Significance
The potato is perhaps the most important of the high Andean crops. Cultivated the length of the Andean cordillera and across disparate ecological zones, it is now also a principal global staple. For this study, we analyzed starch microremains recovered from 14 groundstone tools from Late Archaic to Early Formative period contexts at Jiskairumoko, an early village site in the Titicaca Basin of the south-central Andes of Peru. A total of 50 starches were identified as consistent with cultivated potato. These data are significant because they contribute to the empirical foundation for understanding the development of food production in the study area and underscore the utility of starch analysis in addressing questions relating to geophyte domestication and cultivation.
Keywords: microbotanical starch analysis, Solanum tuberosum, plant domestication, south-central Andes, food production
Abstract
The data presented in this paper provide direct microbotanical evidence concerning the early use of potato (Solanum tuberosum) within its botanical locus of origin in the high south-central Andes. The data derive from Jiskairumoko, an early village site in the western Titicaca Basin dating to the Late Archaic to Early Formative periods (∼3,400 cal y BC to 1,600 cal y BC). Because the site reflects the transition to sedentism and food production, these data may relate to potato domestication and early cultivation. Of 141 starch microremains recovered from 14 groundstone tools from Jiskairumoko, 50 are identified as consistent with cultivated or domesticated potato, based on reference to published materials and a study of wild and cultivated potato starch morphology. Along with macro- and microbotanical evidence for chenopod consumption and grinding tool data reflecting intensive use of this technology throughout site occupation, the microbotanical data reported here suggest the intensive exploitation, if not cultivation, of plant resources at Jiskairumoko. Elucidating the details of the trajectory of potato domestication is necessary for an overall understanding of the development of highland Andean agriculture, as this crop is central to the autochthonous agricultural suite. A paucity of direct botanical evidence, however, has hindered research efforts. The results of the modern and archaeological starch analyses presented here underscore the utility of this method in addressing questions related to the timing, mode, and context of potato origins.
Recent molecular and phytogeographical data indicate the high south-central Andes as the locus of domestication for several crops, including the potato (Solanum tuberosum) (1–3). Archaeological evidence from the region pinpoints the Late Archaic (∼3,400–2,200 cal y BC) and Terminal Archaic periods (∼2,200–1,600 cal y BC) as the time during which the transition from foraging to agro-pastoralism transpired; small-scale farming was in place by the subsequent Early Formative period (∼1,600 cal y BC) (4–7).
Despite the centrality of geophytes to the high-elevation crop suite, however, most of their origins remain elusive. Macrobotanical preservation of this class of plants—and the diagnostic utility of macrogeophyte remains—is limited. Accordingly, beginning in the 1960s, Andeanists Towle (8) and Ugent et al. (9, 10) sought to further their insights through microbotanical analysis of starch morphology from macrogeophytes. Their pioneering work foreshadowed the mounting role of microbotanical studies around the world, including the study area (11–18). Following suit, this study contributes direct microbotanical evidence for potato within its botanical hearth at the onset of food production.
The Study Area: Paleoenvironment and Archaeological Context
Jiskairumoko is an open-air village site situated in the central Ilave Valley of the western Titicaca Basin of southern Peru (Fig. S1). At ∼3,890 m above sea level, Jiskairumoko nears the uppermost limit of montane agriculture. The site was occupied from the Late/Terminal Archaic to Early Formative periods (6, 19) and valley- and site-level data point to significant socioeconomic change during this time, namely, the advent of camelid management and chenopod cultivation, shifts in mobility related to changing subsistence strategies, and the emergence of socioeconomic differentiation. Survey data point to population increase and settlement aggregation at fewer and bigger sites, like Jiskairumoko, near river terraces during the Terminal Archaic period (6, 20). At Jiskairumoko, evidence for sedentarization includes the circular organization of five pithouse structures, labor-intensive wattle-and-daub architecture, the formalized use of space and maintenance of residential structures, secondary refuse patterning, characteristics of storage areas and grinding tools, and faunal and macrobotanical evidence for occupational duration (6). Moreover, the presence of a gold-bead necklace in a Terminal Archaic burial suggests the emergence of socioeconomic differentiation (6, 19).
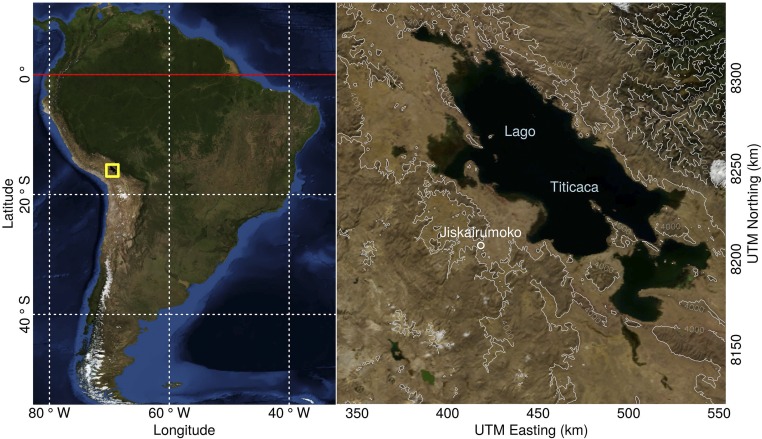
Fig. S1.
Map of Jiskairumoko and study area. Both maps reference the WGS84 datum. The right map is projected to UTM zone 19S. Base imagery is courtesy of NASA (92). Topographic contours are derived from Global Multiresolution Terrain Elevation Data 2010 (93). Cartography by William (Randy) Haas using R statistical computing language and associated mapping packages (94–96).
Based on Paduano et al.’s (21) study of pollen and charcoal lake core data, Craig et al. (20) observe that the rise in the fine charcoal fraction in the Titicaca Basin after ∼2,000 cal y BC “represents the first time in the history of the lake” that fire frequency increased during a more humid period. Craig et al. (20) concur with Paduano et al. (21) that this pattern likely reflects agricultural clearing rather than fires resulting from high aridity and accumulated woody biomass. Together, the archaeological and paleoenvironmental data seem to reflect a dramatic transformation in regional land-use patterns, specifically, the onset of agro-pastoralism at this time. This study adds to our picture of Titicaca Basin developments by elucidating aspects of geophyte use at Jiskairumoko.
Methods and Materials
Twenty unwashed grinding tools were tested for starch grains; tools encompass the span of Jiskairumoko's occupation and represent distinct morphological types and modes of design (Table S1). Standard protocols were used to process artifacts in order to avoid postexcavation contamination and recover starch grains (SI Methods and Materials). Adhering residues were recovered from tools using sonication and starches were extracted using a heavy liquid flotation solution of cesium chloride. An Olympus BHM metallurgical transmitted light microscope equipped with polarization lens and a Nikon Coolpix 990 digital camera was used to analyze and photograph starches. Starch granules were described in terms of a standard set of morphological and metric characteristics (22).
Table S1.
Jiskairumoko grinding tools tested for starch; handstones/manos one-handed unless otherwise noted
| Block no./structure 14C dates | Sample no. | Tool ID/provenience | Tool type | Subtype | Recycled* | Multiple concomitant use | No. Solanum starch | No. non-Solanum starch | Total no. starch |
| Late Archaic period | |||||||||
| Block 9/Pithouse 1 | 1 | Tool 57/x27a8vii | Mano | F-c/basin | X | — | 16 | 19 | 35 |
| 4,562 ± 73 14C y BP (AA58476) | 2 | Tool 232/aa28c7vii | Mano | F-c | X | — | 6 | 8 | 14 |
| 3 | Tool 1/z26c7vi | Mano | F-c | — | — | 1 | — | 1 | |
| 4 | Tool 2/bb27b3vi | Mano | F-c | — | — | 1 | 12 | 13 | |
| 5 | Tool 17/y25c11v | Mano/abrader/pecking stone | Flat/f-c | X | X | 14 | 10 | 24 | |
| 6 | Tool 05/x25dix11 | Pecking stone/ flat-faceted abrader | — | — | — | — | — | — | |
| 7 | Tool 378/z27axiii1 | Handstone | — | — | — | — | — | — | |
| Total no. Late Archaeic starches | 38 | 49 | 87 | ||||||
| Terminal Archaic period | |||||||||
| Block 3/Pithouse 3 | 8 | Tool 131/w36d7viii | Mano/ flat-faceted abrader | F-c | — | X | — | 2 | 2 |
| 3,448 ± 44 14C y BP (AA43382) | 9 | Tool 132/w36d7viii | Mano/ flat-faceted abrader, fragment | F-c | X | X | — | 7 | 7 |
| 10 | Tool 174/ x37c7viiia | Metate frag. | F-c | — | — | 1 | 5 | 6 | |
| 11 | Tool 103/y34aviii10 | Mano frag. | F-c | X | — | — | — | — | |
| 12 | Tool 106/y36cvii7 | Handstone/ poss. mano | Basin | — | — | — | — | — | |
| 13 | Tool 163/z36bvclayfl | Handstone/ pecking stone | — | — | X | — | — | — | |
| Total no. Terminal Archaic starches | 1 | 14 | 15 | ||||||
| Early Formative period | |||||||||
| Block 4/Rectangular Structure 1 | 14 | Tool 107/ ff22c0av | Mano | F-c | — | — | 1 | — | 1 |
| 3,410 ± 70 14C y BP (Beta-97320) | 15 | Tool 120/jj24dvii8 | Mano | Two-handed mano | — | — | — | 1 | 1 |
| 3,401 ± 45 14C y BP (AA43375) | |||||||||
| 3,330 ± 45 14C y BP (AA43376) | |||||||||
| 3,240 ± 70 14C y BP (Beta-97321) | |||||||||
| Block 6/Rectangular Structure 2 and | 16 | Tool 105/ | Mano | Basin | — | — | — | 1 | 1 |
| Block 7/Semi-Subterranean Structure 1 | 17 | Tool 125/ hh32d0aiv | Mano | F-c | — | — | 7 | 23 | 30 |
| 3,235 ± 58 14C y BP (AA45952) | 18 | Tool 198/ jj30d1iv | Mano | Basin | — | — | 3 | 2 | 5 |
| 3,208 ± 58 14C y BP (AA58475) | 19 | Tool 54/rr25a/bxii23 | Mano frag. | F-c | — | — | — | 1 | 1 |
| 20 | Tool 63/rr25av10 | Handstone/ pecking stone | Flat/f-c | — | X | — | — | — | |
| Total no. Early Formative starches | 11 | 28 | 39 | ||||||
| Total no. starches for all time periods | 50 | 91 | 141 | ||||||
F-c, flat-concave.
Recycled manos/handstones are thought to have been recycled from (worn out or fragmented) metates/netherstones.
Taxonomic identification of archaeological starch was based on published reference works and a preliminary comparative study (9, 10, 15, 23–30). Reference taxa include Andean pseudocereal, seed, legume, fruit, and geophyte crops like chenopods, amaranth, maize, beans, chiles, manioc, achira, oca, ulluco, and potato (9, 10, 15, 16, 23, 25, 28, 30–33). Key objectives of the comparative analysis were to discern the degree to which (i) starches from oca, potato, and ulluco are distinctive and (ii) starches from wild and cultivated potato differ from one another (Fig. 1). Study results constitute much of the basis for our interpretation of the Jiskairumoko Solanum, which are the focus of our discussion of the archaeological starches. A more detailed description of the grinding toolkit, sample processing, microscope analysis, and reference study can be found in SI Methods and Materials.
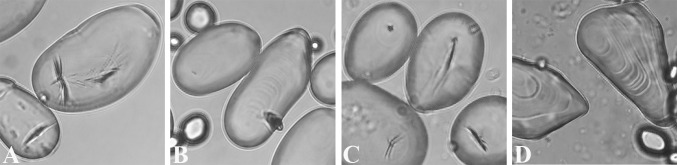
Fig. 1.
Modern Solanum starch grains from freeze-dried (A and B) and fresh tubers (C and D) at 40× magnification. (A) Traditional cultivar, Imilla negra (size range 9–96 µm, mean length 38 µm); (B) traditional cultivar, Paula (size range 12–100 µm, mean length 48 µm); (C) traditional cultivar, Paula (size range 12–75 µm, mean length 41 µm); (D) wild ancestor, Solanum bukasovii (= S. candolleanum) (size data unavailable).
Results
A total of 141 starch grains were recovered from 14 grinding tools; of these, 50 (35%) were identified as potato (Solanum sp.) and derive from nine artifacts spanning occupation of the site (Table 1). Radiocarbon dates derive from associated cultural features and are corroborated by architectural characteristics or a lack of evidence for mixing/disturbance (6, 34). In addition to Solanum, the starch assemblage includes various distinctive unidentified morphological types, along with Phaseolus. Artifacts yielding more than one starch grain have a mix of Solanum and other starch types, indicating that the tools were used to process diverse plants, rather than having specialized uses. A pattern of generalized use fits with the somewhat ad hoc morphology of the grinding toolkit, comprising single and multiple concomitant use, expediently and strategically designed, and recycled tools (Figs. S2 and S3 and Table S1).
Table 1.
Radiocarbon dates and proveniences for tools yielding Solanum starches
| Block no./structure/14C dates | Tool ID/provenience | Tool type/subtype/other attributes | No. Solanum starch |
| Late Archaic | |||
| Block 9/Pithouse 1/4,562 ± 73 14C y BP (AA58476) | Tool 57/x27a8vii | Mano, flat-concave/ basin subtype, recycled from metate | 16 |
| Tool 232/aa28c7vii | Mano, flat-concave, recycled from metate | 6 | |
| Tool 1/z26c7vi | Mano, flat-concave | 1 | |
| Tool 2/bb27b3vi | Mano, flat-concave | 1 | |
| Tool 17/y25c11v | Multiple concomitant use tool (mano, abrader, pecking stone), flat/flat-concave mano, recycled | 14 | |
| Terminal Archaic | |||
| Block 3/Pithouse 3/3,448 ± 44 14C y BP (AA43382) | Tool 174/x37c7viiia | Flat-concave metate fragment | 1 |
| Early Formative | |||
| Block 4/Rectangular Structure 1/3,410 ± 70 14C y BP (Beta-97320) | Tool 107/ff22c0av | Mano, flat-concave | 1 |
| 3,401 ± 45 14C y BP (AA43375) | Tool 125/hh32d0aiv | Mano, flat-concave | 7 |
| 3,330 ± 45 14C y BP (AA43376) | Tool 198/jj30d1iv | Mano, basin | 3 |
| 3,240 ± 70 14C y BP (Beta-97321) | |||
| Total no. Solanum | 50 | ||
Fig. S2.
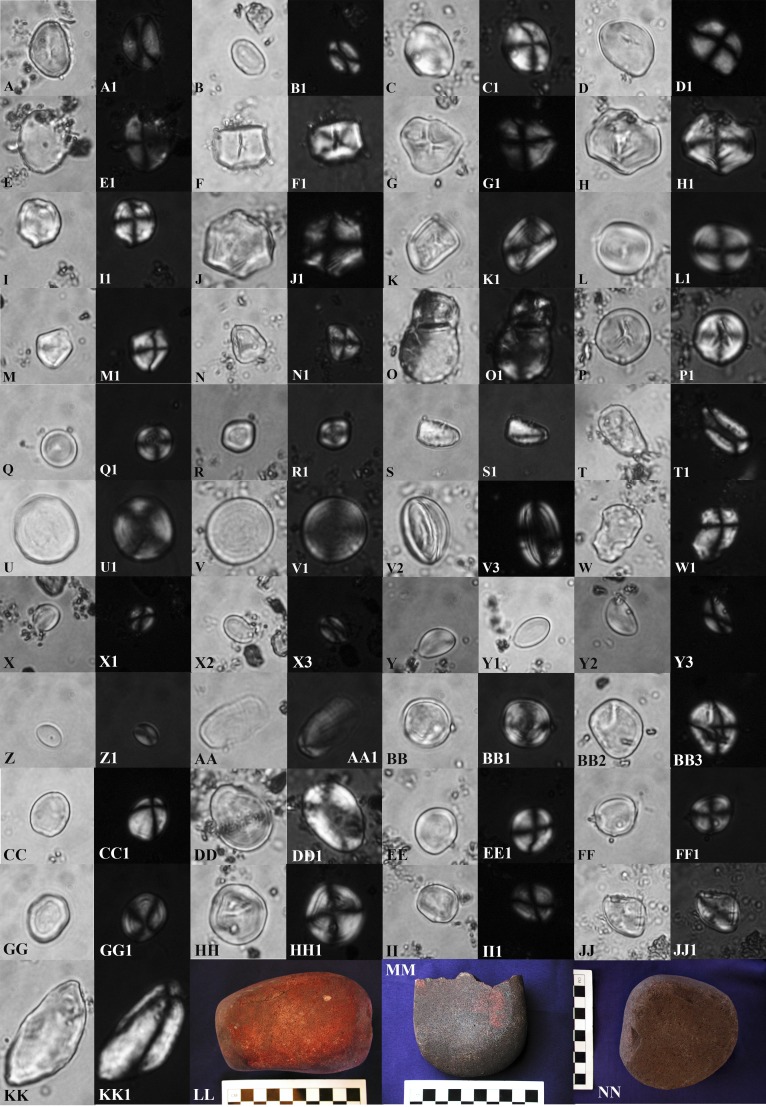
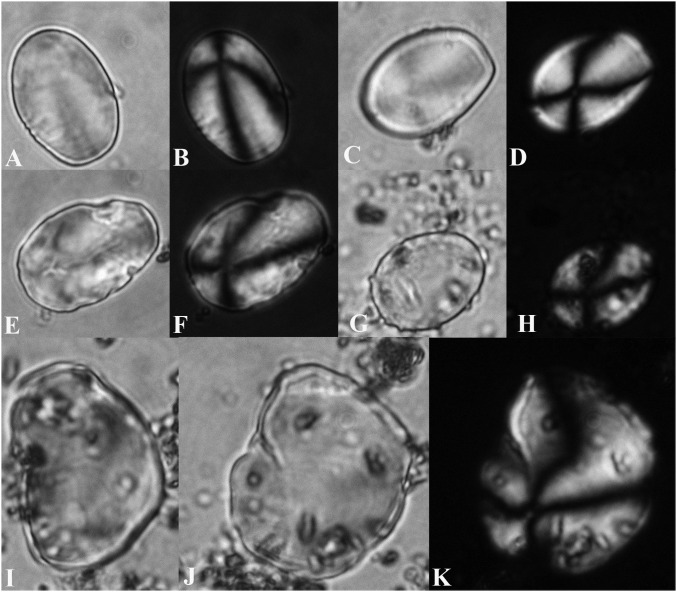
Representative selection of 37 non-Solanum and/or unidentified archaeological starch grains from Jiskairumoko and three groundstone tools yielding starch microremains. (A) T02G01: 23 × 21 µm, Phaseolus sp.; (A1) same grain, polarized; (B) T57G18: 11 × 9 µm, Phaseolus sp.; (B1) same grain, polarized; (C) T232G04: 15 × 15 µm; (C1) same grain, polarized; (D) T02G09: 17 × 15 µm; (D1) same grain, polarized; (E) T105G01: 19 × 15 µm, cf Phaseolus sp.; (E1) same grain, polarized; (F) T02G11: 13 × 15 µm; (F1) same grain, polarized; (G) T57G32: 19 × 17 µm; (G1) same grain, polarized; (H) T57G31: 21 × 19 µm; (H1) same grain, polarized; (I) T57G12: 15 × 19 µm; (I1) same grain, polarized; (J) T57G13: 21 × 21 µm; (J1) same grain, polarized; (K) T57G19: 17 × 13 µm; (K1) same grain, polarized; (L) T57G21: 15 × 15 µm, Phaseolus sp.; (L1) same grain, polarized; (M) T232G06: 14 × 13 µm; (M1) same grain, polarized; (N) T57G24: 13 × 11 µm; (N1) same grain, polarized; (O) T57G26&27: 17 × 17 µm and 13 × 11 µm; (O1) same grains, polarized; (P) T232G09: 17 × 15 µm; (P1) same grain, polarized; (Q) T57G04: 11 × 11 µm; (Q1) same grain, polarized; (R) T57G16: 11 × 11; (R1) same grain, polarized; (S) T57G09: 15 × 9 µm; (S1) same grain, polarized; (T) T57G10: 17 × 11 µm, cf Phaseolus sp.; (T1) same grain (alternate view) polarized; (U) T02G10: 23 × 21 µm; (U1) same grain, polarized; (V) T232G10: 21 × 18 µm; (V1) same grain, polarized; (V2) same grain, side view; (V3) same grain (alternate view), polarized; (W) T57G28: 17 × 12 µm (polarization consistent with Solanum, but too damaged for secure identification); (W1) same grain, polarized; (X) T17G10: 9 × 6 µm; (X1) same grain, polarized; (X2) alternate view of same grain; (X3) alternate view of same grain, polarized; (Y) T17G09: 13 × 9 µm; (Y1) same grain, polarized; (Y2) alternate view of same grain; (Y3) alternate view of same grain, polarized; (Z) T17G11: 9 × 5 µm, spot of foreign matter on grain in eccentric position; (Z1) same grain, polarized; (AA) T57G01: 23 × 13 µm; (AA1) same grain, polarized; (BB) T17G04: 17 × 15 µm; (BB1) same grain, polarized (lamellae very evident in this view); (BB2) alternate view of same grain; (BB3) alternate view of same grain, polarized; (CC) T17G02: 13 × 11 µm; (CC1) same grain, polarized view; (CC2) same grain, semipolarized; (DD) T232G03: 21 × 15 µm; (DD1) same grain, polarized; (EE) T232G13: 13 × 12 µm; (EE1) same grain, polarized; (FF) T232G14: 13 × 11 µm; (FF1) same grain, polarized; (GG) T198G05: 16 × 13 µm; (GG1) same grain, polarized; (HH) T232G12: 17 × 15 µm; (HH1) same grain, polarized; (II) T57G23: 13 × 11 µm;17 (II1) same grain, polarized; (JJ) T02G05: 15 × 13 µm; (JJ1) same grain, polarized; (KK) T57G15: 37 × 19 µm; (KK1) same grain, polarized; (LL) Tool 120 (jj24dvii8): Early Formative period two-handed mano; (MM) Tool 132 (w36d7viii): Terminal Archaic period flat to flat-concave mano fragment; (NN) Tool 2 (bb27b3vi): Late Archaic period flat to flat-concave mano.
Fig. S3.
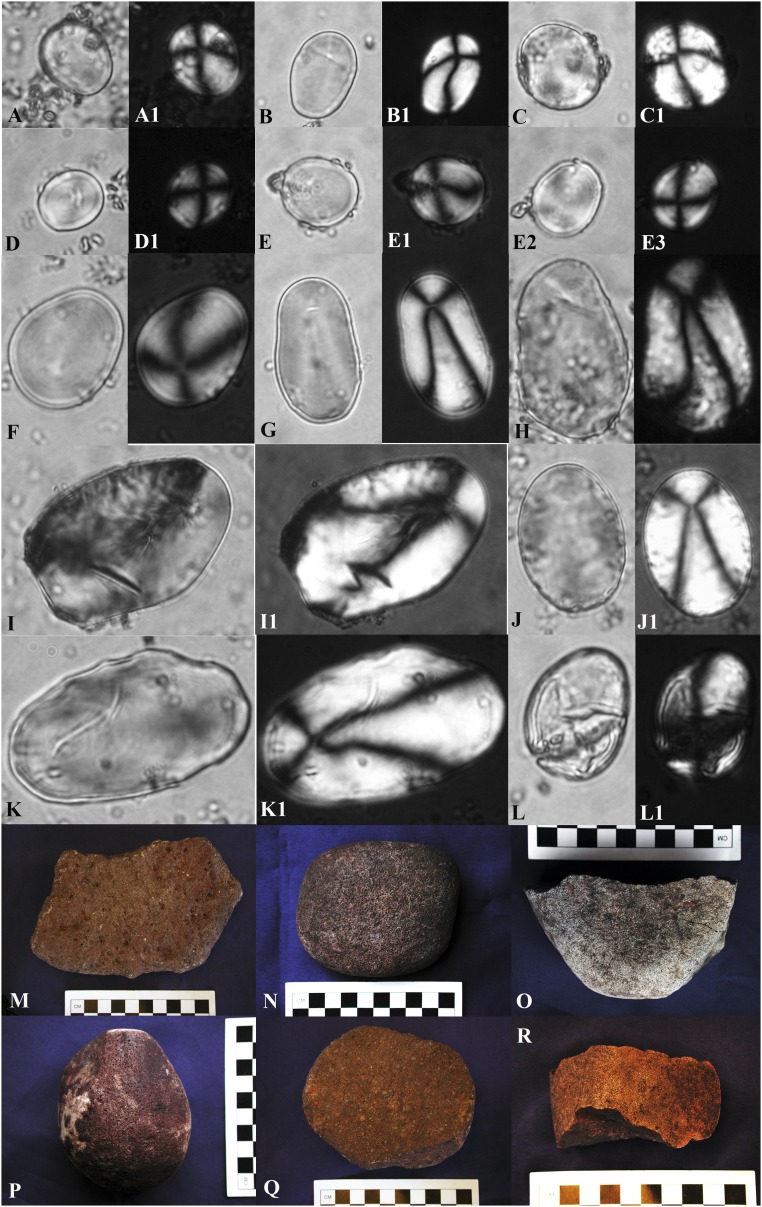
Archaeological Solanum starch grains and six tools tested for starch: (A) T107G01: 19 × 15 µm; (A1) same grain, polarized; (B) T57G06: 22 × 15 µm; (B1) same grain, polarized; (C) T01G01: 23 × 21 µm; (C1) same grain, polarized; (D) T232G01: 15 × 13 µm; (D1) same grain, polarized; (E) T17G05: 17 × 13 µm; same grain, polarized; (E1) same grain, polarized, displaying asymmetrical extinction cross; (E2) alternate view of same grain, (E2) alternate view of same grain, polarized, showing symmetrical extinction cross; (F) T232G05: 26 × 21 µm; (F1) same grain, polarized; (G) T57G07: 34 × 21 µm; (G1) same grain, polarized; (H) T232G07: 38 × 26 µm; (H1) same grain, polarized; (I) T02G07: 53 × 36 µm; damaged grain; (I1) same grain, polarized; (J) 57G08: 37 × 26 µm; battered grain; (J1) same grain, polarized; (K) T57G25: 53 × 32 µm; battered grain; (K1) same grain, polarized; (L) T57G14: 30 × 21 µm; damaged grain; (L1) same grain, polarized; (M) Tool 17 (y25c11v): Late Archaic recycled, flat/flat-concave mano (and pecking stone, abrader); (N) Tool 125 (hh32d0aiv): Early Formative flat-concave mano; (O) Tool 103 (y34aviii10): Terminal Archaic flat/ flat-concave mano; (P) Tool 131 (w36d7viii): Terminal Archaic mano (and flat-faceted abrader); (Q) Tool 1 (z26c7vi): Late Archaic flat-concave mano; (R) Tool 232 (aa28c7vii): Late Archaic recycled flat-concave mano.
Non-Solanum Archaeological Starches.
The Jiskairumoko starch assemblage highlights the necessity for further reference work in this area. Fig. S2 depicts a representative sample of the diverse grain types present; most of these cannot be securely identified at this time, as the focus of this work was identifying Solanum. Four to five starches appear consistent with bean (Phaseolus sp.), based on size, an oblong-reniform shape, visible lamellae, centric hila, and a pale longitudinal fissure consistent with heat treatment (16, 31) (Fig. S2: A–B1, E, E1, L, L1; possibly T and T1); three derive from Late Archaic Tool 57. The presence of Phaseolus at Jiskairumoko would not be not surprising, as this taxon has been identified in Andean contexts dating as early as 8,210–6,970 14C y BP (16, 35).
Andean Geophyte Starch Morphology.
With respect to the high-elevation Andean geophytes, maca and añu starches are significantly smaller than those of oca, potato, and ulluco (Table S2) and are easily distinguished based on other traits as well (23, 28, 36). Potato overlaps with—but exceeds—ulluco and oca with respect to size. Unique grain types comprised of a combination of traits typify all three taxa (9, 10, 23, 28, 30, 37); moreover, a comparison of starches from wild and cultivated Solanum indicates that these may be distinct from one another (Fig. 1, SI Methods and Materials, and Tables S3 and S4).
Table S2.
| Crop name | Taxonomic identification | Size range (μm) |
| Oca | Oxalis tuberosa | 8–55 |
| Ulluco | Ullucus tuberosum | 4–30 |
| Añu | Tropaeolum tuberosum | 4–16 |
| Maca | Lepidium meyenii | 7–15 |
| Potato | Solanum spp. | 4–100 |
Table S3.
Solanum starch granule 2D form: Comparison of modern wild and cultivated taxa and Jiskairumoko archaeological grains
| Starch source | No. starch grains analyzed | Symmetrical oval-ovoid form (%) | Bell shape (%) | Asymmetrical oval with protuberance (%) | Asymmetrical angular (%) |
| Modern | |||||
| Seven cultivated | 350 (50 per taxon) | 64 | 12 | 22 | 1 |
| Three wild taxa | 150 (50 per taxon) | 40 | 2 | 28 | 31 |
| Archaeological | |||||
| Late Archaic | 38 | 88 | NA* | 2 | 2 |
| Terminal Archaic | 1 | 100 | NA | 0 | 0 |
| Early Formative | 12 | 91 | NA | 0 | 0 |
Small bells occur in Solanum, Oxalis, and Ullucus—among other taxa—and are therefore not diagnostic in the case of archaeological Solanum.
Table S4.
Modern Solanum starch granule lamellae: comparison of wild and cultivated taxa
| Status/ no. taxa analyzed | No. starch grains analyzed (50 granules per taxon) | Visible, fine lamellae (%) | Lamellae very visible—ridged or dark/ light bands (%) | Not visible (%) |
| Cultivated (seven taxa) | 350 | 73 | 3 | 24 |
| Wild (three taxa) | 150 | 17 | 83 | 0 |
Archaeological Solanum.
Based on published data and comparative study results, 50 Jiskairumoko starches were identified as Solanum. The archaeological starches are consistent with cultivated potato with regard to 2D and 3D form and polarization, surface, and lamellae characteristics. As illustrated in Figs. 1 and 2 and Fig. S3, symmetrical, oval-ovoid grains predominate (84%), followed by asymmetrical, oblong grains (14%), and a single, asymmetrical, angular grain (2%) (Table S3).
Fig. 2.
Jiskairumoko Solanum starch grains, transmitted and polarized view: (A) T17G21, 23 × 16 μm; (B) same grain, polarized; (C) T57G03: 23 × 17 μm; (D) same grain, polarized; (E) T57G22: 25 × 17 μm; (F) same grain, polarized; (G) T57G30: 19 × 15 μm; (H) same grain, polarized; (I) T17G22: 34 × 21 μm; (J) same grain: alternate view; (K) same grain: alternate, polarized view.
The Solanum starches fall within the size range documented for modern, cultivated potato starches; the latter range from 3–100 μm, with a mean of 35 μm, versus wild potato starches, which range from 9–45 μm, with a mean of 28 μm (Table S5). The 38 Late Archaic period Solanum grains, for example, range in size from 11–58 μm along the longest axis, with a mean length of 29 μm. However, results of the reference study are inconclusive as to the link between domestication and increased starch grain size in potato (37). In crops such as maize, manioc, and Capsicum pepper, a marked increase in starch grain size accompanies domestication (15, 25, 33, 38). By contrast, size differences not correlated with domesticatory status occur among the modern Solanum taxa analyzed (Table S5). Factors such as tuber developmental stage/size, growing environment, and taxonomic identity affect starch grain size (39–42); further comparative study of potato starch morphology controlling for these factors may clarify this relationship.
Table S5.
Solanum starch grain size range (length equaling dimension along longest axis)
| Taxonomic identification/starch source | Type of tuber material | Status | No. starches analyzed | Mean length (μm) | SD | Range (µm) |
| Modern cultivated | ||||||
| Paula variety (Solanum sp.)/Puno market* | Freeze-dried tunta | Cultivated | 50 | 48 | 23 | 12–100 |
| Paula variety (Solanum sp.)/Puno market* | Fresh tuber | Cultivated | 50 | 41 | 20 | 12–75 |
| Paula variety (Solanum sp.)/Puno market* | Freeze-dried chuño | Cultivated | 50 | 25 | 15 | 4–66 |
| Imilla negra variety/Puno market* | Freeze-dried chuño | Cultivated | 50 | 38 | 24 | 9–96 |
| S. tuberosum ssp. andigenum/GRIN/USDA PI No. 258865) | Fresh | Cultivated/ domesticated | 50 | 25 | 12 | 3–46 |
| Modern wild† | ||||||
| Solanum megistacrolobum/GRIN/USDA (PI No. 435072) | Fresh | Wild (relative) | 50 | 27 | 11 | 9–45 |
| Solanum infundibuliforme/GRIN/ USDA (PI No. 472858) | Fresh | Wild (relative) | 50 | 30 | 10 | 10–45 |
| Archaeological | ||||||
| Late Archaic starches | Unknown | Unknown | 38 | 29 | 12 | 11–58 |
| Terminal Archaic | Unknown | Unknown | 1 | 36 x 38 µm | NA | NA |
| Early Formative | Unknown | Unknown | 12 | 22 | 17 | 13–43 |
C.R. purchased tubers at main market in Puno, Peru.
Size data not available for S. candolleanum.
A total of 88% of the Jiskairumoko Solanum starches have open hila or fissuring; 36% are partly-gelatinized, damaged (e.g., cracked) or appear “battered” (Fig. 2 E and F and Fig. S3 I–L1). Compared with the profile of modern Solanum analyzed, these percentages align more closely with those seen in freeze-dried, rather than fresh, tubers. Specifically, closed hila and an absence of fissures predominate in fresh tubers (wild and domesticated), whereas open hila and fissures predominate in freeze-dried tubers (Table S6). Various experimental studies have found a positive correlation between processing and the increased incidence of wear or damage to starches, including cracking and gelatinization (43–45); the modern Solanum data are consistent with such findings.
Table S6.
Solanum hilum and fissure characteristics: Comparison of fresh and freeze-dried and modern and archaeological tubers
| Starch source | Type of tuber material | No. starch grains analyzed | Hilum open (%) | Fissure present (%) | Indeterminate (%) |
| Modern | |||||
| Seven cultivated and wild taxa | Fresh | 350 (50 per taxon) | 33 | 6 | NA |
| Three cultivated taxa | Freeze-dried | 150 (50 per taxon) | 91 | 53 | NA |
| Archaeological | |||||
| Late Archaic | Unknown | 38 | 87 | 50 | 5 |
| Terminal Archaic | Unknown | 1 | 0 | 0 | 100 |
| Early Formative | Unknown | 12 | 92 | 58 | 0 |
As Collins and Copeland (46) observe, however, it can be difficult to distinguish anthropogenic wear from that resulting from unknown taphonomic factors when dealing with ancient starches. Assuming, nevertheless, that the damage to the Jiskairumoko Solanum starches is in fact a result of culinary processing, given the starches’ provenance, we propose that the wear reflects the grinding of the ancient tubers (47), likely for detoxification purposes. Following Johns (48–50), we consider a technology as involved as freeze-drying to have developed later, with the secondary expansion of agriculture into higher elevations and the development of frost-resistant potatoes at that time.
Discussion
Perhaps because of its status as a global staple, Emshwiller (51) observes, the phylogeny of the domesticated potato has been subject to more investigation and debate than that of its Andean geophyte counterparts. Nevertheless, elucidating potato’s taxonomy, distribution, and origins has proven challenging. As Johns and Keen (52) observe, high rates of gene flow among wild, weedy, and cultivated potatoes result in the ongoing creation of new taxa (52); this factor and a high degree of morphological variability have made pinpointing the progenitor species and number and places of domestication difficult (53–57).
Until recently, explanations for potato’s origins favored multiple, independent domestications of several members of a group of wild taxa known collectively as the Solanum brevicaule complex, comprising a northern and southern clade and found in southern Peru, northwestern Bolivia and Bolivia, and northern Argentina, respectively (54, 55, 57–61). Recent phylogenetic research, however, points to a reduction in the number of species in the S. brevicaule complex and to a monophyletic origin: the one-time domestication of a single wild ancestor, Solanum candolleanum (56, 61–64). The proposed progenitor includes 31 taxa previously identified as distinct species/subspecies and belonging to the northern clade of the S. brevicaule complex (56), suggesting that initial domestication transpired somewhere in southern Peru/northwestern Bolivia (2, 56, 65).
In discussing the geography of the potato, Hawkes (55) notes that where Solanum species are found today, they have been present since the last glaciation circa 10,000 y ago. Hawkes (55) observes that taxa adapted to cold, high environments—including ancestral S. candolleanum—“have been able to extend much further than those restricted to isolated medium altitude valleys”—hence the progenitor’s broad distribution throughout southern Peru/northwestern Bolivia. Botanical and paleoenvironmental data indicate that Jiskairumoko residents would likely have encountered S. candolleanum in their immediate environment, among other weedy, herbaceous plants (3, 5, 21, 36, 55, 66–69). First, Hawkes (55) documents the occurrence of Solanum canasense and Solanum multidissectum (both = S. candolleanum) in the Department of Puno, Peru, where Jiskairumoko is located. Furthermore, as a pioneer or “camp-follower” species, in the sense of Anderson (70), wild potatoes are especially attracted to—and flourish in—disturbed habitats (71). Of particular interest in this respect are the river terraces associated with Jiskairumoko. The site itself is situated upon a gravel knoll; as described by Rigsby et al. (72) in their documentation of the Ilave Valley’s fluvial history, the knoll is a remnant of an ancient river terrace, one of five created as a result of rising and falling lake levels associated with regional paleaoclimatic shifts. The terrace upon which Jiskairumoko sits (T5) was created circa 18,000 cal y BP; a nearby lower terrace (T2) was created more recently, ∼2,300 cal y BC (21, 72–74). At the time of Jiskairumoko’s occupation, T2 would have been found within the river floodplain and subject to frequent inundation and aggradation resulting from then-current high rainfall and lake levels. Floodplain disturbance would have provided for the increased productivity of camp-followers like potato and chenopods, perhaps setting the stage for their heightened exploitation and experimentation (20).
Macro- and microremain data at Jiskairumoko point to the Late/Terminal Archaic period exploitation of chenopods and potato, respectively. Although both are locally available resources that could have been gathered, from a morphological perspective the data are consistent with domesticated plants. Flotation data for Jiskairumoko are incomplete; however, using scanning electron microscopy, Andrea Murray identified seven chenopods from a Terminal Archaic burial as having thin seed coats, indicating their domesticated status (6). Chevalier’s identification of chenopod phytoliths from the dental calculus of Terminal Archaic skeletons corroborates exploitation of this taxon at this time (6, 19). Furthermore, as discussed, the archaeological Solanum starch morphology is consistent with that of cultivated potato. A scenario of Late Archaic cultivation fits well with regional paleoenvironmental data, indicating broad-scale agricultural clearing by ∼2,000 cal y BC and contemporaneous valley- and site-level data pointing to camelid management, sedentarization, and the advent of socioeconomic inequality, discussed above (6, 19, 21).
As Johns and Alonso (75) relate, however, wild potatoes contain varying levels of glycoalkaloids that cannot be neutralized by heat (cooking) alone, or by simply removing the peel. High-toxicity species, which include ancestral S. candolleanum, would have required some extra processing to permit initial, regular exploitation. The authors suggest that domestication of such taxa likely involved selection for a decrease in tuber toxicity. With this in mind, the presence of potato in culinary contexts at Jiskairumoko suggests either that toxicity had already been reduced through domestication or that site residents used some other means to reduce toxicity. Johns (48) proposes a chemical–ecological model in which geophagy (specifically, the consumption of phyllosilicate clays to neutralize glycoalkaloids) facilitated potato’s domestication. The chemical profile of Jiskairumoko soils is unknown, such that testing the applicability of the model is not possible at this time. However, as Browman and Gundersen (76) relate, comestible earths have been recovered at archaeological sites in the altiplano, attesting to the antiquity of this practice.
Based on the toxicity of wild potatoes and the recovery of Solanum starch from Jiskairumoko groundstone, we propose that these tools were used not only for seed/pseudocereal plants, but may also have figured into the detoxification of potato at Jiskairumoko, perhaps alongside geophagy. As Stahl (77) points out, grinding—along with processes like milling, grating, and pounding (collectively referred to as “comminution”)—often play a role in detoxification, as in the ethnographically well-known examples of bitter manioc (Manihot esculenta) and acorn (Quercus spp.). Furthermore, Johns (49, 50) observes that in making freeze-dried potatoes (e.g., chuño, moray, tunta), freezing causes tuber cell walls to burst, which facilitates the leaching of glycoalkaloids; we submit that grinding could achieve this same end. Although we find no documented examples of raw potato grinding, Cobo (78) reports the making of very fine flour out of rehydrated chuño, which was first toasted and then ground; Towle (8) describes a similar process of grinding flour from moray: both are added to stews.
At this point, we cannot say with certainty whether the Jiskairumoko Solanum derive from cultivated/domesticated or wild potato. Such a determination hinges on future reference work, wherein: (i) selection of accessions is informed by the history of study of the genus and most recent taxonomic/phylogenetic treatments; (ii) the number of grains studied per taxon and type of morphological characters analyzed take into consideration recent methodological developments and new standards in the field (79–84); (iii) variables such as tuber developmental stage/size and growing environment are controlled for; and (iv) the morphological implications of hybridization among wild/weedy/domesticated potatoes are understood. Such hybridization is commonplace and Andean farmers today, for example, typically welcome volunteer seedlings. Johns and Keen (52) furthermore suggest that, in the past, at times of crop failure, “weed potatoes would be obvious sources of tubers for food and seed.” Just as Bruno and Whitehead (5) consider the macrobotanical ramifications of quinoa crop/weed relationships, so must we develop expectations for the microbotanical repercussions of domesticated potatoes’ ongoing relationships with wild/weedy Solanum, in the context of both early plant cultivation and later, more established, farming practices.
Conclusions
The results of this study illustrate the utility of starch microbotanical analysis in addressing questions relating to the timing, mode, and context of potato domestication. The data presented here add to the empirical dataset for potato use within its domesticatory hearth during the Late Archaic to Early Formative periods. Moreover, preliminary reference work described here suggests the potential of using a population signature approach to distinguish between domesticated and wild Solanum (SI). More comprehensive reference data are necessary to permit full realization of the potential of this method in the study area; such datasets provide the foundation for recent strides made in understanding ancient subsistence regimes around the world (22, 38, 85).
SI Methods and Materials
Grinding Tools.
The twenty artifacts selected for starch analysis represent all periods of occupation of Jiskairumoko. Table S1 describes these grinding tools in terms of proveniences, select attributes, and the number of starches recovered; Figs. S2 and S3 depict nine tools yielding various types of starches.
As noted, multiple characteristics of the grinding tool assemblage indicate intensive use of this technology throughout occupation of Jiskairumoko (37). For example, for all time periods, more than half of the tools show some evidence of wear-management strategies (such as pecking to rerough use surfaces, rotating use-ends, and creating multiple use facets). As Adams (86) relates, such strategies are used to extend the use-life of a tool and are associated with more intensive (versus more limited or extensive) use. Second, the percentage of tools with a use facet area greater than or equal to 75 cm2 increases from 37% in the Late Archaic to 79% in the Early Formative period. Increased use facet area generally results in greater grinding efficiency and is therefore taken to signal the increased importance of grinding (86–88). Third, the percentage of strategically designed tools also increases somewhat over time, from 31% in the Late Archaic to 51% by the Early Formative period. Strategically designed tools are shaped to increase comfort and efficiency, factors that become more salient with longer periods of use or increased reliance on the technology (86). Taken together, these characteristics suggest not only intensive use of grinding tools throughout site occupation, but also intensification of use over time (37).
Starch Residue Recovery.
C.U.R. conducted starch recovery at the Programa Collasuyo field house in Puno, Peru, where the groundstone artifacts were stored. All processing took place in the annex, a closed room located atop the roof of the field house. No modern plant materials were housed in the annex or artifact storage area.
Before artifact processing, several steps were taken to control for postexcavation contamination (79, 81). All surfaces were cleaned with bleach and all work surfaces then covered with aluminum foil; no food was permitted in the room. All items used for processing (e.g., beakers, capped test tubes for transport to the University of California, Santa Barbara, pipettes, and plastic containers) were sterilized with boiling water; items were then covered/wrapped in new aluminum foil until use. New or newly sterilized items were used for the processing of each artifact, including a new pair of “Diamond Grip,” “unpowdered” latex gloves (these were checked and did not contain potato or other starches). We are confident that these procedures controlled for postexcavation contamination.
Sonication was carried out to recover starch residues from the grinding tools. One-hundred percent of each tool was sonicated. In some cases, one part of a tool was sonicated and then flipped to sonicate the other part; Rumold (37) describes the details of this process. As noted, the tools were unwashed, having been collected with starch analysis in mind (6). Accordingly, the starch grains recovered via sonication derive from both the “outermost” and “innermost” layer of the artifact surface, to use Hart’s (89) terminology. Whereas some current archaeological starch analyses use control samples and undertake in situ sampling from tool use facets (e.g., using pipettes or wet/dry brushing) in some contexts (13, 32, 89, 90), the bulk data recovered in the fashion just described are considered sufficient to address the main focus of this paper, despite the loss of data specific to individual use surfaces.
Chemical Starch Extraction.
C.U.R. conducted the chemical extraction of starch grains from their sediment matrix in M.S.A.’s laboratory at the University of California, Santa Barbara. Before beginning analysis, the laboratory room in which samples were to be processed was thoroughly cleaned and all items used in processing were sterilized using boiling water (beakers, test tubes for centrifuging, pipettes, and so forth). New, sterilized items were used for each sample; no food was permitted in the room.
The aqueous sediment samples were concentrated using a centrifuge; test tubes were covered with Parafilm brand paraffin wax film. A heavy liquid solution using cesium chloride (CsCl) was used to extract starches from the sediment pellets; no sodium hexametaphosphate (e.g., Calgon) was used as deflocculant in processing the samples. Following Pearsall et al. (13), all samples were “floated” twice to increase starch numbers, which had been somewhat low in the initial samples processed.
Microscope Analysis.
Microscope analysis of archaeological starches took place in the same University of California, Santa Barbara laboratory as the chemical extraction, using an Olympus BHM metallurgical transmitted light microscope equipped with polarization lens; a Nikon Coolpix 990 digital camera mounted on the microscope was used to photograph microremains. As described above, the workspace was cleaned and all materials were sterilized before use. Furthermore, no modern plant materials were analyzed in this laboratory; the modern reference study was conducted at the Smithsonian Tropical Research Institute in Panama.
Slides were prepared in one of two ways: (i) using a pipette to place a droplet of starch residue on a slide, adding a droplet of distilled water, and affixing a coverslip with clear nail polish at the corners; or (ii) by placing a droplet of the starch residue on a slide, adding a droplet of glycerol mounting medium, and affixing the edges of the coverslip with Permount (using a sterilized glass rod) after scanning. Use of the second method began partway into analysis, as a way to mitigate difficulties associated with relocating starches on water-mounted slides (e.g., “lost” and split starches). At this point, starch granules were also photographed from as many angles as possible upon first encounter/analysis, to avoid having to rehydrate slides and relocate starches.
The entirety of each slide was systematically scanned at 200× power with darkfield illumination. Once a starch grain was encountered, 400× power and brightfield and darkfield illumination were used. Each grain was cataloged, photographed (from various angles, when possible), and described in terms of multiple characteristics. Attributes described include: grain type (simple, compound, semicompound), length and width (i.e., dimension along the longest axis and the widest dimension perpendicular to the length); grain form in two and three dimensions; hilum type (e.g., closed, open vacuole) and hilum position (centric, eccentric, highly eccentric); presence/absence and form of fissures; surface and margin features (e.g., presence/absence of pressure facets); distinctiveness of lamellae; degree and character of polarization (e.g., degree of perpendicularity of arms of extinction cross). Published reference materials and the preliminary comparative dataset described below were used to identify starch grains, when possible (9, 10, 15, 23–25, 27, 29, 30).
Characterization of Modern Starch Morphologies for Solanum, Oxalis, and Ullucus.
C.U.R. conducted a comparative study of starches from potato, oca, and ulluco, in Dolores Piperno’s laboratory at Smithsonian Tropical Research Institute. All oca and ulluco specimens, as well as 4 of the 10 potato specimens, were purchased at the Puno market, in Peru. The remaining six potato specimens were acquired from United States Department of Agriculture Germplasm Resources Information Network (USDA/GRIN). The species designation and status (wild or domesticated) of USDA accessions are known (37); by contrast, given the diversity of potato species cultivated in the Andes and farmers’ high rates of acceptance of weedy or hybrid volunteers (52), market specimens are designated as species unknown (sp.) and as “cultivated” rather than “domesticated.”
Methods of modern starch preparation, slide scanning, and starch grain documentation are described in more detail elsewhere (37). In the case of oca and ulluco, observations are based on analysis of 50 starch grains each from two specimens, or 100 grains total (37). In the case of potato, 50 grains each were analyzed for 10 specimens, totaling 500 grains. All starch grains were described in terms of the same set of characteristics described above for the archaeological starches; one discrepancy is that size data are available for only 7 of the 10 Solanum taxa. Table S5 therefore refers to 7 taxa total, whereas Tables S3, S4, and S6 refer to 10.
As is currently emphasized in discussions of starch analysis methodology (22, 79, 80), systematic reference data are critical for secure starch identifications. Because only 100 grains each were analyzed for oca and ulluco, percentages are not given and conclusions regarding typical grain morphology are considered more tentative than those for potato. Nevertheless, the findings described below are in line with Cortella and Pochettino’s (23) conclusions regarding the distinctiveness of these taxa. Typical grain types and diagnostic attributes are described below.
Modern Potato.
Cultivated Solanum starch grains are typified by a symmetrical, oval-ovoid form in two dimensions (i.e., top view) and spherical form in three dimensions (Table S3); eccentric hilum; distinct, fine lamellae; a smooth surface; and a strongly polarizing extinction cross (37). Symmetrical, oval-ovoid forms predominate in cultivated Solanum (64%), but asymmetrical oval grains with protuberances (22%) and asymmetrical, angular grains (1%) occur as well. Symmetrical oval-ovoid forms also occur in wild Solanum (40%), but are more closely followed in importance by asymmetrical angular grains (31%) and then asymmetrical oval grains with protuberances (28%). Very visible lamellae, which often appear ridged or as alternating dark/light bands, are characteristic of wild Solanum (83%); these may be contrasted with finer, more delicate lamellae typical of cultivated taxa (70–77%) (Table S4) (37). Fig. 1 A–C depicts starches from cultivated Solanum taxa and Fig. 1D, from wild ancestral Solanum candolleanum.
Two-dimensional form and lamellae characteristics may be particularly important in distinguishing cultivated and wild Solanum (37). Although, as Spooner et al. (42) note, there is overlap in the range of morphological expression in wild and cultivated taxa, Rumold (37) proposes that the frequency of occurrence of specific characteristics differs and that wild and cultivated potato starch grains may be distinguishable using a population signature approach, in which secure taxonomic identification rests on the presence of a significant number of starches sharing a set of diagnostic traits, rather than on a single, diagnostic grain (45, 91).
Modern Ulluco.
Whereas smaller ulluco grains are often ovoid and symmetrical in form, larger grains are irregular in form (conoidal, prismatic, pear-shaped), typified by a significant side protuberance that is clearly reflected in the form of the extinction cross (23, 37). Polarization arms are sinuous, sometimes fuzzy, reflecting the irregular form of the grain (23, 37). A few lamellae, midway between hilum and grain margin, are especially salient (37).
Modern Oca.
With regard to form, irregular, single grains predominate in oca (conoidal, prismatic, and pear-shaped) (23, 30, 37). Even when oca grains are symmetrical, however, they are marked by a “swollen” quality, much like a water balloon, that distinguishes them from potato (37). Polarization arms are undulating and irregular, often broken and crossing at more than one point. Although Rumold (37) defines the arms as appearing fuzzy at points, Cortella and Pochettino do not (23). A small, papillose projection or protuberance is frequently found at the base or side of these grains (as seen at the point of attachment of a lemon, for example); Rumold (37) suggests that these may be remnant pressure facets. Other salient characteristics are a very eccentric hilum (versus an eccentric hilum in potato) and many very distinct lamellae that are fine, closely spaced, and appear almost etched into the grain surface, which has a metallic opacity to it.
Acknowledgments
We thank Albino Pilco for his able assistance in the field; the people of Jachicachi, the comunidad campesina in which the site is located, for their friendship and efforts to preserve the site; Dolores Piperno, Barbara Voorhies, and two anonymous reviewers and the editor for helpful criticisms and suggestions on various drafts of this manuscript; William (Randy) Haas for the map of Jiskairumoko and the study area; and the Ministerio de Cultura, Peru for permission to conduct excavations at Jiskairumoko. Field research was supported by a grant from the H. John Heinz III Charitable Trust (to M.S.A.); National Geographic Society Grant 5245-94 (to M.S.A.); National Science Foundation Grants SBR-9816313 and SBR 9978006 (to M.S.A.); National Science Foundation Dissertation Improvement Grant 0130421 (to C.U.R.); and an Andrew Mellon Fellowship (to C.U.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.B. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604265113/-/DCSupplemental.
References
- 1.Emshwiller E, Doyle JJ. Origins of domestication and polyploidy in oca (Oxalis Tuberosa: Oxalidaceae). 2. Chloroplast-expressed glutamine synthetase data. Am J Bot. 2002;89(7):1042–1056. doi: 10.3732/ajb.89.7.1042. [DOI] [PubMed] [Google Scholar]
- 2.Spooner DM, McLean K, Ramsay G, Waugh R, Bryan GJ. A single domestication for potato based on multilocus amplified fragment length polymorphism genotyping. Proc Natl Acad Sci USA. 2005;102(41):14694–14699. doi: 10.1073/pnas.0507400102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson HD. Quinoa and relatives (Chenopodium sect. Chenopodium subsect. Cellulata) Econ Bot. 1990;44(3):92–110. [Google Scholar]
- 4.Aldenderfer MS. High elevation foraging societies. In: Silverman H, Isbell WH, editors. Handbook of South American Archaeology. Springer; New York: 2008. pp. 131–143. [Google Scholar]
- 5.Bruno MC, Whitehead WT. Chenopodium cultivation and Formative Period agriculture at Chiripa, Bolivia. Lat Am Antiq. 2003;14(3):339–355. [Google Scholar]
- 6.Craig NM. 2005. The formation of early settled villages and the emergence of leadership: A test of three theoretical models in the Rio Ilave, Lake Titicaca Basin, southern Peru. PhD dissertation (University of California, Santa Barbara)
- 7.Pearsall DM. Plant domestication and the shift to agriculture in the Andes. In: Silverman H, Isbell WH, editors. Handbook of South American Archaeology. Springer; New York: 2008. pp. 105–120. [Google Scholar]
- 8.Towle MA. 1962. The Ethnobotany of Pre-Columbian Peru (distributed through Current Anthropology for the Wenner-Gren Foundation for Anthropological Research, New York)
- 9.Ugent D, Dillehay TD, Ramirez C. Potato remains from a late Pleistocene settlement in southcentral Chile. Econ Bot. 1987;41(1):17–27. [Google Scholar]
- 10.Ugent D, Pozorski S, Pozorski T. Archaeological potato tuber remains from the Casma Valley of Peru. Econ Bot. 1982;36:182–192. [Google Scholar]
- 11.Grobman A, et al. Preceramic maize from Paredones and Huaca Prieta, Peru. Proc Natl Acad Sci USA. 2012;109(5):1755–1759. doi: 10.1073/pnas.1120270109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logan AL, Hastorf CA, Pearsall DM. Let’s drink together: Early ceremonial use of maize in the Titicaca Basin. Lat Am Antiq. 2012;23(3):235–258. [Google Scholar]
- 13.Pearsall DM, Chandler-Ezell K, Zeidler JA. Maize in ancient Ecuador: results of residue analysis of stone tools from the Real Alto site. J Archaeol Sci. 2004;31(4):423–442. [Google Scholar]
- 14.Perry L, et al. Early maize agriculture and interzonal interaction in southern Peru. Nature. 2006;440(7080):76–79. doi: 10.1038/nature04294. [DOI] [PubMed] [Google Scholar]
- 15.Perry L, et al. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science. 2007;315(5814):986–988. doi: 10.1126/science.1136914. [DOI] [PubMed] [Google Scholar]
- 16.Piperno DR, Dillehay TD. Starch grains on human teeth reveal early broad crop diet in northern Peru. Proc Natl Acad Sci USA. 2008;105(50):19622–19627. doi: 10.1073/pnas.0808752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossen J, Dillehay TD. Plant-use schedules, decreased mobility, and social differentiation: Hunter-gatherers in forested Chile. In: Crothers GM, editor. Hunters and Gatherers in Theory and Archaeology. Center for Archaeological Investigations; Carbondale, IL: 2004. pp. 316–339. [Google Scholar]
- 18.Zarrillo S, Pearsall DM, Raymond JS, Tisdale MA, Quon DJ. Directly dated starch residues document early formative maize (Zea mays L.) in tropical Ecuador. Proc Natl Acad Sci USA. 2008;105(13):5006–5011. doi: 10.1073/pnas.0800894105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aldenderfer M, Craig NM, Speakman RJ, Popelka-Filcoff R. Four-thousand-year-old gold artifacts from the Lake Titicaca basin, southern Peru. Proc Natl Acad Sci USA. 2008;105(13):5002–5005. doi: 10.1073/pnas.0710937105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig NM, Aldenderfer MS, Baker PA, Rigsby C. Terminal Archaic settlement pattern and land cover change in the Rio Ilave, southwestern Lake Titicaca Basin, Peru. In: Dean RM, editor. The Archaeology of Anthropogenic Environments. Center for Archaeological Investigations; Carbondale, IL: 2009. [Google Scholar]
- 21.Paduano GM, Bush MB, Baker PA, Fritz SC, Seltzer GO. A vegetation and fire history of Lake Titicaca since the last glacial maximum. Palaeogeogr Palaeoclimatol Palaeoecol. 2003;194(1-3):259–279. [Google Scholar]
- 22.Lentfer CJ. Going bananas in Papua New Guinea: A preliminary study of starch granule morphotypes in Musaceae fruit. Ethnobotany Research and Applications. 2009;7:217–238. [Google Scholar]
- 23.Cortella AR, Pochettino ML. Comparative morphology of starch of three Andean tubers. Starke. 1995;47(12):455–461. [Google Scholar]
- 24.Giovannetti MA, Lema VS, Bartoli CG, Capparelli A. Starch grain characterization of Prosopis chilensis (Mol.) Stuntz and P. flexuosa DC, and the analysis of their archaeological remains in Andean South America. J Archaeol Sci. 2008;35(11):2973–2985. [Google Scholar]
- 25.Perry L. Starch granule size and the domestication of manioc (Manihot esculenta) and sweet potato (Ipomoea batatas) Econ Bot. 2002;56(4):335–349. [Google Scholar]
- 26.Perry L. Starch analyses reveal the relationship between tool type and function: an example from the Orinoco valley of Venezuela. J Archaeol Sci. 2004;31(8):1069–1081. [Google Scholar]
- 27.Reichert ET. The Differentiation and Specificity of Starches in Relation to Genera, Species, etc., Stereochemistry Applied to Protoplasmic Processes and Products, and as a Strictly Scientific Basis for the Classification of Plants and Animals. Carnegie institution of Washington; Washington, DC: 1913. [Google Scholar]
- 28.Rondán-Sanabria GG, Finardi-Filho F. Physical–chemical and functional properties of maca root starch (Lepidium meyenii Walpers) Food Chem. 2009;114(2):492–498. [Google Scholar]
- 29.Seidemann J. Stärke-Atlas. Paul Parey; Berlin: 1966. [Google Scholar]
- 30.Valcárcel-Yamani B, Rondán-Sanabria GG, Finardi-Filho F. The physical, chemical and functional characterization of starches from Andean tubers: Oca (Oxalis tuberosa Molina), olluco (Ullucus tuberosus Caldas) and mashua (Tropaeolum tuberosum Ruiz & Pavón) Braz J Pharm Sci. 2013;49:453–464. [Google Scholar]
- 31.Babot Md P, Oliszewski N, Grau A. Análisis de caracteres macroscópicos y microscópicos de Phaseolus vulgaris (Fabaceae, Faboideae) silvestres y cultivados del noroeste argentino: Una aplicación en arqueobotánica. Darwiniana. 2007;45(2):149–162. [Google Scholar]
- 32.Piperno DR, Holst I. The presence of starch grains on prehistoric stone tools from the humid neotropics: Indications of early tuber use and agriculture in Panama. J Archaeol Sci. 1998;25(8):765–776. [Google Scholar]
- 33.Piperno DR. Identifying manioc (Manihot esculenta Crantz) and other roots in Pre-Columbian tropical America through starch grain analysis: A case study from Central America. In: Zeder MA, Bradley DG, Emshwiller E, Smith BD, editors. Documenting domestication: New Genetic and Acrchaeological Paradigms. Univ of California Press; Berkeley, CA: 2006. pp. 46–67. [Google Scholar]
- 34.Bronk Ramsey C. Bayesian analysis of radiocarbon dates. Radiocarbon. 2009;51(1):337–360. [Google Scholar]
- 35.Duncan NA, Pearsall DM, Benfer RA., Jr Gourd and squash artifacts yield starch grains of feasting foods from preceramic Peru. Proc Natl Acad Sci USA. 2009;106(32):13202–13206. doi: 10.1073/pnas.0903322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toledo J, et al. Genetic variability of Lepidium meyenii and other Andean Lepidium species (Brassicaceae) assessed by molecular markers. Ann Bot (Lond) 1998;82(4):523–530. [Google Scholar]
- 37.Rumold CU. 2010. Illuminating women’s work and the advent of plant cultivation in the highland Titicaca Basin of South America: New evidence from grinding tool and starch grain analyses. PhD dissertation (University of California, Santa Barbara)
- 38.Holst I, Moreno JE, Piperno DR. Identification of teosinte, maize, and Tripsacum in Mesoamerica by using pollen, starch grains, and phytoliths. Proc Natl Acad Sci USA. 2007;104(45):17608–17613. doi: 10.1073/pnas.0708736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geddes R, Greenwood CT, Mackenzie S. Studies on the biosynthesis of starch granules. Part III. The properties of the components of starches from the growing potato tuber. Carbohydr Res. 1965;1(1):71–82. [Google Scholar]
- 40.Haase NU, Plate J. Properties of potato starch in relation to varieties and environmental factors. Starke. 1996;48(5):167–170. [Google Scholar]
- 41.Noda T, et al. The effect of harvest dates on the starch properties of various potato cultivars. Food Chem. 2004;86(1):119–125. [Google Scholar]
- 42.Spooner D, Jansky S, Clausen A, del Herrera MR, Ghislain M. The enigma of Solanum maglia in the origin of the Chilean cultivated potato, Solanum tuberosum Chilotanum Group. Econ Bot. 2012;66(1):12–21. [Google Scholar]
- 43.Babot MdP 2003. Starch grain damage as an indicator of food processing. Phytolith and Starch Research in the Australian-Pacific-Asian Regions: The State of the Art: Papers from a Conference held at the ANU, August 2001, Canberra, Australia, eds Hart DM Wallis LA (Pandanus Press: The Australian National University, Canberra, Australia), pp 69-81.
- 44.Henry AG, Hudson HF, Piperno DR. Changes in starch grain morphologies from cooking. J Archaeol Sci. 2009;36(3):915–922. [Google Scholar]
- 45.Messner TC, Schindler B. Plant processing strategies and their affect upon starch grain survival when rendering Peltandra virginica (L.) Kunth, Araceae edible. J Archaeol Sci. 2010;37(2):328–336. [Google Scholar]
- 46.Collins MJ, Copeland L. Ancient starch: Cooked or just old? Proc Natl Acad Sci USA. 2011;108(22):E145–author reply E146. doi: 10.1073/pnas.1103241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, Perry L. Identification of ancient starch grains from the tribe Triticeae in the North China Plain. J Archaeol Sci. 2013;40(8):3170–3177. [Google Scholar]
- 48.Johns T. A chemical-ecological model of root and tuber domestication in the Andes. In: Harris DR, Hillman GC, editors. Foraging and Farming: The Evolution of Plant Exploitation. Unwin Hyman; London: 1989. [Google Scholar]
- 49.Johns T. With Bitter Herbs They Shall eat It: Chemical Ecology and the Origins of Human Diet and Medicine. Univ of Arizona Press; Tucson, AZ: 1990. [Google Scholar]
- 50.Johns T. The Origins of Human Diet and Medicine: Chemical Ecology. Univ of Arizona Press; Tucson, AZ: 1996. [Google Scholar]
- 51.Emshwiller E. Ploidy levels among species in the ‘Oxalis tuberosa alliance’ as inferred by flow cytometry. Ann Bot (Lond) 2002;89(6):741–753. doi: 10.1093/aob/mcf135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johns T, Keen SL. Ongoing evolution of the potato on the Altiplano of western Bolivia. Econ Bot. 1986;40(4):409–424. [Google Scholar]
- 53.Brücher H. New contributions to an ancient problem on the origin of the tetraploid potato (Solanum tuberosum L.) Naturwissenschaften. 1990;77(10):497–498. [Google Scholar]
- 54.Bukasov SM. Systematics of the potato. Trudy po Prikladnoj Botanike Genetike i Selekcii. 1978;62:3–35. [Google Scholar]
- 55.Hawkes JG. The Potato. Evolution, Biodiversity, and Genetic Resources. Smithsonian Institution Press; Washington, DC: 1990. [Google Scholar]
- 56.Spooner D, Ghislain M, Simon R, Jansky S, Gavrilenko T. Systematics, diversity, genetics, and evolution of wild and cultivated potatoes. Bot Rev. 2014;80(4):283–383. [Google Scholar]
- 57.Ugent D. The Potato: What is the botanical origin of this important crop plant, and how did it first become domesticated? Science. 1970;170(3963):1161–1166. doi: 10.1126/science.170.3963.1161. [DOI] [PubMed] [Google Scholar]
- 58.Brücher H. Kritische Betrachtungen zur Nomenklatur argentinischer Wildkartoffeln. VII. Solanum setulosistylum Bitter, eine seit 50 Jahren falsch interpretierte Species der Serie Commersoniana. Der Züchter. 1964;34(1):27–32. [Google Scholar]
- 59.Hosaka K. Successive domestication and evolution of the Andean potatoes as revealed by chloroplast DNA restriction endonuclease analysis. Theor Appl Genet. 1995;90(3-4):356–363. doi: 10.1007/BF00221977. [DOI] [PubMed] [Google Scholar]
- 60.Ochoa CM. The Potatoes of South America. Cambridge Univ Press; Cambridge, UK: 1990. [Google Scholar]
- 61.Vandenberg R, et al. Collapse of morphological species in the wild potato Solanum brevicaule complex (Solanaceae: sect. Petota) Am J Bot. 1998;85(1):92–109. [PubMed] [Google Scholar]
- 62.Miller JT, Spooner DM. Collapse of species boundaries in the wild potato Solanum brevicaule complex (Solanaceae, S. sect. Petota): Molecular data. Plant Syst Evol. 1999;214(1-4):103–130. [Google Scholar]
- 63.Spooner DM, et al. Ecogeography of ploidy variation in cultivated potato (Solanum sect. Petota) Am J Bot. 2010;97(12):2049–2060. doi: 10.3732/ajb.1000277. [DOI] [PubMed] [Google Scholar]
- 64.Spooner DM, Fajardo D, Bryan GJ. Species limits of Solanum berthaultii Hawkes and S. tarijense Hawkes and the implications for species boundaries in Solanum sect. Petota. Taxon. 2007;56(4):987–999. [Google Scholar]
- 65.Sukhotu T, Hosaka K. Origin and evolution of Andigena potatoes revealed by chloroplast and nuclear DNA markers. Genome. 2006;49(6):636–647. doi: 10.1139/g06-014. [DOI] [PubMed] [Google Scholar]
- 66.Bruno MC. A morphological approach to documenting the domestication of Chenopodium in the Andes. In: Zeder MA, Bradley DG, Emshwiller E, Smith BD, editors. Documenting Domestication: New Genetic and Archaeological Paradigms. Univ of California Press; Berkeley, CA: 2006. pp. 34–45. [Google Scholar]
- 67.Ochoa C, Ugent D. Maca (Lepidium meyenii Walp.; Brassicaceae): A nutritious root crop of the central Andes. Econ Bot. 2001;55(3):344–345. [Google Scholar]
- 68.Pearsall DM. Adaptation of prehistoric hunter-gatherers to the high Andes: The changing role of plant resources. In: Harris DR, Hillman GC, editors. Foraging and Farming: The Evolution of Plant Exploitation. Unwin Hyman; London: 1989. pp. 318–332. [Google Scholar]
- 69.Quirós CF, Cárdenas RA. Maca (Lepidium meyenii Walp.) In: Hermann M, Heller J, editors. Andean Roots and Tubers: Ahipa, Arracacha, Maca, and Yacon. Institute of Plant Genetics and Crop Research; Gatersleben, Germany: 1997. pp. 173–197. [Google Scholar]
- 70.Anderson E. Plants, Man and Life. Little Brown; Boston: 1952. [Google Scholar]
- 71.De Wet JMJ, Harlan JR. Weeds and domesticates: Evolution in the man-made habitat. Econ Bot. 1975;29(2):99–108. [Google Scholar]
- 72.Rigsby CA, Baker PA, Aldenderfer MS. Fluvial history of the Rio Ilave Valley, Peru, and its relationship to climate and human history. Palaeogeogr Palaeoclimatol Palaeoecol. 2003;194(1-3):165–185. [Google Scholar]
- 73.Baker PA, et al. The history of South American tropical precipitation for the past 25,000 years. Science. 2001;291(5504):640–643. doi: 10.1126/science.291.5504.640. [DOI] [PubMed] [Google Scholar]
- 74.Tapia PM, Fritz SC, Baker PA, Seltzer GO, Dunbar RB. A late quaternary diatom record of tropical climatic history from Lake Titicaca (Peru and Bolivia) Palaeogeogr Palaeoclimatol Palaeoecol. 2003;194(1-3):139–164. [Google Scholar]
- 75.Johns T, Alonso JG. Glycoalkaloid change during the domestication of the potato, Solanum Section Petota. Euphytica. 1990;50(3):203–210. [Google Scholar]
- 76.Browman DL, Gundersen JN. Altiplano comestible earths: Prehistoric and historic geophagy of Highland Peru and Bolivia. Geoarchaeology. 1993;8(5):413–425. [Google Scholar]
- 77.Stahl AB. Plant-food processing: Implications for dietary quality. In: Harris DR, Hillman GC, editors. Foraging and Farming: The Evolution of Plant Exploitation. Unwin Hyman; London: 1989. pp. 171–194. [Google Scholar]
- 78.Cobo B. Historia del Nuevo Mundo. Ediciones Atlas; Madrid: 1964. [Google Scholar]
- 79.Barton H, Torrence R. Cooking up recipes for ancient starch: Assessing current methodologies and looking to the future. J Archaeol Sci. 2015;56:194–201. [Google Scholar]
- 80.Coster ACF, Field JH. What starch grain is that? A geometric morphometric approach to determining plant species origin. J Archaeol Sci. 2015;58:9–25. [Google Scholar]
- 81.Crowther A, Haslam M, Oakden N, Walde D, Mercader J. Documenting contamination in ancient starch laboratories. J Archaeol Sci. 2014;49:90–104. [Google Scholar]
- 82.Field J. Reference collections. In: Torrence R, Barton H, editors. Ancient Starch Research. Left Coast Press; Walnut Creek, CA: 2006. pp. 95–113. [Google Scholar]
- 83.Liu L, Ma S, Cui J. Identification of starch granules using a two-step identification method. J Archaeol Sci. 2014;52:421–427. [Google Scholar]
- 84.Thoms AV, Laurence AR, Short L, Kamiya M. Baking geophytes and tracking microfossils: Taphonomic implications for earth-oven and paleodietary research. J Archaeol Method Theory. 2014;22:1038–1070. [Google Scholar]
- 85.Piperno DR, Holst I, Winter K, McMillan O. Teosinte before domestication: Experimental study of growth and phenotypic variability in Late Pleistocene and early Holocene environments. Quat Int. 2015;363:65–77. [Google Scholar]
- 86.Adams J. Groundstone Analysis: A Technological Approach. Univ of Utah Press; Salt Lake City: 2002. [Google Scholar]
- 87.Buonasera TY. Modeling the costs and benefits of manufacturing expedient milling tools. J Archaeol Sci. 2015;57:335–344. [Google Scholar]
- 88.Mauldin R. The relationship between ground stone and agricultural intensification in western New Mexico. Kiva. 1993;58(3):317–330. [Google Scholar]
- 89.Hart TC. Evaluating the usefulness of phytoliths and starch grains found on survey artifacts. J Archaeol Sci. 2011;38(12):3244–3253. [Google Scholar]
- 90.Piperno DR, Ranere AJ, Holst I, Hansell P. Starch grains reveal early root crop horticulture in the Panamanian tropical forest. Nature. 2000;407(6806):894–897. doi: 10.1038/35038055. [DOI] [PubMed] [Google Scholar]
- 91.Piperno DM, Holst I. Crop domestication in the American tropics: Starch grain analysis. In: Goodman R, editor. Encyclopedia of Plant and Crop Science. Marcel Dekker; New York: 2004. pp. 330–332. [Google Scholar]
- 92.Stöckli R, Vermote E, Saleous N, Simmon R, Herring D. 2005 The blue marble next generation—A true color earth dataset including seasonal dynamics from MODIS. Available at visibleearth.nasa.gov. Accessed July 28, 2016.
- 93.Danielson JJ, Gesch DB. 2011. Global Multi-resolution Terrain Elevation Data 2010 (GMTED2010) (US Geological Survey, Reston, VA). Available at https://lta.cr.usgs.gov/GMTED2010. Accessed August 18, 2016.
- 94.Team RC. 2013 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria) Available at: www.R-project.org/. Accessed June 21, 2016.
- 95.Hijmans R. 2015 Raster: Geographic Data Analysis and Modeling. Available at cran.r-project.org/web/packages/raster/index.html. Accessed December 19, 2015.
- 96.Bivand RS, Pebesma EJ, Gomez-Rubio V. 2008 Applied spatial data analysis with R. 1st Ed (Springer, New York). Available at public.eblib.com/choice/publicfullrecord.aspx?p=364296. Accessed April 8, 2016.