SUMMARY
The Hippo pathway is important for regulating tissue homeostasis and its dysregulation has been implicated in human cancer. However, it is not well understood how the Hippo pathway becomes dysregulated because few mutations in core Hippo pathway components have been identified. Therefore, much work in the Hippo field has focused on identifying upstream regulators, and a complex Hippo interactome has been identified. Nevertheless, it is not always clear which components are the most physiologically relevant in regulating YAP/TAZ. To provide an overview of important Hippo pathway components, we created knockout cell lines for many of these components and compared their relative contributions to YAP/TAZ regulation in response to a wide range of physiological signals. By this approach, we provide an overview of the functional importance of many Hippo pathway components and demonstrate NF2 and RHOA as important regulators of YAP/TAZ and TAOK1/3 as direct kinases for LATS1/2.
Graphical abstract
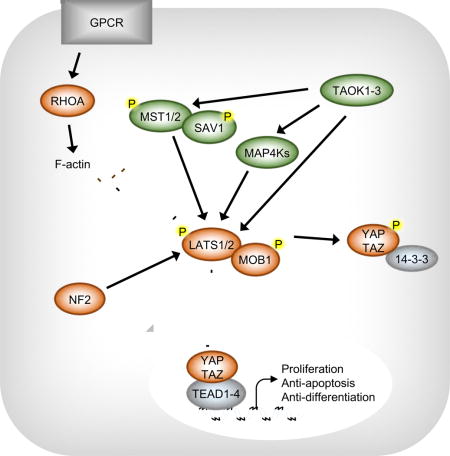
INTRODUCTION
The Hippo pathway is a well-established regulator of tissue homeostasis (Yu et al., 2015b). The mammalian Hippo pathway consists of a kinase cascade of Mammalian STE20-like 1/2 (MST1/2) and Large Tumor Suppressor 1/2 (LATS1/2), which inhibit the primary effectors of the Hippo pathway, Yes Associated Protein (YAP) and WW Domain-containing Transcription Factor (TAZ). When the Hippo pathway is activated, MST1/2 phosphorylates its adaptor protein Salvador 1 (SAV1), which facilitates MST1/2-LATS1/2 interaction (Callus et al., 2006; Tapon et al., 2002). MST1/2 then phosphorylates LATS1/2 at its hydrophobic motif (HM: threonines 1079 on LATS1 and 1041 on LATS2), which promotes LATS1/2 auto-phosphorylation at its activation loop. MST1/2 also phosphorylates MOB Kinase Activator 1A/B (MOB1A/B) at threonine 35, enabling MOB1A/B to bind the auto-inhibitory region of LATS1/2 and promote full LATS1/2 activation (Chan et al., 2005; Praskova et al., 2008). Once activated, LATS1/2 can directly phosphorylate YAP/TAZ.
LATS1/2-dependent phosphorylation of YAP serine 127 results in YAP sequestration in the cytoplasm by binding to 14-3-3, ubiquitination, and degradation (Dong et al., 2007; Liu et al., 2010; Zhao et al., 2010b; Zhao et al., 2007). LATS1/2 also regulate TAZ protein localization and stability in a similar manner, although phosphorylation of TAZ occurs on different residues and TAZ is more unstable due to an additional phosphodegron. Dephosphorylated YAP/TAZ translocate to the nucleus where they act as transcriptional co-activators, interacting with transcription factors to induce expression of genes regulating cell proliferation, apoptosis, and differentiation (Zhao et al., 2008).
Disrupting the Hippo pathway results in the loss of tissue homeostasis. For example, deleting Hippo or Warts (the Drosophila homologs of MST1/2 and LATS1/2, respectively) is sufficient to cause aberrant Yorki (the Drosophila homolog of YAP/TAZ) activity and uncontrolled growth in both eye and wing (Huang et al., 2005; Pan, 2010). Similarly, conditionally deleting MST1/2 or LATS1/2 in the mouse liver results in YAP/TAZ accumulation and massive hepatomegaly and tumors (Chen et al., 2015; Yu et al., 2015a).
Not surprisingly, dysregulation of the Hippo pathway has been implicated in many human diseases (Plouffe et al., 2015). YAP amplification and increased YAP/TAZ nuclear localization have been correlated with an increased risk of metastasis and decreased survival in lung, colorectal, and breast cancers, to name a few (Wang et al., 2012; Wang et al., 2010; Wierzbicki et al., 2013). However, the mechanisms by which the Hippo pathway becomes dysregulated are not fully understood; few mutations in core Hippo pathway components have been identified in human cancers (Harvey et al., 2013).
Therefore, much work has focused on identifying upstream regulators of the Hippo pathway which may contribute to aberrant YAP/TAZ activity in disease. Several studies recently identified the Mitogen-Activated Protein Kinase kinase kinase kinase (MAP4K) family as direct activators of LATS1/2, acting in parallel to MST1/2 (Li et al., 2014a; Meng et al., 2015; Zheng et al., 2015). Other work has greatly expanded the Hippo interactome to include Ras Association Domain Family Member 1A (RASSF1A), Tao Kinases 1–3 (TAOK1/2/3), AMPK (PRKKA1/PRKKA2), Protein Kinase A (PRKACA/PRKCB), Ras Homology Family Member A (RHOA), Neurofibromin 2 (NF2), Angiomotin (AMOT), Catenin Alpha 1 (CTNNA1), and Ajuba Lim Protein (AJUBA) (Figure 1A). While the functions of these components in regulating the Hippo pathway have been well studied by either knockdown or knockout, most studies have focused only on individual components, emphasizing only the importance of the component of interest to that particular study. Thus, it is not always clear how the contribution of each component compares relative to the others, nor which are the most physiologically relevant in regulating YAP/TAZ. To promote a fuller understanding of the Hippo pathway, we created knockout cell lines for each of these components in HEK293A cells using CRISPR/Cas9 and compared their relative contributions in regulating YAP/TAZ phosphorylation and localization. Our study provides an overarching view of known Hippo pathway components in YAP/TAZ regulation in response to a wide range of physiological signals and identifies components which, when deleted, are sufficient to cause significant YAP/TAZ dysregulation.
Figure 1. Using CRISPR to target the Hippo pathway.
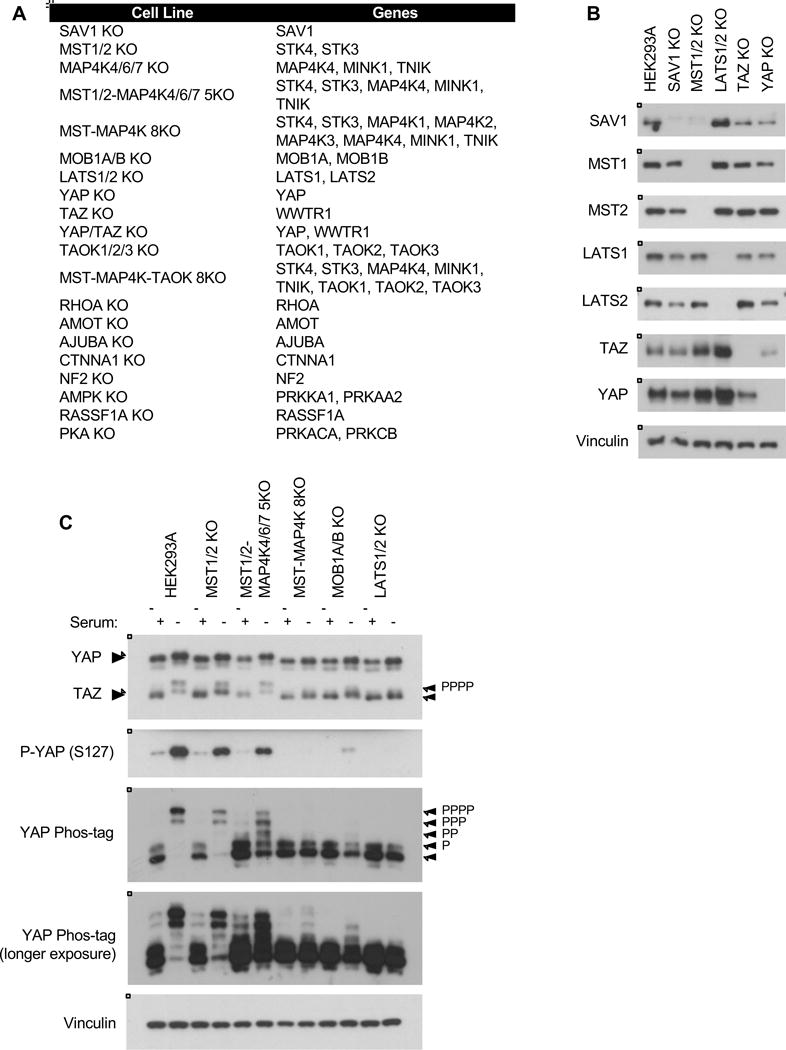
A. List of cell lines created and genes deleted in this study. See Table S1 and Figure S1 for sequences and immunoblots. B. Immunoblots showing CRISPR-mediated deletion of core Hippo pathway components. C. Overnight serum starvation induces YAP/TAZ phosphorylation and degradation in wild-type HEK293A cells. See Figure S2 for quantification. See Figure S7 for a schematic of the Hippo pathway.
RESULTS
Role of Hippo pathway components in regulating YAP/TAZ phosphorylation in response to serum starvation
First, to demonstrate that this approach is sufficient to identify the most critical regulators of YAP/TAZ, we generated knockout cell lines of the Hippo pathway core components (Figures 1A–1B, S1, S7, and Table S1). One of the most robust signals to regulate YAP/TAZ activity is serum; lysophosphatidic acid (LPA) and sphingosine 1-phosphate in serum act through G-protein coupled receptors (GPCRs) to inactivate the Hippo pathway (Miller et al., 2012; Yu et al., 2012). When the Hippo pathway is inactive, YAP/TAZ are dephosphorylated and translocate to the nucleus to induce transcription. During serum starvation, the Hippo pathway is activated and YAP/TAZ are phosphorylated and sequestered in the cytoplasm. YAP/TAZ phosphorylation can be detected by phosphorylation-specific antibodies and by mobility shift (Figure 1C).
As previously reported, deleting either MST1/2 or the MAP4K family alone did not significantly disrupt YAP/TAZ regulation, but deleting both MST1/2 and the MAP4Ks compromised YAP/TAZ phosphorylation in response to serum starvation (Figure 1C and S2). Deleting LATS1/2 blocked nearly all YAP/TAZ phosphorylation, and deleting MOB1A/B severely compromised YAP/TAZ phosphorylation as well. The effect of deleting MOB1A/B was more dramatic than deleting MST1/2 but less than LATS1/2, as trace levels of phosphorylated YAP (S127) were still present in the MOB1A/B KO cells (Figure 1C). These results demonstrate that LATS1/2 has some intrinsic activity to phosphorylate YAP/TAZ independent of MOB1A/B, although MOB1A/B is necessary to promote full LATS1/2 activation (Chan et al., 2005; Praskova et al., 2008). This is consistent with reported animal studies which found that deletion of MOB1A and a homozygous-null mutation in MOB1B resulted in increased YAP activity and hyperproliferation (Nishio et al., 2012). In the absence of MOB1A/B, the cell is unable to fully activate LATS1/2 to inhibit YAP/TAZ.
While the YAP mobility shift by phos-tag is consistent with YAP (S127) phosphorylation, the phos-tag also provides more quantitative information for all YAP phosphorylation sites (Figure 1C). For this reason we used phos-tag as the primary readout to compare overall YAP phosphorylation for subsequent experiments.
To better compare the contribution of each component, we performed a time course of serum starvation (Figure 2 and S3). Upon starvation, YAP phosphorylation occurred rapidly after 15–30 minutes, with no observed intermediate bands of partially-phosphorylated YAP in the wild-type cells. This suggests that virtually all YAP sites undergo phosphorylation in all cells synchronously, revealing an extremely tight regulation of YAP phosphorylation. TAZ phosphorylation also occurred after 15–30 minutes, although phosphorylation of TAZ was less efficient (Figure 2A); TAZ was only partially phosphorylated after 90 minutes of starvation, indicating that endogenous YAP is more efficiently phosphorylated than TAZ. TAZ phosphorylation was also confirmed by phos-tag (Figure S2C–E).
Figure 2. YAP/TAZ phosphorylation in response to serum starvation.
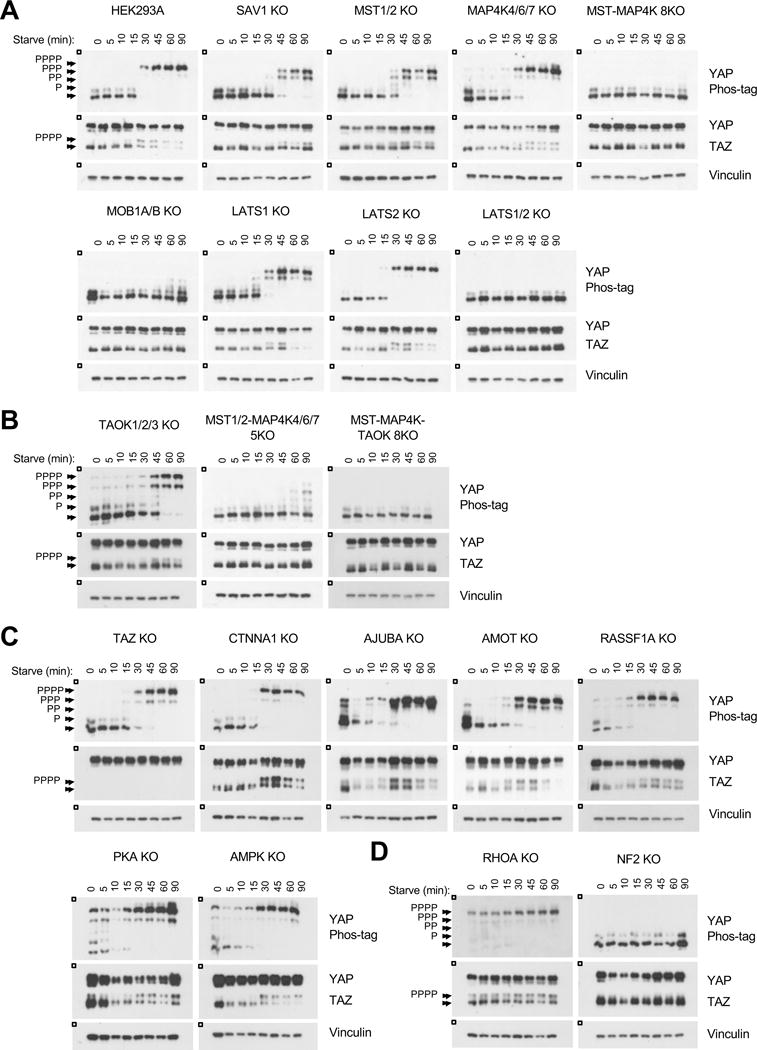
A–D. Immunoblots showing YAP/TAZ phosphorylation status following serum starvation. The HEK293A cells in A are the relevant control for all panels; the figure is subdivided for readability. See Figure S3 for quantification.
Deleting SAV1 or MST1/2 resulted in a significant delay of YAP/TAZ phosphorylation compared to the wild type cells, although interestingly, the SAV1 KO cells showed a greater delay in YAP/TAZ phosphorylation than the MST1/2 KO cells (Figure 2A and S3A). In addition, even after 90 minutes of starvation, YAP was not fully phosphorylated in either cell line. We next compared the effect of deleting LATS1 or LATS2. YAP phosphorylation was modestly compromised in the LATS1 KO cells but unaffected in the LATS2 KO cells. However, deleting both LATS1/2 abolished YAP/TAZ phosphorylation. This response could be rescued by re-expressing LATS1/2 (Moroishi et al., 2015). As previously discussed, the MST1/2-MAP4K1/2/3/4/6/7 8KO (MST-MAP4K 8KO), MOB1A/B KO, and LATS1/2 KO cells all exhibited severe defects in starvation-induced YAP/TAZ phosphorylation; without these core components, the cell was unable to inactivate YAP/TAZ. Together, the above indications confirm the utility of this approach to compare and identify Hippo pathway components which are the most physiologically important in regulating YAP/TAZ.
TAOK has been reported to phosphorylate and activate MST1/2 (Boggiano et al., 2011; Poon et al., 2011). We attempted deleting TAOK1/2/3. Deletion of TAOK1/2 was confirmed by immunoblot and sequencing (Figure S1A, S1W–X). However, we were unable to verify complete deletion of TAOK3 due to the relatively low quality of the TAOK3 antibody (Figure S1J). Sequencing the sgRNA target site was hindered due to multiple genomic copies of TAOK3 in HEK293A cells. Nevertheless, the TAOK1/2/3 KO cell lines showed a significant delay in YAP/TAZ phosphorylation upon serum starvation, similar to that of the MST1/2 KO cells (Figure 2B and S3B). Deleting TAOK1/2/3 on top of the MST1/2-MAP4K4/6/7 5KO cells resulted in complete YAP/TAZ dephosphorylation, while the MST1/2-MAP4K4/6/7 5KO cells retained some YAP phosphorylation. This data suggests that TAOK1/2/3 may have additional activity independent of MST1/2 to activate LATS1/2, which will be further examined in Figure 6.
Figure 6. TAOK1/3 are direct kinases for LATS1/2.
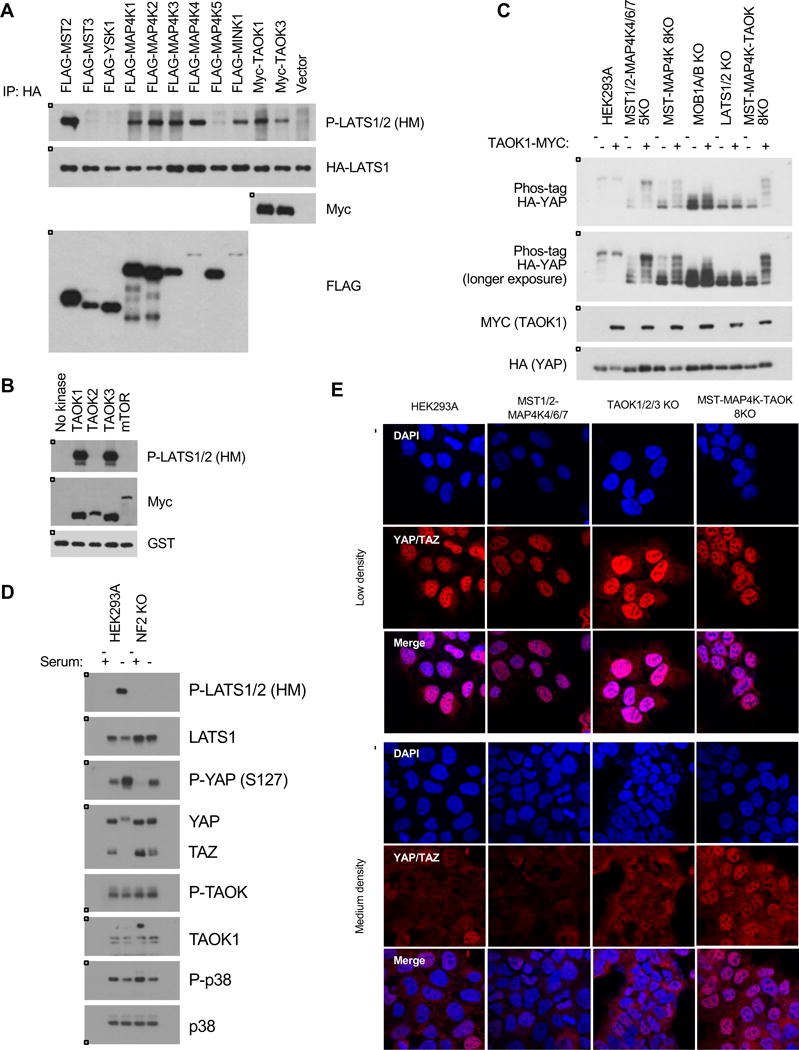
A. Overexpression of TAOK1/3 induces LATS1/2 (HM) phosphorylation in wild-type cells. Cells were transfected with HA-LATS1 and various kinases including MST2, TAOK1, and TAOK3, and HA-LATS1 was immunoprecipitated and immunoblotted for HM phosphorylation. B. TAOK1/3 can directly phosphorylate LATS1/2 (HM) in an in vitro kinase assay. TAOK1, TAOK2, and TAOK3 were transfected into wild-type cells and immunoprecipitated, and an in vitro kinase assay was performed with a truncated form of LATS1 (AA 638–1,130). C. Overexpression of TAOK1 induces YAP phosphorylation in the MST-MAP4K 8KO but not the LATS1/2 KO cells. Cells were seeded at 0.7 × 105 cells/well in a 6-well plate, transfected with TAOK1-MYC, and harvested 24 hours later. D. TAOK kinase activity is unaffected in the NF2 KO cells. P38 is a downstream target of TAOK. E. The MST1/2-MAP4K-TAOK 8KO cells are resistant to cell-cell contact. Immunofluorescence staining for YAP/TAZ (red) and DAPI (blue) at low and medium densities. See Figure S4D for quantification.
The CTNNA1 and TAZ KO cells showed no difference in YAP phosphorylation relative to the wild-type cells. The AJUBA, AMOT, and RASSF1A KO cells were more sensitive to serum starvation (Figure 2C and S3C). The AJUBA KO cells phosphorylated YAP within 10 minutes of starvation, and the AMOT and RASSF1A KO cells showed phosphorylated YAP within 15 minutes of starvation. AJUBA is localized at adherens junctions and interacts with SAV1 and LATS1/2 to promote YAP/TAZ phosphorylation, so its role in response to serum starvation is not clear (Das Thakur et al., 2010). The contribution of AMOT to the Hippo pathway is also not well understood. Although AMOT can induce LATS2-mediated phosphorylation of YAP and sequester YAP/TAZ to tight junctions (Chan et al., 2011; Paramasivam et al., 2011; Wang et al., 2011; Zhao et al., 2010a), AMOT is also reported to activate YAP by binding YAP in the cytoplasm to prevent LATS1/2-mediated phosphorylation and to promote transcription (Yi et al., 2013). Moreover, AMOT binds to and activates NF2 (Li et al., 2015). Here, in response to serum starvation, the AMOT KO cells were slightly more sensitive but overall did not show much difference from the wild-type cells. However, Angiomotin family members AMOTL1 and AMOT2 remained intact in the AMOT KO cells. Because they are closely related, AMOTL1 and AMOTL2 may be functionally redundant with AMOT, which may explain why we did not observe much change in the AMOT KO cells (Chan et al., 2011; Zhao et al., 2010a). RASSF1A acts upstream of MST1/2, but the observation that YAP/TAZ were highly phosphorylated in the RASSF1A KO cells indicates that the RASSF1A contribution to the Hippo pathway in response to serum starvation is not indispensable.
Surprisingly, the AMPK and PKA KO cells had higher basal levels of phosphorylated YAP even in the presence of serum (Figure 2C and S3C). AMPK and PKA are known to promote YAP phosphorylation. Thus, it was unexpected that deleting AMPK or PKA would result in higher basal levels of phosphorylated YAP, although this may reflect a cellular adaptation.
Of all the components tested, NF2 and RHOA showed the most significant, though contrasting, dysregulation of YAP/TAZ. In the NF2 KO cells, YAP/TAZ remained dephosphorylated following serum starvation, while YAP/TAZ were fully phosphorylated in the RHOA KO cells even in the presence of serum (Figure 2D and S3D). These data demonstrate that NF2 and RHOA play dominant roles in LATS1/2 regulation, NF2 in positively regulating LATS1/2 activity and RHOA in negatively regulating LATS1/2 activity. These will be further examined in Figures 5 and 7.
Figure 5. Deletion of NF2 results in hyper-activated YAP/TAZ.
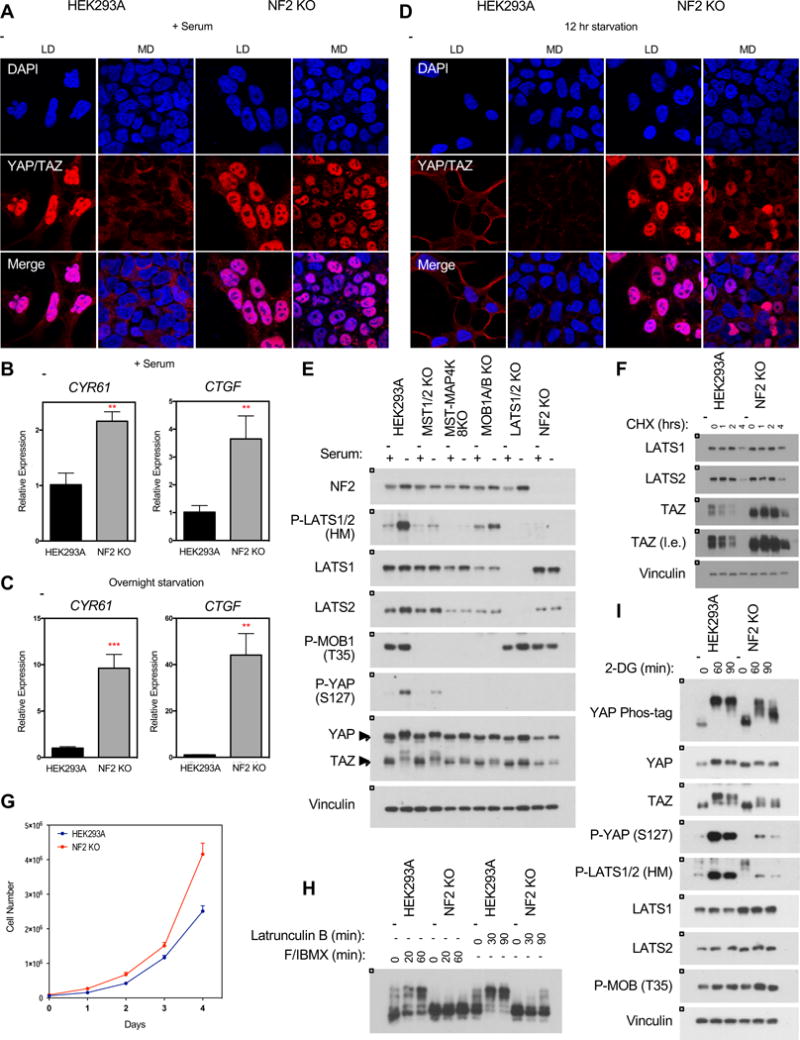
A. Immunofluorescence staining for YAP/TAZ (red) and DAPI (blue) in wild-type and NF2 KO cells at low (LD) and medium (MD) densities in the presence of serum. See Figure S4C for quantification. B and C. Relative expression of CYR61 and CTGF in the presence of serum (B) or following overnight serum starvation (C), as quantified by qPCR. Data represented as mean +/− S.D. D. Immunofluorescence staining for YAP/TAZ (red) and DAPI (blue) in wild-type and NF2 KO cells at low and medium densities following 12 hour serum starvation. See Figure S4C for quantification. E. Deletion of NF2 prevents LATS1/2 (HM) phosphorylation in response to overnight serum starvation. F. Protein stability of LATS1/2 is not affected in NF2 KO cells following treatment with 100 ug/ml cycloheximide. G. Deletion of NF2 confers a growth advantage in HEK293A cells. Cells were plated at 7 × 104 cells/well in a 6-well plate with fresh media and counted after 0, 24, 48, 72, and 96 hours. Data represented as mean +/− S.D. H. NF2 KO cells are sensitive to actin disruption by Latrunculin B. See Figure S5 for YAP/TAZ phosphorylation response of other cell lines. I. NF2 KO cells are sensitive to cellular energy stress.
Figure 7. RHOA is an important mediator of growth signals to activate YAP/TAZ.
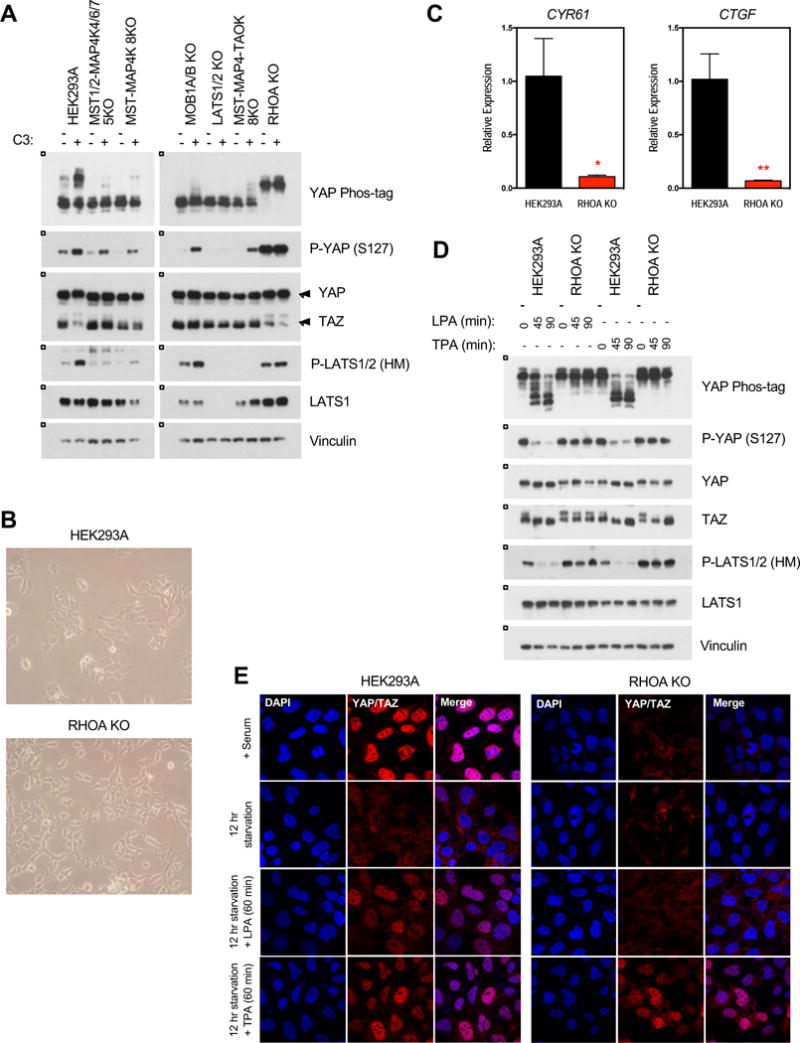
A. Treatment with the Rho inhibitor C3 exoenzyme (C3) induces YAP/TAZ phosphorylation. Cells were treated with C3 (1 ug/ml) for 4 hours. B. The RHOA KO cells showed altered morphology compared to the wild-type cells. C. Relative expression of CYR61 and CTGF in the presence of serum, as quantified by qPCR. Data represented as mean +/− S.D. D. LPA fails to induce YAP/TAZ dephosphorylation while TPA can induce TAZ dephosphorylation in the RHOA KO cells. Cells were starved overnight before treatment with LPA (0.5 uM) or TPA (5 nM). See Figure S6 for YAP/TAZ phosphorylation response of other cell lines. E. Immunofluorescence staining for YAP/TAZ (red) and DAPI (blue) in the HEK293A wild-type and RHOA KO cells. See Figure S4E for quantification.
Role of Hippo pathway components in regulating YAP/TAZ localization and transcriptional activity in response to serum starvation
Phosphorylated YAP/TAZ are sequestered in the cytoplasm by binding to 14-3-3, while dephosphorylated YAP/TAZ translocate to the nucleus to induce transcription. YAP/TAZ protein localization was examined by immunofluorescence and found to be consistent with the observed YAP/TAZ phosphorylation status. For all cell lines shown, in the presence of serum, YAP/TAZ were nuclear (Figure 3A and S4A). Following prolonged serum starvation, YAP/TAZ were primarily cytoplasmic in the wild-type, SAV1 KO, MST1/2 KO, and MAP4K4/6/7 KO cells. Weak YAP/TAZ staining following starvation was due to YAP/TAZ degradation and diffuse cytoplasmic localization. However, in the MST-MAP4K 8KO, MOB1A/B KO, and LATS1/2 KO cells, YAP/TAZ remained nuclear even following prolonged serum starvation. These data further demonstrate that, without these core components, the cell is unable to sequester and inactivate YAP/TAZ in the cytoplasm.
Figure 3. Dysregulation of YAP/TAZ phosphorylation results in aberrant YAP/TAZ localization and transcriptional activity.
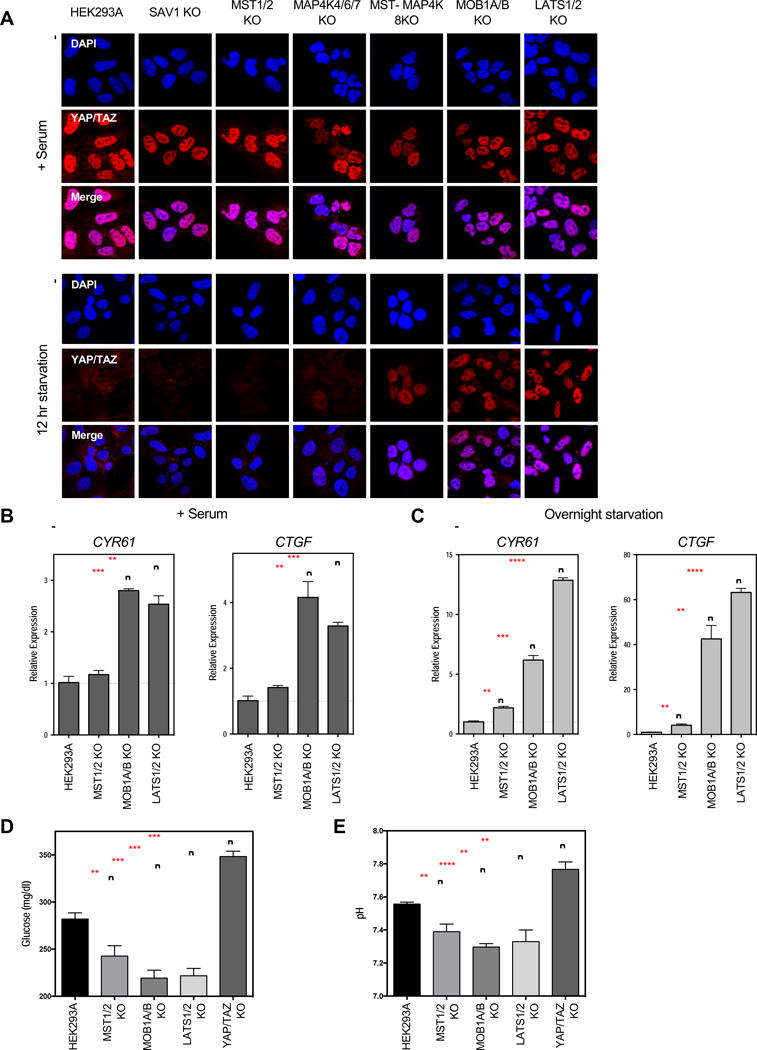
A. Immunofluorescence staining for YAP/TAZ (red) and DAPI (blue) in the presence of serum or following 12 hours of serum starvation. See Figure S4A for quantification. B and C. Relative expression of YAP/TAZ downstream target genes CYR61 and CTGF in the presence of serum (B), or following overnight serum starvation (C), as quantified by qPCR. Data represented as mean +/−S.D. D and E. Glucose levels (D) and pH (E) of the culture media following a 6 hour incubation under normal culture conditions. Data represented as mean +/− S.D.
Dysregulation of YAP/TAZ phosphorylation and localization is sufficient to drive changes in YAP/TAZ transcriptional activity. Cysteine Rich Angiogenic Inducer 61 (CYR61) and Connective Tissue Growth Factor (CTGF) are two well-established target genes induced by YAP/TAZ (Zhao et al., 2008). Even in the presence of serum, MOB1A/B KO and LATS1/2 KO cells had higher CYR61 and CTGF expression than the wild-type cells (Figure 3B). This was consistent with previous observations that, even in the presence of serum, YAP/TAZ are not completely dephosphorylated in the wild-type cells (Figure 1C). This difference became more pronounced following serum starvation (Figure 3C). Following starvation, CYR61 and CTGF expression were strongly reduced in the wild-type cells as YAP/TAZ were sequestered in the cytoplasm. However, YAP/TAZ remained nuclear in the MOB1A/B and LATS1/2 KO cells and CYR61 and CTGF were not repressed. The low level of MOB1A/B-independent LATS1/2 activity towards YAP/TAZ may explain why expression of CYR61 and CTGF expression were greater in the LATS1/2 KO cells than the MOB1A/B KO cells.
We next examined the effect of dysregulated YAP/TAZ on cellular metabolism. Rapidly growing cells metabolize glucose in the culture medium and release lactic acid via glycolysis, which lowers the medium’s pH. To compare the relative rates of metabolism across the different cell lines, cells were given fresh media for 6 hours, after which we measured the culture medium’s remaining glucose levels and pH (Figure 3D and 3E). YAP/TAZ KO cells had a lower metabolic rate than the wild-type cells, as indicated by their higher culture medium’s higher glucose levels and pH. These results support that YAP/TAZ activity stimulates glucose uptake and glycolysis, consistent with the cell growth-promoting activity of YAP/TAZ. The MST1/2 KO cells had lower glucose levels and pH than the wild-type cells, consistent with a negative role for MST1/2 in YAP/TAZ regulation. The MOB1A/B and LATS1/2 KO cells had even lower glucose levels and pH, which is consistent with their increased YAP/TAZ transcriptional activity. Together, these data indicate that aberrant Hippo pathway activity is sufficient to cause metabolic changes in the cell.
Cells were additionally treated with Latrunculin B to disrupt the actin cytoskeleton and Forskolin/3-isobutyl-1-methylxanthine (F/IBMX) to activate PKA. Consistent with previous studies, Latrunculin B and F/IBMX activate the Hippo pathway and induce YAP phosphorylation (Figure S5A and S5B). Even under these conditions, YAP/TAZ remained dephosphorylated in the MST-MAP4K 8KO, MOB1A/B KO, and LATS1/2 KO cells.
Energy starvation is also known to induce YAP/TAZ phosphorylation. We used 2-Deoxy-D-glucose (2-DG), which inhibits glucose metabolism, to induce cellular energy stress. Deletion of SAV1, MST1/2, or MAP4K4/6/7 did not abolish 2-DG-induced YAP/TAZ phosphorylation (Figure S5C). Notably, in these cells, YAP/TAZ phosphorylation peaked after 60 minutes of 2-DG treatment before declining, while no such decline was evident in the wild-type cells. This indicates that the dynamics of the cellular energy stress response are altered in these KO cells. In the MST1/2-MAP4K4/6/7 KO, MST-MAP4K 8KO, MOB1A/B KO, and LATS1/2 KO cells, 2-DG induced partial but not full YAP phosphorylation. This slight upward shift was likely due to LATS1/2-independent YAP phosphorylation by AMPK (Mo et al., 2015; Wang et al., 2015). However, when AMPK was deleted, YAP/TAZ were still fully phosphorylated in response to energy stress, suggesting an AMPK-independent mechanism of LATS1/2 activation. Deletion of PKA partially blocked the effect of 2-DG on YAP phosphorylation. Although YAP was already highly phosphorylated in the RHOA KO cells, phosphorylation of TAZ was still clearly induced in the RHOA KO cells, indicating that RHOA deletion does not block the energy stress response. Collectively, our data indicate that many upstream components may play a role in regulating YAP/TAZ in response to cellular energy stress.
Different mechanisms may regulate YAP/TAZ in response to cell-cell contact than serum starvation
The Hippo pathway is also strongly regulated by cell-cell contact. In response to cell-cell contact, the Hippo pathway is activated and YAP/TAZ are phosphorylated and inactivated to prevent further growth and proliferation. To test the mechanisms of YAP regulation in response to cell-cell contact, cells were plated at three densities and YAP phosphorylation status was examined (Figure 4A). When comparing the core Hippo pathway component knockout cells, high density induced YAP/TAZ phosphorylation in all except the LATS1/2 KO cells (Figure 4B and 4C). Intriguingly, density-induced YAP phosphorylation was significantly compromised in the MST1/2-MAP4K4/6/7 KO, MST-MAP4K 8KO, and MOB1A/B KO cells, although these cells were still clearly able to phosphorylate YAP at high density. YAP/TAZ protein localization was also consistent with these observations (Figure 4D and S4B). At low density, YAP/TAZ were nuclear in all cells. At medium density in the wild-type, SAV1 KO, MST1/2 KO, MAP4K4/6/7 KO, and MST-MAP4K 8KO cells, YAP/TAZ were cytoplasmic. However, YAP/TAZ showed partial and complete nuclear localization in the MOB1A/B KO and LATS1/2 KO cells, respectively.
Figure 4. Inactivation of YAP/TAZ in response to cell-cell contact.
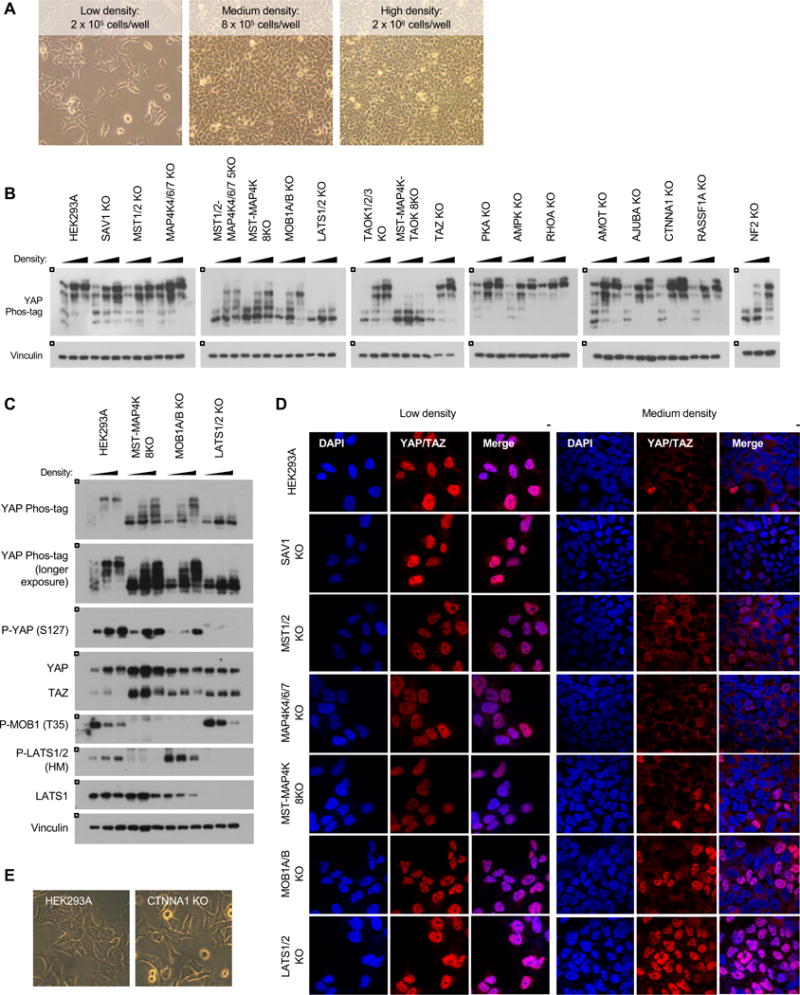
A. Cells were plated at low, medium, and high densities in a 6-well plate. Images show wild-type cells at each of the respective densities. B. Cell-cell contact induces YAP phosphorylation. Cells were plated at each of the respective densities and harvested 24 hours later. C. YAP/TAZ phosphorylation in the wild-type, MST-MAP4K 8KO, MOB1A/B KO, and LATS1/2 KO cells in response to cell-cell contact. D. Immunofluorescence staining for YAP/TAZ (red) and DAPI (blue) at low and medium densities. See Figure S4B for quantification. E. Images show cellular morphologies of wild-type and CTNNA1 KO cells. Cells were plated at low density and images were taken 24 hours later.
From the above data, it is clear that the signal transduction regulating YAP/TAZ in response to cell-cell contact is either different or more severe than those in response to serum starvation. It is important to note that cells grown at high density may also experience limited nutrients, although we already observed that even overnight starvation is not sufficient to induce YAP phosphorylation in the MST-MAP4K 8KO or MOB1A/B KO cells (Figure 1C). However, LATS1/2 are the primary kinases for YAP/TAZ in response to cell-cell contact because deleting LATS1/2 abolished YAP/TAZ phosphorylation and cytoplasmic localization, even at high density.
Besides the LATS1/2 KO cells, the only cell line which showed dramatic disruption of YAP/TAZ phosphorylation was the MST1/2-MAP4K4/6/7-TAOK1/2/3 8KO (MST-MAP4K-TAOK 8KO) cells (Figure 4B). Although trace levels of phosphorylated YAP were present, the majority of YAP remained dephosphorylated even at high density. This was a striking difference when compared to both the TAOK1/2/3 KO and MST1/2-MAP4K4/6/7 KO lines. These data again indicate that TAOK1/2/3 must have additional activity apart from acting upstream of MST1/2 or the MAP4K family.
Many components have been implicated in regulating LATS1/2 kinase activity or YAP/TAZ localization in response to cell-cell contact, including AJUBA, CTNNA1, and AMOT. However, each of these KO cells showed strong YAP phosphorylation at both medium and high densities (Figure 4B). Notably, the NF2 KO cells showed reduced phosphorylation, particularly at medium density, in support of previous reports that NF2 is an important mediator for cell-cell contact-induced YAP/TAZ phosphorylation (Yin et al., 2013). Nevertheless, our data clearly show NF2-independent activation of the Hippo pathway as YAP was still highly phosphorylated at high density in the NF2 KO cells. Deleting some cell junction-associated proteins resulted in altered cellular morphology, which may also influence the Hippo pathway. CTNNA1 is required for adherens junctions, and the CTNNA1 KO cells appeared incapable of forming adherens junctions (Figure 4E). Nevertheless, the CTNNA1 KO cells remained sensitive to cell-cell contact, which could be due to other cell-cell junctions or changes in cell shape at higher densities.
NF2 knockout cells have hyper-activated YAP/TAZ
NF2 is a well-established tumor suppressor and is often mutated in neurofibromatosis and mesothelioma. In patients with neurofibromatosis type 2, inactivating mutations in NF2 have been correlated with increased YAP expression and nuclear localization (Schulz et al., 2014; Striedinger et al., 2008). As discussed previously, deleting NF2 severely compromised YAP/TAZ phosphorylation in response to serum starvation (Figure 2D). Accordingly, serum starvation also induced YAP/TAZ cytoplasmic localization in wild-type cells but not the NF2 KO cells (Figure 5A and S4C). This dysregulation of YAP/TAZ phosphorylation and localization was consistent with the increased expression of YAP/TAZ target genes CYR61 and CTGF in the NF2 KO cells (Figure 5B and 5C). The NF2 KO cells were also resistant to YAP/TAZ cytoplasmic localization when grown at increased density in the presence of serum. However, the combination of increased density and prolonged serum starvation was able to induce YAP/TAZ cytoplasmic localization in the NF2 KO cells (Figure 5D and S4C). Thus, while YAP/TAZ seem largely resistant to regulation when NF2 is deleted, in response to stronger signals (such as the combination of increased density and prolonged serum starvation), the cell is able to override loss of NF2 and inactivate YAP/TAZ. Re-expressing NF2 was sufficient to rescue YAP phosphorylation (Moroishi et al., 2015).
NF2 has been reported to promote LATS1/2 activation by inhibiting LATS1/2 ubiquitination and degradation in the nucleus (Li et al., 2014b). We found that LATS1 protein levels were relatively unchanged in the NF2 KO cells compared to the wild-type cells, although LATS2 expression was decreased slightly (Figure 5E). Nor did we observe any difference in LATS1/2 protein stability following treatment with the translation-inhibitor cycloheximide (Figure 5F). These data suggest that, at least in HEK293A cells, NF2 affects LATS1/2 localization or kinase activity but not protein stability. MST1/2 are direct kinases for MOB1A/B and LATS1/2, and deleting MST1/2 was sufficient to disrupt all MOB1A/B phosphorylation. However, deleting NF2 dramatically reduced LATS1/2 (HM) phosphorylation but had no effect on MOB1A/B phosphorylation (Figure 5E), suggesting that NF2 KO does not affect MST1/2 kinase activity but affects MST1/2-dependent phosphorylation of LATS1/2 by potentially altering LATS1/2 localization.
In addition to increased transcription of YAP/TAZ target genes, deleting NF2 confers a growth advantage as well; NF2 KO cells proliferated at a faster rate than the wild-type cells (Figure 5G). Nevertheless, in addition to combined density and prolonged starvation, there remained some conditions in which YAP/TAZ could be weakly regulated in the NF2 KO cells. Actin disruption by Latrunculin B was able to induce weak YAP phosphorylation (Figure 5H), and the NF2 KO cells showed altered response to energy stress. Although 2-DG treatment induced some YAP/TAZ phosphorylation after 60 minutes, the YAP/TAZ phosphorylation in the NF2 KO cells appeared less robust and recovered quicker than the wild-type cells, as significant YAP dephosphorylation was present after 90 minutes of 2-DG treatment (Figure 5I). Furthermore, phosphorylation of LATS1/2 (HM) and YAP (S127) remained severely compromised in the NF2 KO cells, supporting a critical role for NF2 in Hippo pathway regulation.
TAOK1/3 act upstream of MST1/2 and MAP4K to phosphorylate and activate LATS1/2
In Drosophila, Tao kinase 1 (Tao-1) directly phosphorylates Hippo, and the mammalian TAOK1 can phosphorylate and activate MST2 in vitro (Boggiano et al., 2011; Poon et al., 2011). Deleting TAOK1/2/3 had a slight effect on YAP/TAZ phosphorylation in response to serum starvation, as YAP/TAZ phosphorylation was delayed and less robust than in the wild-type cells (Figure 2B). However, as discussed previously, deleting TAOK1/2/3 in the MST1/2-MAP4K4/6/7 5KO cells almost completely abolished YAP/TAZ phosphorylation in response to serum starvation and cell-cell contact (Figure 2B and 4B). These data suggest that TAOK1/2/3 may also act in parallel to MST1/2 and MAP4K4/6/7 to activate LATS1/2 and induce YAP/TAZ phosphorylation. Consistently, overexpressing TAOK1 was sufficient to induce LATS1/2 (HM) phosphorylation (Figure 6A). In addition, TAOK1 and TAOK3 directly phosphorylated LATS1/2 (HM) in an in vitro kinase assay (Figure 6B). Therefore, TAOK1/3 may act both upstream of and in parallel to MST1/2 and MAP4K to phosphorylate and activate LATS1/2. To test this, we overexpressed TAOK1 in various knockout cell lines. Overexpressing TAOK1 was sufficient to induce YAP phosphorylation even in the MST1/2-MAP4K4/6/7 5KO and MST-MAP4K 8KO cells, but not the MOB1A/B KO or LATS1/2 KO cells, thereby confirming that MST1/2 and the MAP4K family are not required for TAOK1 to induce YAP phosphorylation (Figure 6C). We further examined the effect of TAOK on YAP/TAZ localization. Density-induced YAP/TAZ cytoplasmic localization was observed in the MST1/2-MAP4K4/6/7 5KO and TAOK1/2/3 KO cells, but not in the MST-MAP4K-TAOK 8KO cells (Figure 6E and S4D). These data further support a model that TAOK1/3 can inhibit YAP/TAZ independent of MST1/2 and the MAP4Ks, and can directly phosphorylate and activate LATS1/2.
A prominent role for RHOA in YAP/TAZ activation
RHOA plays an important role in transducing signals from GPCRs to regulate F-actin. Treating cells with the RHO inhibitor C3 exoenzyme (C3) strongly induced LATS1/2 (HM) and YAP (S127) phosphorylation (Figure 7A). C3 also activated, though weakly, LATS1/2 and induced YAP phosphorylation in the MST-MAP4K 8KO, MOB1A/B KO, and MST-MAP4K-TAOK 8KO cells. Although RHOA, RHOB, and RHOC all share significant homology, they have distinct localizations and functions, with RHOA primarily acting to regulate the actin cytoskeleton. By RNA-seq, the expression of RHOB and RHOC in HEK293A cells are significantly lower than that of RHOA (Sultan et al., 2008) (Figure S1Z). Therefore, we hypothesized that the effect of C3 on YAP phosphorylation was primarily through RHOA. Deleting RHOA altered the cellular morphology, and the RHOA KO cells appeared to have increased filopodia (Figure 7B). Furthermore, deleting RHOA resulted in hyper-phosphorylated LATS1/2 and YAP/TAZ (Figure 7A). Accordingly, CYR61 and CTGF expression were also decreased in the RHOA KO cells (Figure 7C).
Neither serum nor LPA could induce YAP/TAZ dephosphorylation in the RHOA KO cells (Figure 2D, 7D, and S6A). These data support a critical role for RHOA in mediating LPA-induced GPCR signaling to the Hippo pathway. Recently, we reported that activation of conventional PKC by 12-O-tetradecanoylphorbol-13-acetate (TPA) could induce YAP/TAZ activation (Figure S6B) (Gong et al., 2015). Interestingly, TPA failed to induce YAP dephosphorylation, but TAZ was clearly dephosphorylated in the RHOA KO cells in response to TPA stimulation (Figure 7D). TPA, but not serum or LPA, also induced significant YAP/TAZ nuclear localization (Figure 7E and S4E). This observation raises the possibility that PKC acts partly via RHOA to regulate YAP/TAZ activity.
DISCUSSION
The Hippo pathway plays an important role in maintaining tissue homeostasis by regulating cell proliferation, apoptosis, and differentiation. Although dysregulation of the Hippo pathway has been associated with many types of human disease, how the Hippo pathway becomes dysregulated is not well understood. In this study we created knockout cell lines for many different Hippo pathway components and compared their response to multiple stimuli to determine which components are the most physiologically important in regulating YAP/TAZ.
Comparing the core Hippo pathway components, it is clear that in response to all stimuli and conditions tested, in HEK293A cells, LATS1/2 are the primary direct kinases for YAP/TAZ. Although LATS1/2 may have some intrinsic kinase activity towards YAP/TAZ, MOB1A/B are required for full phosphorylation and activation of LATS1/2. MST1/2 are the primary kinases for MOB1A/B, but in the MST1/2 KO cells, YAP/TAZ are still significantly phosphorylated following serum starvation even without phosphorylated MOB1A/B (T35) (Figure 5E), indicating that MOB1A/B phosphorylation is not essential for LATS1/2 activation. Moreover, MOB1A/B phosphorylation is not sufficient to induce LATS1/2 phosphorylation as LATS1/2 (HM) phosphorylation is absent in the NF2 KO cells even though MOB1A/B (T35) phosphorylation is high (Figure 5E). In addition, YAP phosphorylation is severely compromised in the MOB1A/B KO cells despite relatively high LATS1/2 (HM) phosphorylation, suggesting that LATS1/2 (HM) phosphorylation by itself is not sufficient and MOB1A/B plays a critical role in LATS1/2 phosphorylation of YAP. Together, these findings demonstrate that while MOB1A/B is critical for full LATS1/2 phosphorylation and activation, phosphorylation of MOB1A/B (T35) is not essential. Furthermore, phosphorylation of LATS1/2 (HM) is not sufficient to predict LATS1/2 activity. The MAP4K family and TAOK1/3 can directly interact with and phosphorylate LATS1/2 (HM) (Li et al., 2014a; Meng et al., 2015; Zheng et al., 2015), but this is still dependent on MOB1A/B to phosphorylate YAP/TAZ. These data fit with the model that MOB1A/B promotes YAP/TAZ phosphorylation by LATS1/2 and is required for auto-phosphorylation of the LATS1/2 activation loop. Thus, LATS1/2 are not fully active even though the HM is phosphorylated when MOB1A/B is absent.
The small increase in phosphorylated YAP in the MOB1A/B KO cells in response to cell-cell contact may be due to enhanced intrinsic activity of LATS1/2 (for example, if LATS1/2 and YAP/TAZ are both localized near adherens junctions at high density to further facilitate YAP/TAZ phosphorylation). Of note, with increasing density, MOB1A/B (T35) phosphorylation is significantly decreased while phosphorylated LATS1/2 (HM) and YAP (S127) are increased (Figure 4C). These observations again demonstrate that phosphorylated MOB1A/B (T35) is not required for LATS1/2 activation.
NF2 deletion is sufficient to significantly disrupt YAP/TAZ phosphorylation. Although we did not observe any change in LATS1/2 protein stability, it is clear that NF2 plays a critical role in LATS1/2 phosphorylation and activation. Deleting NF2 abolished YAP/TAZ phosphorylation in response to serum starvation. The current model for LATS1/2 activation begins when MST1/2 is recruited to the plasma membrane and phosphorylated. Phosphorylated MST1/2 binds and phosphorylates MOB1A/B, and NF2 recruits LATS1/2 to plasma membrane where LATS1/2 joins the MST1/2-MOB1A/B complex and are phosphorylated and activated by MST1/2 (Yin et al., 2013). The mechanism of how NF2 recruits LATS1/2 to the plasma membrane is not fully understood, but as our data demonstrate, NF2 plays a key role in LATS1/2 activation but not MST1/2 kinase activity because MOB1A/B (T35) was fully phosphorylated in the NF2 KO cells. NF2 probably plays an important role in MAP4K and TAOK-mediated activation of LATS1/2 as well because deleting NF2 had a more severe effect on YAP phosphorylation than deleting MST1/2. This role is also likely due to localization, since deletion of NF2 did not affect TAOK1 kinase activity (Figure 6D). There is not a reliable phospho-MAP4K antibody available to determine endogenous MAP4K phosphorylation. NF2 is one of a few instances where a mutation in a Hippo pathway component has a direct link to human disease. However, the observation that YAP/TAZ can still be phosphorylated and that NF2 KO cells retain some sensitivity to certain types of stress gives hope that there may be ways to therapeutically inhibit YAP/TAZ in NF2-mutant patients.
We have identified TAOK1/3 as direct kinases for LATS1/2. Previous studies have shown that TAOK phosphorylates and activates MST1/2 (Boggiano et al., 2011; Poon et al., 2011). Together, we propose that TAOK acts not only upstream of but in parallel to MST1/2 and the MAP4Ks to stimulate LATS1/2. Deleting TAOK1/2/3 in the MST1/2-MAP4K4/6/7 5KO cells significantly reduced YAP/TAZ phosphorylation and cytoplasmic localization in response to serum starvation and cell-cell contact. Although MST1/2 and the MAP4K family account for the majority of LATS1/2 phosphorylation and activation in response to most stimuli tested, TAOK1/3 played a significant role in response to cell-cell contact. The MST-MAP4K-TAOK 8KO and LATS1/2 KO cells are the only cell lines tested resistant to YAP phosphorylation by cell-cell contact. This raises the possibility that TAOK may have a significant role in regulating LATS1/2 activity in response to other stress conditions as well. That there are at least three distinct kinase families (MST, MAP4K, TAOK) capable of activating LATS1/2 highlights: (1) how critical regulation of LATS1/2 and YAP/TAZ are, and (2) how complex regulation of the Hippo pathway is that the cell has evolved so many mechanisms to tightly regulate YAP/TAZ in response to many different stimuli and contexts.
Finally, we establish a clear role for RHOA in mediating growth signals from GPCRs to activate YAP/TAZ. When we deleted RHOA, YAP/TAZ remained highly phosphorylated, sequestered in the cytoplasm, and transcriptionally inactive even in the presence of serum. However, TPA stimulation induced TAZ dephosphorylation and YAP/TAZ nuclear localization, raising the possibility that there are conditions in which YAP and TAZ may be differentially regulated (Figure 7D and 7E). To our knowledge, this is the first genetic data supporting a clear role for RHOA in mediating growth signals to the Hippo pathway, and illustrates how activating or inactivating mutations in components upstream of the Hippo pathway can result in pathological disruption of YAP/TAZ.
In conclusion, we compared and identified which Hippo pathway components have the greatest impact on regulating YAP/TAZ, and clarified their relationships with the core Hippo pathway kinase cascade (Figure S7). In addition, this study provides many useful resources for those studying the Hippo field, cell growth and survival, or the mechanisms of action of drugs targeting the Hippo pathway. It should be noted that our studies were only conducted in HEK293A cells, and it is possible that some components are not essential in HEK293A cells but are essential in other cell types. While we have clarified the role of NF2, TAOK1/3, and RHOA, there remain many other potential components whose deletion or overexpression may contribute to dysregulation of YAP/TAZ in human disease. Understanding these relationships will be critical to grasp how the Hippo pathway becomes disrupted and to identify potential therapeutic targets.
EXPERIMENTAL PROCEDURES
Generation of knockout cell lines
pSpCas9(BB)-2A-Puro (PX459; Addgene plasmid #48139) was a gift from Dr. Feng Zhang (Sanjana et al., 2014). Gene-specific sgRNAs were designed using the CRISPR design tool at http://www.genome-engineering.org/crispr. HEK293A cells were transfected, selected with puromycin for 2–3 days, and single-cell sorted by FACs into 96-well plate format. Single clones were expanded and screened by protein immunoblot and confirmed by sequencing (Figure S1). All sgRNA sequences are listed in Table S1.
Cell culture
HEK293A cells were grown in DMEM with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were plated at 1.5 × 105 cells per well into 6-well plates, unless otherwise noted. 24 hours after plating, cells were given fresh media for 2 hours before treatment with serum-free DMEM media, Latrunculin B (250 ng/ml), F/IBMX (10 uM Forskolin, 100 uM IBMX), or 2-DG (25 mM, in glucose-free DMEM with 10% dialyzed FBS). Cells were starved in serum-free DMEM overnight before treatment with LPA (0.5 uM) or TPA (5 nM). To measure glucose and pH, cells were plated at 8 × 105 cells per well into 6-well plates and given fresh DMEM with 10% FBS for 6 hours. Glucose was measured using a FreeStyle Precision Neo glucose monitoring system.
Immunoblot
Immunoblots were performed as previously described (Meng et al., 2015). 7.5% phos-tag gels were used to compare YAP and TAZ phosphorylation levels. Immunoprecipitation and in vitro kinase assays were performed as previous described (Meng et al., 2015). Antibodies used are listed in Table S2.
Immunofluorescence
Coverslips were pretreated with 0.0005% Poly-L-ornithine solution (Sigma, P4957) in 12-well plates at 37°C for 15 minutes and washed wi th PBS prior to plating cells. Cells were plated 24 hours prior to treatment: medium cell density (1.0 × 105), low cell density (0.5 × 105), serum starvation (12 hours), LPA (0.5 uM), or TPA (5 nM). Cells were fixed in 4% paraformaldehyde for 15 minutes, followed by permeabilization with 0.1% Triton-X for 5 minutes and blocking in 3% BSA for 1 hour. Primary antibody was incubated in 3% BSA overnight at 4°C. Secondary antibodies were diluted in 3% BSA and incubated for 1 hour. Slides were mounted with prolong gold anti-fade reagent with DAPI.
Statistical analysis
Where indicated, experiments were repeated at least three times and statistical analysis was performed using unpaired t tests. ns: P>0.05; *: P≤0.05; **: P≤0.01; ***: P≤0.001; ****: P≤0.0001.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (R35CA196878, R01GM51586, and R01DE015964) to K.L.G. S.W.P., K.C.L., and A.W.H. were supported by a UCSD Pharmacology training grant (T32 GM007752). K.L.G. co-founded but receives no direct financial support from Vivace Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
S.W.P. and K.L.G. conceived and performed experiments and wrote the manuscript. Z.M. performed experiments and provided reagents. K.C.L., B.L., and J.V.C. performed experiments. A.W.H. provided reagents.
References
- Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell. 2011;21:888–895. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. The Journal of biological chemistry. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Xie R, Wang W, Cai J, Choi KS, David KK, Huang B, Yabuta N, Nojima H, et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes & development. 2015;29:1285–1297. doi: 10.1101/gad.264234.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr Biol. 2010;20:657–662. doi: 10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Hong AW, Plouffe SW, Zhao B, Liu G, Yu FX, Xu Y, Guan KL. Opposing roles of conventional and novel PKC isoforms in Hippo-YAP pathway regulation. Cell Res. 2015;25:985–988. doi: 10.1038/cr.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, Amcheslavsky A, Nie Y, Kaneko S, Yao X, Chen X, et al. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell. 2014a;31:291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D, Snuderl M, Ladanyi M, Hanemann CO, Zhou P, et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer cell. 2014b;26:48–60. doi: 10.1016/j.ccr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhou H, Li F, Chan SW, Lin Z, Wei Z, Yang Z, Guo F, Lim CJ, Xing W, et al. Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway. Cell Res. 2015;25:801–817. doi: 10.1038/cr.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. The Journal of biological chemistry. 2010;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, Liu J, Peters EC, Wu X. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, et al. A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes & development. 2015;29:1271–1284. doi: 10.1101/gad.262816.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio M, Hamada K, Kawahara K, Sasaki M, Noguchi F, Chiba S, Mizuno K, Suzuki SO, Dong Y, Tokuda M, et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J Clin Invest. 2012;122:4505–4518. doi: 10.1172/JCI63735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramasivam M, Sarkeshik A, Yates JR, 3rd, Fernandes MJ, McCollum D. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Molecular biology of the cell. 2011;22:3725–3733. doi: 10.1091/mbc.E11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med. 2015;21:212–222. doi: 10.1016/j.molmed.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell. 2011;21:896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A, Zoch A, Morrison H. A neuronal function of the tumor suppressor protein merlin. Acta Neuropathol Commun. 2014;2:82. doi: 10.1186/s40478-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedinger K, VandenBerg SR, Baia GS, McDermott MW, Gutmann DH, Lal A. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia. 2008;10:1204–1212. doi: 10.1593/neo.08642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, Seifert M, Borodina T, Soldatov A, Parkhomchuk D, et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321:956–960. doi: 10.1126/science.1160342. [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DCR, Schiripo TA, Haber DA, Hariharan IK. salvador Promotes Both Cell Cycle Exit and Apoptosis in Drosophila and Is Mutated in Human Cancer Cell Lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. The Journal of biological chemistry. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur J Cancer. 2012;48:1227–1234. doi: 10.1016/j.ejca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer science. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki PM, Adrych K, Kartanowicz D, Stanislawowski M, Kowalczyk A, Godlewski J, Skwierz-Bogdanska I, Celinski K, Gach T, Kulig J, et al. Underexpression of LATS1 TSG in colorectal cancer is associated with promoter hypermethylation. World J Gastroenterol. 2013;19:4363–4373. doi: 10.3748/wjg.v19.i27.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C, Shen Z, Stemmer-Rachamimov A, Dawany N, Troutman S, Showe LC, Liu Q, Shimono A, Sudol M, Holmgren L, et al. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Science signaling. 2013;6:ra77. doi: 10.1126/scisignal.2004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Meng Z, Plouffe SW, Guan KL. Hippo Pathway Regulation of Gastrointestinal Tissues. Annu Rev Physiol. 2015a;77:8.1–8.27. doi: 10.1146/annurev-physiol-021014-071733. [DOI] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015b;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes and Development. 2010a;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes & development. 2010b;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & development. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes & development. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Dev Cell. 2015;34:642–655. doi: 10.1016/j.devcel.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


