The 1918 “Spanish” influenza pandemic holds a particular place in medical history; it wiped out an estimated ∼1% of the global population, or ∼20–50 million deaths worldwide, and earned the dubious honor of being coined the “mother of all pandemics” (1). Fortunately, important strides have been made in elucidating the emergence of novel pandemic influenza viruses and their impact on human populations since the 1918 pandemic. The field of archeo-epidemiology has been particularly active in recent years, thanks to coordinated interdisciplinary efforts involving epidemiologists, virologists, demographers, and medical historians. Their work has paid off to unearth, digitize, and analyze archival disease records sitting in dusty old books, providing a careful description of the mortality and transmissibility patterns of the 1918 influenza pandemic in several areas of the world where little prior quantitative information existed (e.g., refs. 2–5). In PNAS, Grantz et al. (6) contribute to the growing interest in applying modern analytical tools to epidemiological archives, through the lens of socioeconomic disparities affecting the 1918 influenza pandemic.
The role of socioeconomic factors on influenza mortality and morbidity goes back to the early work of Robert Pearl in the years immediately following the 1918 pandemic (7), but has only regained traction among epidemiologists and sociologists in the last decade. In 2006, a seminal study revealed more than 30-fold variation in 1918 pandemic excess mortality rates across a sample of 20 countries, with socioeconomic factors explaining a significant fraction of the observed variation (2). In Latin America, pandemic excess mortality rates varied from 0.4 to 2.9% in national and province-level data, a greater than sevenfold variation (4, 8, 9). In contrast, United States and European populations fared relatively well during the pandemic, despite intense disruption at the end of World War I (excess mortality rates of 0.5–1.1%) (3, 5).
Such geographic disparities in 1918 pandemic death rates remain puzzling, particularly as pandemic viruses are transmitted by direct contact and aerosols in large swaths of naïve populations, akin to a “universal disease” little affected by sanitation or background health. Further, in 1918 the arsenal available to treat primary influenza infection—and their frequent corollary, secondary bacterial infections—was rudimentary and limited to basic supportive care. Population-level interventions to control the spread of the disease were equally scarce, involving school closures and cancellation of large gatherings. Hence, the role of socioeconomic disparities on influenza mortality has remained a subject of debate in the literature, and is often confounded by the timing of arrival of the pandemic virus in a given locale, climatic conditions, or population density. For example, population density and background death rates explained 70% of the variation in cumulative pandemic excess mortality rates in province-level data from Chile (10). In another study, latitude, population density, and the proportion of children explained about 40% of between-province variation in cumulative excess death rates in Spain (11). Very little, however, has been done to understand the drivers of pandemic-related mortality rates at the fine scale of households or census tracts, an issue tackled by Grantz et al. (6).
Grantz et al. (6) painstakingly pieced together historical maps of pneumonia and influenza deaths reported during the lethal wave of the pandemic in Chicago in October–November 1918, together with archival census tract data, to analyze the relationship between pandemic mortality and sociodemographic variables (including illiteracy rate, homeownership, unemployment, population density, and age). Pneumonia and influenza mortality rates were found to increase on average by 32% for every 10% increase in illiteracy rates, after controlling for other sociodemographic variables that had a lesser effect. The findings align with contemporary studies demonstrating how limited literacy and educational achievement hamper access to preventive services (12). The exact mechanisms underlying Grantz et al.’s (6) findings remain unclear however, as the influenza virus does not discriminate between patients who can read or not. Instead, illiteracy rates and other socio-economic variables could be a proxy for the effect of poor nutritional status, weak immune condition, increased risk of secondary infection, or limited access to care on the risk of severe influenza outcomes.
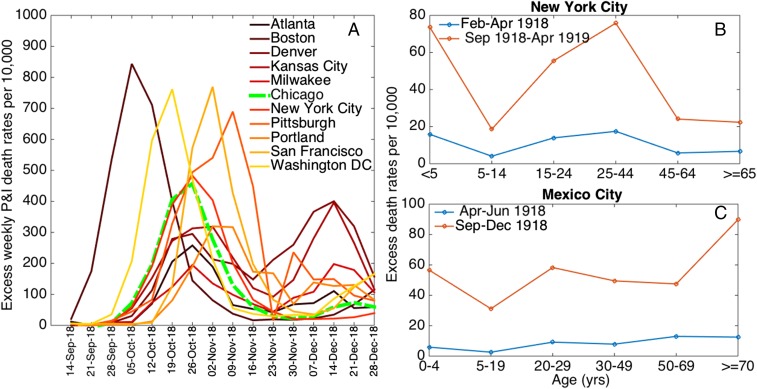
The atypical age patterns of influenza-related deaths remains a long-lasting mystery of the 1918 pandemic, which sets it apart from any other influenza outbreak. An unusual peak of death was seen in young adults aged ∼28 y in all populations that have been studied thus far (5), whereas individuals aged 45 y and over were protected against mortality in much of the United States and Europe (Fig. 1) (3, 5, 13). Whether the relationship between illiteracy and influenza-related mortality evidenced by Grantz et al. (6) holds across age groups whose experience with the 1918 pandemic virus differed profoundly remains a crucial question. The authors note the role of population size over 45 y of age as a predictor of mortality rates in multivariate analyses, and the potential for residual confounding between the demographic and socioeconomic structure of Chicago’s neighborhoods at the time.
Fig. 1.
(A) Excess weekly pneumonia and influenza (P&I) death rates per 10,000 for a few representative large cities in the United States (20), and (B and C) age-specific excess-death rates per 10,000 population associated with the 1918–1919 pandemic waves in New York City (based on all-cause deaths) (3) and Mexico City (based on pneumonia and influenza deaths) (4).
An important issue in quantifying influenza-related death rates is one of specificity. Although the full mortality burden of influenza cannot be captured by analysis of deaths ascribed to influenza alone, because of the importance of secondary bacterial infections, analysis of aggregated pneumonia and influenza outcomes requires caution. Excess mortality models are convenient approaches for subtracting seasonal background noise and remove the many pneumonia deaths that occur year-round and are not a result of influenza. If senior age groups die frequently of noninfluenza causes, but happen to be protected from influenza infection, as was reported during the 1918 pandemic, use of “raw” pneumonia and influenza deaths as in Grantz et al. (6) could considerably dilute the influenza-specific signal. Indeed, socioeconomic factors predicted mortality rates for a number of other infections in 1918 Chicago, as well as all-cause mortality in prepandemic years. Taken together with the role of the over 45-y-old population size in their analysis (an age group typically protected against 1918 influenza), these are telltale signs that the relationship with socio-economic factors may be partly nonspecific of influenza. Analyses restricted to age groups who have low background mortality rates, such as young adults, or use of excess mortality approaches, would alleviate this issue. Unfortunately, long-time series data of the type needed for careful excess mortality approaches are rarely available in historical populations. Further sensitivity analyses could also concentrate on periods when influenza does not circulate, such as summer months or the year before the pandemic, to test the specificity of the reported association.
Another complicating feature of the 1918 pandemic is the occurrence of a mild “herald” wave during May–July of 1918 in North America and Europe, several months before the onslaught of the fall wave, as attested by a number of epidemiological studies (3, 13, 14). In parallel, virological presence of the pandemic A/H1N1 virus has been confirmed in archival samples of soldiers who died in May 1918 in the United States (15). Here, Grantz et al. (6) focus on the fall wave of the pandemic, relying on simulation studies to show that their findings were largely unaffected by a putative herald wave, particularly if the transmissibility of the herald wave virus was low. It is worth noting that influenza transmissibility and mortality estimates were low in the fall of 1918 in Chicago (Fig. 1) (6), relative to previous estimates (8), pointing to the possibility of a large fraction of the susceptible population having been depleted by an earlier wave. Analysis of incidence data would help assess the role of spring wave and dissect the effects of socioeconomic disparities on the risk of influenza infection vs. the severity of infection. Availability of incidence data is scarce in historical population, but this would be an important area for future work addressing the biological mechanisms at play.
Interestingly, Grantz et al. (6) find a tight spatial kernel for influenza dissemination in Chicago, with distance signature declining up to 200 m. To our knowledge, theirs is the first point-source study of influenza diffusion; other contemporary studies at coarser spatial scales have shown much broader kernels in the order of several hundred kilometers, in line with the scale of modern work commutes or air travel (e.g., ref. 16). Whether the intracity diffusion of influenza is still tightly constrained at 200 m in our well-connected societies as it was in 1918 Chicago remains unclear. Deep and geographically comprehensive surveillance records now available in the Big Data Era (17) would offer a fruitful testbed to revisit this question in the context of recent outbreaks.
In conclusion, Grantz et al. (6) should be commended for their spatially refined study on the effect of socioeconomic disparities on influenza mortality, an issue that has attracted attention in the context of old and new pandemics (18, 19). The jury is still out as to the relative contribution of socioeconomic status, however, on balance with other disparities, such as prior immunity or regional factors (18). As we approach the 100th anniversary of the 1918 pandemic, the fascination with this ferocious infectious disease event does not abate, and it is refreshing to see there is yet much to be learned from old treasure troves of data. Moving forward, we will need a clearer picture of the exact mechanisms driving the risk of influenza-related mortality, which are often proxied by broad socioeconomic indicators. Once the immunologic, demographic, economic, and behavioral drivers of influenza mortality are pinned down, better projections of pandemic disease burden on local, national, and global scales will be achieved.
Acknowledgments
The authors received support from the Multinational Influenza Seasonal Mortality Study, an on-going international collaborative effort to understand influenza epidemiological and evolutionary patterns, led by the Fogarty International Center, National Institutes of Health (misms.net/index.php).
Footnotes
The authors declare no conflict of interest.
See companion article on page 13839.
References
- 1.Morens DM, Fauci AS. The 1918 influenza pandemic: Insights for the 21st century. J Infect Dis. 2007;195(7):1018–1028. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918-20 pandemic: A quantitative analysis. Lancet. 2006;368(9554):2211–2218. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 3.Olson DR, Simonsen L, Edelson PJ, Morse SS. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci USA. 2005;102(31):11059–11063. doi: 10.1073/pnas.0408290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowell G, Viboud C, Simonsen L, Miller MA, Acuna-Soto R. Mortality patterns associated with the 1918 influenza pandemic in Mexico: Evidence for a spring herald wave and lack of preexisting immunity in older populations. J Infect Dis. 2010;202(4):567–575. doi: 10.1086/654897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viboud C, et al. Age- and sex-specific mortality associated with the 1918-1919 influenza pandemic in Kentucky. J Infect Dis. 2013;207(5):721–729. doi: 10.1093/infdis/jis745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grantz KH, et al. Disparities in influenza mortality and transmission related to sociodemographic factors within Chicago in the pandemic of 1918. Proc Natl Acad Sci USA. 2016;113:13839–13844. doi: 10.1073/pnas.1612838113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearl R. Influenza studies: Further data on the correlation of explosiveness of outbreak of the 1918 epidemic. Public Health Rep. 1921;36:273–298. [Google Scholar]
- 8.Chowell G, et al. The 1918-19 influenza pandemic in Boyacá, Colombia. Emerg Infect Dis. 2012;18(1):48–56. doi: 10.3201/eid1801.101969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowell G, et al. The 1918-1920 influenza pandemic in Peru. Vaccine. 2011;29(Suppl 2):B21–B26. doi: 10.1016/j.vaccine.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowell G, Simonsen L, Flores J, Miller MA, Viboud C. Death patterns during the 1918 influenza pandemic in Chile. Emerg Infect Dis. 2014;20(11):1803–1811. doi: 10.3201/eid2011.130632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowell G, Erkoreka A, Viboud C, Echeverri-Davila B. Spatial-temporal excess mortality patterns of the 1918-1919 influenza pandemic in Spain. BMC Infect Dis. 2014;14:371. doi: 10.1186/1471-2334-14-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinberg I, et al. Examining associations between health information seeking behavior and adult education status in the U.S.: An analysis of the 2012 PIAAC Data. PLoS One. 2016;11(2):e0148751. doi: 10.1371/journal.pone.0148751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: Implications for pandemic control strategies. J Infect Dis. 2008;197(2):270–278. doi: 10.1086/524065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammon CE. Spanish flu epidemic in 1918 in Geneva, Switzerland. Euro Surveill. 2002;7(12):190–192. doi: 10.2807/esm.07.12.00391-en. [DOI] [PubMed] [Google Scholar]
- 15.Sheng Z-M, et al. Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci USA. 2011;108(39):16416–16421. doi: 10.1073/pnas.1111179108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viboud C, et al. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science. 2006;312(5772):447–451. doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
- 17. Simonsen L, Gog JR, Olson DR, Viboud C, Infectious Disease Surveillance in the “Big Data” era: Towards faster, locally-relevant and more accurate systems. J Infect Dis, in press. [DOI] [PMC free article] [PubMed]
- 18.Simonsen L, et al. GLaMOR Collaborating Teams Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: A modeling study. PLoS Med. 2013;10(11):e1001558. doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viboud C, et al. Global mortality impact of the 1957-1959 influenza pandemic. J Infect Dis. 2016;213(5):738–745. doi: 10.1093/infdis/jiv534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins S, Frost WH, Gover M, Sydenstricker E. Mortality from Influenza and Pneumonia in 50 Largest Cities of the United States 1910-1929. Public Health Rep. 1930;45(39):2277–2328. [Google Scholar]