The beauty of snowflakes originates from the very different ways the ice crystals grow in different environments (1, 2). The amazing variety of snowflake crystal shapes occurs despite the fact that the crystalline structure of snow is always the same: the thermodynamic equilibrium shape is a simple hexagonal crystal. Therefore, the path taken in the nucleation and growth of the ice appears to be more important in determining the final result than the thermodynamic equilibrium state. In PNAS, Lee et al. (3) show that the nucleation pathway for salts precipitating from aqueous solutions is also rather more complicated than the simple formation of a crystalline nucleus that spontaneously forms and subsequently grows. There is still a lot of debate on the conditions under which perfect single crystals are formed, as opposed to the formation of “imperfect” or multiple crystals of different shapes, sizes, and crystalline structures (4–14). Crystallization is traditionally very important for many processes, from the production of steel to the purification of chemicals. In the pharmaceutical and chemical industry high purity is a requirement and is achieved through multiple recrystallizations. For crystallography, the structure of many important biological molecules, such as DNA, and many proteins was unraveled by X-ray diffraction that necessitates good-quality single crystals; all this necessitates a good comprehension of crystal nucleation and growth.
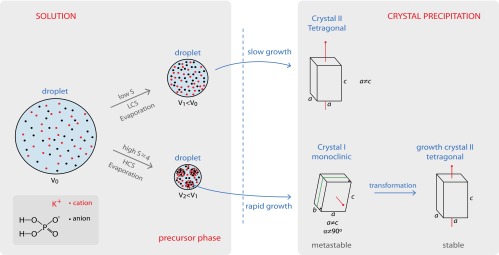
Lee et al. (3) follow the crystallization of potassium dihydrogen phosphate (KDP) in evaporating, levitated droplets of aqueous solutions of this salt using a very clever experimental set-up that integrates electrostatic levitation with in situ micro Raman spectroscopy and wide-angle X-ray scattering. In this way, the authors are able to investigate the evolution of salt solutions before and during crystal precipitation and reveal the sequence of events that lead to the formation of crystals. Lee et al.’s results show that the crystallization behavior of KDP solutions deviates from what is expected from the classic view on nucleation and growth. Highly supersaturated solutions of up to four (!) times the equilibrium solubility can be reached before precipitation. The authors show that in these highly supersaturated solutions, nucleation precursors can form that do not have the crystalline structure of the salt. These precursors subsequently transform into salt crystals, and act as a type of “reaction intermediate” that is energetically favored over the direct formation of the equilibrium crystal structure (Fig. 1).
Fig. 1.
Schematic of the crystallization pathways reported by Lee et al. (3). The top path is the “normal” crystallization of the equilibrium crystal shape directly from solution; in the experiments this happens for low concentration solutions (LCS). The lower path happens for high concentrations (HCS): a three-step nucleation process with first the formation of a precursor “phase” consisting of regions that are more concentrated in salt, followed first by the nucleation of a metastable monoclinic crystal before the equilibrium tetragonal crystal appears. S, supersaturation; V, droplet volume.
The observations of Lee at al. (3) and others (4–13) of multistep nucleation processes of course raise many questions as to the nature of the intermediate state. The intermediate can be: (i) disordered, resulting from a dynamical liquid–liquid phase separation that leads to the formation of clusters (e.g., “dense liquid” droplets with a high salt concentration that act as prenucleation clusters); or (ii) ordered, because of the formation of crystallites of an intermediate crystal structure: the intermediate stage could be a different polymorph of the same crystal. It is interesting to note that in the few instances where such metastable clusters in a two-step nucleation process were reported experimentally, the salts involved not only have different (metastable) crystalline forms, but possibly also hydrated and amorphous forms (7–11).
The first hypothesis is the dynamic formation of a new and dense liquid-like phase. Lee et al. (3) follow the drop during its evaporation; they first observe the appearance of a second Raman peak that occurs while the solution remains transparent and no X-ray diffraction is observed. This new Raman vibration is shifted to higher wavelengths than in the solution (i.e., it is closer to the corresponding peak of the crystalline phases). This result is attributed to the formation of a different liquid state with a different local structure that is perhaps similar to liquid-like ionic polymers (12). Lee et al.’s (3) Raman results therefore suggest the appearance of a dynamic coexistence of regions highly concentrated in salt with more water-rich ones, similarly to what has been reported in simulations for CaCO3 (12). True liquid–liquid phase separations in aqueous systems have been reported in the presence of organic molecules and proteins (4, 5, 14–16); however little is known about such phase separation occurring in aqueous solutions of inorganic salts (11, 13, 17). Extended simulations suggest that univalent or bivalent salts should not show a phase separation (18). However, experimentally, such phase separations have in fact been observed for different salts, among which is the monovalent salt LiCl (19). For proteins that can carry multiple charges, liquid–liquid phase separation and two-step nucleation are indeed observed both in simulations and experiments (4, 5, 14, 15, 20). However, the nature of the intermediate phase remains a subject of vivid discussion; it appears obvious that the intermediate is stabilized by attractive forces that counter the entropic free-energy cost of creating a region more concentrated in salt; this, however, does not necessarily have to be a first-order liquid–liquid transition.
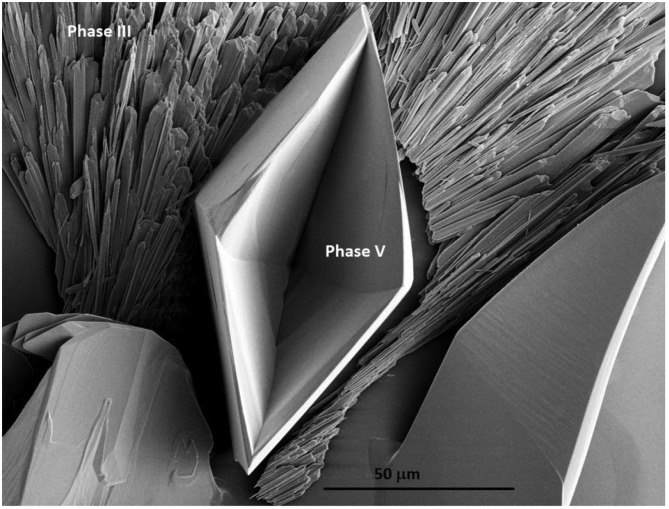
The second hypothesis is the formation of an intermediate crystal structure. It is well known that nonequilibrium crystal structures may form upon crystallization by evaporation, cooling or in confinement, that subsequently transform into the equilibrium form. In the experiments of Lee et al. (3), depending on the degree of supersaturation reached, the evaporation can lead either directly to the precipitation of the stable tetragonal anhydrous KDP crystal or show the rapid precipitation of a metastable monoclinic KDP, which then transforms to the stable tetragonal form (Fig. 1). Similarly, it has been shown that solutions of sodium sulfate (Na2SO4), upon evaporation at room temperature, can reach high supersaturation, which subsequently leads to the precipitation of two anhydrous structures with, again, distinct Raman signatures (21–23). Very similar to what Lee et al. (3) report for KDP, the bulk crystallization starts with a very rapid growth of the metastable phase III (dendritic shape), which then converts into the stable phase V (rhombohedral shape); Fig. 2 shows the two different crystal structures that result if the conversion is unable to complete because of lack of solution.
Fig. 2.
Formation of anhydrous sodium sulfate crystals after evaporation of the salt solution; dynamically, the dendritic metastable phase III forms first, and then transforms into the stable rhombohedral phase V in solution. In this experiment the evaporation was too fast to allow for complete transformation.
It is worthwhile mentioning that, both for KDP and sodium sulfate, achieving such high concentrations is again surprising, especially because both have hydrated polymorphs that should be the first phase to appear according to the phase diagram (23, 24). Generally, the solubility of hydrated forms of a specific salt is smaller than that of its anhydrous form at a given temperature. This means that upon evaporation at a given temperature, the hydrated polymorph with the lowest solubility is in principle the less stable in solution, and one might anticipate its precipitation first. Nevertheless, in the evaporation experiments on KDP and sodium sulfate, this does not happen, implying that such thermodynamic equilibrium considerations cannot explain the dynamic process. Apparently the interfacial free-energy barriers for the formation of the hydrated crystals are sufficiently high to inhibit their formation; the result is that the solutions achieve high supersaturations upon further evaporation. The whole process is therefore clearly dominated by kinetics, and not by thermodynamics.
The results of the kinetically governed nucleation dynamics then appear to be that of both types of intermediate stages: different crystal structure or dynamic phase separation have been reported in different experiments. Lee et al. (3) in fact report a combination of both: first the formation of a dense liquid phase followed by the formation of metastable crystal structure, which then turns into the equilibrium crystalline polymorph. In agreement with the above observations, this only happens for very high supersaturations; for moderate ones, a simple one-step nucleation and growth process immediately yields the equilibrium crystal structure. Thus, the high supersaturations appear to be a prerequisite for multistep nucleation. When the nucleation then finally takes place, the droplet becomes completely opaque, which suggests that many small crystallites form. These detailed in situ observations then suggest the following scenario for salt nucleation from highly supersaturated solutions. If indeed concentrated salt solutions are prone to a liquid–liquid type phase separation, such a transition could also have a spinodal, where small drops of dense liquid spontaneously start to form. Because these domains are denser in salt, the supersaturation in the drops is even higher than the average one, allowing crystals to nucleate very rapidly; the energy barrier for nucleation is of course lowered upon increasing the supersaturation. Which crystalline form appears is then again dictated kinetically, but eventually the equilibrium crystal will appear. This gives a plausible reason for the formation of many crystals, and has now been observed in evaporating sessile (11) and levitating drops (3). The existence of a spinodal in the nucleation process was already discussed by Filobelo et al. (15), who define the spinodal as the point where the generation of the new phase is only limited by the kinetics of growth of its clusters. Indeed, in the experiments of Lee et al. (3), in which the crystallization passes through the pathway with two intermediates (i.e., a three-step process), once the spinodal is reached, the transformation of the solution that has remained metastable for many hours takes place in seconds.
Unfortunately for the crystallographers, such multistep nucleation processes, which might have yielded novel ways to control nucleation and growth, then lead to the formation of many small crystals. It appears that independently of the type of intermediate stage, the observed multistep nucleation processes happen invariably at very high supersaturations. This leads to the formation of a large number of crystals that grow very rapidly and are consequently prone to growth instabilities (11, 25). To form a perfect single crystal, it is therefore preferable to start with a single seed in an only slightly supersaturated solution to have a slow and controlled growth; this also avoids subsequent growth instabilities that give snowflake-type crystals their beautiful characteristic shapes.
Footnotes
The authors declare no conflict of interest.
See companion article on page 13618.
References
- 1.Thompson DW. On Growth and Form. Cambridge Univ Press; Cambridge, UK: 1945. [Google Scholar]
- 2.Nittmann J, Stanley HE. Non-deterministic approach to anisotropic growth patterns with continuously tunable morphology: The fractal properties of some real snowflakes. J Phys Math Gen. 1987;20:L1185–L1191. [Google Scholar]
- 3.Lee S, et al. Multiple pathways of crystal nucleation in extremely supersaturated aqueous potassium dihydrogen phosphate (KDP) solution droplet. Proc Natl Acad Sci USA. 2016;113:13618–13623. doi: 10.1073/pnas.1604938113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galkin O, Vekilov PG. Control of protein crystal nucleation around the metastable liquid-liquid phase boundary. Proc Natl Acad Sci USA. 2000;97(12):6277–6281. doi: 10.1073/pnas.110000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vekilov PG. Dense liquid precursor for the nucleation of ordered solid phases from solution. Cryst Growth Des. 2004;4:671–685. [Google Scholar]
- 6.Sear RP. Nucleation: Theory and applications to protein solutions and colloidal dispersions. J Phys Condens Matter. 2007;19:033101. [Google Scholar]
- 7.Gebauer D, Völkel A, Cölfen H. Stable prenucleation calcium carbonate clusters. Science. 2008;322(5909):1819–1822. doi: 10.1126/science.1164271. [DOI] [PubMed] [Google Scholar]
- 8.Pouget EM, et al. The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science. 2009;323(5920):1455–1458. doi: 10.1126/science.1169434. [DOI] [PubMed] [Google Scholar]
- 9.Wang YW, Kim YY, Christenson HK, Meldrum F. A new precipitation pathway for calcium surlfate dehydrate (gypsum) via amorphous and hemihydrate intermediates. Chem Commun (Camb) 2012;48(4):540–506. doi: 10.1039/c1cc14210k. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner J, et al. Nucleation and growth of magnetite from solution. Nat Mater. 2013;12(4):310–314. doi: 10.1038/nmat3558. [DOI] [PubMed] [Google Scholar]
- 11.Shahidzadeh N, Schut MFL, Desarnaud J, Prat M, Bonn D. Salt stains from evaporating droplets. Sci Rep. 2015;5:10335. doi: 10.1038/srep10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demichelis R, Raiteri P, Gale JD, Quigley D, Gebauer D. Stable prenucleation mineral clusters are liquid-like ionic polymers. Nat Commun. 2011;2:590. doi: 10.1038/ncomms1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty D, Patey GN. Evidence that crystal nucleation in aqueous NaCl solution occurs by the two step mechanism. Chem Phys Lett. 2013;587:25–29. [Google Scholar]
- 14.Sleutel M, Van Driessche AES. Role of clusters in nonclassical nucleation and growth of protein crystals. Proc Natl Acad Sci USA. 2014;111(5):E546–E553. doi: 10.1073/pnas.1309320111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filobelo LF, Galkin O, Vekilov PG. Spinodal for the solution-to-crystal phase transformation. J Chem Phys. 2005;123(1):014904. doi: 10.1063/1.1943413. [DOI] [PubMed] [Google Scholar]
- 16.Harano K, et al. Heterogeneous nucleation of organic crystals mediated by single-molecule templates. Nat Mater. 2012;11(10):877–881. doi: 10.1038/nmat3408. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Chou IM, Hu WX, Burruss RC. In situ observations of liquid-liquid phase separation in aqueous MgSO4 solutions: Geological and geochemical implications. Geochim Cosmochim Acta. 2013;103:1–10. [Google Scholar]
- 18.Orkoulas G, Panagiotopoulos AZ. Phase behavior of the restricted primitive model and square-well fluids from Monte Carlo simulations in the grand canonical ensemble. J Chem Phys. 1999;110:1581–1590. [Google Scholar]
- 19.Suzuki Y, Mishima O. Two distinct raman profiles of glassy dilute LiCl solution. Phys Rev Lett. 2000;85(6):1322–1325. doi: 10.1103/PhysRevLett.85.1322. [DOI] [PubMed] [Google Scholar]
- 20.Erdemir D, Lee AY, Myerson AS. Nucleation of crystals from solution: Classical and two-step models. Acc Chem Res. 2009;42(5):621–629. doi: 10.1021/ar800217x. [DOI] [PubMed] [Google Scholar]
- 21.Linnow K, Steiger M, Lemster C, De Clercq H, Jovanovic M. In situ Raman observation of the crystallization in NaNO3–Na2SO4–H2O solution droplets. Environ Earth Sci. 2013;69(5):1609–1620. [Google Scholar]
- 22.Shahidzadeh N, Desarnaud J. Damage in porous media: Role of the kinetics of salt (re)crystallization. Eur Phys J Appl Phys. 2012;60(2):24205. [Google Scholar]
- 23.Linnow K, Zeunert A, Steiger M. Investigation of sodium sulfate phase transitions in a porous material using humidity- and temperature-controlled X-ray diffraction. Anal Chem. 2006;78(13):4683–4689. doi: 10.1021/ac0603936. [DOI] [PubMed] [Google Scholar]
- 24.Syed KA, Pang SF, Zhang Y, Zhang YH. Micro-Raman observation on the H2PO4(-) association structures in a supersaturated droplet of potassium dihydrogen phosphate (KH2PO4) J Chem Phys. 2013;138(2):024901. doi: 10.1063/1.4773585. [DOI] [PubMed] [Google Scholar]
- 25.Desarnaud J, Derluyn H, Carmeliet J, Bonn D, Shahidzadeh N. Metastability limit for the nucleation of NaCl crystals in confinement. J Phys Chem Lett. 2014;5(5):890–895. doi: 10.1021/jz500090x. [DOI] [PubMed] [Google Scholar]