Abstract
Primary ovarian insufficiency (POI) is a rare but important cause of ovarian hormone deficiency and infertility in women. In addition to causing infertility, POI is associated with multiple health risks, including bothersome menopausal symptoms, decreased bone density and increased risk of fractures, early progression of cardiovascular disease, psychological impact that may include depression, anxiety, and decreased perceived psychosocial support, potential early decline in cognition, and dry eye syndrome. Appropriate hormone replacement therapy to replace premenopausal levels of ovarian sex steroids is paramount to increasing quality of life for women with POI and ameliorating associated health risks. In this review, we discuss POI and complications associated with this disorder, as well as safe and effective hormone replacement therapy options. To decrease morbidity associated with POI, we recommend using HRT formulations that most closely mimic normal ovarian hormone production and continuing HRT until the normal age of natural menopause, ~50 years. We address special populations of women with POI, including women with Turner Syndrome, women with increased risk of breast or ovarian cancer, women approaching the age of natural menopause, and breastfeeding women.
Keywords: Primary Ovarian Insufficiency, Premature Ovarian Failure, Premature Menopause, Early Menopause, Estrogen, Progestin, Androgen, Hormone Replacement Therapy, Menopausal Hormone Therapy, Management, Morbidity, Mortality
Introduction
Primary ovarian insufficiency (POI) is a rare but important cause of sex steroid deficiency and infertility in pre-menopausal women. POI is characterized by menopausal levels of follicle stimulating hormone (FSH) and absent or irregular menstrual cycles prior to age 40. Because the average age of natural menopause is 50–51 years, women exhibiting these findings after age 40 but prior to age 45 are said to have early menopause (1). Spontaneous POI affects ~1% of women prior to age 40, and ~0.1% prior to age 30. An estimated 5% of women undergo early menopause prior to age 45 (2). Many of the health complications associated with POI are directly related to ovarian hormone deficiency, primarily estrogen deficiency. This underscores the importance of physiologic hormone replacement therapy in women with POI. Unfortunately, data regarding adverse effects from the Women’s Health Initiative (WHI) trial, a study of older post-menopausal women, has dissuaded many from using estrogen therapy (ET) or estrogen/progestin therapy (EPT) in young women with POI or early menopause (3). The WHI showed multiple increased health risks related to use of EPT, including increased risk of stroke, breast cancer, and cardiovascular disease (4). This is unfortunate because in contrast to women with normal menopause, the situation in young women with POI and early menopause is in fact a pathologic state of estrogen deficiency compared to their peers with normal ovarian function. In women with POI and early menopause the term Hormone Replacement Therapy (HRT) is entirely accurate because the prescribed hormones are replacing hormones that would normally be present.
Health complications of POI include menopausal symptoms (hot flashes, night sweats, insomnia, dyspareunia, decreased sexual desire, and vaginal dryness); decreased bone mineral density (BMD) and increased risk of fracture; infertility; increased risk of mood disorders, namely depression and anxiety; cognitive decline; sexual dysfunction; increased rates of auto-immune disease; increased risk of cardiovascular disease; increased risk of Type 2 diabetes mellitus (T2DM) or pre-DM; and dry eye syndrome (1). Physiologic EPT ameliorates many of these health risks and is considered standard of care for women with POI or early menopause (1, 5, 6). It is generally recommended to continue EPT until ~age 50 (the average age of natural menopause), unless a specific contra-indication exists such as an estrogen-dependent malignancy. In this review, we will discuss the use of HRT in women with POI and early menopause, including benefits and risks; HRT formulations available in the U.S.; management of HRT after age 50 in these women; and HRT use in special populations with POI. Women who experience ovarian insufficiency as a result of oophorectomy present a unique situation which will be addressed in a separate review.
There are multiple etiologies for POI, including genetic, autoimmune, iatrogenic related to chemotherapy or radiation, surgical, and spontaneous presentation. Spontaneous 46,XX POI (sPOI) refers to ovarian insufficiency prior to age 40 in women with a normal 46,XX karyotype for whom the condition develops spontaneously. In 90% of cases of sPOI, a specific underlying cause cannot be identified. Approximately 4% of sPOI cases are due to lymphocytic auto-immune oophoritis caused by auto-immunity against steroidogenic cells, a process that may affect function of both the ovary and the adrenal glands (1, 7). A pre-mutation in the Fragile X Mental Retardataion-1 (FMR1) gene is responsible for an estimated 2–5% of cases of isolated sPOI and 14% of familial sPOI cases (8, 9). The FMR1 gene contains a polymorphic trinucleotide (CGG) repeat, normally present in <45 copies, at the 5′ untranslated region. A full mutation of the FMR1 gene occurs when >200 CGG repeats exist and is the cause of Fragile X Syndrome, the most common heritable form of mental retardation. An FMR1 gene pre-mutation, which may expand to the full mutation across generations, contains 55–199 CGG repeats, and incurs ~24% risk of developing sPOI in carriers (9). The most common genetic cause of POI is Turner Syndrome, which is most commonly related to 45,X karyotype. Turner Syndrome affects ~1 in 2,500 girls (10).
Benefits and Risks of HRT in women with POI and Early Menopause
Menopausal symptoms and sexual function
Women with POI and early menopause commonly complain of bothersome menopausal symptoms, which may present gradually or suddenly. The symptoms these women experience are identical to those experienced by women who proceed through menopause naturally, and may include hot flashes, night sweats, insomnia, and sexual dysfunction due to vaginal dryness, dyspareunia, and loss of libido (11–14). A decline in ovarian estradiol production, and likely to some extent ovarian testosterone production, is responsible for these symptoms (12, 14, 15). Menopause symptoms should be taken seriously by affected women and their physicians, as these symptoms can affect quality of life and signal hormone deficiencies that may contribute to disease (16).
Appropriate physiologic estrogen replacement alleviates menopausal symptoms, and may improve sexual dysfunction that is related to vaginal dryness, dyspareunia, and decreased libido. A role for testosterone replacement in treating menopausal symptoms, particularly those related to sexual dysfunction, has not been clearly established. There are currently no approved testosterone formulations for women in the US. Further, androgen deficiency in women is not well-defined and thus should not be diagnosed until normative data on testosterone levels across a woman’s lifespan have been established (17). That said, some reports have demonstrated that testosterone replacement enhances the beneficial effects of estrogen therapy on sexual function in women with POI following oophorectomy (18–22).
Bone Mineral Density (BMD) and fracture risk
Multiple studies have shown that the lower BMD seen in women with POI or early menopause (< age 45) due to any etiology is associated with significantly increased risk for fracture (23–29). Several of these studies further demonstrated that fracture rates are reduced among women with POI or early menopause who are treated with HRT (23, 24, 26, 29). Peak bone mass is attained by ~age 30 in women; prolonged estrogen deficiency prior to this age results in decreased peak bone mass accrual, and estrogen deficiency after this age results in early bone loss. Early BMD loss or failure to attain peak bone mass results in increased fracture risk and is a primary health concern among young women with POI, particularly if appropriate treatment with HRT is not initiated soon after disease onset (1, 30–33). Compared to regularly-menstruating, similarly-aged women, a cohort of young women with 46,XX sPOI (mean age 32 years, range 20–39) had significantly lower BMD z-scores. Importantly, 21% of women with sPOI in this cohort had BMD z-scores < −2.0, indicative of low BMD for age and a fracture risk factor (33). Fully 67% of these women with sPOI had femoral neck BMD scores of < −1.0, and among women with sPOI who were within 1.5 years of diagnosis, almost half (47%) had femoral neck z-scores < −1.0. Progressive decreases in ovarian hormone production occurring well before the diagnosis of sPOI may perhaps contribute to the high rates of low BMD in women who were recently diagnosed.
It is important to address modifiable risk factors that may contribute to reduced bone mass (Table 1)(33). Delay in diagnosis is a contributing risk factor for women with POI. In one study of POI over 50% of women had to visit three or more different clinicians with a complaint of menstrual abnormality before an FSH level was measured (34). It is important to view the menstrual cycle as a vital sign of bone health and investigate abnormalities aggressively. To support bone health, women with POI need to assure adequate calcium and vitamin D intake and maintain a routine of regular weight-bearing exercise. In a group of women with POI, Popat et al found that 58% had inadequate serum 25(OH)D levels, 49% had inadequate calcium intake, and nearly 1 in 4 had no regular exercise program (33). Clinicians should maintain serum 25-hydroxy-vitamin D levels in the normal range (> 30 ng/mL)(35). Women with POI should take 1,000 to 2,000 IU of vitamin D3 (cholecalciferol) daily, along with 1200 mg of elemental calcium, either through dietary sources or supplements to optimize bone health (1). Additional risk factors for low BMD in women with POI include older age, younger age at POI diagnosis (particularly diagnosis prior to age of peak bone mass accrual), and lower body mass index (BMI) (32). Interestingly, race other than Caucasian was a risk factor for reduced BMD in Black, Asian, and Hispanic women with POI (Table 2)(33). These differences disappeared when corrected for other factors, a distressing fact pointing out problems with racially biased health disparities (Table 3)(33). Altogether, women with POI need physiologic HRT and healthy lifestyle habits to maintain bone density and minimize fracture risk.
Table 1.
Risk factors for Z-score <−2 at any site as assessed by PPR in women with POI (n=442) (adapted with permission from (Popat, 2009 #248)).
| Risk Factor | PPR | 95% Confidence interval | P |
|---|---|---|---|
| Onset of menstrual irregularity before age 20 yr | 2.72 | 1.74, 4.33 | <0.0001 |
| Delay in diagnosis >1 yr | 1.96 | 1.14, 3.35 | 0.018 |
| Serum 25(OH) Vit D < 32 ng/mL | 2.89 | 1.47, 5.69 | 0.002 |
| No regular exercise | 1.93 | 1.22, 3.06 | 0.005 |
| Weight <55 kg | 2.8 | 1.47, 5.36 | 0.002 |
| Daily calcium intake <1000 mg | 2.8 | 1.37, 5.75 | 0.005 |
| Smoking >2 cigarrettes/day | 0.91 | 0.25, 3.39 | 0.84 |
PPR = Prevalence proportional ratio.
Table 2.
Risk for serum 25 (OH)-Vit D deficiency (<32 ng/mL) and BMD Z-score < −2 by race/ethnicity in women with POI (n=442) (adapted with permission from (Popat, 2009 #248))
| Race/ethnicity | PPR | 95% Confidence interval | P |
|---|---|---|---|
| Caucasian | 1.94 | 0.88, 4.28 | 0.099 |
| Afican-American | 6.74 | 3.17, 14.3 | <0.0001 |
| Asian | 9.07 | 4.1, 20.04 | <0.0001 |
| Hispanic | 3.88 | 1.35, 11.2 | 0.012 |
PPR = Prevalence proportional ratio.
Table 3.
Serum 25(OH)-Vitamin D levels, calcium intake, and compliance to HRT by race/ethnicity (adapted with permission from (Popat, 2009 #248)).
| Serum 25(OH)-Vit D (ng/mL) | Calcium Intake (mg/day) | HRT Compliance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Race/ethnicity | N (429) | Mean (SEM) | P | N (250) | Mean (SEM) | P | N (423) | Compliant (%) | P |
| Caucasian | 337 | 33.6 (0.8) | — | 205 | 1947 (49) | — | 336 | 75 | — |
| Afican - American | 48 | 19.3 (2.3) | <0.001 | 25 | 1716 (144) | <0.001 | 44 | 66 | 0.20 |
| Asian | 18 | 17.1 (3.7) | <0.001 | 7 | 1258 (142) | 0.016 | 18 | 44 | 0.01 |
| Hispanic | 26 | 24.8 (3) | 0.01 | 13 | 1803 (241) | <0.001 | 25 | 64 | 0.24 |
The NIH Intramural Research Program conducted a 3-year prospective randomized controlled trial in young women with 46,XX sPOI to investigate the effectiveness of a standardized regimen of HRT on BMD. The study employed treatment with physiologic estradiol replacement with cyclic oral progestin (transdermal estradiol 100 μg/day with oral medroxyprogesterone 10 mg daily for 12 days/month). This replacement therapy improved lumbar spine and femoral neck BMD, such that at the end of the 3-year intervention, BMD did not differ between women with sPOI and a group of contemporaneously recruited normally-cycling control women (Figure 1)(36). The addition of transdermal testosterone replacement to the regimen of transdermal estradiol and oral medroxyprogesterone acetate provided no additional beneficial effect on BMD (36).
Figure 1.
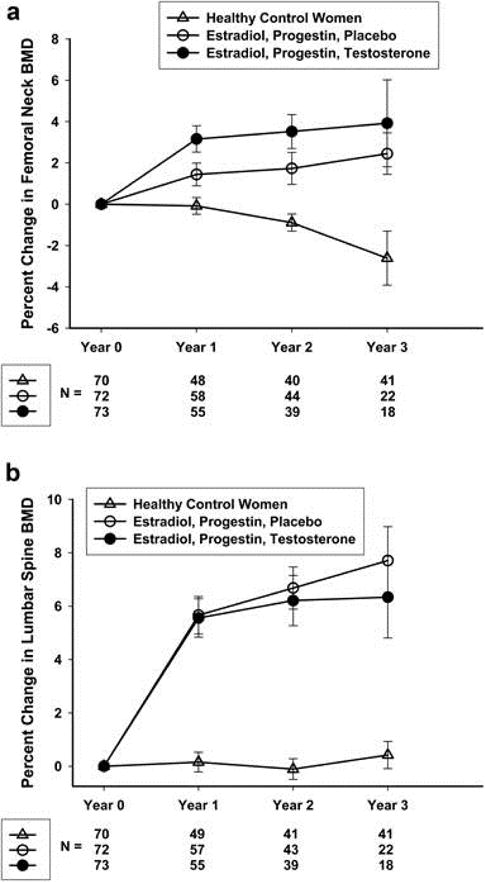
Percentage change over 3 years in femoral neck (a) and lumbar spine (b) BMD in healthy control women and women with 46,XX sPOI treated with E+P or E+P+T (with permission from (Popat, 2014 #85)).
Important evidence is accumulating to support a conclusion that physiologic HRT (transdermal estradiol and cyclic progestin) is more effective in maintaining bone health in young women with POI than continuous combined therapy with oral contraceptive pills. For example, a study in women with POI comparing the efficacy of 12 months of physiologic HRT (transdermal estradiol 100–150 μg/day plus cyclic progestin) to 12 months of a combined oral contraceptive pill (OCP)(ethinyl estradiol 30 μg and norethisterone 1.5 mg daily for 3 weeks per month) demonstrated that physiologic HRT was superior to OCPs in protecting and improving BMD (37). Another 2-year open-label randomized trial in women with POI compared physiologic estradiol replacement (2 mg oral estradiol and 0.075 mg levonorgestrel per day) with combined OCP (0.030 mg ethinylestradiol and 0.150 mg levonorgestrel taken daily for 21 days followed by a 7 day break). The findings demonstrated a significantly greater increase in lumbar spine BMD with physiologic estradiol replacement (38). Together, these data support the use of physiologic estradiol and progestin HRT over continuous combined contraceptive steroids for maintaining bone health in women with POI.
Cardiovascular disease
Evidence points to estrogen deficiency as a driver of increased CVD risk associated with POI (39). A recent meta-analysis demonstrated that women who experienced menopause prior to age 45 have a higher risk of coronary heart disease, cardiovascular mortality, and overall mortality compared to women who experienced menopause after age 50 (40). Compared to age-matched normal women, women with sPOI have reduced vascular endothelial function, an early sign of atherosclerosis. Treatment with HRT for 6 months significantly improved endothelial function in these women (41). Women with POI, regardless of the etiology, have an increased risk of cardiovascular disease (42–47) and ischemic stroke (48). Taken together, regular cardiovascular disease risk assessments and risk reduction measures are indicated. These will include lifestyle modifications, management of lipid levels and hypertension, and early initiation of physiologic HRT. These are paramount for the long-term cardiovascular health of women with POI. That said, benefits of HRT on cardiovascular health have largely been shown in studies of naturally post-menopausal women; no long-term data exist on cardiovascular outcomes in young women with POI treated with HRT. Therefore, one can only extrapolate the cardiovascular benefit of HRT among women with POI using outcome data from older, naturally menopausal populations, along with evidence that HRT improves risk markers in women with POI.
Emotional Health
POI is associated with an increased risk of depression and anxiety, in large part due to the diagnosis of infertility as well as lack of perceived psychosocial support (49–51). A study comparing 154 women with 46,XX sPOI to a control group showed significant decreases in perceived social support and self-esteem. These negative psycho-social perceptions were present irrespective of a woman’s marital status, whether or not she had children, or the length of time since her diagnosis of sPOI (49). Similarly, women with sPOI report reduced self-esteem, increased social anxiety and shyness, and more symptoms of depression compared to controls (50). Indeed, the prevalence of clinical depression is greater among women with sPOI compared to controls and seems to be associated with the onset of menstrual irregularity. Interestingly, in one study rates of depression in women with sPOI were higher than rates seen in young women with Turner Syndrome, suggesting that an unexpected onset of ovarian dysfunction later in adulthood rather than during childhood may increase the psychological impact of ovarian insufficiency in young women (51).
The role estrogen deficiency plays in the development of depression during the menopausal transition or in women with ovarian insufficiency is controversial (52). The risk for new onset depression is heightened by more severe vasomotor symptoms (53). Physiologic HRT, in particular the estradiol component, has been shown to alleviate symptoms of depression and even lead to remission when initiated during peri-menopause or in very early menopause (54–56). Androgen replacement therapy has also been shown to contribute to improved mood symptoms among postmenopausal women (55); however, among women with spontaneous 46,XX POI, treatment for twelve months with physiologic androgen replacement therapy did not worsen or improve quality of life, self-esteem, or mood(57).
Cognitive function
Evidence suggests that estrogen is neuro-protective and thus estrogen deficiency at an early age would theoretically heighten a woman’s risk for cognitive decline and dementia. Animal studies demonstrate neuro-protective effects of estrogen, including enhancement of synaptic plasticity and reduced production of β-amyloid, the protein associated with Alzheimer’s disease development (58). Neuroimaging studies in humans suggest that estrogen enhances brain activity related to memory processing (59). Further, several studies in older postmenopausal women indicate that estrogen replacement therapy is protective against development of dementia, especially when started early in the menopausal transition and used for >10 years duration (60, 61). Data on cognitive benefits of HRT, however, come exclusively from older postmenopausal populations, and no data exist showing direct cognitive benefits of HRT in young women with POI. Therefore, potential cognitive benefits of HRT in women with POI can only be extrapolated from existing evidence in older women and from animal data.
Infertility
The most common terms women use to describe how they feel when they first hear about their diagnosis of POI are “devastated,” “shocked,” and “confused” (34) For many women with POI, infertility is the most devastating aspect of the diagnosis. Women with POI do not respond to traditional fertility treatments. Their options for child-raising include adoption, a small potential for spontaneous pregnancy, donor embryo, or egg donation using in vitro fertilization. Spontaneous pregnancy will occur in about 5 to 10% of women with 46,XX sPOI (62).
Nearly 3 out of 4 women with POI have ovarian follicles remaining in the ovary (63). It is clear that POI in most cases is not a “failure” of the ovary, but rather intermittent and unpredictable ovarian function that can persist for decades. However, the tonic elevation in serum LH levels causes premature luteinization of growing antral follicles, which diminishes the chances for spontaneous ovulation or response to ovarian stimulation (64). Theoretically, treatment with physiologic HRT, such as transdermal estradiol plus cyclic medroxyprogesterone, may enhance the ability of ovarian follicles to avoid premature luteinization and respond to an endogenous or exogenous stimulus from gonadotropins, undergo follicular maturation, and ovulate. This theoretical benefit of HRT stems from its ability to suppress serum LH levels into the pre-menopausal range (65), potentially reducing the inappropriate luteinization of follicles caused by chronically elevated LH levels, and thereby improving ovulation rates (64). A second proposed mechanism by which estradiol may improve fertility rates is by suppressing chronically elevated FSH levels, which have been shown to down-regulate granulosa cell FSH receptors. Estradiol treatment may allow for restoration of FSH receptors and thereby enhance the response to exogenous gonadotropins in the remaining ovarian follicle pool (66). Despite this theoretical fertility-enhancing effect of HRT, clinical investigations have demonstrated little or no benefit in practice.
In a randomized, controlled trial investigating effects of physiologic estrogen replacement on fertility in women with sPOI, 6 weeks of oral estradiol 2 mg daily suppressed serum LH levels and increased estradiol concentrations appropriately; however, estradiol had no effect on folliculogenesis, ovulation rates, or pregnancy rates during this short trial (67). In another randomized, placebo-controlled study investigating the effects of pre-treatment with estrogen on the ovarian response to gonadotropin therapy in women with POI, treatment with ethinyl estradiol 0.05 mg three times daily for two weeks prior to ovulation induction resulted in significantly higher ovulation rates compared to placebo (32% vs 0%, respectively). Follicular development and ovulation occurred only in women who achieved serum FSH levels ≤15 mIU/mL, suggesting that suppression of endogenous gonadotropins by estradiol improved response rates. Among the eight women who ovulated in that study, four achieved pregnancy, all after estradiol pre-treatment followed by ovulation induction with gonadotropins (66). In another study of 100 women with POI, pre-treatment with estradiol prior to ovarian stimulation with exogenous gonadotropins resulted in ovulation in 19% of cycles, a pregnancy rate of ~5%, and a live-birth rate of 2% (68). This study, however, was not placebo-controlled, and the pregnancy rate was similar to the rate of spontaneous pregnancy seen in women with sPOI, thus the positive impact of estradiol on fertility cannot be determined.
Dry Eye syndrome
Women with POI suffer from dry eye syndrome significantly more than age-matched controls with normal ovarian function (20% vs 3%) (69). Dry eye syndrome in women with POI is not associated with reduced tear production, as is typically seen in older individuals (>65 years) who are more commonly affected by this ocular surface disorder. There are sex hormone receptors in ocular surface tissues, providing a potential mechanism by which ovarian hormones could alter function (69). Further, there is a link between dry eye syndrome and androgen deficiency in other patient populations (70, 71); however, no investigations to date have explored a role for androgen or estrogen replacement therapy in ameliorating dry eye symptoms in women with POI.
Hormone Replacement Therapy
Transdermal or Transvaginal Estradiol
The Women’s Health Initiative study involved menopausal women who on average were 63 years old (4). These results should not be applied to young women with POI or early menopause. POI is a pathologic condition in which young women have low serum estradiol levels as compared with their peers. For young women with estradiol deficiency, hormone therapy is indeed “replacement,” whereas in women with normal menopause, hormone therapy is in fact hormone “extension.” It is important to make this distinction clear to patients. Unfortunately, a recent study showed that more than half (52%) of young women with POI either never take HRT, start HRT many years after their diagnosis, and/or discontinue HRT use prior to age 45 (72).
The weight of evidence now favors transdermal or transvaginal estradiol therapy as the first line of HRT for young women with POI or early menopause. Young women who develop POI require long-term ovarian sex steroid replacement. Some will require this therapy for decades. Current therapies are prescribed to control symptoms and help prevent disease related to estradiol deficiency.
Ideally, replacement would mimic normal ovarian function. The thought experiment solution to this dilemma is to develop an artificial ovary that would be designed to deliver a constant parenteral infusion of the right mix of hormones to mimic endogenous ovarian production throughout a menstrual cycle. The transdermal patch and the vaginal ring that deliver 0.100 mg of estradiol per day are a first rudimentary step in this direction. These formulations mimic the daily ovarian production rate of estradiol and achieve average serum estradiol levels of 100 pg/ml; this is the average level women with normal ovarian function experience across the menstrual cycle (73). An equivalent dose of oral estradiol is also effective replacement, however, the transdermal and transvaginal routes of administration deliver hormone directly into the circulation, which avoids complications associated with the first pass effect on the liver when estrogen is given orally (74). Risk of venous thromboembolism is increased by oral estrogen compared to transdermal estrogen use (74–77). In the multi-center Estrogen and Thromboembolism Risk (ESTHER) study performed in postmenopausal women, the odd ratio (OR) for venous thromboembolism in women using oral estrogens was 4.2 (95% CI, 1.5–11.6) compared to 0.9 (95% CI, 0.4–2.1) in women using transdermal estrogen preparations (75). Additionally, unlike oral estrogens, transdermal HRT does not adversely alter cardiovascular disease or thromboembolic risk markers (78, 79). In women with underlying obesity or clotting disorders, the venous thromboembolism risk associated with oral estrogen is heightened further, to 5–8 times the risk seen in non-users or users of transdermal estrogen preparations (76). Also, relative risk of stroke is increased in postmenopausal women prescribed oral estrogen compared to transdermal estrogen as part of their hormone therapy regimen. (80).
Steroidal hormone agents developed as contraceptives provide supra-physiologic levels of synthetic estrogen and progestin in order to suppress ovulation in normally-cycling women. Thus, by definition these agents provide more steroid hormone than is required to replace ovarian production rates. Contraceptive steroid hormone agents have been associated with increased risk of thromboembolism, stroke, subarachnoid hemorrhage, and worsening cardio metabolic risk, including increase in blood pressure, and unfavorable lipid profiles. In particular, drosperinone-containing formulations are associated with ~2-fold increased risk of venous thromboembolism and arterial thrombotic events, including acute myocardial infarction and stroke (81). Also, oral contraceptives typically have a 1 week ‘pill free’ period each month (or every 3 months with 3-month preparations), resulting in a regular temporary estrogen deficient state. For some women with POI, this may mean return of unwanted menopausal symptoms during that interval.
A recent randomized, controlled, cross-over trial in young women with POI compared the cardiovascular effects of treatment with transdermal estradiol plus cyclic progestin to treatment with a combination oral contraceptive (Figures 2 and 3)(82). Compared with the oral contraceptive, twelve months of transdermal physiologic HRT resulted in significantly lower blood pressure, better renal function, and reduced activation of the renin-angiotensin-aldosterone system. This evidence suggests that transdermal physiologic HRT is superior to the combined oral contraceptive in promoting cardiovascular health in young women with POI.
Figure 2.
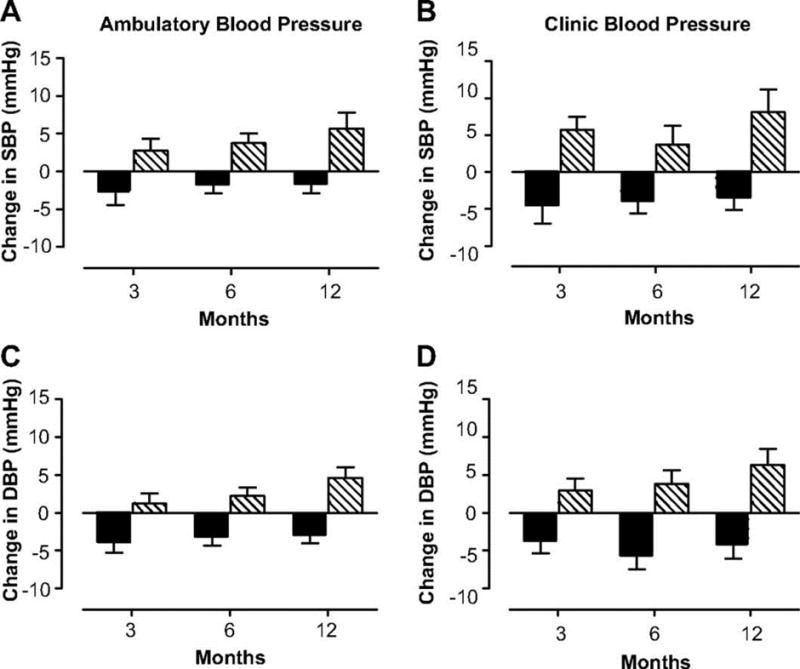
Change in 24-hr ambulatory (A) and clinic (B) systolic and diastolic blood pressure in women with POI treated with physiologic HRT (
 ) or standard OCPs (
) or standard OCPs (
 ). (with permission from (Langrish, 2009 #33)).
). (with permission from (Langrish, 2009 #33)).
Figure 3.
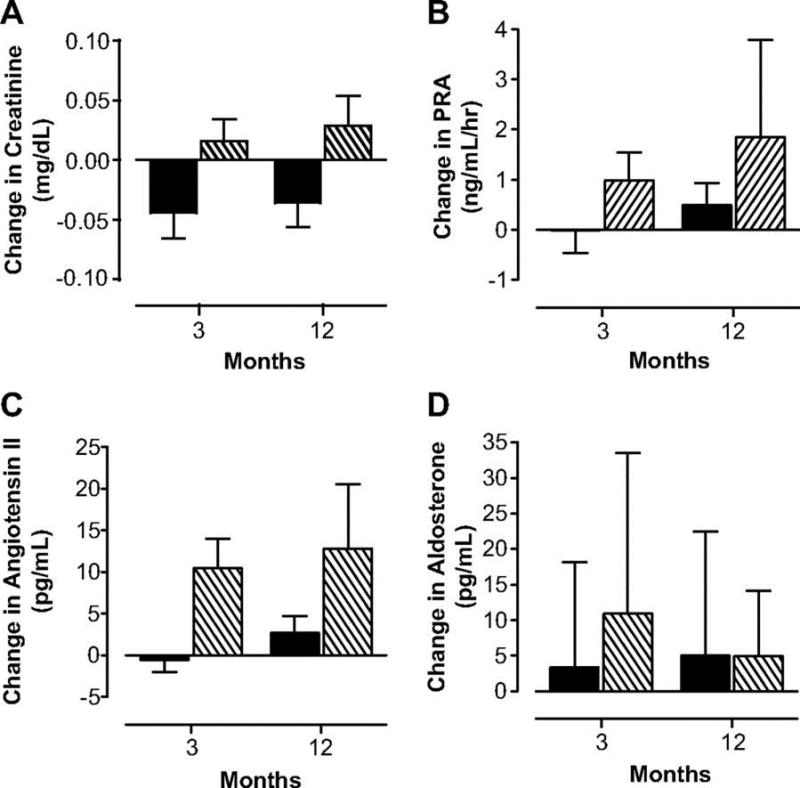
Changes in serum creatinine (A), plasma renin activity (B), angiotensin II (C) and aldosterone (D) concentrations in women with POI treated with physiologic HRT (
 ) or standard OCPs (
) or standard OCPs (
 ). (with permission from (Langrish, 2009 #33)).
). (with permission from (Langrish, 2009 #33)).
Progestins
To date, an NIH Intramural Research Program study provides the only long term controlled data published regarding HRT for young women with POI (36). Most women with POI have an intact uterus, thus the recommended hormone replacement is both estrogen and progestin. Cyclical progestin is recommended for endometrial protection. The NIH study of HRT in POI used transdermal estradiol (100 ug/day) with oral medroxyprogesterone acetate (10mg/day for 12 days/month). This regimen was tolerated well. Medroxyprogesterone acetate is the only progestin for which available evidence demonstrates capability to fully induce secretory endometrium in conjunction with a full replacement dose of estrogen when used in regular monthly cycles (83, 84). Cycling progestin courses given less frequently than monthly (termed “long –cycle HRT”) is not recommended as this approach increases the risk of endometrial hyperplasia and potentially of endometrial cancer (84). Medroxyprogesterone acetate is not derived from testosterone and is a 21-carbon “pure” progestin, as compared with the 19 nor-progestins which may have associated androgenic effects (85).
The NIH study on HRT in POI employed medroxyprogesterone acetate as the progestin due to concerns regarding the lack of evidence for effectiveness of oral micronized progesterone to protect the endometrium when used in conjunction with full replacement doses of estradiol. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Group did not investigate the efficacy of oral micronized progesterone to effectively induce secretory endometrium in conjunction with a full replacement dose of estrogen. The group only investigated lower doses of estrogen, so the effectiveness of oral micronized progestin to induce full secretory endometrium in this context remains an open question (86). Likewise, use of a progestin-containing intra-uterine device (PIUD) as the progestin component of an HRT regimen in young women with POI is not recommended given that PIUD-induced endometrial suppression has not been studied in this population, but only in older postmenopausal women with intact uteri who require a progestin along with low, postmenopausal doses of estradiol (87). Furthermore, the PIUD would prevent pregnancy and lead to cessation of regular menses, undesirable effects for many young women with POI who wish for their HRT regimen to help ‘normalize’ their reproductive lives (88).
Furthermore, there are concerns regarding the pharmacokinetics of oral micronized progesterone. After administration of a 200 mg dose of oral micronized progesterone, the mean serum progesterone level peaks after only three hours and, importantly, returns to baseline by 24 hours. The progesterone peak level, as validated independently by mass spectrometry, is only at the lower progesterone level of the range defined for a normal functional corpus luteum (89). This suggests that with this dose of oral micronized progesterone, the integrated progestin effect at the level of the endometrium would be inadequate to induce full maturation in the face of a full replacement dose of estradiol. Some young women with POI will take hormone replacement therapy for decades. More supporting evidence regarding the effects of oral micronized progesterone at the level of the endometrium in the face of a full replacement dose of estradiol is needed before this can be recommended as the first line progestin for this clinical situation. There is insufficient evidence to determine if medroxyprogesterone acetate and micronized progesterone therapy differ significantly with regard to association with the development of breast cancer. Women who cannot tolerate medroxyprogesterone acetate may be prescribed micronized progesterone but should be monitored for endometrial suppression on at least an annual basis.
With regard to effect on cardiovascular risk, in combination with estradiol, both medroxyprogesterone acetate and micronized progesterone improve cardiovascular risk markers, including serum HDL and LDL levels, blood pressure, and fibrinogen levels. However, micronized progesterone may be superior in improving HDL levels compared to medroxyprogesterone acetate (90). In the Kronos Early Estrogen Prevention Study (KEEPS) performed in post-menopausal women, the addition of micronized progesterone to estradiol replacement had a neutral impact on coronary artery calcium scores, carotid intima media thickness, blood pressure, lipids, and insulin resistance (91). The addition of micronized progesterone or medroxyprogesterone acetate to HRT regimens does not alter venous thromboembolism risk (75, 76). However, the use of norpregnane derivatives (nomegestrol acetate, promegestone), which are rarely used in the U.S., increases venous thromboembolism risk up to 4-fold (75).
Testosterone
In pre-menopausal women, endogenous testosterone production is ~300 mcg daily, with approximately 50% produced by the adrenal glands and 50% by the ovaries (92). Therefore, women with POI have deficiencies not only in ovarian estrogen and progesterone production, but also in ovarian testosterone production. Testosterone deficiency may contribute to the symptomatology of POI, thus there has been interest in investigating the risks and benefits of testosterone replacement therapy in this population. Currently, there is insufficient evidence to recommend the diagnosis or treatment of testosterone deficiency in women, even those with POI (93).
There was no benefit of testosterone replacement on quality of life, self-esteem, or mood demonstrated by a 12-month, randomized, placebo-controlled trial of physiologic testosterone replacement in women with 46,XX sPOI (already receiving HRT with estradiol and medroxyprogesterone acetate) (57). In another small study in young women with Turner Syndrome (n=14, ages 17–27 years), treatment with 1.5 mg oral methyl testosterone for 1 year resulted in improvements in BMD. There were also benefits in cardio- metabolic risk factors (improved lipid profiles, decreased fat mass and increased lean body mass), improvements in neurocognitive measures (attention and memory); improved libido; and improved overall quality of life, and with a favorable safety profile (94). Taken together, and given their deficient ovarian androgen production, it appears likely that with more research testosterone replacement in physiologic doses may ultimately prove of benefit to women with POI.
Dehydroepiandrosterone
Dehydroepiandrosterone (DHEA) is an endogenous androgen produced by the ovaries and adrenal glands, and plays a role in ovarian folliculogenesis. Women with POI have lower levels of androstenedione compared to normally-cycling women (95). DHEA is available over-the-counter in the U.S. because it is considered a food supplement. Available evidence does not support routine replacement use of DHEA for women with POI (93). However, treatment with DHEA has been used in a few fertility centers to improve ovarian response in women with ovarian insufficiency (96–98). Yilmaz et al demonstrated in a prospective study that 6 weeks of DHEA supplementation (25 mg orally three times daily) in women with occult ovarian insufficiency (“diminished ovarian reserve”) improves markers of ovarian response, including serum FSH, AMH, and Inhibin B levels, and leads to small increases in antral follicle counts (97). Gleicher et al (96) reported in a meta-analysis of use of DHEA that women with occult ovarian insufficiency or overt POI had improved reproductive outcomes on the treatment. Taken together, however, the findings regarding fertility-enhancing effects of DHEA in women with ovarian insufficiency are still controversial and show minimal clinical benefit at most.
Custom compounding
Although customized, compounded HRT has become more popular for women who require estrogen, progestin, and/or androgen replacement therapy, these formulations are not recommended because they are not regulated by the U.S. Food and Drug Administration (FDA). Therefore, neither the safety nor the efficacy of any compounded HRT regimen has been appropriately evaluated. Actual levels of hormones achieved with compounded HRT formulations are not readily known, thus the risks outweigh potential benefits (5).
Special Populations
Turner Syndrome
Turner Syndrome, which is due to loss of part or all of one X chromosome (45,X), affects approximately 1 in 2,500 girls and women. It is the most common genetic cause of POI. Although there is wide phenotypic variation among girls and women with Turner Syndrome, ~90% will develop POI (10). Ovarian function is most commonly lost early in life, such that many girls with Turner Syndrome require estrogen replacement for induction of puberty and menarche, to promote bone accrual early in life and later, and to promote maintenance of bone density. Girls and women with Turner Syndrome have an increased prevalence of low BMD for age and an increased fracture risk due to chronic estrogen deficiency, particularly if diagnosis and initiation of optimal estrogen treatment are delayed (10, 99, 100). Thus, it is paramount that HRT be initiated in a timely fashion in this population. In girls with Turner Syndrome who do not enter puberty spontaneously, estrogen replacement therapy for puberty induction should start at approximately age 12, with gradually increasing doses until full physiologic replacement doses are achieved, usually over ~2 years of titration. Previous guidelines recommended waiting to start estrogen therapy until age 15 to avoid estrogen-induced epiphyseal closure and reduction in height potential. However, it has been demonstrated that starting ovarian hormone replacement this late has detrimental effects on accrual of bone mass (10, 101). Women with Turner Syndrome should be educated about the importance of compliance with the prescribed HRT regimen to maintain normal BMD. Of note, BMD measurements should be adjusted for skeletal size in women with Turner Syndrome because short statue leads to falsely low BMD readings if not corrected (100). Women with Turner Syndrome who are appropriately treated with HRT starting in adolescence have normal BMD after adjustment for skeletal size as adults (102).
Adult women with Turner Syndrome should be treated with physiologic replacement doses of estradiol and cyclic progestin, as recommended for women with other forms of POI. The preferred HRT regimen for women with Turner Syndrome is transdermal or transvaginal estradiol 100 μg daily with cyclic medroxyprogesterone acetate 10 mg daily for 12 days per month. This regimen is clinically available, supported by the best evidence, and currently best mimics physiologic patterns of normal ovarian function. Benefits of HRT in women with Turner Syndrome include bone protection, relief of menopausal symptoms related to estrogen deficiency, and likely protection from cardiovascular disease.
Breast and Ovarian Cancer
In women with a history of breast cancer or ovarian cancer, HRT is considered unsafe and alternative measures should be employed to reduce the risks and symptoms associated with POI (103, 104). These may include 1) low dose vaginal estrogens or selective estrogen receptor modulators (SERMS) for vulvovaginal atrophy associated with estrogen deficiency, 2) healthy lifestyle changes to reduce cardiometabolic risk, 3) calcium and vitamin D supplementation and regular weight-bearing exercise to promote bone health, 4) and possibly anti-depressants and/or psychotherapy if indicated for depressed mood (103, 104).
HRT and breastfeeding
According to FDA-approved drug labels, neither estradiol in oral or transdermal forms, nor hormonal contraceptives, are recommended to be used during breastfeeding. Small amounts of hormone are transmitted via breastmilk, which may cause jaundice or breast enlargement in the neonate (105). Additionally, estrogen use may interfere with lactation by decreasing the quantity and quality of breast milk (106). A nursing mother with POI should be advised not to use HRT, including contraceptive steroids, until she has completely weaned her child (107).
HRT after age 50
The mean age of natural menopause is 50±4 years (108). The decision of when and how to discontinue HRT in women with POI needs to be individualized; each woman has her own particular set of health needs. The fact that the occurrence of natural menopause encompasses a broad range of ages provides flexibility for the patient and clinician to decide when to stop HRT. For example, women with a strong family history of breast cancer might decide to stop HRT at age 45. Early age of menopause is associated with a reduced risk of breast cancer (109). Nevertheless, women with BRCA 1 and 2 gene mutations treated with estrogen until age 50 do not show any increase in risk for developing breast cancer (110, 111). On the other hand, a woman with a strong family history of osteoporosis or cardiovascular disease might wish to continue HRT until age 55. Later age of menopause is associated with a reduced risk of osteoporosis and cardiovascular disease (43, 112–115). Women with POI can be reassured that lower, post-menopausal doses of HT, when initiated within 10 years after menopause onset, have been associated with overall favorable risk-benefit profiles, including decreases in menopausal symptoms, fractures, cardiovascular disease, and type II diabetes mellitus, and reduced mortality (116).
Summary/Conclusions
Physiologic HRT is paramount to the health and quality of life of women with POI or early menopause. The choice of HRT should closely mimic normal ovarian steroid hormone production and provide sufficient levels of estradiol to reduce menopausal symptoms, maintain bone density, minimize psychological impacts of estrogen deficiency, and protect against early progression of cardiovascular disease and dementia. The progestin component of HRT for women with POI should be cyclical and will protect the endometrium by inducing regular withdrawal bleeds. HRT should be continued until the age of natural menopause, at which time the dose may be tapered to postmenopausal levels or stopped, depending on a woman’s specific risks and needs. Clinicians need to be aware of how to diagnose and treat POI so that women affected by this disorder do not encounter unnecessary health risks later in life.
Acknowledgments
This work was supported in part by the Intramural Research Program, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD (LMN)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shannon D. Sullivan, Medical Officer, U.S. Food and Drug Administration, 10903 New Hampshire Ave., Silver Spring, MD 20993, Phone: 202-701-5174, Fax: 301-796-9712.
Philip M. Sarrel, Emeritus Professor, Obstetrics, Gynecology and Reproductive Sciences and Psychiatry, Yale University, New Haven, Connecticut, Correspondence to: Philip Sarrel, 161 Center Road, Woodbridge, Ct. 06525, Phone: 203-389-5809.
Lawrence M. Nelson, Medical Investigator, CAPT US Public Health Service, Intramural Research Program on Reproductive and Adult Endocrinology, The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, CRC, Room 1-3330, 10 Center Drive, MSC-1109, Bethesda, MD 20892-1103, Phone (direct): 301 402 6608; (cell): 703 304 4152, FAX: 301 402 0574.
References
- 1.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–14. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–6. [PubMed] [Google Scholar]
- 3.Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: results from the National Health and Nutrition Examination Survey, 1999–2010. Obstet Gynecol. 2012;120:595–603. doi: 10.1097/AOG.0b013e318265df42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, et al. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100:3975–4011. doi: 10.1210/jc.2015-2236. [DOI] [PubMed] [Google Scholar]
- 6.Neves ECM, Birkhauser M, Samsioe G, Lambrinoudaki I, Palacios S, Borrego RS, et al. EMAS position statement: The ten point guide to the integral management of menopausal health. Maturitas. 2015;81:88–92. doi: 10.1016/j.maturitas.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18:107–34. doi: 10.1210/edrv.18.1.0291. [DOI] [PubMed] [Google Scholar]
- 8.Rafique S, Sterling EW, Nelson LM. A new approach to primary ovarian insufficiency. Obstet Gynecol Clin North Am. 2012;39:567–86. doi: 10.1016/j.ogc.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray A, Schoemaker MJ, Bennett CE, Ennis S, Macpherson JN, Jones M, et al. Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genet Med. 2014;16:19–24. doi: 10.1038/gim.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bondy CA. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 11.Graziottin A, Basson R. Sexual dysfunction in women with premature menopause. Menopause. 2004;11:766–77. doi: 10.1097/01.gme.0000139926.02689.a1. [DOI] [PubMed] [Google Scholar]
- 12.Kalantaridou SN, Vanderhoof VH, Calis KA, Corrigan EC, Troendle JF, Nelson LM. Sexual function in young women with spontaneous 46,XX primary ovarian insufficiency. Fertil Steril. 2008;90:1805–11. doi: 10.1016/j.fertnstert.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Almeida DM, Benetti-Pinto CL, Makuch MY. Sexual function of women with premature ovarian failure. Menopause. 2011;18:262–6. doi: 10.1097/gme.0b013e3181f4318d. [DOI] [PubMed] [Google Scholar]
- 14.van der Stege JG, Groen H, van Zadelhoff SJ, Lambalk CB, Braat DD, van Kasteren YM, et al. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause. 2008;15:23–31. doi: 10.1097/gme.0b013e3180f6108c. [DOI] [PubMed] [Google Scholar]
- 15.Kalantaridou SN, Calis KA, Vanderhoof VH, Bakalov VK, Corrigan EC, Troendle JF, et al. Testosterone deficiency in young women with 46,XX spontaneous premature ovarian failure. Fertil Steril. 2006;86:1475–82. doi: 10.1016/j.fertnstert.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Gold E, Sternfeld B, et al. Are vasomotor symptoms associated with alterations in hemostatic and inflammatory markers? Findings from the Study of Women’s Health Across the Nation Menopause. 2011;18:1044–51. doi: 10.1097/gme.0b013e31821f5d39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wierman ME, Basson R, Davis SR, Khosla S, Miller KK, Rosner W, et al. Androgen therapy in women: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab. 2006;91:3697–710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- 18.Nachtigall L, Casson P, Lucas J, Schofield V, Melson C, Simon JA. Safety and tolerability of testosterone patch therapy for up to 4 years in surgically menopausal women receiving oral or transdermal oestrogen. Gynecol Endocrinol. 2011;27:39–48. doi: 10.3109/09513590.2010.487597. [DOI] [PubMed] [Google Scholar]
- 19.Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682–8. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- 20.Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, et al. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165:1582–9. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- 21.Davis SR, van der Mooren MJ, van Lunsen RH, Lopes P, Ribot C, Rees M, et al. Efficacy and safety of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Menopause. 2006;13:387–96. doi: 10.1097/01.gme.0000179049.08371.c7. [DOI] [PubMed] [Google Scholar]
- 22.Floter A, Nathorst-Boos J, Carlstrom K, von Schoultz B. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric. 2002;5:357–65. [PubMed] [Google Scholar]
- 23.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14:567–71. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- 24.Mallmin H, Ljunghall S, Persson I, Bergstrom R. Risk factors for fractures of the distal forearm: a population-based case-control study. Osteoporos Int. 1994;4:298–304. doi: 10.1007/BF01622186. [DOI] [PubMed] [Google Scholar]
- 25.Vega EM, Egea MA, Mautalen CA. Influence of the menopausal age on the severity of osteoporosis in women with vertebral fractures. Maturitas. 1994;19:117–24. doi: 10.1016/0378-5122(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 26.Tuppurainen M, Kroger H, Honkanen R, Puntila E, Huopio J, Saarikoski S, et al. Risks of perimenopausal fractures–a prospective population-based study. Acta Obstet Gynecol Scand. 1995;74:624–8. doi: 10.3109/00016349509013475. [DOI] [PubMed] [Google Scholar]
- 27.Johansson C, Mellstrom D. An earlier fracture as a risk factor for new fracture and its association with smoking and menopausal age in women. Maturitas. 1996;24:97–106. doi: 10.1016/0378-5122(95)01024-6. [DOI] [PubMed] [Google Scholar]
- 28.van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int. 2003;14:525–30. doi: 10.1007/s00198-003-1408-1. [DOI] [PubMed] [Google Scholar]
- 29.van der Klift M, de Laet CE, McCloskey EV, Johnell O, Kanis JA, Hofman A, et al. Risk factors for incident vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res. 2004;19:1172–80. doi: 10.1359/JBMR.040215. [DOI] [PubMed] [Google Scholar]
- 30.Anasti JN, Kalantaridou SN, Kimzey LM, Defensor RA, Nelson LM. Bone loss in young women with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1998;91:12–5. doi: 10.1016/s0029-7844(97)00583-8. [DOI] [PubMed] [Google Scholar]
- 31.Ohta H, Sugimoto I, Masuda A, Komukai S, Suda Y, Makita K, et al. Decreased bone mineral density associated with early menopause progresses for at least ten years: cross-sectional comparisons between early and normal menopausal women. Bone. 1996;18:227–31. doi: 10.1016/8756-3282(95)00480-7. [DOI] [PubMed] [Google Scholar]
- 32.Leite-Silva P, Bedone A, Pinto-Neto AM, Costa JV, Costa-Paiva L. Factors associated with bone density in young women with karyotypically normal spontaneous premature ovarian failure. Arch Gynecol Obstet. 2009;280:177–81. doi: 10.1007/s00404-008-0881-3. [DOI] [PubMed] [Google Scholar]
- 33.Popat VB, Calis KA, Vanderhoof VH, Cizza G, Reynolds JC, Sebring N, et al. Bone mineral density in estrogen-deficient young women. J Clin Endocrinol Metab. 2009;94:2277–83. doi: 10.1210/jc.2008-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alzubaidi NH, Chapin HL, Vanderhoof VH, Calis KA, Nelson LM. Meeting the needs of young women with secondary amenorrhea and spontaneous premature ovarian failure. Obstet Gynecol. 2002;99:720–5. doi: 10.1016/s0029-7844(02)01962-2. [DOI] [PubMed] [Google Scholar]
- 35.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 36.Popat VB, Calis KA, Kalantaridou SN, Vanderhoof VH, Koziol D, Troendle JF, et al. Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J Clin Endocrinol Metab. 2014;99:3418–26. doi: 10.1210/jc.2013-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crofton PM, Evans N, Bath LE, Warner P, Whitehead TJ, Critchley HO, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf) 2010;73:707–14. doi: 10.1111/j.1365-2265.2010.03868.x. [DOI] [PubMed] [Google Scholar]
- 38.Cartwright B, Robinson J, Seed PT, Fogelman I, Rymer J. Hormone replacement therapy versus the combined oral contraceptive pill in premature ovarian failure: a randomised controlled trial of the effects on bone mineral density. J Clin Endocrinol Metab. 2016:jc20154063. doi: 10.1210/jc.2015-4063. [DOI] [PubMed] [Google Scholar]
- 39.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–10. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 40.Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016 doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- 41.Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab. 2004;89:3907–13. doi: 10.1210/jc.2004-0015. [DOI] [PubMed] [Google Scholar]
- 42.Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–6. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 43.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52:303–7. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 44.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD, Jr, Roger VL, Melton LJ, 3rd, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lokkegaard E, Jovanovic Z, Heitmann BL, Keiding N, Ottesen B, Pedersen AT. The association between early menopause and risk of ischaemic heart disease: influence of Hormone Therapy. Maturitas. 2006;53:226–33. doi: 10.1016/j.maturitas.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Archer DF. Premature menopause increases cardiovascular risk. Climacteric. 2009;12(Suppl 1):26–31. doi: 10.1080/13697130903013452. [DOI] [PubMed] [Google Scholar]
- 47.van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714–8. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 48.Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD., Jr Premature menopause or early menopause and risk of ischemic stroke. Menopause. 2012;19:272–7. doi: 10.1097/gme.0b013e31822a9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orshan SA, Ventura JL, Covington SN, Vanderhoof VH, Troendle JF, Nelson LM. Women with spontaneous 46,XX primary ovarian insufficiency (hypergonadotropic hypogonadism) have lower perceived social support than control women. Fertil Steril. 2009;92:688–93. doi: 10.1016/j.fertnstert.2008.07.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt PJ, Cardoso GM, Ross JL, Haq N, Rubinow DR, Bondy CA. Shyness, social anxiety, and impaired self-esteem in Turner syndrome and premature ovarian failure. JAMA. 2006;295:1374–6. doi: 10.1001/jama.295.12.1374. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt PJ, Luff JA, Haq NA, Vanderhoof VH, Koziol DE, Calis KA, et al. Depression in women with spontaneous 46, XX primary ovarian insufficiency. J Clin Endocrinol Metab. 2011;96:E278–87. doi: 10.1210/jc.2010-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 53.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–90. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 54.Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529–34. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 55.Zweifel JE, O’Brien WH. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology. 1997;22:189–212. doi: 10.1016/s0306-4530(96)00034-0. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414–20. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 57.Guerrieri GM, Martinez PE, Klug SP, Haq NA, Vanderhoof VH, Koziol DE, et al. Effects of physiologic testosterone therapy on quality of life, self-esteem, and mood in women with primary ovarian insufficiency. Menopause. 2014;21:952–61. doi: 10.1097/GME.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–48. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maki PM, Resnick SM. Effects of estrogen on patterns of brain activity at rest and during cognitive activity: a review of neuroimaging studies. Neuroimage. 2001;14:789–801. doi: 10.1006/nimg.2001.0887. [DOI] [PubMed] [Google Scholar]
- 60.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–95. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 61.LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA. 2001;285:1489–99. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- 62.van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–92. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 63.Hubayter ZR, Popat V, Vanderhoof VH, Ndubizu O, Johnson D, Mao E, et al. A prospective evaluation of antral follicle function in women with 46,XX spontaneous primary ovarian insufficiency. Fertil Steril. 2010;94:1769–74. doi: 10.1016/j.fertnstert.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson LM, Anasti JN, Kimzey LM, Defensor RA, Lipetz KJ, White BJ, et al. Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab. 1994;79:1470–5. doi: 10.1210/jcem.79.5.7962345. [DOI] [PubMed] [Google Scholar]
- 65.Popat VB, Vanderhoof VH, Calis KA, Troendle JF, Nelson LM. Normalization of serum luteinizing hormone levels in women with 46,XX spontaneous primary ovarian insufficiency. Fertil Steril. 2008;89:429–33. doi: 10.1016/j.fertnstert.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tartagni M, Cicinelli E, De Pergola G, De Salvia MA, Lavopa C, Loverro G. Effects of pretreatment with estrogens on ovarian stimulation with gonadotropins in women with premature ovarian failure: a randomized, placebo-controlled trial. Fertil Steril. 2007;87:858–61. doi: 10.1016/j.fertnstert.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 67.Taylor AE, Adams JM, Mulder JE, Martin KA, Sluss PM, Crowley WF., Jr A randomized, controlled trial of estradiol replacement therapy in women with hypergonadotropic amenorrhea. J Clin Endocrinol Metab. 1996;81:3615–21. doi: 10.1210/jcem.81.10.8855811. [DOI] [PubMed] [Google Scholar]
- 68.Check JH, Nowroozi K, Chase JS, Nazari A, Shapse D, Vaze M. Ovulation induction and pregnancies in 100 consecutive women with hypergonadotropic amenorrhea. Fertil Steril. 1990;53:811–6. doi: 10.1016/s0015-0282(16)53514-6. [DOI] [PubMed] [Google Scholar]
- 69.Smith JA, Vitale S, Reed GF, Grieshaber SA, Goodman LA, Vanderhoof VH, et al. Dry eye signs and symptoms in women with premature ovarian failure. Arch Ophthalmol. 2004;122:151–6. doi: 10.1001/archopht.122.2.151. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol. 2002;120:1689–99. doi: 10.1001/archopht.120.12.1689. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan BD, Evans JE, Krenzer KL, Reza Dana M, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab. 2000;85:4866–73. doi: 10.1210/jcem.85.12.7066. [DOI] [PubMed] [Google Scholar]
- 72.Hipp HS, Charen KH, Spencer JB, Allen EG, Sherman SL. Reproductive and gynecologic care of women with fragile X primary ovarian insufficiency (FXPOI) Menopause. 2016;23:993–9. doi: 10.1097/GME.0000000000000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mishell DR, Jr, Nakamura RM, Crosignani PG, Stone S, Kharma K, Nagata Y, et al. Serum gonadotropin and steroid patterns during the normal menstrual cycle. Am J Obstet Gynecol. 1971;111:60–5. doi: 10.1016/0002-9378(71)90927-6. [DOI] [PubMed] [Google Scholar]
- 74.Scarabin PY, Oger E, Plu-Bureau G. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428–32. doi: 10.1016/S0140-6736(03)14066-4. [DOI] [PubMed] [Google Scholar]
- 75.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–5. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 76.Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336:1227–31. doi: 10.1136/bmj.39555.441944.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohammed K, Abu Dabrh AM, Benkhadra K, Al Nofal A, Carranza Leon BG, Prokop LJ, et al. Oral vs Transdermal Estrogen Therapy and Vascular Events: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2015;100:4012–20. doi: 10.1210/jc.2015-2237. [DOI] [PubMed] [Google Scholar]
- 78.Hemelaar M, van der Mooren MJ, Rad M, Kluft C, Kenemans P. Effects of non-oral postmenopausal hormone therapy on markers of cardiovascular risk: a systematic review. Fertil Steril. 2008;90:642–72. doi: 10.1016/j.fertnstert.2007.07.1298. [DOI] [PubMed] [Google Scholar]
- 79.Post MS, Christella M, Thomassen LG, van der Mooren MJ, van Baal WM, Rosing J, et al. Effect of oral and transdermal estrogen replacement therapy on hemostatic variables associated with venous thrombosis: a randomized, placebo-controlled study in postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23:1116–21. doi: 10.1161/01.ATV.0000074146.36646.C8. [DOI] [PubMed] [Google Scholar]
- 80.Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- 81.Sidney S, Cheetham TC, Connell FA, Ouellet-Hellstrom R, Graham DJ, Davis D, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87:93–100. doi: 10.1016/j.contraception.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Langrish JP, Mills NL, Bath LE, Warner P, Webb DJ, Kelnar CJ, et al. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension. 2009;53:805–11. doi: 10.1161/HYPERTENSIONAHA.108.126516. [DOI] [PubMed] [Google Scholar]
- 83.Gibbons WE, Moyer DL, Lobo RA, Roy S, Mishell DR., Jr Biochemical and histologic effects of sequential estrogen/progestin therapy on the endometrium of postmenopausal women. Am J Obstet Gynecol. 1986;154:456–61. doi: 10.1016/0002-9378(86)90690-3. [DOI] [PubMed] [Google Scholar]
- 84.Bjarnason K, Cerin A, Lindgren R, Weber T. Adverse endometrial effects during long cycle hormone replacement therapy. Scandinavian Long Cycle Study Group. Maturitas. 1999;32:161–70. doi: 10.1016/s0378-5122(99)00033-x. [DOI] [PubMed] [Google Scholar]
- 85.Darney PD. The androgenicity of progestins. Am J Med. 1995;98:104S–10S. doi: 10.1016/s0002-9343(99)80067-9. [DOI] [PubMed] [Google Scholar]
- 86.Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1996;275:370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 87.Wildemeersch D, Janssens D, Weyers S. Continuous combined parenteral estrogen substitution and intrauterine progestogen delivery: the ideal HST combination? Maturitas. 2005;51:207–14. doi: 10.1016/j.maturitas.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Wildemeersch D, Andrade A, Goldstuck N. Femilis((R)) 60 Levonorgestrel-Releasing Intrauterine System-A Review of 10 Years of Clinical Experience. Clin Med Insights Reprod Health. 2016;10:19–27. doi: 10.4137/CMRH.S40087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nahoul K, Dehennin L, Scholler R. Radioimmunoassay of plasma progesterone after oral administration of micronized progesterone. J Steroid Biochem. 1987;26:241–9. doi: 10.1016/0022-4731(87)90078-1. [DOI] [PubMed] [Google Scholar]
- 90.Barrett-Connor E, Slone S, Greendale G, Kritz-Silverstein D, Espeland M, Johnson SR, et al. The Postmenopausal Estrogen/Progestin Interventions Study: primary outcomes in adherent women. Maturitas. 1997;27:261–74. doi: 10.1016/s0378-5122(97)00041-8. [DOI] [PubMed] [Google Scholar]
- 91.Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161:249–60. doi: 10.7326/M14-0353. [DOI] [PubMed] [Google Scholar]
- 92.Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab. 1974;39:340–6. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- 93.Wierman ME, Arlt W, Basson R, Davis SR, Miller KK, Murad MH, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489–510. doi: 10.1210/jc.2014-2260. [DOI] [PubMed] [Google Scholar]
- 94.Zuckerman-Levin N, Frolova-Bishara T, Militianu D, Levin M, Aharon-Peretz J, Hochberg Z. Androgen replacement therapy in Turner syndrome: a pilot study. J Clin Endocrinol Metab. 2009;94:4820–7. doi: 10.1210/jc.2009-0514. [DOI] [PubMed] [Google Scholar]
- 95.Elias AN, Pandian MR, Rojas FJ. Serum levels of androstenedione, testosterone and dehydroepiandrosterone sulfate in patients with premature ovarian failure to age-matched menstruating controls. Gynecol Obstet Invest. 1997;43:47–8. doi: 10.1159/000291817. [DOI] [PubMed] [Google Scholar]
- 96.Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR) Reprod Biol Endocrinol. 2011;9:67. doi: 10.1186/1477-7827-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yilmaz N, Uygur D, Inal H, Gorkem U, Cicek N, Mollamahmutoglu L. Dehydroepiandrosterone supplementation improves predictive markers for diminished ovarian reserve: serum AMH, inhibin B and antral follicle count. Eur J Obstet Gynecol Reprod Biol. 2013;169:257–60. doi: 10.1016/j.ejogrb.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Narkwichean A, Maalouf W, Campbell BK, Jayaprakasan K. Efficacy of dehydroepiandrosterone to improve ovarian response in women with diminished ovarian reserve: a meta-analysis. Reprod Biol Endocrinol. 2013;11:44. doi: 10.1186/1477-7827-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hanton L, Axelrod L, Bakalov V, Bondy CA. The importance of estrogen replacement in young women with Turner syndrome. J Womens Health (Larchmt) 2003;12:971–7. doi: 10.1089/154099903322643893. [DOI] [PubMed] [Google Scholar]
- 100.Bakalov VK, Chen ML, Baron J, Hanton LB, Reynolds JC, Stratakis CA, et al. Bone mineral density and fractures in Turner syndrome. Am J Med. 2003;115:259–64. doi: 10.1016/s0002-9343(03)00364-4. [DOI] [PubMed] [Google Scholar]
- 101.Hogler W, Briody J, Moore B, Garnett S, Lu PW, Cowell CT. Importance of estrogen on bone health in Turner syndrome: a cross-sectional and longitudinal study using dual-energy X-ray absorptiometry. J Clin Endocrinol Metab. 2004;89:193–9. doi: 10.1210/jc.2003-030799. [DOI] [PubMed] [Google Scholar]
- 102.Bertelloni S, Cinquanta L, Baroncelli GI, Simi P, Rossi S, Saggese G. Volumetric bone mineral density in young women with Turner’s syndrome treated with estrogens or estrogens plus growth hormone. Horm Res. 2000;53:72–6. doi: 10.1159/000023517. [DOI] [PubMed] [Google Scholar]
- 103.Deniz G, Antoine C, Liebens F, Carly B, Pastijn A, Rozenberg S. Treatment of premature menopause in breast cancer patients. Acta Chir Belg. 2007;107:263–6. doi: 10.1080/00015458.2007.11680053. [DOI] [PubMed] [Google Scholar]
- 104.Nappi RE, Cassani C, Rossi M, Zanellini F, Spinillo A. Dealing with premature menopause in women at high-risk for hereditary genital and breast cancer. Minerva Ginecol. 2016 [PubMed] [Google Scholar]
- 105.Which pill to choose if breastfeeding. Fam Plann Inf Serv. 1978;1:17. [PubMed] [Google Scholar]
- 106.Tankeyoon M, Dusitsin N, Chalapati S, Koetsawang S, Saibiang S, Sas M, et al. Effects of hormonal contraceptives on milk volume and infant growth. WHO Special Programme of Research, Development and Research Training in Human Reproduction Task force on oral contraceptives. Contraception. 1984;30:505–22. doi: 10.1016/0010-7824(84)90001-5. [DOI] [PubMed] [Google Scholar]
- 107.Kelsey JJ. Hormonal contraception and lactation. J Hum Lact. 1996;12:315–8. doi: 10.1177/089033449601200419. [DOI] [PubMed] [Google Scholar]
- 108.van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68:95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]
- 109.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–51. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marchetti C, Iadarola R, Palaia I, di Donato V, Perniola G, Muzii L, et al. Hormone therapy in oophorectomized BRCA1/2 mutation carriers. Menopause. 2014;21:763–8. doi: 10.1097/GME.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 111.Rebbeck TR, Friebel T, Wagner T, Lynch HT, Garber JE, Daly MB, et al. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–10. doi: 10.1200/JCO.2004.00.8151. [DOI] [PubMed] [Google Scholar]
- 112.Svejme O, Ahlborg HG, Nilsson JA, Karlsson MK. Early menopause and risk of osteoporosis, fracture and mortality: a 34-year prospective observational study in 390 women. BJOG. 2012;119:810–6. doi: 10.1111/j.1471-0528.2012.03324.x. [DOI] [PubMed] [Google Scholar]
- 113.Sioka C, Fotopoulos A, Georgiou A, Xourgia X, Papadopoulos A, Kalef-Ezra JA. Age at menarche, age at menopause and duration of fertility as risk factors for osteoporosis. Climacteric. 2010;13:63–71. doi: 10.3109/13697130903075337. [DOI] [PubMed] [Google Scholar]
- 114.de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339–45. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 115.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162:1089–97. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 116.Lobo RA. What the future holds for women after menopause: where we have been, where we are, and where we want to go. Climacteric. 2014;17(Suppl 2):12–7. doi: 10.3109/13697137.2014.944497. [DOI] [PubMed] [Google Scholar]


