Significance
The brain’s default mode network (DMN) is comprised of regions that are highly active during wakeful rest. In the past 15 y, the DMN has been a target of investigation in thousands of basic and clinical neuroscience studies, yet the fundamental role of this network remains debated and unknown. Some studies suggest that DMN activity increases with self-reported mind-wandering away from the present sensory environment, a state in which task performance tends to be highly unstable. However, we show that DMN activity increases with stable, rather than variable, behavior, independent from increases with mind-wandering. Our work urges reinterpretation of the significance of DMN activity fluctuations in daily life and DMN disruption in disease.
Keywords: daydreaming, default mode network, sustained attention, spontaneous thought, resting state
Abstract
The brain’s default mode network (DMN) is highly active during wakeful rest when people are not overtly engaged with a sensory stimulus or externally oriented task. In multiple contexts, increased spontaneous DMN activity has been associated with self-reported episodes of mind-wandering, or thoughts that are unrelated to the present sensory environment. Mind-wandering characterizes much of waking life and is often associated with error-prone, variable behavior. However, increased spontaneous DMN activity has also been reliably associated with stable, rather than variable, behavior. We aimed to address this seeming contradiction and to test the hypothesis that single measures of attentional states, either based on self-report or on behavior, are alone insufficient to account for DMN activity fluctuations. Thus, we simultaneously measured varying levels of self-reported mind-wandering, behavioral variability, and brain activity with fMRI during a unique continuous performance task optimized for detecting attentional fluctuations. We found that even though mind-wandering co-occurred with increased behavioral variability, highest DMN signal levels were best explained by intense mind-wandering combined with stable behavior simultaneously, compared with considering either single factor alone. These brain–behavior–experience relationships were highly consistent within known DMN subsystems and across DMN subregions. In contrast, such relationships were absent or in the opposite direction for other attention-relevant networks (salience, dorsal attention, and frontoparietal control networks). Our results suggest that the cognitive processes that spontaneous DMN activity specifically reflects are only partially related to mind-wandering and include also attentional state fluctuations that are not captured by self-report.
The brain’s default mode network (DMN) has been described as a distributed set of regions in association cortices showing increased activity during undirected, awake “resting” states relative to a wide variety of states that commonly involve externally oriented attention (1, 2). During undirected, awake life, humans frequently engage in mind-wandering, self-generated thoughts unrelated to the immediate sensory world (3). Based on such observations, researchers have considered that increased spontaneous DMN activation could be a neurophysiological correlate of mind-wandering (4, 5).
Neuroimaging studies that have incorporated self-report measures suggest a role of the DMN in spontaneous cognition. Converging evidence from tasks that elicit mind-wandering (6, 7), interindividual differences in mind-wandering tendencies (8), and intraindividual fluctuations in self-reports (9–12) suggests that DMN activity is increased during stimulus-independent, task-unrelated thought. The DMN is also engaged when subjects actively think about the past, the future, and the perspectives of other people, all of which constitute the types of thoughts that commonly occur during mind-wandering (4).
However, theoretical considerations pose serious challenges to the notion that increased spontaneous DMN activity reflects mind-wandering exclusively. The correlational neuroimaging evidence described above does not imply that instances of increased spontaneous DMN activity signify mind-wandering (the “reverse inference” problem) (13). Behavioral and self-report outcomes are imperfect measures of cognition, and the fundamental function of the DMN may not be captured by any single measure.
Empirical considerations also suggest that a sole focus on studying mind-wandering is unlikely to unveil a comprehensive account of DMN function (14). For example, although the DMN is commonly deactivated during externally oriented tasks requiring cognitive control (15), older adult populations report low levels of mind-wandering during task performance yet exhibit attenuated DMN deactivation (16). Additionally, in certain contexts, increased DMN activity is time-locked to stimulus changes in the external environment (17, 18).
Although spontaneous increases of DMN activity are postulated to reflect mind-wandering, a highly consistent finding is that DMN activity is higher when ongoing behavior is stable rather than variable (19–21). This finding would be counterintuitive if there were a one-to-one mapping between mind-wandering intensity and DMN activity. Mind-wandering occurrence is consistently associated with variable, rather than stable, behavior (10, 22, 23). Both variable behavior and mind-wandering are associated with errors in continuous performance tasks (CPTs) (incorrect responses), or “attention lapses” (20, 24), and DMN activity is elevated preceding such lapses (20, 25). Thus, it remains mysterious how DMN activity is associated with both mind-wandering (presumably an error-prone, behaviorally variable state) and behavioral stability.
Here, using a unique task paradigm, we simultaneously assessed fluctuating levels of behavioral variability, self-reported mind-wandering, and brain activity with fMRI during a CPT optimized for detecting these fluctuations. Doing so, we introduce a platform for going beyond understanding whether increased DMN activity simply co-occurs with mind-wandering or with behavioral stability. Considering both factors simultaneously and dynamically, we test whether DMN activity fluctuations reflect cognitive processes that are captured only when both self-reported and behavioral indices of attentional states are jointly considered.
Results
Linking Self-Reported Attention with Behavior.
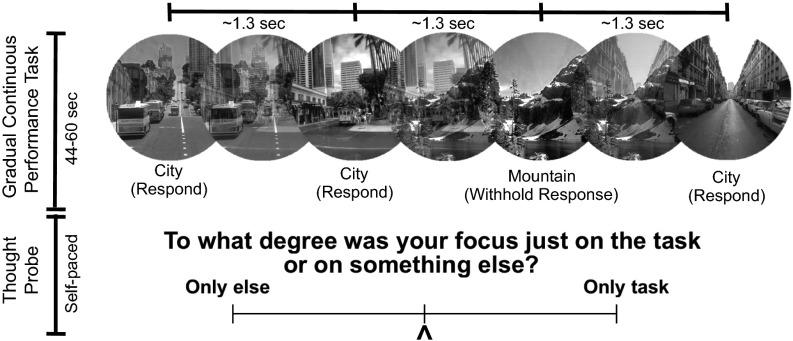
We recruited 28 healthy adults to perform a modified version of the gradual CPT (gradCPT) (Fig. S1), known to elicit marked fluctuations of attention (20, 26). Participants viewed gradually changing images of scenes and were instructed to respond with a button press to city but not mountain scenes. It was previously shown that slower, relative to faster, rates of stimulus presentation in other cognitive tasks are associated with increased mind-wandering and associated neural activity (27, 28), and so we presented scene transitions at a slower rate than in previous gradCPT studies (∼1,300 ms instead of ∼800 ms). An experience sampling approach (9) was used to detect mind-wandering. Blocks of the task lasted 44–60 s, with a “thought-probe” appearing at the end of the block consisting of a self-report rating of the degree to which attention was on- or off-task in the immediately preceding period. In contrast to previous fMRI studies (9–11), the rating scale was graded rather than discrete, ranging from 0 (focus was completely on task) to 100 (focus was completely on something else). Thus, we assessed a wide spectrum of self-reported attentional states and linear relationships with behavior and brain activity (Fig. S2A). Subjects completed 36 total thought-probes in four gradCPT runs (each ∼9 min) with short breaks between runs (summary behavioral data in Table S1).
Fig. S1.
Task paradigm. Subjects viewed gradually changing images of (frequent) city and (rare) mountain scenes. Subjects were instructed to respond with a button press to city but not mountain events. After 44–60 s, a self-paced thought-probe (bottom) appeared. Using a continuous scale (100, only else; 0, only task), subjects evaluated the degree to which they were just focused on the task or on something else just before appearance of the thought-probe.
Fig. S2.
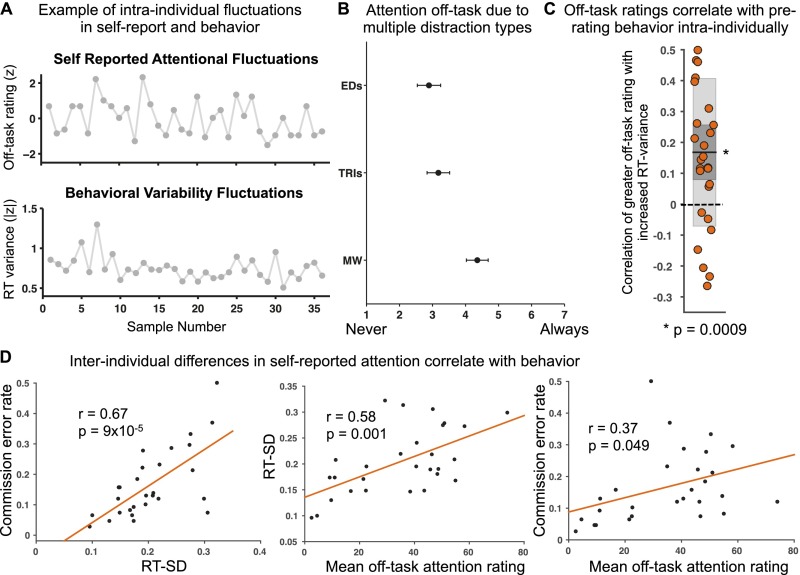
Behavioral results. (A) Single-subject example of spontaneous fluctuations in self-reported attention (normalized off-task rating shown) and in prerating RT variance (absolute z averaged across 30-s prerating windows) across 36 thought-probes. (B) Average postscan ratings of the degree to which subjects reported attention off-task due to external distractions, task-related interferences, and mind-wandering (error bars denote SEM). (C) Within-subject Fisher-transformed Pearson correlations between off-task rating and ∼30-s prerating RT variance across 36 trial blocks within each subject (shaded dark gray denotes SEM; shaded light gray denotes SD; dark line denotes mean across subjects; dotted line demarcates zero value). Data points are slightly jittered along the x axis solely for visualization purposes. *P < 0.05, two-tailed Wilcoxon signed rank test. EDs, external distractions; MW, mind-wandering; RT, reaction time; TRIs, task-related interferences. (D) Correlations of interindividual differences in RT SD across all trial blocks vs. commission error rate across all trials (Left) and mean off-task rating across all thought-probes vs. average RT SD across all trial blocks (Middle) and vs. commission error rate across all trials (Right). RT, reaction.
Table S1.
Group-level (n = 28) summary statistics of behavioral and self-report data
| Metric | Mean | SD |
| Mean RT, s | 1.24 | 0.12 |
| RT SD, s | 0.20 | 0.06 |
| Commission error rate | 0.17 | 0.11 |
| Off-task rating—0, on-task; 100, off-task | 34.8 | 18.8 |
Commission error rate is defined as the number of incorrect button presses to target mountain events divided by the total number of mountain events. At the within-subject level, mean RT and RT SD statistics are based on 36 samples, each of combined RTs in ∼30-s pre–thought-probe time windows.
Upon task completion, subjects were interviewed about the degree to which their reports of attention off-task were due to (a) external/sensory distractions (e.g., sounds), (b) task-related interferences (e.g., task strategizing), and (c) mind-wandering (i.e., task-unrelated and stimulus-independent thoughts) (1, never; 7, always) (11, 24). Although there was substantial interindividual variability in these ratings (see Specificity to Mind-Wandering), mean ratings for mind-wandering (mean ± SD = 4.4 ± 1.7) were higher than those for external distractions (2.9 ± 1.9) and task-related interferences (3.2 ± 1.8) (Fig. S2B). Participants reported high confidence in their abilities to accurately indicate on- or off-task focus (mean ± SD rating = 5.8 ± 0.96; 0, “not confident at all”; 7, “extremely confident”).
We next sought to provide behavioral validation of these off-task self-reports. We predicted that greater off-task attention ratings would be associated with increased prerating behavioral variability in the gradCPT, similar to results shown with other cognitive tasks (10, 29). We focused on reaction time (RT) variability in the 30-s prerating period (except where indicated) because relative to shorter prerating time windows, we could use more samples to calculate RT metrics (23 trials) and acknowledge uncertainty in the duration of preprobe periods to which participants’ thought-probe responses referred (but results from shorter prerating periods were largely similar; Table S2). For consistency with previous studies on the DMN and RT variability (19, 20), we report our main analyses based on RT variance (absolute deviance from the mean) across trials preceding thought probes, but we also report results with RT coefficient of variation (CoV).
Table S2.
Mean, SD, and P values (two-tailed Wilcoxon signed rank test) for within-subject correlations (n = 28) of off-task rating, prerating RT variance, and prerating DMN activity using 20- and 10-s prerating periods
| Correlation | Prerating period | |||
| ∼20 s | ∼10 s | |||
| r, mean ± SD | P | r, mean ± SD | P | |
| Off-task rating vs. prerating RT variance | 0.14 ± 0.24 | 0.004 | 0.12 ± 0.23 | 0.007 |
| Prerating DMN activity vs. off-task rating | 0.13 ± 0.17 | 0.0005 | 0.10 ± 0.16 | 0.005 |
| Prerating DMN activity vs. RT variance | –0.11 ± 0.17 | 0.004 | –0.05 ± 0.18 | 0.22 |
Before Wilcoxon signed rank tests, within-subject Pearson’s r values were Fisher-transformed to z values. For RT variance, 15 and 8 prerating trials were used, respectively, for 20- and 10-s periods. For DMN activity, 19 and 9 prerating brain volumes were averaged, respectively, for 20- and 10-s periods.
The mean within-subject correlation of off-task rating with RT variance was positive and significantly greater than zero (P = 0.0009, two-tailed Wilcoxon signed rank test; Fig. S2C), supporting the hypothesized relationship (similar results were obtained with RT CoV; see Table S3). In contrast, off-task rating was not significantly correlated with mean RT (P = 0.19), and RT variance was also not significantly correlated with mean RT (P = 0.42), suggesting that RT variance is an independent and better marker of self-reported attentional state compared with RT speed.
Table S3.
Mean, SD, and P values (two-tailed Wilcoxon signed rank test) for within-subject correlations (n = 28) of RT CoV with off-task rating and with DMN signal in 30-s prerating periods
| Correlation | r, mean ± SD | P |
| Off-task rating vs. prerating RT CoV | 0.18 ± 0.23 | 0.001 |
| Prerating DMN activity vs. RT CoV | −0.13 ± 0.17 | 0.0014 |
Before Wilcoxon signed rank tests, within-subject Pearson’s r values were Fisher-transformed to z values.
At the interindividual level, RT variability was positively correlated with rate of attention lapses (commission errors; r = 0.67, P = 9 × 10−5; Fig. S2D), replicating previous work and suggesting that individuals with increased RT variability have heightened levels of “out-of-the-zone” attention (20, 26). We extend those results here, showing that such individuals also report experiencing greater off-task attention during task performance; the mean off-task rating across all thought-probes within subjects was positively correlated with both RT variability (r = 0.58, P = 0.001) and attention lapse rate (r = 0.37, P = 0.049; Fig. S2D) but not with mean RT (r = 0.24, P = 0.22).
DMN Fluctuations Reflect Both Self-Reported Attention and Behavioral Variability.
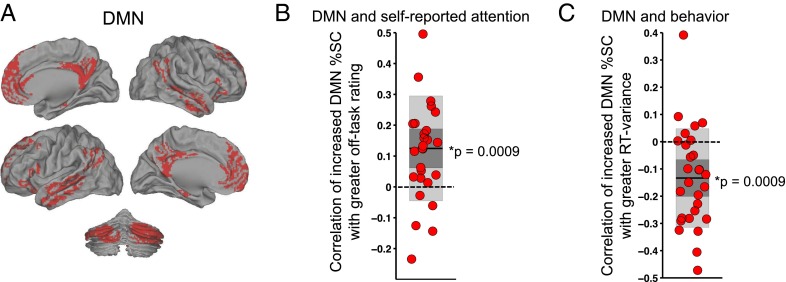
Here, we have shown that increased RT variability is associated with self-reports of greater off-task attention at both intra- and interindividual levels, yet decreased RT variability and off-task self-reports have separately been previously associated with increased DMN activity (9, 10, 12, 19, 20). To evaluate each of those relationships in our paradigm, we extracted mean activity level (percent signal change, %SC) in 30-s prerating periods from the DMN, defined from a network atlas developed in an independent, large cohort of subjects, and including gray matter in the medial prefrontal cortex, posterior cingulate cortex, lateral parietal areas, portions of the temporal lobe, and cerebellar components (Fig. 1A) (30, 31). We refer to fluctuations in this extracted brain activity, and in associated self-reported attention and behavioral variability, as “spontaneous” (32) because each sample within a given subject occurs during performance of the same task without any explicit change in cognitive demand or in the nature of the presented stimuli across prerating periods (the number of target mountain events was matched).
Fig. 1.
DMN activity correlates positively with self-reported off-task attention and negatively with behavioral variability. (A) The DMN mask used for extracting mean activity, including cortical and cerebellar regions. (B) Within-subject Fisher-transformed Pearson correlations between prerating DMN %SC and off-task rating across 36 trial-blocks within each subject. (C) Within-subject Fisher-transformed Pearson correlations between prerating DMN %SC and prerating RT variance across 36 trial blocks within each subject. In B and C, shaded dark gray denotes SEM, shaded light gray denotes SD, dark line denotes mean across subjects, and dotted line demarcates zero value. *P < 0.05, two-tailed Wilcoxon signed rank test. DMN, default mode network; RT, reaction time; SC, signal change.
The mean within-subject correlation of off-task rating with prerating spontaneous DMN activity was positive and significantly greater than zero (P = 0.0009, two-tailed Wilcoxon signed rank test; Fig. 1B). Additionally, the mean within-subject correlation of prerating RT variance with DMN activity was negative and significantly less than zero (P = 0.0009) (Fig. 1C; similar results for RT CoV are shown in Table S3). Thus, our results suggest that simultaneous relationships of DMN activity with self-reported off-task attention and with behavioral variability are in opposite directions.
Multiple Cognitive Factors Explain DMN Fluctuations.
Our results thus far show that spontaneous DMN activity is increased both during self-reported off-task attention and during periods of stable behavior, even though off-task attention is associated with greater behavioral variability. How can DMN activity simultaneously relate to two seemingly opposing processes? We empirically addressed this question with linear mixed-effects model analyses (subjects as random effects and within-subject variables as fixed effects; see SI Methods) that had several possible outcomes. For example, DMN activity that is explained by off-task rating could be dependent on or interacting with behavioral variability (or vice versa), or DMN activity could be explained by additive variance of off-task rating and RT variability, with relative independence.
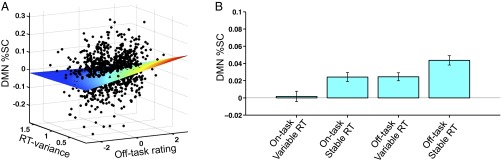
Our model fits provided unequivocal support for independent, additive effects of the variances contributed by off-task rating and RT variability to DMN activity. Compared each to models only including off-task rating (β = 0.11, t = 3.6, P = 0.0004) or only including RT variability (β = –0.12, t = –3.8, P = 0.0001), the combination of off-task rating (β1 = 0.13, t = 4.1, P = 0.0002) and RT variability (β2 = –0.14, t = –4.3, P = 8.9 × 10−5) in a single model afforded an improved explanation of DMN signal, with more than double the variance explained (R2 for combined fixed effects = 0.031) relative to the inclusion of only one predictor (R2 = 0.012 for off-task rating, R2 = 0.014 for RT variability; see SI Results for outcomes with RT CoV and mean RT modeled). No significant off-task rating by RT variability interaction was found (P = 0.15), further supporting independence of the variances contributed to DMN activity. A 3D plot (for visualization only) of trial blocks across all subjects is shown in Fig. 2A (and Fig. 2B for alternative representation based on median splits of trial block types). These plots show that the highest DMN activity trial blocks were those that occurred during combined low RT variance with high self-reported off-task attention, whereas the lowest DMN activity trial blocks were those that occurred during combined high RT variance with high self-reported on-task focus.
Fig. 2.
Self-reported attention and RT variability additively account for DMN activity. (A) 3D plot showing all trial blocks in all subjects with values for off-task rating (x1; within-subject normalized off-task rating), RT variance (x2), and DMN %SC (y). Color is proportional to mesh surface height, with red areas highest (high DMN activity) and blue areas lowest (low DMN activity). (B) Bar plots showing mean DMN %SC in trial blocks with four combinations of on-/off-task attention and high and low RT variance, with on/off (low/high) categories defined based on median split of all trial blocks of all subjects for within-subject normalized off-task ratings and RT variance. DMN, default mode network; RT, reaction time; SC, signal change.
Specificity to Mind-Wandering.
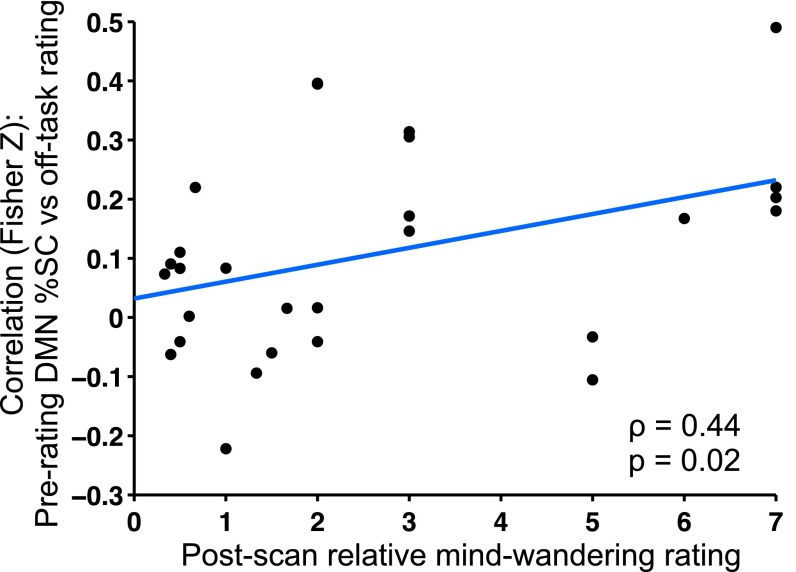
Previous studies show that before thought-probe onsets, increased DMN activation levels are most strongly associated with mind-wandering compared with external distractions and task-related interferences (10, 11). Mind-wandering and external distraction represent internally and externally oriented attention, respectively, and opposite relationships with DMN activity have been shown (11). Thus, using interindividual differences in postscan ratings of the degree to which off-task attention reports were due to different factors, we sought to determine whether the relationship between off-task rating and DMN activity was driven by participants who reported highest relative levels of mind-wandering (calculated as the ratio of mind-wandering to external distraction rating). Focusing on the 10-s prerating period (9, 10), we found a positive correlation between relative mind-wandering and the correlation strength of off-task rating vs. DMN activity (ρ = 0.44, P = 0.02) (Fig. 3). To illustrate the time dependence of this positive relationship, we repeated the analysis using activity from single whole-brain volumes (acquired every 1.08 s) (SI Results and Fig. S3). These results suggest temporal specificity of the relationship between DMN activity and mind-wandering to the period that participants referred to when they were evaluating their attentional state.
Fig. 3.
DMN off-task relationship is driven most strongly by individuals with high relative levels of self-reported mind-wandering. The degree to which subjects reported off-task attention due to mind-wandering (relative to external distractions) is positively correlated with the within-subject Fisher-transformed Pearson correlation between off-task rating and prerating DMN %SC averaged within the 10-s prerating period. DMN, default mode network; SC, signal change.
Fig. S3.
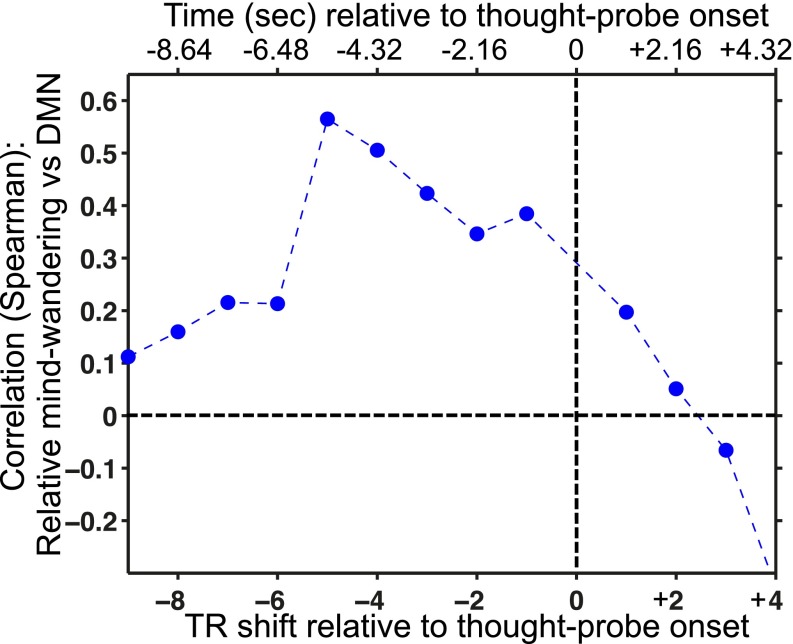
Spearman’s ρ values for the analysis shown in Fig. 3 (based on DMN activity in 10 s), here repeated using single whole-brain volumes before and after the thought-probe onset (rather than averaging across brain volumes). Whole-brain volumes were acquired every 1.08 s. Because thought-probes were self-paced, the time between thought-probe onset and brain volume acquisition varied slightly from probe to probe. Thus, the TR numbers and corresponding time (s) on the x axis denote the upper limit of the possible time between thought-probe onset and volume acquisition (e.g., –2.16 s indicated on the plot denotes a range of –1.08 s to –2.16 s). DMN, default mode network; SC, signal change; TR, repetition time.
Generalizability Across DMN Subcomponents.
Our results reveal that mean activity within the whole DMN is explained by a combination of self-reported off-task attention and behavioral stability levels, but the DMN is comprised of anatomical and functional subsystems and subregions that could exhibit distinct profiles (4, 33, 34). For example, a given DMN subsystem/subregion could relate to self-reported attention but not behavioral variability, or vice versa, an effect that would be washed out when analyzing only average whole-DMN activity. We thus repeated our analyses of within-subject correlation of off-task rating vs. DMN activity within three known subsystems [i.e., the DMN core, the dorsomedial prefrontal cortex (dmPFC) subsystem, and the medial temporal lobe (MTL) subsystem (30, 33)].
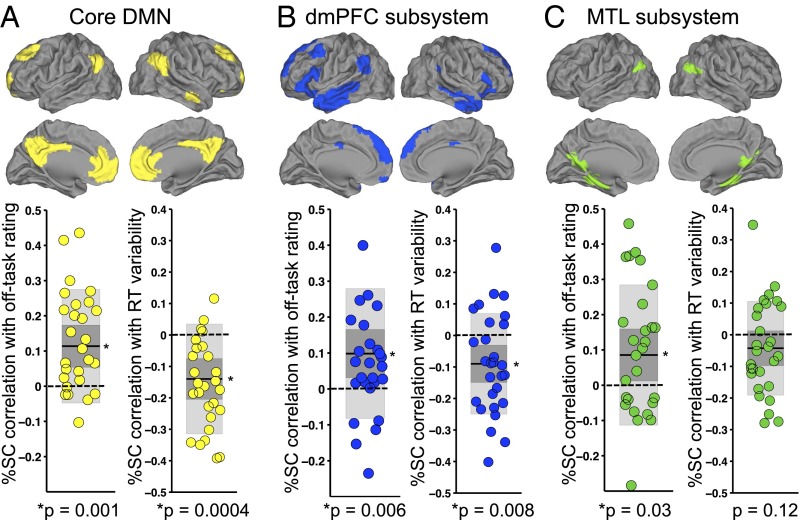
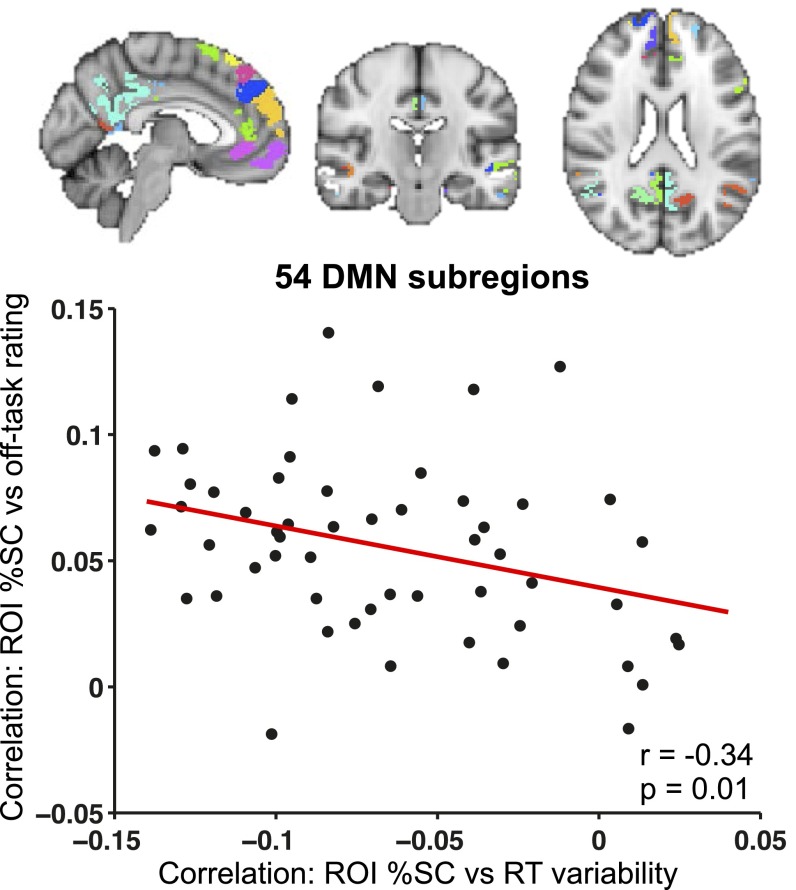
Within each subsystem, relationships of DMN activity with off-task attention vs. those with behavioral variability were of similar magnitude and, as in the whole-DMN analysis, were in opposite directions (Fig. 4). Higher off-task rating was significantly associated with activity in the DMN core (P = 0.001), dmPFC subsystem (P = 0.006), and MTL subsystem (P = 0.03). Lower RT variance was significantly associated with activity in the DMN core (P = 0.0004) and dmPFC subsystem (P = 0.008), whereas the association with MTL subsystem activity was in the same direction but was not significant (P = 0.12). An additional analysis with a more fine-grained parcellation of the DMN (54 subregions) revealed that subregions that relate most strongly (and positively) to off-task attention are similar to those that relate most strongly (and negatively) to behavioral variability (SI Results and Fig. S4).
Fig. 4.
Subsystems of the DMN are associated with self-reported attention and RT variance. For each subsystem, we show within-subject Fisher-transformed Pearson correlations of prerating %SC with off-task rating (Left) and RT variance (Right) across 36 trial blocks within each subject. (A) The core DMN regions (yellow). (B) The dmPFC subsystem regions (blue). (C) The MTL subsystem regions (green). In all plots, shaded dark gray denotes SEM, shaded light gray denotes SD, dark line denotes mean across subjects, and dotted line demarcates zero value. *P < 0.05, two-tailed Wilcoxon signed rank test. DMN, default mode network; dmPFC, dorsomedial prefrontal cortex; MTL, medial temporal lobe; RT, reaction time; SC, signal change.
Fig. S4.
Correspondence of DMN areas associated with off-task attention and those associated with behavioral variability. (Top) Subregions of the DMN shown in different colors (54 total subregions). (Bottom) Plot of the correlation of %SC in a subregion with RT variance vs. the correlation of %SC with within-subject normalized off-task rating (r = –0.34, P = 0.01). Correlation values for each subregion were based on all trial blocks concatenated across all subjects. DMN, default mode network; ROI, region of interest; SC, signal change.
Specificity Relative to Other Brain Networks.
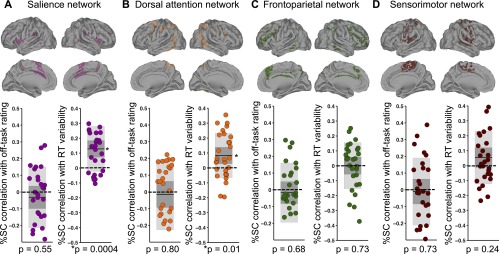
Other networks besides the DMN could be involved in self-reportable attention and/or behavioral variability. Increased behavioral variability has previously been associated with higher activity in dorsal attention and salience networks (opposite to the DMN pattern) (19–21), and some but not all previous studies (35, 36) have found increased mind-wandering associated with activity in the frontoparietal control network. Additionally, because our measurements occurred in tandem with sensorimotor processes (due to button pressing), it was important to ensure that we had captured effects that were specific to cognitive processes in the DMN. Thus, to test for specificity of effects in the DMN, we performed control analyses testing for relationships of self-reported attention and behavioral variability with (a) dorsal attention, salience, and frontoparietal network activity and (b) sensorimotor network activity.
Consistent with our predictions, within-subject correlations of prerating RT variance with salience network (P = 0.0004, two-tailed Wilcoxon signed rank test) and dorsal attention network activity (P = 0.01) were significantly greater than zero at the group level, but there was no significant association between RT variance and activity in sensorimotor (P = 0.24) or frontoparietal control (P = 0.73) networks. There were no significant within-subject correlations of off-task rating with salience (P = 0.55), dorsal attention (P = 0.80), sensorimotor (P = 0.73), or frontoparietal control (P = 0.68) network activity (Fig. S5).
Fig. S5.
Correlations of salience, dorsal attention, frontoparietal control, and sensorimotor network activity with off-task ratings and with RT variance. The figure shows brain regions and plots of within-subject Fisher-transformed Pearson correlations of prerating network %SC with off-task rating (Left) and RT variance (Right) across 36 trial blocks within each subject for the (A) salience network (purple), (B) dorsal attention network (orange), (C) frontoparietal network (green), and (D) sensorimotor network (brown). In all plots, shaded dark gray denotes SEM, shaded light gray denotes SD, dark line denotes mean across subjects, dotted line demarcates zero value, and data points are slightly jittered along the x axis solely for visualization purposes. RT, reaction time; SC, signal change.
SI Methods
Exclusion Criteria.
Although 29 subjects in total had participated, 1 had no variation in his or her level of reported task focus (selected the extreme end of “only task” focus in response to all 36 thought-probes) and thus could not be included in analyses. Subjects were screened by phone and at an initial visit before the day of neuroimaging, where subjects were also trained on performing the gradCPT. Exclusion criteria were as follows: current mood, psychotic, anxiety (excluding simple phobias) or attention-deficit/hyperactivity disorder, current use of psychotropic medication, full-scale IQ less than 80, neurological disorders, sensorimotor handicaps, current alcohol or substance abuse/dependence, and claustrophobia.
MRI Acquisition.
Functional and anatomical MRIs were acquired on the 3T Siemens CONNECTOM scanner with a custom-made 64-channel phased array head coil (54), housed at the Athinoula A. Martinos Center for Biomedical Imaging. Subjects completed the following sequence of runs with short breaks separating each (lasting a total of 1.5–2 h): one multi-echo T1-weighted run, one resting-state fMRI run, one task fMRI (gradCPT) run followed by four task fMRI gradCPT runs with thought-probes, and four runs of diffusion MRI. In the present study, only the four gradCPT runs with thought-probes were included for analysis, and the T1-weighted run was used for anatomical coregistration. The T2*-weighted whole-brain fMRI runs were performed with multiband, echo-planar imaging (simultaneous multislice factor of 4) and the following parameters: repetition time (TR), 1.08 s; echo time (TE), 30 ms; flip angle, 60°; field of view (FoV), 110 mm2; 68 transverse slices; 2 mm isotropic voxels. The T1-weighted scan parameters were as follows: TR, 2,530 ms; TE, 1.15 ms; inversion time (TI), 1,100 ms; flip angle, 7°; FoV, 256 mm2; 1 mm isotropic voxels.
Task Paradigm and Presentation.
In four fMRI runs, participants performed the gradCPT (20, 26), modified here to include thought-probes. The following script was used during training on a computer (outside the scanner) to instruct participants in how to respond to the thought-probes:
“While you’re doing this task, you may find that you’re sometimes very focused but at other times your thoughts may have wandered elsewhere and you were distracted. So now we’ll do the same thing, except at some point, the task will be interrupted with 2 questions on the screen. The first question will ask you what you were just thinking of (immediately before the question came up), and you’ll use the right and left arrow keys to indicate on a continuous scale to what degree you were focused on the task or focused on something else. Use the left and right arrow keys to choose your response and hit the space bar to enter the response. The second question will ask you to what degree you were aware of what you were just thinking of. For example, sometimes you may notice that your mind wandered away from the task, but other times you may not have been aware until you were asked.”
Stimuli were round, continuously changing grayscale images of city and mountain scenes presented with the Psychophysics Toolbox (55) in Matlab on an Apple MacBook Pro connected to a projector. Subjects viewed stimuli within the scanner bore through a mirror back-projecting the images. Using linear pixel-by-pixel interpolation, scene images from a set of 10 city and mountain photographs, respectively, gradually transitioned between one another for the duration of the task. Each transition lasted ∼1.3 s on average. Scenes were presented randomly, but the same scene could not repeat on consecutive trials. Using an MRI-compatible button box, subjects were instructed to respond with a single press of their right index finger each time they saw a city scene but to withhold a response when they saw mountain scenes, which occurred pseudorandomly in ∼10% of trials.
Thought-probes appeared pseudorandomly every 44–60 s (three possible block durations of 44, 52, and 60 s). Rather than gradually transitioning into another scene image, the last scene before the thought-probe faded into a scrambled image (to give subjects a similar amount of time to respond as in other trials). Upon the thought-probe, a question was displayed: “To what degree was your focus just on the task or on something else?” A continuous scale appeared below the question text with far-right and far-left anchors of only task and only else, respectively. Subjects pressed buttons with their middle and ring fingers to move the scale left and right, respectively, and with their thumb to enter their response. Responses were recorded on a graded scale of integers (not visible to the subjects) ranging from 0 (only task) to 100 (only else). A second self-paced question screen about meta-awareness of task-related focus (“To what degree were you aware of where your focus was?”) appeared after the thought-probe, but responses for this second question were not included in the present analyses. The gradCPT immediately resumed after subjects entered their question responses (except for the last thought-probe in the run). Scanning was manually stopped after each gradCPT thought-probe run. Across all runs and all subjects, the mean number of volumes collected per run was 489.1 (i.e., 8 min, 48 s). The four first volumes were deleted before preprocessing and analyses.
Because brain activity before thought-probes could be influenced by rare mountain (target) events, we matched thought-probes for the number of mountains presented in the periods immediately preceding each probe. In the 12 s immediately preceding thought-probes, no targets were presented so that probe responses were minimally influenced by attentional capture due to these rare events. In the 20 s before the 12-s preprobe period (i.e., –12 to –32 s), the target rate was 8% (average of one mountain). Before –32 s prepreprobe, the target rate was 13% (number of mountains varying, depending on total block length).
Postscan Oral Interview.
After scanning was completed, a postscan oral interview was conducted using 1–7 Likert-type scales as done previously (11). Subjects were asked how confident they were that during thought-probes they were able to accurately assess whether their attention was on task or on something else (1, not confident at all; 7, extremely confident). Additionally, they were asked overall to what degree their reports of attention off-task were due to (a) external/sensory distractions (e.g., hearing, sensations within body), (b) task-related interferences, and (c) mind-wandering (e.g., thinking about what happened yesterday, plans for later, what other people you know might be doing now) (1, never; 7, always) (24). Subjects were also encouraged to give qualitative examples of the types of thoughts/distractions that led them to rate their attention as off-task.
RT Calculation.
The RTs were assigned relative to the beginning of each image transition (so an RT of ∼1.3 s would indicate a button press when an image was 100% coherent and not mixed with the previous or following image). For rare trials with highly deviant fast (before 70% image coherence for current trial’s scene) or slow (after 40% coherence of the following trial’s scene) RTs or multiple button presses, an iterative algorithm was used to calculate RT (20). First, for these rare trials, the algorithm assigned unambiguous correct responses. Second, ambiguous trials that could have indicated response to the previous or current trial were assigned to one of the two trials if one had no response. If both adjacent trials had no response, the RT was assigned to the trial closest in time (unless that trial was a mountain image, where we did not assign “error” button presses in these ambiguous situations). Finally, when multiple presses could be assigned to a given trial, the fastest RT was assigned to that trial.
Behavioral Data Analysis.
We assessed the link between block-by-block subjective task-focus ratings and behavior at both intra- and interindividual levels. The RT variance was calculated by subtracting the mean RT across all four gradCPT thought-probe runs from each RT value and then dividing by the RT SD across the same runs. Each RT variance value was then converted to an absolute value (thus assuming that both very slow and very fast presses were considered to be variable behavior). We present all intraindividual analyses based on these absolute RT variance values for consistency with previous work on DMN activity associated with RT variability. Those previous studies included versions of the gradCPT that were not interrupted with thought-probes, and it was advantageous to use the variance time course (VTC) as a method to assess continuous changes in RT variability time-locked to BOLD activity (19, 20). However, we repeated analyses here using RT CoV (a more common metric of variability) and obtained highly similar results (Table S3). The mean ± SD within-subject correlation of trial block-by-trial block RT variance vs. RT CoV across 30-s prethought periods (36 per subject) was r = 0.85 ± 0.09.
At the intraindividual level, across 36 total blocks, we performed our main analyses based on RTs in the ∼30-s preprobe period. However, we also present results based on RTs in ∼20-s and ∼10-s preprobe periods (Table S2). Within subjects, we calculated Pearson’s correlation of 36 thought-probe ratings with mean preprobe absolute RT variance as well as mean preprobe RT. We then converted these values from r to z using the Fisher transformation and submitted the z values to a Wilcoxon signed rank test (two-tailed) across subjects (significance set at P < 0.05) to test whether subjective thought-probe ratings were associated with RT behavior within subjects.
At the interindividual level, we calculated Pearson’s correlation coefficient of mean thought-probe rating vs. the following behavioral metrics: mean RT variability (SD) and mean RT in 30-s preprobe periods and commission error rate (i.e., percentage of mountain trials in which subjects incorrectly pressed a button). Additionally we correlated RT variability and mean RT with commission error rate. Significance was set at P < 0.05 (two-tailed).
fMRI Data Preprocessing.
Using FSL v5.0.7 (56), for each fMRI run we performed brain extraction (BET), realignment of each volume to the middle volume (MCFLIRT), and spatial smoothing (5-mm full-width at half-maximum kernel). We then submitted the data to ICA-AROMA, an automated tool for motion-artifact removal (57, 58). This involved running independent components analysis (ICA, with automatic dimensionality estimation using FSL’s MELODIC tool), identification of motion-relevant components, and regression of the motion-relevant components out of the data. To further reduce the impact of physiological and scanner-related noise, we then regressed out the mean time series within white matter (WM) and within cerebrospinal fluid (CSF) masks. These nongray matter masks were individually derived from automated segmentation using FSL’s FAST applied to the T1-weighted images. The WM and CSF masks were then linearly registered to fMRI space and thresholded to retain the top 198 cm3 and 20 cm3, respectively, consistent with previous work (11, 59). We finally applied a high-pass (0.01 Hz cutoff) temporal filter to remove intrinsic scanner-related low-frequency signal drift. The preprocessed data were then transformed to standard space using linear registration (FLIRT) to T1-weighted space (6 df) followed by linear registration to MNI152 space (12 df). Voxel intensity values were then converted to %SC (subtracted then divided by the mean within-run voxel intensity, then multiplied by 100).
fMRI Analysis.
We defined the DMN as voxels within cortical and cerebellar areas that are parts of a seven-network brain parcellation based on resting-state correlated activity from an independent, large sample of healthy adults (30, 31). We extracted the mean time course across all DMN voxels from all fMRI runs. For each pre–thought-probe period, we calculated the mean %SC. We focus on ∼30-s preprobe periods (see Behavioral Data Analysis), based on 28 TRs, but we also present results from ∼20-s and ∼10-s preprobe periods (19 TRs and 9 TRs, respectively) (see Table S2). We did not convolve brain activity with a hemodynamic response function because we wanted to avoid any possible contamination of the signal with activity evoked by thought-probe onsets as well as any assumptions about the time courses of self-reported attention. Within each subject, we calculated Pearson’s correlation of mean preprobe DMN %SC with mean off-task rating and mean preprobe RT variance (based on RT variance time-course values). After Fisher r-to-z transformation, we submitted within-subject correlation values to two-tailed Wilcoxon signed rank tests (separately for DMN–off-task and DMN–RT variance relationships) (significance set at P < 0.05).
The possible simultaneous contributions of off-task attention and behavioral variability to DMN activity was assessed with linear mixed-effects models implemented in R (60), with subjects as random effects, DMN %SC as the dependent variable, and within-subject normalized off-task rating, RT variance, and within-subject normalized mean RT (mean subtracted then divided by SD) in ∼30-s prerating periods as fixed effects. We tested five models with variations of these fixed effects as inputs: (a) off-task rating only; (b) RT variance only; (c) both off-task rating and RT variance; (d) off-task rating, RT variance, and their interaction; and (e) off-task rating, RT variance, and mean RT (significance set at P < 0.05). We also tested alternative models with RT variance replaced by RT CoV (normalized within subjects; see SI Results).
To assess whether DMN–off-task relationships were driven by individuals with off-task attention due to mind-wandering, we computed relative mind-wandering as the postscan mind-wandering rating divided by external distraction rating. We correlated relative mind-wandering vs. the within-subject Fisher z-transformed correlation between off-task rating and DMN %SC within the 10-s prerating period. Consistent with previous work (11), we used Spearman’s rank correlation with significance set at P < 0.05. Furthermore, to evaluate the temporal specificity of the correlation to the prerating period, we redid this correlation using a few single TRs (i.e., without averaging across TRs) before and after the thought-probe onset, up to and including the fourth postprobe TR. For some subjects, in the last thought-probe of a run, the scanner was manually stopped before collection of the third and/or fourth postprobe TR, so those thought-probes were excluded from the temporal specificity analysis (for 6 and 3 subjects, respectively, 34 and 35 thought-probes were used instead of 36).
fMRI Analysis: DMN Subregions.
Using the same methods as described above for assessing whole-DMN activity vs. within-subject variations in off-task ratings and RT variances (i.e., Wilcoxon signed rank test on Fisher z values, significance set at P < 0.05), we performed analyses within three DMN subsystems. The subsystems were defined using a 17-network cortical atlas defined based on intrinsic functional connectivity in healthy adults (30). These bilateral subsystems were (a) core DMN (including posterior cingulate cortex/precuneus, medial prefrontal cortex, and posterior temporoparietal areas), (b) dorsomedial prefrontal subsystem (including dmPFC, anterior temporal lobe, and temporoparietal areas), and (c) medial temporal lobe subsystem (including medial temporal lobe, retrosplenial cortex, and lateral parietal–occipital areas).
We used a more fine-grained, newly developed, cortical functional atlas of 333 regions (61) to account for the possibility that the DMN includes further subdivisions. We multiplied this atlas by the binary mask of the whole-DMN gray matter mask used in the main analyses described above. We then thresholded the overlay between the two atlases such that only regions containing 50 or more voxels were retained (to remove regions that were likely to be at borders with other networks), resulting in 53 distinct DMN regions. We added the cerebellar DMN as an additional region, so the total number of DMN regions was 54. In each DMN region, we correlated %SC vs. normalized off-task rating and %SC vs. RT variance across all subjects and all trial blocks. We then performed Pearson’s correlation, across the 54 regions, on the correlation of %SC with off-task rating vs. the correlation of %SC vs. RT variance (significance set at P < 0.05).
fMRI Analysis: Other Networks.
Using the same methods as described above for assessing whole-DMN activity vs. within-subject variations in off-task ratings and RT variances (i.e., Wilcoxon signed rank test on Fisher z values, significance set at P < 0.05), we performed analyses within four distinct networks using the seven-network gray matter version of the atlas described in other analyses (30). These bilateral networks were (a) the salience network (including dorsal anterior insula, midcingulate cortex, and anterior temporoparietal junction), (b) the dorsal attention network (including inferior parietal lobule, frontal eye fields, and ventral temporal–occipital areas), (c) the sensorimotor network (including primary motor areas, primary somatosensory areas, and posterior insula), and (d) the frontoparietal control network (including dorsolateral prefrontal cortex, superior parietal lobule, and inferior temporal gyrus).
SI Results
RT CoV, Off-Task Rating, and DMN Activity.
We repeated our tests of all linear mixed-effects models described in the main text with RT variance replaced by RT CoV as a metric for behavioral variability. A model including RT CoV as the only fixed effect explaining DMN activity was significant (β = –0.12, t = –3.9, P = 0.0002). Furthermore, including both off-task (β1 = 0.14, t = 4.3, P = 0.0002) and RT CoV (β2 = –0.15, t = –4.5, P = 3.3 × 10−5) suggested additive, independent variance explaining DMN activity (R2 for combined fixed effects = 0.033 compared with R2 = 0.015 for RT CoV alone), consistent with results based on RT variance. There was no significant interaction between off-task rating and RT CoV (P = 0.21) explaining DMN activity.
Mean RT as a Factor Explaining DMN Activity.
Notably, although we report in the main text that mean pre–thought-probe RT was not significantly associated with self-reported off-task attention or with RT variance, it remained possible that fluctuations of mean RT could relate to DMN activity. Thus, in a supplementary analysis, we tested a linear mixed effects model that included mean RT as an additional fixed effect. Interestingly, over and above the effects of off-task rating (β1 = 0.12, t = 3.8, P = 0.0004) and RT variance (β2 = –0.15, t = –4.4, P = 3.9 × 10−5), mean RT accounted for a small but significant (β3 = –0.08, t = –2.1, P = 0.04) amount of additional variance in DMN signal (R2 for combined fixed effects = 0.037, compared with R2 = 0.031 when not including mean RT). This effect, indicating that slower RTs are reflected in lower DMN activity (or increased DMN suppression), is consistent with previous work assessing RT speed alone, potentially reflecting a time-on-task effect (62). Importantly, DMN relationships with RT variability and off-task ratings were reliable even when accounting for mean RT, perhaps not surprisingly given the nonsignificant relationships of mean RT with these metrics of attentional state.
Time Dependence of DMN–Mind-Wandering Relationship.
To illustrate the temporal specificity of the positive relationship between interindividual differences in relative mind-wandering and DMN activity (shown in Fig. 3), we repeated the analysis using activity from single whole-brain volumes just before and just after the onset of thought-probes. As the hemodynamic brain response to thought-probe onset would be expected to begin quickly (and then peak a few seconds later) (37), we expected a gradual decline of the relationship after thought-probe onset. Indeed, using any single-brain volume in the seconds leading up to the thought-probe onset, the correlation with relative mind-wandering was positive, but immediately after thought-probe onset, the correlation gradually reduced to values near zero (Fig. S3). The relationship between relative mind-wandering and DMN activity was strongest in the time window immediately before thought-probe onset (∼5–6 s) relative to the few seconds prior (∼6–7 s and further back). These results suggest temporal specificity of the relationship between DMN activity and mind-wandering to the period that participants referred to when they were evaluating their attentional state.
Generalizability Across DMN Subregions.
To break the DMN down into fine-grained possible subdivisions, we used a cortical functional atlas consisting of an optimal set of regions that show strong within-region homogeneity of intrinsic functional connectivity profile with the rest of the brain (61). This resulted in 54 total DMN regions (53 cortical regions from the functional atlas that mapped to the DMN, plus the cerebellar component of the DMN). For each DMN subregion, across all trial blocks and all subjects, we correlated both off-task rating and RT variance with prerating activity level. Across the 54 DMN subregions, there was a negative correlation between the degree to which subregions had activity that correlated with off-task rating vs. activity that correlated with behavioral variability (r = –0.34, P = 0.01) (Fig. S4). Taken together, these results suggest that the DMN subsystems and subregions that relate most strongly (and positively) to off-task attention are similar to those that relate most strongly (and negatively) to behavioral variability.
Discussion
Here we present a unique account of the behavioral relevance of spontaneous DMN activity. We found that simultaneous consideration of both self-reported attentional fluctuations and behavioral variability provides a better explanation of DMN activity than either factor does alone. Spontaneous DMN activity was greatest during intense mind-wandering coupled with stable behavior, even though mind-wandering was associated with greater behavioral variability. These results were consistent across DMN subsystems and subregions. Furthermore, activity of the salience, dorsal attention, frontoparietal control, and sensorimotor networks showed opposite or no such relationships, suggesting specificity of DMN function in mind-wandering and behavioral variability. Our results reveal that the cognitive processes reflected by spontaneous DMN activity are only partially related to mind-wandering and include also attentional state fluctuations that are not captured by self-report.
Critically, our findings challenge the notion that increased spontaneous DMN activity predominantly reflects internal mentation. Moreover, our findings can be used to inform new possible hypotheses of DMN function that may not have been realized from studies of mind-wandering or behavioral variability alone. One possible hypothesis is that spontaneous DMN activity is associated with both mind-wandering and stable behavior, each independently, because of separate neurophysiological processes. Increased BOLD activations are associated with local field potentials and broadband gamma-wave activity (37, 38), whereas the basis of BOLD deactivations is complex and less well understood (39, 40). Mind-wandering may be time-locked to DMN activation, as supported by findings indicating that autobiographical memory processes (typically engaged during mind-wandering) are associated with increased broadband gamma activity (41). Conversely, stable behavior may be reflected in reduced DMN deactivation. Spontaneous states of variable behavior could be associated with DMN deactivation due to transient “perceived” increases in cognitive demand, consistent with observed activation in dorsal attention and salience networks. Thus, our findings here may be explained by additive effects of increased DMN activation (mind-wandering) and reduced DMN deactivation (behavioral stability).
Our findings speak to the significance of the “baseline” activity often studied in neuroimaging experiments, typically a wakeful resting state (39). During rest, attention may fluctuate among states comparable to those characterized here. In aging populations exhibiting cognitive decline or deficits (e.g., in Alzheimer’s disease) (4), a common finding is baseline-level DMN hypometabolism while patients are at rest. Such populations also exhibit increased behavioral variability (42) and decreased self-reported mind-wandering (43), both of which were associated with decreased DMN activity in our study, thus providing conceivable behavioral correlates of low baseline DMN activity with cognitive decline. Another common finding in such populations is reduced DMN deactivation (relative to baseline) during tasks requiring active cognitive control (15). Whereas increased cognitive demand typically results in greater DMN deactivation in healthy individuals (44), a testable hypothesis is that this deactivation is suppressed in populations with a high propensity for behavioral variability and decreased (or distorted) mind-wandering because a chronic baseline state of low DMN activity could decrease the range of deactivation responsiveness to cognitive demand (45) (see also ref. 16).
Notably, our findings do not rule out the possibility that DMN fluctuations reflect an overarching function that is indicated by both stable behavior and mind-wandering. For example, both factors may be relevant to memory consolidation and/or retrieval. Simultaneous electrophysiology with fMRI suggests that spontaneous hippocampal ripples are followed by selective activation of the DMN (46). Hippocampal ripples after learning have been shown to be predictive of subsequent memory performance (47). States of mind-wandering often involve rehearsal of learned information to prepare for the future. Although a link between stable behavior and memory remains speculative, states of stable behavior could be associated with a readiness to consolidate information.
A common feature across many contexts is that the DMN is activated when attention is likely focused away from the immediate sensory environment and toward internally oriented thoughts (15). We confirm here that DMN activity is increased with greater mind-wandering intensity. However, the finding that stable behavior is associated with increased DMN activity, over and above the association with mind-wandering, is not easily reconcilable with an exclusive role of spontaneous DMN activity in internally focused attention. Consistent with previous studies (22–24, 29), we show that variable, rather than stable, behavior is associated with self-reported off-task attention both at intra- and interindividual levels. It remains possible that stable behavior reflects “in the zone” periods where task performance is high and attentional resources are available for internal mentation that is not reportable because subjects are not aware of their attentional state.
Conversely, stable behavior and associated DMN activity may reflect an aspect of externally oriented attention. Several lines of evidence point toward a nonexclusive role of the DMN in internally oriented attention. First, as in all other brain networks, DMN activity does not stop fluctuating during loss of consciousness (48). Second, intracranial electrophysiology studies of DMN areas have shown increased activity immediately at the offset of task performance, perhaps too rapidly to reflect mind-wandering (49). Third, fMRI studies with unique task paradigms suggest that under certain contexts, increased DMN activity occurs during cognitive processes that may involve aspects of externally oriented attention (17, 18, 50). Finally, studies of spontaneous prestimulus activity suggest nuanced relationships of DMN activity with attentional performance (51, 52). Thus, roles in intrinsic function, internally oriented attention, and externally oriented attention may need to be reconciled to provide a full account of DMN function.
Although our results shed light on the functional significance of fluctuations in DMN activation/deactivation, further work is needed to uncover the behavioral relevance of dynamic communication within the DMN and with other networks. Despite the relationships with activity presented here, mind-wandering and stable behavior could each have unique relationships with functional network connectivity. Connectivity of the DMN is highly relevant to attentional fluctuations (12, 21, 34). Simultaneous consideration of both self-reported mind-wandering and behavior could yield insights into the significance of time-varying network dynamics.
Virtually every known brain disorder has been associated with altered DMN (de)activation and/or intrinsic DMN functional connectivity (15, 53). Often the behavioral significance of these DMN disruptions is inferred from what is known about the role of a healthy DMN in cognition. Although our results confirm that the DMN is engaged during mind-wandering, they also may point toward a more fundamental function in cognition that should be considered in healthy as well as clinical populations. Further research into the relationship between the DMN and cognition in the healthy brain is thus much needed so that a better understanding of DMN dysfunction in disease can be achieved.
Methods
Twenty-eight healthy, right-handed adults (13 males, 15 females; mean age ± SD = 26.2 ± 3.8) were included for all final analyses. Participants provided written informed consent for procedures approved by the Partners Human Research Institutional Review Board. Subjects were trained to perform the gradCPT on an initial visit and then returned on another day to complete neuroimaging on the 3T Siemens CONNECTOM scanner with 64-channel head coil. In each of four fMRI runs, the gradCPT was presented with nine self-paced thought-probes (see SI Methods). Procedures for behavioral and neuroimaging data preprocessing and analyses are detailed in SI Methods.
Acknowledgments
We thank Jonathan Smallwood and Michael Hove for study design advising, Aya Hamadeh for data collection, and Francesca Fortenbaugh, David Rothlein, and Joke Durnez for analysis support. This work was performed at Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital. This work was supported by a Canadian Institutes of Health Research fellowship award (to A.K.), NIH Grant R01 HD067744-01A1 (to E.M.V.), the Athinoula A. Martinos Center for Biomedical Imaging, and National Center for Research Resources Grants P41RR14075 and P41 EB015896. M.E. was supported by a Veterans Affairs Clinical Science R&D Career Development Award 1IK2CX000706-01A2.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611743113/-/DCSupplemental.
References
- 1.Shulman GL, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 2.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132(6):946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- 4.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 5.Christoff K, Irving ZC, Fox KC, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: A dynamic framework. Nat Rev Neurosci. 2016;17(11):718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- 6.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 7.Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010;104(1):322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: Both task-unrelatedness and stimulus-independence are related to default network activity. PLoS One. 2011;6(2):e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA. 2013;110(46):18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittner M, et al. When the brain takes a break: A model-based analysis of mind wandering. J Neurosci. 2014;34(49):16286–16295. doi: 10.1523/JNEUROSCI.2062-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poldrack RA. Inferring mental states from neuroimaging data: From reverse inference to large-scale decoding. Neuron. 2011;72(5):692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 15.Anticevic A, et al. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maillet D, Schacter DL. Default network and aging: Beyond the task-negative perspective. Trends Cogn Sci. 2016;20(9):646–648. doi: 10.1016/j.tics.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crittenden BM, Mitchell DJ, Duncan J. Recruitment of the default mode network during a demanding act of executive control. eLife. 2015;4:e06481. doi: 10.7554/eLife.06481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simony E, et al. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat Commun. 2016;7:12141. doi: 10.1038/ncomms12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esterman M, Rosenberg MD, Noonan SK. Intrinsic fluctuations in sustained attention and distractor processing. J Neurosci. 2014;34(5):1724–1730. doi: 10.1523/JNEUROSCI.2658-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esterman M, Noonan SK, Rosenberg M, Degutis J. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb Cortex. 2013;23(11):2712–2723. doi: 10.1093/cercor/bhs261. [DOI] [PubMed] [Google Scholar]
- 21.Kucyi A, Hove MJ, Esterman M, Hutchison RM, Valera EM. Dynamic brain network correlates of spontaneous fluctuations in attention. Cereb Cortex. 2016:bhw029. doi: 10.1093/cercor/bhw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McVay JC, Kane MJ. Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. J Exp Psychol Learn Mem Cogn. 2009;35(1):196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastian M, Sackur J. Mind wandering at the fingertips: Automatic parsing of subjective states based on response time variability. Front Psychol. 2013;4:573. doi: 10.3389/fpsyg.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stawarczyk D, Majerus S, Maj M, Van der Linden M, D’Argembeau A. Mind-wandering: Phenomenology and function as assessed with a novel experience sampling method. Acta Psychol (Amst) 2011;136(3):370–381. doi: 10.1016/j.actpsy.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg M, Noonan S, DeGutis J, Esterman M. Sustaining visual attention in the face of distraction: A novel gradual-onset continuous performance task. Atten Percept Psychophys. 2013;75(3):426–439. doi: 10.3758/s13414-012-0413-x. [DOI] [PubMed] [Google Scholar]
- 27.Smallwood J, McSpadden M, Luus B, Schooler J. Segmenting the stream of consciousness: The psychological correlates of temporal structures in the time series data of a continuous performance task. Brain Cogn. 2008;66(1):50–56. doi: 10.1016/j.bandc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 28.McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage. 2006;29(4):1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seli P, Cheyne JA, Smilek D. Wandering minds and wavering rhythms: Linking mind wandering and behavioral variability. J Exp Psychol Hum Percept Perform. 2013;39(1):1–5. doi: 10.1037/a0030954. [DOI] [PubMed] [Google Scholar]
- 30.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 33.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kucyi A, Davis KD. Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage. 2014;100:471–480. doi: 10.1016/j.neuroimage.2014.06.044. [DOI] [PubMed] [Google Scholar]
- 35.Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 36.Stawarczyk D, D’Argembeau A. Neural correlates of personal goal processing during episodic future thinking and mind-wandering: An ALE meta-analysis. Hum Brain Mapp. 2015;36(8):2928–2947. doi: 10.1002/hbm.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002;3(2):142–151. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- 38.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 39.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 40.Ramot M, et al. A widely distributed spectral signature of task-negative electrocorticography responses revealed during a visuomotor task in the human cortex. J Neurosci. 2012;32(31):10458–10469. doi: 10.1523/JNEUROSCI.0877-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster BL, Dastjerdi M, Parvizi J. Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing. Proc Natl Acad Sci USA. 2012;109(38):15514–15519. doi: 10.1073/pnas.1206580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57(2):101–115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- 43.Maillet D, Schacter DL. From mind wandering to involuntary retrieval: Age-related differences in spontaneous cognitive processes. Neuropsychologia. 2016;80:142–156. doi: 10.1016/j.neuropsychologia.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Čeko M, et al. Is a responsive default mode network required for successful working memory task performance? J Neurosci. 2015;35(33):11595–11605. doi: 10.1523/JNEUROSCI.0264-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner GR, Spreng RN. Prefrontal engagement and reduced default network suppression co-occur and are dynamically coupled in older adults: The default-executive coupling hypothesis of aging. J Cogn Neurosci. 2015;27(12):2462–2476. doi: 10.1162/jocn_a_00869. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan R, et al. Hippocampal sharp-wave ripples influence selective activation of the default mode network. Curr Biol. 2016;26(5):686–691. doi: 10.1016/j.cub.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131(Pt 7):1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 48.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 49.Dastjerdi M, et al. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc Natl Acad Sci USA. 2011;108(7):3023–3028. doi: 10.1073/pnas.1017098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spreng RN, et al. Goal-congruent default network activity facilitates cognitive control. J Neurosci. 2014;34(42):14108–14114. doi: 10.1523/JNEUROSCI.2815-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29(42):13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sali AW, Courtney SM, Yantis S. Spontaneous fluctuations in the flexible control of covert attention. J Neurosci. 2016;36(2):445–454. doi: 10.1523/JNEUROSCI.2323-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 54.Keil B, et al. A 64-channel 3T array coil for accelerated brain MRI. Magn Reson Med. 2013;70(1):248–258. doi: 10.1002/mrm.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 56.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Pruim RH, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- 58.Pruim RH, et al. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 59.Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59(2):1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 61.Gordon EM, et al. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26(1):288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Decoupling of reaction time-related default mode network activity with cognitive demand. Brain Imaging Behav. March 22, 2016 doi: 10.1007/s11682-016-9543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]