Significance
Over the past few decades, natural products, or chemical compounds derived from plants, animals, or microbes have greatly inspired drug discovery. Because natural products often have more complex and architecturally unique scaffolds than available man-made drugs, characterization of natural product biosynthetic pathways often reveals unprecedented chemistry and enzymatic platforms. Oxetanocin-A (OXT) is a natural product nucleoside analog that has an unusual four-membered oxetane ring connected to an adenine base. Prior to this work, there were no details available about OXT biosynthesis. Here, we not only elucidate a scheme for the two-enzyme catalyzed production of OXT, but also reveal modifications to an HD domain phosphohydrolase enzyme scaffold that expand the catalytic repertoire of this enzyme superfamily.
Keywords: X-ray crystallography, phosphohydrolase, metalloenzymes, natural products, nucleosides
Abstract
HD domain phosphohydrolase enzymes are characterized by a conserved set of histidine and aspartate residues that coordinate an active site metallocenter. Despite the important roles these enzymes play in nucleotide metabolism and signal transduction, few have been both biochemically and structurally characterized. Here, we present X-ray crystal structures and biochemical characterization of the Bacillus megaterium HD domain phosphohydrolase OxsA, involved in the biosynthesis of the antitumor, antiviral, and antibacterial compound oxetanocin-A. These studies reveal a previously uncharacterized reaction for this family; OxsA catalyzes the conversion of a triphosphorylated compound into a nucleoside, releasing one molecule of inorganic phosphate at a time. Remarkably, this functionality is a result of the OxsA active site, which based on structural and kinetic analyses has been tailored to bind the small, four-membered ring of oxetanocin-A over larger substrates. Furthermore, our OxsA structures show an active site that switches from a dinuclear to a mononuclear metal center as phosphates are eliminated from substrate.
Oxetanocin-A (OXT) is a nucleoside analog produced by Bacillus megaterium NK84-0128 (1) that contains an unusual four-membered oxetane ring connected to an adenine base through an N-glycosidic linkage (2). Clinical interest in OXT stems from the observed activity against hepatitis B virus (3), herpes simplex virus (1), HIV (4), and human cytomegalovirus (5). This antimicrobial and antiviral activity may be a consequence of the reported inhibition of cellular and viral DNA polymerases by triphosphorylated OXT compounds (6). Four genes located within the BglII-D fragment (6.8 kb) of the pOXT1 plasmid isolated from the producing strain have been hypothesized to encode proteins involved in OXT production (OxsA and OxsB) and OXT resistance (OxrA and OxrB) (7).
OxsA is a member of the HD domain superfamily of enzymes. This superfamily is divided into classes of enzymes that use a His-Asp doublet of residues and an additional series of conserved His and Asp residues to coordinate a single divalent metal (HXnHDXnD motif) (8, 9), a dinuclear metal active site (HXnHDXnHXnHXnD motif) (10, 11), or a recently described trinuclear iron metal-center (12). Although in many cases the identity of the relevant catalytic metal required for chemistry in vivo is unclear, there are examples of mononuclear enzymes that can use magnesium (13), cobalt (14), or manganese (8, 13, 15), and dinuclear enzymes that can use nickel (16), manganese (17), or iron (10, 18–20).
Most of the biochemically characterized HD domain enzymes catalyze phosphoester bond hydrolysis and are annotated as phosphohydrolases (8, 9). Their reactions are reasonably diverse and include hydrolysis of a phosphate from 2′-deoxyadenosine monophosphate (dAMP) by the Escherichia coli enzyme YfbR (14), hydrolysis of pyrophosphate from (p)ppGpp (13) or a deoxyribonucleoside triphosphate (15), and hydrolysis of inorganic triphosphate from deoxyadenosine triphosphate (dATP) (21, 22). These enzymes can also cleave the phosphodiester bond of cyclic-di-GMP (11, 23). Finally, two HD domain enzymes, myo-inositol oxygenase and PhnZ, are not hydrolases at all. They bind mixed-valent Fe2+/Fe3+ metal centers and operate as oxygenases, indicating the chemical diversity possible with the HD domain fold (10, 18, 20, 24–26).
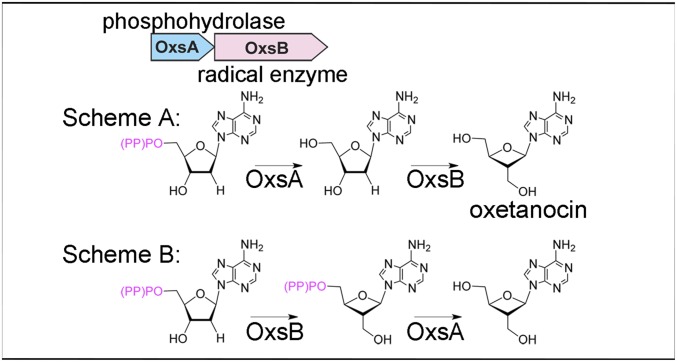
Here we investigate two plausible mechanistic proposals for the biosynthesis of OXT through the biochemical and structural characterization of OxsA (Fig. 1). In the first proposal, OxsA catalyzes the removal of one or multiple phosphates from a phosphorylated 2′-deoxyadenosine derivative, and OxsB, a cobalamin-dependent S-adenosylmethionine (AdoMet) radical enzyme, catalyzes a radical rearrangement to form the oxetane ring of OXT. In the second proposal, OxsB catalyzes ring contraction first, and OxsA works second to hydrolyze one or multiple phosphates from the phosphorylated OXT product.
Fig. 1.
Proposed schemes for OXT biosynthesis using the proteins encoded by the oxsA and oxsB production genes, which are annotated as an HD domain phosphohydrolase and a cobalamin-dependent AdoMet radical enzyme, respectively.
Results
OxsA Has a Mononuclear HD Domain Phosphohydrolase Fold.
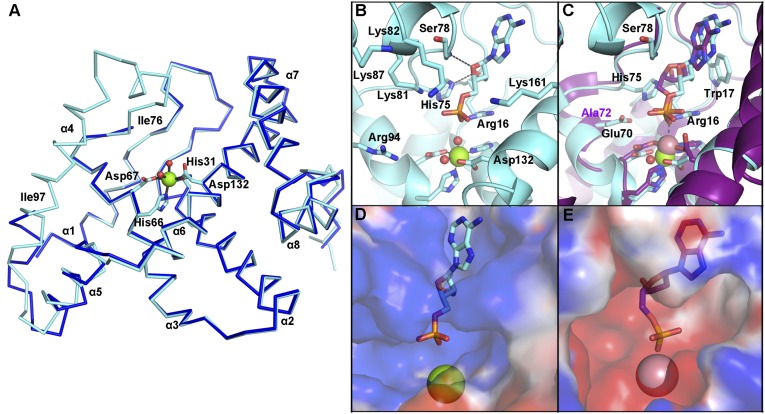
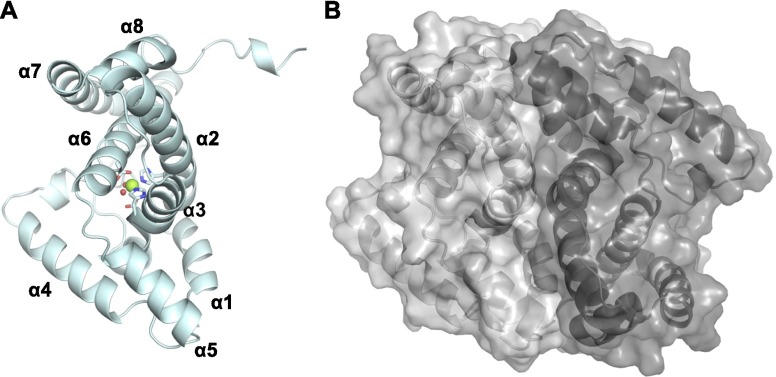
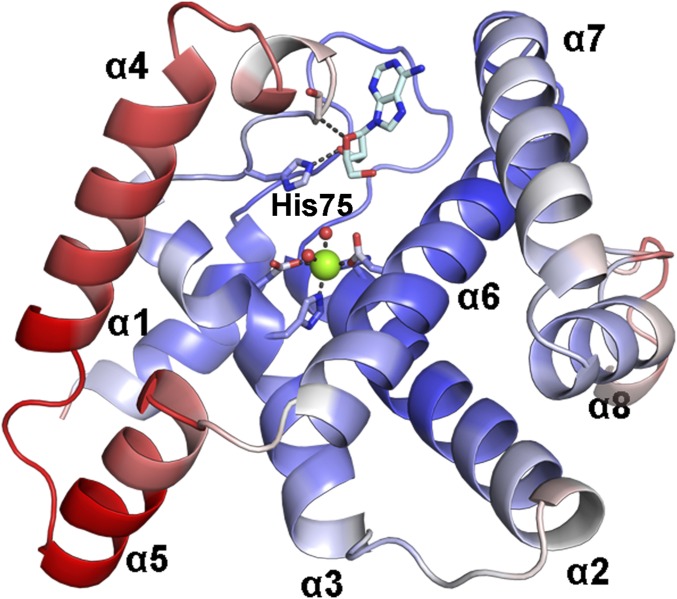
The crystal structure of OxsA was determined to 2.05-Å resolution using the E72A variant of the E. coli 5′-nucleotidase, YfbR (14), as a search model. The data collection and refinement statistics for all determined structures are listed in Table S1. Similar to the structure of YfbR, OxsA has a globular fold composed of eight α-helices (Fig. 2A). There is one monomer of OxsA per asymmetric unit, but based on an approximate 2,600-Å2 interface (23% of the total monomeric surface area) between the OxsA monomer and a symmetry-related molecule, it appears the biological unit of OxsA is a dimer (Fig. S1). This interface maps to the dimer interface previously identified in YfbR, formed predominantly between α-helices 1 and 3 (14). An active site Mg2+ from the crystallization solution is found at the core of the α-helices, octahedrally coordinated by His31, His66, Asp67, and Asp132 of the characteristic HXnHDXnD motif, and two water molecules. Residues Met1, Ile76–Ile97, and Arg191–Asn194 were too disordered to build and are therefore missing from the refined structure of OxsA.
Table S1.
Data collection and refinement statistics
| Data collection and refinement | OxsA | OxsAOXT | OxsAOXT-P | OxsAOXT-PP | OxsAOXT-PPP |
| Data collection | |||||
| Space group | H32 | H32 | H32 | H32 | H32 |
| Cell dimensions | |||||
| a, b, c (Å) | 143.0, 143.0, 53.8 | 143.0, 143.0, 53.8 | 143.0, 43.0, 53.8 | 142.6, 142.6, 53.8 | 143.0, 143.0, 53.8 |
| α, β, γ (°) | 90.0, 90.0, 120.0 | 90.0, 90.0, 120.0 | 90.0, 90.0, 120.0 | 90.0, 90.0, 120.0 | 90.0, 90.0, 120.0 |
| Resolution (Å)* | 28.95–2.05 (2.12–2.05) | 49.33–1.85 (1.92–1.85) | 41.29–1.64 (1.70–1.64) | 21.41–1.92 (2.00–1.92) | 49.33–1.90 (1.97–1.90) |
| Rmerge* | 0.055 (0.61) | 0.077 (0.93) | 0.045 (0.841) | 0.068 (0.903) | 0.054 (0.803) |
| I/σI* | 40 (1.9) | 37.9 (4.3) | 55.8 (3.1) | 26.2 (1.8) | 26.2 (1.6) |
| Completeness (%)* | 99.7 (97.0) | 100 (100) | 99.7 (99.5) | 99.4 (99.7) | 99.5 (98.4) |
| Redundancy* | 8.9 (4.7) | 13.5 (12.1) | 17.3 (13.9) | 7.3 (6.9) | 4.8 (3.6) |
| CC1/2* | (0.724) | (0.922) | (0.886) | (0.699) | (0.595) |
| Refinement | |||||
| Resolution (Å) | 2.05 | 1.85 | 1.64 | 1.93 | 1.90 |
| No. reflections* | 13,290 (1,276) | 18,194 (1,801) | 25,785 (2,551) | 15,836 (1,591) | 16,408 (1,593) |
| Rwork/Rfree (%) | 21.30, 25.44 | 17.87, 21.95 | 17.82, 20.37 | 17.69, 21.85 | 18.46, 22.95 |
| No. atoms | |||||
| Protein | 1,416 | 1,665 | 1,733 | 1,648 | 1,642 |
| Ligand | 4 | 18 | 22 | 26 | 30 |
| Mg2+ | 1 | 1 | 1 | 2 | 2 |
| Water | 56 | 90 | 125 | 80 | 58 |
| B-factors (Å2) | |||||
| Protein | 47.96 | 42.37 | 39.28 | 44.35 | 51.25 |
| Ligand | 48.01 | 43.90 | 37.91 | 52.15 | 60.81 |
| Mg2+ | 32.46 | 41.56 | 44.82 | 53.56 | |
| Water | 50.34 | 43.03 | 43.69 | 42.99 | 49.47 |
| Rmsd | |||||
| Bond lengths (Å) | 0.004 | 0.009 | 0.011 | 0.015 | 0.011 |
| Bond angles (°) | 0.37 | 0.81 | 1.26 | 1.03 | 0.95 |
Values in parentheses are for highest-resolution shell.
Fig. 2.
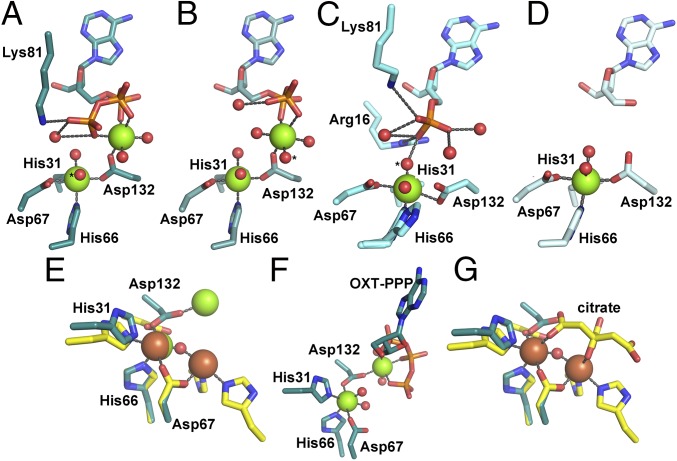
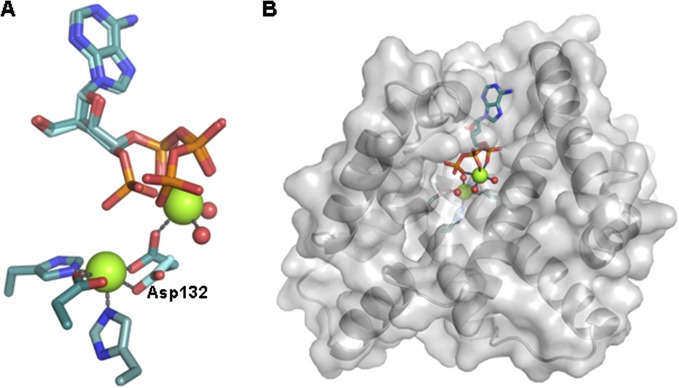
OxsA has a mononuclear HD domain phosphohydrolase protein fold that appears designed to bind nucleoside analogs with four-membered rings. (A) The structure of OxsA (blue) is missing residues Ile76–Ile97, which become ordered in the OxsAOXT-P structure (cyan). (B) OXT-P binds within the HD domain active site and interacts with His75 and the backbone of Ser78. Arg16 orients the 5′-phosphate of OXT-P near Mg2+ and within a positively charged pocket. (C) Despite sharing similar architectures, an overlay of E72A-YfbR (purple) with bound dAMP reveals that the 3′-carbon of dAMP would be located unfavorably close (1.4 Å) to the ε-nitrogen of His75. (D) The overall positive electrostatic potential of the OxsA active site. (E) The electrostatic potential measured for the equivalent pocket of E72A-YfbR (14) shows an overall negative charge. In D and E, the electrostatic potential surfaces are displayed on an equivalent scale. Green and pink spheres represent the divalent metal cocrystallized with OxsA (Mg2+) and E72A-YfbR (Co2+).
Fig. S1.
The biological unit of OxsA is likely to be a dimer. (A) The structure of the OxsA monomer that is present in the asymmetric unit. (B) Space filling view of OxsA dimer; 2,600 Å2 of surface area is buried by α-helices 1–3 of one monomer (light gray) and its symmetry related monomer (dark gray).
Structure of OxsA with Oxetanocin 5′-Monophosphate Reveals a Tailored Active Site Architecture.
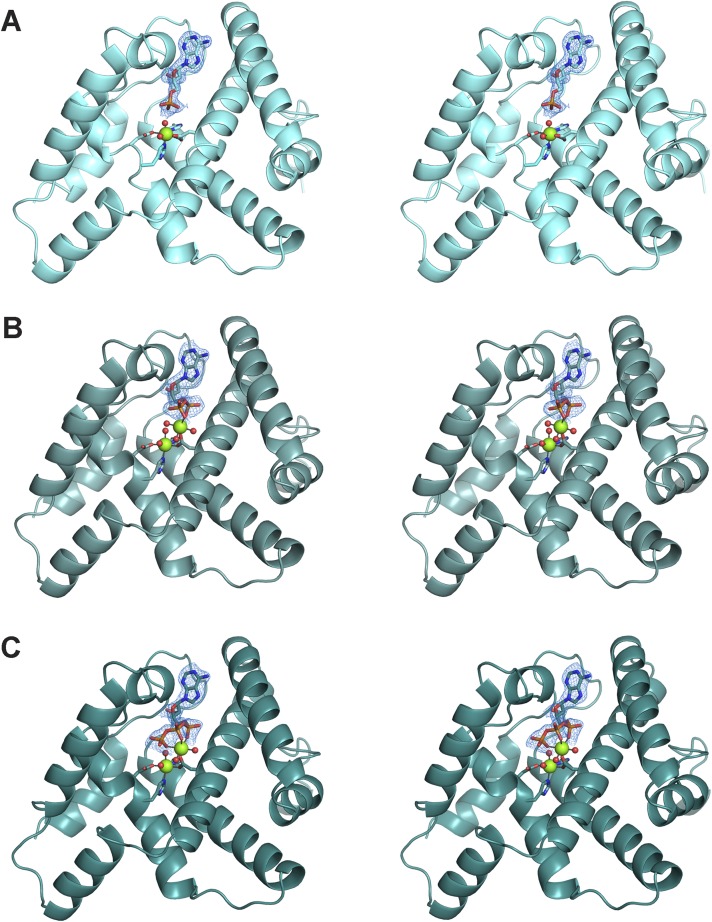
The structure of OxsA cocrystallized with chemically synthesized oxetanocin-5′-monophosphate (OxsAOXT-P) was determined to 1.64-Å resolution. The structure of OxsAOXT-P is similar to the ligand-free structure with an rmsd determined by PyMOL of 0.22 Å for 1,014 atoms. The main difference between the ligand-bound structure and the ligand-free structure is the ability to model residues Ile76–Ile97 (Fig. 2A). Upon binding OXT-P, these residues order to form a short helical loop following α3 and all of α4, such that only residues 1 and 193–194 are absent from the OxsAOXT-P structure.
Binding of OXT-P in the active site of OxsA involves interactions between the ε-nitrogen of His75 and the C3′-hydroxymethyl group of OXT, as well as a hydrogen bond between the backbone amide of Ser78 and the endocyclic oxygen of OXT (Fig. 2B and Fig. S2A). The active site of OxsAOXT-P is similar to that of YfbR (Fig. 2C) and contains previously identified important YfbR residues, including: Trp17 (OxsA numbering), a residue proposed to sterically occlude ribonucleotide binding; Glu70, a residue hypothesized to be involved in protonation of the nucleoside product; and Arg16, which positions the 5′-phosphate of the YfbR substrate, dAMP, for catalysis (14). Consistent with this role, the guanidinium group of Arg16 of OxsA orients the 5′-phosphate of OXT-P near Mg2+ and within a positively charged pocket formed by residues Arg16, Lys81, Lys82, Lys87, Arg94, and Lys161 (Fig. 2B).
Fig. S2.
OxsA has a globular fold composed entirely of α-helices. (A) Stereoview of OxsAOXT-P. (B) Stereoview of OxsAOXT-PP. (C) Stereoview of OxsAOXT-PPP. Each stereo figure is shown with a 2F(o)–F(c) simulated annealing composite omit electron density map contoured at 1.0 σ around the bound OXT substrate.
Notably, because OxsA and YfbR share only 30% sequence identity, there are also active site differences. Specifically, His75 of OxsA, the residue that contacts the C3′-hydroxymethyl group of OXT, is a catalytically essential Asp in YfbR (Asp77) (14). The smaller Asp is ideal for accommodating the larger deoxyribose ring of the YfbR substrate (dAMP) and for hydrogen bonding to the 3′-hydroxyl moiety of dAMP. In contrast, the larger His residue in OxsA nicely accommodates the smaller four-membered ring of OXT (Fig. 2C) and hydrogen bonds to Glu70, the equivalent of which is the proposed catalytic acid in YfbR (14). Interestingly, although the phosphates of dAMP and OXT-P occupy almost identical active site positions (Fig. 2C), the metal positions are slightly different, resulting in direct interaction between dAMP and the closer Co2+ in YfbR and a through-water interaction for OXT-P and Mg2+ in OxsA. Although this variation may be a result of the difference in the metal that was cocrystallized with the proteins (Mg2+ in OxsA or Co2+ in YfbR), it should be noted that an inactive E72A-variant was used to determine the structure of Co2+-YfbR with dAMP-bound (14), and that E72 (E70 in OxsA) is very close to the substrate and metal center (Fig. 2C). As described below, Co2+ may be the catalytic metal in this site for both OxsA and YfbR.
Additional differences in the active site of OxsA and YfbR become evident upon comparison of the electrostatic potential surfaces; the aforementioned positively charged pocket of OxsA appears poised to accommodate one or multiple phosphates (Fig. 2 B and D). In contrast, the active site of YfbR demonstrates an overall negative charge (Fig. 2E) and, consistent with this observation, YfbR lacks activity for deoxyribonucleoside di- and triphosphorylated substrates (14).
OxsA Activity Assays Show Higher in Vitro Activity with Co2+ Than Mg2+.
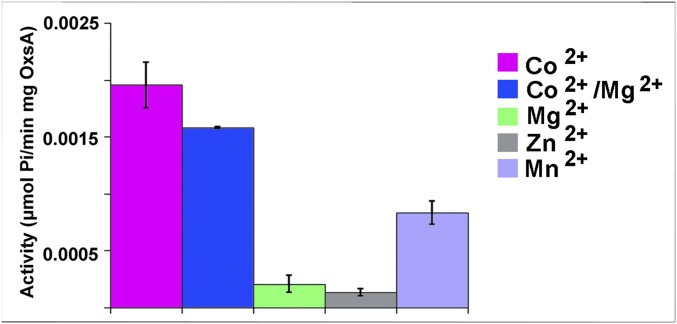
The phosphatase activity of OxsA was tested against OXT-P using the malachite green assay, as previously described (27). Briefly, the assay measures activity using the absorbance of the complex formed between ammonium molybdate, malachite green, and free inorganic phosphate produced from the hydrolysis reaction. Activity was measured in the presence of Zn2+, Mn2+, Mg2+, or Co2+ (Fig. 3). Similar to YfbR, Co2+-OxsA is most active (14, 28), whereas Zn2+-, Mn2+-, and Mg2+-bound OxsA showed lower levels of activity.
Fig. 3.
OxsA activity as measured in the presence of 20 μM OXT-P and 0.6 mM of the indicated divalent metal ions. Co2+-OxsA shows the highest level of activity. Error bars represent ± SD of three independent experiments.
Crystal Structures of OxsA with Multiply Phosphorylated OXT Molecules Reveal a Change in the Active Site Metal Content.
To investigate whether OxsA is able to bind and turnover multiply phosphorylated OXT molecules, as we predicted above based on the positive electrostatic surface of the OxsA active site, a protocol, which involved the use of the chemically synthesized OXT-P and the commercially available enzymes myokinase and pyruvate kinase, was developed to generate OXT diphosphate (OXT-PP) and OXT triphosphate (OXT-PPP) (Fig. S3). Using this material, the structures of OxsAOXT-PP and OxsAOXT-PPP were determined to 1.92- and 1.90-Å resolution, respectively. Similar to the structure of OxsAOXT-P, residues Ile76–Ile97 are ordered and accommodate the bound OXT derivatives. In comparison with the OxsAOXT-P structure, the rmsds determined by PyMOL for the OxsAOXT-PP and OxsAOXT-PPP structures are 0.16 Å for 1,243 atoms and 0.20 Å for 1,184 atoms, respectively.
Fig. S3.
OXT-PP and OXT-PPP were enzymatically synthesized and then purified using HPLC. (A) The synthesis of OXT-PP was accomplished using a combination of myokinase, Mg2+, ATP, and OXT-P. (B) OXT-PPP was enzymatically generated through combination of the isolated OXT-PP, pyruvate kinase, phosphoenol pyruvate, and Mg2+.
Surprisingly, despite remarkable similarities in the position of the oxetane ring and adenine base in the active sites of OxsAOXT-P, OxsAOXT-PP, and OxsAOXT-PPP, the structures with OXT-PP and OXT-PPP have a second active site Mg2+ (Fig. 4 A–C and Fig. S2). Because all crystals were grown with the same concentration of Mg2+, the presence of the second Mg2+ would seem to be due to the binding of di- and triphosphorylated substrates. As these compounds likely exist in the cell as Mg2+-complexes, OXT-PP and OXT-PPP could deliver the second Mg2+ upon binding. Accordingly, the structure of OxsA with OXT bound (OxsAOXT), also determined in this work to 1.85-Å resolution, which similar to OXT-P cannot deliver a metal ion to the active site, shows only one Mg2+ in the canonical HD-site (Fig. 4D).
Fig. 4.
The metal content of the OxsA active site is modulated through substrate binding. (A) OxsAOXT-PPP has a dinuclear center with one Mg2+ found in the HD-site and the other ligated primarily by substrate. (B) A similar dinuclear site is observed in OxsAOXT–PP. (C) OxsAOXT–P has a single Mg2+ ligated by the mononuclear HD domain residues. His66 exhibits two conformations. (D) A mononuclear site is also observed in the structure of OxsAOXT. (E) Overlay of OxsAOXT-PPP (dark cyan) with PhnZ (yellow) reveals use of a different Asp as the bridging ligand of the dinuclear site. (F) Mg2+ in the second site in OxsAOXT-PPP is 5.2-Å from Mg2+ in the HD-site and has only one protein ligand. (G) The Fe ions in PhnZ are 3.7 Å apart, bridged by a μ-oxo moiety and a citrate molecule from the crystallization buffer that binds in place of substrate (18). The second Fe site has three protein ligands (18). In A–C, the proposed nucleophilic water is indicated by an asterisk (*). Green, brown, and red spheres correspond to magnesium, iron, and water molecules, respectively. Stereoviews of OxsAOXT-P, OxsAOXT-PP, and OxsAOXT-PPP can be found in Fig. S2.
In the structure of OxsAOXT-P introduced above, HD residue Asp132 is found bent 2.8 Å toward the active site metal ion and away from the negative charge of the 5′-phosphate of OXT-P, and His66 exhibits two conformations, where in one it serves as a ligand to Mg2+ and in the other it is flipped out away from the metal center, replaced by a water molecule (Fig. 4C). In contrast, structures with OXT-PP and OXT-PPP show His66 in one conformation, ligating the lower HD-site metal ion of the dinuclear center, and Asp132 serving as a bridging ligand between the two metal ions (Fig. 4 A and B). The second active site metal ion of the OxsAOXT-PP structure exhibits an octahedral coordination geometry, coordinated by Asp132, one oxygen atom of each the α- and β- phosphate groups, and three water molecules (Fig. 4B). For the OxsAOXT-PPP structure, the six-coordinate geometry is fulfilled by Asp132, one oxygen atom of each the α-, β-, and γ-phosphate groups, and two water molecules (Fig. 4A). The two metal centers are separated by 5.3- and 5.2-Å for the OxsAOXT-PP and OxsAOXT-PPP structures, respectively, which is different from other HD domain dinuclear sites, which are separated by 3.4–3.8 Å [PDB ID codes 3TM8 (11), 2IBN (29), 3CCG 2O08, 2OGI, 2PQ7, and 4N6W (18)] and bridged by the HD doublet Asp residue (Fig. 4 E–G).
OxsA Shows Activity with Di- and Triphosphorylated OXT.
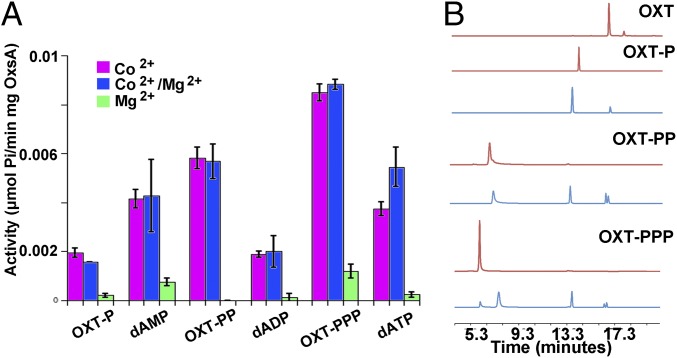
The phosphatase activity of OxsA was tested against OXT-PP, OXT-PPP, 2′-deoxyadenosine diphosphate (dADP), and dATP using the malachite green assay described above. Activity is much lower in the presence of Mg2+ compared with Co2+ (Fig. 5A), explaining why we did not observe turnover in our crystals. As mentioned above, capturing the structure of YfbR with substrate and Co2+ required use of inactive mutant protein. Interestingly, a 50:50 Mg2+/Co2+ mixture does not reduce the activity compared with 100% Co2+ (Fig. 5A), suggesting that the highest level of activity requires Co2+ to be present in only one of the two metal sites.
Fig. 5.
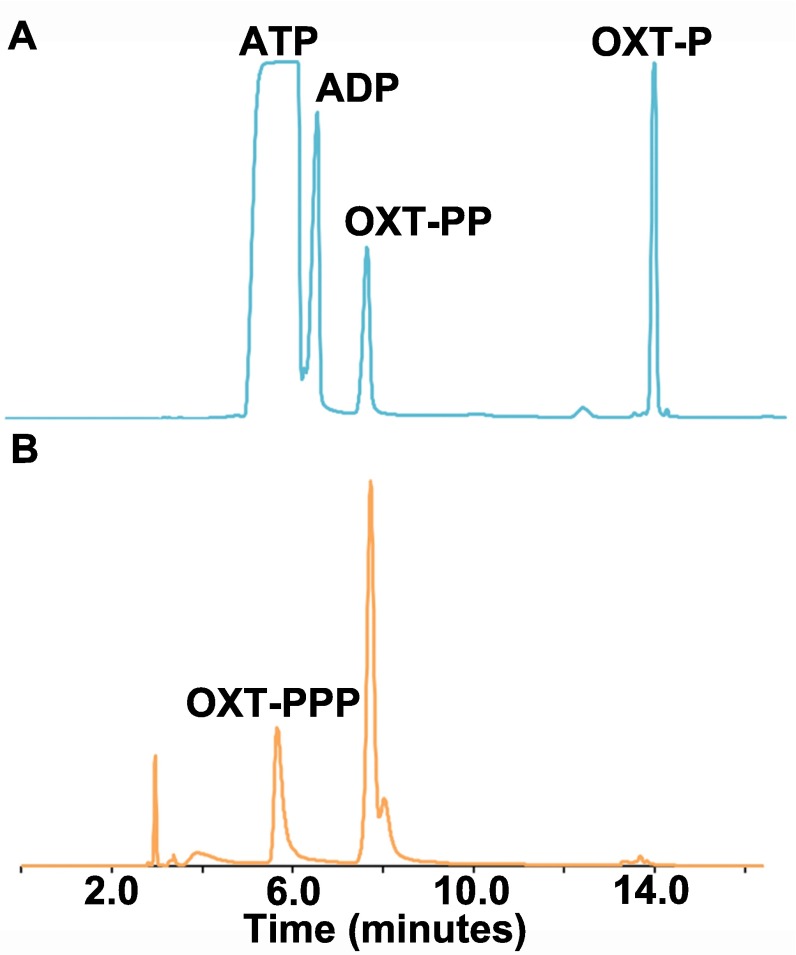
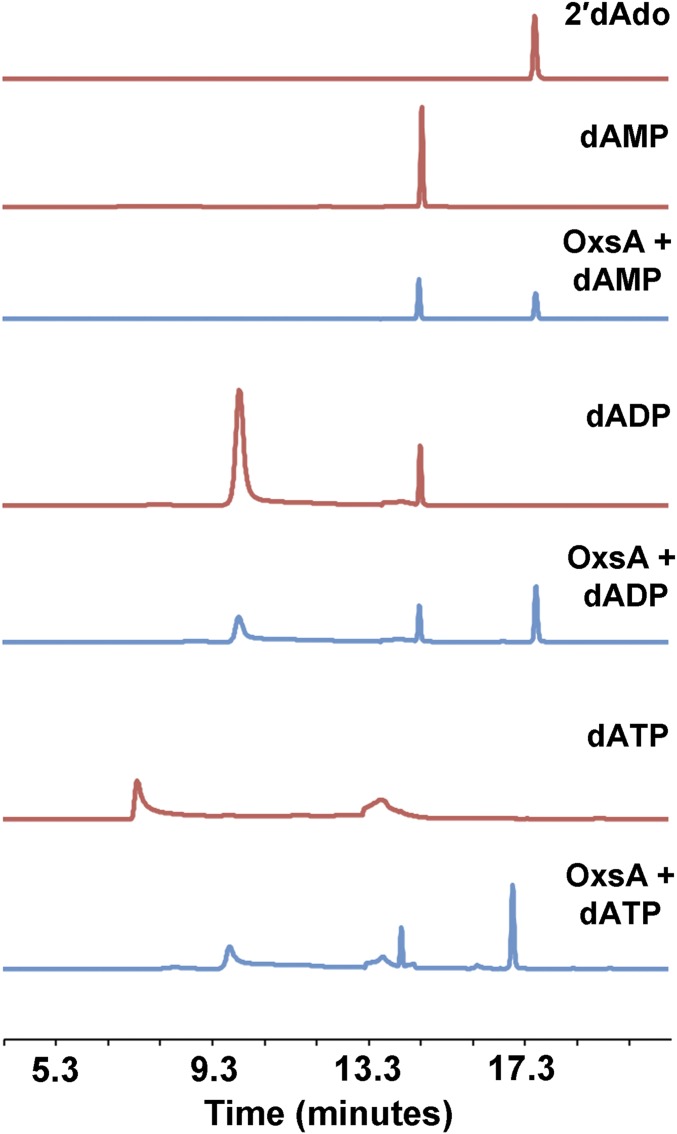
OxsA activity on phosphorylated OXT and deoxyadenosine compounds. (A) The activity of OxsA is plotted as a function of tested substrate (10 molar equivalents) in the presence of Co2+, Mg2+, or a Co2+/Mg2+ mixture. Error bars represent the ± SD of three independent experiments. (B) HPLC analysis of OxsA hydrolysis products when incubated with OXT derivatives (OXT-PPP, OXT-PP, or OXT-P). Each red trace corresponds to an OXT derivative standard. The blue traces correspond to hydrolysis products after incubation of the OXT derivative with OxsA. Current LC-MS data indicates splitting of the nucleoside peak is because of hydroxylation that occurs under the HPLC assay conditions.
The kcat of OxsA with OXT-PPP and OXT-PP was 3.8- and 2.4-fold higher, respectively, than was measured with OXT-P (Table 1). This trend is reflective of what is observed in solution; phosphate esters are more stable to hydrolysis than phosphoanhydrides. Thus, it does not appear that OxsA has evolved to be a much better catalyst for one of the phosphorylated forms of OXT over another. The KM values are similar for all three phosphorylated forms of OXT, and are between 8 and 13 μM (Table 1). Therefore, for the OXT molecules, each added phosphate equates to an increase in the kcat of OxsA and a corresponding increase in the kcat/KM. The same is not true for the phosphorylated deoxyadenosine substrates, for which OxsA activity on dADP was lower than for both dATP and dAMP (Fig. 5A and Table 1). This difference could be due in part to the fact that the commercially available deoxyribonucleotide compounds are less pure (98–100%, ≥95%, and ≥97% purity for dAMP, dADP, and dATP, respectively) than both our chemically and enzymatically synthesized OXT standards. Importantly, the KM values for dAMP, dADP, and dATP are 2- to 20-fold higher (22–155 μM) than the KM values for OXT-P(PP) (Table 1). In the case of dATP (155 ± 18 μM), the KM is almost equivalent to what has previously been measured as the concentration in bacterial cells (175–200 μM) (30, 31), indicating OxsA is not a good catalyst for dATP hydrolysis.
Table 1.
Summary of kinetic parameters for OxsA with OXT- and deoxyribonucleotide compounds
| Tested substrate | KM (μM) | Vmax × 10−3 (U/mg)* | kcat × 10−3 (s−1) | kcat/KM (M−1⋅s−1) |
| OXT-P | 11 ± 3 | 3.2 ± 0.20 | 1.2 ± 0.077 | 106 ± 30 |
| dAMP | 22 ± 2 | 9.8 ± 0.24 | 3.7 ± 0.094 | 167 ± 16 |
| OXT-PP | 13 ± 3 | 7.8 ± 0.46 | 2.9 ± 0.17 | 221 ± 47 |
| dADP | 53 ± 11 | 7.1 ± 0.35 | 2.6 ± 0.13 | 51 ± 10 |
| OXT-PPP | 8 ± 2 | 12 ± 0.86 | 4.5 ± 0.33 | 525 ± 121 |
| dATP | 155 ± 18 | 58 ± 2.4 | 22 ± 0.83 | 142 ± 17 |
See SI Materials and Methods for experimental details.
U/mg = μmol/min mg of OxsA.
A similar study of activity was pursued using HPLC to investigate production of OXT and deoxyribonucleotide hydrolysis products. In both cases, the diphosphate, monophosphate, and nucleoside products were detected consistent with OxsA being able to release more than one molecule of phosphate from the provided substrates until the nucleoside products, OXT or 2′-deoxyadeonosine, are produced (Fig. 5B and Fig. S4).
Fig. S4.
OxsA sequentially hydrolyzes inorganic phosphate molecules from OXT and deoxyribonucleotide molecules. HPLC analysis of OxsA hydrolysis products when incubated with dATP, dADP, or dAMP. Each red trace corresponds to a deoxyribonucleotide standard. The blue traces correspond to hydrolysis products after incubation of the deoxyribonucleotide derivative with OxsA.
The observed substrate tolerance of OxsA prompted an investigation into the B-factors of the OxsA structures with OXT molecules bound. Within each determined structure, similar B-factors are observed in the core helices (α1–α3, and α6), which contribute the HD-metal binding residues. The highest B-factors are found on the loop following the His75 helix, and on α4-α5 (Fig. S5). Both His75 and Ser78, which form the two principle interactions with all OXT derivatives in the active site, fall in a region where the average B-factor is 10–15 Å2 higher than the average for the entire structure. This flexibility likely allows for nonoptimal substrates to be accommodated in the active site, albeit giving rise to higher KM values. Unfortunately, molecular details of this accommodation are not available, as crystals grown or soaked with a 5- to 100-fold molar excess of the deoxyribonucleotide molecules do not reveal any electron density for bound ligands.
Fig. S5.
The observed promiscuous activity of OxsA is likely due to flexibility in the His75 region of OxsA. B-factors are plotted on a scale of 20–65 Å2 with increasing values colored blue-white-red. Although His75 should favor the smaller four-membered ring, the flexibility in the His75 region that may allow for active site rearrangements, and thus the observed activity on deoxyribonucleotide molecules.
Discussion
To study the mechanism by which OxsA and OxsB catalyze the synthesis of OXT, we have determined the structures of five states of OxsA (OxsAOXT-PPP, OxsAOXT-PP, OxsAOXT-P, OxsAOXT, and OxsA) using X-ray crystallography. These structural studies coupled with the malachite green assay of detecting inorganic phosphate production, the Michaelis–Menten kinetic measurements, and the HPLC analysis of hydrolysis product formation are consistent with the proposed scheme B for OXT biosynthesis (shown in Fig. 1). In this scheme, OxsA catalyzes the hydrolysis of a phosphorylated OXT molecule to generate the nucleoside product of the pathway.
Because of the simple nature of the OXT biosynthetic gene cluster, first contracting the more thermodynamically stable five-membered ring of a purine nucleoside coopted from a primary metabolic pathway to generate the strained, four-membered ring of OXT, followed by dephosphorylation of an OXT phosphate may serve as a mechanism to trap the OXT product and thus prevent its OxsB-catalyzed equilibration with a purine nucleoside phosphate. Interestingly, it appears that regardless of whether dAMP, dADP, or dATP is contracted by OxsB, OxsA can catalyze hydrolysis of the resultant OXT-derivative down to OXT, one phosphate at a time. The KM values for each of the OXT derivatives are similar, potentially allowing OxsA to hold on to the phosphorylated forms of OXT until final conversion to OXT is achieved. From the perspective of chemical warfare between microbes, the phosphorylated forms of OXT are likely the toxic species. Indeed, OXT-PPP has been shown to inhibit cellular and viral DNA polymerases (6). Therefore, it appears that the combined action of OxsA and OxsB generate the warfare agent, OXT, but renders the compound inert for transport out of the cell. Consequently, OxsA can be thought of as having a dual function: OxsA is involved in both completing the biosynthesis of OXT and imparting resistance to the producing strain. Presumably, OXT is then taken up by a neighboring organism and rephosphorylated, thus reacquiring its toxicity. In line with this theory are our results that promiscuous kinases can be used to enzymatically generate OXT-PP and OXT-PPP with reasonable yields.
The X-ray crystal structures of OxsA presented in this work add another level of diversity to the HD domain superfamily; the active site appears to be designed to bind the secondary metabolite OXT and also decrease the binding affinity of substrates with larger deoxyribose rings. This design is accomplished by the use of a bulky His residue near the HD-active site metal ion in place of the equivalent Asp found in YfbR. Notably, however, this enzyme redesign places His75 in a highly flexible region of the protein, which when not bound with OXT, affords enzymatic promiscuity, allowing hydrolysis of deoxyribonucleotide substrates, albeit with lower catalytic efficiency (a consequence of the presumed decrease in substrate affinity illustrated by the measured KM values). The enzyme YedJ, which also has a His residue in the equivalent position, similarly shows poor activity with primary metabolic phosphatase and phosphodiesterase substrates (14).
Perhaps even more impressive is the revelation from this structural work that an HD domain scaffold can accommodate more than one type of metal center depending on which substrate is bound. Before this work, there were characterized HD domain enzymes, which use mono‐ (14, 15, 21, 22, 32, 33), di- (10, 11, 16–18, 23, 24, 29, 34), and trinuclear (12) metal centers, but no examples that use “switchable” metallocenters nor examples of dinuclear sites that resemble the one that we observe here. We hypothesize that this second metal center is introduced as a consequence of binding a Mg2+-OXT di- or triphosphate molecule. As discussed below, the use of a second metal site extends the hydrolytic capabilities of the active site, allowing OxsA to catalyze three-phosphate elimination reactions at the α-, β-, and γ-positions of its substrate. The observed mono- to di-nuclear metal switching mechanism of OxsA is vaguely reminiscent of RNA polymerase II, which requires two Mg2+ ions that are bridged by an Asp and located 5.8 Å apart; one Mg2+ remains bound to the enzyme and one Mg2+ is only stably bound in the presence of a nucleotide substrate (35, 36). Unlike OxsA, however, in RNA polymerase II the stable Mg2+ has three Asp ligands and coordinates the α-phosphate of the substrate, and the transient Mg2+ has additional protein ligands (35, 36).
Mechanistically, the vast majority of phosphate elimination reactions proceed through an in-line attack by a nucleophile, at the phosphorus atom, opposite the position of the leaving group (37). This type of reaction is one proposal for YfbR, where hydrolysis requires that the 5′-phosphate of dAMP coordinates the HD-metal center, and is thereby positioned for nucleophilic attack by a Co2+ bound hydroxide ion, followed by, or with concomitant protonation of the leaving group by Glu72 (Glu70 in OxsA numbering) (14). Based on the X-ray crystal structures determined here, it can be intuited how OxsA could use a similar mechanism to catalyze three sequential hydrolysis reactions with the involvement of three different water molecules (starred in Fig. 4) and a flexible dinuclear motif that has only one protein-based ligand to the second metal ion.
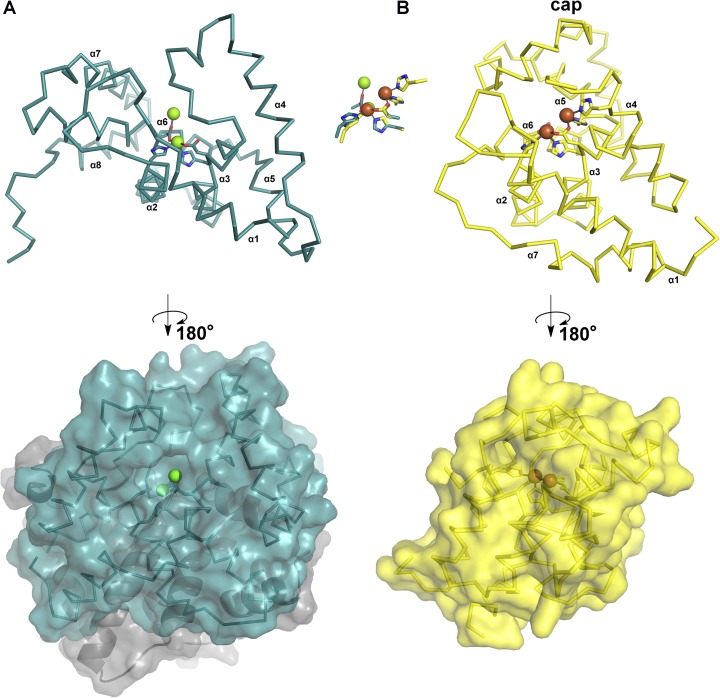
With respect to hydrolysis of the γ-phosphate of OXT-PPP, a water molecule bound to the metal ion in the canonical HD-site is positioned correctly to serve as the nucleophile (Fig. 4A). Metal ions act as Lewis acids, lowering the pKa of bound water molecules and activating them for nucleophilic attack on phosphorylated compounds. For hydrolysis of the β-phosphate, a water molecule bound to the metal ion in the noncanonical site meets the requisite geometric requirement (Fig. 4B). Accordingly, even if this second metal ion binds as part of the substrate, the active site does require its presence for hydrolysis of OXT-PP. Loss of this second metal site is expected to occur along with or following hydrolysis of the β-phosphate. The remaining phosphate of OXT-P moves down to occupy the second metal site, repositioning Asp132 (Fig. S6A). An exit route for the metal ion can be visualized in a surface representation of the dinuclear OxsA protein, which reveals that the second site is much more solvent accessible than the HD-site (Fig. S6B) as well as a typical HD domain second metal site (Fig. S7).
Fig. S6.
The modularity of the second metal binding site in OxsA permits multifunctionality. (A) An overlay of the OxsAOXT-PPP (dark teal), OxsAOXT-PP (aquamarine), and OxsAOXT-P (light cyan) active sites reveal that the second metal site and the sidechain of the bridging Asp132 clash with the α–phosphate group of the bound OXT-P molecule. (B) Loss of the second metal binding site could be facilitated by the solvent accessibility of the site.
Fig. S7.
Comparison of OxsA with the characteristic HD domain dinuclear enzyme PhnZ reveals differences in the accessibility of the second metal site. (A) The second metal site in OxsA is solvent-exposed. Ribbon drawing is shown above and space-filing model of the OxsA dimer is shown below. (B) The second metal site in PhnZ (PDB ID code 4N6W) (18) is found below the protein surface, protected by additional protein structure (labeled cap). A ribbon drawing for PhnZ is shown above and a space-filing model below. The superposition of the dinuclear sites from these two proteins is also shown with Mg2+ ions in green spheres and Fe ions in brown spheres.
In part because of the transient nature of the second metal site in OxsA, we propose that Mg2+ and not Co2+ is the relevant noncanonical metal in vivo. Our data show that Co2+-OxsA and Co2+/Mg2+-OxsA have similarly high levels of activity, indicating that Co2+ need not occupy both sites for maximum turnover. Additionally, Co2+ decreases cellular growth rate and becomes toxic to B. megaterium at levels above 50 μM (38), whereas Mg2+ is typically present in the millimolar range in cells (39) and commonly associated with phosphorylated molecules. Although it is possible that the physiologically relevant metal in both sites is Mg2+, B. megaterium is a major producer of cobalamin (40), requiring a cellular flux of Co2+ that should be sufficient to fill a canonical and stable metal site. For these reasons, we suggest that the dinuclear site is composed of Co2+ in the canonical HD-site and Mg2+ in the noncanonical, transient secondary site, which is introduced with OXT-PP and OXT-PPP substrates.
Finally, for hydrolysis of the α-phosphate, a water bound to the metal in the HD-site is positioned appropriately (3.6 Å from the phosphorus atom) (Fig. 4C). In addition to metal-catalyzed water activation, the enzymatic mechanism may involve modulation of the electrophilicity of the phosphate to be hydrolyzed, as our structures show an overall positive active site electrostatic potential (Fig. 2D) created by a number of Lys and Arg residues in addition to the metal ions that are present (Fig. 2C).
Although OxsA appears tailored to perform hydrolysis reactions on three different forms of the secondary metabolite OXT, likely using three different water molecules bound to metal ions in two different metal-binding sites, OxsA is neither highly specific nor highly active. For example, the specific activity of Co2+-OxsA is ∼85 times slower than that observed for YfbR with its native substrate dAMP (14). Even though primary metabolic enzymes are often faster than ones involved in secondary metabolism, OxsA rates of 0.0012–0.022 s−1 (0.072–1.3 min−1) (Table 1) still seem modest. Although turnover numbers for OxsA’s partner enzyme, OxsB, have not yet been reported, AdoMet radical enzymes are notoriously slow. A recent review article reports that rates are typically on the order of 0.018–1.6 min−1 (41). If OxsB turns out to be a typical AdoMet radical enzyme, it seems likely that there would have been little evolutionary pressure to evolve OxsA to turn over phosphorylated OXT molecules much faster than they are being produced. A biochemical characterization of OxsB will be informative in this regard.
There are currently ∼100,000 protein X-ray crystal structures deposited in the PDB. Despite this large number, it has been estimated that fewer than 1,000 unique protein folds exist (42). This discrepancy means that protein scaffolds must be reused and reinvented for new catalytic functions through divergent or convergent evolution (8, 43). One theory for how Nature repurposes protein architectures is that it selects from a pool of enzymes with mechanisms that would lend a strategy for catalysis of a new reaction (43). Often, this approach is at the expense of a lower enzymatic rate and catalytic promiscuity for the original substrate (43). Similar to the production of OXT, a toxic compound, the redesign of an enzyme has the ability to give the host a competitive advantage (43). These concepts are exemplified by the HD domain enzyme superfamily whose catalytic repertoire is just starting to be unveiled. Here, we report a customized HD domain active site architecture and a switchable metallocenter, which is regulated by the binding of OXT substrates. These studies reveal the function of OxsA and serve to elucidate a scheme for a two-enzyme catalyzed biosynthesis of the nucleoside analog OXT.
Materials and Methods
The oxsA gene was synthesized by Mr. Gene with codon optimization for E. coli expression, subcloned into the pET24b(+) vector, and transformed into E. coli BL21-star-(DE3). OxsA was purified using ammonium sulfate precipitation and a DEAE-Sepharose CL-6B column. OXT and OXT-P were chemically synthesized (Scheme S1), whereas OXT-PP and OXT-PPP were enzymatically generated. Inorganic phosphate production was measured using the absorbance of malachite green at 630 nm (27). The OxsA reaction products were detected at 260 nm following separation on an analytical C-18 reverse-phase HPLC column. The structure of OxsA was solved using the structure of YfbR (14) as a molecular replacement model. The structure of OxsA was used to determine the structure of OxsAOXT, which was used to determine the structures of OxsAOXT-P, OxsAOXT-PP, and OxsAOXT-PPP. Detailed protocols can be found in SI Materials and Methods.
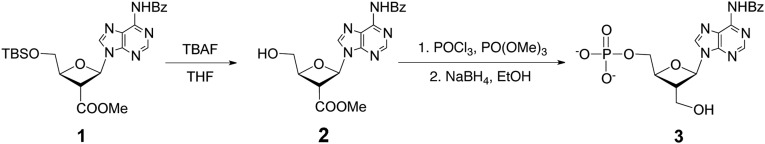
Scheme S1.
SI Materials and Methods
Protein Overexpression and Purification of OxsA.
The oxsA gene was commercially synthesized by Mr. Gene with codon optimization for Escherichia coli expression and subcloned into the pET24b(+) vector. The resultant pET24b-OxsA plasmid was transformed into E. coli BL21 star (DE3) and cells were cultured in LB medium with kanamycin (20 mg/L) at 37 °C. Once the OD600 reached 0.6, expression of OxsA was induced with ∼0.15 mM isfopropyl-β-d-thiogalactopyranoside (IPTG) and the temperature was decreased to 18 °C. After a 20-h incubation, the cells were harvested and sonicated. The supernatant was subjected to a 70% ammonium sulfate precipitation. The ammonium sulfate precipitate was collected by centrifugation and the protein pellet was resuspended in 50 mM Tris pH 8.0 and purified by a DEAE-Sepharose CL-6B column with a linear gradient of 0–1 M NaCl in 50 mM Tris buffer (pH 8.0). The eluent containing OxsA was dialyzed against storage buffer [50 mM Hepes, 100 mM NaCl, 5% (vol/vol) glycerol, pH 8.0].
Synthesis of OXT.
OXT was prepared as previously reported and characterized using MS and NMR (44).
Synthesis of OXT-P.
The synthesis of OXT-P (3) is outlined in Scheme S1 and described below.
Preparation of methyl(2R,3R,4S)-2-(6-benzamido-9H-purin-9-yl)-4-(hydroxymethyl)oxetane-3-carboxylate, or compound 2.
First, compound 1 was prepared from adenosine as previously reported (44). To yield compound 2, tetrabutylammonium fluoride (TBAF, 0.6 mL, 1 M in THF, 0.6 mmol) was added to a solution of compound 1 (150 mg, 0.3 mmol) in 10 mL THF, and the resulting mixture was stirred for 1 h before concentration under reduced pressure. Concentration resulted in the appearance of an oily residue that was purified by flash chromatography on silica gel with CH2Cl2/MeOH (9:1) as the eluting solvent to give compound 2 as a white solid. Compound 2 was characterized using 1H NMR and MS performed at the NMR and Mass Spectrometry Facilities in the Department of Chemistry at the University of Texas at Austin. The yield of the reaction was 99%. 1H NMR (CDCl3) δ 8.85 (s, 1 H), 8.47 (s, 1 H), 8.06 (m, 2 H), 7.63 (m, 1 H), 7.64 (m, 2 H), 6.70 (d, J = 5.5 Hz, 1 H, 1'-H), 4.96 (m, 2 H, 2'-H and 4'-H), 4.15 (m, 1 H, 5′-H), 3.89 (m, 1 H, 5′-H), 3.80 (s, 3 H, OMe).
Preparation of OXT-A 5′-monophosphate, or compound 3.
POCl3 (0.1 mL, 1.0 mmol) was added to a solution of compound 2 (75 mg, 0.2 mmol) in 1 mL of PO(OMe)3 at −20 °C, following which, the reaction mixture was stirred at 4 °C overnight. After the overnight incubation, 10 mL of saturated sodium bicarbonate aqueous solution was added to the reaction mixture and then extracted three times with 10 mL of chloroform. The aqueous phase was collected and lyophilized. The powder obtained after lyophilization was dissolved in 6 mL of water and 6 mL of ethanol. Sodium borohydride (500 mg, 20.8 mmol) was added to the solution and was stirred for 5 h at room temperature. Following the room temperature step, the reaction was allowed to proceed at −20 °C, in a freezer, for another 70 h. Finally, the pH of the resultant solution was adjusted to 7.0 with 1.0 M HCl. The solution containing crude compound 3 was lyophilized and purified by HPLC using a reverse-phase column (Agilent ZORBAX ODS 250 × 9.4 mm). The samples were eluted by using 1% NH4OAc in water (wt/vol) (pH 5.1) as mobile phase A and CH3CN as mobile phase B. The separation was achieved at a flow rate of 1 mL/min with the following gradient program: 0–5 min 2% B, 5–23 min 2–10% B, 23–32 min 10–35% B, 32–35 min 35% B, 35–37 min 35–2% B, 37–47 min 2% B. The elution was monitored at 260 nm using a UV detector. Compound 3 was characterized using 1H NMR and MS performed at the NMR and Mass Spectrometry Facilities in the Department of Chemistry at the University of Texas at Austin. The yield of the reaction was 50%. 1H NMR (D2O) δ 8.65 (s, 1 H), 8.09 (s, 1 H), 6.41 (d, J = 6.0 Hz, 1 H, 1'-H), 4.77 (dddd, J = 6.8, 2.4, 2.4, 2.4 Hz, 1 H, 4'-H), 4.05 (ddd, J = 12.7, 4.9, 2.2 Hz, 1 H, 5′-H), 4.00 (ddd, J = 12.7, 5.8, 2.9 Hz, 1 H, 5′-H), 3.82 (dd, J = 12.0, 5.0 Hz, 1 H, 3′-H), 3.79 (dd, J = 12.0, 5.9 Hz, 1 H, 3′-H), 3.74 (dddd, J = 6.0, 6.0, 6.0, 6.0 Hz, 1 H, 2'-H); 31P NMR (D2O) δ 0.49; 13C NMR (D2O) δ 154.2, 151.0, 148.3, 141.2, 118.2, 81.1, 77.8 (d, J = 34.6 Hz), 65.5, 58.8, 44.9.
Enzymatic generation of OXT-PP and OXT-PPP.
Standard compounds, ATP, ADP, AMP, phosphoenol pyruvate, and enzymes, myokinase from chicken muscle, and pyruvate kinase from rabbit muscle, were all purchased from Sigma Aldrich. OXT-PP was enzymatically generated by incubating 4 mM OXT-P, 80 mM ATP, 1 unit of myokinase, and 0.2 M magnesium chloride hexahydrate, in a final volume of 25 μL for 1 h at 37 °C. After incubation, the reaction was diluted to 100 μL with H2O, applied to a 10-kDa molecular weight cut-off (MWCO) spin column, and centrifuged at 4 °C at 14,000 × g for 40 min. The resultant ∼75-μL filtrate was analyzed for OXT-PP production using an Agilent 1200 series HPLC system equipped with an analytical C-18 reverse phase column pre-equilibrated in H2O containing 0.1% (vol/vol) trifluouroacetic acid (TFA). The reaction components were eluted using a linear gradient of 0–30% acetonitrile in 0.1% (vol/vol) TFA over 30 min. The multiwavelength detector was set at 220, 260, and 280 nm. Standards of ATP, ADP, AMP, and OXT-P were similarly run and analyzed. The peak corresponding to OXT-PP (2.8 min, corresponding to 2.8% acetonitrile) was collected, frozen in liquid nitrogen, and fractions totaling ∼10 mL were lyophilized for 16 h. Once lyophilized, OXT-PP was resuspended in Buffer A [50 mM Hepes (pH 8.0), 100 mM NaCl, 2 mM MgCl2] with a yield of 25–35%.
OXT-PPP was made using the myokinase reaction conditions described above to first generate and purify OXT-PP. Once lyophilized, 1 mM OXT-PP was combined with three units of pyruvate kinase, 2 mM phosphoenol pyruvate, and 0.2 M magnesium chloride hexahydrate in a final volume of 20 μL. The reaction was incubated for 2 h at 37 °C, and terminated by dilution of the sample to 100 μL with H2O, and removal of the enzyme using a 10-kDa MWCO spin column centrifuged at 4 °C, 14,000 × g for 40 min. The resultant sample was applied to the analytical C-18 reverse-phase column pre-equilibrated in H2O containing 0.1% (vol/vol) TFA, and the reaction products were eluted identically to that described above. Here, the peak corresponding to OXT-PPP (1.1 min, corresponding to 1.1% acetonitrile) was collected, frozen in liquid nitrogen, and fractions totaling ∼10 mL were lyophilized for 16 h. Once lyophilized, OXT-PPP was resuspended in Buffer A with an approximate 65% yield.
Detection of Inorganic Phosphate Production.
The same enzyme preparation was used for all activity measurements (>95% purity determined based on SDS/PAGE). A fresh aliquot of protein was removed from the freezer each day that experiments were performed, and each new aliquot was assayed with OXT-P and dAMP to ensure a consistent level of activity was observed between experiments performed on different days. Standard compounds, dATP (≥97% purity), dADP (≥95% purity), and dAMP (98–100% purity) were purchased from Sigma Aldrich. The malachite green assay was performed as previously described (27). In short, for individual measurements, a 160-μL reaction was prepared to contain 6 μg OxsA, 0.6 mM magnesium (II) chloride hexahydrate, or 0.6 mM cobalt (II) chloride, or 0.6 mM manganese (II) chloride, or 0.6 mM zinc (II) chloride, and Buffer B [20 mM Hepes (8.0), and 100 mM NaCl]. The reactions to test metal dependence were initiated by the addition of 20 μM OXT-P, whereas the individual reactions for Micahelis–Menten kinetics were initiated by the addition of varying amounts of substrate (dAMP, dADP, dATP, OXT-P, OXT-PP, OXT-PPP). The individual measurements made for Michaelis–Menten constant determination with dADP, dATP, OXT-PP, or OXT-PPP, were performed with an equimolar mixture of magnesium (II) chloride hexahydrate and cobalt (II) chloride, whereas the individual measurements made for Michaelis–Menten constant determination with OXT-P and dAMP were performed with cobalt (II) chloride.
Phosphate production was linear for at least the first hour of the reaction at 37 °C, and therefore reaction times of either 1 h, 30 min, or 15 min were chosen based on the substrate and included metal to generate a sufficient amount of product for measurement. In each case, after incubation at 37 °C, 40 μL of an acidic malachite green quenching solution was used to terminate the 160-μL phosphatase reaction samples. Each individual reaction was performed in at least triplicate and the absorbance was measured at 630 nm. The absorbance of malachite green was converted into inorganic phosphate production based on the use of a standard curve. For each individual measurement, a control reaction including all of the reaction components except OxsA was run. The measured absorbance of the control was subtracted from the corresponding reactions as a way to correct for the hydrolysis of the phosphorylated nucleosides in solution over time. The reaction rates were plotted as a function of substrate (dAMP, dADP, dATP, OXT-P, OXT-PP, OXT-PPP) concentration and fit to the Michaelis–Menten equation using KaleidaGraph (Synergy Software). The reported errors in Table 1 were calculated using Kaleidagraph software to reflect the error in data fitting.
HPLC Activity Assays.
HPLC assays were prepared in a final volume of 50 μL and contained 10 μM OxsA, 5 mM cobalt (II) chloride, 100 μM substrate (dAMP, dADP, dATP, OXT-P, OXT-PP, OXT-PPP), and Buffer B. Following incubation of each reaction sample at 37 °C for 30 min, samples were diluted to 100 μL with H2O, applied to a 10-kDa MWCO spin column, and centrifuged twice at 4 °C, 14,000 × g for 40 min. Standard nucleosides OXT and 2′-deoxyadenosine were prepared similarly minus the inclusion of OxsA. The resultant ∼75-μL filtrate was analyzed for product formation using HPLC equipped with an analytical C-18 reverse-phase column pre-equilibrated in H2O containing 0.1% (vol/vol) TFA. The reaction components were eluted using a linear gradient of 0–30% acetonitrile in 0.1% (vol/vol) TFA over 30 min.
Crystallization of OxsA.
Crystallization conditions for OxsA were identified aerobically at room temperature within 20 min of mixing 1 μL protein solution [15 mg/mL OxsA in 50 mM Hepes (pH 8.0), 100 mM NaCl, and 5% (vol/vol) glycerol] with 1 μL precipitant solution [0.1 M Tris hydrochloride (pH 8.5), 0.2 M magnesium chloride hexahydrate, 15% (wt/vol) PEG 4000] over a 500-μL reservoir solution. OxsA crystals were soaked in cryoprotectant [0.1 M Tris hydrochloride (pH 8.5), 0.2 M magnesium chloride hexahydrate, 15% (wt/vol) PEG 4000, 20% (vol/vol) ethylene glycol] for 5 min, harvested aerobically at room temperature, and cryocooled in liquid nitrogen.
Crystals of OxsAOXT were grown similarly to the OxsA crystals with a modified protein solution [50 mM Hepes (pH 8.0), 100 mM NaCl, and 5% (vol/vol) glycerol, and 5 mM OXT], and an optimized crystallization condition [0.1 M Tris hydrochloride (pH 8.5), 0.2 M magnesium chloride hexahydrate, 15% (wt/vol) PEG monomethyl ether 2000]. Crystals of OxsAOXT were soaked in cryoprotectant, which was composed of the well solution and an added 25% (vol/vol) ethylene glycol for 5 min, harvested aerobically at room temperature, and cryocooled in liquid nitrogen. Crystals of OxsAOXT-P were grown with an altered protein solution [50 mM Hepes (pH 8.0), 100 mM NaCl, and 5% (vol/vol) glycerol, and 5 mM OXT-P] and a modified precipitant condition [0.1 M Tris hydrochloride (pH 8.5), 0.2 M magnesium chloride hexahydrate, 30% (wt/vol) PEG 400]. Crystals of OxsAOXT-PP were obtained after growing crystals of OxsA with an altered precipitant condition [0.1 M Tris hydrochloride (pH 8.5), 0.2 M magnesium chloride hexahydrate, 30% (wt/vol) PEG 400] and soaked in a solution of 3 mM OXT-PP in 30% (wt/vol) PEG400 for 1 h. Finally, crystals of OxsAOXT-PPP were grown as described for OxsAOXT-PP, with an altered soaking solution that contained 1 mM OXT-PPP in 30% wt/vol PEG400, and an altered soaking time of 2.5 h. Crystals of OxsAOXT-P, OxsAOXT-PP, and OxsAOXT-PPP were harvested aerobically at room temperature, and cryocooled in liquid nitrogen without additional cryoprotectant.
Data Processing and Phasing.
All crystals of OxsA indexed as H32 with one molecule in the asymmetric unit. Indexing, integration, and scaling were performed in HKL2000 (45). The initial dataset of OxsA with no ligands bound was collected at the Stanford Synchrotron Radiation Lightsource (Menlo Park, CA), beamline 12–2 (PILATUS 6M) at a wavelength of 0.9795 Å. The remaining datasets of OxsAOXT, OxsAOXT-P, OxsAOXT-PP, and OxsAOXT-PPP were collected at the Advanced Photon Source (Argonne, IL), beamline 24ID-C (Pilatus 6M) at wavelengths of 0.9792, 0.9791, 0.9792, and 0.9795 Å, respectively. All datasets were collected at a temperature of 100K. Data statistics are summarized in Table S1.
The structure of OxsA was solved to 2.05-Å resolution using molecular replacement. As OxsA shares 30% sequence identity, and 51% sequence similarity with the E. coli 5′-nucleotidase, YfbR (PDB ID code 2PAR), a molecular replacement model without ligands, water molecules, and with side chains pruned to the most common atom was generated using Phenix Sculptor (46). With the Phenix Sculptor generated model, a molecular replacement solution was determined using Phenix AutoMR (47), and resulted in a log-likelihood gain (LLG) value of 400, a z-score of 15.9, and a correlation coefficient of 0.4242.
The resulting structure of OxsA lacking water molecules was used to determine the structure of OxsAOXT to 1.85-Å resolution. The higher resolution, more complete, resultant structure of OxsAOXT was then used minus water and OXT to determine the structures of OxsAOXT-P, OxsAOXT-PP, and OxsAOXT-PPP to 1.64-, 1.93-, and 1.90-Å resolution, respectively. For each dataset, iterative rounds of positional refinement and individual B-factor refinement were performed in Phenix (47). Model building, manual adjustment of the model, and the addition of water molecules were performed in COOT (48). The structures of OxsA with OXT derivatives were built and refined with similar protocols, incorporating five cycles of simulated annealing to reduce model bias during refinement in Phenix and using identical Rfree test sets (5% originating from the OxsA dataset). COOT was used to add small molecules and water in the late stages of refinement. Small-molecule parameter files were generated using the electronic Ligand Builder and Optimization workbench, eLBOW (49), in Phenix for OXT, OXT-P, OXT-PP, and OXT-PPP. For each dataset, simulated annealing composite omit maps were generated to verify the structures. The OxsA structure is missing residues 1, 76–97, and 191–194 and analysis of the Ramachandran statistics using the MolProbity program showed that 96.3%, 3.1%, and 0.6% of residues are in the favored, allowed, and disallowed regions, respectively. The OxsAOXT structure is missing residues 1 and 194 at the N- and C-termini and similar analysis of the Ramachandran statistics revealed that 98.9%, 1.1%, and 0% of residues are in the favored, allowed, and disallowed regions, respectively. The OxsAOXT-P structure is likewise missing residues 1 and 194 and the Ramachandran statistics show that 97.9%, 2.1%, and 0% of residues are in the favored, allowed, and disallowed regions, respectively. The OxsAOXT-PP structure is missing residues 1 and 193–194, with 98.4%, 1.6%, and 0% in the favored, allowed, and disallowed regions, respectively. Finally, the structure of OxsA with OXT-PPP bound is missing residues 1 and 193–194 and analysis of the Ramachandran statistics using the MolProbity program showed that 96.8%, 3.2%, and 0% of residues are in the favored, allowed, and disallowed regions, respectively. Refinement statistics are also listed in Table S1 and all figures were made in PyMOL. Software used in the project was installed and configured by SBGrid (50).
Electrostatic Surface Diagrams.
The missing residues (82 to 89) of YfbR E72A (PDB ID code 2PAU) were built using the Chimera interface to MODELLER (51). The resultant structure of YfbR was used in the electrostatic calculations, which were performed using the PDB2PQR (52, 53) server. In brief, the server was used to generate the OxsA and YfbR PQR files, which contain information about charge and radius. These PQR files were then used with the Adaptive Poisson-Boltzmann Server plugin (54) for PyMOL to generate the electrostatic surface diagrams and display them on an equivalent scale. Electrostatic calculations were performed in the absence of metal ions and substrates, to only reflect the protein environment.
Acknowledgments
This work was supported by National Institute of Health Grants F32-GM108189 (to J.B.-R.) and GM035906 (to H.-w.L.), and Welch Foundation Grant F-1511 (to H.-w.L.). C.L.D. is a Howard Hughes Medical Institute Investigator. This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (P41 GM103403). The Pilatus 6M detector on 24-ID-C beam line is funded by a NIH-Office of Research Infrastructure Programs High-End Instrumentation Grant S10 RR029205. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357. This work also includes research conducted at the Stanford Synchrotron Radiation Lightsource. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US DOE, Office of Science, Office of Basic Energy Sciences under Contract DE-AC02-76SF00515. The Stanford Synchrotron Radiation Lightsource Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.W.C. is a Guest Editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5TK6, 5TK7, 5TK8, 5TK9, and 5TKA).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613610113/-/DCSupplemental.
References
- 1.Shimada N, et al. Oxetanocin, a novel nucleoside from bacteria. J Antibiot (Tokyo) 1986;39(11):1623–1625. doi: 10.7164/antibiotics.39.1623. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura H, et al. The X-ray structure determination of oxetanocin. J Antibiot (Tokyo) 1986;39(11):1626–1629. doi: 10.7164/antibiotics.39.1626. [DOI] [PubMed] [Google Scholar]
- 3.Ueda K, Tsurimoto T, Nagahata T, Chisaka O, Matsubara K. An in vitro system for screening anti-hepatitis B virus drugs. Virology. 1989;169(1):213–216. doi: 10.1016/0042-6822(89)90057-3. [DOI] [PubMed] [Google Scholar]
- 4.Hoshino H, Shimizu N, Shimada N, Takita T, Takeuchi T. Inhibition of infectivity of human immunodeficiency virus by oxetanocin. J Antibiot (Tokyo) 1987;40(7):1077–1078. doi: 10.7164/antibiotics.40.1077. [DOI] [PubMed] [Google Scholar]
- 5.Nishiyama Y, Yamamoto N, Takahash K, Shimada N. Selective inhibition of human cytomegalovirus replication by a novel nucleoside, oxetanocin G. Antimicrob Agents Chemother. 1988;32(7):1053–1056. doi: 10.1128/aac.32.7.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izuta S, et al. Inhibitory effects of triphosphate derivatives of oxetanocin G and related compounds on eukaryotic and viral DNA polymerases and human immunodeficiency virus reverse transcriptase. J Biochem. 1992;112(1):81–87. doi: 10.1093/oxfordjournals.jbchem.a123870. [DOI] [PubMed] [Google Scholar]
- 7.Morita M, et al. Cloning of oxetanocin A biosynthetic and resistance genes that reside on a plasmid of Bacillus megaterium strain NK84-0128. Biosci Biotechnol Biochem. 1999;63(3):563–566. doi: 10.1271/bbb.63.563. [DOI] [PubMed] [Google Scholar]
- 8.Galperin MY, Koonin EV. Divergence and convergence in enzyme evolution. J Biol Chem. 2012;287(1):21–28. doi: 10.1074/jbc.R111.241976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aravind L, Koonin EV. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci. 1998;23(12):469–472. doi: 10.1016/s0968-0004(98)01293-6. [DOI] [PubMed] [Google Scholar]
- 10.Brown PM, et al. Crystal structure of a substrate complex of myo-inositol oxygenase, a di-iron oxygenase with a key role in inositol metabolism. Proc Natl Acad Sci USA. 2006;103(41):15032–15037. doi: 10.1073/pnas.0605143103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovering AL, Capeness MJ, Lambert C, Hobley L, Sockett RE. The structure of an unconventional HD-GYP protein from Bdellovibrio reveals the roles of conserved residues in this class of cyclic-di-GMP phosphodiesterases. MBio. 2011;2(5):e00163-11. doi: 10.1128/mBio.00163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellini D, et al. Crystal structure of an HD-GYP domain cyclic-di-GMP phosphodiesterase reveals an enzyme with a novel trinuclear catalytic iron centre. Mol Microbiol. 2014;91(1):26–38. doi: 10.1111/mmi.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinemeyer EA, Richter D. In vitro degradation of guanosine tetraphosphate (ppGpp) by an enzyme associated with the ribosomal fraction from Escherichia coli. FEBS Lett. 1977;84(2):357–361. doi: 10.1016/0014-5793(77)80724-2. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman MD, Proudfoot M, Yakunin A, Minor W. Structural insight into the mechanism of substrate specificity and catalytic activity of an HD-domain phosphohydrolase: The 5′-deoxyribonucleotidase YfbR from Escherichia coli. J Mol Biol. 2008;378(1):215–226. doi: 10.1016/j.jmb.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon YJ, et al. Structural and biochemical characterization of bacterial YpgQ protein reveals a metal-dependent nucleotide pyrophosphohydrolase. J Struct Biol. 2016;195(1):113–122. doi: 10.1016/j.jsb.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Yakunin AF, et al. The HD domain of the Escherichia coli tRNA nucleotidyltransferase has 2′,3′-cyclic phosphodiesterase, 2′-nucleotidase, and phosphatase activities. J Biol Chem. 2004;279(35):36819–36827. doi: 10.1074/jbc.M405120200. [DOI] [PubMed] [Google Scholar]
- 17.Sultan SZ, et al. Analysis of the HD-GYP domain cyclic dimeric GMP phosphodiesterase reveals a role in motility and the enzootic life cycle of Borrelia burgdorferi. Infect Immun. 2011;79(8):3273–3283. doi: 10.1128/IAI.05153-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wörsdörfer B, et al. Organophosphonate-degrading PhnZ reveals an emerging family of HD domain mixed-valent diiron oxygenases. Proc Natl Acad Sci USA. 2013;110(47):18874–18879. doi: 10.1073/pnas.1315927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miner KD, Klose KE, Kurtz DM., Jr An HD-GYP cyclic di-guanosine monophosphate phosphodiesterase with a non-heme diiron-carboxylate active site. Biochemistry. 2013;52(32):5329–5331. doi: 10.1021/bi4009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing G, et al. A coupled dinuclear iron cluster that is perturbed by substrate binding in myo-inositol oxygenase. Biochemistry. 2006;45(17):5393–5401. doi: 10.1021/bi0519607. [DOI] [PubMed] [Google Scholar]
- 21.Goldstone DC, et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011;480(7377):379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 22.Vorontsov II, et al. Characterization of the deoxynucleotide triphosphate triphosphohydrolase (dNTPase) activity of the EF1143 protein from Enterococcus faecalis and crystal structure of the activator-substrate complex. J Biol Chem. 2011;286(38):33158–33166. doi: 10.1074/jbc.M111.250456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan RP, et al. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc Natl Acad Sci USA. 2006;103(17):6712–6717. doi: 10.1073/pnas.0600345103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Bollinger JM, Jr, Diao Y, Matthews ML, Xing G, Krebs C. myo-Inositol oxygenase: A radical new pathway for O(2) and C-H activation at a nonheme diiron cluster. Dalton Trans. 2009;(6):905–914. doi: 10.1039/b811885j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McSorley FR, et al. PhnY and PhnZ comprise a new oxidative pathway for enzymatic cleavage of a carbon-phosphorus bond. J Am Chem Soc. 2012;134(20):8364–8367. doi: 10.1021/ja302072f. [DOI] [PubMed] [Google Scholar]
- 26.Xing G, et al. Oxygen activation by a mixed-valent, diiron(II/III) cluster in the glycol cleavage reaction catalyzed by myo-inositol oxygenase. Biochemistry. 2006;45(17):5402–5412. doi: 10.1021/bi0526276. [DOI] [PubMed] [Google Scholar]
- 27.Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988;171(2):266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 28.Proudfoot M, et al. General enzymatic screens identify three new nucleotidases in Escherichia coli. Biochemical characterization of SurE, YfbR, and YjjG. J Biol Chem. 2004;279(52):54687–54694. doi: 10.1074/jbc.M411023200. [DOI] [PubMed] [Google Scholar]
- 29.Thorsell AG, et al. Structural and biophysical characterization of human myo-inositol oxygenase. J Biol Chem. 2008;283(22):15209–15216. doi: 10.1074/jbc.M800348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckstein MH, He J, Rubin H. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J Bacteriol. 2008;190(2):718–726. doi: 10.1128/JB.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bochner BR, Ames BN. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982;257(16):9759–9769. [PubMed] [Google Scholar]
- 32.Kondo N, et al. Structure of dNTP-inducible dNTP triphosphohydrolase: Insight into broad specificity for dNTPs and triphosphohydrolase-type hydrolysis. Acta Crystallogr D Biol Crystallogr. 2007;63(Pt 2):230–239. doi: 10.1107/S0907444906049262. [DOI] [PubMed] [Google Scholar]
- 33.Hogg T, Mechold U, Malke H, Cashel M, Hilgenfeld R. Conformational antagonism between opposing active sites in a bifunctional RelA/SpoT homolog modulates (p)ppGpp metabolism during the stringent response [corrected] Cell. 2004;117(1):57–68. Erratum in Cell (2004) 117(3):415. doi: 10.1016/s0092-8674(04)00260-0. [DOI] [PubMed] [Google Scholar]
- 34.van Staalduinen LM, et al. Crystal structure of PhnZ in complex with substrate reveals a di-iron oxygenase mechanism for catabolism of organophosphonates. Proc Natl Acad Sci USA. 2014;111(14):5171–5176. doi: 10.1073/pnas.1320039111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cramer P, Bushnell DA, Kornberg RD. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science. 2001;292(5523):1863–1876. doi: 10.1126/science.1059493. [DOI] [PubMed] [Google Scholar]
- 36.Westover KD, Bushnell DA, Kornberg RD. Structural basis of transcription: Nucleotide selection by rotation in the RNA polymerase II active center. Cell. 2004;119(4):481–489. doi: 10.1016/j.cell.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Frey PA. Stereochemistry of enzymatic-reactions of phosphates. Tetrahedron. 1982;38(11):1541–1567. [Google Scholar]
- 38.Moore SJ, Mayer MJ, Biedendieck R, Deery E, Warren MJ. Towards a cell factory for vitamin B12 production in Bacillus megaterium: Bypassing of the cobalamin riboswitch control elements. N Biotechnol. 2014;31(6):553–561. doi: 10.1016/j.nbt.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Sissi C, Palumbo M. Effects of magnesium and related divalent metal ions in topoisomerase structure and function. Nucleic Acids Res. 2009;37(3):702–711. doi: 10.1093/nar/gkp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raux E, Lanois A, Warren MJ, Rambach A, Thermes C. Cobalamin (vitamin B12) biosynthesis: Identification and characterization of a Bacillus megaterium cobI operon. Biochem J. 1998;335(Pt 1):159–166. doi: 10.1042/bj3350159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broderick JB, Duffus BR, Duschene KS, Shepard EM. Radical S-adenosylmethionine enzymes. Chem Rev. 2014;114(8):4229–4317. doi: 10.1021/cr4004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf YI, Grishin NV, Koonin EV. Estimating the number of protein folds and families from complete genome data. J Mol Biol. 2000;299(4):897–905. doi: 10.1006/jmbi.2000.3786. [DOI] [PubMed] [Google Scholar]
- 43.Gerlt JA, Babbitt PC. Divergent evolution of enzymatic function: mechanistically diverse superfamilies and functionally distinct suprafamilies. Annu Rev Biochem. 2001;70:209–246. doi: 10.1146/annurev.biochem.70.1.209. [DOI] [PubMed] [Google Scholar]
- 44.Norbeck DW, Kramer JB. Synthesis of (-)-Oxetanocin. J Am Chem Soc. 1988;110(21):7217–7218. [Google Scholar]
- 45.Otwinowski Z, et al. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 46.Bunkóczi G, Read RJ. Improvement of molecular-replacement models with Sculptor. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):303–312. doi: 10.1107/S0907444910051218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 49.Moriarty NW, Grosse-Kunstleve RW, Adams PD. Electronic Ligand Builder and Optimization Workbench (eLBOW): A tool for ligand coordinate and restraint generation. Acta Crystallogr D Biol Crystallogr. 2009;65(Pt 10):1074–1080. doi: 10.1107/S0907444909029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morin A, et al. Collaboration gets the most out of software. eLife. 2013;2:e01456. doi: 10.7554/eLife.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 52.Dolinsky TJ, et al. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007;35(Web Server issue):W522–525. doi: 10.1093/nar/gkm276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32(Web Server issue):W665–667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98(18):10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]