 20.2 and 8.9%, respectively, Fig. 6 ▸. Despite this, not all of these contacts result in meaningful interactions based on the comparison between d e + d i contact distances and the sum of the van der Waals radii. This sequence is followed by O⋯H/ H⋯O contacts which form the fourth most dominant interactions with a contribution of approximately 4.6% to the overall Hirshfeld surface. In general, there is not much deviation of the topological distribution between (I) and (I)·CHCl3 except that the contribution from H⋯Cl/Cl⋯H increases by nearly twofold upon the inclusion of the solvent molecule in (I)·CHCl3. As for the chloroform molecule, H⋯Cl/Cl⋯H makes the major contribution at 74.4%, followed by 8.9% from H⋯H and 8.4% from H⋯Cl/Cl⋯H; the remaining contributions from other minor contacts.
Figure 6.
Percentage contributions of the different close contacts to the Hirshfeld surface of (I) in (I)·CHCl3, (I)·CHCl3 and CHCl3.
As mentioned previously, Cl⋯π(arene) and S⋯Cl interactions are formed by the chloroform molecule. In order to gain insight into the charge distribution and rationalize these close contacts, the electrostatic potential (ESP) was mapped over the Hirshfeld surface by ab initio Hartree–Fock (HF) quantum modelling with the 6-31G(d) basis set, as this represents the best possible level of theory and basis set functions in this study so as to keep the accuracy and computational cost at manageable level.
As shown in Fig. 7 ▸ a, a phenyl ring of the complex molecule exhibits mild electronegative character as evidenced from the pale-red spot on the ESP map in contrast to the strong electropositive character about CHCl3, being intense-blue. The electropositive character of the methine group extends slightly beyond the chloro atom approaching its equatorial ring of the negative charge region, hence establishing the weak Cl⋯π(arene) interaction with d e + d i ∼ 3.3 Å being slightly less than the sum of van der Waals radii of 3.45 Å. The S⋯Cl halogen bond, on the other hand, is established through the highly directional interaction between the electronegative sulfur of (I) and the σ-hole of the chloro atom of CHCl3 with weak electropositive character, Fig. 7 ▸ b. The electropositive character of the σ-hole results from the electron deficiency in the outer lobe of the p orbital (non-bonded) when a half-filled p orbital of a halogen participates in the formation of covalent bond (Clark et al., 2007 ▸).
Figure 7.
Electrostatic potential (ESP) mapped over the Hirshfeld surfaces of the complex molecule (I) (left) and CHCl3 (right), showing the attraction between the electronegative (red) and electropositive (blue) sites for (a) Cl⋯π(arene) and (b) S⋯Cl interactions, respectively. The ESP was mapped onto the Hirshfeld surface within the isocharge value of −0.119 to 0.164 a.u. by the ab initio Hartree–Fock (HF) quantum modelling approach with the 6-31G(d) basis set.
NMR Study
FT NMR spectra were recorded on a Bruker AVANCE III 400 MHz spectrometer, operating at 400.13, 100.61 and 161.98 MHz, respectively, for 1H, 13C and 31P. Spectra were indirectly referenced to the solvent deuterium lock shift; chemical shifts are quoted relative to TMS and 85% H3PO4. Probe temperatures were controlled by a standard variable temperature unit and are considered accurate to within ±1 K. Spectra were acquired on approximately 14 mmol solutions of (I) in each of CD2Cl2, d 6-DMSO and CDCl2CDCl2.
The ambient temperature (298 K) 1H NMR spectra of (I) display the expected signals due to the triphenylphosphine and dithiocarbamate ligands. The spectra are qualitatively identical in all three solvents, with the only significant differences being the position of the –OH signal of the dithiocarbamate ligand.
The aromatic region of the 1H spectrum in d 6-DMSO shows two multiplets at ca 7.39 ppm (6 H) and 7.28 ppm (24 H) attributable to Ph-H atoms of the triphenylphosphine ligands. A sharp singlet observed at 8.32 ppm (1 H) was assigned to CHCl3, as seen in the X-ray crystal structure analysis. The dithiocarbamate moiety displays a single set of resonances, indicating the two –CH2CH2OH groups are chemically equivalent. The –OH groups display a triplet at 4.80 ppm (3 J HH = 5.3 Hz), which disappears on the addition of D2O. The methylene hydrogen atoms display a triplet at 3.96 ppm (3 J HH = 6.4 Hz) and a pseudo quartet at 3.65 ppm, assignable to NCH2– and –CH2OH, respectively. On the addition of D2O, the quartet collapses to a triplet.
The 13C{1H} spectra in each of the solvents are also qualitatively identical. In d 6-DMSO solution, the carbon atoms of the triphenylphospine ligands give rise to four resonances at 134.6 ppm (very weak, d, 1 J PC ∼22 Hz, Cipso), 133.6 ppm (d, 2 J PC = 12 Hz, Cortho), 130.1 ppm (s, Cpara) and 128.9 ppm (d, 3 J PC = 5.70 Hz, Cmeta). The dithiocarbamate ligand shows two signals due to the methylene carbon atoms at 58.7 ppm (NCH2—) and 56.0 ppm (—CH2OH), respectively. The quaternary carbon atom of the dithiocarbamate was not observed.
The ambient temperature 31P{1H} spectrum in CD2Cl2 displays as single, broad resonance at −1.55 ppm (Δv1/2 = 280 Hz). The line broadening is attributed to rapid relaxation of Cu via the quadrupole relaxation (QR) mechanism. Quadrupole relaxation is strongly temperature dependent: the rate of relaxation increases as the temperature decreases. On cooling, the signal sharpens progressively: Δv1/2 (203 K) ∼35 Hz. The sharpening presumably arises because of the effective ‘decoupling’ of the 65Cu–31P and 63Cu–31P scalar couplings as the rate of (Cu) relaxation increases (Grace et al., 1970 ▸). The addition of ca 2 mg (0.9 equivalents) of triphenylphosphine at ambient temperature, to putatively give (I)·PPh3, gives a single, broad peak at ca −3 ppm, which is between the chemical shifts of pure (I) and free PPh3 (ca −6 ppm), indicating rapid exchange of the triphenylphosphine ligands.
In an attempt to resolve the Cu-P J couplings, 31P{1H} spectra were recorded in CDCl2CDCl2 solution at elevated temperatures (to reduce the rate of QR). However, no significant changes were observed in the line widths on elevating the temperature to 328 K, and any Cu–P coupling, if not lost through reversible ligand dissociation, remained unresolved. There was no evidence of decomposition at higher temperatures in this solvent.
There are two key conclusions from the foregoing. Firstly, the experiments with D2O proving exchange of the hydroxy-H atom indicates that this atom is labile, suggesting functionalization at this group should, in principle, be feasible. Secondly, the presence of additional Ph3P in solution does not result in displacement of the dithiocarbamate ligand nor force a monodentate mode of coordination proving the stability of complex (I) in each of (I)·CHCl3 and (I)·PPh3, and in solution.
Database survey
The structural chemistry of (R 3P)2Cu(S2CNR′R′′) compounds was summarized very recently (Jamaludin et al., 2016 ▸). In all, there are eight examples now available in the literature, namely {(Ph3P)2Cu[S2CN(Me)(CH2CH2OH)]}·CH2Cl2 Jamaludin et al., 2016 ▸), [(Ph3P)2Cu{S2CN(CH2CH2OH)2}]·PPh3 (Jian et al., 2000 ▸), [(Ph3P)2Cu{S2CN(n-Pr)2}]·CH2Cl2 (Xu et al., 2001 ▸), [(Ph3P)2Cu{S2CN(CH2CH2)2S}]·CH2Cl2 (Gupta et al., 2013 ▸), [(Ph3P)2Cu{S2CN(CH2CH2)2NPh}] (Gupta et al., 2013 ▸), [(Ph3P)2Cu{S2CN(Me)CH2Ph}]·CH2Cl2 (Kumar et al., 2009 ▸) and [(Ph3P)2Cu{S2CN(CH2Ph)(CH2py-4)}]·2H2O (Rajput et al., 2012 ▸). Interestingly, all but one structure co-crystallizes with another molecule, solvent or otherwise, perhaps indicating inefficient molecular packing for these molecules. The P2S2 donor sets all eight compounds approximate tetrahedral angles with the range of τ4 values being a low 0.78 in {(Ph3P)2Cu[S2CN(Me)(CH2CH2OH)]}·CH2Cl2 (Jamaludin et al., 2016 ▸) to a high of 0.85 in [(Ph3P)2Cu{S2CN(CH2CH2)2S}]·CH2Cl2 (Gupta et al., 2013 ▸), the narrow range emphasizing the similarity in the molecular structures/geometries.
Synthesis and crystallization
All chemicals and solvents were used as purchased without purification, and all reactions were carried out under ambient conditions. The melting point was determined on a Biobase automatic melting point apparatus MP450. The IR spectrum was obtained on a Perkin Elmer Spectrum 400 FT Mid-IR/Far-IR spectrophotometer from 4000 to 400 cm−1; abbreviations: br, broad; m, medium; s, strong.
Preparation of (I)·CHCl3: triphenylphosphine (Alfa Aesar, 2 mmol, 0.524 g) in acetonitrile (Merck, 10 ml) was added to copper(I) chloride (Sigma Aldrich, 1 mmol, 0.099 g) in acetonitrile (10 ml), followed by addition of a dispersion of potassium bis(2-hydroxyethyl)dithiocarbamate (1 mmol, 0.219 g) in acetonitrile (15 ml), prepared from the standard procedures (Jamaludin et al., 2016 ▸). The resulting mixture was stirred for 2 h at room temperature. Chloroform (Merck, 35 ml) was added to the reaction mixture and it was left for slow evaporation at room temperature. Yellow blocks of (I)·CHCl3 were obtained after one day. Yield: 0.699 g (91%). M.p. 423.8–424.5 K. IR (cm−1): 3268 (br) (OH), 1433 (s) (C—N), 1168 (m), 990 (s) (C—S).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. Carbon-bound H atoms were placed in calculated positions (C—H = 0.95–1.00 Å) and were included in the refinement in the riding-model approximation, with U
iso(H) set to 1.2U
eq(C). Refinement of the O-bound H atoms proved unstable so these atoms were fixed in the model in the positions revealed by a difference Fourier map, with U
iso(H) = 1.5U
eq(O). The maximum and minimum residual electron density peaks of 1.97 and 1.93 e Å−3, respectively, were located 0.78 and 0.62 Å from the Cl1 atom. While this feature of the difference map might indicate disorder, additional peaks that might be anticipated for the other atoms in the disordered component of chloroform molecule were not evident. This, plus the observation that the anisotropic displacement parameters of the atoms comprising the chloroform molecule exhibited no unusual features, suggest the residual electron densities have limited chemical significance. Finally, owing to poor agreement, four reflections, i.e. (326), (1 5), (666) and (1
5), (666) and (1 2) , were omitted from the final cycles of refinement.
2) , were omitted from the final cycles of refinement.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [Cu(C5H5NO2S2)(C18H15P)2]·CHCl3 |
| M r | 887.71 |
| Crystal system, space group | Triclinic, P

|
| Temperature (K) | 100 |
| a, b, c (Å) | 10.7271 (2), 13.5412 (2), 15.9361 (3) |
| α, β, γ (°) | 67.747 (2), 87.126 (2), 72.826 (2) |
| V (Å3) | 2041.92 (7) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.95 |
| Crystal size (mm) | 0.44 × 0.24 × 0.19 |
| Data collection | |
| Diffractometer | Rigaku SuperNova, Dual Mo at zero, AtlasS2 |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku Oxford Diffraction, 2015 ▸) |
| T min, T max | 0.928, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 78295, 11363, 10195 |
| R int | 0.029 |
| (sin θ/λ)max (Å−1) | 0.708 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.045, 0.117, 1.03 |
| No. of reflections | 11363 |
| No. of parameters | 478 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.97, −1.93 |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989016017837/hb7632sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016017837/hb7632Isup2.hkl
CCDC reference: 1515483
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Sunway University for support of biological and crystal engineering studies of metal dithiocarbamates.
supplementary crystallographic information
Crystal data
| [Cu(C5H5NO2S2)(C18H15P)2]·CHCl3 | Z = 2 |
| Mr = 887.71 | F(000) = 916 |
| Triclinic, P1 | Dx = 1.444 Mg m−3 |
| a = 10.7271 (2) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 13.5412 (2) Å | Cell parameters from 38605 reflections |
| c = 15.9361 (3) Å | θ = 3.1–30.1° |
| α = 67.747 (2)° | µ = 0.95 mm−1 |
| β = 87.126 (2)° | T = 100 K |
| γ = 72.826 (2)° | Prism, colourless |
| V = 2041.92 (7) Å3 | 0.44 × 0.24 × 0.19 mm |
Data collection
| Rigaku SuperNova, Dual Mo at zero, AtlasS2 diffractometer | 11363 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, SuperNova (Mo) X-ray Source | 10195 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.029 |
| Detector resolution: 5.2303 pixels mm-1 | θmax = 30.2°, θmin = 2.4° |
| ω scans | h = −14→14 |
| Absorption correction: multi-scan (CrysAlis PRO; Rigaku Oxford Diffraction, 2015) | k = −19→18 |
| Tmin = 0.928, Tmax = 1.000 | l = −22→21 |
| 78295 measured reflections |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.045 | H-atom parameters constrained |
| wR(F2) = 0.117 | w = 1/[σ2(Fo2) + (0.0542P)2 + 3.7972P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.002 |
| 11363 reflections | Δρmax = 1.97 e Å−3 |
| 478 parameters | Δρmin = −1.93 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cu | 0.36192 (2) | 0.77121 (2) | 0.71076 (2) | 0.01300 (7) | |
| S1 | 0.19989 (5) | 0.72501 (4) | 0.81318 (3) | 0.01687 (10) | |
| S2 | 0.18227 (5) | 0.94266 (4) | 0.66887 (3) | 0.01434 (10) | |
| P1 | 0.51698 (5) | 0.80568 (4) | 0.77705 (3) | 0.01311 (10) | |
| P2 | 0.40667 (5) | 0.66266 (4) | 0.62988 (3) | 0.01279 (10) | |
| O1 | −0.0657 (2) | 1.09492 (18) | 0.86819 (14) | 0.0388 (5) | |
| H1O | −0.0221 | 1.1248 | 0.8899 | 0.058* | |
| O2 | −0.0552 (2) | 0.89051 (18) | 0.99446 (14) | 0.0379 (5) | |
| H2O | −0.0317 | 0.9473 | 0.9646 | 0.057* | |
| N1 | 0.00449 (17) | 0.90924 (16) | 0.79337 (12) | 0.0179 (3) | |
| C1 | 0.11675 (19) | 0.86434 (16) | 0.76152 (13) | 0.0138 (3) | |
| C2 | −0.0662 (2) | 1.02862 (19) | 0.74859 (15) | 0.0222 (4) | |
| H2A | −0.1600 | 1.0404 | 0.7600 | 0.027* | |
| H2B | −0.0588 | 1.0517 | 0.6822 | 0.027* | |
| C3 | −0.0154 (3) | 1.1018 (2) | 0.78121 (17) | 0.0274 (5) | |
| H3A | 0.0814 | 1.0761 | 0.7870 | 0.033* | |
| H3B | −0.0444 | 1.1802 | 0.7370 | 0.033* | |
| C4 | −0.0538 (2) | 0.8356 (2) | 0.86728 (15) | 0.0244 (5) | |
| H4A | −0.0415 | 0.7655 | 0.8574 | 0.029* | |
| H4B | −0.1491 | 0.8725 | 0.8635 | 0.029* | |
| C5 | 0.0018 (2) | 0.8061 (2) | 0.96199 (16) | 0.0276 (5) | |
| H5A | −0.0136 | 0.7356 | 1.0035 | 0.033* | |
| H5B | 0.0974 | 0.7937 | 0.9613 | 0.033* | |
| C11 | 0.4522 (2) | 0.90455 (17) | 0.83239 (13) | 0.0155 (4) | |
| C12 | 0.3587 (2) | 0.88068 (19) | 0.89530 (15) | 0.0193 (4) | |
| H12 | 0.3316 | 0.8164 | 0.9064 | 0.023* | |
| C13 | 0.3054 (2) | 0.9506 (2) | 0.94159 (15) | 0.0223 (4) | |
| H13 | 0.2421 | 0.9338 | 0.9842 | 0.027* | |
| C14 | 0.3446 (2) | 1.0450 (2) | 0.92569 (16) | 0.0239 (4) | |
| H14 | 0.3080 | 1.0928 | 0.9572 | 0.029* | |
| C15 | 0.4374 (3) | 1.0687 (2) | 0.86363 (16) | 0.0264 (5) | |
| H15 | 0.4645 | 1.1330 | 0.8528 | 0.032* | |
| C16 | 0.4912 (2) | 0.99887 (18) | 0.81703 (15) | 0.0214 (4) | |
| H16 | 0.5547 | 1.0158 | 0.7746 | 0.026* | |
| C21 | 0.6312 (2) | 0.86383 (16) | 0.69950 (13) | 0.0140 (3) | |
| C22 | 0.5816 (2) | 0.96097 (17) | 0.62185 (14) | 0.0186 (4) | |
| H22 | 0.4900 | 0.9973 | 0.6120 | 0.022* | |
| C23 | 0.6653 (2) | 1.00436 (18) | 0.55932 (15) | 0.0221 (4) | |
| H23 | 0.6308 | 1.0706 | 0.5072 | 0.027* | |
| C24 | 0.7997 (2) | 0.95133 (19) | 0.57246 (15) | 0.0212 (4) | |
| H24 | 0.8568 | 0.9818 | 0.5298 | 0.025* | |
| C25 | 0.8497 (2) | 0.85403 (19) | 0.64806 (14) | 0.0196 (4) | |
| H25 | 0.9411 | 0.8171 | 0.6568 | 0.024* | |
| C26 | 0.7660 (2) | 0.81042 (18) | 0.71115 (14) | 0.0169 (4) | |
| H26 | 0.8009 | 0.7436 | 0.7627 | 0.020* | |
| C31 | 0.6275 (2) | 0.68849 (17) | 0.86749 (13) | 0.0162 (4) | |
| C32 | 0.6884 (2) | 0.70275 (19) | 0.93539 (15) | 0.0218 (4) | |
| H32 | 0.6666 | 0.7743 | 0.9389 | 0.026* | |
| C33 | 0.7811 (2) | 0.6126 (2) | 0.99804 (16) | 0.0268 (5) | |
| H33 | 0.8220 | 0.6228 | 1.0444 | 0.032* | |
| C34 | 0.8138 (3) | 0.5084 (2) | 0.99318 (17) | 0.0300 (5) | |
| H34 | 0.8781 | 0.4474 | 1.0356 | 0.036* | |
| C35 | 0.7531 (3) | 0.4929 (2) | 0.9266 (2) | 0.0347 (6) | |
| H35 | 0.7752 | 0.4211 | 0.9236 | 0.042* | |
| C36 | 0.6593 (2) | 0.58267 (19) | 0.86396 (18) | 0.0267 (5) | |
| H36 | 0.6170 | 0.5716 | 0.8187 | 0.032* | |
| C41 | 0.48854 (19) | 0.51623 (16) | 0.69995 (14) | 0.0156 (4) | |
| C42 | 0.5952 (2) | 0.45035 (19) | 0.67222 (16) | 0.0220 (4) | |
| H42 | 0.6255 | 0.4805 | 0.6136 | 0.026* | |
| C43 | 0.6577 (2) | 0.3406 (2) | 0.73001 (18) | 0.0272 (5) | |
| H43 | 0.7300 | 0.2961 | 0.7105 | 0.033* | |
| C44 | 0.6151 (2) | 0.29608 (19) | 0.81547 (18) | 0.0273 (5) | |
| H44 | 0.6593 | 0.2219 | 0.8552 | 0.033* | |
| C45 | 0.5079 (3) | 0.3600 (2) | 0.84291 (17) | 0.0273 (5) | |
| H45 | 0.4773 | 0.3288 | 0.9012 | 0.033* | |
| C46 | 0.4446 (2) | 0.46945 (18) | 0.78592 (16) | 0.0223 (4) | |
| H46 | 0.3711 | 0.5127 | 0.8055 | 0.027* | |
| C51 | 0.26885 (19) | 0.65508 (16) | 0.57220 (13) | 0.0135 (3) | |
| C52 | 0.2709 (2) | 0.55962 (17) | 0.55691 (14) | 0.0169 (4) | |
| H52 | 0.3427 | 0.4937 | 0.5812 | 0.020* | |
| C53 | 0.1684 (2) | 0.56083 (18) | 0.50627 (15) | 0.0196 (4) | |
| H53 | 0.1702 | 0.4958 | 0.4960 | 0.024* | |
| C54 | 0.0630 (2) | 0.65717 (19) | 0.47064 (15) | 0.0207 (4) | |
| H54 | −0.0067 | 0.6581 | 0.4355 | 0.025* | |
| C55 | 0.0595 (2) | 0.75232 (18) | 0.48645 (15) | 0.0192 (4) | |
| H55 | −0.0125 | 0.8181 | 0.4622 | 0.023* | |
| C56 | 0.16193 (19) | 0.75072 (17) | 0.53791 (14) | 0.0158 (4) | |
| H56 | 0.1588 | 0.8151 | 0.5496 | 0.019* | |
| C61 | 0.51483 (19) | 0.69669 (16) | 0.53792 (14) | 0.0150 (4) | |
| C62 | 0.4884 (2) | 0.70661 (17) | 0.44993 (14) | 0.0179 (4) | |
| H62 | 0.4136 | 0.6900 | 0.4363 | 0.021* | |
| C63 | 0.5711 (2) | 0.74078 (18) | 0.38150 (16) | 0.0225 (4) | |
| H63 | 0.5526 | 0.7468 | 0.3218 | 0.027* | |
| C64 | 0.6801 (2) | 0.7659 (2) | 0.40050 (18) | 0.0266 (5) | |
| H64 | 0.7356 | 0.7901 | 0.3538 | 0.032* | |
| C65 | 0.7076 (2) | 0.7553 (2) | 0.4883 (2) | 0.0295 (5) | |
| H65 | 0.7827 | 0.7716 | 0.5018 | 0.035* | |
| C66 | 0.6258 (2) | 0.7211 (2) | 0.55654 (17) | 0.0237 (5) | |
| H66 | 0.6454 | 0.7141 | 0.6164 | 0.028* | |
| C6 | 0.0552 (3) | 0.4509 (2) | 0.7262 (2) | 0.0354 (6) | |
| H6 | 0.0901 | 0.4682 | 0.6646 | 0.042* | |
| Cl1 | 0.14649 (11) | 0.31507 (7) | 0.79474 (7) | 0.0593 (3) | |
| Cl2 | −0.10822 (9) | 0.46142 (12) | 0.71310 (9) | 0.0740 (4) | |
| Cl3 | 0.07466 (6) | 0.55046 (5) | 0.76719 (4) | 0.03169 (13) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu | 0.01265 (12) | 0.01232 (11) | 0.01406 (12) | −0.00179 (8) | 0.00184 (8) | −0.00664 (9) |
| S1 | 0.0200 (2) | 0.0124 (2) | 0.0169 (2) | −0.00543 (18) | 0.00520 (18) | −0.00421 (17) |
| S2 | 0.0153 (2) | 0.0118 (2) | 0.0124 (2) | −0.00113 (16) | 0.00266 (16) | −0.00311 (16) |
| P1 | 0.0136 (2) | 0.0124 (2) | 0.0123 (2) | −0.00270 (17) | 0.00066 (17) | −0.00431 (17) |
| P2 | 0.0117 (2) | 0.0117 (2) | 0.0150 (2) | −0.00122 (17) | 0.00111 (17) | −0.00678 (18) |
| O1 | 0.0411 (11) | 0.0450 (12) | 0.0303 (10) | 0.0017 (9) | 0.0000 (8) | −0.0252 (9) |
| O2 | 0.0432 (11) | 0.0421 (11) | 0.0300 (10) | −0.0052 (9) | 0.0066 (8) | −0.0216 (9) |
| N1 | 0.0131 (8) | 0.0227 (9) | 0.0159 (8) | −0.0025 (7) | 0.0033 (6) | −0.0078 (7) |
| C1 | 0.0134 (8) | 0.0163 (9) | 0.0125 (8) | −0.0043 (7) | 0.0015 (7) | −0.0065 (7) |
| C2 | 0.0161 (9) | 0.0255 (11) | 0.0198 (10) | 0.0043 (8) | −0.0011 (8) | −0.0107 (8) |
| C3 | 0.0300 (12) | 0.0218 (11) | 0.0257 (11) | 0.0060 (9) | −0.0041 (9) | −0.0136 (9) |
| C4 | 0.0171 (10) | 0.0363 (13) | 0.0199 (10) | −0.0105 (9) | 0.0076 (8) | −0.0097 (9) |
| C5 | 0.0244 (11) | 0.0366 (13) | 0.0182 (10) | −0.0062 (10) | 0.0046 (8) | −0.0090 (9) |
| C11 | 0.0166 (9) | 0.0161 (9) | 0.0135 (9) | −0.0027 (7) | −0.0002 (7) | −0.0069 (7) |
| C12 | 0.0188 (10) | 0.0219 (10) | 0.0200 (10) | −0.0076 (8) | 0.0039 (8) | −0.0103 (8) |
| C13 | 0.0220 (10) | 0.0269 (11) | 0.0194 (10) | −0.0055 (9) | 0.0044 (8) | −0.0119 (9) |
| C14 | 0.0300 (12) | 0.0234 (11) | 0.0194 (10) | −0.0039 (9) | 0.0027 (9) | −0.0126 (9) |
| C15 | 0.0400 (13) | 0.0209 (10) | 0.0233 (11) | −0.0130 (10) | 0.0068 (10) | −0.0117 (9) |
| C16 | 0.0284 (11) | 0.0194 (10) | 0.0190 (10) | −0.0094 (8) | 0.0055 (8) | −0.0090 (8) |
| C21 | 0.0178 (9) | 0.0127 (8) | 0.0120 (8) | −0.0049 (7) | 0.0004 (7) | −0.0050 (7) |
| C22 | 0.0230 (10) | 0.0134 (9) | 0.0152 (9) | −0.0008 (7) | 0.0012 (8) | −0.0046 (7) |
| C23 | 0.0335 (12) | 0.0135 (9) | 0.0153 (9) | −0.0054 (8) | 0.0040 (8) | −0.0025 (7) |
| C24 | 0.0311 (11) | 0.0200 (10) | 0.0169 (10) | −0.0132 (9) | 0.0084 (8) | −0.0083 (8) |
| C25 | 0.0191 (10) | 0.0245 (10) | 0.0171 (9) | −0.0087 (8) | 0.0029 (8) | −0.0085 (8) |
| C26 | 0.0167 (9) | 0.0187 (9) | 0.0137 (9) | −0.0050 (7) | 0.0002 (7) | −0.0047 (7) |
| C31 | 0.0153 (9) | 0.0159 (9) | 0.0132 (9) | −0.0052 (7) | 0.0003 (7) | −0.0006 (7) |
| C32 | 0.0241 (11) | 0.0226 (10) | 0.0153 (9) | −0.0067 (8) | −0.0009 (8) | −0.0035 (8) |
| C33 | 0.0274 (12) | 0.0311 (12) | 0.0164 (10) | −0.0105 (10) | −0.0048 (8) | −0.0009 (9) |
| C34 | 0.0268 (12) | 0.0241 (11) | 0.0247 (12) | −0.0076 (9) | −0.0063 (9) | 0.0073 (9) |
| C35 | 0.0373 (14) | 0.0151 (10) | 0.0410 (15) | −0.0023 (10) | −0.0130 (12) | −0.0012 (10) |
| C36 | 0.0292 (12) | 0.0164 (10) | 0.0299 (12) | −0.0050 (9) | −0.0087 (9) | −0.0038 (9) |
| C41 | 0.0146 (9) | 0.0137 (8) | 0.0183 (9) | −0.0020 (7) | −0.0018 (7) | −0.0072 (7) |
| C42 | 0.0199 (10) | 0.0190 (10) | 0.0232 (11) | 0.0006 (8) | 0.0000 (8) | −0.0085 (8) |
| C43 | 0.0213 (11) | 0.0186 (10) | 0.0339 (13) | 0.0049 (8) | −0.0025 (9) | −0.0093 (9) |
| C44 | 0.0259 (11) | 0.0158 (10) | 0.0322 (12) | −0.0018 (8) | −0.0085 (9) | −0.0027 (9) |
| C45 | 0.0308 (12) | 0.0188 (10) | 0.0263 (12) | −0.0079 (9) | 0.0009 (9) | −0.0017 (9) |
| C46 | 0.0218 (10) | 0.0170 (10) | 0.0249 (11) | −0.0042 (8) | 0.0041 (8) | −0.0062 (8) |
| C51 | 0.0126 (8) | 0.0146 (8) | 0.0125 (8) | −0.0039 (7) | 0.0032 (6) | −0.0049 (7) |
| C52 | 0.0156 (9) | 0.0154 (9) | 0.0198 (9) | −0.0023 (7) | 0.0013 (7) | −0.0083 (7) |
| C53 | 0.0198 (10) | 0.0201 (10) | 0.0227 (10) | −0.0072 (8) | 0.0031 (8) | −0.0116 (8) |
| C54 | 0.0156 (9) | 0.0260 (11) | 0.0214 (10) | −0.0068 (8) | 0.0002 (8) | −0.0096 (8) |
| C55 | 0.0138 (9) | 0.0177 (9) | 0.0219 (10) | −0.0013 (7) | −0.0002 (7) | −0.0054 (8) |
| C56 | 0.0143 (9) | 0.0137 (9) | 0.0186 (9) | −0.0030 (7) | 0.0035 (7) | −0.0064 (7) |
| C61 | 0.0131 (8) | 0.0117 (8) | 0.0209 (9) | −0.0018 (7) | 0.0043 (7) | −0.0087 (7) |
| C62 | 0.0174 (9) | 0.0152 (9) | 0.0195 (10) | −0.0038 (7) | 0.0037 (7) | −0.0060 (7) |
| C63 | 0.0245 (11) | 0.0178 (10) | 0.0205 (10) | −0.0044 (8) | 0.0066 (8) | −0.0043 (8) |
| C64 | 0.0239 (11) | 0.0217 (11) | 0.0373 (13) | −0.0098 (9) | 0.0165 (10) | −0.0142 (10) |
| C65 | 0.0198 (11) | 0.0349 (13) | 0.0484 (15) | −0.0146 (10) | 0.0147 (10) | −0.0284 (12) |
| C66 | 0.0177 (10) | 0.0309 (12) | 0.0332 (12) | −0.0088 (9) | 0.0075 (9) | −0.0231 (10) |
| C6 | 0.0351 (14) | 0.0380 (14) | 0.0356 (14) | −0.0135 (12) | −0.0031 (11) | −0.0143 (12) |
| Cl1 | 0.0739 (6) | 0.0376 (4) | 0.0688 (6) | −0.0043 (4) | −0.0159 (5) | −0.0297 (4) |
| Cl2 | 0.0320 (4) | 0.1170 (9) | 0.1089 (9) | −0.0168 (5) | −0.0041 (5) | −0.0847 (8) |
| Cl3 | 0.0340 (3) | 0.0258 (3) | 0.0323 (3) | −0.0095 (2) | −0.0041 (2) | −0.0067 (2) |
Geometric parameters (Å, º)
| Cu—P2 | 2.2380 (5) | C26—H26 | 0.9500 |
| Cu—P1 | 2.2602 (6) | C31—C32 | 1.392 (3) |
| Cu—S1 | 2.3791 (6) | C31—C36 | 1.394 (3) |
| Cu—S2 | 2.4213 (5) | C32—C33 | 1.391 (3) |
| S1—C1 | 1.714 (2) | C32—H32 | 0.9500 |
| S2—C1 | 1.717 (2) | C33—C34 | 1.381 (4) |
| P1—C31 | 1.824 (2) | C33—H33 | 0.9500 |
| P1—C21 | 1.825 (2) | C34—C35 | 1.383 (4) |
| P1—C11 | 1.827 (2) | C34—H34 | 0.9500 |
| P2—C51 | 1.827 (2) | C35—C36 | 1.395 (3) |
| P2—C61 | 1.828 (2) | C35—H35 | 0.9500 |
| P2—C41 | 1.828 (2) | C36—H36 | 0.9500 |
| O1—C3 | 1.442 (3) | C41—C42 | 1.395 (3) |
| O1—H1O | 0.8576 | C41—C46 | 1.398 (3) |
| O2—C5 | 1.397 (3) | C42—C43 | 1.394 (3) |
| O2—H2O | 0.8400 | C42—H42 | 0.9500 |
| N1—C1 | 1.348 (3) | C43—C44 | 1.380 (4) |
| N1—C2 | 1.468 (3) | C43—H43 | 0.9500 |
| N1—C4 | 1.476 (3) | C44—C45 | 1.382 (4) |
| C2—C3 | 1.513 (3) | C44—H44 | 0.9500 |
| C2—H2A | 0.9900 | C45—C46 | 1.390 (3) |
| C2—H2B | 0.9900 | C45—H45 | 0.9500 |
| C3—H3A | 0.9900 | C46—H46 | 0.9500 |
| C3—H3B | 0.9900 | C51—C56 | 1.393 (3) |
| C4—C5 | 1.512 (3) | C51—C52 | 1.397 (3) |
| C4—H4A | 0.9900 | C52—C53 | 1.389 (3) |
| C4—H4B | 0.9900 | C52—H52 | 0.9500 |
| C5—H5A | 0.9900 | C53—C54 | 1.391 (3) |
| C5—H5B | 0.9900 | C53—H53 | 0.9500 |
| C11—C16 | 1.391 (3) | C54—C55 | 1.393 (3) |
| C11—C12 | 1.400 (3) | C54—H54 | 0.9500 |
| C12—C13 | 1.391 (3) | C55—C56 | 1.394 (3) |
| C12—H12 | 0.9500 | C55—H55 | 0.9500 |
| C13—C14 | 1.391 (3) | C56—H56 | 0.9500 |
| C13—H13 | 0.9500 | C61—C62 | 1.393 (3) |
| C14—C15 | 1.386 (3) | C61—C66 | 1.398 (3) |
| C14—H14 | 0.9500 | C62—C63 | 1.397 (3) |
| C15—C16 | 1.395 (3) | C62—H62 | 0.9500 |
| C15—H15 | 0.9500 | C63—C64 | 1.387 (3) |
| C16—H16 | 0.9500 | C63—H63 | 0.9500 |
| C21—C26 | 1.399 (3) | C64—C65 | 1.390 (4) |
| C21—C22 | 1.402 (3) | C64—H64 | 0.9500 |
| C22—C23 | 1.386 (3) | C65—C66 | 1.389 (3) |
| C22—H22 | 0.9500 | C65—H65 | 0.9500 |
| C23—C24 | 1.394 (3) | C66—H66 | 0.9500 |
| C23—H23 | 0.9500 | C6—Cl2 | 1.733 (3) |
| C24—C25 | 1.386 (3) | C6—Cl1 | 1.748 (3) |
| C24—H24 | 0.9500 | C6—Cl3 | 1.771 (3) |
| C25—C26 | 1.392 (3) | C6—H6 | 1.0000 |
| C25—H25 | 0.9500 | ||
| P2—Cu—P1 | 123.65 (2) | C24—C25—H25 | 120.0 |
| P2—Cu—S1 | 109.81 (2) | C26—C25—H25 | 120.0 |
| P1—Cu—S1 | 110.96 (2) | C25—C26—C21 | 120.80 (19) |
| P2—Cu—S2 | 123.17 (2) | C25—C26—H26 | 119.6 |
| P1—Cu—S2 | 103.74 (2) | C21—C26—H26 | 119.6 |
| S1—Cu—S2 | 75.264 (18) | C32—C31—C36 | 119.2 (2) |
| C1—S1—Cu | 84.12 (7) | C32—C31—P1 | 122.25 (16) |
| C1—S2—Cu | 82.75 (7) | C36—C31—P1 | 118.39 (16) |
| C31—P1—C21 | 101.95 (9) | C33—C32—C31 | 120.2 (2) |
| C31—P1—C11 | 101.78 (9) | C33—C32—H32 | 119.9 |
| C21—P1—C11 | 104.44 (9) | C31—C32—H32 | 119.9 |
| C31—P1—Cu | 118.06 (7) | C34—C33—C32 | 120.3 (2) |
| C21—P1—Cu | 114.57 (7) | C34—C33—H33 | 119.9 |
| C11—P1—Cu | 114.16 (7) | C32—C33—H33 | 119.9 |
| C51—P2—C61 | 102.10 (9) | C33—C34—C35 | 120.0 (2) |
| C51—P2—C41 | 103.13 (9) | C33—C34—H34 | 120.0 |
| C61—P2—C41 | 103.77 (9) | C35—C34—H34 | 120.0 |
| C51—P2—Cu | 117.50 (6) | C34—C35—C36 | 120.0 (2) |
| C61—P2—Cu | 116.28 (7) | C34—C35—H35 | 120.0 |
| C41—P2—Cu | 112.27 (7) | C36—C35—H35 | 120.0 |
| C3—O1—H1O | 105.7 | C31—C36—C35 | 120.2 (2) |
| C5—O2—H2O | 109.4 | C31—C36—H36 | 119.9 |
| C1—N1—C2 | 120.12 (18) | C35—C36—H36 | 119.9 |
| C1—N1—C4 | 119.48 (18) | C42—C41—C46 | 118.73 (19) |
| C2—N1—C4 | 120.09 (18) | C42—C41—P2 | 122.71 (17) |
| N1—C1—S1 | 120.50 (15) | C46—C41—P2 | 118.53 (16) |
| N1—C1—S2 | 122.12 (15) | C41—C42—C43 | 120.4 (2) |
| S1—C1—S2 | 117.38 (11) | C41—C42—H42 | 119.8 |
| N1—C2—C3 | 113.01 (18) | C43—C42—H42 | 119.8 |
| N1—C2—H2A | 109.0 | C44—C43—C42 | 120.3 (2) |
| C3—C2—H2A | 109.0 | C44—C43—H43 | 119.8 |
| N1—C2—H2B | 109.0 | C42—C43—H43 | 119.8 |
| C3—C2—H2B | 109.0 | C43—C44—C45 | 119.7 (2) |
| H2A—C2—H2B | 107.8 | C43—C44—H44 | 120.1 |
| O1—C3—C2 | 108.3 (2) | C45—C44—H44 | 120.1 |
| O1—C3—H3A | 110.0 | C44—C45—C46 | 120.5 (2) |
| C2—C3—H3A | 110.0 | C44—C45—H45 | 119.7 |
| O1—C3—H3B | 110.0 | C46—C45—H45 | 119.7 |
| C2—C3—H3B | 110.0 | C45—C46—C41 | 120.3 (2) |
| H3A—C3—H3B | 108.4 | C45—C46—H46 | 119.8 |
| N1—C4—C5 | 114.9 (2) | C41—C46—H46 | 119.8 |
| N1—C4—H4A | 108.5 | C56—C51—C52 | 119.51 (18) |
| C5—C4—H4A | 108.5 | C56—C51—P2 | 118.08 (15) |
| N1—C4—H4B | 108.5 | C52—C51—P2 | 122.29 (15) |
| C5—C4—H4B | 108.5 | C53—C52—C51 | 120.22 (19) |
| H4A—C4—H4B | 107.5 | C53—C52—H52 | 119.9 |
| O2—C5—C4 | 111.6 (2) | C51—C52—H52 | 119.9 |
| O2—C5—H5A | 109.3 | C52—C53—C54 | 120.1 (2) |
| C4—C5—H5A | 109.3 | C52—C53—H53 | 119.9 |
| O2—C5—H5B | 109.3 | C54—C53—H53 | 119.9 |
| C4—C5—H5B | 109.3 | C53—C54—C55 | 120.0 (2) |
| H5A—C5—H5B | 108.0 | C53—C54—H54 | 120.0 |
| C16—C11—C12 | 119.11 (19) | C55—C54—H54 | 120.0 |
| C16—C11—P1 | 124.41 (16) | C54—C55—C56 | 119.85 (19) |
| C12—C11—P1 | 116.47 (16) | C54—C55—H55 | 120.1 |
| C13—C12—C11 | 120.4 (2) | C56—C55—H55 | 120.1 |
| C13—C12—H12 | 119.8 | C51—C56—C55 | 120.29 (19) |
| C11—C12—H12 | 119.8 | C51—C56—H56 | 119.9 |
| C14—C13—C12 | 120.2 (2) | C55—C56—H56 | 119.9 |
| C14—C13—H13 | 119.9 | C62—C61—C66 | 118.8 (2) |
| C12—C13—H13 | 119.9 | C62—C61—P2 | 123.81 (16) |
| C15—C14—C13 | 119.6 (2) | C66—C61—P2 | 117.29 (16) |
| C15—C14—H14 | 120.2 | C61—C62—C63 | 120.5 (2) |
| C13—C14—H14 | 120.2 | C61—C62—H62 | 119.7 |
| C14—C15—C16 | 120.4 (2) | C63—C62—H62 | 119.7 |
| C14—C15—H15 | 119.8 | C64—C63—C62 | 120.2 (2) |
| C16—C15—H15 | 119.8 | C64—C63—H63 | 119.9 |
| C11—C16—C15 | 120.3 (2) | C62—C63—H63 | 119.9 |
| C11—C16—H16 | 119.9 | C63—C64—C65 | 119.6 (2) |
| C15—C16—H16 | 119.9 | C63—C64—H64 | 120.2 |
| C26—C21—C22 | 118.63 (19) | C65—C64—H64 | 120.2 |
| C26—C21—P1 | 122.28 (15) | C66—C65—C64 | 120.3 (2) |
| C22—C21—P1 | 118.91 (16) | C66—C65—H65 | 119.8 |
| C23—C22—C21 | 120.4 (2) | C64—C65—H65 | 119.8 |
| C23—C22—H22 | 119.8 | C65—C66—C61 | 120.6 (2) |
| C21—C22—H22 | 119.8 | C65—C66—H66 | 119.7 |
| C22—C23—C24 | 120.4 (2) | C61—C66—H66 | 119.7 |
| C22—C23—H23 | 119.8 | Cl2—C6—Cl1 | 111.63 (17) |
| C24—C23—H23 | 119.8 | Cl2—C6—Cl3 | 111.63 (17) |
| C25—C24—C23 | 119.7 (2) | Cl1—C6—Cl3 | 111.60 (15) |
| C25—C24—H24 | 120.1 | Cl2—C6—H6 | 107.2 |
| C23—C24—H24 | 120.1 | Cl1—C6—H6 | 107.2 |
| C24—C25—C26 | 120.0 (2) | Cl3—C6—H6 | 107.2 |
| C2—N1—C1—S1 | −179.88 (15) | P1—C31—C32—C33 | −174.02 (18) |
| C4—N1—C1—S1 | −6.2 (3) | C31—C32—C33—C34 | 0.3 (4) |
| C2—N1—C1—S2 | 0.1 (3) | C32—C33—C34—C35 | −0.9 (4) |
| C4—N1—C1—S2 | 173.79 (16) | C33—C34—C35—C36 | 0.4 (4) |
| Cu—S1—C1—N1 | −173.40 (17) | C32—C31—C36—C35 | −1.3 (4) |
| Cu—S1—C1—S2 | 6.57 (10) | P1—C31—C36—C35 | 173.7 (2) |
| Cu—S2—C1—N1 | 173.49 (17) | C34—C35—C36—C31 | 0.7 (4) |
| Cu—S2—C1—S1 | −6.48 (10) | C51—P2—C41—C42 | 96.01 (19) |
| C1—N1—C2—C3 | −84.6 (2) | C61—P2—C41—C42 | −10.2 (2) |
| C4—N1—C2—C3 | 101.8 (2) | Cu—P2—C41—C42 | −136.55 (17) |
| N1—C2—C3—O1 | −77.9 (2) | C51—P2—C41—C46 | −85.86 (18) |
| C1—N1—C4—C5 | 83.8 (3) | C61—P2—C41—C46 | 167.95 (17) |
| C2—N1—C4—C5 | −102.5 (2) | Cu—P2—C41—C46 | 41.58 (19) |
| N1—C4—C5—O2 | 82.1 (3) | C46—C41—C42—C43 | −1.0 (3) |
| C31—P1—C11—C16 | 103.55 (19) | P2—C41—C42—C43 | 177.13 (18) |
| C21—P1—C11—C16 | −2.2 (2) | C41—C42—C43—C44 | −0.3 (4) |
| Cu—P1—C11—C16 | −128.12 (17) | C42—C43—C44—C45 | 1.6 (4) |
| C31—P1—C11—C12 | −75.31 (18) | C43—C44—C45—C46 | −1.4 (4) |
| C21—P1—C11—C12 | 178.89 (16) | C44—C45—C46—C41 | 0.1 (4) |
| Cu—P1—C11—C12 | 53.01 (17) | C42—C41—C46—C45 | 1.1 (3) |
| C16—C11—C12—C13 | 0.2 (3) | P2—C41—C46—C45 | −177.09 (19) |
| P1—C11—C12—C13 | 179.10 (17) | C61—P2—C51—C56 | −94.07 (16) |
| C11—C12—C13—C14 | 0.0 (3) | C41—P2—C51—C56 | 158.48 (16) |
| C12—C13—C14—C15 | −0.2 (4) | Cu—P2—C51—C56 | 34.40 (18) |
| C13—C14—C15—C16 | 0.2 (4) | C61—P2—C51—C52 | 82.03 (18) |
| C12—C11—C16—C15 | −0.2 (3) | C41—P2—C51—C52 | −25.42 (19) |
| P1—C11—C16—C15 | −179.03 (18) | Cu—P2—C51—C52 | −149.50 (15) |
| C14—C15—C16—C11 | 0.0 (4) | C56—C51—C52—C53 | 1.3 (3) |
| C31—P1—C21—C26 | 8.01 (19) | P2—C51—C52—C53 | −174.71 (16) |
| C11—P1—C21—C26 | 113.67 (18) | C51—C52—C53—C54 | −0.1 (3) |
| Cu—P1—C21—C26 | −120.71 (16) | C52—C53—C54—C55 | −0.6 (3) |
| C31—P1—C21—C22 | −176.85 (16) | C53—C54—C55—C56 | 0.1 (3) |
| C11—P1—C21—C22 | −71.19 (18) | C52—C51—C56—C55 | −1.9 (3) |
| Cu—P1—C21—C22 | 54.43 (17) | P2—C51—C56—C55 | 174.34 (16) |
| C26—C21—C22—C23 | −1.6 (3) | C54—C55—C56—C51 | 1.2 (3) |
| P1—C21—C22—C23 | −176.94 (17) | C51—P2—C61—C62 | −2.56 (19) |
| C21—C22—C23—C24 | 0.6 (3) | C41—P2—C61—C62 | 104.40 (18) |
| C22—C23—C24—C25 | 0.6 (3) | Cu—P2—C61—C62 | −131.80 (16) |
| C23—C24—C25—C26 | −0.9 (3) | C51—P2—C61—C66 | 173.12 (17) |
| C24—C25—C26—C21 | −0.2 (3) | C41—P2—C61—C66 | −79.91 (18) |
| C22—C21—C26—C25 | 1.4 (3) | Cu—P2—C61—C66 | 43.89 (18) |
| P1—C21—C26—C25 | 176.57 (16) | C66—C61—C62—C63 | −0.3 (3) |
| C21—P1—C31—C32 | 79.67 (19) | P2—C61—C62—C63 | 175.35 (16) |
| C11—P1—C31—C32 | −28.1 (2) | C61—C62—C63—C64 | −0.4 (3) |
| Cu—P1—C31—C32 | −153.85 (16) | C62—C63—C64—C65 | 0.9 (3) |
| C21—P1—C31—C36 | −95.22 (19) | C63—C64—C65—C66 | −0.7 (4) |
| C11—P1—C31—C36 | 157.05 (19) | C64—C65—C66—C61 | 0.0 (4) |
| Cu—P1—C31—C36 | 31.3 (2) | C62—C61—C66—C65 | 0.5 (3) |
| C36—C31—C32—C33 | 0.8 (3) | P2—C61—C66—C65 | −175.44 (18) |
Hydrogen-bond geometry (Å, º)
Cg1 is the ring centroid of (C51–C56).
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2O···O1 | 0.84 | 1.95 | 2.710 (3) | 150 |
| O1—H1O···O2i | 0.86 | 1.97 | 2.697 (3) | 142 |
| C6—Cl3···Cg1 | 1.77 (1) | 3.81 (1) | 3.798 (3) | 76 (1) |
Symmetry code: (i) −x, −y+2, −z+2.
References
- Biersack, B., Ahmad, A., Sarkar, F. H. & Schobert, R. (2012). Curr. Med. Chem. 19, 3949–3956. [DOI] [PubMed]
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Cavallo, G., Metrangolo, P., Milani, R., Pilati, T., Priimagi, A., Resnati, G. & Terraneo, G. (2016). Chem. Rev. 116, 2478–2601. [DOI] [PMC free article] [PubMed]
- Chen, B.-J., Jamaludin, N. S., Khoo, C.-H., See, T.-H., Sim, J.-H., Cheah, Y.-K., Halim, S. N. A., Seng, H.-L. & Tiekink, E. R. T. (2016). J. Inorg. Biochem. 163, 68–80. [DOI] [PubMed]
- Clark, T., Hennemann, M., Murray, J. S. & Politzer, P. (2007). J. Mol. Model. 13, 291–296. [DOI] [PubMed]
- Dance, I. & Scudder, M. (1995). J. Chem. Soc. Chem. Commun. pp. 1039–1040.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Gans, J. & Shalloway, D. (2001). J. Mol. Graphics Modell. 19, 557–559. [DOI] [PubMed]
- Grace, M., Beall, H. & Bushweller, C. H. (1970). J. Chem. Soc. D, p. 701.
- Gupta, A. N., Singh, V., Kumar, V., Rajput, A., Singh, L., Drew, M. G. B. & Singh, N. (2013). Inorg. Chim. Acta, 408, 145–151.
- Hogarth, G. (2012). Mini Rev. Med. Chem. 12, 1202–1215. [DOI] [PubMed]
- Jamaludin, N. S., Halim, S. N. A., Khoo, C.-H., Chen, B.-J., See, T.-H., Sim, J.-H., Cheah, Y.-K., Seng, H.-L. & Tiekink, E. R. T. (2016). Z. Kristallogr 231, 341–349.
- Jian, F., Bei, F., Lu, L., Yang, X., Wang, X., Razak, I. A., Shanmuga Sundara Raj, S. & Fun, H.-K. (2000). Acta Cryst. C56, e288–e289. [DOI] [PubMed]
- Kumar, A., Mayer-Figge, H., Sheldrick, W. S. & Singh, N. (2009). Eur. J. Inorg. Chem. pp. 2720–2725.
- Losasso, C., Belluco, S., Cibin, V., Zavagnin, P., Mičetić, I., Gallocchio, F., Zanella, M., Bregoli, L., Biancotto, G. & Ricci, A. (2014). Front. Microbiol 5, 1–9. [DOI] [PMC free article] [PubMed]
- Onwudiwe, D. C., Ekennia, A. C. & Hosten, E. (2016). J. Coord. Chem. 69, 2454–2468.
- Rajput, G., Singh, V., Singh, S. K., Prasad, L. B., Drew, M. G. B. & Singh, N. (2012). Eur. J. Inorg. Chem. pp. 3885–3891.
- Rigaku Oxford Diffraction (2015). CrysAlis PRO. Agilent Technologies Inc., Santa Clara, CA, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Sim, J. H., Jamaludin, N. S., Khoo, C. H., Cheah, Y. K., Halim, S. N. B. A., Seng, H. L. & Tiekink, E. R. T. (2014). Gold Bull. 47, 225–236.
- Skrott, Z. & Cvek, B. (2012). Mini Rev. Med. Chem. 12, 1184–1192. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Verma, S. K. & Singh, V. K. (2015). RSC Adv. 5, 53036–53046.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Xu, L. Z., Lin, J. H., Zhang, S. S., Jiao, K. & Jian, F. F. (2001). Pol. J. Chem 75, 755–757.
- Yang, L., Powell, D. R. & Houser, R. P. (2007). Dalton Trans. pp. 955–964. [DOI] [PubMed]
- Yeo, C. I., Tan, S. L. & Tiekink, E. R. T. (2016). Acta Cryst. E72, 1446–1452. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989016017837/hb7632sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016017837/hb7632Isup2.hkl
CCDC reference: 1515483
Additional supporting information: crystallographic information; 3D view; checkCIF report









