Significance
Pathogenic or symbiosis islands are mobile genetic elements that can provide recipient bacteria the capacity to establish intimate interactions with eukaryotic hosts. For example, legume symbionts have evolved via horizontal transfer of symbiotic plasmids or genomic islands. Here, we show that the transfer of the symbiosis island of the Sesbania rostrata symbiont, Azorhizobium caulinodans, to other rhizobia is enhanced by plant flavonoids that also serve as signals to initiate the symbiotic process. These data suggest that eukaryotic hosts are involved in bacterial horizontal gene transfer to promote symbiotic interactions between rhizobia and legumes.
Keywords: horizontal gene transfer, host-range, integrative and conjugative element, naringenin, nodulation
Abstract
Horizontal gene transfer (HGT) of genomic islands is a driving force of bacterial evolution. Many pathogens and symbionts use this mechanism to spread mobile genetic elements that carry genes important for interaction with their eukaryotic hosts. However, the role of the host in this process remains unclear. Here, we show that plant compounds inducing the nodulation process in the rhizobium-legume mutualistic symbiosis also enhance the transfer of symbiosis islands. We demonstrate that the symbiosis island of the Sesbania rostrata symbiont, Azorhizobium caulinodans, is an 87.6-kb integrative and conjugative element (ICEAc) that is able to excise, form a circular DNA, and conjugatively transfer to a specific site of gly-tRNA gene of other rhizobial genera, expanding their host range. The HGT frequency was significantly increased in the rhizosphere. An ICEAc-located LysR-family transcriptional regulatory protein AhaR triggered the HGT process in response to plant flavonoids that induce the expression of nodulation genes through another LysR-type protein, NodD. Our study suggests that rhizobia may sense rhizosphere environments and transfer their symbiosis gene contents to other genera of rhizobia, thereby broadening rhizobial host-range specificity.
Horizontal gene transfer (HGT) plays a major role in the biodiversity and ecology of bacteria by contributing to their adaptability, fitness, and competitiveness (1). Gene acquisition involves mobile genetic elements (MGEs), such as plasmids and genomic islands. Large MGEs can provide complex novel traits, such as virulence and mutualistic traits that enable bacteria to interact with eukaryotic hosts (2). However, the role of the host in the transfer of MGE is poorly documented. One type of MGE that is widespread in prokaryotic genomes is called integrative and conjugative elements (ICEs) (3). ICEs are self-transmissible mobile elements that carry genes encoding the machinery for DNA processing and transferring. These genes allow ICEs to excise themselves from the chromosome and form a closed circular molecule, conjugate, and site-specifically recombine into recipient chromosomes. ICEs have been found to render a diverse range of characteristics on the bacteria that carry them, including resistance to antibiotics and heavy metals, the ability to degrade aromatic compounds, and promotion of biofilm formation. Some ICEs carry traits such as virulence and symbiosis.
Rhizobia are facultative endosymbionts that are able to form nitrogen-fixing nodules on the root of legume hosts (4). Some tropical legumes, such as Sesbania rostrata, are also nodulated on the stem by their rhizobial partners (5). Initiation of symbiosis requires a two-way signal exchange program involving plant compounds and bacterial nodulation (nod) genes (6). The conserved LysR-family transcriptional regulator NodD perceives flavonoid signals released by the host legume, and activates nod genes that encode enzymes responsible for the synthesis of signaling Nod factors, which trigger the plant developmental program leading to nodule organogenesis (7). Nod factors are lipochito-oligosaccharides of diverse structures and the major determinants of host specificity. Rhizobia have evolved via horizontal transfer of essential nodulation and nitrogen fixation genes that are carried by either large plasmids or genomic islands. Exchange of symbiotic material between rhizobial species and genera has been frequent during evolution (8). The symbiosis islands of the Mesorhizobium, Azorhizobium, and Bradyhizobium species are mainly located on the chromosome (9–11). For example, the rhizobium strain Mesorhizobium loti R7A has a 502-kb ICE called ICEMlSymR7A that contains genes required for nitrogen-fixing symbiosis with Lotus corniculatus (12). The ICEMlSymR7A is inserted downstream of a phe-tRNA gene in the M. loti chromosome and can be transferred to nonsymbiotic mesorhizobia both in the laboratory and in the field, converting these strains into symbionts of L. corniculatus (12, 13). The transfer of ICEMlSymR7A is mediated by quorum sensing and is controlled by a complex multipartite regulatory system involving an excisionase and an excision activator produced via a programmed ribosomal frameshift (14–16).
Here, we provide evidence that, in the S. rostrata-Azorhizobium caulinodans system, the symbiotic process and the symbiosis island transfer are triggered by the same plant compound. The symbiosis island of A. caulinodans is an 87.6-kb integrative and conjugative element (ICEAc) that is able to excise, form a circular DNA, and conjugatively transfer to a specific site of gly-tRNA gene of various rhizobial species, allowing them to stem nodulate S. rostrata. The ICEAc transfer is enhanced by an ICEAc-located LysR-family protein, AhaR, in response to the plant flavonoid compounds that also induce the expression of the A. caulinodans nodulation genes, implying that rhizobia may sense rhizosphere environments and modulate their genetic programs to induce both symbiotic and HGT machineries.
Results
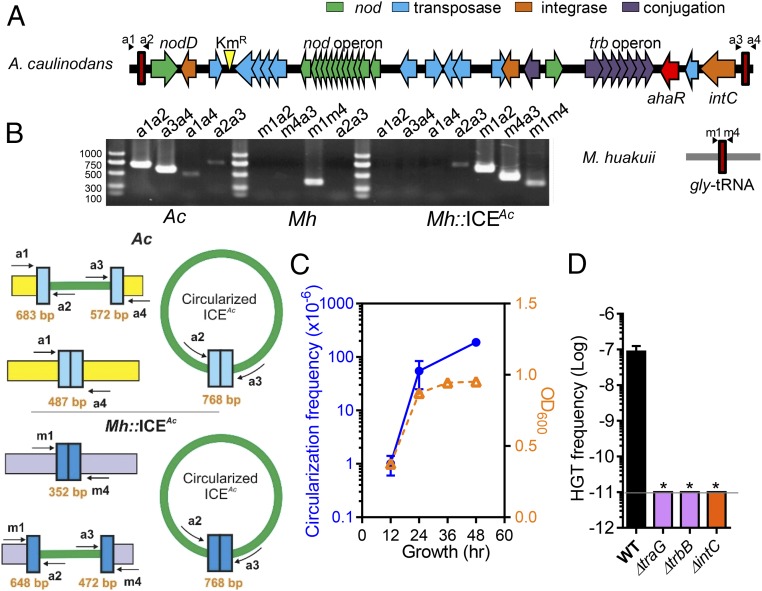
A. caulinodans Symbiosis Island ICEAc Can Horizontally Transfer to Other Rhizobial Genera.
Analysis of the A. caulinodans ORS571 genome (10) revealed that its 87.6-kb symbiosis region, which contains three nodulation loci that are involved in the biosynthesis of Nod factors, has typical characteristics of a symbiosis island: It has a lower GC content and is inserted adjacent to a gly-tRNA gene. This region also contains genes related to conjugal transfer, transposases, and integrases (Fig. 1A and SI Appendix, Fig. S1), suggesting that it may facilitate horizontal gene transfer events. To examine whether this symbiosis island (ICEAc) is able to transfer to other rhizobia, we inserted a kanamycin-resistant cassette (KmR) into an intergenic region of ICEAc in wild-type A. caulinodans ORS571 (Fig. 1A). We then mixed the resulting strain with other rhizobial species and examined whether recipient strains acquired kanamycin resistance. We found that the KmR marker could transfer from A. caulinodans into three Mesorhizobium species (Mesorhizobium huakuii, M. loti, and Mesorhizobium tianshanense) tested with ∼10−7 transfer frequency (SI Appendix, Table S1). A Sinorhizobium species could also acquire kanamycin resistance but the Rhizobium and Bradyrhizobium strains examined could not, indicating that the transfer spectrum is limited.
Fig. 1.
The genetic structure and requirement for ICEAc HGT. (A) The genetic structure of A. caulinodans symbiosis island. Gene functions are shown in different colors. Primers used to detect the excision and integration in A. caulinodans and M. huakuii are indicated. (B) PCR amplicons obtained from the donor A. caulinodans (Ac), recipient M. huakuii (Mh), and transconjugants Mh::ICEAc by using primer pairs indicated. Diagrams of circularized ICE products and predicted length of PCR products are shown. (C) ICEAc excision. Wild-type A. caulinodans was grown in TY medium at 28 °C. DNA was extracted at the time points indicated, and qPCR was performed to determine the percentage of circular ICEAc (shown in blue). Growth curve (OD600) is shown in orange. (D) Genes required for ICEAc HGT. Wild-type or mutant A. caulinodans was mixed with M. huakuii and incubated at 28 °C. At the time points indicated, colony-forming unit (cfu) of transconjugants was determined by plating on selective agar plates. HGT frequency was calculated by dividing the number of transconjugants by the number of recipient. Data are mean ± SD of three independent experiments. Statistical analysis was performed by using Student’s t test. *P < 0.05.
We then selected an M. huakuii strain as the recipient to further study ICEAc HGT. To confirm that the entire predicted ∼87-kb ICEAc region was transferred, we first applied arbitrary PCR to determine the insertion site of ICEAc in the recipient. We found that ICEAc was integrated adjacent to the 3′-end of M. huakuii gly-tRNA gene, which has a similar sequence to that of A. caulinodans (SI Appendix, Fig. S2). Interestingly, some nonrecipients of ICEAc also have similar gly-tRNA gene sequences, suggesting that having ICEAc insertion sites is necessary but not sufficient for the HGT events. We then “spot-checked” the transfer of ICEAc genes in transconjugant M. huakuii by PCR amplification and found that all of the ICEAc genes examined were present. To study the excision and integration of ICEAc, the hallmark characteristics of diverse integrative and conjugative elements (3), we designed a set of primers that are specific for sequences of either the donor or the recipients (Fig. 1 A and B and SI Appendix, Table S2). Using primer pairs m1-a2 and m4-a3, PCR products were detected in M. huakuii harboring ICEAc (Mh::ICEAc), but not in wildtype M. huakuii (Mh) (Fig. 1B), indicating the integration of ICEAc into the M. huakuii genome. We also detected an ICE circular form in both A. caulinodans and Mh::ICEAc by using the primer pair a2-a3 (Fig. 1B), indicative of excision events. The effect of growth phase on the excision of ICEAc was investigated by quantitative PCR (qPCR) of DNA purified from A. caulinodans cultures at different growth stages. Fig. 1C shows that excision products were present at low frequency in log-phase growth cells but increased in the stationary phase. Collectively, these data suggest that the A. caulinodans symbiosis island is an integrative and conjugative element that can excise from the chromosome to form a circular intermediate and conjugally transfer to different genera of rhizobia.
Conjugation Apparatus and an Integrase Are Required for ICEAc HGT.
To identify genes required for ICEAc HGT, we constructed in-frame deletion mutants of individual ICEAc genes putatively involved in integration, because an integrase is required for excision and integration of ICEs (3), as well as in nodulation, transposition, and conjugation (indicated in Fig. 1A). We then compared their HGT frequency with M. huakuii to that of the wild-type A. caulinodans strain (SI Appendix, Table S3). We found that neither the nodDBZ, nolK, and noeC nodulation mutants, nor five of the six transposase mutants tested significantly affected ICEAc HGT (SI Appendix, Table S3). Deletion of the putative transposase AZC_3801 displayed ∼10-fold decrease in HGT frequency. Further study is required to understand the role of this transposase in ICEAc HGT. Deletion of genes encoding putative conjugation-related genes traG (AZC_3827) and trbB (AZC_3858) completely abolished the ICEAc transfer (Fig. 1D). Deletion of integrase gene A (intA, AZC_3793) and intB (AZC_3849) did not alter HGT, whereas intC (AZC_3882) is critical for ICEAc HGT because an intC mutant of A. caulinodans failed to transfer its ICEAc to M. huakuii (Fig. 1D and SI Appendix, Table S3). Thus, these data provide insight into the molecular basis of the ICEAc HGT: Conjugation-related genes traG, trbB, and integrase gene intC are critical.
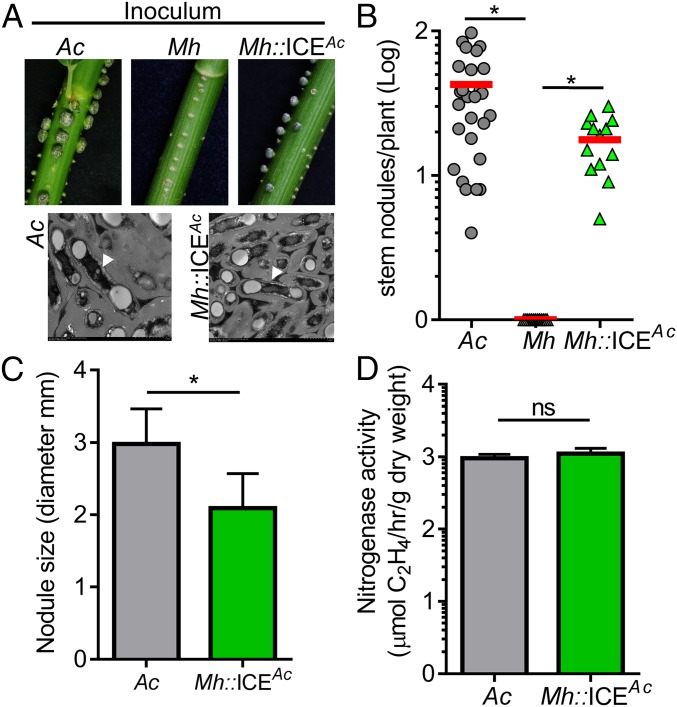
Rhizobial Recipients of ICEAc Display Expanded Host-Range Specificity.
Because the ICEAc harbors the nodD gene encoding a flavonoid-activating transcriptional regulator of the nod loci (17) and three nod loci that encode most of the enzymatic machinery for Nod factor synthesis (10, 18), we tested whether rhizobia that have acquired the ICEAc have gained the ability to nodulate the host of A. caulinodans, Sesbania rostrata. We applied cultures of wild-type A. caulinodans, M. huakuii, and its derivative Mh::ICEAc to the stems of S. rostrata. We found that after 30 d, as expected, wild-type A. caulinodans produced well-developed stem nodules, whereas no nodules were formed by M. huakuii (Fig. 2A). Strikingly, when Mh::ICEAc was inoculated, a significant amount of stem nodules were formed (Fig. 2A and SI Appendix, Fig. S3). Although the number of nodules formed by Mh::ICEAc was lower and average size of the nodules was smaller than those formed by A. caulinodans (Fig. 2 B and C), Mh::ICEAc-induced nodules had a similar microscopic structure (Fig. 2A, Lower) and could fix N2 as efficiently as those of A. caulinodans (Fig. 2D). Moreover, the acquisition of ICEAc did not alter M. huakuii symbiosis ability with its native plant host because Mh::ICEAc strains also induced root nodule formation on Astragalus sinicus (SI Appendix, Fig. S4). In addition to M. huakuii, the transfer of ICEAc to M. loti, M. tianshanense, and S. medicae conferred on these strains the ability to form stem nodules on S. rostrata (SI Appendix, Figs. S3 and S5). These data show that horizontal transfer of ICEAc between rhizobia has the potential to expand their host-range specificity.
Fig. 2.
Expansion of host range by ICEAc HGT. (A) Stem nodule formation. Stationary phase cultures of A. caulinodans (Ac), M. huakuii (Mh) and ICEAc-acquired M. huakuii (Mh::ICEAc) were applied on the surface of 6-wk-old S. rostrata stems and nodule formation was observed 30 d after inoculation (top panels). Bottom panels, transmission electron micrographs of stem nodules. Arrows, bacteroid. (B) The number and (C) the size of stem nodules formed 30 d after inoculation. (D) Nitrogenase activity in stem nodules. Nodules formed by inoculation of Ac, Mh and Mh::ICEAc were harvested and acetylene reduction activity was determined and the nitrogenase activity was calculated as % of acetylene production per gram of nodule dry weight. Data are mean ± SD of at least four independent experiments. ns, no significance; *P < 0.05.
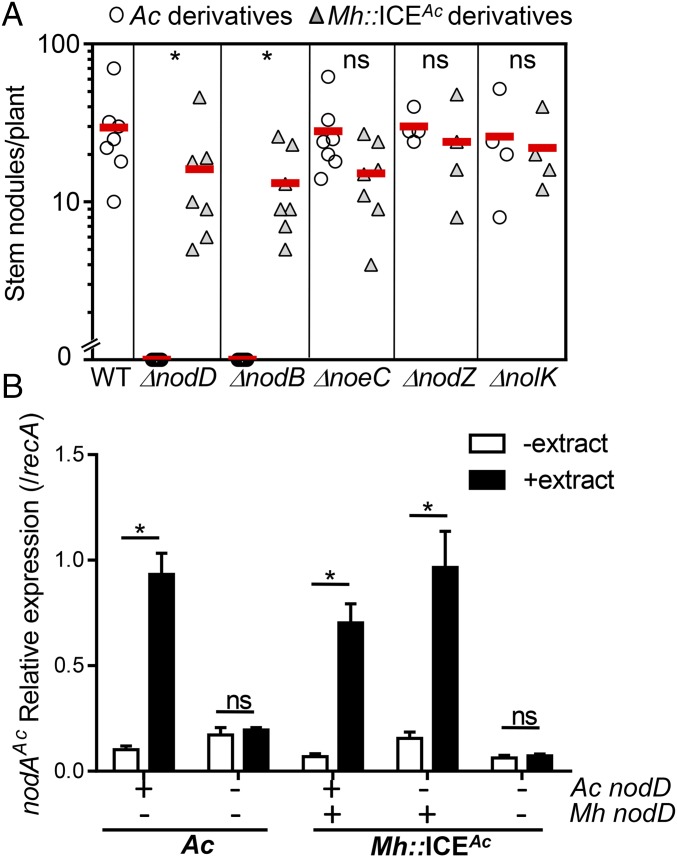
To further investigate the possible interactions between nodulation genes on the symbiosis island and on the recipient chromosomes, we constructed in-frame deletions in various nod genes of ICEAc and compared the nodulation efficiencies of these mutants to M. huakuii harboring corresponding mutated forms of ICEAc. Fig. 3A shows that as expected in A. caulinodans, mutations in nodD, the key nod activator (17), and nodB, encoding a chitooligosaccharide deacetylase involved in Nod factor synthesis (19), abolished nodulation ability of A. caulinodans, whereas mutations in nodZ, noeC, or nolK had little effect on nodulation, consistent with previous reports (20). Interestingly, M. huakuii strains that acquired ICEAc containing nodD or nodB mutations preserved part of the nodulation ability on S. rostrata, (Fig. 3A), implying that NodB and NodD are functionally conserved between these two strains. To confirm that M. huakuii NodD is functionally exchangeable with A. caulinodans NodD, we examined the expression of NodD-regulated nodA gene in different strains by using RT-qPCR. In the presence of seed exudates of S. rostrata, nodA was induced in wild-type A. caulinodans, but not in nodD mutants (Fig. 3B). However, in M. huakuii, deletion of ICEAc nodD did not affect nodA expression, but deletion in both nodD genes abolished the induction (Fig. 3B), indicating that NodD of M. huakuii and A. caulinodans are functionally equivalent.
Fig. 3.
Interchangeable nodulation genes between ICEAc and rhizobial recipients. (A) Stem nodule formation of nod gene mutants of A. caulinodans and Mh::ICEAc. Stationary cultures of A. caulinodans nod mutants (circles) and Mh::ICEAc harboring different nod mutations (triangles) were applied on the surface of 6-wk-old stems, and the number of nodules were numerated 30 d after inoculation. (B) nodA expression. Wild-type and nodD mutants of A. caulinodans and ICEAc-acquired M. huakuii or ICEAc∆nodD were grown in TY medium in the absence and presence of S. rostrata root extracts at 28 °C to OD600 ∼ 0.5. RNA was then harvested, and RT-qPCR was performed by using the primers specific to A. caulinodans nodA. The data were normalized against recA. Data are mean ± SD of four independent experiments. ns, no significance; *P < 0.05.
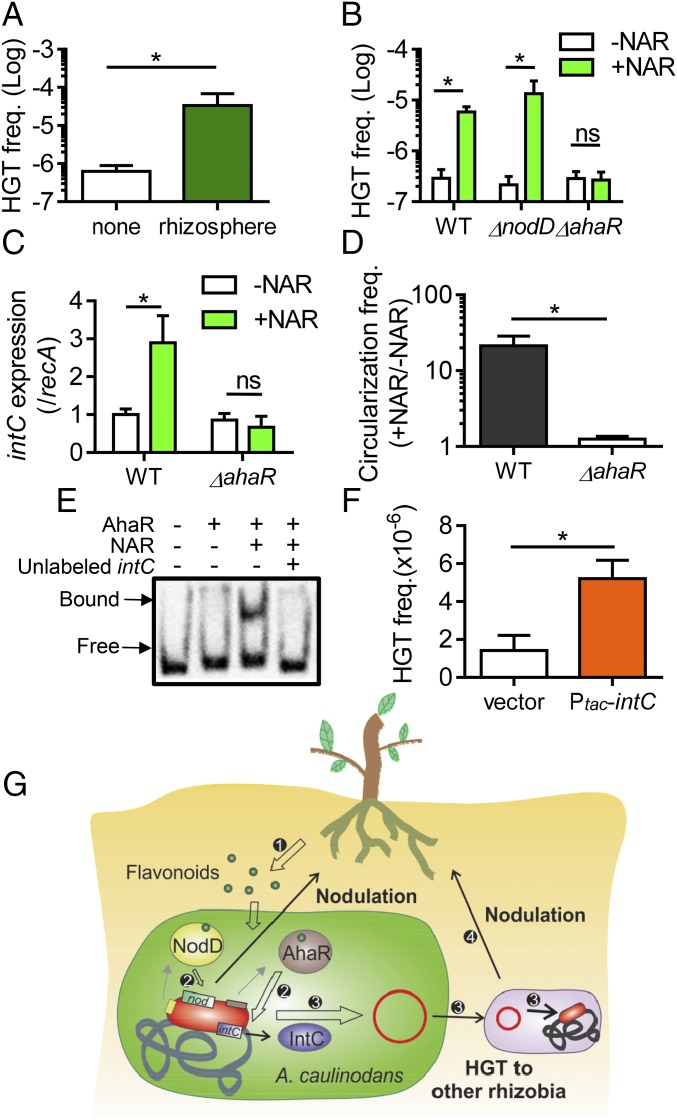
Rhizosphere Promotes ICEAc HGT.
To examine the potential impact of the plant on ICEAc HGT, we inoculated both A. caulinodans and M. huakuii into vermiculite with S. rostrata or without plant (as negative control). After 2 d, HGT frequency was measured. We found that HGT of ICEAc occurred with frequency over 50-fold higher in the rhizosphere compared with the negative control (Fig. 4A). We also tested whether this phenotype is plant-specific by performing conjugation around the roots of Astragalus, Zea, and Lotus. We found that the HGT frequency also increased significantly compared with no-plant controls (SI Appendix, Fig. S6A). These data suggest that there may be a common plant signal involved. As integrase is the key enzyme for ICE excision and integration (3) and IntC is critical for ICEAc HGT (Fig. 1D), we examined intC expression in the rhizosphere and found that the transcription of intC was significantly increased (SI Appendix, Fig. S6B). These data suggest that A. caulinodans may sense certain plant compounds that enhance the transfer of the symbiosis island.
Fig. 4.
Host plant signals induce ICEAc HGT. (A) HGT in rhizosphere. Approximately 109 A. caulinodans and M. huakuii were mixed and inoculated in 1 g of autoclaved vermiculite without and with S. rostrata for 24 h. Samples were withdrawn to determine the HGT frequency. *P < 0.05. (B–D) Flavonoid-induced HGT (B), intC expression (C), and circularization (D). Approximately 109 A. caulinodans wild-type, nodD, and ahaR mutants were mixed with M. huakuii on cellulose filter membranes in the absence and presence of 20 µM naringenin (NAR) and incubated for 24 h. Bacterial cells were then recovered, and cfu of transconjugants and recipients were determined by plating on selective agar plates. HGT frequency was calculated by dividing the number of transconjugants by the number of recipient (B). Bacterial RNA was also extracted and RT-qPCR was performed by using intC specific primers and normalized against recA (C). Bacterial DNA was extracted at the time points indicated, and qPCR was performed to determine the percentage of circular ICEAc (D). ns, not significant (E) EMSA. AhaR-His6 specifically retarded the mobility of biotin-labeled 265-bp DNA containing intC promoters. When indicated, 100 µM naringenin was included in the reaction mix. Over-excess amount of unlabeled intC promoter DNA was used as a competitive DNA. (F) IntC-dependent HGT induction. Approximately 109 A. caulinodans wild type containing either vector or Plac-intC plasmid were mixed with M. huakuii on filters for 24 h. HGT frequency was then determined. Data are mean ± SD of at least three independent experiments. Student’s t test. (G) Working model. Host plants produce flavonoid signals to induce nodulation process through NodD and to induce HGT process through AhaR. ICEAc HGT results in nodulation of plants by other rhizobia. Nonhost plants may also exude flavonoids to induce HGT, generating nodulation proficient bacteria.
Plant-Secreted Flavonoid Compounds Induce HGT Through a NodD Hhomolog.
To identify the possible plant inducers, we extracted the root exudates of S. rostrata with different organic solvents and assayed the fractions for induction of ICEAc HGT. SI Appendix, Fig. S7A shows that the ethyl acetate-extracted fraction could strongly stimulate HGT. HPLC-MS analysis (SI Appendix, Fig. S7B) showed that this fraction contained naringenin, a common flavonoid compound, which has been shown to induce nod gene expression in A. caulinodans (18). We thus selected naringenin to examine the role of plant signals in HGT. Strikingly, we found that naringenin could strongly induce ICEAc HGT in the wild-type strain (Fig. 4B and SI Appendix, Table S4). Because transcriptional activator NodD senses flavonoids and activates nodulation genes in most rhizobia (6, 7) (SI Appendix, Fig. S8A), we initially hypothesized that A. caulinodans may use the same mechanism to induce HGT in response to the presence of naringenin. We tested the HGT frequency in nodD deletion mutants with or without the compound. We found that mutation of nodD did not affect naringenin-enhanced ICEAc HGT (Fig. 4B), suggesting that NodD is not involved in the HGT processes. Interestingly, there are at least two other LysR-family homologs on the A. caulinodans symbiosis island (SI Appendix, Fig. S8B). Like NodD, many LysR-family proteins are able to bind small molecule ligands and regulate transcription (21). We therefore disrupted each of these two lysR-like genes and examined their effects on naringenin-mediated HGT. We found that AZC_3803 had no impact on ICEAc HGT (SI Appendix, Fig. S8C). By contrast, deletion of AZC_3869 abolished flavonoid-mediated induction of HGT (Fig. 4B and SI Appendix, Fig. S8C), suggesting that this protein may regulate ICEAc HGT in response to host plant signals. We annotated this gene as ahaR (Azorhizobium HGT activator R). To investigate the mechanism of AhaR activation of HGT in the presence of naringenin, we examined expression of the key integrase IntC in wild-type and ahaR mutants because intC was induced when A. caulinodans interacted with plant roots (SI Appendix, Fig. S6B). The expression of intC (determined by either RT-qPCR or LacZ transcriptional reporters) was induced by naringenin, and this induction was AhaR-dependent (Fig. 4C and SI Appendix, Fig. S9). Moreover, we found that the ICEAc circularization was induced by naringenin and the induction was AhaR-dependent (Fig. 4D), suggesting that AhaR may sense naringenin to enhance ICEAc transfer. To examine whether AhaR directly regulates intC, we purified AhaR protein and performed gel shift assays. We found that AhaR could specifically bind intC promoter in the presence of naringenin (Fig. 4E), suggesting that AhaR may activate intC expression directly. To ensure AhaR binding of intC is specific, we show that neither naringenin alone nor an unrelated regulatory protein could bind the intC promoter DNA (SI Appendix, Fig. S10). Furthermore, overexpression of intC on a plasmid could elevate ICEAc HGT in the absence of naringenin (Fig. 4F), suggesting that AhaR-naringenin induction of HGT acts through IntC. Interestingly, although integrases are required for both ICE integration and excision (22, 23), overexpression of IntC may not lead to more ICEAc excision. It has been reported that additional small DNA-binding proteins RDFs (recombination directionality factors) are often required to bias the process toward excision (24). We identified AZC_3835 on the ICEAc as the homolog of ICEMlSymR7A RDF (25), and the role of AZC_3835 in ICEAc HGT is under investigation. Of note, the induction of HGT by overexpression of intC did not reach the level that was induced in the rhizosphere or by flavonoid compounds. It is possible that additional components of the HGT machinery may also be affected by the host plant. Taken together, these results show that plant signals that initiate the nodulation process can also enhance the spread of symbiosis genetic materials through horizontal transfer.
Discussion
The plant root-soil interface, also called rhizosphere, is the theater of a large variety of relationships between higher plants and microbes, ranging from loose associations to complex intimate mutualistic symbioses (26). The ability of bacteria to sense and respond to plant signals is crucial for achieving these interactions. In rhizobium-legume symbioses, specific plant secondary metabolites called flavonoids play a major role in the initiation of the symbiotic process by attracting bacteria and inducing the expression of bacterial nod genes encoding Nod factors that unlock the development of nodules on the roots of compatible legumes (27). This induction occurs through interaction with the LysR family NodD regulatory protein. Here, we provide evidence that nodulation-inducing flavonoid compounds enhance horizontal transfers of symbiosis islands and play a major role in the spread of symbiotic proficiency to different taxa. We found that the symbiosis island of the S. rostrata symbiont, A. caulinodans, is an 87.6-kb integrative and conjugative element (ICEAc) that is able to excise, form a circular DNA, and conjugatively transfer to a specific site of the gly-tRNA gene of various bacterial species. This transfer is enhanced by the flavanone naringenin that induces A. caulinodans nod genes. Naringenin induces the expression of the key integrase IntC, and this induction depends on a LysR transcriptional regulator AhaR located within the ICEAc, which binds the IntC promoter in the presence of naringenin (Fig. 4G). Naringenin is widespread in legumes and nonlegumes (28), which probably explains why transfer is enhanced in the rhizosphere of several nonhost plants, and largely involved in communication with plant-associated bacteria. In addition to its crucial role in the nodulation process, it was shown to stimulate wheat and Arabidopsis thaliana colonization by A. caulinodans (29, 30). It was previously reported that opines produced by crown gall tumors initiated on plants by the pathogen Agrobacterium tumefaciens are required for Ti plasmid conjugal transfer among agrobacteria (31) and that root exudates enhance bacterial conjugal gene transfer in the rhizosphere (32, 33). Our findings provide another example of the role of the host in the transfer of bacterial functions involved in interactions with eukaryotes, including bacteria-animal associations (34, 35).
MGEs, such as pathogenicity or symbiosis plasmids and genomic islands, are known to have contributed to genome evolution by providing in a single event a whole set of functions, resulting in profound modifications in the recipient lifestyle. HGT of MGEs is thought to have largely contributed to ecological transitions, yet the success of transfer may be limited or favored by genetic and environmental conditions. It was demonstrated that the symbiosis island of M. loti can transfer to different Mesorhizobium species and, possibly, Rhizobium (13). Our study shows that ICEAc can transfer to several species of Mesorhizobium and Sinorhizobium, confirming that ICE have a wide host range (3). However, transfer to Rhizobium and Bradyrhizobium was unsuccessful in our experimental conditions (SI Appendix, Table S1). The reasons behind this host restriction are not clear. HGTs mostly occur between closely related species from the same taxonomic group or with similar genomic GC content (<5% difference in most cases) (36). However, neither the phylogenetic distance (Azorhizobium is close to Bradyrhizobium; ref. 37) nor the difference in GC content (SI Appendix, Fig. S11) meet those criteria. Host range determinants such as DNA restriction-modification systems and CRISPR systems might be involved in transfer limitation (36). Interestingly, the Sesbania symbionts S. saheli bv sesbaniae and S. teranga bv sesbaniae bear a symbiotic plasmid and harbor nodulation genes phylogenetically unrelated to Azorhizobium nod genes (tree in ref. 38), suggesting they have a completely different origin. This finding is surprising, because these Sesbania azorhizobia and sinorhizobia have the same geographical origin and host, and suggests that other factors determine the success of the transfer. Bacteria that share the same ecological niche frequently exchange genetic material (39). Different ecologies might explain this apparent contradiction or discrepancy. A. caulinodans was isolated from stem nodules and is thought to be epiphytic bacteria (40), whereas S. rostrata sinorhizobia were isolated from root nodules and are abundant in the soil and in the rhizosphere (41). Transfer of the ICEAc into another rhizobium results in the coexistence of different symbiotic modules on two different replicons of the same strain, a situation already observed in natura (11). ICEAc transfer allows the recipient rhizobial genome to diversify its range of symbiotic hosts and form nitrogen-fixing nodules on S. rostrata, in addition to its natural symbiotic partner.
MGEs can be viewed as elements with independent evolutionary trajectories that allow them to increase their own fitness, in addition to spreading functions useful for the host bacterium. This point of view looks evolutionarily more attractive than altruistic behavior of transferring beneficial traits to competing bacteria, such as antibiotic resistance genes (42) or symbiotic functions. Propagation of symbiosis modules occurs both via multiplication of their bacterial hosts and colonization of new recipient genomes. We found that plant compounds enhance HGT and initiate the nodulation process that ensures massive and specific multiplication, through the interaction with ICE-located regulatory genes. Interestingly, it was recently shown that the dissemination of symbiotic plasmids to different taxa is assisted by error-prone DNA polymerases encoded in the transferred element, which are more active in the plant environment (43). Altogether, these observations support the view that both the plant and the symbiosis element manipulate in concert with the bacterium (8).
Materials and Methods
Bacterial Strains and Plasmids.
Strains and plasmids used in this study are listed in SI Appendix, Table S5. The detailed information for these deletion constructs are listed in SI Appendix, Table S6.
HGT of ICEAc in Vitro and in Rhizosphere.
A. caulinodans with a kanamycin-resistant cassette inserted in ICEAc was used as donor. Spontaneous spectinomycin-resistant mutants were isolated for all rhizobia strains and were used as recipients for examining ICEAc transfer. Donor and recipient cells were mixed and filtered onto 0.22-μm nitrocellulose filters, which were incubated on tryptone-yeast extract (TY) plates at 28 °C for 24 h. When indicated, 20 µM naringenin was included in the medium. Cells were then recovered from the filters, and the number of transconjugants were determined by serial dilution and selected on TY plates containing appropriate antibiotics. To confirm ICEAc HGT to M. huakuii, colony PCR was performed by using primers m1/a2 and m4/a3. To confirm whether the ICEAc was excised and circularized, primers pairs a2/a3 and PCR products were sequenced to confirm the excision positions. To determine the ICEAc insertion site in transconjugants, arbitrary PCR was performed (44) to amplify the flanking sequences of ICEAc and the PCR product was then sequenced. To measure the circularization frequency, qPCR was performed by using DNA from cultures of A. caulinodans grown to different time points indicated as templates and RT-a2/RT-a3 as primers. Amplification of a gene outside of ICEAc (AZC_3912) was used as an internal control in all samples. The standard curves using serially diluted template DNA were used to validate the efficiency of the PCRs.
To determine ICEAc HGT in rhizosphere, ∼109 cells per g vermiculite of A. caulinodans or its derivatives and M. huakuii were inoculated in the rhizosphere of Sesbania, Astragalus, Zea, and Lotus, which were planted in autoclaved vermiculite, or empty vermiculite (supplied with TY media) as controls. Plants were grown in a plant growth chamber at 28 °C with a 12h/12h day/night cycle, and sterile nitrogen-free plant nutrient solution (44) was provided. Vermiculite samples (0.5 g) around the roots (after removing 1 cm surface vermiculite) were withdrawn at the time points indicated and resuspended in sterilized H2O. HGT events were quantified by determining cfus of transconjugants and recipients on selective plates as described above.
AhaR Electrophoretic Mobility Shift Assays.
DNA fragments (265-bp) containing the intC promoter were amplified by PCR using biotin-labeled primers (SI Appendix, Table S2). Binding reactions contained 100 ng of AhaR-His6, 0.2 pmol labeled DNA with or without 20 pmol of unlabeled probes in a buffer consisting of 20 mM Tris⋅HCl (pH 8.0), 50 mM KCl, 1 mM DTT, 0.2 mM EDTA, and 5% (vol/vol) glycerol. When indicated, 100 µM naringenin was included in the reaction mix. After 20 min of incubation at room temperature, samples were size-fractionated and the band shifts were detected and analyzed by using a Chemiluminescent Nucleic Acid Detection Module kit (Thermo) according to the manufacturer’s instructions. The images were then scanned.
Other methods used in this paper are described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Toshihiro Aono for providing S. rostrata seeds. This study was supported by Natural Science Foundation of China (NSFC) Awards 31470202, 81371763, and 31170077; 973 Projects 2015CB150600 and 2010CB126502; and NIH Grant R01 AI120489.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615121113/-/DCSupplemental.
References
- 1.Polz MF, Alm EJ, Hanage WP. Horizontal gene transfer and the evolution of bacterial and archaeal population structure. Trends Genet. 2013;29(3):170–175. doi: 10.1016/j.tig.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ochman H, Moran NA. Genes lost and genes found: Evolution of bacterial pathogenesis and symbiosis. Science. 2001;292(5519):1096–1099. doi: 10.1126/science.1058543. [DOI] [PubMed] [Google Scholar]
- 3.Wozniak RA, Waldor MK. Integrative and conjugative elements: Mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat Rev Microbiol. 2010;8(8):552–563. doi: 10.1038/nrmicro2382. [DOI] [PubMed] [Google Scholar]
- 4.Masson-Boivin C, Giraud E, Perret X, Batut J. Establishing nitrogen-fixing symbiosis with legumes: How many rhizobium recipes? Trends Microbiol. 2009;17(10):458–466. doi: 10.1016/j.tim.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Boivin TNI, et al. Stem nodulation in legumes: Diversity, mechanisms, and unusual characteristics. Crit Rev Plant Sci. 1997;16(1):1–30. [Google Scholar]
- 6.Fisher RF, Long SR. Rhizobium--plant signal exchange. Nature. 1992;357(6380):655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Yang S, Tang F, Zhu H. Symbiosis specificity in the legume: Rhizobial mutualism. Cell Microbiol. 2012;14(3):334–342. doi: 10.1111/j.1462-5822.2011.01736.x. [DOI] [PubMed] [Google Scholar]
- 8.Remigi P, Zhu J, Young JP, Masson-Boivin C. Symbiosis within symbiosis: Evolving nitrogen-fixing legume symbionts. Trends Microbiol. 2016;24(1):63–75. doi: 10.1016/j.tim.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Servín-Garcidueñas LE, et al. Complete genome sequence of Bradyrhizobium sp. strain CCGE-LA001, isolated from field nodules of the enigmatic wild bean Phaseolus microcarpus. Genome Announc. 2016;4(2):e00126–16. doi: 10.1128/genomeA.00126-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KB, et al. The genome of the versatile nitrogen fixer Azorhizobium caulinodans ORS571. BMC Genomics. 2008;9:271. doi: 10.1186/1471-2164-9-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, et al. Whole-genome sequencing of Mesorhizobium huakuii 7653R provides molecular insights into host specificity and symbiosis island dynamics. BMC Genomics. 2014;15:440. doi: 10.1186/1471-2164-15-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan JT, Patrick HN, Lowther WL, Scott DB, Ronson CW. Nodulating strains of Rhizobium loti arise through chromosomal symbiotic gene transfer in the environment. Proc Natl Acad Sci USA. 1995;92(19):8985–8989. doi: 10.1073/pnas.92.19.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan JT, Ronson CW. Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. Proc Natl Acad Sci USA. 1998;95(9):5145–5149. doi: 10.1073/pnas.95.9.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsay JP, et al. A widely conserved molecular switch controls quorum sensing and symbiosis island transfer in Mesorhizobium loti through expression of a novel antiactivator. Mol Microbiol. 2013;87(1):1–13. doi: 10.1111/mmi.12079. [DOI] [PubMed] [Google Scholar]
- 15.Ramsay JP, et al. A LuxRI-family regulatory system controls excision and transfer of the Mesorhizobium loti strain R7A symbiosis island by activating expression of two conserved hypothetical genes. Mol Microbiol. 2009;73(6):1141–1155. doi: 10.1111/j.1365-2958.2009.06843.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramsay JP, et al. Ribosomal frameshifting and dual-target antiactivation restrict quorum-sensing-activated transfer of a mobile genetic element. Proc Natl Acad Sci USA. 2015;112(13):4104–4109. doi: 10.1073/pnas.1501574112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goethals K, Van den Eeede G, Van Montagu M, Holsters M. Identification and characterization of a functional nodD gene in Azorhizobium caulinodans ORS571. J Bacteriol. 1990;172(5):2658–2666. doi: 10.1128/jb.172.5.2658-2666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mergaert P, et al. Fucosylation and arabinosylation of Nod factors in Azorhizobium caulinodans: Involvement of nolK, nodZ as well as noeC and/or downstream genes. Mol Microbiol. 1996;21(2):409–419. doi: 10.1046/j.1365-2958.1996.6451366.x. [DOI] [PubMed] [Google Scholar]
- 19.John M, Röhrig H, Schmidt J, Wieneke U, Schell J. Rhizobium NodB protein involved in nodulation signal synthesis is a chitooligosaccharide deacetylase. Proc Natl Acad Sci USA. 1993;90(2):625–629. doi: 10.1073/pnas.90.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Haeze W, Mergaert P, Promé JC, Holsters M. Nod factor requirements for efficient stem and root nodulation of the tropical legume Sesbania rostrata. J Biol Chem. 2000;275(21):15676–15684. doi: 10.1074/jbc.275.21.15676. [DOI] [PubMed] [Google Scholar]
- 21.Maddocks SE, Oyston PC. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology. 2008;154(Pt 12):3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- 22.Johnson CM, Grossman AD. Identification of host genes that affect acquisition of an integrative and conjugative element in Bacillus subtilis. Mol Microbiol. 2015;98(6):1222. doi: 10.1111/mmi.13282. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CM, Grossman AD. Integrative and conjugative elements (ICEs): What they do and how they work. Annu Rev Genet. 2015;49:577–601. doi: 10.1146/annurev-genet-112414-055018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis JA, Hatfull GF. Control of directionality in integrase-mediated recombination: Examination of recombination directionality factors (RDFs) including Xis and Cox proteins. Nucleic Acids Res. 2001;29(11):2205–2216. doi: 10.1093/nar/29.11.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsay JP, Sullivan JT, Stuart GS, Lamont IL, Ronson CW. Excision and transfer of the Mesorhizobium loti R7A symbiosis island requires an integrase IntS, a novel recombination directionality factor RdfS, and a putative relaxase RlxS. Mol Microbiol. 2006;62(3):723–734. doi: 10.1111/j.1365-2958.2006.05396.x. [DOI] [PubMed] [Google Scholar]
- 26.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: The microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013;11(11):789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 27.Perret X, Staehelin C, Broughton WJ. Molecular basis of symbiotic promiscuity. Microbiol Mol Biol Rev. 2000;64(1):180–201. doi: 10.1128/mmbr.64.1.180-201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil. 2010;329(1-2):1–25. [Google Scholar]
- 29.Webster G, et al. The flavonoid naringenin stimulates the intercellular colonization of wheat roots by Azorhizobium caulinodans. Plant Cell Environ. 1998;21(4):373–383. [Google Scholar]
- 30.Gough C, et al. Specific flavonoids promote intercellular root colonization of Arabidopsis thaliana by Azorhizobium caulinodans ORS571. Mol Plant Microbe Interact. 1997;10(5):560–570. doi: 10.1094/MPMI.1997.10.5.560. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, et al. The bases of crown gall tumorigenesis. J Bacteriol. 2000;182(14):3885–3895. doi: 10.1128/jb.182.14.3885-3895.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mølbak L, Molin S, Kroer N. Root growth and exudate production define the frequency of horizontal plasmid transfer in the Rhizosphere. FEMS Microbiol Ecol. 2007;59(1):167–176. doi: 10.1111/j.1574-6941.2006.00229.x. [DOI] [PubMed] [Google Scholar]
- 33.Kroer N, Barkay T, Sørensen S, Weber D. Effect of root exudates and bacterial metabolic activity on conjugal gene transfer in the rhizosphere of a marsh plant. FEMS Microbiol Ecol. 1998;25(4):375–384. [Google Scholar]
- 34.Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272(5270):1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 35.Peterson G, Kumar A, Gart E, Narayanan S. Catecholamines increase conjugative gene transfer between enteric bacteria. Microb Pathog. 2011;51(1-2):1–8. doi: 10.1016/j.micpath.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Popa O, Dagan T. Trends and barriers to lateral gene transfer in prokaryotes. Curr Opin Microbiol. 2011;14(5):615–623. doi: 10.1016/j.mib.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Haukka K, Lindström K, Young JP. Three phylogenetic groups of nodA and nifH genes in Sinorhizobium and Mesorhizobium isolates from leguminous trees growing in Africa and Latin America. Appl Environ Microbiol. 1998;64(2):419–426. doi: 10.1128/aem.64.2.419-426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Normand P, et al. Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Res. 2007;17(1):7–15. doi: 10.1101/gr.5798407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smillie CS, et al. Ecology drives a global network of gene exchange connecting the human microbiome. Nature. 2011;480(7376):241–244. doi: 10.1038/nature10571. [DOI] [PubMed] [Google Scholar]
- 40.Adebayo A, Watanabe I, Ladha JK. Epiphytic occurrence of Azorhizobium caulinodans and other rhizobia on host and nonhost legumes. Appl Environ Microbiol. 1989;55(9):2407–2409. doi: 10.1128/aem.55.9.2407-2409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bulgarelli D, et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 2012;488(7409):91–95. doi: 10.1038/nature11336. [DOI] [PubMed] [Google Scholar]
- 42.Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405(6784):299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- 43.Remigi P, et al. Transient hypermutagenesis accelerates the evolution of legume endosymbionts following horizontal gene transfer. PLoS Biol. 2014;12(9):e1001942. doi: 10.1371/journal.pbio.1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai T, et al. Host legume-exuded antimetabolites optimize the symbiotic rhizosphere. Mol Microbiol. 2009;73(3):507–517. doi: 10.1111/j.1365-2958.2009.06790.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.