Significance
One of the greatest challenges in projecting future shifts in the global climate is understanding how soil respiration rates will change with warming. Multiple experimental warming studies have explored this response, but no consensus has been reached. Based on a global synthesis of 27 experimental warming studies spanning nine biomes, we find that although warming increases soil respiration rates, there is limited evidence for a shifting respiration response with experimental warming. We also note a universal decline in the temperature sensitivity of respiration at soil temperatures >25 °C. Together, our data indicate that future respiration rates are likely to follow the current temperature response function, but higher latitudes will be more responsive to warmer temperatures.
Keywords: soil respiration, climate change, experimental warming, temperature sensitivity, biome
Abstract
The respiratory release of carbon dioxide (CO2) from soil is a major yet poorly understood flux in the global carbon cycle. Climatic warming is hypothesized to increase rates of soil respiration, potentially fueling further increases in global temperatures. However, despite considerable scientific attention in recent decades, the overall response of soil respiration to anticipated climatic warming remains unclear. We synthesize the largest global dataset to date of soil respiration, moisture, and temperature measurements, totaling >3,800 observations representing 27 temperature manipulation studies, spanning nine biomes and over 2 decades of warming. Our analysis reveals no significant differences in the temperature sensitivity of soil respiration between control and warmed plots in all biomes, with the exception of deserts and boreal forests. Thus, our data provide limited evidence of acclimation of soil respiration to experimental warming in several major biome types, contrary to the results from multiple single-site studies. Moreover, across all nondesert biomes, respiration rates with and without experimental warming follow a Gaussian response, increasing with soil temperature up to a threshold of ∼25 °C, above which respiration rates decrease with further increases in temperature. This consistent decrease in temperature sensitivity at higher temperatures demonstrates that rising global temperatures may result in regionally variable responses in soil respiration, with colder climates being considerably more responsive to increased ambient temperatures compared with warmer regions. Our analysis adds a unique cross-biome perspective on the temperature response of soil respiration, information critical to improving our mechanistic understanding of how soil carbon dynamics change with climatic warming.
Compared with anthropogenic emissions, roughly nine times more carbon dioxide (CO2) is released from soils to the atmosphere via soil respiration on an annual basis (1). Both plant root respiration and microbial respiration during the decomposition of organic matter contribute to this efflux of carbon (C) from soils, cumulatively estimated at ∼90 Pg C⋅yr−1 (2). Rising temperatures are expected to stimulate soil respiration (3), both by accelerating rates of C cycling via autotrophic respiration and by providing a potentially powerful positive feedback to climatic warming via heterotrophic decomposition of organic matter. However, due to a suite of factors beyond temperature that control soil respiration rates (e.g., soil moisture, C substrate quality and quantity, and nutrient availability), the interaction between temperature and respiration remains uncertain (3–5). As such, soil respiration is a major and poorly understood flux in the global C cycle.
Experimental warming of soils is one approach used to understand the complex relationship between respiration and temperature because it allows scientists to separate the effects of warming from confounding environmental variation (e.g., soil type and plant species composition). Results of experimental studies reveal a range of responses of soil respiration to warming, with few unifying trends observed across biomes (6–8). Although warming has been shown to stimulate soil respiration within many sites, several studies show neutral or even negative responses to warming, often attributed to moisture limitation (9, 10), shifts in microbial physiological response or composition (11–13), or depletion of labile C pools (14–17). As such, multiple single-site analyses find evidence of acclimation (sometimes termed thermal adaptation) of soil respiration to experimental warming (10–14, 16, 17), although others report no evidence for such shifts in respiration response over time (18–20). Moreover, the response of soil respiration to temperature is not consistent across all temperature ranges, because the temperature sensitivity of respiration typically decreases under warmer conditions (21, 22). As a result, the interaction between soil respiration and climate warming remains one of the greatest sources of uncertainty in climate projections, despite being an important boundary condition in current Earth system models (ESMs) (4, 23, 24).
Current understanding of how soil respiration responds to experimental warming stems from single-site warming experiments or traditional metaanalyses based on average or cumulative soil respiration values in control versus warmed plots. To date, no cross-biome synthesis efforts of experimental warming have evaluated how temperature and moisture interact at high temporal frequencies to determine rates of soil respiration. Therefore, the goals of this study were to (i) synthesize the results of experimental warming studies to understand how the temperature response function of soil respiration changes with experimental warming treatments across biomes, with respect to both warming duration and seasonality; (ii) investigate the role of soil moisture in driving these responses; and (iii) examine whether a uniform model exists that can describe the response of soil respiration to temperature across all biomes. To do this, we generated an unprecedented global dataset of >3,800 observations of instantaneous soil respiration, soil temperature, and soil moisture based on data from 27 individual warming experiments spanning nine biomes and up to 22 y of experimental warming. Our analysis is unique among soil respiration synthesis efforts focused on warming experiments, in that we used instantaneous observations (i.e., plot-scale measurements of soil respiration averaged from individual sampling events) rather than annual or monthly averaged values to evaluate the temperature response function of soil respiration and the interaction with soil moisture at the global scale.
Results and Discussion
Evaluating Differences in Temperature Response Function with Experimental Warming.
We first sought to determine whether respiration responses from experimentally warmed plots paralleled those of control plots over the seasonal range of temperature variation at the biome scale. After evaluating multiple functional forms, we used a log-quadratic temperature response function because this was the best supported model for most biomes (SI Appendix, Table S3):
| [1] |
where R is soil respiration (µmol C⋅m2⋅s−1) and T is soil temperature (°C). Using this basic model, we included warming treatment as an interaction term to evaluate differences in the temperature response between warmed versus control plots (Table 1). We used this log-quadratic model for all biomes (model d in SI Appendix, Table S3), except the boreal forest and northern shrublands, where a log-linear model [ln(R) = γ0 + γ1T] was the better fit when including the warming treatment interaction term (model c in SI Appendix, Table S3). We evaluated two specific features of the temperature response function: (i) the temperature sensitivity (i.e., the shape of the curve denoted by the first derivative of Eq. 1: ; Table 1) and (ii) the magnitude of the respiration response when T = 0 (i.e., the y intercept of Eq. 1: γ0; Table 1).
Table 1.
Model parameters of soil respiration (natural log, in µmol C⋅m−2⋅s−1) (R) as a function of soil temperature (T) (°C), evaluating the interaction with warming treatment
| Parameters for model: ln(R) ∼ γ0 + γ1T + γ2T2 | ||||||
| Model | γ0 ± SE | γ1 ± SE | γ2 ± SE | n | R2 | T at R max |
| All biomes except desert | 0.39 | |||||
| Control treatment | −1.292 ± 0.079 | 0.204 ± 0.011 | −0.0042 ± 0.0003 | 1075 | 24.2 | |
| Warming treatment | −1.309 ± 0.119 | 0.205 ± 0.015 | −0.0040 ± 0.0005 | 1268 | 25.3 | |
| Desert | 0.42 | |||||
| Control treatment | −2.571 ± 0.062 | 0.019 ± 0.008 | 0.0004 ± 0.0002 | 737 | na | |
| Warming treatment | −3.431 ± 0.088 | 0.072 ± 0.011 | −0.0007 ± 0.0003 | 737 | 55.4 | |
| Boreal forest | 0.84 | |||||
| Control treatment | −0.063 ± 0.045 | 0.109 ± 0.0035 | ns | 160 | na | |
| Warming treatment | −0.010 ± 0.059 | 0.093 ± 0.0043 | ns | 306 | na | |
| Temperate forest | 0.54 | |||||
| Control treatment | −0.813 ± 0.166 | 0.160 ± 0.024 | −0.0025 ± 0.0008 | 239 | 32.0 | |
| Warming treatment | −1.485 ± 0.349 | 0.197 ± 0.042 | −0.0031 ± 0.0012 | 258 | 31.8 | |
| Northern shrubland | 0.63 | |||||
| Control treatment | −1.188 ± 0.081 | 0.142 ± 0.008 | ns | 172 | na | |
| Warming treatment | −1.153 ± 0.115 | 0.141 ± 0.012 | ns | 172 | na | |
| Southern shrubland | 0.25 | |||||
| Control treatment | −1.420 ± 0.421 | 0.157 ± 0.040 | −0.0027 ± 0.0009 | 51 | 29.1 | |
| Warming treatment | −0.485 ± 0.642 | 0.066 ± 0.061 | −0.0010 ± 0.0013 | 51 | 34.4 | |
| Grassland | 0.51 | |||||
| Control treatment | −1.517 ± 0.166 | 0.200 ± 0.024 | −0.0036 ± 0.0006 | 269 | 27.8 | |
| Warming treatment | −1.558 ± 0.244 | 0.205 ± 0.030 | −0.0036 ± 0.0008 | 297 | 28.7 | |
| Temperate agriculture | 0.73 | |||||
| Control treatment | −3.012 ± 0.173 | 0.305 ± 0.030 | −0.0066 ± 0.0012 | 131 | 23.3 | |
| Warming treatment | −3.091 ± 0.291 | 0.313 ± 0.046 | −0.0065 ± 0.0016 | 131 | 24.2 | |
Models run with data from both treatments, with parameters for each treatment calculated using the model equation. Model equation: , with γi = αi + βi. warming treatment (W = 1) or control treatment (W = 0). n, sample size; na, not applicable; ns, not significant; R2, correlation coefficient; and T at R max, soil temperature (°C) when . Parameter units: γ0, ln µmol C⋅m−2⋅s−1; γ1, °C−1; and γ2, °C−2. Bold biome names indicate significant interactions with treatment. All models are significant (P < 0.001). For comparison of model fits, see SI Appendix, Table S3. For model parameters including moisture, see SI Appendix, Table S2.
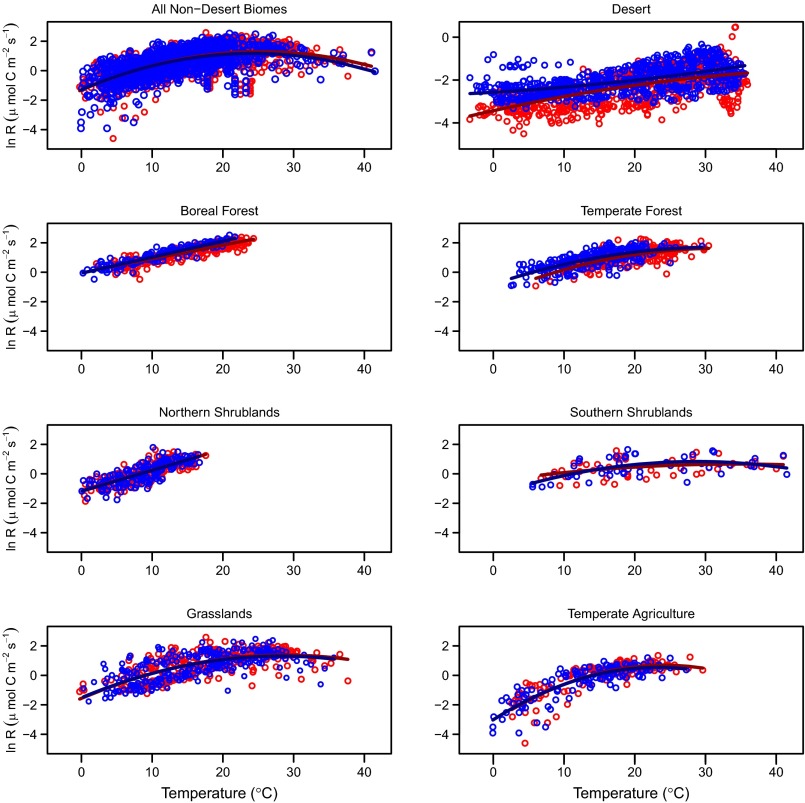
Including data from all warming durations and seasons, we observed no significant differences in the temperature sensitivity of soil respiration between warmed or control treatments within each individual biome, with the exception of boreal forest and desert (Table 1 and Fig. 1). In the boreal forest and desert biomes, where significant differences in the temperature sensitivities between warmed versus control plots were observed, trends between treatments were not consistent; compared with control plots, warmed plots in the boreal forest had consistently lower temperature sensitivity, whereas in the desert, warmed plots had slightly higher temperature sensitivity at temperatures <24 °C but lower sensitivity at temperatures >24 °C (SI Appendix, Fig. S1, and Fig. 2).
Fig. 1.
Ln respiration (µmol C⋅m−2⋅s−1) as a function of soil temperature (°C) across biome types. Data are instantaneous measurements from control (blue circles) and warmed (red circles) treatments, with best fit regression lines fitted through control and warmed values (for coefficients, see Table 1). Temperature sensitivity in control versus warmed plots was not significantly different, except in desert and boreal forest biomes (Table 1). Note that y axis scales are all equal, except for desert, which had lower respiration rates compared with all other biomes (SI Appendix, Fig. S4). For partial regression plots of respiration on temperature and moisture, see SI Appendix, Fig. S7.
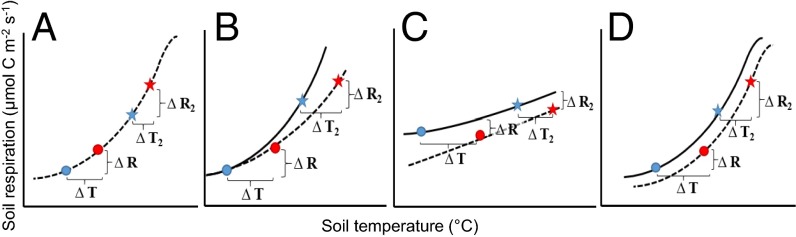
Fig. 2.
Conceptual diagram of instantaneous delta respiration (∆R) and temperature (∆T) response between warmed (red symbols) and control (blue symbols) treatments on a given day of measurements at the lower end of the temperature range (<25 °C). Circles represent sampling date in spring, whereas stars represent sampling date in summer. (A) All nondesert biomes, except boreal forests. Despite the increase of respiration with warming on a given day of measurements, the temperature response function (the dashed line) across the different colors (the warming effect) is similar to that across the different symbols (the seasonal temperature variation). (B) Boreal forests. Warmed plots (dashed line) had lower sensitivity compared with control plots (solid line). However, no significant differences in the y intercept were observed. (C) Desert. Warmed plots (dashed line) had a lower y intercept but higher sensitivity compared with control plots (solid line). (D) Temperate forest. Despite displaying similar temperature sensitivities, y intercepts of warmed plots (dashed line) were marginally (P = 0.06) lower than control plots (solid line). Delta response was always calculated as warmed value minus control value.
The lack of difference in the temperature sensitivity of respiration between control and warmed plots in all biomes except the desert and boreal forests cannot be attributed to an insufficient magnitude of warming. Across our studies, the desert plots were subjected to a relatively small degree of warming (0.34 °C on average) but showed the largest differences in sensitivity between treatments. By contrast, grasslands experienced larger amounts of experimental warming (1.9 °C on average) (SI Appendix, Table S1) but did not display altered sensitivity between treatments.
In addition to evaluating changes in the temperature sensitivities with respiration (i.e., the shape of the temperature response function denoted by γ1 and γ2 in Table 1), we also evaluated differences in the magnitude of respiration rates between treatments (denoted by the y intercept, γ0, in Table 1). The desert was the only biome to display a significantly different y intercept between warmed versus control plots, with warmed plots having a lower y intercept than control plots. Thus, compared with desert control plots, warmed plots emitted less CO2 at a given temperature, despite being generally more sensitive to changes in soil temperature (Fig. 2C). Similar to the desert, temperate forests showed a marginally significant (P = 0.06) trend of emitting less CO2 from warmed plots compared with control plots at a given temperature (γ0 in Table 1 and Fig. 2D). Therefore, although the shapes of the temperature response functions with and without experimental warming were similar in temperate forests, the magnitude of respiration from warmed plots was typically lower than from control plots. In turn, despite little difference in temperature sensitivities between treatments, the reduced fluxes from warmed plots provide evidence of acclimation to experimental warming in the temperate forest.
The lack of difference in temperature response between warmed and control plots in most biomes persists regardless of warming duration or season. For example, by partitioning the observations into categories of warming duration (<2, 2–5, 5–10, and >10 y) and season (growing, nongrowing, and shoulder) and running the model described by Eq. 1, we continued to find no differences in the temperature response function between warmed and control plots, except in the boreal forest and desert. We then ran two additional multivariate regression models that added duration or season as predictors of soil respiration with interactions with warming treatment to our temperature response functions (SI Appendix, Table S3). Here we found similar outcomes, with significant interactions between season and warming treatment observed only in the boreal forest and desert. Significant interactions between duration and warming treatment were also observed in the boreal forest and desert, in addition to the temperate forest and northern shrubland. Thus, over time, respiration from warmed plots appears to respond differently to temperature compared with respiration from control plots in these four biomes (SI Appendix).
Together, our results show a similar temperature response of soil respiration from warmed and control plots across several major biome types, providing limited support of acclimation with experimental warming at the biome scale, across seasons and often independent of warming duration. However, the pronounced difference in the temperature response of respiration between treatments in the boreal forest and desert ecosystems suggests that acclimation of soil communities to warmer conditions is likely to have greater consequences for soil C dynamics in these biomes.
Changes in Soil Moisture with Experimental Warming.
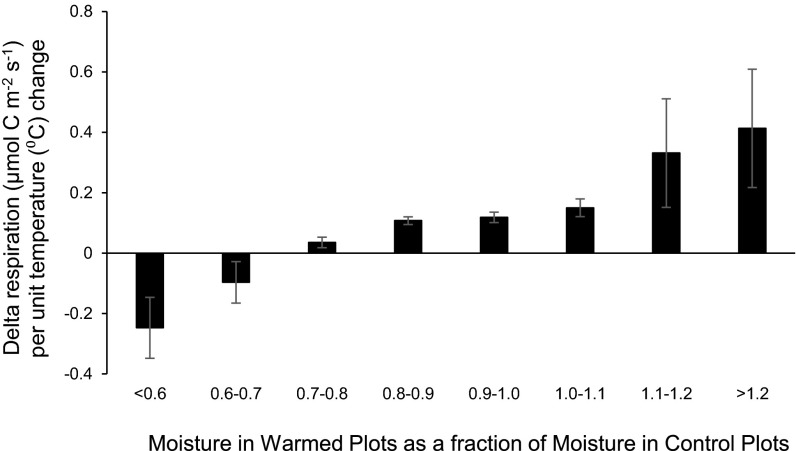
Reductions in soil moisture that accompany experimental warming can influence the soil respiration response to elevated temperatures (25, 26). Using log response ratios as our index of effect size, we found that soil moisture was significantly (P < 0.05) reduced in warmed plots across all sites, with the magnitude of this soil drying being weakly correlated to the amount of soil warming at each site (P = 0.08; r = −0.32; SI Appendix, Fig. S2A). In situations of severe soil drying, we found evidence that soil respiration becomes limited by moisture, which in turn changes the respiration–temperature relationship. For example, not only are the lowest moisture quartiles typically associated with a depressed temperature response function (SI Appendix, Fig. S3; γ0, γ1, and γ2 in SI Appendix, Table S4), but the magnitude of the respiration response to warming decreased linearly with the degree of soil drying across our entire dataset (P < 0.05; Fig. 3). In fact, when moisture of warmed plots dropped by at least 30% relative to control plots, respiration rates were actually lower from warmed plots, despite experiencing higher soil temperatures (Fig. 3 and SI Appendix).
Fig. 3.
Difference in respiration (µmol C⋅m−2⋅s−1) between warmed and control plots normalized by degree of warming (∆T, °C), binned by amount of soil desiccation with warming (soil moisture content warmed plots divided by soil moisture content control plots) across the entire dataset. x axis values <1 indicate warmed plots have less moisture available than control plots. y axis values <0 indicate that respiration rates were lower from warmed plots, despite warmer soil temperatures. Respiration data were not log transformed. Delta respiration was always calculated as warmed values minus control values.
A Universal Decline in Temperature Sensitivity at Seasonally Elevated Temperatures.
Our dataset of instantaneous soil respiration and temperature measurements allowed us to evaluate the temperature response function of soil respiration across biomes. We observed a similar Gaussian response pattern (expressed as a log-quadratic function; Eq. 1) in the soil respiration response across temperature gradients in most nondesert biomes, with respiration rates increasing with temperature up to ∼25 °C (23–34 °C, depending on the biome), above which respiration rates level off and decrease (Table 1; Fig. 1; and SI Appendix, Fig. S4). This common functional form applies to all of the nondesert biomes that reach temperatures above 25 °C (thus excluding boreal forests and northern shrublands), despite variation in temperature response function parameters among biomes (Table 1 and SI Appendix, Fig. S4). Low soil moisture at high temperatures partially explains this decreasing sensitivity at elevated temperatures (SI Appendix, Fig. S3). Nevertheless, respiration rates continue to reach a plateau or even slightly decrease at elevated soil temperatures, even under the wettest conditions in most biomes (SI Appendix, Fig. S3 and Table S4). In turn, we hypothesize that decreased autotrophic demand for ATP and enzyme capacity (27), in addition to microbial enzymatic activities reaching their physiological thermal limit (13, 28), play important roles in the reduced temperature sensitivity under warmer conditions. The desert was again unique among biomes in that control plots did not display decreased sensitivity at such high temperatures, and warmed plots displayed dramatically higher temperature threshold for reduced respiration (55 °C) (Table 1 and Fig. 1). The fundamentally different response of soil respiration to temperature in deserts could be due to several factors, namely, higher respiration temperature optima and maxima of plant and microbial communities in the desert compared with other ecosystems (28) or the importance of abiotic (i.e., UV-driven) decomposition as a major component of litter decomposition in deserts (29).
Regionally Variable Response to Global Change.
The reversal in the direction of the temperature response at temperatures greater than ∼25 °C observed in most nondesert biomes suggests that warmer global temperatures will result in regionally variable responses in soil respiration rates because different regions occupy different positions on the shared temperature–response function. Compared with lower latitudes, higher-latitude sites more often experience soil temperatures <25 °C, where the relationship between soil respiration and temperature is nearly exponential. As such, our data indicate that higher-latitude sites will be more responsive to increased ambient temperatures compared with warmer regions that more frequently experience soil temperatures >25 °C. Our results also support the idea that models of soil respiration based on fixed parameters (e.g., fixed Q10 in an exponential function) are inadequate for describing the respiration response across the full temperature range (4, 21, 22). Without accounting for reduced temperature sensitivity at elevated temperatures, ESMs will likely overestimate soil respiration rates in response to climate warming, particularly from lower-latitude regions.
Limited Evidence of Acclimation of Soil Respiration to Experimental Warming.
Acclimation of soil respiration to soil warming can manifest itself in different ways, both via changing the shape of the temperature response curve (i.e., temperature sensitivity) and position of the curve on the y axis (i.e., y intercept). Our analyses addressed both of these factors, finding evidence of shifting sensitivities only in the desert and boreal forest biomes and lower fluxes at a given temperature (i.e., y intercepts) from warmed plots in the desert (P < 0.01) and temperate forest (P = 0.06) biomes. Such reduced fluxes from warmed plots in the desert and temperate forests could be a consequence of soil drying because desert and temperate forest warmed plots had less soil moisture than control plots (3% and 13% difference in soil moisture between warmed and control plots in desert and temperate forests, respectively). However, reduced C substrate supply (14) and microbial acclimation (11, 13) could be factors contributing to reduced fluxes at a given temperature in these biomes.
The lack of difference in the respiration temperature response functions that we observe between warmed versus control treatments within most biomes highlights a commonality among treatments often not observed in single-site studies (10–14, 16, 17). This finding suggests that in many regions of the globe, simply measuring ambient respiration rates across a seasonal temperature gradient within a site will yield a similar temperature response to measurements made in a soil warming experiment (Fig. 2A). That is, seasonally driven soil respiration–temperature response curves appear to be largely adequate at predicting how future warming will alter fluxes of CO2 from soils to the atmosphere. Nevertheless, the relative roles of autotrophic versus heterotrophic soil respiration and how these processes change with warming remains poorly defined but critical to understanding the strength of soil respiration feedbacks to climate change (30). In addition, it is unclear if the lack of difference in respiration response between control versus warmed treatments that we observe here will persist over the long term because the majority of the extant experiments have a relatively short duration (<5 y). Considering that significant interactions between experiment duration and warming treatment were observed in several biome types, long-term studies are necessary to fully disentangle interactions between warming, soil respiration, and other ecosystem components (e.g., C substrate quality and quantity, nutrient and water availability, and shifts in microbial community) (31).
Our conclusions are based on the largest and highest-resolution global dataset of soil respiration response to experimental warming in existence, to our knowledge. The scale and magnitude of our dataset provide a unique opportunity to enhance our understanding of the sensitivity of global C stocks to warming. However, current understanding of how soil respiration will respond to warmer temperatures is restricted to the types of biomes where experimental warming studies occur, predominantly in North America and Europe. We stress the importance of expanding experimental warming studies to underrepresented regions, specifically the Arctic and the tropics. Northern latitudes are warming faster than other parts of the globe (32) and store extremely large amounts of C in soils (33). However, measurements of ecosystem respiration are far more common than those of soil respiration in the Arctic, making it challenging to tackle the roles of plant versus microbial responses to global change in these systems. Plant and microbial communities in tropical latitudes, where no experimental warming manipulations have been published, may be pushed past their physiological temperature optima with even slight warming. As we demonstrate here, major changes to the shape of the seasonal response curve at higher ambient temperatures are common but not well defined. Thus, exploring the biome-specific responses of soil respiration as temperatures shift beyond the historical range of variability is critical to understanding soil C dynamics in a warmer world.
Methods
Data for this study were obtained from a combination of unpublished data and published literature values (SI Appendix). Our synthesis generated a dataset that includes 3,817 observations, from control (n = 1,812), first-level (i.e., lowest-level or sole) warming (n = 1,812), second- (higher-) level warming (n = 179, four studies), and third-level warming (n = 14, one study) (SI Appendix, Table S1).
Evaluating Temperature Response Functions.
Our models investigated the role of warming treatment, moisture, season, and warming duration in controlling the temperature response function of soil respiration across biomes (SI Appendix). Individual biomes represented by >100 data points were analyzed individually, which excluded montane meadow and tundra ecosystems from being analyzed in isolation. Different multivariate models (SI Appendix, Table S3) were used to investigate different questions (SI Appendix). To evaluate whether respiration responses from the warmed plots paralleled those from control plots, we used multiple linear regression to model respiration as a function of soil temperature, with temperature as a continuous variable and warming treatment as a binary categorical variable (Table 1) (models c and d in SI Appendix, Table S3). The categorical term was accompanied by an interaction with soil temperature, which allowed us to analyze the influence of warming treatment on soil respiration while taking into account the influence of temperature. Our criteria for the warming treatment interaction model selection (model c vs. d in SI Appendix, Table S3) were to (i) include only significant temperature terms and (ii) in models with significant temperature terms, use the Akaike information criterion (AIC) for model selection. We examined differences in the temperature sensitivity between warmed and control plots using the first derivative of Eq. 1 (Table 1). This model is equivalent to R = exp(γ0 + γ1T + γ2T2). However, for boreal forest and northern shrubland data, we used a log-linear model [i.e., R = exp(γ0 + γ1T)] because the second-order temperature term was not significant in models including the treatment interaction for these biomes (Fig. 1 and SI Appendix, Table S3). These two models nearly approximate one another when T is <25 °C, as in the cases of the boreal and northern shrubland. Thus, the better fit of the monotonic log-linear model in the boreal forest and northern shrubland biomes verifies our model choice of the log-quadratic function because the log-quadratic function shows a decreasing trend in soil respiration when temperature is higher than 25 °C. We calculated the temperature threshold of maximum respiration in each biome by setting the derivate of Eq. 1 equal to zero (Table 1). We also compared the AICs of model c or d with models excluding warming treatment as a predictor (model a or b) to further investigate whether warming treatments had an effect on the respiration response (SI Appendix, Table S3); lower AICs for models without the warming treatment term indicate that experimental warming does not alter the shape of the curve to a large degree. One southern shrubland site (Hungary; SI Appendix, Table S1) (34) contained limited data across its temperature gradient and therefore was not included in our analysis of southern shrubland temperature response functions, although the model results with and without inclusion of this site are included in SI Appendix, Table S3, for comparison. To test for a difference in sensitivity between biomes, we ran a multiple linear regression with biome type as a predictor and as an interaction term with temperature (model j in SI Appendix, Table S3).
Data Transformation and Model Diagnostics.
Respiration data were transformed using natural log (which transforms exponential functions into linear functions) to meet assumptions of regression models and to minimize the role of outliers in altering the response functions. In turn, model outputs must be transformed to represent the actual values (i.e., y intercepts in Table 1 should be antilogged to represent the soil respiration flux at 0 °C). All model residuals fit the assumption of normal distributions, except the models of all nondesert biomes together and the temperate agriculture biome in isolation, where residuals were left-tail skewed. Because the desert had significantly lower respiration rates compared with all other biomes (SI Appendix, Fig. S4), models were never run with all data together, because combined residuals were distinctly bimodal. For all models included in our analysis, colinearity between soil moisture and soil temperature was evaluated by calculating variance inflation factors (35), which were always <1.5, indicating extremely limited colinearity. Power analysis (36) revealed power = 1 for all models, except multivariate regression of the southern shrubland warming interaction, where power = 0.95.
Metaanalysis.
We used metaanalysis to quantify (i) how warming altered the magnitude of soil respiration and moisture across sites (SI Appendix, Fig. S2) and (ii) whether first-order temperature sensitivities were different between warmed and control plots at the site level (SI Appendix, Fig. S8). We used the log response ratio (RR) as our index of effect size (37) in determining how warming altered the magnitudes of temperature, respiration, and moisture, which was calculated as the natural log proportional change in the means of the treatment (XT) and the control (XC) groups:
| [2] |
and a random effect model (38). We used the standardized mean difference (raw mean difference divided by pooled SD) and random effect model to determine differences in temperature sensitivities between treatments across sites. All metaanalysis was done using the metafor package in R (39). Effect sizes with 95% confidence intervals overlapping zero indicate no significant effect of warming on the factor in question. Values greater than zero indicate that warming increased soil temperature, soil moisture, soil respiration, and/or temperature sensitivity, whereas values lower than zero indicate that warming decreased these values. In studies with multiple levels of warming treatment (four studies; SI Appendix, Table S1), data from the warmest treatment were used to compute effect sizes. Data from site ID 17 (40) were excluded from SI Appendix, Fig. S2, due to extremely high effect size (RR = 0.95) and small difference in temperature between treatments (∆T = 0.5). All tests of significance level used alpha (α) of 0.05. All analysis and statistics were done in R (version 3.2.0) (41).
Supplementary Material
Acknowledgments
We thank Jill Baron and other Powell Center staff for their support and encouragement for this research. This work was primarily funded by the US Geological Survey (USGS) John Wesley Powell Center for Analysis and Synthesis Award G13AC00193 (to J.T., P.H.T., and K.D.K.). Additional support for J.C.C. was provided by the USGS LandCarbon Program. J.P. and M.E. acknowledge the financial support from the European Research Council Synergy Grant ERC-SyG-2013-610028 IMBALANCE-P, the Spanish Government Grant CGL2013-48074-P, and the Catalan Government Grant SGR 2014-274. B.E., S.R., G.d.D., K.S.L., I.K.S., and A.T. acknowledge the Integrated Network on Climate Research Activities on Shrubland Ecosystems infrastructural project funded by the EC FP7-Infrastructure-2008–1 Grant Agreement 227628. S.B., B.R.J., L.P.-M., and L.L.R. were supported by the Office of Biological and Environmental Research, US Department of Energy, Grant DE-FG02-09ER604719. T.W.C. was supported by a grant from Marie Skłodowska Curie and the British Ecological Society. P.B.R. and W.C.E. acknowledge the financial support by the Office of Biological and Environmental Research, US Department of Energy Grant DE-FG02-07ER64456, and the US National Science Foundation (NSF) Long-Term Ecological Research Program (DEB-1234162), Long-Term Research in Environmental Biology (DEB-1242531), and Ecosystem Sciences (NSF DEB-1120064) Programs. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data have been published by the US Geological Survey (USGS) Powell Center and have been published on USGS ScienceBase (http://dx.doi.org/10.5066/F7MK6B1X) and reviewed by the USGS Woods Hole Coastal & Marine Science Center.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605365113/-/DCSupplemental.
References
- 1.Schlesinger WH, Bernhardt E. Biogeochemistry, An Analysis of Global Change. 3rd Ed Elsevier; Waltham, MA: 2013. [Google Scholar]
- 2.Hashimoto S, et al. Global spatiotemporal distribution of soil respiration modeled using a global database. Biogeosciences. 2015;12(13):4121–4132. [Google Scholar]
- 3.Bond-Lamberty B, Thomson A. Temperature-associated increases in the global soil respiration record. Nature. 2010;464(7288):579–582. doi: 10.1038/nature08930. [DOI] [PubMed] [Google Scholar]
- 4.Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440(7081):165–173. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- 5.Friedlingstein P, et al. Climate–carbon cycle feedback analysis: Results from the C 4 MIP model intercomparison. J Clim. 2006;19(14):3337–3353. [Google Scholar]
- 6.Rustad L, et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia. 2001;126(4):543–562. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- 7.Lu M, et al. Responses of ecosystem carbon cycle to experimental warming: A meta-analysis. Ecology. 2013;94(3):726–738. doi: 10.1890/12-0279.1. [DOI] [PubMed] [Google Scholar]
- 8.Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob Change Biol. 2011;17(2):927–942. [Google Scholar]
- 9.Suseela V, Conant RT, Wallenstein MD, Dukes JS. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob Change Biol. 2012;18(1):336–348. [Google Scholar]
- 10.Reynolds LL, Johnson BR, Pfeifer-Meister L, Bridgham SD. Soil respiration response to climate change in Pacific Northwest prairies is mediated by a regional Mediterranean climate gradient. Glob Change Biol. 2015;21(1):487–500. doi: 10.1111/gcb.12732. [DOI] [PubMed] [Google Scholar]
- 11.Luo Y, Wan S, Hui D, Wallace LL. Acclimatization of soil respiration to warming in a tall grass prairie. Nature. 2001;413(6856):622–625. doi: 10.1038/35098065. [DOI] [PubMed] [Google Scholar]
- 12.Bradford MA, et al. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett. 2008;11(12):1316–1327. doi: 10.1111/j.1461-0248.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- 13.Crowther TW, Bradford MA. Thermal acclimation in widespread heterotrophic soil microbes. Ecol Lett. 2013;16(4):469–477. doi: 10.1111/ele.12069. [DOI] [PubMed] [Google Scholar]
- 14.Melillo JM, et al. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298(5601):2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- 15.Kirschbaum MUF. Soil respiration under prolonged soil warming: Are rate reductions caused by acclimation or substrate loss? Glob Change Biol. 2004;10(11):1870–1877. [Google Scholar]
- 16.Knorr W, Prentice IC, House JI, Holland EA. Long-term sensitivity of soil carbon turnover to warming. Nature. 2005;433(7023):298–301. doi: 10.1038/nature03226. [DOI] [PubMed] [Google Scholar]
- 17.Hartley IP, Heinmeyer A, Ineson P. Effects of three years of soil warming and shading on the rate of soil respiration: Substrate availability and not thermal acclimation mediates observed response. Glob Change Biol. 2007;13(8):1761–1770. [Google Scholar]
- 18.Vicca S, et al. No signs of thermal acclimation of heterotrophic respiration from peat soils exposed to different water levels. Soil Biol Biochem. 2009;41(9):2014–2016. [Google Scholar]
- 19.Jing X, et al. No temperature acclimation of soil extracellular enzymes to experimental warming in an alpine grassland ecosystem on the Tibetan Plateau. Biogeochemistry. 2014;117(1):39–54. [Google Scholar]
- 20.Hartley IP, Hopkins DW, Garnett MH, Sommerkorn M, Wookey PA. Soil microbial respiration in arctic soil does not acclimate to temperature. Ecol Lett. 2008;11(10):1092–1100. doi: 10.1111/j.1461-0248.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd J, Taylor JA. On the temperature dependence of soil respiration. Funct Ecol. 1994;8(3):315–323. [Google Scholar]
- 22.Tjoelker MG, Oleksyn J, Reich PB. Modelling respiration of vegetation: Evidence for a general temperature-dependent Q10. Glob Change Biol. 2001;7(2):223–230. [Google Scholar]
- 23.Exbrayat J-F, Pitman AJ, Zhang Q, Abramowitz G, Wang Y-P. Examining soil carbon uncertainty in a global model: Response of microbial decomposition to temperature, moisture and nutrient limitation. Biogeosciences. 2013;10(11):7095–7108. [Google Scholar]
- 24.Crowther TW, et al. Biotic interactions mediate soil microbial feedbacks to climate change. Proc Natl Acad Sci USA. 2015;112(22):7033–7038. doi: 10.1073/pnas.1502956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu W, et al. A meta-analysis of the response of soil moisture to experimental warming. Environ Res Lett. 2013;8(4):044027. [Google Scholar]
- 26.Sierra CA, Trumbore SE, Davidson EA, Vicca S, Janssens I. Sensitivity of decomposition rates of soil organic matter with respect to simultaneous changes in temperature and moisture. J Adv Model Earth Syst. 2015;7(1):335–356. [Google Scholar]
- 27.Atkin OK, Edwards EJ, Loveys BR. Response of root respiration to changes in temperature and its relevance to global warming. New Phytol. 2000;147(1):141–154. [Google Scholar]
- 28.Balser TC, Wixon DL. Investigating biological control over soil carbon temperature sensitivity. Glob Change Biol. 2009;15(12):2935–2949. [Google Scholar]
- 29.Austin AT, Vivanco L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature. 2006;442(7102):555–558. doi: 10.1038/nature05038. [DOI] [PubMed] [Google Scholar]
- 30.Tang J, Misson L, Gershenson A, Cheng W, Goldstein AH. Continuous measurements of soil respiration with and without roots in a ponderosa pine plantation in the Sierra Nevada Mountains. Agric Meteorol. 2005;132(3):212–227. [Google Scholar]
- 31.Melillo JM, et al. Soil warming, carbon-nitrogen interactions, and forest carbon budgets. Proc Natl Acad Sci USA. 2011;108(23):9508–9512. doi: 10.1073/pnas.1018189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serreze MC, Barry RG. Processes and impacts of Arctic amplification: A research synthesis. Global Planet Change. 2011;77(1-2):85–96. [Google Scholar]
- 33.Tarnocai C, et al. Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochem Cycles. 2009;23(2):GB2023. [Google Scholar]
- 34.Lellei-Kovács E, et al. Experimental warming does not enhance soil respiration in a semiarid temperate forest-steppe ecosystem. Community Ecol. 2008;9(1):29–37. [Google Scholar]
- 35.Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87(417):178–183. [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Ed Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 37.Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80(4):1150–1156. [Google Scholar]
- 38.Curtis PS, Wang X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia. 1998;113(3):299–313. doi: 10.1007/s004420050381. [DOI] [PubMed] [Google Scholar]
- 39.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 40.Flanagan LB, Sharp EJ, Letts MG. Response of plant biomass and soil respiration to experimental warming and precipitation manipulation in a Northern Great Plains grassland. Agric For Meteorol. 2013;173:40–52. [Google Scholar]
- 41.R Core Team 2015 R: A language and environment for statistical computing. www.r-project.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.