Synopsis
The long-term use of calcium hydroxide and the recent increase in the use of hydraulic calcium-silicate cements as direct pulp-capping materials provide important clues in terms of how reparative dentin may be induced to form a “biological seal” to protect the underlying pulp tissues. In this review article, we will discuss clinical and molecular perspectives of reparative dentin formation based on evidence learned from the use of these pulp-capping materials. We will also discuss the emerging role of calcium as an odontoinductive component in these pulp-capping materials.
Keywords: Reparative dentin, calcium hydroxide, hydraulic calcium-silicate cements, calcium, odontoinductive, odontoconductive, ORAI1
I. INTRODUCTION
Dental caries is the most prevalent infectious oral diseases experienced by more than 90% of adults in the United States 1,2. A quarter of U.S. populations do not have dental insurance 3, and more than 60% of underserved areas are still in need of dentists 4. Considering these potentially unidentified individuals, it is expected that almost all individuals may have experienced dental caries at least once in their lifetime.
Due to its high prevalence, removing dental caries is one of the most common procedures performed in routine dental practices. During caries removal, deep caries penetrating through the enamel and dentin frequently leads to either indirect or direct pulp-capping procedures in order to induce tertiary (reactionary or reparative, respectively) dentin formation 5. Several pulp capping materials, including calcium hydroxide [Ca(OH)2 or CH] and hydraulic calcium silicate cements (HCSCs) such as mineral trioxide aggregate (MTA), are used for this purpose. For indirect pulp capping, these materials are placed on the “unexposed” pulp to enhance reactionary dentin formation from the existing odontoblasts at the dentino-pulpal complex (DPC). In contrast, direct pulp capping refers to placing the pulp-capping materials on the “exposed” pulp – where odontoblast layers are breached – to enhance reparative dentin formation mediated by odontoblast-like cells differentiated from dental pulp stem cells (DPSCs) at the materio-pulpal complex (MPC).
Unlike indirect pulp capping which usually has predictable clinical outcomes, direct pulp capping has outcomes that are often variable depending on the operator technique, the material properties, and the host pulpal responses. In direct pulp capping the ultimate goal is to preserve the underlying pulp and maintain pulp vitality by regenerating reparative dentin at the MPC, which functions as a “biological seal” to protect the underlying pulp tissues, to increase the life expectancy of the tooth, and to improve the overall oral health. A successful pulp capping procedure can avoid more invasive and expensive dental treatment such as root canal therapy. Therefore, it is important to optimize direct pulp-capping techniques, improve biocompatibility of the materials, and enhance biological responses of the pulp tissues in order to maximize regeneration of reparative dentin.
Here, we will discuss the current status of different types of direct pulp-capping materials with specific focuses on CH and HCSCs due to their extensive clinical utilization and substantial amounts of available studies. We will then attempt to delineate molecular mechanisms by which reparative dentin forms based on the common properties of these pulp-capping materials, as well as known bone-grafting materials. Finally, we will suggest possible roles of calcium ions (Ca2+) in the formation of mineralized tissues including reparative dentin and bone.
II. CLINCAL PERSPECTIVES: PULP-CAPPING MATERIALS IN PULPAL THERAPY
1) Calcium hydroxide (CH)
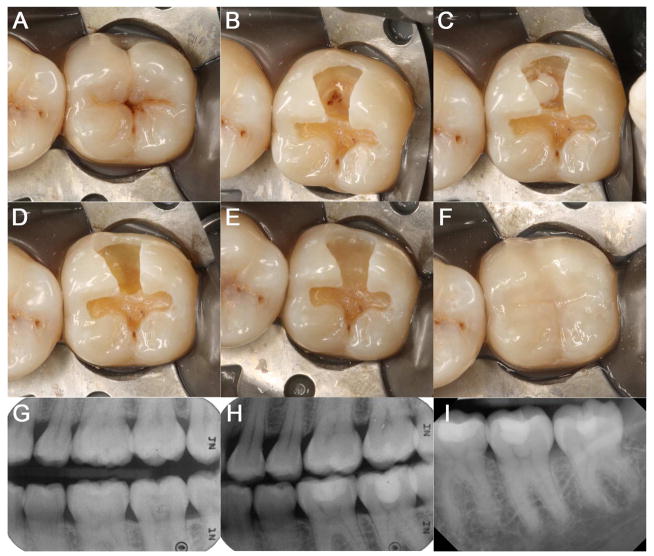
CH was first introduced to the dental profession in 1920s, and has long been recognized as the gold standard pulp-capping material 6–8. Early clinical studies including over 2,300 cases of direct pulp capping showed 80–90% success rate 6. Recent review of literature revealed that the overall success rates, as mostly determined by an asymptomatic tooth without radiographic lesions, fall between 68.5%–80.1% within a two-year follow-up period but drops to 58.7–76.3% after ten years 9–12. As such, the use of CH as a direct pulp-capping material is frequently performed (Figure 1).
Figure 1. Direct pulp capping on #18 using CH.
(A) Pre-operative clinical photograph of #18. (B) OB preparation with exposed MB pulp horn. (C) Dycal placement on the exposed pulp. (D) Fuji Lining LC placement as a liner directly on the Dycal. (E) Fuji II LC placed as a base on #18. (F) Composite restoration on #18. (G) Pre-operative radiograph of #18. (H) Post-operative radiograph of #18. (I) Periapical radiograph of #18 at follow-up after 2½ years.
One of the most clinically relevant functions of CH is the facilitation of reparative dentin formation. Histological studies of pulp-capped teeth revealed a thin layer of coagulation necrosis in the pulp due to the irriation from high pH 13,14. Mild inflammation, cellular debris and calcium-protein granules were observed below the superficial necrotic layer onto which the eventual dentin bridge formed. While detailed mechanisms remain elusive, the superficial necrosis was believed to be vital for initiation of dentin-bridge formation 7,14–16. It was also suggested that CH facilitates reparative dentin formation through the creation of an alkaline environment and an increased availability of calcium ions 7,14,17,18.
However, there are several disadvantages. CH dressings dissolve clinically within 1–2 years 19,20. The susceptibility to dissolution by acid and tissue fluid presented a problem during subsequent acid-etch resin restoration 21. CH lacks inherent adhesion to dentin, but in newer formulations, may bind to dentin via urethane dimethacrylate, which also partially adds resistance to acid dissolution 22. The most prominent issue with CH is that 89% of dentin bridges formed contained tunnel defects 20,23. Considering that the dissolution of the dressing leaving a void beneath the restoration, these “tunnel defects” present a high risk for microleakage, leading to bacterial reinfection, persisting pulpal inflammation and necrosis. These disadvantages may potentially be linked to notable variation in outcomes and a decrease in clinical success rate over follow-up time 9,10. Consistent with such notions, numerous studies reported that the success rate of pulp capping with CH varies significantly, ranging from 13 to 95% 24–26.
In summary, CH remains favored by practitioners for pulpal therapy due to its excellent antimicrobial activity and capacity to form reparative dentin. However, tunnel defects and progressive dissolution compromise the integrity of reparative dentin, and these shortfalls call into questions about the use of CH as a long-term pulp therapeutic agent.
2) Hydraulic calcium-silicate cements (HCSCs)
Mineral trioxide aggregate (MTA), a prototype HCSC, was first introduced in early 1990s by Torabinejad and his coworkers 27,28. It was initially recommended as a root-end filling material but subsequently used in various clinical applications such as pulp capping, pulpotomy, apexogenesis, apical barrier formation, and repair of root perforations 29. The main composition of MTA is tricalcium silicate, dicalcium silicate, tricalcium aluminate, bismuth oxide and calcium sulfate dehydrate. MTA is hygroscopic; its setting requires and is not adversely affected by the presence of water 30, which is a central and unique advantage that contrasts with existing dental materials.
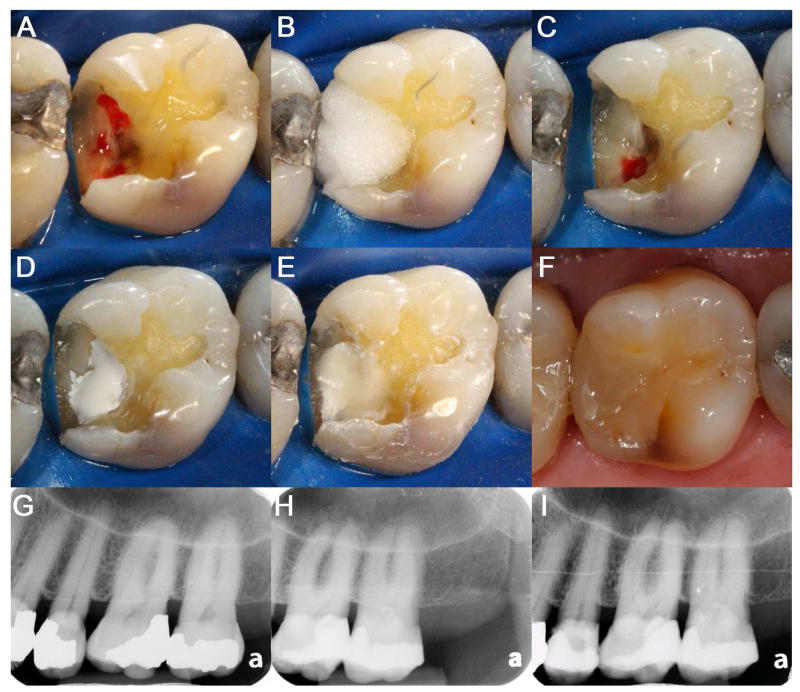
Increasing lines of evidence support a notion that MTA confers superior clinical outcomes. Although some reports showed insignificant clinical outcomes with MTA when compared to CH 31, other clinical studies showed more favorable success rates with MTA than CH 12,32–34. In some of these studies, the success rates of direct pulp capping using MTA have been reported to exceed more than 90% 35–37. In addition, success rates seem to be maintained over long follow-up periods with MTA 33,37,38, which was different from CH’s showing time-dependent decline in success rates 39,40. Therefore, more pulp capping procedures are being performed using HCSCs (Figure 2).
Figure 2. Direct pulp capping on #14 using HCSCs.
(A) DO preparation with exposed pulp. (B) Cotton pellet soaked with 3.5% NaOCl. (C) Hemostasis achieved at the pulp-exposed area. (D) Placement of bioceramics on the exposed pulp. (E) Fuji Plus placement as a base directly on the HCSCs. (F) Composite restoration on #14. (G) Pre-operative radiograph of #14. (H) Post-operative radiograph of #14. (I) Periapical radiograph of #14 at the follow-up after 1½ years.
The superior clinical outcomes of MTA are attributed to its physical properties: flexural strength, compressive strength, and push-out strength as well as antimicrobial effect, radiopacity, dimensional stability, and tolerance to moisture 41. In addition, MTA has shown to have better sealing properties 30,42, biocompatibility 43,44 and osteogenic/odontogenic differentiation capacity 45,46. As the type of pulp capping material was shown to be the single most important factor influencing the pulpal survival rate among others 34, these properties contribute to its favorable clinical outcomes.
Similar to other biomaterials, however, MTA displays some limitations: discoloration, long setting time, difficulty in manipulation, and high cost 29. A long setting-time (e.g., 2 h 45min) is one of the major drawbacks of MTA, which may delay the completion of the treatment in a single appointment in the clinic. Indeed, the prototype MTA requires temporization of the tooth to achieve the proper hardness before final restoration, which creates another potential problem of microleakage or reinfection while the temporary is in place 47. Discoloration is another major drawback especially when it is used on the anterior teeth, requiring additional treatment such as bleaching 48. A tooth-colored white MTA was developed to overcome this limitation; however, unexpected tooth discoloration has also been reported by this white MTA 49,50. Furthermore, handling of MTA is another challenge for clinicians, which potentially discourages its routine use in clinical applications.
Since the expiration of the MTA patent in 2013, a number of different HCSCs were introduced. Similar to MTA, these materials share common physicochemical and biocompatible properties but are claimed to have improved characteristics 51. For example, Biodentine (Septodont, Saint-Maur-des-Fosses, France) and Endocem (Maruchi, Wonju, Korea) are fast-setting HCSCs with the setting time of 12 min and 4.5 min, respectively. Biodentine has a shortened setting time with the addition of a setting accelerator (CaCl2) and the removal of the liquid component 52, whereas Endocem has small particle sizes that increase surface contact with water, resulting in fast setting and ease of manipulation 53. Another improvement is the replacement of the opacifier with bismuth oxide to zirconium oxide 54,55. Because bismuth oxide causes MTA discoloration after light irradiation in an oxygen-free environment 56, the zirconium oxide-containing HCSCs, such as Endocem, RetroMTA (BioMTA, Seoul, Korea), Biodentine and Endosequence (Brasseler USA, Savannah, Ga.) are suggested as better choices for esthetic reasons. Additional improvement includes better handling properties; Endosequence (Brasseler USA, Savannah, Ga.) is a premixed, ready-to-use syringeable paste that is condensable, which make them user-friendly with an ease of handling and application. It also has a demonstrated strength and biologic effect similar to MTA 57.
Despite the rapid increase in newly developed HCSCs with multiple modifications in their compositions, there are insufficient studies to experimentally support whether they are superior or even comparable alternatives to MTA. Although HCSCs are evidently promising for pulp-capping materials, more clinical, pre-clinical, and molecular studies are needed to define the clinical efficacy for regenerating the pulp for reparative dentin formation.
3) Emdogain
Emdogain is an enamel matrix derivative (EMD) product extracted from the Hertwig’s root sheath during porcine tooth development. Originally developed to promote regeneration of periodontium 58, Emdogain has been proposed in several recent studies as a potential pulp capping material 59,60. Amelogenins, the major components of EMD, can mimic epithelial-mesenchymal interactions by triggering the release of various growth factors and cytokines such as BMP and TGF-β, which in turn promote differentiation of mesenchymal stem cells in the pulp into odontoblast-like cells 61,62. In addition, EMD may also enhance dentin mineralization since osteoblast-like cells reportedly upregulate mineralization-inducing genes upon treatment with Emdogain 63. In the context of pulp capping, EMD may facilitate formation of thicker reparative dentin barrier compared to CH, as was indeed proven in several animal studies 64,65. However, with the limited number of human studies available, the efficacy of Emdogain is debatable in comparison to CH 66–68. A blinded randomized clinical study on experimentally exposed human pulp showed ineffective hard tissue barrier formation by Emdogain, although post-operative symptoms were less frequent compared to CH 67. More pulpal inflammation was also associated with Emdogain treatment. Two other studies on primary teeth 68, and partial pulpotomy in permanent teeth 66 failed to establish superiority of reparative dentin formation by Emdogain. Of note, these studies consisted of a limited sample size (<45 subjects) and short follow-up period (3–12 months). Hence, the clinical efficacy and safety of Emdogain as a pulp capping material is inconclusive at best and requires further investigation.
4) Growth factors and matrix-derived proteins
Since the turn of the century, several bioactive materials for pulp capping alternatives have been proposed and have been reviewed elsewhere 69. Growth factors (BMPs, IGF-1, EGF, FGF, TGF, PDGF-BB) and matrix-derived proteins (BSP) may stimulate reparative dentin formation that is comparable or even superior to CH, but appropriate delivery carrier and dosage need to be considered for controlled reparative dentin regeneration. The limited half-life also necessitates the need for multiple applications, which may incur high cost. As such, studies for efficacies of these bioactive molecules in pulp capping remain to be determined in the in vitro and animal study stages.
III. MOLECULAR PERSPECTIVES: COMMON PROPERTIES OF PULP-CAPPING MATERIALS
Pulpal wound healing including reparative dentin formation is a multi-factorial, complex process that is orchestrated by discrete but overlapping steps of migration, proliferation, and mineralization of pulp cells 70. Unlike reactionary dentin, which is formed by existing odontoblasts, reparative dentin is formed by odontoblast-like cells presumably differentiated from DPSCs when the pulp becomes exposed and the existing odontoblastic layers are breached. At the cellular level, these DPSCs are expected to migrate to and proliferate on the MPC, ultimately undergoing odontogenic differentiation to form reparative dentin.
Although both CH and HCSCs are thought to be odontoconductive by functioning as scaffolds for proper execution of these processes by DPSCs, clinical and molecular studies suggests evidently that they are also odontoinductive such that these materials can stimulate DPSCs to undergo odontogenic differentiation and mineralization. Therefore, identifying the factors released from pulp-capping agents that regulate DPSCs to form reparative dentin and defining the fundamental molecular mechanisms by which DPSCs respond to these pulp-capping agents are critically important for regenerating reparative dentin and creating a “biological seal.”
As was discussed previously, there exist a number of pulp-capping materials that are clinically proven to induce reparative dentin formation in human. Based on this historical evidence, some of the properties are suggested to play key roles in regenerating reparative dentin. These properties include:
high pH,
anti-bacterial activity, and
calcium ion release.
1) High pH
Alkaline environment (high pH) is known to promote osteogenic differentiation and bone formation 71,72. Conversely, acidic environment is demonstrated to inhibit bone formation 73. Alkaline phosphatase, an important enzyme in initiating calcification, allows for the increase in local concentration of inorganic phosphate at an alkaline pH 74. Because CH is a water-soluble compound that dissolves upon contact with tissue fluid and releases hydroxyl anions (OH−) to increase pH to 12–13, it was suggested that such alkaline pH attributes to reparative dentin formation by CH 75–77. Similarly, HCSCs were also demonstrated to create a high-pH environment as the end product of the chemical reaction is CH 78–80.
Although an alkaline environment is essential for creating an osteoinductive environment, mineralization is highly sensitive to pH change; alkaline phosphatase activity peaks at pH 7.37 and significantly diminished above this physiologic level 73. Furthermore, pH above 8.0 was shown to inhibit mineralization process both in vitro and in vivo 81,82. Because measuring the precise pH at or around the interfaces between pulp-capping materials and pulp tissue is technically challenging, further investigation is needed to clarify the actual effects of high pH on reparative dentin formation in vivo.
2) Anti-microbial activity
One of the clinical advantages of CH as a pulp medicament largely derives from its antimicrobial activity due to OH− release (pH up to 12.5). The highly reactive hydroxyl radicals along with the raised pH can cause damage to the cytoplasmic membrane and DNA of bacterial microorganisms 83,84. In addition, the high pH may also provide anti-inflammatory effect, via denaturation of proinflammatory cytokines 85 and stimulation of regulatory IL-10 86. Sustained attenuation of bacterial irritation and inflammatory response in turn may provide a conductive environment for reparative dentin formation. However, such effects are indirect; the removal of bacterial organisms does not actively contribute to reparative dentin formation. Therefore, it remains to be elucidated whether anti-microbial activity of the dental pulp-capping materials is a molecular determinant in reparative dentin formation.
3) Calcium ions (Ca2+)
Although Ca2+ is one of the major constituents released by both CH and HCSCs 78,87, the role of Ca2+ in reparative dentin formation is largely underexplored. An earlier study demonstrated that, when CH with radiolabeled Ca2+ was used as a direct pulp-capping material on pulp-exposed teeth in dogs, radiocalcium was not found in the reparative dentin area, suggesting that Ca2+ necessary for formation of reparative dentin matrix itself is not derived from CH but from the pulp 88. However, emerging evidence supports a notion that Ca2+ plays indispensable roles not only in formation of mineralized matrixes but also in transduction of the intracellular signaling pathway that are involved in maintaining and regulating normal biological processes 89,90. Indeed, recent studies suggested that Ca2+ released from biomaterials is one of the key factors mediating mineralization process 7,87 and that Ca2+ released from pulp-capping materials may be an active component in reparative dentin formation.
Unlike its role in dentin formation, Ca2+ in bone formation is well documented. High amounts of extracellular Ca2+ induced expression of alkaline phosphatase (ALP), osteocalcin (OC) and osteopontin (OP) in pre-osteoblast cells 91,92. Furthermore, extracellular Ca2+ also induced osteoblast differentiation and mineralization both in vitro and in vivo 93–95, indicating that Ca2+ alone has a de novo characteristic to induce osteogenic differentiation.
To enhance bone formation in vivo, biomaterials such as hydroxyapatite (HA), tricalcium phosphates (TCP), or biphasic calcium phosphates (BCP), a mixture of HA and TCP, are frequently used as scaffolds for bone grafting 96. Although these materials are known to be osteoconductive in nature by serving as scaffold for bone growth, they are also suggested to be osteoinductive; these materials by themselves actively stimulate differentiation of pre-osteoblastic cells to osteoblasts and formation of new bone 97,98. Interestingly, all of these materials are highly enriched with Ca2+ 99, and their capacity to induce bone formation seems to differ depending on the amount of Ca2+. Indeed, Ca/P ratio of TCP is 1.67 when that of HA is 1.5, and TCP is capable of releasing more Ca2+ than HA 100. Furthermore, Barradas et al. demonstrated that TCP induces more bone formation than HA both in vitro and in vivo, and such difference is primarily attributed to high solubility of TCP to release Ca2+ 101.
Similar to osteoblasts, extracellular Ca2+ also induce odontogenic differentiation of dental mesenchymal cells. Ca2+ treatment alone induced osteogenic gene expression, such as osteopontin and BMP2 in dental pulp cells 102,103. Mizuno et al. also showed that Ca2+ released from CH stimulated fibronectin gene expression in dental pulp cells, a mechanism that may induce differentiation of these cells to become mineralized tissue forming cells 104. Elevated Ca2+ is also known to stimulate differentiation and mineralization of other dental mesenchymal cells such as cementoblasts by increasing fgf-2 expression 105.
At the molecular level, extracellular Ca2+ level is detected by the calcium sensing receptor (CaSR), a seven-transmembrane homodimer receptor that belongs to the C family of the G-protein-coupled receptor superfamily. An activated CaSR elicits intracellular signaling pathways that ultimately lead to migration, proliferation and differentiation of cells 106. Recently, it was shown that CaSR mediates osteogenic differentiation and mineralization of bone marrow mesenchymal stromal cells 107. However, the presence of CaSR in bone-forming cells is controversial 108, and osteoblasts derived from CaSR-null mice remained to possess osteogenic differentiation potential 109,110. Further, another study demonstrated that inhibition of CaSR further induced, rather than suppressed, Ca2+-mediated osteogenic differentiation 111, suggesting that CaSR is dispensable in osteogenic differentiation and mineralization.
Ca2+ itself is an important intracellular signaling molecule, and there exist different types of Ca2+ channels that regulate intracellular Ca2+ level 112. Among them, L-type voltage-gated calcium channel was shown to be associated with Ca2+-mediated osteogenic differentiation and mineralization 113–116. Similarly, recent studies showed that L-type calcium channel plays a key role in differentiation of dental pulp stem cells and periodontal ligament cells 111,117. However, L-type voltage-gated calcium channel is a large transmembrane multi-protein complex that mediates Ca2+ influx in response to membrane depolarization via voltage differences 112. As such, it remains to be elucidated as to how membrane depolarization links to differentiation and mineralization of osteoblasts and odontoblasts.
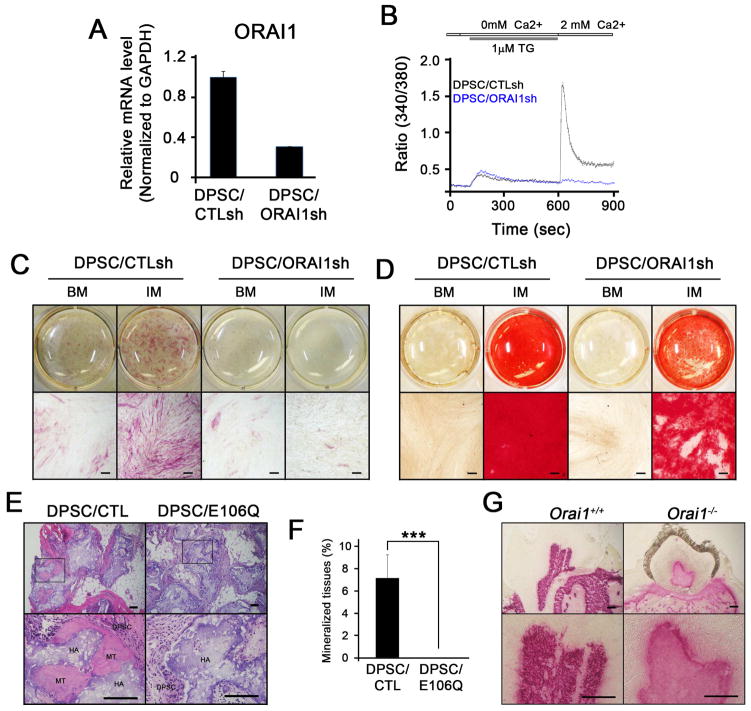
Recent studies identified another class of calcium channel, Orai1, that regulates intracellular Ca2+ level and Ca2+-mediated signaling pathway in most non-excitable cells 118. Orai1 is an essential subunit of Ca2+ release-activated Ca2+ (CRAC) channel that mediates Ca2+ influx via the store-operated Ca2+ entry (SOCE) mechanism. Although Orai1 is extensively studied and characterized in immune cells 119, recent studies showed that it plays a critical role in mediating bone formation. In particular, Orai1-null mice exhibited osteoporotic phenotypes, and disruption of Orai1 function in osteoblasts suppressed osteogenic differentiation and mineralization 120–122. Similarly, Sohn et al. recently demonstrated that Orai1 also plays an indispensable role in odontogenic differentiation and mineralization 123. When Orai1 was knocked down in dental pulp stem cells (DPSCs), these cells exhibited not only incompetent Ca2+ influx (Fig. 3) but also inability to undergo odontogenic differentiation and mineralization as demonstrated by alkaline phosphatase staining and activity as well as alizarin red staining (Fig. 3). More importantly, transplantation of DPSCs harboring Orai1/E106Q, a dominant negative form of Orai1, caused no formation of mineralized nodules in vivo, indicating that Orai1 is required for odontogenic differentiation and mineralization both in vitro and in vivo. Further studies on the role of Orai1 in reparative dentin formation warrant closer examination.
Figure 3. The calcium channel, Orai1, plays an indispensable role in odontogenic differentiation and mineralization.
(A) Quantitative real-time polymerase chain reaction (qRT-PCR) of ORAI1 expression following knockdown experiment in DPSCs, showing efficient suppression of ORAI1 in DPSC/ORAI1sh cells but not in DPSC/CTLsh cells. (B) Measurement of intracellular Ca2+ level in DPSCs, confirming inhibition of Ca2+ influx when ORAI1 expression is suppressed in DPSCs. (C) Alkaline phosphatase (ALP) staining of DPSCs following treatment with basal medium (BM) and bone-forming induction medium (IM) for 5 days, demonstrating inhibition of ALP activity important for odontogenic differentiation. (D) Alizarin red staining of DPSCs following treatment with basal medium (BM) and bone-forming induction medium (IM) for 14 days, demonstrating inhibition of odontogenic mineralization. (E) Ectopic mineralized-tissue formation of DPSC/CTL cells but not DPSC/E106Q cells harboring dominant negative form of ORAI1, demonstrating indispensable role of ORAI1 in vivo. (F) Quantification of ectopic mineralized-tissue formation in vivo. (G) ALP staining of a tooth prepared from Orai1+/+ and Orai1−/− mice. From Sohn S, Park Y, Srikanth S, et al. The Role of ORAI1 in the Odontogenic Differentiation of Human Dental Pulp Stem Cells. Journal of Dental Research 2015;94(11); with permission.
IV. FUTURE PERSPECTIVES AND CONCLUSIONS
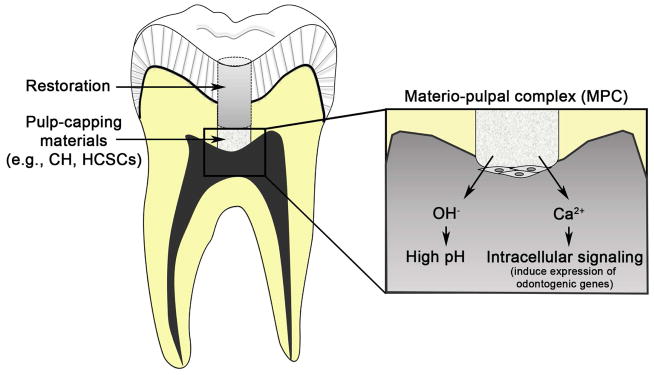
Although a substantial numbers of clinical and molecular studies support the use of CH and HCSCs for direct pulp capping, achieving clinically successful outcomes in a reproducible and reliable manner still requires more investigations. Such shortfalls may be due, in part, to the lack of thorough understanding in the fundamental mechanisms of pulpal wound healing and reparative dentin formation. Many pulp-capping animal models were previously used to better understand the mechanisms of reparative dentin formation 124–126; however, pulp-capping studies in large animals are usually observational in nature. For this reason, transgenic or knockout mice that have an overexpressed or deleted gene of interest in an inducible and cell-type specific manner would help in expediting our understanding in reparative dentin formation at the molecular level (Figure 4) 127,128.
Figure 4. Schematic diagram of molecular mechanisms that govern reparative dentin formation by CH or HCSCs at the MPC.
CH and HCSCs release hydroxyl group (OH−) and increase local pH at the MPC, creating alkaline environment that induces anti-bacterial activity and potentially promotes odontogenic mineralization. CH and HCSCs also release calcium ions (Ca2+), eliciting intracellular signaling pathways that ultimately promote odontogenic differentiation and mineralization.
Due to the favorable clinical outcomes with the prototype MTA, its derivative products are widely available with modifications to their compositions. Nonetheless, their relative efficacy, or even their toxicity, is still far from complete understanding. Further comparative studies on validating and standardizing the effects of different HCSCs also warrant closer examination.
CH and HCSCs are odontoconductive by functioning as scaffolds onto which DPSCs migrate, proliferate, and differentiate to form reparative dentin. Clinical and preclinical studies support a notion that they are also odontoinductive as they also stimulate DPSCs to form reparative dentin. It would be beneficial and effective to incorporate bioactive materials as one of their constituents to further potentiate odontoconductive and odontoinductive properties of the pulp-capping materials.
Historically, CH has been used as a pulp-capping material, and its efficacy has proven to protect the exposed pulp based on clinical experiences for many decades. Recently introduced HCSCs have increasingly gained popularity due to improved physical and chemical properties to enhance reparative dentin formation. Nonetheless, many practitioners still prefer complete removal of the pulp once exposed rather than pulp-capping placement, primarily due to apprehension that they would be perceived as an “incompetent dentist” for the unsuccessful outcomes and multiple subsequent visits should the pulp capping fails. Because successful direct pulp capping is largely dependent on operator technique, material properties, and the host pulpal responses, it is important to recognize the importance of, and to optimally maximize the merits of each component so that reparative dentin can be regenerated in a predictable and reproducible manner. In this regard, more studies are needed at the clinical, preclinical, and molecular levels to improve each component.
KEY POINTS.
Direct pulp capping is often performed on the exposed pulp after deep caries removal in order to induce reparative dentin, a physical barrier that functions as a “biological seal” to protect the underlying pulp tissues and maintain pup vitality.
Although calcium hydroxide (CH) has been used as the “gold standard” pulp-capping material for many decades, recently introduced hydraulic calcium-silicate cements (HCSCs) such as mineral trioxide aggregate (MTA) have increasingly gained popularity due to their superior material properties that are biocompatible, odontoconductive, and to certain degree, odontoinductive.
These pulp-capping materials confer capacity to induce reparative dentin by providing an alkaline environment and anti-bacterial activity; however, increasing lines of evidence support a notion that the release of calcium ions (Ca2+) actively induces in reparative dentin formation by eliciting intracellular Ca2+ signaling pathways.
Among the intracellular Ca2+ regulators, ORAI1 protein was recently shown to have an indispensable role in odontogenic differentiation and mineralization in dental pulp stem cells by regulating Ca2+ influx.
Successful clinical outcomes of direct pulp capping depend on the operator technique, the material properties, and the host pulpal responses. Therefore, it is important to develop strategies that maximize the efficacy of each component for regenerating reparative dentin in a predictable and reproducible manner.
Acknowledgments
This study was supported by the grants from NIDCR/NIH R01DE023348, UCLA Faculty Research Grant, and Dean’s Faculty Research Seed grant to RHK.
Footnotes
Disclosure statement: The authors declare that there is no conflict of interest related to this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Minju Song, Email: minju81s@gmail.com.
Bo Yu, Email: boyu@ucla.edu.
Sol Kim, Email: solkim1010@gmail.com.
Marc Hayashi, Email: mhayashi@dentistry.ucla.edu.
Colby Smith, Email: csmith@dentistry.ucla.edu.
Suhjin Sohn, Email: sohns74@gmail.com.
Euiseong Kim, Email: andyendo@yuhs.ac.
James Lim, Email: jlimdds@gmail.com.
Richard G. Stevenson, Email: rstevenson@dentistry.ucla.edu.
References
- 1.Dye B, Thornton-Evans G, Li X, Iafolla T. Dental caries and tooth loss in adults in the United States, 2011–2012. NCHS Data Brief. 2015;(197):197. [PubMed] [Google Scholar]
- 2.Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat. 2007;11(248):1–92. [PubMed] [Google Scholar]
- 3.Bloom B, Cohen RA. Dental insurance for persons under age 65 years with private health insurance: United States, 2008. NCHS Data Brief. 2010;(40):1–8. [PubMed] [Google Scholar]
- 4.Statistics DHPSA. First Quarter of Fiscal Year 2016 Designated HPSA Quarterly Summary. Bureau of Health Workforce Health Resources and Services Administration (HRSA) U.S. Department of Health & Human Services; 2015. [Google Scholar]
- 5.Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 2011;3:711–735. doi: 10.2741/e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baume LJ, Holz J. Long term clinical assessment of direct pulp capping. Int Dent J. 1981;31(4):251–260. [PubMed] [Google Scholar]
- 7.Sangwan P, Sangwan A, Duhan J, Rohilla A. Tertiary dentinogenesis with calcium hydroxide: a review of proposed mechanisms. Int Endod J. 2013;46(1):3–19. doi: 10.1111/j.1365-2591.2012.02101.x. [DOI] [PubMed] [Google Scholar]
- 8.Hilton TJ. Keys to clinical success with pulp capping: a review of the literature. Oper Dent. 2009;34(5):615–625. doi: 10.2341/09-132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willershausen B, Willershausen I, Ross A, Velikonja S, Kasaj A, Blettner M. Retrospective study on direct pulp capping with calcium hydroxide. Quintessence Int. 2011;42(2):165–171. [PubMed] [Google Scholar]
- 10.Dammaschke T, Leidinger J, Schafer E. Long-term evaluation of direct pulp capping--treatment outcomes over an average period of 6.1 years. Clin Oral Investig. 2010;14(5):559–567. doi: 10.1007/s00784-009-0326-9. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo T, Nakanishi T, Shimizu H, Ebisu S. A clinical study of direct pulp capping applied to carious-exposed pulps. J Endod. 1996;22(10):551–556. doi: 10.1016/S0099-2399(96)80017-3. [DOI] [PubMed] [Google Scholar]
- 12.Hilton TJ, Ferracane JL, Mancl L. Northwest Practice-based Research Collaborative in Evidence-based D. Comparison of CaOH with MTA for direct pulp capping: a PBRN randomized clinical trial. J Dent Res. 2013;92(7 Suppl):16S–22S. doi: 10.1177/0022034513484336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilton TJ. Keys to Clinical Success with Pulp Capping: A Review of the Literature. Operative dentistry. 2009;34(5):615–625. doi: 10.2341/09-132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrela C, Sydney GB, Bammann LL, Felippe O., Junior Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J. 1995;6(2):85–90. [PubMed] [Google Scholar]
- 15.Estrela C, Pimenta FC, Ito IY, Bammann LL. Antimicrobial evaluation of calcium hydroxide in infected dentinal tubules. J Endod. 1999;25(6):416–418. doi: 10.1016/S0099-2399(99)80269-6. [DOI] [PubMed] [Google Scholar]
- 16.Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabe PF, Dezan E., Junior Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod. 1999;25(3):161–166. doi: 10.1016/s0099-2399(99)80134-4. [DOI] [PubMed] [Google Scholar]
- 17.Okabe T, Sakamoto M, Takeuchi H, Matsushima K. Effects of pH on mineralization ability of human dental pulp cells. J Endod. 2006;32(3):198–201. doi: 10.1016/j.joen.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Foreman PC, Barnes IE. Review of calcium hydroxide. Int Endod J. 1990;23(6):283–297. doi: 10.1111/j.1365-2591.1990.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 19.Stanley HR, Pameijer CH. Pulp capping with a new visible-light-curing calcium hydroxide composition (Prisma VLC Dycal) Oper Dent. 1985;10(4):156–163. [PubMed] [Google Scholar]
- 20.Cox CF, Bergenholtz G, Heys DR, Syed SA, Fitzgerald M, Heys RJ. Pulp capping of dental pulp mechanically exposed to oral microflora: a 1–2 year observation of wound healing in the monkey. J Oral Pathol. 1985;14(2):156–168. doi: 10.1111/j.1600-0714.1985.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 21.Olmez A, Oztas N, Basak F, Sabuncuoglu B. A histopathologic study of direct pulp-capping with adhesive resins. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(1):98–103. doi: 10.1016/s1079-2104(98)90157-3. [DOI] [PubMed] [Google Scholar]
- 22.Pameijer CH, Stanley HR. The disastrous effects of the “total etch” technique in vital pulp capping in primates. Am J Dent. 1998;11(Spec No):S45–54. [PubMed] [Google Scholar]
- 23.Cox CF, Subay RK, Ostro E, Suzuki S, Suzuki SH. Tunnel defects in dentin bridges: their formation following direct pulp capping. Oper Dent. 1996;21(1):4–11. [PubMed] [Google Scholar]
- 24.Al-Hiyasat AS, Barrieshi-Nusair KM, Al-Omari MA. The radiographic outcomes of direct pulp-capping procedures performed by dental students: a retrospective study. J Am Dent Assoc. 2006;137(12):1699–1705. doi: 10.14219/jada.archive.2006.0116. [DOI] [PubMed] [Google Scholar]
- 25.Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. 2000;26(9):525–528. doi: 10.1097/00004770-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Cvek M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J Endod. 1978;4(8):232–237. doi: 10.1016/S0099-2399(78)80153-8. [DOI] [PubMed] [Google Scholar]
- 27.Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127(10):1491–1494. doi: 10.14219/jada.archive.1996.0058. [DOI] [PubMed] [Google Scholar]
- 28.Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19(12):591–595. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 29.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root end filling materials: Effects of blood contamination. J Endod. 1994;20(4):159–163. doi: 10.1016/S0099-2399(06)80326-2. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto CE, Adachi E, Pameijer CH, Barnes D, Romberg EE, Jefferies S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am J Dent. 2006;19(2):85–90. [PubMed] [Google Scholar]
- 32.Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with Mineral Trioxide Aggregate: a randomized controlled trial. 2008. Int Endod J. 2009;42(5):422–444. doi: 10.1111/j.1365-2591.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- 33.Mente J, Geletneky B, Ohle M, et al. Mineral trioxide aggregate or calcium hydroxide direct pulp capping: an analysis of the clinical treatment outcome. J Endod. 2010;36(5):806–813. doi: 10.1016/j.joen.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Cho SY, Seo DG, Lee SJ, Lee J, Jung IY. Prognostic factors for clinical outcomes according to time after direct pulp capping. J Endod. 2013;39(3):327–331. doi: 10.1016/j.joen.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Marques MS, Wesselink PR, Shemesh H. Outcome of Direct Pulp Capping with Mineral Trioxide Aggregate: A Prospective Study. J Endod. 2015;41(7):1026–1031. doi: 10.1016/j.joen.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Farsi N, Alamoudi N, Balto K, Al Mushayt A. Clinical assessment of mineral trioxide aggregate (MTA) as direct pulp capping in young permanent teeth. J Clin Pediatr Dent. 2006;31(2):72–76. doi: 10.17796/jcpd.31.2.n462281458372u64. [DOI] [PubMed] [Google Scholar]
- 37.Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate: an observational study. J Am Dent Assoc. 2008;139(3):305–315. doi: 10.14219/jada.archive.2008.0160. quiz 305–315. [DOI] [PubMed] [Google Scholar]
- 38.Jang Y, Song M, Yoo IS, Song Y, Roh BD, Kim E. A Randomized Controlled Study of the Use of ProRoot Mineral Trioxide Aggregate and Endocem as Direct Pulp Capping Materials: 3-month versus 1-year Outcomes. J Endod. 2015 doi: 10.1016/j.joen.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Horsted P, Sandergaard B, Thylstrup A, El Attar K, Fejerskov O. A retrospective study of direct pulp capping with calcium hydroxide compounds. Endod Dent Traumatol. 1985;1(1):29–34. doi: 10.1111/j.1600-9657.1985.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 40.Haskell EW, Stanley HR, Chellemi J, Stringfellow H. Direct pulp capping treatment: a long-term follow-up. J Am Dent Assoc. 1978;97(4):607–612. doi: 10.14219/jada.archive.1978.0356. [DOI] [PubMed] [Google Scholar]
- 41.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part I: chemical, physical, and antibacterial properties. J Endod. 2010;36(1):16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Torabinejad M, Smith PW, Kettering JD, Pitt Ford TR. Comparative investigation of marginal adaptation of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod. 1995;21(6):295–299. doi: 10.1016/S0099-2399(06)81004-6. [DOI] [PubMed] [Google Scholar]
- 43.Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005;38(11):834–842. doi: 10.1111/j.1365-2591.2005.01028.x. [DOI] [PubMed] [Google Scholar]
- 44.Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review--part II: leakage and biocompatibility investigations. J Endod. 2010;36(2):190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Seo MS, Hwang KG, Lee J, Kim H, Baek SH. The effect of mineral trioxide aggregate on odontogenic differentiation in dental pulp stem cells. J Endod. 2013;39(2):242–248. doi: 10.1016/j.joen.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Yan M, Fan Z, Ma L, Yu Y, Yu J. Mineral trioxide aggregate enhances the odonto/osteogenic capacity of stem cells from inflammatory dental pulps via NF-kappaB pathway. Oral Dis. 2014;20(7):650–658. doi: 10.1111/odi.12183. [DOI] [PubMed] [Google Scholar]
- 47.Song M, Kang M, Kim HC, Kim E. A randomized controlled study of the use of ProRoot mineral trioxide aggregate and Endocem as direct pulp capping materials. J Endod. 2015;41(1):11–15. doi: 10.1016/j.joen.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Jang JH, Kang M, Ahn S, et al. Tooth Discoloration after the Use of New Pozzolan Cement (Endocem) and Mineral Trioxide Aggregate and the Effects of Internal Bleaching. J Endod. 2013;39(12):1598–1602. doi: 10.1016/j.joen.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 49.Belobrov I, Parashos P. Treatment of Tooth Discoloration after the Use of White Mineral Trioxide Aggregate. J Endod. 2011;37(7):1017–1020. doi: 10.1016/j.joen.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G. Tooth discoloration induced by endodontic materials: a laboratory study. Int Endod J. 2012;45(10):942–949. doi: 10.1111/j.1365-2591.2012.02053.x. [DOI] [PubMed] [Google Scholar]
- 51.Dawood AE, Parashos P, Wong RHK, Reynolds EC, Manton DJ. Calcium silicate-based cements: composition, properties, and clinical applications. Journal of investigative and clinical dentistry. 2015 doi: 10.1111/jicd.12195. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 52.Nayak G, Hasan MF. Biodentine-a novel dentinal substitute for single visit apexification. Restorative dentistry & endodontics. 2014;39(2):120–125. doi: 10.5395/rde.2014.39.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi Y, Park SJ, Lee SH, Hwang YC, Yu MK, Min KS. Biological effects and washout resistance of a newly developed fast-setting pozzolan cement. J Endod. 2013;39(4):467–472. doi: 10.1016/j.joen.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 54.Kohli MR, Yamaguchi M, Setzer FC, Karabucak B. Spectrophotometric Analysis of Coronal Tooth Discoloration Induced by Various Bioceramic Cements and Other Endodontic Materials. J Endod. 2015;41(11):1862–1866. doi: 10.1016/j.joen.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Kang S-H, Shin Y-S, Lee H-S, et al. Color Changes of Teeth after Treatment with Various Mineral Trioxide Aggregate–based Materials: An Ex Vivo Study. J Endod. 2015;41(5):737–741. doi: 10.1016/j.joen.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 56.Vallés M, Mercadé M, Duran-Sindreu F, Bourdelande JL, Roig M. Influence of Light and Oxygen on the Color Stability of Five Calcium Silicate–based Materials. J Endod. 2013;39(4):525–528. doi: 10.1016/j.joen.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Machado J, Johnson JD, Paranjpe A. The Effects of Endosequence Root Repair Material on Differentiation of Dental Pulp Cells. J Endod. 2016;42(1):101–105. doi: 10.1016/j.joen.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Lyngstadaas SP, Wohlfahrt JC, Brookes SJ, Paine ML, Snead ML, Reseland JE. Enamel matrix proteins; old molecules for new applications. Orthod Craniofac Res. 2009;12(3):243–253. doi: 10.1111/j.1601-6343.2009.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyngstadaas SP, Lundberg E, Ekdahl H, Andersson C, Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001;28(2):181–188. doi: 10.1034/j.1600-051x.2001.028002181.x. [DOI] [PubMed] [Google Scholar]
- 60.Al-Hezaimi K, Al-Tayar BA, BaJuaifer YS, Salameh Z, Al-Fouzan K, Tay FR. A Hybrid Approach to Direct Pulp Capping by Using Emdogain with a Capping Material. Journal of Endodontics. 2011;37(5):667–672. doi: 10.1016/j.joen.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Schlueter SR, Carnes DL, Cochran DL. In vitro effects of enamel matrix derivative on microvascular cells. J Periodontol. 2007;78(1):141–151. doi: 10.1902/jop.2007.060111. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki S, Nagano T, Yamakoshi Y, et al. Enamel matrix derivative gel stimulates signal transduction of BMP and TGF-{beta} J Dent Res. 2005;84(6):510–514. doi: 10.1177/154405910508400605. [DOI] [PubMed] [Google Scholar]
- 63.Weishaupt P, Bernimoulin JP, Trackman P, Hagewald S. Stimulation of osteoblasts with Emdogain increases the expression of specific mineralization markers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(2):304–308. doi: 10.1016/j.tripleo.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura Y, Hammarstrom L, Lundberg E, et al. Enamel matrix derivative promotes reparative processes in the dental pulp. Adv Dent Res. 2001;15:105–107. doi: 10.1177/08959374010150010201. [DOI] [PubMed] [Google Scholar]
- 65.Igarashi R, Sahara T, Shimizu-Ishiura M, Sasaki T. Porcine enamel matrix derivative enhances the formation of reparative dentine and dentine bridges during wound healing of amputated rat molars. J Electron Microsc (Tokyo) 2003;52(2):227–236. doi: 10.1093/jmicro/52.2.227. [DOI] [PubMed] [Google Scholar]
- 66.Kiatwateeratana T, Kintarak S, Piwat S, Chankanka O, Kamaolmatyakul S, Thearmontree A. Partial pulpotomy on caries-free teeth using enamel matrix derivative or calcium hydroxide: a randomized controlled trial. Int Endod J. 2009;42(7):584–592. doi: 10.1111/j.1365-2591.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- 67.Olsson H, Davies JR, Holst KE, Schroder U, Petersson K. Dental pulp capping: effect of Emdogain Gel on experimentally exposed human pulps. Int Endod J. 2005;38(3):186–194. doi: 10.1111/j.1365-2591.2004.00932.x. [DOI] [PubMed] [Google Scholar]
- 68.Garrocho-Rangel A, Flores H, Silva-Herzog D, Hernandez-Sierra F, Mandeville P, Pozos-Guillen AJ. Efficacy of EMD versus calcium hydroxide in direct pulp capping of primary molars: a randomized controlled clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):733–738. doi: 10.1016/j.tripleo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 69.Qureshi A, ES, Nandakumar, Pratapkumar, Sambashivarao Recent advances in pulp capping materials: an overview. Journal of clinical and diagnostic research: JCDR. 2014;8(1):316–321. doi: 10.7860/JCDR/2014/7719.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldberg M. Pulp healing and regeneration: more questions than answers. Adv Dent Res. 2011;23(3):270–274. doi: 10.1177/0022034511405385. [DOI] [PubMed] [Google Scholar]
- 71.Arnett TR. Extracellular pH regulates bone cell function. J Nutr. 2008;138(2):415S–418S. doi: 10.1093/jn/138.2.415S. [DOI] [PubMed] [Google Scholar]
- 72.Kohn DH, Sarmadi M, Helman JI, Krebsbach PH. Effects of pH on human bone marrow stromal cells in vitro: implications for tissue engineering of bone. J Biomed Mater Res. 2002;60(2):292–299. doi: 10.1002/jbm.10050. [DOI] [PubMed] [Google Scholar]
- 73.Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005;77(3):167–174. doi: 10.1007/s00223-004-0285-8. [DOI] [PubMed] [Google Scholar]
- 74.Golu EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18:444–448. [Google Scholar]
- 75.Carrotte P. Endodontics: Part 9. Calcium hydroxide, root resorption, endo-perio lesions. Br Dent J. 2004;197(12):735–743. doi: 10.1038/sj.bdj.4811897. [DOI] [PubMed] [Google Scholar]
- 76.Staehle HJ, Pioch T, Hoppe W. The alkalizing properties of calcium hydroxide compounds. Endod Dent Traumatol. 1989;5(3):147–152. doi: 10.1111/j.1600-9657.1989.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 77.Glass RL, Zander HA. Pulp healing. J Dent Res. 1949;28(2):97–107. doi: 10.1177/00220345490280021101. [DOI] [PubMed] [Google Scholar]
- 78.Tanomaru-Filho M, Chaves Faleiros FB, Sacaki JN, Hungaro Duarte MA, Guerreiro-Tanomaru JM. Evaluation of pH and calcium ion release of root-end filling materials containing calcium hydroxide or mineral trioxide aggregate. J Endod. 2009;35(10):1418–1421. doi: 10.1016/j.joen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Fridland M, Rosado R. MTA solubility: a long term study. J Endod. 2005;31(5):376–379. doi: 10.1097/01.don.0000140566.97319.3e. [DOI] [PubMed] [Google Scholar]
- 80.Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J Endod. 2003;29(12):814–817. doi: 10.1097/00004770-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Monfoulet LE, Becquart P, Marchat D, et al. The pH in the microenvironment of human mesenchymal stem cells is a critical factor for optimal osteogenesis in tissue-engineered constructs. Tissue Eng Part A. 2014;20(13–14):1827–1840. doi: 10.1089/ten.TEA.2013.0500. [DOI] [PubMed] [Google Scholar]
- 82.Fliefel R, Popov C, Troltzsch M, Kuhnisch J, Ehrenfeld M, Otto S. Mesenchymal stem cell proliferation and mineralization but not osteogenic differentiation are strongly affected by extracellular pH. J Craniomaxillofac Surg. 2016 doi: 10.1016/j.jcms.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 83.Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44(8):697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 84.Siqueira JF, Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32(5):361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 85.Khan AA, Sun X, Hargreaves KM. Effect of calcium hydroxide on proinflammatory cytokines and neuropeptides. J Endod. 2008;34(11):1360–1363. doi: 10.1016/j.joen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reyes-Carmona JF, Santos AR, Figueiredo CP, Felippe MS, Felippe WT, Cordeiro MM. In vivo host interactions with mineral trioxide aggregate and calcium hydroxide: inflammatory molecular signaling assessment. J Endod. 2011;37(9):1225–1235. doi: 10.1016/j.joen.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 87.Natale LC, Rodrigues MC, Xavier TA, Simoes A, de Souza DN, Braga RR. Ion release and mechanical properties of calcium silicate and calcium hydroxide materials used for pulp capping. Int Endod J. 2015;48(1):89–94. doi: 10.1111/iej.12281. [DOI] [PubMed] [Google Scholar]
- 88.Sciaky I, Pisanti S. Localization of calcium placed over amputated pulps in dogs’ teeth. J Dent Res. 1960;39:1128–1132. doi: 10.1177/00220345600390060601. [DOI] [PubMed] [Google Scholar]
- 89.Apati A, Paszty K, Erdei Z, Szebenyi K, Homolya L, Sarkadi B. Calcium signaling in pluripotent stem cells. Mol Cell Endocrinol. 2012;353(1–2):57–67. doi: 10.1016/j.mce.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 90.Tonelli FM, Santos AK, Gomes DA, et al. Stem cells and calcium signaling. Adv Exp Med Biol. 2012;740:891–916. doi: 10.1007/978-94-007-2888-2_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dvorak MM, Siddiqua A, Ward DT, et al. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci U S A. 2004;101(14):5140–5145. doi: 10.1073/pnas.0306141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsuoka H, Akiyama H, Okada Y, et al. In vitro analysis of the stimulation of bone formation by highly bioactive apatite- and wollastonite-containing glass-ceramic: released calcium ions promote osteogenic differentiation in osteoblastic ROS17/2.8 cells. J Biomed Mater Res. 1999;47(2):176–188. doi: 10.1002/(sici)1097-4636(199911)47:2<176::aid-jbm7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 93.Cheng S, Wang W, Lin Z, et al. Effects of extracellular calcium on viability and osteogenic differentiation of bone marrow stromal cells in vitro. Hum Cell. 2013;26(3):114–120. doi: 10.1007/s13577-012-0041-8. [DOI] [PubMed] [Google Scholar]
- 94.Nakamura S, Matsumoto T, Sasaki J, et al. Effect of calcium ion concentrations on osteogenic differentiation and hematopoietic stem cell niche-related protein expression in osteoblasts. Tissue Eng Part A. 2010;16(8):2467–2473. doi: 10.1089/ten.TEA.2009.0337. [DOI] [PubMed] [Google Scholar]
- 95.Ma S, Yang Y, Carnes DL, et al. Effects of dissolved calcium and phosphorous on osteoblast responses. J Oral Implantol. 2005;31(2):61–67. doi: 10.1563/0-742.1. [DOI] [PubMed] [Google Scholar]
- 96.Barradas AM, Yuan H, van der Stok J, et al. The influence of genetic factors on the osteoinductive potential of calcium phosphate ceramics in mice. Biomaterials. 2012;33(23):5696–5705. doi: 10.1016/j.biomaterials.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 97.Coathup MJ, Samizadeh S, Fang YS, Buckland T, Hing KA, Blunn GW. The osteoinductivity of silicate-substituted calcium phosphate. J Bone Joint Surg Am. 2011;93(23):2219–2226. doi: 10.2106/JBJS.I.01623. [DOI] [PubMed] [Google Scholar]
- 98.Chan O, Coathup MJ, Nesbitt A, et al. The effects of microporosity on osteoinduction of calcium phosphate bone graft substitute biomaterials. Acta Biomater. 2012;8(7):2788–2794. doi: 10.1016/j.actbio.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 99.Yuan H, Fernandes H, Habibovic P, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci U S A. 2010;107(31):13614–13619. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 101.Barradas AM, Monticone V, Hulsman M, et al. Molecular mechanisms of biomaterial-driven osteogenic differentiation in human mesenchymal stromal cells. Integr Biol (Camb) 2013;5(7):920–931. doi: 10.1039/c3ib40027a. [DOI] [PubMed] [Google Scholar]
- 102.Tada H, Nemoto E, Kanaya S, Hamaji N, Sato H, Shimauchi H. Elevated extracellular calcium increases expression of bone morphogenetic protein-2 gene via a calcium channel and ERK pathway in human dental pulp cells. Biochem Biophys Res Commun. 2010;394(4):1093–1097. doi: 10.1016/j.bbrc.2010.03.135. [DOI] [PubMed] [Google Scholar]
- 103.Rashid F, Shiba H, Mizuno N, et al. The effect of extracellular calcium ion on gene expression of bone-related proteins in human pulp cells. J Endod. 2003;29(2):104–107. doi: 10.1097/00004770-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 104.Mizuno M, Banzai Y. Calcium ion release from calcium hydroxide stimulated fibronectin gene expression in dental pulp cells and the differentiation of dental pulp cells to mineralized tissue forming cells by fibronectin. Int Endod J. 2008;41(11):933–938. doi: 10.1111/j.1365-2591.2008.01420.x. [DOI] [PubMed] [Google Scholar]
- 105.Kanaya S, Nemoto E, Ebe Y, Somerman MJ, Shimauchi H. Elevated extracellular calcium increases fibroblast growth factor-2 gene and protein expression levels via a cAMP/PKA dependent pathway in cementoblasts. Bone. 2010;47(3):564–572. doi: 10.1016/j.bone.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 106.Aguirre A, Gonzalez A, Planell JA, Engel E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem Biophys Res Commun. 2010;393(1):156–161. doi: 10.1016/j.bbrc.2010.01.109. [DOI] [PubMed] [Google Scholar]
- 107.Gonzalez-Vazquez A, Planell JA, Engel E. Extracellular calcium and CaSR drive osteoinduction in mesenchymal stromal cells. Acta Biomater. 2014;10(6):2824–2833. doi: 10.1016/j.actbio.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 108.Kim YH, Kim JM, Kim SN, Kim GS, Baek JH. p44/42 MAPK activation is necessary for receptor activator of nuclear factor-kappaB ligand induction by high extracellular calcium. Biochem Biophys Res Commun. 2003;304(4):729–735. doi: 10.1016/s0006-291x(03)00661-2. [DOI] [PubMed] [Google Scholar]
- 109.Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111(7):1029–1037. doi: 10.1172/JCI17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pi M, Garner SC, Flannery P, Spurney RF, Quarles LD. Sensing of extracellular cations in CasR-deficient osteoblasts. Evidence for a novel cation-sensing mechanism. J Biol Chem. 2000;275(5):3256–3263. doi: 10.1074/jbc.275.5.3256. [DOI] [PubMed] [Google Scholar]
- 111.Koori K, Maeda H, Fujii S, et al. The roles of calcium-sensing receptor and calcium channel in osteogenic differentiation of undifferentiated periodontal ligament cells. Cell Tissue Res. 2014;357(3):707–718. doi: 10.1007/s00441-014-1918-5. [DOI] [PubMed] [Google Scholar]
- 112.Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov. 2016;15(1):19–34. doi: 10.1038/nrd.2015.5. [DOI] [PubMed] [Google Scholar]
- 113.Wen L, Wang Y, Wang H, et al. L-type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2012;424(3):439–445. doi: 10.1016/j.bbrc.2012.06.128. [DOI] [PubMed] [Google Scholar]
- 114.Bergh JJ, Shao Y, Puente E, Duncan RL, Farach-Carson MC. Osteoblast Ca(2+) permeability and voltage-sensitive Ca(2+) channel expression is temporally regulated by 1,25-dihydroxyvitamin D(3) Am J Physiol Cell Physiol. 2006;290(3):C822–831. doi: 10.1152/ajpcell.00403.2005. [DOI] [PubMed] [Google Scholar]
- 115.Shin MK, Kim MK, Bae YS, et al. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cell Signal. 2008;20(4):613–624. doi: 10.1016/j.cellsig.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 116.Barradas AM, Fernandes HA, Groen N, et al. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials. 2012;33(11):3205–3215. doi: 10.1016/j.biomaterials.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 117.Ju Y, Ge J, Ren X, et al. Ca1.2 of L-type Calcium Channel Is a Key Factor for the Differentiation of Dental Pulp Stem Cells. J Endod. 2015 doi: 10.1016/j.joen.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 118.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 119.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 120.Lee SH, Park Y, Song M, et al. Orai1 mediates osteogenic differentiation via BMP signaling pathway in bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2016 doi: 10.1016/j.bbrc.2016.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Robinson LJ, Mancarella S, Songsawad D, et al. Gene disruption of the calcium channel Orai1 results in inhibition of osteoclast and osteoblast differentiation and impairs skeletal development. Lab Invest. 2012;92(7):1071–1083. doi: 10.1038/labinvest.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hwang SY, Foley J, Numaga-Tomita T, Petranka JG, Bird GS, Putney JW., Jr Deletion of Orai1 alters expression of multiple genes during osteoclast and osteoblast maturation. Cell Calcium. 2012;52(6):488–500. doi: 10.1016/j.ceca.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sohn S, Park Y, Srikanth S, et al. The Role of ORAI1 in the Odontogenic Differentiation of Human Dental Pulp Stem Cells. J Dent Res. 2015;94(11):1560–1567. doi: 10.1177/0022034515608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koliniotou-Koumpia E, Tziafas D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. J Dent. 2005;33(8):639–647. doi: 10.1016/j.jdent.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 125.Tarim B, Hafez AA, Cox CF. Pulpal response to a resin-modified glass-ionomer material on nonexposed and exposed monkey pulps. Quintessence Int. 1998;29(8):535–542. [PubMed] [Google Scholar]
- 126.Tziafa C, Koliniotou-Koumpia E, Papadimitriou S, Tziafas D. Dentinogenic responses after direct pulp capping of miniature swine teeth with Biodentine. J Endod. 2014;40(12):1967–1971. doi: 10.1016/j.joen.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 127.Saito K, Nakatomi M, Ida-Yonemochi H, Ohshima H. Osteopontin Is Essential for Type I Collagen Secretion in Reparative Dentin. J Dent Res. 2016 doi: 10.1177/0022034516645333. [DOI] [PubMed] [Google Scholar]
- 128.Hunter DJ, Bardet C, Mouraret S, et al. Wnt Acts as a Pro-Survival Signal to Enhance Dentin Regeneration. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2444. [DOI] [PubMed] [Google Scholar]