Significance
Generation of new neurons is maintained in the adult hippocampus throughout life. The process, which is driven by an exhaustible reservoir of neuronal stem cells (NSCs), greatly declines with age, however. We show that even a short, episodic exposure to the angiogenic factor VEGF and a resultant ramification/rejuvenation of the vasculature within the stem cell microenvironment (“niche”) is sufficient for neurogenesis to proceed at a markedly elevated rate for months later without accelerating the rate of NSC depletion. Importantly, this manipulation culminates in marked attenuation of age-dependent neurogenic decline. Long-term neurogenic enhancement via VEGF preconditioning was found to be associated with extensive NSC morphological remodeling resembling a “juvenile” pattern of NSC and blood vessel engagements.
Keywords: adult neurogenesis, VEGF, neurovascular, aging, neural stem cells
Abstract
Several factors are known to enhance adult hippocampal neurogenesis but a factor capable of inducing a long-lasting neurogenic enhancement that attenuates age-related neurogenic decay has not been described. Here, we studied hippocampal neurogenesis following conditional VEGF induction in the adult brain and showed that a short episode of VEGF exposure withdrawn shortly after the generation of durable new vessels (but not under conditions where newly made vessels failed to persist) is sufficient for neurogenesis to proceed at a markedly elevated level for many months later. Continual neurogenic increase over several months was not accompanied by accelerated exhaustion of the neuronal stem cell (NSC) reserve, thereby allowing neurogenesis to proceed at a markedly elevated rate also in old mice. Neurogenic enhancement by VEGF preconditioning was, in part, attributed to rescue of age-related NSC quiescence. Remarkably, VEGF caused extensive NSC remodelling manifested in transition of the enigmatic NSC terminal arbor onto long cytoplasmic processes engaging with and spreading over even remote blood vessels, a configuration reminiscent of early postnatal “juvenile” NSCs. Together, these findings suggest that VEGF preconditioning might be harnessed for long-term neurogenic enhancement despite continued exposure to an “aged” systemic milieu.
The reserve of neuronal stem cells (NSCs) in the hippocampal dentate gyrus (DG) continues to produce neuroblasts maturing into functional neurons after birth. Adult hippocampal neurogenesis, however, rapidly declines with age, and in the rodent brain, it is barely detected beyond 8 mo of age (1–4). Several factors, including BDNF, FGF, insulin-like growth factor, VEGF, and others, were shown to enhance the basal level of hippocampal neurogenesis (reviewed in ref. 5). However, there is no report of a factor capable of inducing sustained neurogenic enhancement lasting months after its withdrawal.
It has been shown that age-related decrease in hippocampal neurogenesis is associated with progressive depletion of the finite NSC pool due to the fact that neuroblast-producing asymmetrical NSC divisions can only take place a limited number of times before their terminal differentiation to astrocytes (2). Supporting the notion of an exhaustible NSC reservoir is also a study showing that experimentally forced accelerated neurogenesis results in premature NSC depletion (6) and a study showing that experimentally induced epilepsy is associated with premature NSC exhaustion (7). Balancing this deterministic view, however, certain genetic manipulations [e.g., experimental phosphatase and tensin homolog (PTEN) deletion] were shown to increase NSC number by symmetrical divisions (8). It thus remains unclear whether there are means to enforce enhanced hippocampal neurogenesis for a long period without necessarily causing premature exhaustion of the NSC reservoir and a resultant accelerated age-related neurogenic decay.
Stem cells (SCs) in adult organs reside in specialized niches and continuous communication with niche cells is required for proper SC function. Physical proximity between SCs and blood vessels (BVs) in different adult organs has prompted the contention that BVs are an indispensable component of SC niches (9–13). Although an adequate vascular support is clearly essential for maintaining proper SC function, whether SC function and output can be further improved through targeted vascular manipulations has not been examined.
In an attempt to produce long-lasting neurogenic increase persisting in aged mice, we herein used a transgenic system designed for brain-specific conditional (and reversible) VEGF gain of function that expands and rejuvenates the niche vasculature (14). VEGF is a highly pleiotropic factor shown to affect brain function in many different ways, including increasing adult hippocampal neurogenesis (reviewed in ref. 15). Here, we made extensive use of a VEGF on/off switchable system for uncoupling VEGF functions, requiring its ongoing signaling from functions primed by VEGF but not requiring its continued presence, primarily functions attributed to vasculatures laid down by VEGF.
Physical proximity between NSCs and BVs was demonstrated per the two major neurogenic niches in the adult rodent brain, namely, the subventricular zone (SVZ) and the hippocampal DG (9–12). Per SVZ neurogenesis, a direct NSC-BV contact was shown to be required (12, 16) to involve extracellular matrix signaling (11, 17) and ephrinB2/Jagged1 (16) or NT-3 signaling (18). Less is known, however, regarding the nature of physical NSC-BV engagements and communication modes in the DG niche.
Prompted by the view that the quiescent state of SCs, in general, constitutes a poised state for activation by extrinsic factors (19) and by findings that young systemic factors can rescue quiescence of aged stem and progenitor cells (20–22), we examined whether hippocampal NSC quiescence can be rescued by cerebral VEGF gain-of-function despite continued exposure to an “aged” systemic milieu.
Results
A Transgenic System for Conditional VEGF Induction Associated with Expansion and Rejuvenation of the DG Vascular SC Niche.
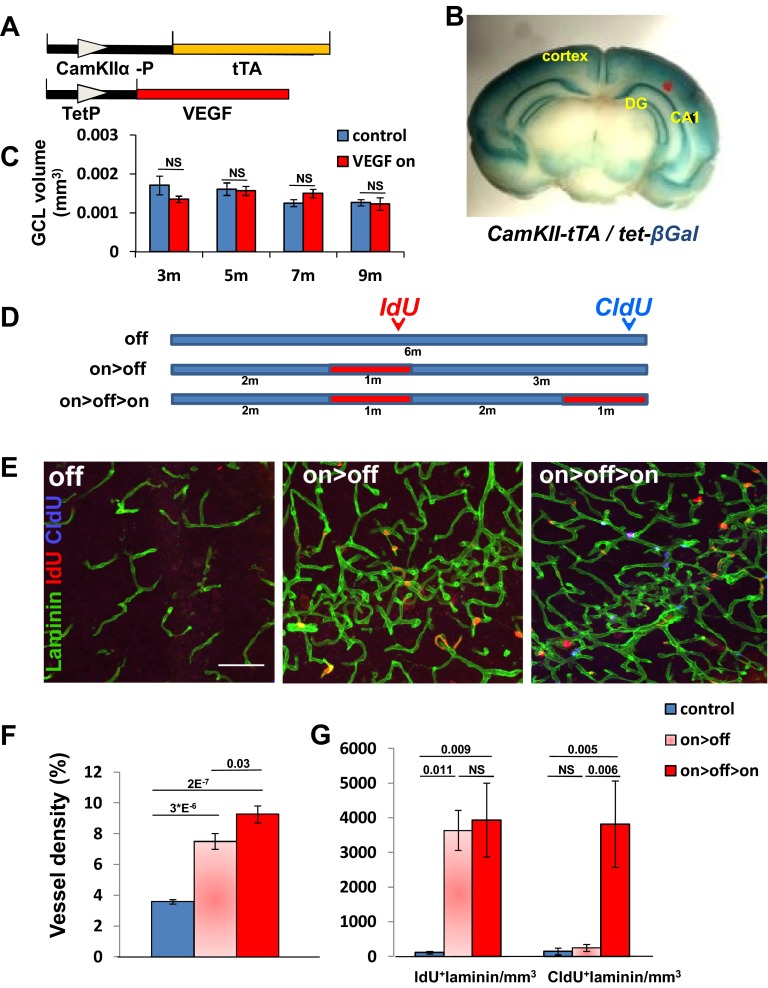
To expand the vasculature in the vicinity of NSCs at the DG subgranular zone (SGZ), VEGF165 was induced in this region. Briefly, calmodulin kinase IIα (CaMKIIα) promoter-driven transactivator protein (23) was used to induce VEGF165 conditionally by withdrawing tetracycline from the drinking water (a scheme of the bitransgenic system used is shown in Fig. S1A). A preparatory experiment using a “responder” transgene encoding βGal reporter corroborated the general utility of the CaMKIIα-tTA (tetracycline-transactivator) driver to promote efficient expression of a responder gene of interest throughout the hippocampus (in addition to other regions in the adult brain) (Fig. S1B).
Fig. S1.
Transgenic system for conditional VEGF-induced expansion and rejuvenation of hippocampal vasculature. (A) Schematic representation of the bitransgenic system used for conditional VEGF induction and deinduction. (B) CaMKIIα-tTA driver transgene induces expression of a tetracycline-regulated lacZ reporter in the hippocampus. CA1, Cornu Ammonis 1. (C) Measurement of the average GCL volume in confocal images of 50-μm thickness representing all areas (in rostral-caudal axis) of the DG. NS, not significant. (D) Experimental protocol for sequential injections of thymidine analogs during VEGF induction and deinduction designed for labeling proliferating cells during the respective VEGF on episodes (red boxes). (E) Representative images of hippocampal sections highlighting vessels (laminin+ cells) and proliferating cells at the two time points. (Scale bar, 50 μm.) Quantification of MVD expressed as a percentage of the total area occupied by vessels (F) and of EC proliferation (G). NS, not significant.
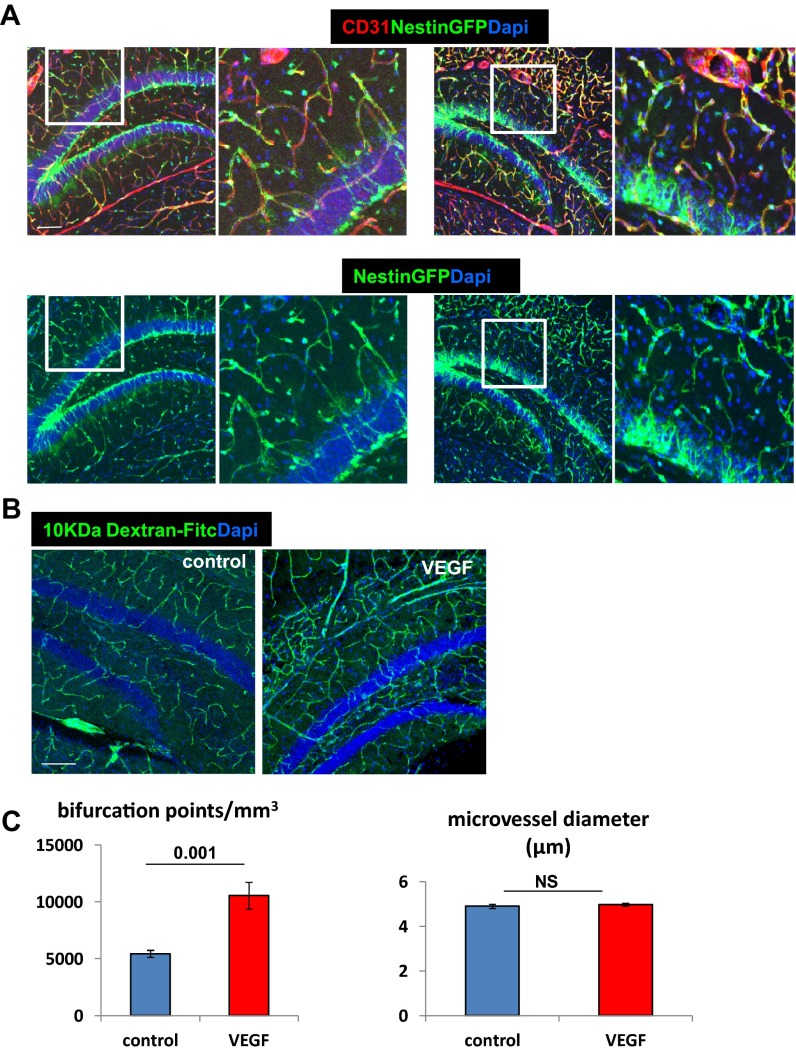
As previously published, VEGF induction elicited a robust angiogenic response evidenced by massive endothelial cell (EC) proliferation and increase of microvascular density (MVD) already detectable within a week from tetracycline withdrawal (14). VEGF-induced added microvessels appeared to have a normal blood–brain barrier (BBB) as judged by their pericyte coverage (Fig. S2A) and a proper BBB function as determined by impermeability to 10 kDa of intracardially injected FITC-labeled dextran (Fig. S2B). Increased MVD was associated with increased vascular complexity (more bifurcation points) without an increase in vessel diameters (Fig. S2C).
Fig. S2.
No change in permeability and vessel diameter in VEGF-induced hippocampal neovessels, although vascular complexity increases. (A) VEGF was induced for 2 wk, and pericytes were visualized with nestin-GFP (detecting both NSCs and pericytes). Note complete pericyte coverage of ECs (CD31+). (Scale bar, 100 μm.) (B) FITC-labeled dextran tracer (10,000 Da) commonly used to detect BBB disruption (53) was injected intracardially before euthanasia of mice in which VEGF was induced 7 d earlier. Luminal dextran reflects an MVD increase, whereas the absence of parenchymal tracer indicates a functional BBB. (Scale bar, 100 μm.) (C) Quantification of the number of microvessel bifurcations and diameter in animals induced by VEGF 1 mo before euthanasia. NS, not significant.
To follow phenotypes resulting from vascular expansion without the confounding factors associated with ongoing ectopic VEGF expression, VEGF was deinduced at the indicated time by readding tetracycline (VEGF on>off protocol; the scheme of a prototypic experiment is shown in Fig. S1D). VEGF ELISA and quantitative PCR measurements secured that transgenic VEGF is rapidly silenced and is undetectable within 2 wk from readding tetracycline (14) (see Fig. 2B). Inspecting the DG vasculature several months after VEGF deinduction indicated that vessels induced by VEGF are durable (Fig. S1E). To provide a quantitative estimate for the durability of VEGF-induced vessels, two successive episodes of VEGF induction were exercised, separated by an “off” period of several months (on>off>on protocol), in conjunction with sequential pulsing with iodo-deoxyuridine (IdU) and chloro-deoxyuridine (CldU) to tag actively proliferating ECs differentially during each of the “on” cycles. Results not only proved the feasibility of repetitive expansion/rejuvenation of hippocampal vasculature but showed that previously generated vessels are fully maintained as quiescent vessels for months (Fig. S1 F and G). Long-term persistence of newly added vessels was further evident by their presence 9 mo after VEGF withdrawal (Fig. S3). Importantly, the ability of VEGF-induced hippocampal vascular reinforcement was not blunted at an old age (discussed below).
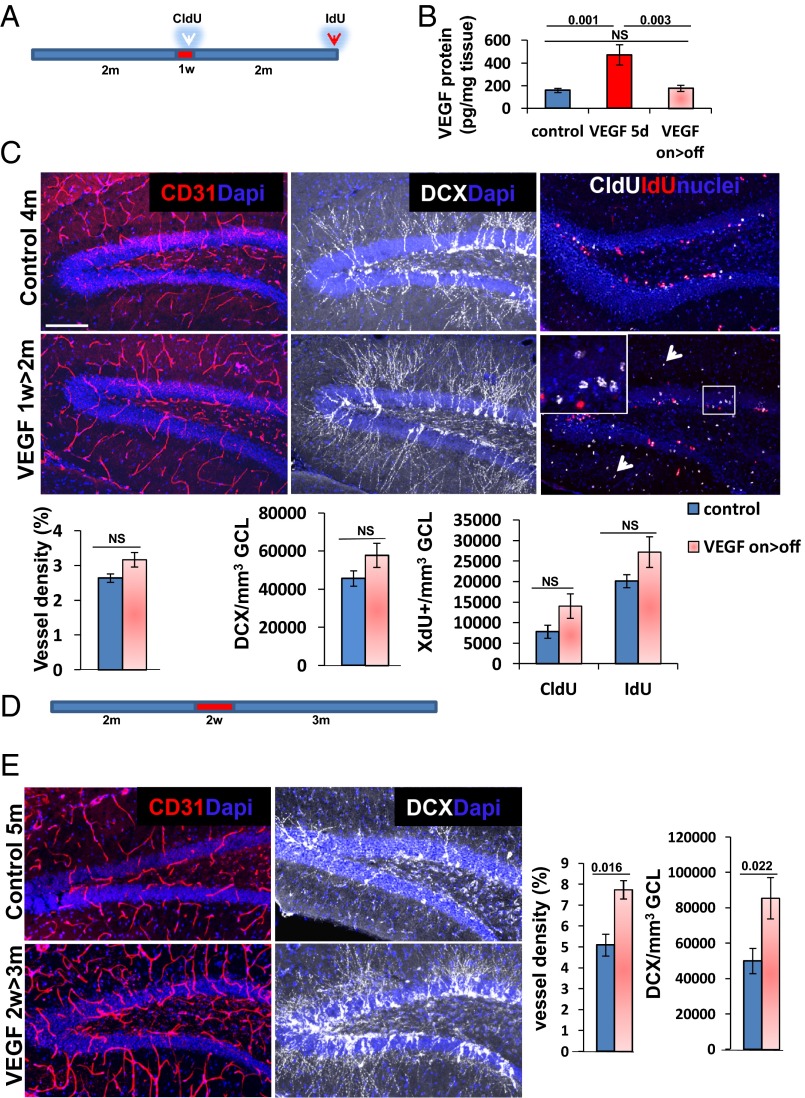
Fig. 2.
VEGF fails to increase neurogenesis in the absence of newly induced durable vessels. (A) VEGF was induced in 2-mo-old mice for 1 wk and then deinduced, and the hippocampus was examined 2 mo thereafter. CldU was injected by the end of the on period, and IdU was injected just before euthanasia. (B) ELISA of hippocampal VEGF in animals killed 5 d postinduction (VEGF 5d) and in animals induced for 2 wk and deinduced for an additional 2 wk (on>off). (C) Immunostaining and quantification for CD31, DCX, and incorporated thymidine analogs. Note no significant differences in MVD and neurogenesis between the controls and on>off group despite a previous exposure to VEGF. Also note the incorporation of CldU in cells in the molecular layer of on>off animals (arrowheads) as an indication of efficient VEGF induction. (D) VEGF was induced for 2 wk and deinduced for 3 mo. (E) CD31 and DCX immunohistochemistry showing that induced vessels and neurogenesis both persist long term after 2 wk of VEGF exposure. NS, not significant. (Scale bar: C and E, 100 μm.)
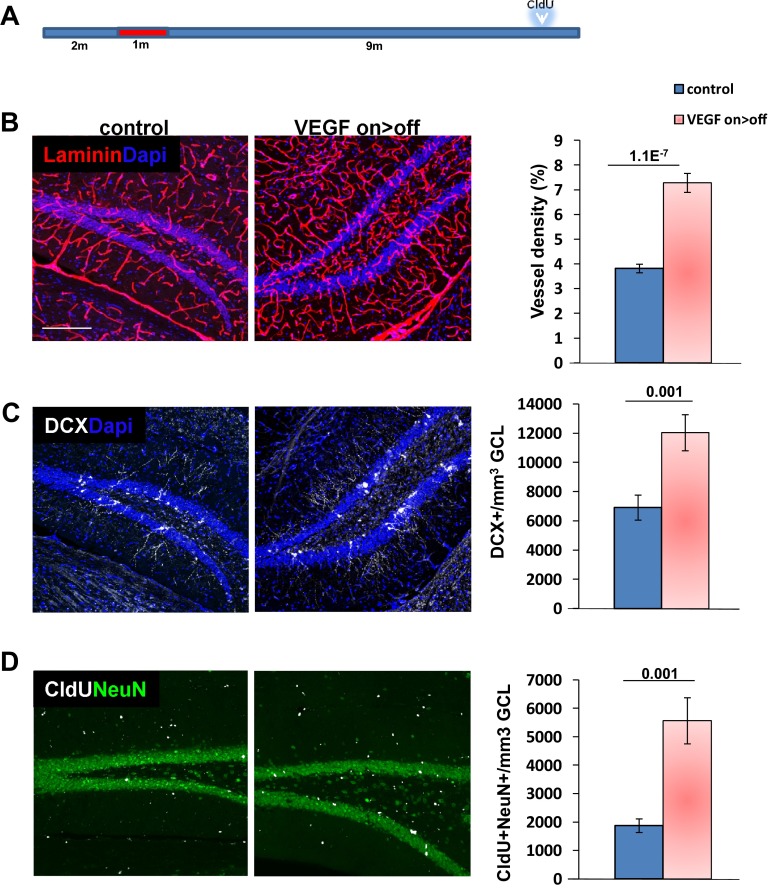
Fig. S3.
Increased rate of neuroblast production and neuronal differentiation is evident 9 mo after VEGF deinduction. (A) VEGF was induced for 1 mo (at the age of 2 mo) and then deinduced for an additional 9 mo. CldU (100 mg/kg, three i.p. injections) was administered 1 mo before euthanasia. (B) Laminin staining for BVs and quantification of vessel density in the DG. (C) DCX staining for neuroblasts and quantification. (D) Cells double-positive for CldU and the mature neuronal marker NeuN highlighting newborn neurons. (Scale bar: B–D, 100 μm.)
VEGF-Induced Neurogenic Increase Is Proportional to the Magnitude of Vascular Increase and Persists Months After VEGF Deinduction.
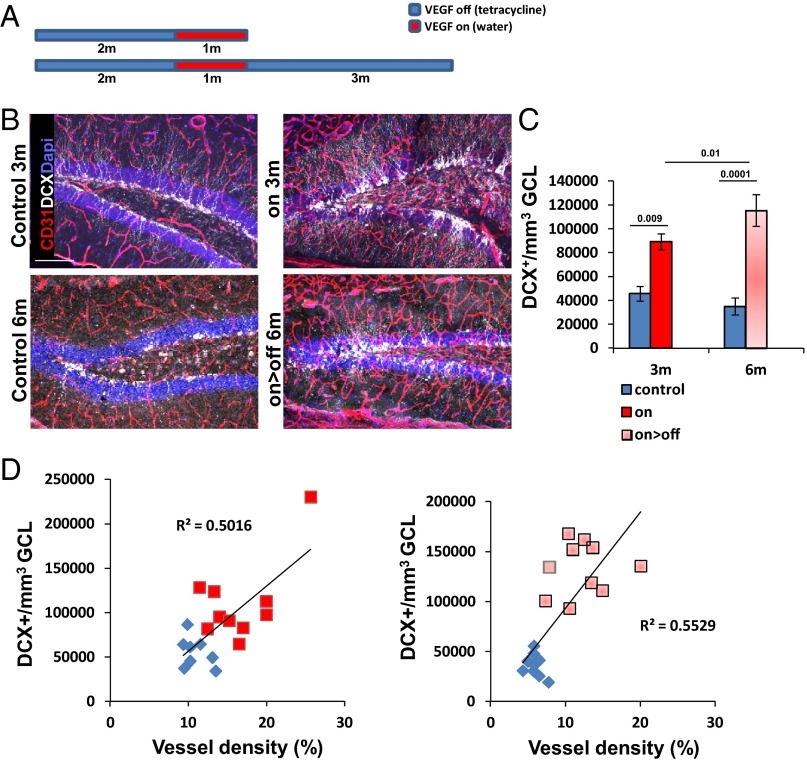
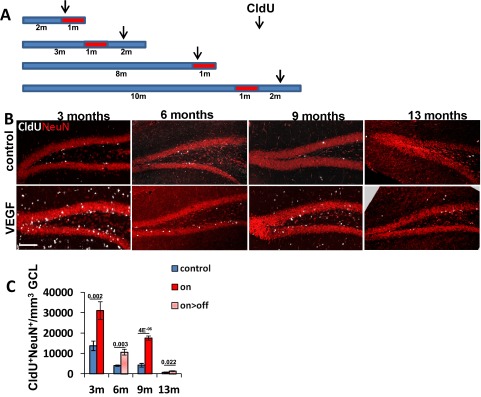
We and others have previously shown that experimental VEGF overexpression leads to an increase in hippocampal neurogenesis (14, 24–26). Our previous findings that VEGF loss of function in the adult hippocampus does not adversely affect hippocampal vessels or neurogenesis and that VEGF is dispensable for basal DG neurogenesis (14, 27) have suggested that the stimulatory effect of VEGF might be attributed to VEGF-induced new vessels rather than to a direct effect of VEGF on ECs and/or on nonvascular cells expressing cognate receptors (24, 28–32). To examine this proposition, we resorted to the use of the VEGF on>off strategy and compared its effect with ongoing VEGF expression (on) (the scheme is shown in Fig. 1A). VEGF led to a robust increase in DG neurogenesis, as evidenced by a large increase in doublecortin (DCX)-positive cells marking newborn neuroblasts. Remarkably, the elevated level of neurogenesis did not subside following the switching off of VEGF and proceeded at a high rate similar to the one observed with ongoing VEGF induction, indicating that ongoing VEGF signaling is not required to maintain an increased neurogenic level (Fig. 1 B and C). In fact, the elevated neurogenic rate measured after VEGF withdrawal was identical to the level measured during its continued presence; in both cases, the magnitude of neurogenic increase was found to be proportional to the increase in MVD (Fig. 1D).
Fig. 1.
VEGF preconditioning associated with increased vessel density is sufficient to promote a dose-dependent increase in DG neurogenesis. (A) VEGF was induced at the indicated time for a duration of 1 mo (red boxes). Depicted ages on the left represent the age at euthanasia. (B and C) Immunostaining for DCX reveals enhanced neurogenesis under both on and on>off experimental regimes. Note also that MVD is increased in both cases, as highlighted by CD31 staining (quantifications are shown in Fig. 3). (Scale bar, 100 μm.) (D) MVD and DCX+ cell density were determined for each mouse participating in this experiment (values presented represent averages calculated from six sections per animal) and plotted as DCX+ cell density versus MVD per each individual mouse. Note that neurogenic increase is proportional to MVD increase regardless of whether VEGF was continuously on (Left) or switched off (Right), respectively. P = 0.0093 for the on group, and P = 0.0004 for the on>off group.
Next, we determined whether augmented neurogenesis would persist even after a more prolonged time following VEGF withdrawal. To this end, VEGF was induced for 1 mo and then deinduced, and neurogenesis was examined 9 mo thereafter. Measuring neurogenesis, either by counting new neurons added 1 mo before euthanasia (identified as cells double-positive for CldU and the panneuronal marker NeuN) or by measuring DCX+ neuroblasts, indicated that up-regulated neurogenesis still exceeds the neurogenic level in littermate controls (Fig. S3).
VEGF Fails to Induce Long-Lasting Neurogenic Enhancement Unless Accompanied by Formation of Durable Vessels.
Data presented above showing that ongoing VEGF expression is dispensable for long-term persistence of enhanced neurogenesis are compatible with the notion that the neurogenesis rate is governed by the added vasculature. Nevertheless, we wished to rule out the possibility of some irreversible, long-lasting effect of VEGF on nonvascular cells expressing cognate VEGF receptors. It should be pointed out, however, that contrary to other reports (24, 31, 32), we failed to detect VEGF receptor 1 (VEGFR1) or VEGFR2 expression in non-ECs within the DG (14). Here, we employed yet another approach of using a VEGFR2-GFP knock-in allele to confirm that expression of this major VEGF signaling receptor is restricted to the DG vasculature (Fig. S4 A–C). Further, EC-restricted VEGFR2 expression (detected by immunostaining) was also evidenced following VEGF induction (Fig. S4D). To prove an essential vascular role, we took advantage of previous findings showing that newly made vessels depend on VEGF for survival and regress if denied VEGF before maturing into a VEGF refractory stage (33, 34). VEGF was therefore induced for 1 wk only, a duration shown as sufficient for new BV formation but insufficient for maintenance, thus resulting in complete regression of newly formed BV shortly after VEGF withdrawal (Fig. 2 A–C). Remarkably, no increase in neurogenesis could be detected despite a week-long VEGF exposure, strongly arguing that neurogenic increase is secondary to VEGF’s angiogenic activity. Delaying VEGF withdrawal for an additional week was indeed sufficient for generating lifelong durable neovessels in the DG and, correspondingly, for markedly increased hippocampal neurogenesis (Fig. 2 D and E).
Fig. S4.
VEGFR2 is expressed exclusively in hippocampal ECs. A knock-in VEGFR2-GFP reporter was used to detect VEGFR2-expressing cells. (A) Costaining with CD31. (Scale bar, 100 μm.) (B) Costaining with GFAP, detecting both astrocytes and NSCs (white, arrows) as well as DCX (red, arrowheads). (Scale bar, 20 μm.) (C) Three-dimensional reconstructions of B (Inset) indicate lack of GFP expression in GFAP+ or DCX+ cells, which are found in close physical proximity to GFP+ capillaries. (D) Immunostaining for VEGFR2 in 3-mo-old VEGF mouse induced for the last month. (Scale bar, 50 μm.)
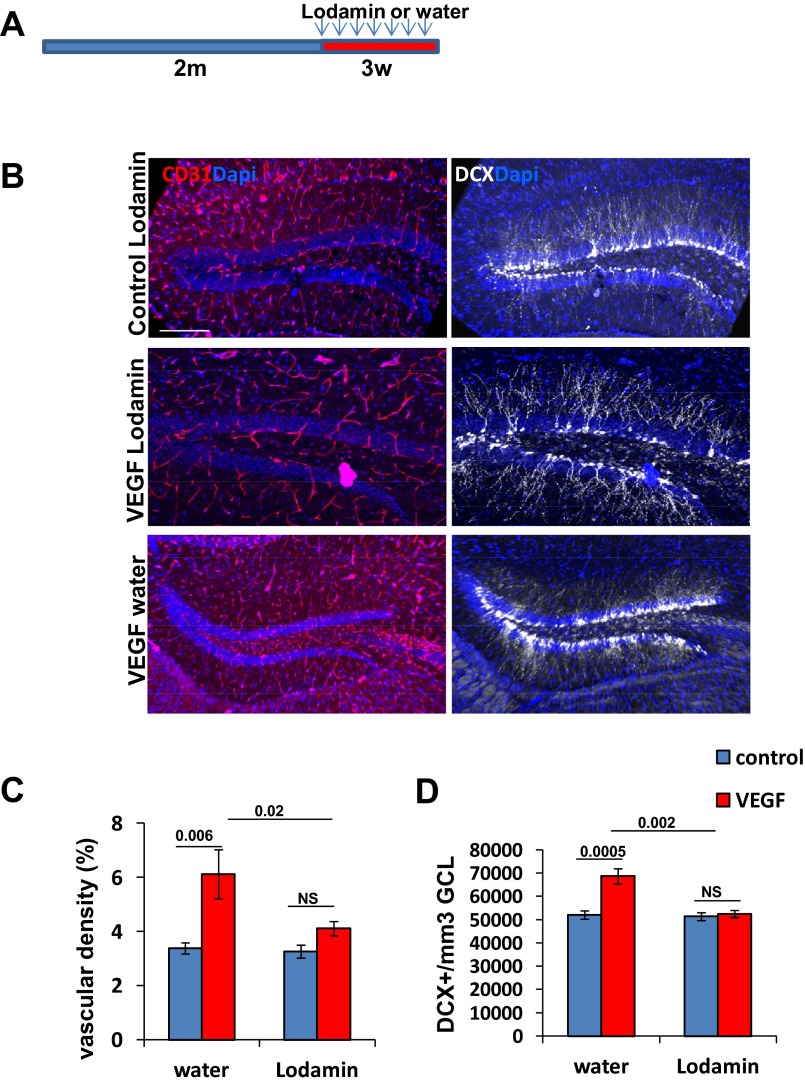
An independent, additional proof that the effects of VEGF on neurogenesis are indeed mediated by the newly formed vessels was obtained using a strategy of a sustained VEGF exposure together with angiogenesis inhibition. Briefly, we used Lodamin, a modified, improved version of TNP-470, a well-known antiangiogenic compound developed by Folkman and coworkers (35). Its mode of action was shown to be irreversible inactivation of methionine animopeptidase-2, leading to EC cycle arrest in late G1 phase (36) in a clearly VEGF-independent mechanism. Lodamin was designed to be impermeable to the BBB; therefore, it has no effect on brain parenchyma (35). As shown in Fig. S5, Lodamin fully inhibited VEGF-induced DG neovascularization and, remarkably, completely negated neurogenic enhancement by VEGF despite a continued 3-wk-long exposure to VEGF. Together, these findings strongly support the conclusion that neurogenic enhancement by VEGF preconditioning is mediated by VEGF-induced vascular changes.
Fig. S5.
VEGF induction together with angiogenesis inhibition fails to increase neurogenesis. (A) VEGF was induced together with Lodamin addition at the age of 2 mo (30 mg/kg in water, administered by oral gavage every other day throughout the induction period of 3 wk). (B) Representative image of CD31 and DCX-stained DG. (Scale bar, 100 μm.) (C and D) Quantification of BVs (C) and neuroblast densities (D). Note efficient inhibition of angiogenesis and no significance increase in DCX+ cells in Lodamin-treated VEGF animals. NS, not significant.
Induction of VEGF in Old Mice and a Resultant Increase in MVD Up-Regulates Already Subsided DG Neurogenesis.
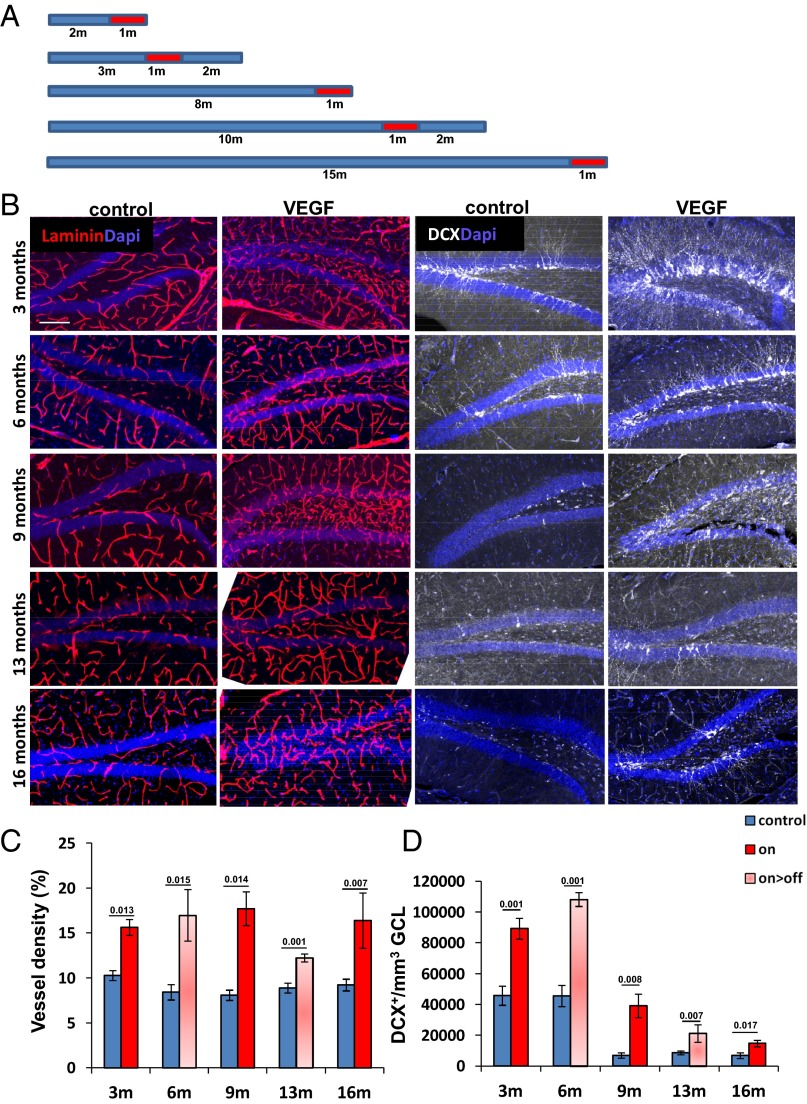
In the experiments described above, we showed that VEGF induction associated with vascular expansion at an early age has a profound effect on DG neurogenesis at later stages, culminating in maintaining a “young” neurogenic level in old mice. We next wished to determine whether the aged hippocampus is still sufficiently plastic to elicit an angiogenic response and to augment neurogenesis in response to VEGF induction. To this end, we delayed the onset of VEGF induction to progressively older ages and measured vascular and neurogenic consequences of these manipulations (an experimental protocol is shown in Fig. 3A). Results showed that VEGF responsiveness has not blunted in the aged hippocampus, as evidenced by an efficient neovascularization response and significant MVD increase (Fig. 3 B and C). Considering that microvascular rarefaction, a process often accompanying natural aging in other organs, is insignificant in the aged DG (Fig. 3C), any contribution of neovessels to neurogenesis might, in principle, be attributed to a vascular rejuvenation effect. VEGF induction at any age examined led to a marked increase in neurogenesis that again persisted after VEGF deinduction, as judged by enumeration of DCX+ neuroblasts (Fig. 3 B and D) or by enumeration of newly generated mature neurons (NeuN+ cells that have incorporated CldU) (Fig. S6). Although the neurogenic output of the young (3–6 mo) hippocampus could not be fully restored in the aged (9–16 mo) hippocampus, presumably due to progressive age-related NSC loss (discussed below), the apparent twofold to fivefold increase in neurogenic levels attained by VEGF induction and preconditioning did not significantly differ between young and old mice.
Fig. 3.
VEGF preconditioning up-regulates DG neurogenesis in old mice. (A) Experimental protocol for VEGF induction at progressive ages, with red boxes indicating the onset of a month-long VEGF induction. (B, Left) Representative images of DG vasculature with or without VEGF induced 1 or 3 mo before euthanasia. Ages indicated on the left represent the age of mice at euthanasia. (B, Right) Serial sections of the above stained with DCX. (Scale bar, 100 μm.) Quantification of vessel densities (C) and DCX+ cell densities (D). Note, in particular, the marked increase in neurogenesis in 13- and 16-mo-old mice with increased vasculature.
Fig. S6.
VEGF preconditioning in old mice increases the production rate of mature neurons. (A) Experimental protocol used was as described for Fig. 4 with the addition of CldU injections (50 mg/kg, three injections at 8-h intervals) 3 wk before euthanasia. (B) Representative images depicting hippocampal cell proliferation. Note a large number of CldU+/NeuN− cells in specimens retrieved when VEGF was still on, representing proliferating ECs but mostly CldU+/NeuN+ cells following VEGF deinduction. (Scale bar, 100 μm.) (C) Quantification of CldU+/NeuN+ cells marking newly formed neurons.
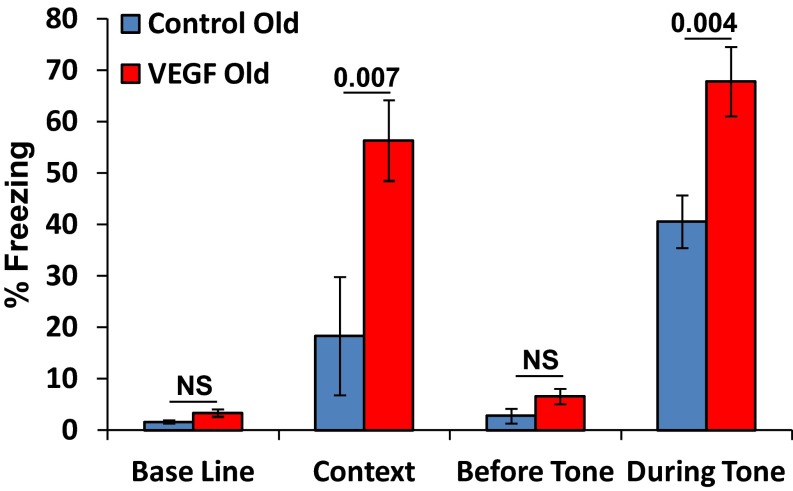
Using the fear-conditioning paradigm, we have previously shown that VEGF overexpression improves memory and that this effect is attributed to VEGF-induced increase of neuronal plasticity rather than to its neurogenic effects (14). To determine whether a short episode of VEGF exposure in aged mice is still capable of enhancing learning, behavioral studies were conducted on 1-y-old mice in which VEGF was preinduced for 2 wk. Results clearly showed that VEGF induction at an old age is still capable of improving learning (Fig. S7).
Fig. S7.
VEGF induction in old mice can improve memory. Twelve-month-old mice were induced by VEGF for 15 d. Memory was tested in the fear-conditioning paradigm. Results show a significant effect of VEGF on the percentage of freezing in the context, an indication of improved hippocampal-dependent memory. No difference was seen in the baseline (before conditioning) between the groups (P = 0.998). There was also a significant effect of VEGF on freezing during the tone, indication of improved cued memory. No difference was seen before the tone between the groups (P = 0.119). NS, not significant.
Neurogenic Enhancement by VEGF Manipulations Is Not Associated with Accelerated NSC Depletion.
Among possible causes for age-related neurogenic decay is a progressive, activity-dependent NSC depletion, reflecting the situation where a finite NSC pool can only yield a correspondingly limited number of new neurons (2). Forced neurogenic increase by either notch manipulation (6) or kainic acid treatment (7) was indeed shown to accelerate NSC depletion. It was of concern, therefore, that increased neurogenesis brought about by VEGF and resultant vascular changes might also come at the expense of premature NSC exhaustion and premature diminution of the neurogenic potential. Results described above showing that maintaining a markedly elevated level of neurogenesis continually for months does not impede neurogenesis at an older age (Figs. 1 and 2 and Fig. S3), to an extent, alleviated this concern.
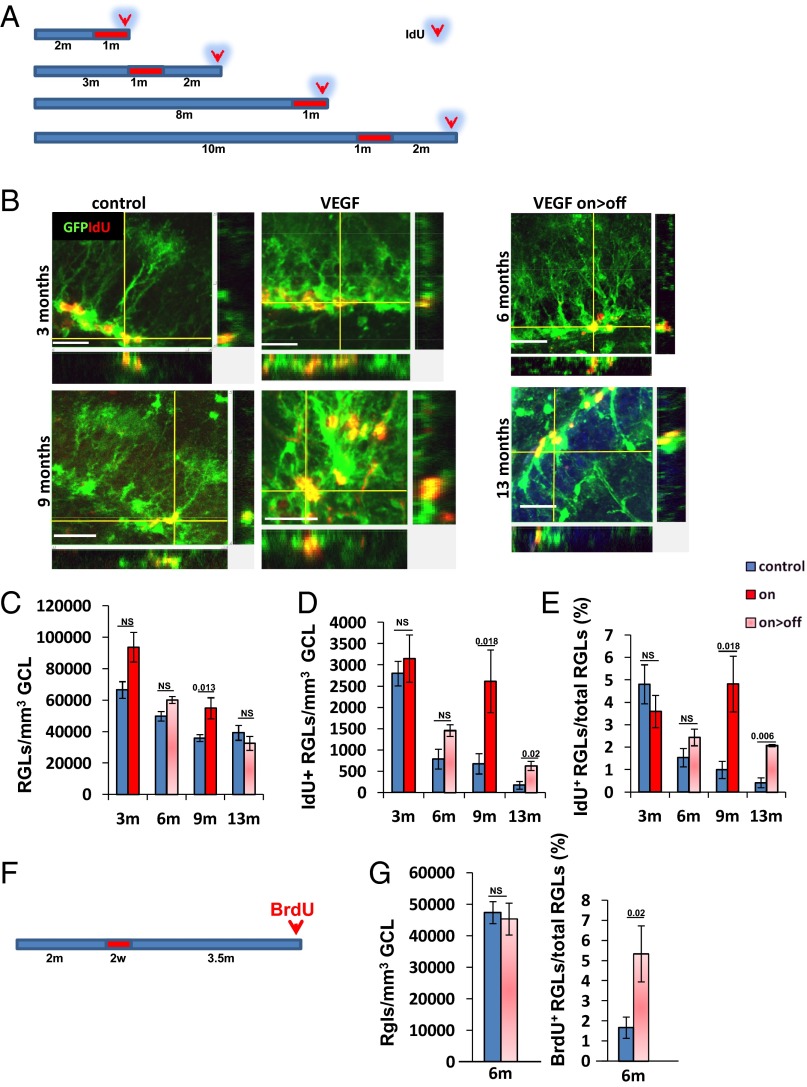
To enumerate NSCs directly, NSCs were visualized with the aid of an NSC transgenic reporter and unequivocally identified in conjunction with their unique morphology (Fig. 4B). Briefly, a nestin-GFP reporter transgene marking both NSCs and intermediate progenitor cells (IPCs) (but not further downstream descendants) (37, 38) was bred onto the VEGF-switchable system, and NSCs were identified in hippocampal sections as GFP+ cells having their cell body embedded in the DG SGZ and their major branch extending toward the granule cell layer (GCL) and terminating with an arbor of dendrite-like branches [a morphology earning these slow-dividing cells the name of radial glia-like cells (RGLs)]. Quantification of RGLs in young (3 mo) and old (13 mo) mice confirmed an age-related decline in total NSC number (66,000 cells per cubic millimeter and 39,000 cells per cubic millimeter, respectively). Remarkably, RGL numbers did not decrease, and even modestly increased, in VEGF-induced mice compared with their age-matched controls (Fig. 4 C and G). These results confirm that increased neurogenesis brought about by VEGF manipulations is not associated with an accelerated NSC depletion.
Fig. 4.
Neurogenic enhancement in old mice is not accompanied by accelerated NSC depletion and is partly attributed to a reduction of age-related NSC quiescence. (A) Schematic representation of the VEGF on>off switching regime (red bar representing on episodes) and IdU labeling (100 mg/kg, three injections 1–2 d before euthanasia) in triple-transgenic mice also harboring a nestin-GFP reporter transgene. (B) Representative z-stack image of dividing RGLs. The x–y and y–z axes also show colocalization of GFP and IdU staining. (Scale bars, 20 μm.) (C and D) Quantification of RGLs per GCL volume unit and of IdU+ RGLs representing actively proliferating RGLs. (E) To adjust for changes in total RGL numbers, data were also plotted as the fraction of actively dividing RGLs in control and manipulated mice of the respective ages. (F) Regime of VEGF on/off switching and BrdU administration (1–2 d before euthanasia) to detect actively proliferating RGLs 4 mo after VEGF induction. (G) Quantification of RGLs per GCL volume unit and of the percentage of dividing RGLs when mice reached 6 mo of age. NS, not significant.
VEGF Preconditioning Reduces Age-Related NSC Quiescence.
Several subprocesses in multistep neurogenesis might, in principle, be affected by VEGF preconditioning and contribute to the apparent overall neurogenic increase. This mechanism may involve processes directly affecting NSCs, as well as downstream processes associated with proliferation and/or survival of their descendants. Here, we examined whether VEGF and associated vascular changes may impact age-related changes in NSC activation status. To this end, IdU was injected (twice a day for 2 d) just before euthanasia, and actively proliferating IdU+/GFP+ RGLs were visualized and quantified in DG sections. Note that in this procedure, asymmetrically dividing RGLs are clearly discernible from symmetrically dividing IPCs (representative examples are shown in Fig. 4B and Fig. S8). In agreement with a previous report (4), a progressive age-related decline in the number of actively proliferating RGLs was observed, enumerated here as 2,800 cells per cubic millimeter at 3 mo but declining to 170 cells per cubic millimeter by 13 mo of age (Fig. 4D). This natural aging process was, however, attenuated following VEGF treatments (Fig. 4D). To adjust for the accompanying process of age-related NSC depletion, results are also presented as the fraction of proliferating NSCs from the total NSC reserve remaining at each respective age. Thus, whereas the fraction of NSCs engaged in active proliferation dropped from a calculated 5% in young mice to 1% by the time control mice have reached 13 mo of age, such a decline was partially prevented in VEGF-induced control littermates (Fig. 4E). Again, the increase in the fraction of actively dividing NSCs was evident regardless of whether VEGF was still on or was withdrawn 2 mo earlier.
Fig. S8.
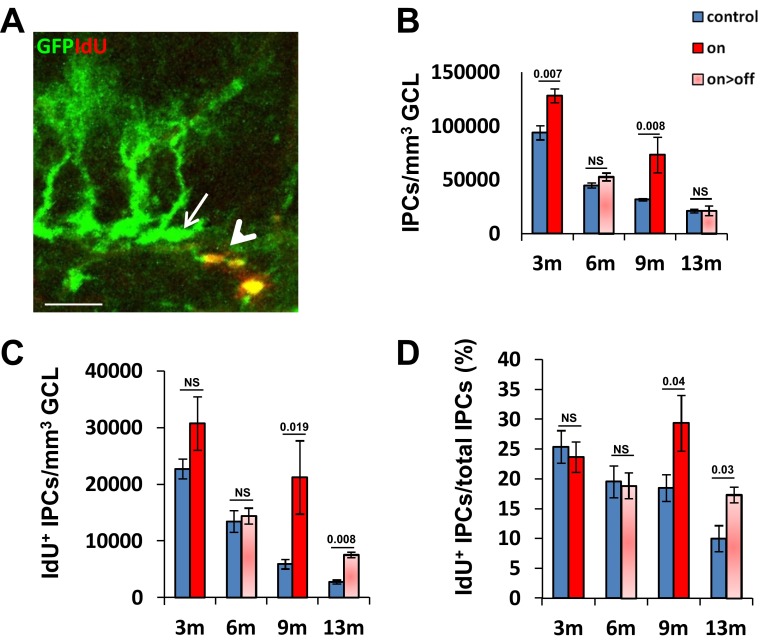
Vascular rejuvenation increases the production rate of neural progenitors (IPCs). The same mice used for visualizing and enumerating RGLs and their proliferation status (Fig. 5) were also used for visualizing total numbers of IPCs and IdU+ IPCs per volume unit. (A) Representative image of IPCs (identified as non-RGL/nestin+ cells, arrow) and symmetrically dividing IPCs (non-RGL nestin+/IdU+ cells, arrowhead). (Scale bar, 20 μm.) (B) Total IPCs number per volume unit. (C) Numbers of dividing IPCs per volume unit. (D) Fraction of proliferating IPCs from total IPCs. NS, not significant.
To determine whether the increase in the percentage of active NSCs persists for even longer after VEGF deinduction, VEGF was induced for 2 wk and the hippocampus was examined 4 mo later. In these 6-mo-old mice, RGL numbers were indistinguishable from the RGL numbers of control littermates, but the percentage of actively dividing RGLs was much higher than in age-matched controls, 5% of total RGLs, the same number observed in young (3-mo-old) animals (Fig. 4 F and G).
As expected, the increase in the fraction of RGLs engaged in production of new neurons in older VEGF-manipulated mice was accompanied by a parallel increase in the number of detected IdU+ IPCs in these mice (Fig. S8). We conclude that augmentation of neurogenesis in aged mice consequent to VEGF preconditioning is, in large part, due to increasing the fraction of active NSCs.
VEGF Preconditioning Induces Changes in NSC Morphology and NSC-BV Engagements.
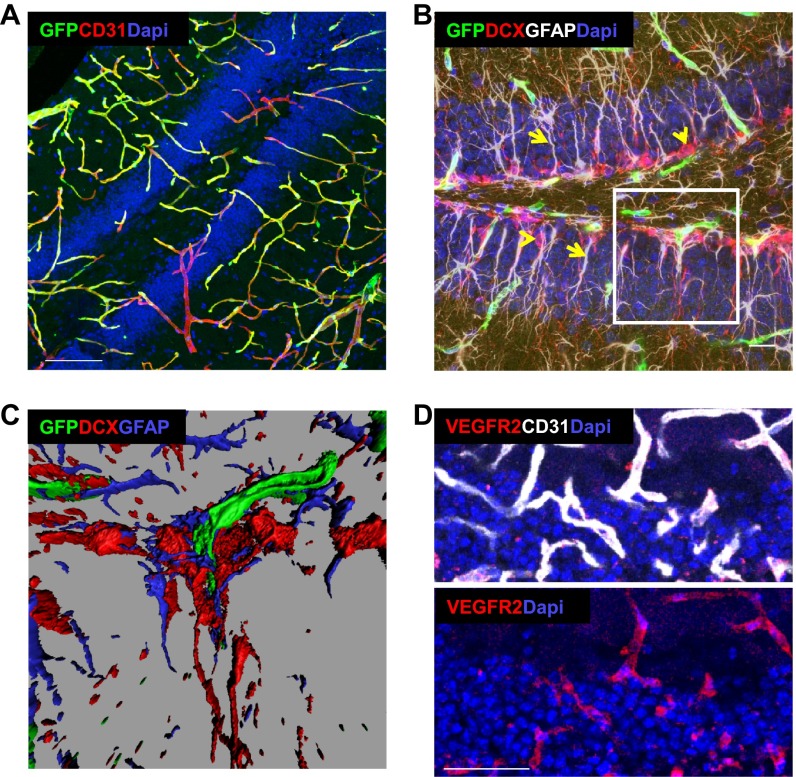
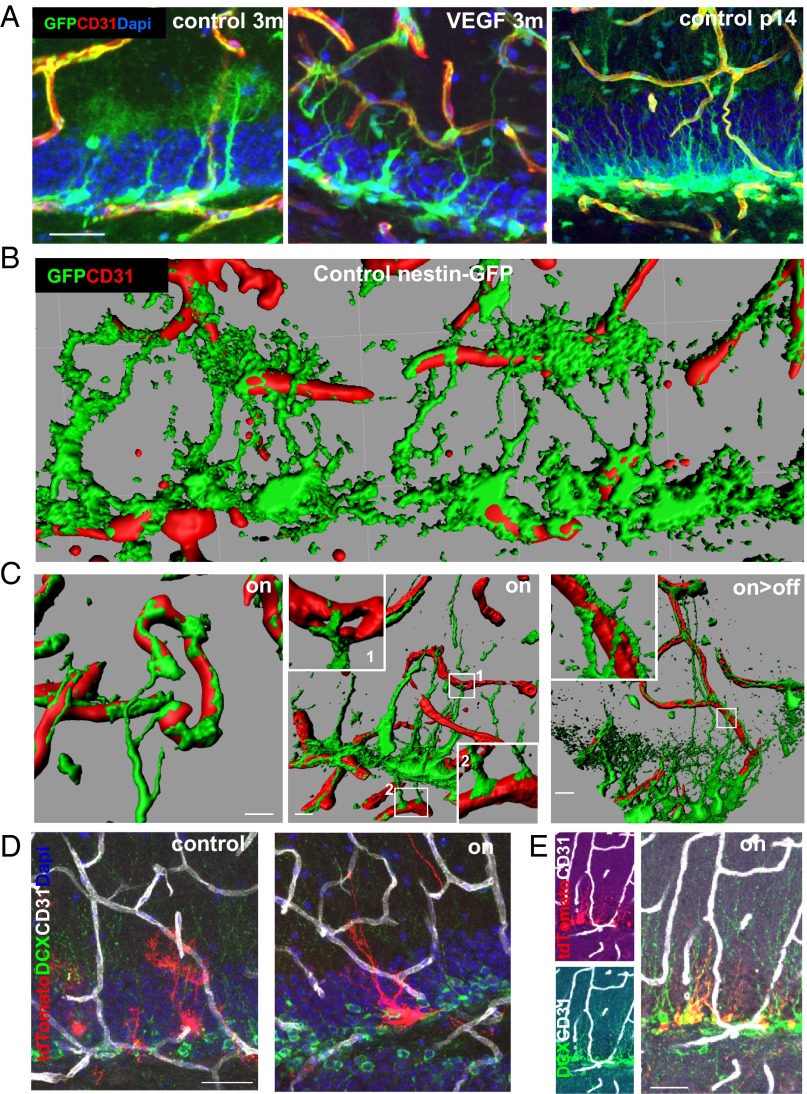
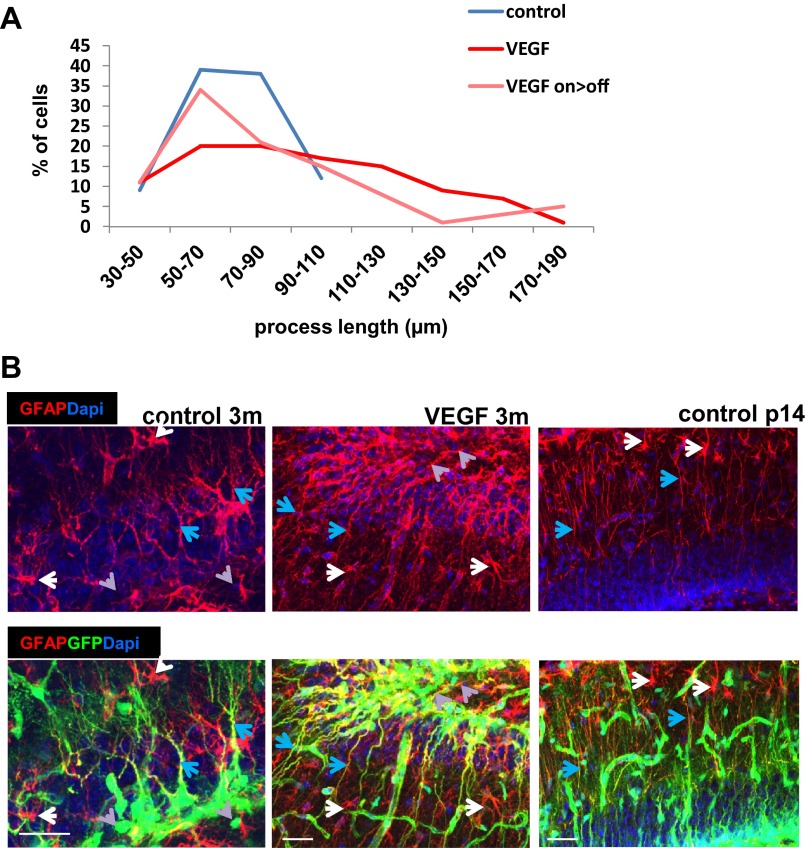
We wished to examine whether the vascular changes induces a change in the unique cellular architecture of hippocampal NSCs. To this end, confocal images of GFP-tagged RGLs, costained with CD31 to mark ECs, were examined. A dramatic change in RGL morphology was noted as early as 5 d from switching on VEGF and was even more pronounced with a longer VEGF exposure. Most striking was a complete disappearance of the RGL terminal arbor normally ending at the inner molecular layer and its replacement with multiple long cytoplasmic extensions, some of which reached further out into the DG molecular layer (Fig. 5A, Left and Center and Fig. S9A). This morphological transition took place in all RGLs, and in 30% of them, it also persisted for months after VEGF withdrawal (Fig. 5C and Fig. S9A). Costaining with GFAP, a marker distinguishing RGLs from IPCs [RGLs are GFAP+, and IPCs are GFAP− (37)], confirmed that these morphological changes indeed represent RGL transitions rather than changes induced in IPCs (Fig. S9B). Intriguingly, this VEGF-induced structural RGL transition resembles the configuration of hippocampal RGLs detected in the postnatal brain (39) (Fig. 5A, Right). Considering that the postnatal hippocampus is engaged in more intense neurogenesis than the mature hippocampus, this result suggests that the RGL configuration induced by VEGF manipulations might be correlated with enhanced neurogenesis.
Fig. 5.
Structural NSC changes resembling a postnatal NSC configuration and NSC-BV contacts after VEGF preconditioning. (A) NSCs were visualized by nestin-GFP expression in tritransgenic mice as described for Fig. 4. RGLs in a control 3-mo-old mouse (Left), RGLs in 3-mo-old VEGF mouse induced for the last month (Center), and RGLs in control postnatal day 14 (p14) mice (Right). BVs are highlighted with CD31 staining. (Scale bar, 20 μm.) (B and C) Apical and distal contact points between NSCs and BVs. Three-dimensional reconstructions of confocal images obtained with the aid of Imaris software highlighting direct contacts between RGL processes (green) and BVs in a control animal (B) and following VEGF induction (C). Note spreading of terminal RGL processes over vessels, creating button-like structures at contact points. RGLs make contacts with vessels residing in the molecular layer (1) and hilar vessels (2). (Right) Also note the persistence of these structures following VEGF withdrawal. (Scale bars, 10 μm.) (D and E) NSC remodeling and NSC-BV contacts visualized using Gli1-creERT2 and Ai9-tdTomato alleles bred into the VEGF switchable system. Mice were killed 1 mo after concomitant VEGF induction and tamoxifen administration allowing to visualize both solitary RGLs (D) and clones of neuroblast descendants (E). Costaining with BVs and neuroblasts is shown. (Scale bars, 30 μm.)
Fig. S9.
VEGF-induced remodeled RGLs encompass longer processes and maintain their GFAP-positive identity. (A) Distribution of NSC processes’ length in control, VEGF on (1-mo switch), and on-off (1 mo on > 2 mo off). One hundred cells of four animals in each group was counted. The P value for control vs. VEGF is 3 * 10−5; for control vs. VEGF on > off, the P value is 0.051; and for VEGF on vs. on > off, the P value is 0.025. (B) Immunostaining for CD31 and GFAP in nestin-GFP animals. Although GFAP labels both NSCs and astrocytes, GFP+GFAP+ RGLs (blue arrows) are readily discernible from GFP−GFAP+ astrocytes (white arrows) and from GFP+GFAP− IPCs (gray arrows). GFAP positivity of postnatal and remodeled GFP+ cells corroborates their NSC identity. (Scale bars, 50 μm.)
Next, we examined whether, similar to what has been reported for SVZ NSCs (9, 16, 40) and, recently, for hippocampal RGLs (41, 42), communication between BVs and hippocampal NSCs involves a direct contact between the two. Many of the long cellular process extended by RGLs consequent to VEGF induction established multiple direct contacts with ECs (Fig. 5 B–D). Newly made RGL-EC engagements involved vessels distanced much further away from the SGZ, where the RGL cell bodies are situated, but occasionally also involved vessels in the opposite direction (Fig. 5C, Center). Strikingly, upon contacting vessels, cytoplasmic extensions extended by RGLs spread over them, creating button-like structures. The functional significance of induced BV-RGL contacts is currently unclear.
These observations were reproduced through tagging RGLs in Gli1-CREERT2::Ai9 tdTomato reporter mice. One month after tamoxifen administration and VEGF induction, the same morphological change and BV association were observed (Fig. 5D), followed by their differentiation to DCX+ cells (Fig. 5E). Of note, the morphological RGL changes induced by VEGF were not associated with the reactive phenotype induced by high-dose kainic acid injection (7), because the latter has multiple short and thick processes and differentiates into astrocytes, whereas BV-induced NSCs have long and thin processes and undergo neuronal differentiation (Fig. 5E).
Discussion
Although several growth factors were shown to be capable of enhancing adult hippocampal neurogenesis, this study describes a case where even an episodic, short exposure to a given factor is sufficient to produce a long-lasting effect of enhanced neurogenesis persisting months after its withdrawal. This unprecedented situation could, in principle, be attributed either to a direct, irreversible proneurogenic effect on NSCs or, alternatively, to a long-lasting effect on the neurogenic microenvironment, which, in the case of VEGF, is likely to involve the niche vasculature. Several lines of evidence argue for the latter case. First, the neurogenic increase is proportional to the increase in MVD regardless of whether exposure to VEGF is episodic or continual. Second, the direct effect on nonvascular cells is incompatible with our failure to detect VEGF receptors, notably, the major signaling receptor VEGFR2, on nonvascular cells in the adult DG. Third, exposure to VEGF under conditions where newly added vessels were made but were not sustained failed to increase neurogenesis. Fourth, prolonged VEGF induction in the face of angiogenesis inhibition (in a VEGF-independent manner) failed to increase neurogenesis. Taken together, these results strongly argue for an indirect effect mediated by neovessels laid down by VEGF. It should be pointed out, however, that contrary to our failure to detect VEGF receptors on nonvascular hippocampal cells, expression of VEGFR2 on hippocampal NSCs has been recently reported (24, 31), arguing for a possible direct communication between VEGF and NSC. In addition, it was shown that VEGF produced by NSCs has a role in regulating neurogenesis (31). Reasons for the discrepancy concerning VEGFR2 expression, presumed to reflect methodological differences, remain unclear. In any case, the status of endogenous VEGFRs does not hamper the conclusion that a short episode of VEGF exposure is sufficient to result in a long-term neurogenic increase.
Interestingly, hippocampal neurogenesis was previously shown to be increased by physical exercise and exposure to an enriched environment (43, 44), stimuli also leading to an increase in MVD (45), but it remains unclear whether these effects are interrelated.
Irrespective of the mechanism by which VEGF elicits a long-lasting increase in neurogenic output, there was no trade-off in the form of an accelerated exhaustion of the NSC reservoir as would have been predicted by the SC disposal model (2), and as was indeed observed when other modalities to increase DG neurogenesis were used (6, 7). Although the basis for this apparent difference is currently unclear, this unique property of VEGF-induced neurogenesis might be advantageous for intended long-term increase of hippocampal neurogenic output.
Here, we show that neurogenic enhancement linked to vascular ramification is associated with a dramatic change in SC morphology. Remodeled NSCs are distinguished by long cytoplasmic extensions terminating at the outer molecular layer instead of the usual terminal arbor hanging loose in the inner molecular layer. The role of the SC terminal arbor, if any, is currently unknown, but it has been argued that it would be surprising for such an elaborate structure not to function in the regulation of hippocampal NSCs (46). The apparent affinity of remodeled cytoplasmic extensions to BVs and the observation that they establish direct, stable contacts with BVs are compatible with a direct vessel-NSC paracrine communication. Notably, this spatial arrangement is also reminiscent of the situation in the SVZ neurogenic niche, where B1 cells extend specialized end-feet stretching out toward the vasculature (9, 16, 40). Intriguingly, the particular NSC configuration induced by vascular manipulations in the mature hippocampus is similar to the configuration of NSCs found in the early postnatal DG (39), and because the latter is engaged in significantly more intense neurogenesis, it is tempting to speculate that this particular NSC configuration is more compatible with a high neurogenic rate.
In principle, BVs may impact SC performance not only by virtue of providing perfusion and distributing blood-borne factors but also in a perfusion-independent manner via production of paracrinically acting “angiocrine” factors (47–49). Could enhanced neurogenesis be due simply to a quantitative increase of the niche vasculature or, alternatively, to some qualitative change associated with vascular “rejuvenation”? Embedded in the latter is the supposition of “vascular aging” (i.e., that the niche vasculature undergoes age-related changes compromising its ability to support proper SC function). A recent study has indeed demonstrated qualitative age-related changes in the vasculature of the bone osteogenic niche that may compromise osteogenesis (50), and an age-related decrease in CD31 expression was noted in SVZ vasculature (22). Our observation that the marked decrease in DG neurogenesis taking place naturally between 3 and 16 mo of age is not associated with a detectable vascular loss suggests that if neurogenic decrease is indeed due to failing vascular support, it must be primarily due to vascular aging rather than to vessel rarefaction. Having shown that rejuvenating the niche vasculature in old mice (in which neurogenesis has already mostly diminished) restores a high neurogenic rate provides a rationale for the potential utility of vascular rejuvenation. A recent study has demonstrated the feasibility of enhancing SVZ neurogenesis in old mice via heterochronic parabiosis. The finding that this procedure was accompanied by vascular remodeling prompted the conjecture by the authors of neurogenic rejuvenation by vascular rejuvenation (22). However, it remains to be determined whether neurogenic and vascular changes are causally related and, importantly, whether neurogenic enhancement can also be achieved by direct vascular rejuvenation without the confounding factor of exposure to systemic factors present in the young circulation. Results presented here demonstrate that neurogenic enhancement at the DG niche can be achieved directly by on-site vascular manipulation and can take place despite continued exposure to an “old” systemic milieu.
Which facets of neurogenesis are positively affected by ectopic VEGF? Several nonmutually exclusive mechanisms to be considered include expansion of the NSC pool, counteracting NSC quiescence, additional rounds of neuroblast division, and increasing neuroblast survival. Here, we only addressed the issue of NSC quiescence and showed that naturally occurring, progressive NSC quiescence can be significantly attenuated by VEGF. The findings that VEGF preconditioning increases the fraction of active NSCs without accelerating NSC disposal may explain the sustained increase in neurogenesis also achievable at later ages.
Methods
Mice.
All animal procedures were approved by the Animal Care and Use Committee of The Hebrew University. The following transgenic mouse lines were used in this study. The CaMKIIα-tTA, Ai9, and Gli1-creERT2 lines were purchased from The Jackson Laboratories. The pTET-VEGF164 responder line was as described previously (14). Nestin-GFP mice (38) were obtained from G. Enikolopov (Cold Spring Harbor Laboratory) (2, 38). VEGFR2-GFP/+ knock-in mice (51) were a generous gift from A. Medvinsky, (University of Edinburgh). For switching off VEGF, water was supplemented by 500 mg/L tetracycline (Tevacycline; Teva, Inc.) and 3% sucrose. For switching on the transgene, tetracycline-supplemented water was replaced by fresh water for the desired time. BrdU (50 mg/kg; Sigma), CldU (100 mg/kg; MP Biomedicals), and IdU (100 mg/kg; Sigma) were injected i.p. three times at 8-h intervals at the indicated time points before euthanasia. Tamoxifen (40 mg/mL in sunflower seed oil; Sigma) was administered orally once daily for 3 d at a dose of ∼8 mg per animal. Lysine-fixable FITC-labeled dextran (10,000 Da; Molecular Probes) was injected into the left ventricle at 0.1 g/kg before euthanasia. Lodamin (30 mg/kg in water) was synthesized as described (35) and given by oral gavage every other day for 3 wk. Animals were housed in specific-pathogen-free housing conditions with irradiated rodent food and water/tetracycline ad libitum. Both males and females were used. Animals harboring CaMKIIα-tTA or pTET-VEGF164 alone served as littermate controls for double-transgenic animals.
Immunofluorescence.
Brains were fixed by immersion in 4% (wt/vol) paraformaldehyde for 5 h, incubated in 30% (wt/vol) sucrose, embedded in OCT (optimal cutting temperature) Tissue-Tec, and cryosectioned to 50-μm floating coronal sections. Sections of the whole hippocampus were pooled but selected before staining to represent all hippocampal areas in the rostral-caudal axis. Staining was done as described (52) with the following: anti-GFP (1:400; Abcam) anti-BrdU (1:200; Amersham), anti-CldU (1:400; Serotec), anti-IdU (1:200; Becton Dickinson), antilaminin (1:400; Neomarkers), anti-DCX (1:3,000; Millipore), anti-CD31 (1:50; Becton Dickinson), anti-NeuN (Cell Signaling 1:600), and anti-VEGFR2 (Cell Signaling 1:200). Cy5-conjugated extravidin, biotinylated anti–guinea-pig, Cy2 anti-rabbit, Cy3 anti-mouse, and Cy2 anti-goat were all from Jackson Immunoresearch (1:400 dilution).
Confocal Microscopy.
Confocal microscopy was done using an Olympus FV-1000 on 20× and 40× air lens, with a 1.46-μm distance between confocal z-slices. Three-dimensional reconstructions of z-stack scans were performed by Bitplane IMARIS 7.6.3 software. Volume and isosurface functions were used.
MVD Quantification.
The z-stacks were processed using IMARIS 7.6.3 software. An area of 318 × 318 × 22.5 μm, including the hilus, GCL, and molecular layer, was analyzed. The surface function of the channel, including BV staining, was conducted, and the total volume was calculated by the software. The ratio between BV volume and the total region of interest was calculated.
Cell Density Quantification.
The volume of the GCL in every image was measured by FV-1000 viewer software (Olympus) using DAPI staining. Quantification of cells within this area (BrdU, CldU, or IdU) was done manually using the same software by a blinded experimenter. Noteworthy, a month-long exposure to VEGF was not associated with a significant change in GCL volume, implying that the relative number of cells calculated per volume unit is not skewed by changes in GCL volume (Fig. S1C).
Statistical Analysis.
All data are presented as mean ± SEM. For comparison between two groups, the Student’s t test was used, and P values were calculated in Excel (Microsoft) and SPSS 21.0 software (IBM) assuming two-tailed distribution and unequal variances. Comparisons between multiple groups were calculated by SPSS using one- or two-way ANOVA. At least four images were taken for each animal, and the average per animal was used to calculate the average of the experimental group. The numbers of animals in every group and all statistical parameters for each experiment are presented in Table S1.
Table S1.
Summary of the statistical test, numbers of animals (left, control; right, VEGF), and statistical information for every experiment undergoing quantification
| Graph | Statistical test | No. of animals (control, VEGF) | df | Test values |
| Fig. 1C | t test | 3 mo: 4,6 | 8 | t = −5.096 |
| 6 mo: 10,5 | 13 | t = −9.663 | ||
| On/on > off: 6,5 | 9 | t = −3.199 | ||
| Fig. 2B | ANOVA | Control: 6 | 2,12 | F = 13.491 |
| VEGF 5 d: 4 | P = 0.00851 | |||
| VEGF on > off: 5 | ||||
| Fig. 2C | t test | 6,5 | 9 | MVD: t = −1.678 |
| DCX: t = −2.303 | ||||
| CldU: t = −1.943 | ||||
| IdU: t = −1.866 | ||||
| Fig. 2E | t test | 7,9 | 14 | MVD: t = −2.740 |
| DCX: t = −2.629 | ||||
| Fig. 3C | t test | 3 mo: 4,5 | 7 | t = −2.880 |
| 6 mo: 5,4 | 7 | t = −3.207 | ||
| 9 mo: 5,4 | 7 | t = −3.133 | ||
| 13 mo: 6,4 | 8 | t = −4.261 | ||
| 16 mo: 13,7 | 18 | t = −3.014 | ||
| Fig. 3D | t test | 3 mo: 4,5 | 7 | t = −5.770 |
| 6 mo: 5,4 | 7 | t = −5.885 | ||
| 9 mo: 5,4 | 7 | t = −3.858 | ||
| 13 mo: 6,4 | 8 | t = −3.049 | ||
| 16 mo: 13,7 | 18 | t = −2.630 | ||
| Fig. 4C | t test | 3 mo: 7,6 | 11 | t = −2.024 |
| 6 mo: 5,4 | 7 | t = −1.326 | ||
| 9 mo: 6,4 | 8 | t = −3.190 | ||
| 13 mo: 7,4 | 9 | t = 1.193 | ||
| Fig. 4D | t test | 3 mo: 7,6 | 11 | t = −0.537 |
| 6 mo: 5,4 | 7 | t = −2.33 | ||
| 9 mo: 6,4 | 8 | t = −2.979 | ||
| 13 mo: 7,4 | 9 | t = −3.383 | ||
| Fig. 4E | t test | 3 mo: 7,6 | 11 | t = −0.537 |
| 6 mo: 5,4 | 7 | t = −2.33 | ||
| 9 mo: 6,4 | 8 | t = −2.979 | ||
| 13 mo: 7,4 | 9 | t = −2.711 | ||
| Fig. 4G, Left | t test | 6,10 | 14 | t = 0.774 |
| Fig. 4G, Right | t test | 4,5 | 7 | t = −1.234 |
| Fig. S1C | t test | 3 mo: 4,6 | 8 | t = 1.742 |
| 5 mo: 6,4 | 8 | t = 0.36 | ||
| 7 m: 6,5 | 9 | t = −1.125 | ||
| 9 mo: 6,4 | 8 | t = 0.200 | ||
| Fig. S1F | ANOVA | 8,4,4 | 2,16 | F = 53.596, P = 8 * 10−8 |
| Fig. S1G | ANOVA | 4,4,5 | 2,10 | CldU: F = 10.026, P = 0.003 |
| IdU: F = 9.148, P = 0.006 | ||||
| Fig. S2C | t test | 10,11 | 19 | Branch points: t = −4.027 |
| Thickness: t = −0.667 | ||||
| Fig. S3B | t test | 17,12 | 27 | t = −7.113 |
| Fig. S3C | t test | 16,10 | 24 | t = −3.825 |
| Fig. S3D | t test | 8,9 | 15 | t = −4.119 |
| Fig. S5C | ANOVA | Water: 6,5 | 1,15 | Lodamin effect: F = 4.045, P = 0.063 |
| Lodamin: 4,4 | VEGF effect: F = 11.511, P = 0.004 | |||
| Fig. S5D | ANOVA | Water: 6,5 | 1,15 | Lodamin effect: F = 11.238, P = 0.006 |
| Lodamin: 4,4 | VEGF effect: F = 19.427, P = 0.001 | |||
| Fig. S6 | t test | 3 mo: 8,5 | 11 | t = −4.015 |
| 6 mo: 4,4 | 6 | t = −2.887 | ||
| 9 mo: 6,4 | 8 | t = −10.995 | ||
| 13 mo: 6,4 | 8 | t = −1.326 | ||
| Fig. S7 | ANOVA | 4,5 | 1,7 | Context: F = 7.050, P = 0.033 |
| Tone: F = 6.422, P = 0.039 | ||||
| Fig. S8B | t test | 3 mo: 8,5 | 11 | t = −2.148 |
| 6 mo: 4,4 | 6 | t = −1.871 | ||
| 9 mo: 6,4 | 8 | t = −3.448 | ||
| 13 mo: 7,4 | 9 | t = −0.159 | ||
| Fig. S8C | t test | 3 mo: 8,5 | 11 | t = −1.646 |
| 6 mo: 4,4 | 6 | t = −0.688 | ||
| 9 mo: 6,4 | 8 | t = −2.937 | ||
| 13 mo: 7,4 | 9 | t = −3.404 | ||
| Fig. S8D | t test | 3 mo: 8,5 | 11 | t = −0.561 |
| 6 mo: 4,4 | 6 | t = −1.021 | ||
| 9 mo: 6,4 | 8 | t = −2.87 | ||
| 13 mo: 7,4 | 9 | t = −3.112 | ||
| Fig. S9 | ANOVA | 4 animals, 100 cells in each group | 2,297 | F = 12.366, P = 7 * 10−6 |
Acknowledgments
We thank Dr. Alexander Medvinsky (University of Edinburgh) for mice and Dr. Avi Merzel for statistical analysis. This work was supported by a European Council Research (ERC) grant [Project VASNICHE (Grant 322692)] and the Britain Israel Research and Academic Exchange Partnership.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609592113/-/DCSupplemental.
References
- 1.Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2010;31(1):151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Encinas JM, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8(5):566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lugert S, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6(5):445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Aimone JB, et al. Regulation and function of adult neurogenesis: From genes to cognition. Physiol Rev. 2014;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehm O, et al. RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30(41):13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sierra A, et al. Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell Stem Cell. 2015;16(5):488–503. doi: 10.1016/j.stem.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaguidi MA, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145(7):1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Licht T, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci USA. 2011;108(12):5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Licht T, Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell Mol Life Sci. 2013;70(10):1727–1737. doi: 10.1007/s00018-013-1280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottone C, et al. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol. 2014;16(11):1045–1056. doi: 10.1038/ncb3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazanis I, et al. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci. 2010;30(29):9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delgado AC, et al. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron. 2014;83(3):572–585. doi: 10.1016/j.neuron.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14(6):329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rando TA, Finkel T. Cardiac aging and rejuvenation--a sense of humors? N Engl J Med. 2013;369(6):575–576. doi: 10.1056/NEJMcibr1306063. [DOI] [PubMed] [Google Scholar]
- 21.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 22.Katsimpardi L, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344(6184):630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayford M, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274(5293):1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 25.Jin K, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schänzer A, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14(3):237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 28.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97(18):10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer MH, Tripps WK, Feldmann RE, Jr, Kuschinsky W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci Lett. 2003;344(3):165–168. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- 30.Mani N, Khaibullina A, Krum JM, Rosenstein JM. Astrocyte growth effects of vascular endothelial growth factor (VEGF) application to perinatal neocortical explants: receptor mediation and signal transduction pathways. Exp Neurol. 2005;192(2):394–406. doi: 10.1016/j.expneurol.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Kirby ED, Kuwahara AA, Messer RL, Wyss-Coray T. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci USA. 2015;112(13):4128–4133. doi: 10.1073/pnas.1422448112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittko IM, et al. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci. 2009;29(27):8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125(9):1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 34.Alon T, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1(10):1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 35.Benny O, et al. An orally delivered small-molecule formulation with antiangiogenic and anticancer activity. Nat Biotechnol. 2008;26(7):799–807. doi: 10.1038/nbt1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sin N, et al. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc Natl Acad Sci USA. 1997;94(12):6099–6103. doi: 10.1073/pnas.94.12.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Encinas JM, et al. Quiescent adult neural stem cells are exceptionally sensitive to cosmic radiation. Exp Neurol. 2008;210(1):274–279. doi: 10.1016/j.expneurol.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469(3):311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 39.Brunne B, et al. Origin, maturation, and astroglial transformation of secondary radial glial cells in the developing dentate gyrus. Glia. 2010;58(13):1553–1569. doi: 10.1002/glia.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacar B, et al. Neural progenitor cells regulate capillary blood flow in the postnatal subventricular zone. J Neurosci. 2012;32(46):16435–16448. doi: 10.1523/JNEUROSCI.1457-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moss J, et al. Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc Natl Acad Sci USA. 2016;113(18):E2536–E2545. doi: 10.1073/pnas.1514652113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Licht T, Keshet E. The vascular niche in adult neurogenesis. Mech Dev. 2015;138(Pt 1):56–62. doi: 10.1016/j.mod.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 43.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 44.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 45.Ekstrand J, Hellsten J, Tingström A. Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neurosci Lett. 2008;442(3):203–207. doi: 10.1016/j.neulet.2008.06.085. [DOI] [PubMed] [Google Scholar]
- 46.Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10(6):698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bautch VL. Stem cells and the vasculature. Nat Med. 2011;17(11):1437–1443. doi: 10.1038/nm.2539. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg JS, Hirschi KK. Diverse roles of the vasculature within the neural stem cell niche. Regen Med. 2009;4(6):879–897. doi: 10.2217/rme.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14(11):1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latroche C, et al. Structural and functional alterations of skeletal muscle microvasculature in dystrophin-deficient mdx mice. Am J Pathol. 2015;185(9):2482–2494. doi: 10.1016/j.ajpath.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Licht T, et al. VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development. 2010;137(2):261–271. doi: 10.1242/dev.039636. [DOI] [PubMed] [Google Scholar]
- 53.Ben-Zvi A, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]