Significance
Kelp forests support diverse and productive ecological communities throughout temperate and arctic regions worldwide, providing numerous ecosystem services to humans. Literature suggests that kelp forests are increasingly threatened by a variety of human impacts, including climate change, overfishing, and direct harvest. We provide the first globally comprehensive analysis of kelp forest change over the past 50 y, identifying a high degree of variation in the magnitude and direction of change across the geographic range of kelps. These results suggest region-specific responses to global change, with local drivers playing an important role in driving patterns of kelp abundance. Increased monitoring aimed at understanding regional kelp forest dynamics is likely to prove most effective for the adaptive management of these important ecosystems.
Keywords: kelp forest, Laminariales, global change, climate change, coastal ecosystems
Abstract
Kelp forests (Order Laminariales) form key biogenic habitats in coastal regions of temperate and Arctic seas worldwide, providing ecosystem services valued in the range of billions of dollars annually. Although local evidence suggests that kelp forests are increasingly threatened by a variety of stressors, no comprehensive global analysis of change in kelp abundances currently exists. Here, we build and analyze a global database of kelp time series spanning the past half-century to assess regional and global trends in kelp abundances. We detected a high degree of geographic variation in trends, with regional variability in the direction and magnitude of change far exceeding a small global average decline (instantaneous rate of change = −0.018 y−1). Our analysis identified declines in 38% of ecoregions for which there are data (−0.015 to −0.18 y−1), increases in 27% of ecoregions (0.015 to 0.11 y−1), and no detectable change in 35% of ecoregions. These spatially variable trajectories reflected regional differences in the drivers of change, uncertainty in some regions owing to poor spatial and temporal data coverage, and the dynamic nature of kelp populations. We conclude that although global drivers could be affecting kelp forests at multiple scales, local stressors and regional variation in the effects of these drivers dominate kelp dynamics, in contrast to many other marine and terrestrial foundation species.
Assessing ecosystem change on a global scale has been instrumental in highlighting the magnitude of human impacts on natural ecosystems. For example, awareness of global declines in fish populations (1), coral reefs (2), and tropical rainforests (3) has substantially increased public interest and subsequent political motivation for environmental conservation. In some cases, global assessments have highlighted complex patterns of change (4, 5), which often reflect variable trajectories among regions (4). However, even where clear global declines have been detected, trajectories of change are often not uniform in direction and magnitude among regions (e.g., ref. 6). Examining patterns of regional change can provide important insights into the mechanisms underlying global change, and lead to more specific recommendations for local and regional management actions aimed at reversing or lessening further ecosystem degradation (7). Such regional insights are particularly useful, as local and regional stressors may be more amenable to management and conservation actions than global stressors (8).
Global declines have been documented in a number of marine habitat-forming or “foundation” species (sensu ref. 9), including seagrasses, corals, and oysters (2, 6, 10). The loss of these species often leads to a direct reduction in ecosystem services critical to human well-being (11–13), and the local demise of taxa that provide those services (14, 15). The brown algae known as kelps (Order Laminariales) are globally important foundation species that occupy 43% of the world’s marine ecoregions (defined in ref. 16) along coastlines of all continents except Antarctica. Kelps are useful sentinels of change because they are highly responsive to environmental conditions (17, 18), and are directly exposed to many human activities that impact the coastal zone (e.g., harvesting, pollution, sedimentation, invasive species, fishing, recreation) (19). Kelps are among the most prolific primary producers on the planet, supporting productivity per unit area rivaling that of intensively cultivated agricultural fields and tropical rainforests (20, 21). Kelps enhance diversity and secondary productivity locally through the formation of biogenic habitat (22, 23), and over broad spatial scales via detrital subsidies (24). Kelp forests support numerous ecosystem services, including commercial fisheries, nutrient cycling, and shoreline protection, valued in the range of billions of dollars annually (19, 25, 26). Consequently, changes in the global abundance of kelps would have far reaching impacts on ecosystem health and services.
Historically, kelp forest ecosystems have demonstrated a high degree of resilience (27, 28), but recent evidence suggests that the capacity of kelp forests to recover from disturbance may be eroding (29, 30). Kelp forest declines have now been documented in many regions in response to a variety of stressors (18, 31–35), but kelp abundances have been stable or increasing in other areas (17, 36, 37). Here, we amass a global database of kelp abundances to provide a comprehensive picture of kelp forest change. Specifically, we assess the evidence for two possible patterns: (i) coherent patterns of change across the global range of kelps, with a global average trend that is large relative to regional variability, or (ii) regional variability that is far larger than any global trend. The former pattern would suggest that global stressors are the main driver of kelp forest dynamics, whereas the latter pattern indicates a dominant influence of regional drivers or region-specific responses to global drivers of change. The results of our analysis provide key insights into the trajectory of one of the world’s most productive ecosystems.
Results
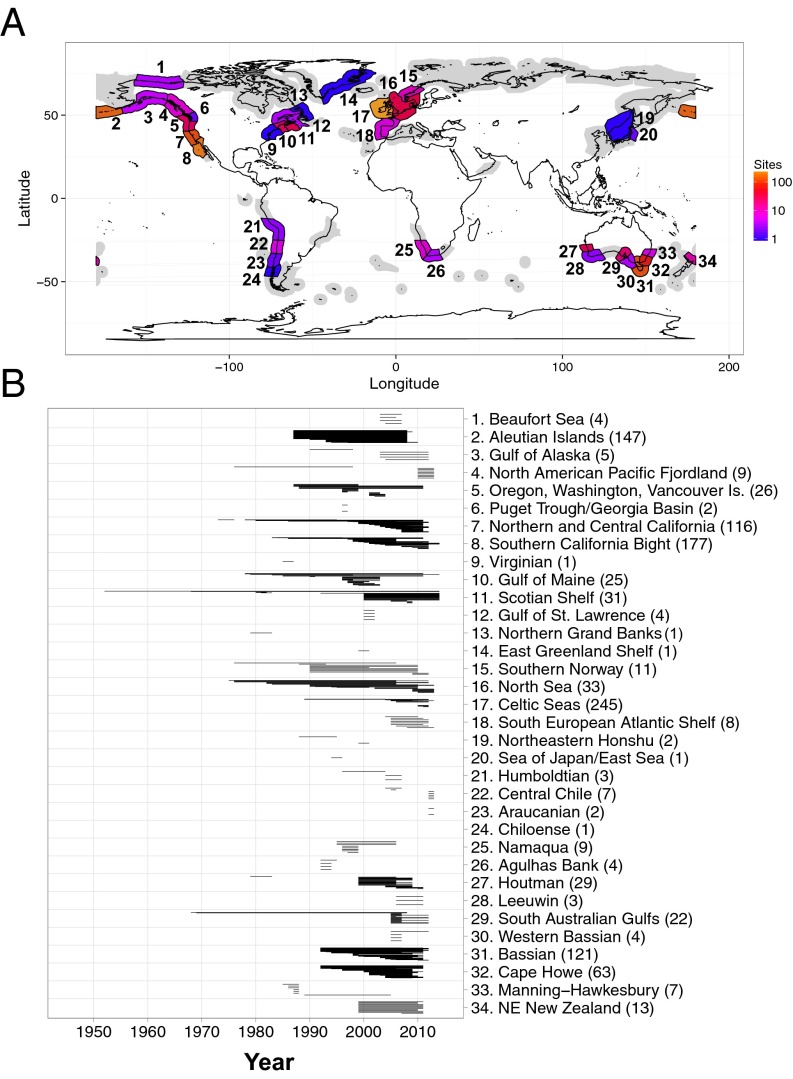
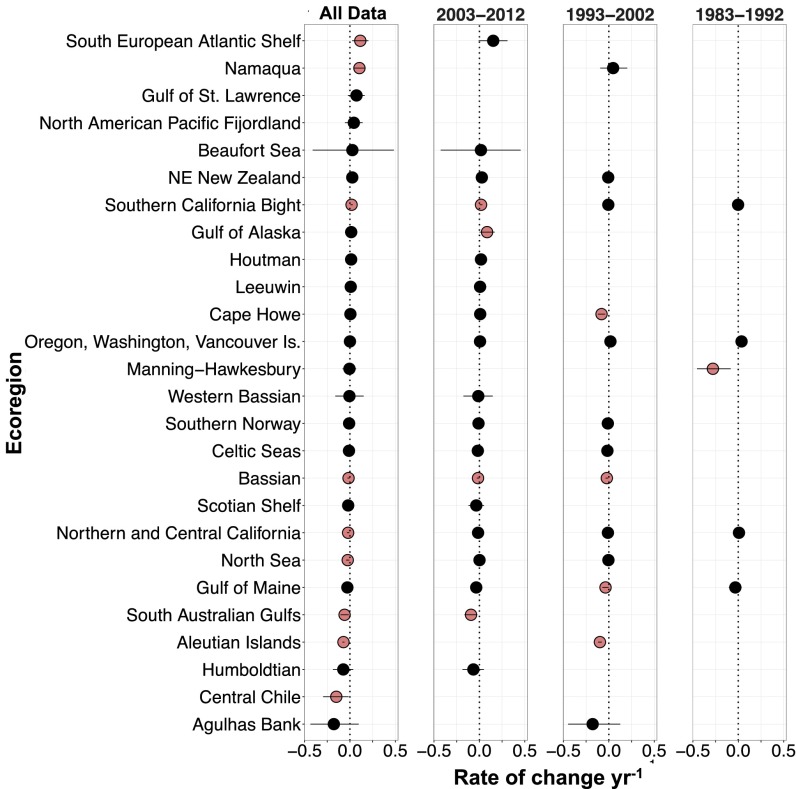
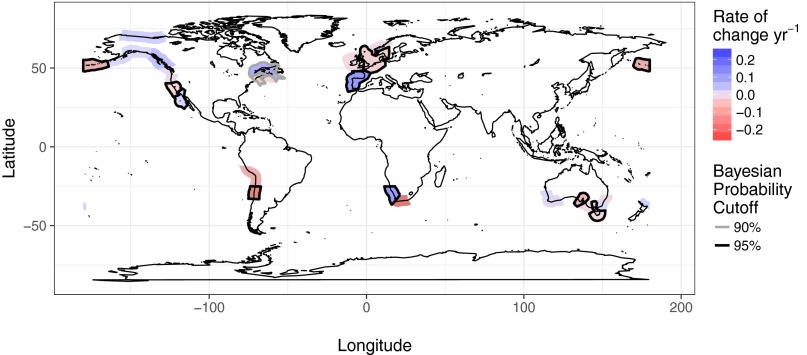
Our database contains data from 34 of the 99 global ecoregions where kelps exist, covering a large portion of the temperate range of kelps as well as a few Arctic areas (Fig. 1). Our analysis revealed that trajectories of change in kelp abundances were highly variable across regions (Figs. 2 and 3 and SI Appendix, Figs. S1 and S2). Using all available data across the greatest time span for each ecoregion, we estimated a high probability of decline (≥95%) in kelp abundances for Central Chile (instantaneous rate of change = −0.150, n = 7 sites from 2004 to 2013), the Aleutian Islands (−0.071, n = 147 sites from 1987 to 2010), the South Australian Gulfs (−0.059, n = 22 sites from 1968 to 2012), the North Sea (−0.024, n = 33 sites from 1975 to 2013), North-Central California (−0.019, n = 116 sites from 1973 to 2012), and the Bassian ecoregion (−0.015, n = 121 sites from 1992 to 2012) (Figs. 2 and 3 and SI Appendix, Figs. S1 and S2). High-magnitude declines were also inferred for the Agulhas Bank (−0.177, n = 4 sites from 1992 to 1995) and Humboldt (−0.073, n = 3 sites from 1996 to 2007) ecoregions, but there is greater uncertainty in these trends. There was also evidence for a small overall decline in the Gulf of Maine (93% probability, −0.028, n = 25 sites from 1978 to 2014) and the Scotian Shelf (83% probability, −0.020, n = 31 sites from 1952 to 2014) ecoregions, but, again, these trends were associated with higher uncertainty (Figs. 2 and 3 and SI Appendix, Figs. S1 and S2). When data were partitioned by decade, our analysis identified a high probability of decline in kelps from 2003 to 2012 in the South Australian Gulfs (−0.092) and the Bassian ecoregion (−0.015); from 1993 to 2002 in the Aleutian Islands (−0.098), Cape Howe (−0.080), Gulf of Maine (−0.036), and Bassian (−0.022) ecoregions; and from 1983 to 1992 in the Manning–Hawkesbury ecoregion (−0.280) (Fig. 2).
Fig. 1.
(A) Number of sites in the dataset (n = 1,454) by ecoregion (16). Gray shading indicates ecoregions where kelps are present but for which no data were available. (B) Range of dates within each study within each ecoregion, with line shading indicating the weight of studies within that range.
Fig. 2.
Modeled ecoregion slopes (instantaneous rate of change per year) and 90% credible intervals for the full dataset (n = 8,846 data points from 26 ecoregions), with variable temporal coverage in each ecoregion, and by decade (1983–1992, 1993–2002, and 2003–2012). Ecoregion means are colored red if the 90% credible intervals (i.e., high probability) do not overlap zero.
Fig. 3.
Modeled slopes (instantaneous rate of change per year) and associated probability (Bayesian probability cutoff) for each ecoregion (n = 8,846 data points from 26 ecoregions). Slope magnitudes are shown as colored shading of ecoregions, whereas probability is demonstrated using the thickness of ecoregion outlines.
We identified three ecoregions where there was a high probability of increases in kelp abundance over the time periods for which we have data (Figs. 2 and 3 and SI Appendix, Figs. S1 and S2): the South European Atlantic Shelf (instantaneous rate of change = 0.114, n = 8 sites from 2004 to 2013), the Southern California Bight (0.018, n = 178 sites from 1980 to 2014), and Namaqua (0.104, n = 9 sites from 1995 to 2006). Relatively high-magnitude increases were also inferred for the Gulf of St. Lawrence (0.072, n = 4 sites from 2000 to 2002), North American Pacific Fjordland (0.043, n = 9 sites from 1976 to 2013), Beaufort Sea (0.027, n = 4 sites from 2003 to 2007), and Northeastern New Zealand (0.026, n = 13 sites from 1999 to 2011) ecoregions, but there was greater uncertainty in these trends (Figs. 2 and 3 and SI Appendix, Figs. S1 and S2). When data were partitioned by decade, high-probability increases were inferred from 2003 to 2012 in the Gulf of Alaska (0.085) and Southern California Bight (0.018) (Fig. 2). Large uncertainty in the posterior rate estimates of the remaining ecoregions precluded conclusions regarding the direction of trends (Figs. 2 and 3 and SI Appendix, Figs. S1 and S2). As a result, 35% of ecoregions were inferred not to exhibit strong evidence of a directional change.
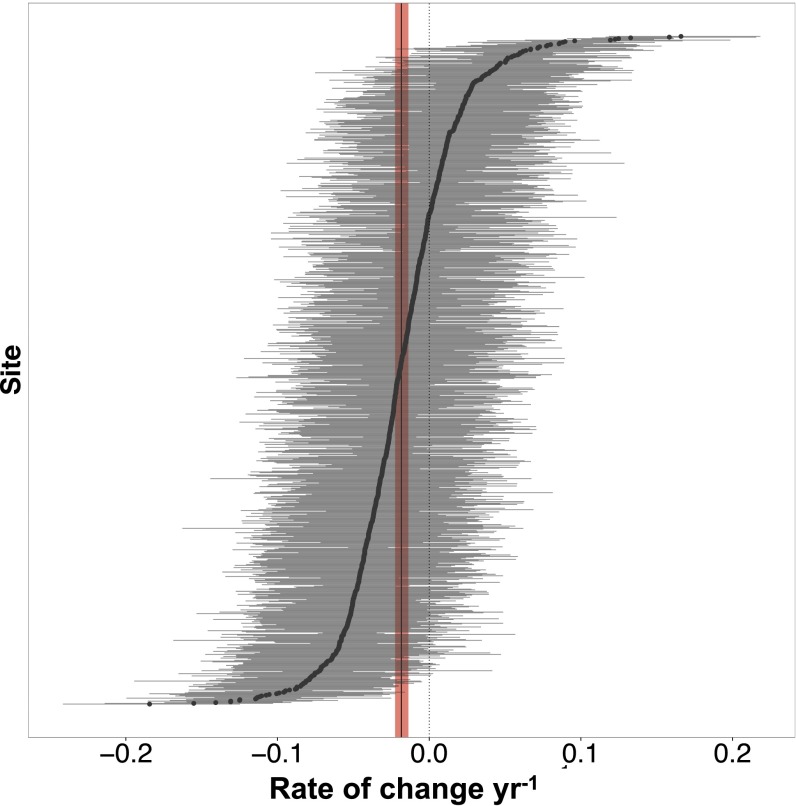
Our analysis detected a global average decline (i.e., the mean of site-level slopes is negative, instantaneous rate of change = −0.018 y−1, n = 1,138 sites from 1952 to 2015) (Fig. 4) that was an order of magnitude smaller than the largest proportional rate of change observed regionally (−0.177 y−1 in Agulhas Bank). Although the majority of the dataset was composed of relatively short-duration studies (71% were <20 y in duration) (SI Appendix, Fig. S3), the negative global trend detected by our analysis was most strongly influenced by declines detected in datasets that were collected over time periods longer than 20 y (SI Appendix, Fig. S4) in 10 ecoregions (Scotian Shelf, South Australian Gulfs, North Sea, Gulf of Maine, North-Central California, Celtic Seas, North Sea, Southern California Bight, Aleutian Islands, and Southern Norway). Slope estimates associated with time series shorter than 20 y had higher variability, and more commonly showed no directional change (SI Appendix, Fig. S4). The global average trend was also most strongly influenced by ecoregions with the most data (i.e., the Aleutian Islands, North-Central California, Southern California Bight, Bassian, Cape Howe, Celtic Seas), and therefore may not be representative of large portions of the range of kelp where data were missing entirely (i.e., Eastern Canadian Arctic and West Greenland Shelf, the Russian Arctic, Cold Temperate Northwest Pacific, Southern Chile and the Patagonian Shelf, Sahara Upwelling, Eastern Bering Sea, Southern New Zealand, Great Australian Bight) (Fig. 1), or where data were sparse through time and across space (Figs. 1 and 2). Furthermore, although the earliest data were collected in 1952, the bulk of available data were collected from the 1990s to the present (Fig. 1 and SI Appendix, Fig. S3), with the temporal distribution of available data differing by ecoregion (Fig. 1).
Fig. 4.
Global distribution of modeled site level slopes (instantaneous rate of change per year) and 90% credible intervals (n = 8,846 data points from 26 ecoregions). The vertical black line shows the global mean slope, with the shaded band indicating the 90% credible interval, which does not overlap zero (i.e., >95% probability of decline).
Discussion
Global declines of a focal species group can indicate that the cumulative effects of single or multiple stressors are overwhelming the resilience of species throughout their range. The potential for this decline is considered more likely for species with slow postdisturbance recovery rates [e.g., corals (38)], those species that display little seasonal variability in abundance [e.g., mangroves (39)], or those species experiencing stressors of sufficiently widespread and high magnitude [e.g., fishes (1)]. However, for many taxa, globally coherent signals of change are unlikely when the identity and magnitude of drivers vary widely on local and regional scales (4, 17, 40), where species and ecotypes within taxonomic groups respond variably to change, or where abiotic and biotic contexts vary widely across geographic locations (41). The global average decline detected by our analysis was modest compared with the large interregional variability in the trajectories of change exhibited by kelps across their range. Many ecoregions did not exhibit evidence of directional change, and for those ecoregions that did, both declines and increases were evident at rates much larger than the global average. These findings suggest region-specific signals of global change, with local factors playing a dominant role in driving kelp forest dynamics.
Many kelp forest ecosystems are naturally highly variable on both seasonal and interannual time scales, and over small spatial scales (42–44), reflecting a high reactivity to environmental drivers and variation in their capacity to resist (45) and recover from both small- and large-scale disturbances (46, 47). This wide temporal variation contrasts with other marine foundation species such as seagrasses (48, 49) and corals (50), which tend to hold space for many years and take decades to recover from disturbance. For kelp, rapid recovery following catastrophic population losses is enabled by frequent recruitment and fast individual growth rates; indeed, kelps have some of the fastest growth rates of any primary producer on the planet (20, 21). The high degree of natural variability among kelp systems may also explain why detecting directional trends in kelp abundances is difficult in some regions of the globe; available time series tend to be short and variable compared with any long-term change that kelp forests may exhibit (51).
The regional variability in change detected by our analysis is also a reflection of the diversity of multiscaled stressors whose effects vary in direction and magnitude across the geographic range of kelp forests (52–54). In some cases, the results of our analysis are consistent with predictions regarding regional trends in temperature, with decreases in abundance in locations where water temperatures are warming (e.g., Bassian ecoregion, Gulf of Maine, Scotian Shelf) (32, 33) and increases where water temperatures are cooling (e.g., Western South Africa, Southern California Bight) (52, 55). In many instances, literature suggests that climate-driven temperature change is acting synergistically with other stressors, such as the fishing of sea urchin predators [Bassian ecoregion (7)], pollution [South Australian Gulfs (56)], and invasive species [Scotian Shelf (32)] to cause the kelp declines detected by our analysis. However, our analyses identified trajectories of change in some regions that were the opposite of the trajectories of change predicted by climate alone (e.g., decreases in central and Northern Chile, the Aleutian Islands), suggesting the overwhelming influences of other drivers. For example, kelp harvesting accounted for recent kelp declines in Central and Northern Chile despite a regional cooling trend (57). In other instances, regional increases in kelp (e.g., west coast of Vancouver Island, Southern California Bight) can be attributed to successful local management efforts, including the recovery of previously exploited sea urchin predators (58) and reductions in local pollution levels (59). Regional variation in the trajectories of kelp change was also influenced by the underlying frequency of sea urchin-driven phase shifts that characterize many kelp forest ecosystems (28, 30). For example, long-term declines in the Aleutians were associated with persistent shifts toward sea urchin barrens since the 1990s (60). Such long-term change in kelp abundances may be obscured in systems that experience more frequent phase shifts, such as the Scotian Shelf (47) and Northern, Central, and Southern California (61–63). In some regions, identified trajectories were opposite to those trajectories documented in local studies (e.g., South European Atlantic Shelf, Western Australia) (64, 65). These discrepancies are likely a result of the short time series available for these regions, the greater ability of focused regional studies to pick up certain types of change (e.g., range changes, shifts in species composition), and/or the occurrence of discrete events that disrupt stability or gradual change (18).
We have compiled and analyzed the most comprehensive database of subtidal kelp abundance time series to date. However, there remains a high degree of uncertainty in the trajectory of change across the global range of kelps, with few to no data in many regions where kelp exists. Even in ecoregions where kelps have been sampled, datasets contain few sites and are characterized by inconsistent sampling over time. In particular, there is a noticeable lack of historical “baseline” data by which to benchmark change, with the majority of our data having been collected within the past 30 y. This data limitation is especially true for Arctic regions, where climate change impacts are anticipated to be highest (52). The decrease in the number of sites in our dataset in the past 5 y is also a potential cause for concern, although this decrease may partially reflect the exclusion of recently initiated monitoring programs that did not meet our criteria for inclusion (≥2 y in duration). Nonetheless, the reality is that the majority of subtidal research programs are short in duration due, in large part, to variable and unpredictable funding. Consequently, in some regions, there has been a clear shift in emphasis away from long-term monitoring for this key group of foundation species (25). Detecting future changes in kelp ecosystems at regional and global scales is best achieved by methodologically consistent, long-term monitoring efforts broadly distributed throughout all ecoregions (51). The use of aerial and satellite data to monitor changes in kelp canopies can help fill gaps in effort (17, 42), but these technologies are useful only for kelps that have surface canopies, whose current geographic distribution represents only about half of the total global extent of kelps.
Overall, our results show a high degree of variability within and among regions in the direction and magnitude of kelp forest change over the past half-century, with declines noted in just over a third of ecoregions included our analysis. This result is strikingly different from that reported for many other marine and terrestrial systems that are experiencing consistent declines in species abundance across the globe (1–3, 6) and, in many ways, highlights the atypical resilience of kelps. However, where kelp resilience is eroding and leading to declines in abundance (7, 18, 54, 64, 66, 67), impacts to ecosystem health and services can be far-reaching (19, 25, 26, 68, 69). Maintaining the resilience of kelp forest ecosystems into the future will rely on the continued monitoring of kelps and management of stressors on local and regional scales.
Materials and Methods
We compiled time series of diver-sampled kelp abundances from published and contributed datasets (SI Appendix, Tables S1 and S2). We also collected published datasets from a Web of Science search conducted in July–August 2013 and repeated in May 2014. The search terms we used were: kelp and loss, abundance, change, recovery, stability, decline, gain, time series, density, biomass, percent cover, and disturbance. We also used the terms increase and decrease in association with abundance, biomass, and density. This search returned a total of 4,490 results. We included studies if they had at least three measurements of diver-sampled abundance (quadrat, transect, or plot-scale) of a kelp species (Order Laminariales) that spanned more than 2 y. This literature search resulted in the inclusion of datasets from 48 published studies (SI Appendix, Table S1). We also requested datasets from the Kelp Ecosystem Ecology Network listserv (www.kelpecosystems.org) and from additional scientists identified by network members, and included these datasets if they met our criteria. Relevant measures of abundance were biomass density, individual density, stipe density, or percent cover. We note that these measures of abundance may not capture some range contractions or losses of kelp bed area, depending on the local and regional sampling regime. However, they were chosen because they represent the longest time series available for the widest range of kelp species. Data using the SACFOR scale (S, superabundant; A, abundant; C, common; F, frequent; O, occasional; R, rare) from Europe were translated to corresponding abundances (70). Only data from subtidal kelps (>3-m depth) were included. Juvenile kelps (independently defined by data collectors) were excluded due to variable reporting of their abundances across datasets. The datasets included and their associated citations are listed in SI Appendix, Tables S1 and S2. Species included in each ecoregion are listed in SI Appendix, Table S1.
We compiled datasets into a single database representing time series of kelp abundance at a particular site (as defined by each author or data contributor). In some datasets, this series represented changes at the level of a population (single species), but, more commonly, this series represented a summed assemblage of multiple kelp species, the composition of which was made to remain consistent throughout the duration of each time series. We note that aggregating across species precludes us from detecting changes in kelp species composition within regions. We averaged abundance values at the site scale across all depths and replicate samples taken during a given sampling event, keeping the number of replicates consistent across sampling dates. For most trajectories, we averaged across sampling events that occurred within 45 d of one another to generate a single value, and treated that average as having occurred on the mean date of the combined sampling events. We aggregated samples from some datasets over time intervals other than 45 d to accommodate sampling regimes specific to particular studies (4 d, Southern Chile; 20 d, Nova Scotia and Northern Ireland; 31 d, Northern Chile; and 65 d Southern California).
We selected only one metric for analysis in instances where studies included multiple metrics of abundance (i.e., biomass density, individual density, stipe density, percent cover). For each trajectory, we analyzed the metric with the greatest number of sampling events. In the case of multiple metrics having the same number of samples, we selected the metric using the following hierarchy: biomass density (per square meter), stipe density (per square meter), individual density (per square meter), and percent cover.
We used Bayesian hierarchical linear models to estimate proportional rates of change in the dataset as a whole (1949–2015) and split by decade (1983–1992, 1993–2002, and 2003–2012). We note that similar results were achieved using a likelihood-based mixed-effects model framework. We discuss trends at the global and ecoregion levels only, because the analytical method used is not well suited to describing trends at sites where data are sparse. We grouped sites by ecoregion according to Spalding et al. (16). In each model, we simultaneously estimated a unique rate for each time series and for each chosen geographic region (with ecoregion or global subdivisions). We achieved this estimation by considering the rate for each unique time series as a random (normal) deviation from the mean rate within that geographic region. To account for study-level differences in uncertainty, we allowed for a unique error variance within each study within each geographic region.
The model describing the abundance of kelps at the ith site within the jth study within the kth geographic grouping (ecoregion or globe) at time t was
| [1] |
| [2] |
where xk,j,i,t is the time in years, β0k and β1k are the geographic group level intercept and slope (on the log-scale), γ0k,i and γ1k,i are the site level deviations from β0k and β1k (respectively), σj is the error variance associated with each study, and yk,j,i,t is the kelp value standardized to the mean of the maximum three values in the specific ecoregion-focal unit combination as follows:
| [3] |
We used a hierarchical framework to account for site-level deviations from the geographic group-level mean parameters. This method is essential to account for among-site variability in trends while also estimating overall geographic group level mean trends (71). Thus, the site level deviations γ0k,i and γ1k,i were assumed to follow a multivariate normal (MVN) distribution:
| [4] |
For numerical stability and efficiency, instead of estimating Σ directly, we used the Cholesky decomposition (L) of the correlation matrix (Ω) and the SD in intercept and slope among sites σγ = [σγ0 σγ1]T with a Lewandowski–Dorota–Joe (LKJ) prior in L (github.com/stan-dev/stan/releases/download/v2.7.0/stan-reference-2.7.0.pdf), where
| [5] |
| [6] |
Priors and hyperpriors were as follows:
| [7] |
| [8] |
| [9] |
| [10] |
| [11] |
Because error variance may differ depending on the type of measurement (biomass density, individual density, stipe density, or percent cover) we allowed for different half-Cauchy hyperpriors (νM) on the study level error variance for each of the four measurement types.
We sampled posteriors within a Bayesian hierarchical framework via the no-U-turn-sampler variant of Hamilton Monte Carlo (72) in Stan via rstan (73, 74) using R (75). Sampling included three chains each of 3,000 iterations and a 1,500-iteration burn-in period (sufficient to produce posterior convergence).
From the numerically generated posterior samples, we calculated the mean and two-tailed quantiles, or 90% symmetrical credible intervals (i.e., Bayesian analog to a confidence interval) for each parameter. We highlight those rate estimates for which the 90% posterior credible interval did not overlap zero as having a high probability of decline or increase. That is, if the upper bound of the 90% credible interval lies on zero, this value equates to a 95% probability of decline. We express the magnitude of change as the mean estimated slope (instantaneous rate of change per year), considering kelps to be declining where instantaneous rates are less than −0.015 y−1 and increasing where rates are greater than 0.015 y−1 (representing rates of change equivalent to a halving or doubling in abundance over a 50-y period).
Supplementary Material
Acknowledgments
We thank Robert Scheibling for comments on a previous draft of this manuscript and Paul Geoghegan and A. Le Gal for contributions to the dataset. This research was conducted as part of the Kelp and Climate Change Working Group supported by the National Center for Ecological Analysis and Synthesis, a center funded by National Science Foundation (Grant DEB-00-72909); the University of California, Santa Barbara; and the State of California. This work was also supported by Massachusetts Institute of Technology SeaGrant 2015-R/RCM-39 (to J.E.K.B.), a Natural Sciences and Engineering Research Council of Canada Discovery Grant (to A.K.S.), the Norwegian Institute for Water Research, and the Institute of Marine Research in Norway.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.H.G. is a Guest Editor invited by the Editorial Board.
Data deposition: The global database of kelp abundance time series has been published on the Kelp Ecosystem Ecology Network website (www.kelpecosystems.org/data/), and on the Temperate Reef Base website (temperatereefbase.imas.utas.edu.au/portal/search?uuid=ecbe5cc3-3fbf-4569-b5e8-07c2201fcb9c). All data processing, analysis, and visualization R code have been made available via GitHub (https://github.com/kelpecosystems/global_kelp_time_series).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606102113/-/DCSupplemental.
References
- 1.Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423(6937):280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301(5635):955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 3.Woodwell GM, et al. Global deforestation: Contribution to atmospheric carbon dioxide. Science. 1983;222(4628):1081–1086. doi: 10.1126/science.222.4628.1081. [DOI] [PubMed] [Google Scholar]
- 4.Boyd PW, Lennartz ST, Glover DM, Doney SC. Biological ramifications of climate-change-mediated oceanic multi-stressors. Nat Commun. 2014;5:71–79. [Google Scholar]
- 5.Condon RH, et al. Recurrent jellyfish blooms are a consequence of global oscillations. Proc Natl Acad Sci USA. 2013;110(3):1000–1005. doi: 10.1073/pnas.1210920110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waycott M, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA. 2009;106(30):12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling SD, Johnson CR, Frusher SD, Ridgway KR. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc Natl Acad Sci USA. 2009;106(52):22341–22345. doi: 10.1073/pnas.0907529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strain EMA, van Belzen J, van Dalen J, Bouma TJ, Airoldi L. Management of local stressors can improve the resilience of marine canopy algae to global stressors. PLoS One. 2015;10(3):e0120837. doi: 10.1371/journal.pone.0120837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dayton PK. Toward an understanding of community resilience and the potential effects of enrichment to the benthos at McMurdo Sound, Antarctica. In: Parker BC, editor. Proceedings of the Colloquium on Conservation Problems in Antarctica. Allen Press; Lawrence, KS: 1972. [Google Scholar]
- 10.Beck MW, et al. Shellfish reefs at risk globally and recommendations for ecosystem revitalization. Bioscience. 2011;61:107–116. [Google Scholar]
- 11.Danielsen F, et al. The Asian tsunami: A protective role for coastal vegetation. Science. 2005;310(5748):643. doi: 10.1126/science.1118387. [DOI] [PubMed] [Google Scholar]
- 12.Grabowski JH, et al. Economic valuation of ecosystem services provided by oyster reefs. Bioscience. 2012;62:900–909. [Google Scholar]
- 13.McGlathery KJ, Sundback K, Anderson IC. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Mar Ecol Prog Ser. 2007;348:1–18. [Google Scholar]
- 14.Jackson EL, Rees SE, Wilding C, Attrill MJ. Use of a seagrass residency index to apportion commercial fishery landing values and recreation fisheries expenditure to seagrass habitat service. Conserv Biol. 2015;29(3):899–909. doi: 10.1111/cobi.12436. [DOI] [PubMed] [Google Scholar]
- 15.Moburg F, Folke C. Ecological goods and services of coral reef ecosystems. Ecol Econ. 1999;29:215–233. [Google Scholar]
- 16.Spalding MD, et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. Bioscience. 2007;57:573–583. [Google Scholar]
- 17.Bell TW, Cavanaugh KC, Reed DC, Siegel DA. Geographic variability in the controls of giant kelp biomass dynamics. J Biogeogr. 2015;42:2100–2021. [Google Scholar]
- 18.Wernberg T, et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat Clim Chang. 2013;3:78–82. [Google Scholar]
- 19.Bennett S, et al. The ‘Great Southern Reef’: Social, ecological and economic value of Australia’s neglected kelp forests. Mar Freshw Res. 2016;67:47–56. [Google Scholar]
- 20.Leith H, Whittaker RH. Primary Productivity of the Biosphere. Springer; New York: 1975. [Google Scholar]
- 21.Mann KH. Seaweeds: Their productivity and strategy for growth: The role of large marine algae in coastal productivity is far more important than has been suspected. Science. 1973;182(4116):975–981. doi: 10.1126/science.182.4116.975. [DOI] [PubMed] [Google Scholar]
- 22.Dayton PK. Ecology of kelp communities. Annu Rev Ecol Syst. 1985;16:215–245. [Google Scholar]
- 23.Duggins DO, Simenstad CA, Estes JA. Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science. 1989;245(4914):170–173. doi: 10.1126/science.245.4914.170. [DOI] [PubMed] [Google Scholar]
- 24.Krumhansl KA, Scheibling RE. Production and fate of kelp detritus. Mar Ecol Prog Ser. 2012;467:281–302. [Google Scholar]
- 25.Smale DA, Burrows MT, Moore P, O’Connor N, Hawkins SJ. Threats and knowledge gaps for ecosystem services provided by kelp forests: A northeast Atlantic perspective. Ecol Evol. 2013;3(11):4016–4038. doi: 10.1002/ece3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vásquez JA, et al. Economic valuation of kelp forests in northern Chile: Values of goods and services of the ecosystem. J Appl Phycol. 2014;26:1081–1088. [Google Scholar]
- 27.Dayton PK, Tegner MJ, Parnell PE, Edwards PB. Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecol Monogr. 1992;62:421–445. [Google Scholar]
- 28.Filbee-Dexter K, Scheibling RE. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar Ecol Prog Ser. 2014;495:1–25. [Google Scholar]
- 29.Wernberg T, et al. Decreasing resilience of kelp beds along a latitudinal temperature gradient: Potential implications for a warmer future. Ecol Lett. 2010;13(6):685–694. doi: 10.1111/j.1461-0248.2010.01466.x. [DOI] [PubMed] [Google Scholar]
- 30.Ling SD, et al. Global regime shift dynamics of catastrophic sea urchin overgrazing. Philos Trans R Soc Lond B Biol Sci. 2015;370:1–10. [Google Scholar]
- 31.Connell SD, et al. Recovering a lost baseline: Missing kelp forests from a metropolitan coast. Mar Ecol Prog Ser. 2008;360:63–72. [Google Scholar]
- 32.Filbee-Dexter K, Feehan C, Scheibling RE. Large-scale degradation of a kelp ecosystem in an ocean warming hotspot. Mar Ecol Prog Ser. 2016;543:141–152. [Google Scholar]
- 33.Johnson CR, et al. Climate change cascades: Shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J Exp Mar Biol Ecol. 2011;400:17–32. [Google Scholar]
- 34.Moy FE, Christie H. Large-scale shift from sugar kelp (Saccharina latissima) to ephemeral algae along the south and west coast of Norway. Mar Biol Res. 2012;8:309–321. [Google Scholar]
- 35.Steneck RS, et al. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ Conserv. 2002;29:436–459. [Google Scholar]
- 36.Blamey LK, et al. Ecosystem change in the southern Benguela and the underlying processes. J Mar Syst. 2015;144:9–29. [Google Scholar]
- 37.Smale DA, Wernberg T, Yunnie ALE, Vance T. The rise of Laminaria ochroleuca in the Western English Channel (UK) and comparisons with its competitor and assemblage dominant Laminaria hyperborea. Mar Ecol (Berl) 2014;36:1033–1044. [Google Scholar]
- 38.Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007;450(7166):98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- 39.Cebrian J. Variability and control of carbon consumption, export, and accumulation in marine communities. Limnol Oceanogr. 2002;47:11–22. [Google Scholar]
- 40.Norderhaug KM, et al. Combined effects from climate variation and eutrophication on the diversity in hard bottom communities on the Skagerrak coast 1990-2010. Mar Ecol Prog Ser. 2015;530:29–46. [Google Scholar]
- 41.Johnson CR, Mann KH. Diversity, patterns of adaptation, and stability of Nova Scotian kelp beds. Ecol Monogr. 1988;58:129–154. [Google Scholar]
- 42.Cavanaugh KC, Seigel DA, Reed DC, Dennison PE. Environmental controls of giant kelp biomass in the Santa Barbara Channel, California. Mar Ecol Prog Ser. 2011;429:1–17. [Google Scholar]
- 43.Gagné JA, Mann KH, Chapman ARO. Seasonal patterns of growth and storage in Laminaria longicruris in relation to differing patterns of availability of nitrogen in the water. Mar Biol. 1982;69:91–101. [Google Scholar]
- 44.Reed DC, et al. Wave disturbance overwhelms top-down and bottom-up control of primary production in California kelp forests. Ecology. 2011;92(11):2108–2116. doi: 10.1890/11-0377.1. [DOI] [PubMed] [Google Scholar]
- 45.Ghedini G, Russell BD, Connell SD. Trophic compensation reinforces resistance: Herbivory absorbs the increasing effects of multiple disturbances. Ecol Lett. 2015;18(2):182–187. doi: 10.1111/ele.12405. [DOI] [PubMed] [Google Scholar]
- 46.Edwards MS. Estimating scale-dependency in disturbance impacts: El Niños and giant kelp forests in the northeast Pacific. Oecologia. 2004;138(3):436–447. doi: 10.1007/s00442-003-1452-8. [DOI] [PubMed] [Google Scholar]
- 47.Scheibling RE, Feehan CJ, Lauzon-Guay JS. Climate change, disease and the dynamics of a kelp-bed ecosystem in Nova Scotia. In: Fernández-Palocios JM, et al., editors. Climate Change: Perspectives from the Atlantic: Past, Present and Future. Servicio de Publicaciones de la Universidad de La Laguna; Tenerife, Canary Islands: 2013. pp. 41–81. [Google Scholar]
- 48.Meehan AJ, West RJ. Recovery times for a damaged Posidonia australis bed in south eastern Australia. Aquat Bot. 2000;67:161–167. [Google Scholar]
- 49.Erftemeijer PLA, Lewis RRR., 3rd Environmental impacts of dredging on seagrasses: A review. Mar Pollut Bull. 2006;52(12):1553–1572. doi: 10.1016/j.marpolbul.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Graham NAJ, Nash KL, Kool JT. Coral reef recovery dynamics in a changing world. Coral Reefs. 2011;30:283–294. [Google Scholar]
- 51.Reed DC, Rassweiler AR, Miller RJ, Page HM, Holbrook SJ. The value of a broad temporal and spatial perspective in understanding dynamics of kelp forest ecosystems. Mar Freshw Res. 2016;67:14–24. [Google Scholar]
- 52.Lima FP, Wethey DS. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat Commun. 2012;3:704. doi: 10.1038/ncomms1713. [DOI] [PubMed] [Google Scholar]
- 53.Shears NT, Ross PM. Toxic cascades: Multiple anthropogenic stressors have complex and unanticipated interactive effects on temperate reefs. Ecol Lett. 2010;13(9):1149–1159. doi: 10.1111/j.1461-0248.2010.01512.x. [DOI] [PubMed] [Google Scholar]
- 54.Strain EMA, Thomson RJ, Micheli F, Mancuso FP, Airoldi L. Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Glob Change Biol. 2014;20(11):3300–3312. doi: 10.1111/gcb.12619. [DOI] [PubMed] [Google Scholar]
- 55.Bolton JJ, Anderson RJ, Smit AJ, Rothman MD. South African kelp moving eastwards: The discovery of Ecklonia maxima (Osbeck) Papenfuss at De Hoop Nature Reserve on the south coast of South Africa. Afr J Mar Sci. 2012;34:147–151. [Google Scholar]
- 56.Russell BD, Thompson JA, Falkenberg LJ, Connell SD. Synergistic effects of climate change and local stressors: CO2 and nutrient-driven change in subtidal rocky habitats. Glob Change Biol. 2009;15:2153–2162. [Google Scholar]
- 57.Vásquez JA. Production, use and fate of Chilean brown seaweeds: Resources for a sustainable fishery. J Appl Phycol. 2008;20:457–467. [Google Scholar]
- 58.Watson J, Estes JA. Stability, resilience, and phase shifts in rocky subtidal communities along the west coast of Vancouver Island, Canada. Ecol Monogr. 2011;81:215–239. [Google Scholar]
- 59.Foster MS, Schiel DR. Loss of predators and the collapse of southern California kelp forests (?): Alternatives, explanations and generalizations. J Exp Mar Biol Ecol. 2010;393:59–70. [Google Scholar]
- 60.Estes JA, Tinker MT, Williams TM, Doak DF. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282(5388):473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- 61.Dayton PK, et al. Patch dynamics and stability of some California kelp communities. Ecol Monogr. 1984;54:253–289. [Google Scholar]
- 62.Ebeling AW, Laur DR, Rowley RJ. Severe storm disturbances and reversal of community structure in a southern California kelp forest. Mar Biol. 1985;84:287–294. [Google Scholar]
- 63.Pearse JS, Hines AH. Expansion of a central California kelp forest following the mass mortality of sea urchins. Mar Biol. 1979;51:83–91. [Google Scholar]
- 64.Wernberg T, et al. Climate-driven regime shift of a temperate marine ecosystem. Science. 2016;353(6295):169–172. doi: 10.1126/science.aad8745. [DOI] [PubMed] [Google Scholar]
- 65.Voerman SE, Llera E, Rico JM. Climate driven changes in subtidal kelp forest communities in NW Spain. Mar Environ Res. 2013;90:119–127. doi: 10.1016/j.marenvres.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Andersen GS, Steen H, Christie H, Fredriksen S, Moy FE. Seasonal patterns of sporophyte growth, fertility, fouling, and mortality of Saccharina latissima in Skagerrak, Norway: Implications for forest recovery. J Mar Biol. 2011;2011:1–8. [Google Scholar]
- 67.Connell SD, Russell BD. The direct effects of increasing CO2 and temperature on non-calcifying organisms: Increasing the potential for phase shifts in kelp forests. Proc Biol Sci. 2010;277(1686):1409–1415. doi: 10.1098/rspb.2009.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krumhansl KA, Scheibling RE. Detrital production in Nova Scotian kelp beds: Patterns and processes. Mar Ecol Prog Ser. 2011;421:67–82. [Google Scholar]
- 69.Costanza R, et al. The value of the world’s ecosystem services and natural capital. Nature. 1997;387:253–260. [Google Scholar]
- 70.Connor DW, Hiscock K. 1996. Data collection methods (with Appendices 5–10). Marine Nature Conservation Review: Rationale and Methods, Coasts and Seas of the United Kingdom. MNCR series, ed Hiscock K (Joint Nature Conservation Committee, Peterborough, UK), pp 51–65, 126–158.
- 71.Gelman A, et al. Bayesian Data Analysis. 3rd Ed Chapman & Hall; London: 2013. [Google Scholar]
- 72.Hoffman MD, Gelman A. The no-U-turn sampler: Adaptively setting path lengths in Hamiltonian Monte Carlo. J Mach Learn Res. 2014;15:1593–1623. [Google Scholar]
- 73.Stan Development Team 2014 RStan: The R interface to Stan. Version 2.5.0. Available from mc-stan.org/interfaces/rstan.html. Accessed October 28, 2016.
- 74.Stan Development Team 2014b Stan: A C++ library for probability and sampling. Version 2.5.0. Available at mc-stan.org. Accessed October 28, 2016.
- 75.R Core Team 2016 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). Available at www.R-project.org. Accessed June 1, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.