Abstract
Diverse antibody effector functions mediated by the Fc domain have been commonly associated with reduced risk of infection in a growing number of nonhuman primate and human clinical studies. This study evaluated the anti-HIV antibody effector activities in polyclonal serum samples from HIV-infected donors, VAX004 vaccine recipients, and healthy HIV-negative subjects using a variety of primary and cell line-based assays, including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cell-mediated viral inhibition (ADCVI), and antibody-dependent cellular phagocytosis (ADCP). Additional assay characterization was performed with a panel of Fc-engineered variants of monoclonal antibody b12. The goal of this study was to characterize different effector functions in the study samples and identify assays that might most comprehensively and dependably capture Fc-mediated antibody functions mediated by different effector cell types and against different viral targets. Deployment of such assays may facilitate assessment of functionally unique humoral responses and contribute to identification of correlates of protection with potential mechanistic significance in future HIV vaccine studies. Multivariate and correlative comparisons identified a set of ADCVI and phagocytosis assays that captured different antibody activities and were distinct from a group of ADCC assays that showed a more similar response profile across polyclonal serum samples. The activities of a panel of b12 monoclonal Fc variants further identified distinctions among the ADCC assays. These results reveal the natural diversity of Fc-mediated antibody effector responses among vaccine recipients in the VAX004 trial and in HIV-infected subjects, and point to the potential importance of polyfunctional antibody responses.
Introduction
Antibodies (Abs) can provide protection against viral infections through a broad array of different mechanisms, including neutralization, agglutination, phagocytosis, and lysis of infected cells. Results of an immune correlates analysis from the RV144 HIV vaccine efficacy trial suggest that non-neutralizing antibodies may have contributed to a reduced risk of infection (1). Several subsequent RV144 follow-up studies have provided evidence to suggest a role for non-neutralizing, Fc receptor (FcR)-mediated antibody effector functions (2, 3), including antibody-dependent cellular cytotoxicity (ADCC) (4–6) and phagocytosis (2). Moreover, previous work has demonstrated that ADCC-inducing Abs are detectable in early HIV infection (7), are enriched in long-term HIV non-progressors (8), are associated with better outcomes in mother-to-child transmission (9, 10), and correlate with enhanced HIV control (11, 12). In animal models, effector functions support the protective activity of neutralizing antibodies (13, 14), can protect neonatal macaques from SIV challenge (15), and have been associated with vaccine-mediated protection in multiple studies (16, 17), suggesting that they may play an important role in providing both protection against infection and better outcomes after infection (reviewed in (18, 19)).
Because FcγR-mediated activities include an array of different mechanisms, mediated by multiple, divergent effector cell types, it is exceedingly difficult to measure the entirety of these activities in a single assay. In recent years, a number of different effector functional assays have been developed to assess ADCC, antibody-dependent cell mediated viral inhibition (ADCVI) and antibody-dependent cellular phagocytosis (ADCP) activities in HIV studies. However, unlike neutralization activities, these Fc-mediated Ab effector functions are less often included in the routine evaluation of the immunogenicity of HIV vaccine candidates. This exclusion is partly due to the developmental challenges associated with well-characterized Fc-effector functional assays, a relative lack of information to prioritize among or differentiate between available assays, and questions as to which mechanisms may predominate in vivo.
To fill this knowledge gap, we systematically evaluated a number of available Fc-effector functional assays using a common set of samples with possible differences in effector function. The main objective of this study was to identify assays that most comprehensively and dependably capture Fc-mediated antibody functions mediated by different effector cell types and against different viral targets. Ultimately, these results may be used to facilitate the understanding of distinctive aspects of the Fc-mediated immune responses with broad and highly functional antiviral activity.
Materials and Methods
Reagents and antibodies
Each laboratory was provided common reagents, as applicable, and performed assays on a total of 140 blinded IgG samples purified from treated chronically HIV infected subjects (n=31), untreated chronically HIV infected subjects (n=28), elite controllers (20) (n=31; all of whom achieved control of HIV infection at plasma HIV RNA levels of <2000 copies/mL and had documented infection for at least one year), recipients of AIDSVAX B/B gp120 (n=30) and placebo (n=10) 2 weeks post fourth inoculation in the VAX004 efficacy trial (21), HIV seronegative subjects (n=10) and control antibodies as described in Table 1. Among the control antibodies, a panel of Fc domain mutated b12 mAb variants with divergent Fc receptor binding (22), and described in Table 2, and four blinded replicates each of IVIG (purified IgG from a pool of plasmas from healthy, HIV-negative subjects)(23), HIVIG (purified IgG from a pool of plasmas from HIV-infected subjects presumed to be infected with clade B virus) (24), and HIVIG-C (purified IgG from a pool of plasmas from HIV-infected subjects confirmed to be infected with clade C virus) were evaluated. Serum antibody samples were purified via melon gel based depletion of prevalent serum proteins such as albumin and the majority of IgA, while passively enriching IgG, as previously described (25). A common stock of BaL virus (Bal.LucR.T2A.ecto produced in 293T.17 cells) or recombinant BaL gp120 was used in each assay, as appropriate when possible. Similarly, a common stock of cryopreserved PBMCs heterozygous for the F/V158 FcγRIIIa allotype (26) obtained by leukapheresis was utilized unless otherwise specified. All studies were approved by appropriate local Institutional Review Boards and each subject gave written informed consent.
Table 1.
Study samples
| Sample Group | Number |
|---|---|
| Infected | |
| Chronically Infected/Treated | 31 |
| Chronically Infected/Untreated | 28 |
| Elite controller | 31 |
| Uninfected | 10 |
| Vaccinee | |
| VAX004 | 30 |
| Placebo | 10 |
| Control (replicates) | |
| IVIG | 4 |
| HIVIG | 4 |
| HIVIG-C | 4 |
| Fc point mutants | |
| b12 | 10 |
Table 2.
b12 point mutants and their relative binding affinities (EC50) determined by ELISA (reproduced from (22))
| Variant | FcγRI | FcγRIIA | FcγRIIIA |
|---|---|---|---|
| R292A | 0.91 | 0.43 | 0.65 |
| D270E | 0.71 | 0.42 | 0.69 |
| S239A | 0.87 | 0.87 | 0.22 |
| S298A | 0.84 | 0.52 | 1.31 |
| K338A | 1.21 | 1.5 | 0.41 |
| S267G | 1.05 | 2.99 | 0.21 |
| G236A | 0.44 | 8.63 | 0.93 |
| I332E | 1.21 | 3.19 | 7.32 |
| SD/IE | 1.29 | 5.99 | 31 |
| SD/IE/AL | 1.06 | 3.41 | 90 |
| SD/IE/GA | 0.91 | 49 | 66 |
| WT | 1 | 1 | 1 |
Functional Assays
Table 3 summarizes different attributes of the 7 functional assays, described briefly below.
Table 3.
Descriptions of the Functional Assays
| Abbreviation | Name | Assay Description | Assay Readout | Ref |
|---|---|---|---|---|
| BVADCC | Bound Virion Antibody Dependent Cellular Cytotoxicity | Inhibition of infection of target cells at bound virion stage | % decrease in infected target cells | (36) |
| GTL ADCC | Antibody Dependent Cellular Cytotoxicity assessed by Granzyme release | Induction of Granzyme B and uptake by target cells | % antigen coated target cells taking up Granzyme B | (27) |
| RFADCC | Rapid Fluorescent Antibody Dependent Cellular Cytotoxicity | Killing of sensitized target cells labeled with vital dyes | % dye loss. | (2, 30) |
| LUC ADCC | Antibody Dependent Cellular Cytotoxicity assessed by luminescence | Reduction of virus-derived luciferase activity in infected target cells | % reduction of virus-derived luciferase activity in infected target cells | (28) |
| ADCVI | Antibody Dependent Cellular Viral Inhibition | Inhibition of virus production by target cells | % decrease of p24 production by target cells (ELISA) | (38) |
| Virion Phagocytosis | Virion Phagocytosis | Phagocytosis of virus (HIV-1 US657) by effector cells (THP-1) | % cells taking up virus x MFI of positive cells (measured by Flow) | (13) |
| ADCP | Antibody Dependent Cellular Phagocytosis | Phagocytosis of antigen coated beads by effector cells (THP-1) | % cells stained x MFI of positive cells (measured by flow) | (39, 53) |
GTL ADCC
The GTL ADCC assay was conducted essentially as previously described (27). For this study, the CEM.NKRCCR5 cell line was coated with the HIV-1 recombinant gp120 representing the BaL isolate. The recombinant gp120 was provided by Dr. George Lewis to match the AT-2 inactivated virus used in his BV-ADCC assay (below). These target cells were labeled with GranToxiLux (GTL; from OncoImmunin, Inc), which becomes fluorescent upon cleavage by Granzyme B (GrB). Upon recognition of the targets mediated by the anti-Env Ab-FcR interactions, the FcγR-bearing effector cells deliver GrB to the HIV-infected target cells. Once internalized, GrB cleaves the peptide and releases a fluorescent signal that can be identified by flow cytometry. The assay readout is the percent of antigen coated target cells, which take up GrB. Both peak and area under the titration curve (AUC) were used as assay data summary measures. The AUC was calculated as the integrated background-subtracted net activity over a range of dilutions using the trapezoidal method and was truncated above zero.
LUC ADCC
The LUC ADCC assay utilized the HIV-1 Infectious Molecular Clone (pNL-LucR.T2A-BaL.ecto, herein referred to as BaL.LucR.T2A.ecto) expressing the BaL envelope (HIV Bal.LucR.T2A.ecto/293T/17; GenBank accession number AY426110 (https://www.ncbi.nlm.nih.gov/nuccore/AY426110)) and the Renilla luciferase reporter gene to infect the CEM.NKRCCR5 cell line used as target cells (28, 29). Whole PBMC, obtained from cryopreserved leukopheresis samples, were used as effectors as previously reported (26). In this assay, IgG-containing samples were incubated with the target cells in the presence of the effector cells for 6 hours. The assay readout is the percent specific killing based on the decrease of virus-derived luciferase activity. Both peak and AUC were used as assay data summary measures.
RFADCC
A modification of the rapid fluorescent ADCC (RFADCC) assay (30) was used, as previously described (2). In brief, the CEM-NKR-CCR5 T cell line was labeled with intracellular CFSE, membrane dye PKH26 and then pulsed with recombinant SF162 gp120 protein. NK cells were enriched directly from fresh healthy donor whole blood using RosetteSep (Stem Cell Technologies). Purified IgG was added to the CEM-NKr cells and incubated with NK cells for 4 hr at 37°C. The cell mix was then fixed and the proportion of PKH26+ cells that lost intracellular CFSE (i.e. lysed target cells) was determined by FACS. Data presented represents activity at a test concentration of 100 μg/ml averaged over 2 replicates.
BVADCC
Replication-defective but entry competent AT-2 inactivated BaL virions (31–33) were generously provided by Dr. Jeff Lifson, FCRC, NIH), permitting the detection of ADCC responses to epitopes that are exposed during viral entry (34–36) as described in (35, 36). Briefly, CEM-NKr-CCR5+ target cells were sensitized by spinoculation (37) at 12°C that is non-permissive for fusion, with at a MOI of approximately 5. Target cells were then sensitized with antibody dilutions, washed to eliminate prozone effects, and used at 37°C in a three hour RFADCC assay. The assay readout represents percent cytotoxicity determined by dual dye loss from the target cells for each antibody dilution (30). Both peak and AUC were used as assay data summary measures. The AUC was calculated as the integrated control-sample normalized readout over a range of dilutions using the trapezoidal method and was truncated above zero.
ADCVI
Briefly, the assay was performed as previously described (38) by co-incubating HIV-infected target cells (CEM.NKR-CCR5 cells) with test antibody and fresh PBMCs at a 10:1 effector:target ratio After seven days, virus yield was measured by p24 ELISA. Antibody ADCVI activity was defined as the average percent decrease of p24 production by target cells across p24 ELISA replicates.
Virion Phagocytosis
HIV-1US657 (GenBank U04908) virus was first incubated with test antibody and then added to THP-1 cells (ATCC) as described (13). The percentage of FITC-positive cells and their fluorescent intensity was determined by flow cytometry. The assay readout was determined by multiplying the percentage of FITC-positive cells by the fluorescent intensity of positive cells. Appropriate controls were used to subtract background (internalization of FITC-labeled virus in the absence of Env-specific antibody). The average readout over replicates was determined and a sample’s response was defined positive if the background-adjusted value was ≥ 116.
ADCP
Briefly, recombinant SF162 gp120 (Immune Technology) coated 1μm fluorescent neutravidin beads (Life Technologies) were incubated with purified IgG samples for 2 hrs at 37°C as previously described (39). THP-1 cells (ATCC) were then added to the bead/antibody mix and incubated overnight to allow for phagocytosis, followed by fixation and analysis of bead uptake by FACS. Data presented represents activity at a test concentration of 100 μg/ml averaged over 2 replicates. The assay readout represents scaled iMFI values (frequency x MFI). No pre-specified positivity criteria were utilized.
IgG Titering Antigen Array Assay
Briefly, an array of select HIV-1 antigens was printed in triplicate onto poly-lysine functionalized glass slides using a robotic microarrayer (Arrayit). After blocking, arrays were incubated with samples containing the antibodies of interest. For purified IgG, the dynamic range was found to be 50 ng/mL to 300 ng/mL using VRC01. Dilution between 1:100 and 1:500 of serum samples allowed for detectable signal with minimal background. After incubation with antibody samples, arrays were blocked, washed, and probed for IgG signal using a fluorescently labeled Goat Anti-Human IgG detection antibody, scanned on a GenePix® 4200AL (Molecular Devices) and analyzed with GenePix® Pro 6.0 (Molecular Devices) for the median fluorescence intensity (MFI) of each spot.
Data analysis
All analyses were performed in R 3.1.1 (40).
Correlation analysis
Spearman’s correlation coefficients were calculated using raw data and visualized in a two-panel R pairs plot. The scatterplots of each pair of assay readouts were display in the upper right. The correlation coefficients were displayed on a gradient color scale, overlaid by statistical significance stars for unadjusted p-values from testing a zero correlation coefficient in the lower left.
Hierarchical clustering of functional assay data
Ward’s method of hierarchical clustering was used to generate dendrograms that visualized correlations and groupings among functional assay readouts using the hclust() function in R (41). Integrated assay data was first mean-centered and scaled by the standard deviation across samples. One minus the Euclidean distances among assay readouts was used as the dissimilarity index in the clustering.
Principal Components Analysis (PCA)
To investigate the relationship between the functional assays and how antibody functions may differentiate different subject classes, PCA was performed on scaled, centered data using the prcomp() function in R. In PCA, the original activity measurements were algebraically converted and combined into a new set of uncorrelated composite variables, ranked in order of their contribution to explaining the variation in the data. These new variables are termed “principal components” (PCs). The first three principal components were visualized in a 3-dimensional scatterplot produced using the scatterplot3D package in R (42). To minimize visual complexity of plots, two plots were produced: one to display PCA scores for individual subjects colored to identify subject class, the other to display coordinates of each functional assay on the projected coordinates. Points and text were colored to identify different functional assays on the triplot.
Results
Antibodies purified from plasma samples from HIV infected subjects, VAX004 participants, and pooled IgG controls were assessed in a series of blinded cell-based assays of antibody effector function. These assays included four measures of antibody-dependent cellular cytotoxicity (ADCC), two measures of antibody-dependent phagocytosis, and one measure of antibody-dependent cell-mediated virus inhibition (Table 3). These assays are based on different principles and measure various aspects of antibody effector function, as follows: 1) The GTL ADCC assay detects the presence of ADCC-mediating antibodies using a flow-based assay to quantify the elimination of target cells that have been coated with recombinant HIV-1 gp120 or infected with HIV-1 (27). 2) The LUC ADCC assay measures the replication in target cells in the presence of effector PBMC and IgG-containing samples of a recombinant HIV-1 provirus that stably expresses Renilla luciferase (28, 29), yielding a readout of percent specific killing of virus-infected cells. 3) The RFADCC assay utilizes co-staining of target cells with a membrane dye and a viability dye before the addition of IgG-containing samples and effector cells, enabling quantification of target cell lysis. 4) The BVADCC is a modified version of the RFADCC assay, such that the target cells are sensitized by highly purified replication-defective but entry competent AT-2 inactivated BaL virions (31–33), permitting the detection of ADCC responses to epitopes that are exposed during viral entry (34–36) as described in (35, 36). 5) The virion phagocytosis assay measures the ability of macrophages or monocytes to internalize antibody-coated FITC-labeled HIV (13). 6) The THP-1 antibody-dependent cellular phagocytosis (ADCP) assay uses an FcγR-expressing monocytic-like cell line to measure monocyte-mediated phagocytosis of IgG antibody-coated fluorescent beads (39). 7) The ADCVI assay is a measure of the ability of antibody, in combination with FcγR-bearing effector cells, to inhibit virus replication (38).
These different assay types were chosen because they have the potential to measure distinct Fc-mediated Ab effector functions or combinations thereof. For example, the ADCVI assay captures a cumulative measure of the role of antibody in reducing viral outgrowth. Thus, ADCVI can encompass ADCC, phagocytosis, and the production of cytokines/chemokines, all of which might be involved in the inhibition of virus outgrowth from infected cells. In contrast, the ADCC assays assess target cell killing, or effector cell degranulation, for example. The four ADCC assays exhibit a number of differences; the LUC ADCC and GTL ADCC assays use whole PBMC as effector cells, whereas the RFADCC assay uses an NK cell-enriched population obtained from whole blood. Thus, these assays might reveal differences in cytotoxicity mediated by NK cells vs other immune cells such as macrophages, neutrophils, and eosinophils. Another difference among the four ADCC assays is that the GTL ADCC, RFADCC, and BVADCC assays enable the analysis of target cell killing on the single cell versus the population level, since these assays are flow cytometry-based. In contrast, the LUC ADCC assay provides a population-level readout of target cell killing. Some assays utilize infected target cells, whereas others coat target cells with gp120. Similarly, although the two phagocytosis assays (virion phagocytosis and ADCP) are similar, they also have an important difference. Specifically, the fluorescent beads used in the ADCP assay (0.1 μm) are approximately 10-fold greater in diameter than a typical HIV-1 virion (0.1 μm)(43). Since phagocytosis is known to be impacted by particle size (44), the virion phagocytosis assay is important in that it measures phagocytosis of a highly physiologically relevant particle (i.e. a virion). However, the ADCP assay has unique advantages in that it does not involve the use of infectious virus and avoids all of the technical hurdles involved therein, and is amenable to isolating the activity of responses toward individual antigens and epitopes.
Purified serum IgG was utilized in order to reduce the potential impact of other soluble factors known to modulate effector cell activity (45). Purified serum IgG from uninfected placebo recipients in VAX004 were used as additional controls for assay specificity. A uniform batch of BaL virus or recombinant BaL gp120 antigen, as dictated by assay format, was used in order to match the antibodies evaluated across assays as much as possible. In addition, unfractionated samples from a subset of the study cohort were also used for comparisons with purified serum IgG samples.
Assay specificity and reproducibility
Replicated assessments of the activity of pooled polyclonal plasma IgG from healthy subjects (IVIG), HIV-infected subjects (HIVIG and HIVIG-C) were utilized to determine intra-assay reproducibility and ability to distinguish positive from negative samples (Figure 1). Generally, functional assays reliably differentiated positive from negative control samples, and the coefficients of variation (%CV) among these blinded replicates ranged from 1–50% among positive controls, with an average of 13% across all assays. In most cases, wide CVs could be attributed to 1 of 4 replicates giving a dramatically different readout, rather than a broad distribution among all replicates. Variability up to 30% of CV is often considered acceptable in functional assays such as those evaluated here.
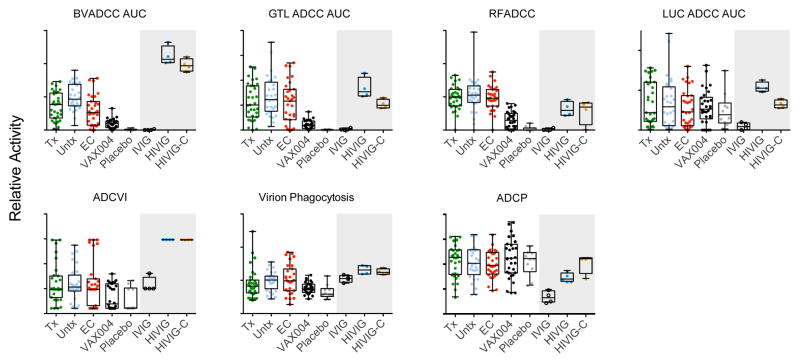
Figure 1. Antibody functional activity across subject groups and control samples.
The functional activity of purified antibodies from HIV infected treated (Tx), untreated (Untx), or elite controllers (EC), Vax004 vaccinees (VAX004), and VAX004 placebo recipients (Placebo) was assessed across a suite of cell-based assays of antibody effector function including ADCC activity (BVADCC, GTL ADCC, RFADCC, LUC ADCC), ADCVI, and phagocytic activity (Virion Phagocytosis, ADCP). Replicates of polyclonal IVIG, HIVIG, and HIVIG-C were used as negative and positive controls and to assess assay reproducibility, and were evaluated at a different concentration (gray shaded region). AUC denotes area under the titration curve. Activity magnitudes (y-axis scales) are described in the materials and methods and should not be compared across assays.
Interestingly, HIVIG-C was observed to exhibit equivalent or somewhat lower activity relative to HIVIG in a number of assays, with the exception of phagocytosis activity detected by the ADCP assay, suggesting a distinction among assays. Notably, these control antibody samples were evaluated at a higher test concentration than those from infected and vaccinated subjects, and thus should not be directly compared to the latter two sample sets. In addition, we observed good reproducibility based on replicates performed on the same purified IgG samples or unfractionated samples in the ADCVI, Virion Phagocytosis, RFADCC and ADCP assays (Supplemental Figure 1), and apparent differentiation among positive and negative control samples, as previously reported in other blinded studies (1, 2, 12, 17).
Fc-effector functions detected in HIV-infected and VAX004 samples
We first examined the activity levels of polyclonal antibodies purified from subjects who were HIV-infected or vaccinated with AIDSVAX B/B gp120 and observed that they exhibited high inter-subject and in some cases high inter-group variability (Figure 1), indicating that Fc effector function responses likely differ between vaccinated and infected subjects and are probably affected by a multitude of factors such as host genetics, sex, and/or infection duration. We next compared activity levels among the different assays between HIV infected subjects (treated, untreated, and elite controllers) versus HIV uninfected subjects (VAX004 placebo recipients, whose serum demonstrated a lack of non-specific activity) and did not observe high activity among the subject groups in the ADCVI, virion phagocytosis, ADCP, or LUC ADCC (AUC) assays. However, activity levels in the BVADCC (AUC), GTL ADCC (AUC), and RFADCC assays were generally higher in HIV-infected subjects than in HIV-uninfected subjects. Though many previous studies have reported potentiated or broadened antibody activity present among HIV controllers or long-term non-progressors (7, 8, 46–48), these findings, which differ from the present study in assays used and populations analyzed, were not confirmed here, consistent with a similar recent study where subjects were balanced for age, gender, and presence or absence of protective HLA class I alleles (49). We next narrowed the scope of this comparison to placebo versus vaccine participants in VAX004 and observed that placebo recipients exhibited little to no activity in most assays, while vaccine recipients exhibited higher activity than placebo recipients in the BVA ADCC, GTL ADCC and RFA ADCC assays, albeit reduced relative to that of infected subjects, consistent with the decreased prevalence of BaLgp120-reactive antibodies in the vaccinated population (Supplemental Figure 2). However, striking differences in activity were not observed between placebo and vaccine recipient samples in the LUC ADCC, ADCVI, Virion Phagocytosis, or ADCP assays. In a previous study in which the virus tested was more closely matched to the vaccine envelope, ADCVI activity was observed to be associated with reduced risk of infection in VAX004 recipients (12), suggesting that the relatively low response observed among vaccine recipients may result from our choice of virus strain. Overall, these findings indicate that the different assays used in this study generally did not capture redundant information, but instead captured relatively distinct immunological functions.
Correlations among assays in HIV-infected subjects
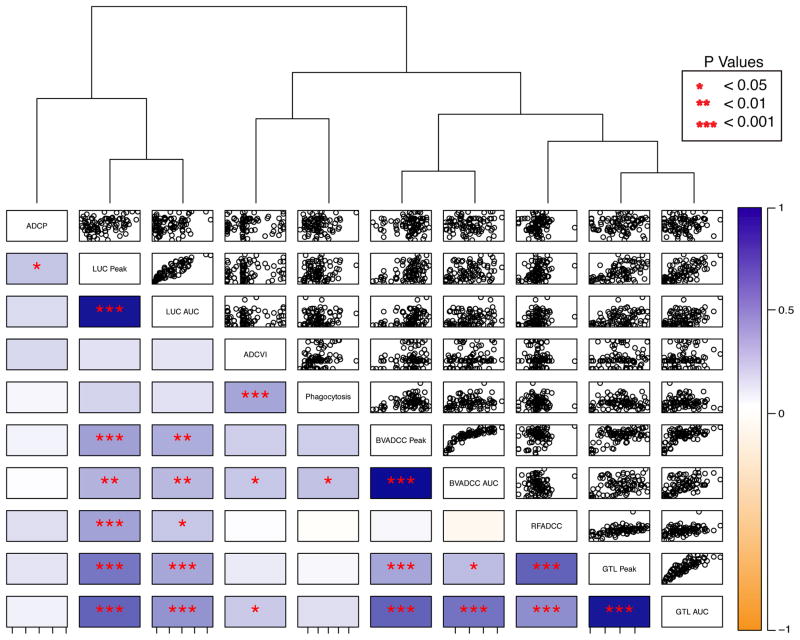
To more directly compare across functional assays, a correlation analysis for each pair of assays was performed across the cohort of 90 infected subjects. The functional activity of antibodies from each infected subject was plotted across each pair of effector assays to identify whether individual subjects had responses of similar magnitude across assays, or whether some subjects possessed antibodies with discrepant activity magnitudes, such as being highly phagocytic but with low ADCC potency (Figure 2). Hierarchical clustering of all seven assay readouts based on the infected subjects further captured the relative similarity and dissimilarities between functional assessments. Relatively higher correlations were observed between summary values such as peak activity and AUC within a given assay when both measures were available, e.g. BVADCC Peak versus BVADCC AUC. By contrast, low to moderate correlation was observed between different ADCC assays that utilized either virus or antigen-coated target cells, and no to very low correlation was observed for the other assays either among themselves or with the ADCC assays (e.g. ADCP vs ADCVI). This pattern was confirmed by the dendrogram, in which a number of ADCC assays (BVADCC, RFADCC, and GTL ADCC) clustered together in a single subclade on the right side of the dendrogram, whereas the LUC ADCC, ADCVI, Virion Phagocytosis, and ADCP assays clustered separately in individual subclades on the left side of the dendrogram. This analysis suggests that these assays capture distinct functional activities, as evidenced by the lower correlations among the readouts of these assays as compared to the more tightly clustered ADCC assays on the right side of the dendrogram.
Figure 2. Functional correlations across HIV-infected subjects.
Correlation plots presenting the activity of Abs from each subject across pairs of functional assays are shown. The direction and strength of Spearman correlation coefficients between functional assessments and their significance values (lower left), and corresponding scatterplots (upper right) are presented. Correlation strength is represented via the color scale, where dark orange indicates perfect negative correlation and dark blue signifies perfect positive correlation. Unadjusted p values are reported as: p<0.05 = *; p<0.01 = **; and p<0.001 = ***. The correlation matrix is ordered based on hierarchical clustering. For assays in which multiple summary values were available (e.g. Peak activity and AUC), both measurements are presented.
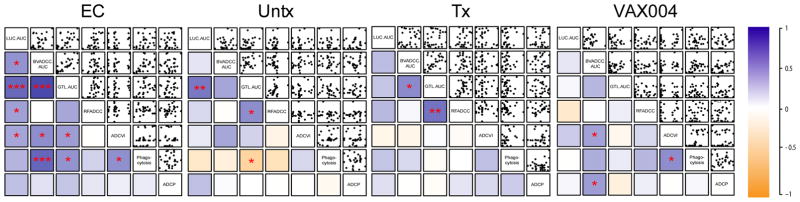
Because functional coordination across multiple assays may differ among subject groups (2, 49), the correlation analysis was repeated within each subject group (Figure 3). This analysis indicated that a higher degree of functional correlation was present in elite controllers than any other group. As previously observed in VAX003 (2), antibodies from the VAX004 subjects generally exhibited reduced functional correlation. While the limited correlations between antibody activities across different assays could be driven by the generally lower activity observed among vaccinees, antibodies from treated and untreated subjects, who generally exhibited higher activity, were similarly less coordinated.
Figure 3. Functional correlations within subject groups.
Correlation plots presenting scatterplots among subjects by class across each pair of functional assays, organized by hierarchical clustering (LUC ADCC AUC, BVADCC AUC, GTL ADCC AUC, RFADCC, ADCVI, Virion Phagocytosis, and ADCP). AUC denotes area under the titration curve. Strength of Spearman correlation coefficients is presented in color scale, where dark orange indicates perfect negative correlation and dark blue is perfect positive correlation. Unadjusted p values are reported as: p<0.05 = *; p<0.01 = **; and p<0.001 = ***.
Principal component analysis in HIV infected and VAX004 subjects
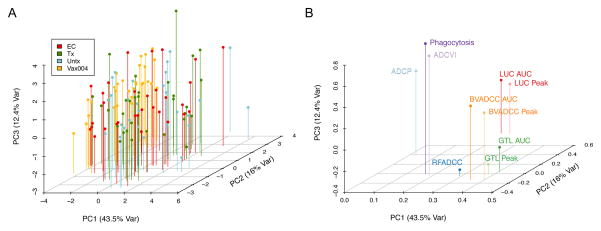
To further assess the differences among subject groups and assays, a principal components analysis (PCA) of all functional assay readouts was conducted based on the cohort of 90 HIV-infected subjects and 30 VAX004 vaccine recipients. We found that the first three lead principal components of the assay readouts explained a total of 72% variation of the data (Figure 4A), with GTL AUC and BVADCC Peak contributing the most to the first principal component (Figure 4B). Consistent with the box plots presented in Figure 1, subjects were generally well mixed across the lead PCs, with the exception of VAX004 subjects, which were distinguished from infected subjects by PC1 (Figure 4A). Across assays, summary values (peak or AUC) from the same assay were again strongly grouped together across all 3 lead PCs. Similar to the clustering results, a number of ADCC assays differentiated themselves from ADCVI, ADCP, and phagocytosis assays in PC1. However, these grouped ADCC assays contributed to variation captured in PC2 and PC3 (Figure 4B), which points toward distinctions among them in terms of the antibody activities that they capture. This analysis confirms similarities among the ADCC assays and also highlights distinctions between these Ab activities and those assessed in the ADCVI, Virion Phagocytosis and ADCP assays. Similarly, it also recapitulates the absence of strong activity differences among the different groups of HIV-infected subjects.
Figure 4. Principal component triplots.
A. Subjects are displayed as pinned dots in the 3D space defined by the three lead principal components (representing 72% of the variation in the data). B. Contributions of each assay to PC1, PC2, and PC3 are displayed as pinned dots.
Fc-effector functions detected in b12 mutants
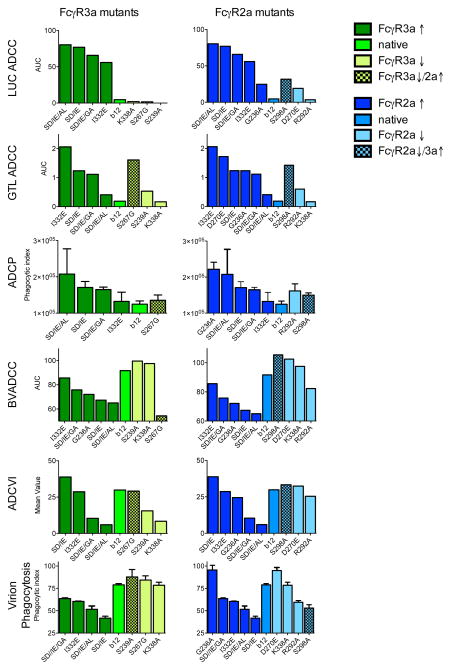
Lastly, the relative activity of b12 Fc domain point mutants (22) was assessed across assay types. These antibody variants were designed to have selectively increased or decreased binding affinities to the two major activating FcγR2s, particularly FcγR2a and FcγR3a (Table 2, reproduced from (22)). While all antibody variants have been shown to bind Env-gp120 and neutralize HIV-1 virions as well as their wild-type counterpart, the function of an antibody variant in the ADCVI, ADCP, ADCC, and other similar assays was shown to be generally correlated with its FcγR binding ability (22, 39). Thus, we predicted that the b12 antibody variants with decreased FcγR affinity would have lower activity in these assays, whereas the affinity-enhanced b12 antibody variants would exhibit higher activity. Consistent with this hypothesis, the LUC ADCC, GTL ADCC, and the ADCP assays exhibited differentiation among variants, where affinity-enhanced mutants were generally associated with increased antibody activity, and affinity-compromised mutants with reduced activity (Figure 5). However, not all assays exhibited this predictable FcγR-affinity-dependent activity in the context of the b12 panel. For example, no strong activity patterns related to FcγR binding affinity were observed in the ADCVI assay, which may reflect the fact that this assay measures multiple antibody functions simultaneously. While FcγR2a affinity did not appear to be strongly associated with activity in the Virion Phagocytosis assay, the set of FcγR3a affinity-enhanced mutants demonstrated reduced activity. Additionally, FcγR2a and FcγR3a affinity mutants appeared to exhibit the opposite effect as might have been expected on BVADCC assay activity. Further, the nature of the immune complexes formed are likely to be a key variable that may drive differences between monomeric Fc-FcγR interactions and observed activity in biological assays. Collectively, these observations point to the complexity of FcγR-mediated antibody activity, indicating that in many assays, activity is not a simple result of affinity for a single FcγR, but rather may reflect multiple and competing interactions, and that these differing dependencies provide further distinctions between the activities measured by each assay.
Figure 5. Functional activities across a b12 Fc domain point mutant panel.
Functional activities of b12 Fc point mutants with enhanced (dark color) or reduced (light color) binding to FcγRs. Mutants are binned into groups representing those with altered binding to FcγR3a (green) or FcγR2a (blue). Checkered bars represent point mutants whose impact on binding affinity to FcγR3a opposes the impact on binding affinity to FcγR2a, or vice versa. RFADCC results are not shown because they were negative.
Discussion
Evidence has accumulated that beyond prevalence and magnitude of antigen-specific binding antibodies, there are specific qualitative features of the humoral immune response other than virus neutralization that may contribute to reduced risk of HIV infection. A growing number of human and NHP studies have identified correlates of reduced risk of infection related to antibody effector function (reviewed in (18)). Because these studies have often used diverse methods to characterize effector function, we sought to compare a set of cell-based assays of ADCC and phagocytic function for the purpose of identifying key similarities and differences between assays. Given the value of some of these assays to vaccine development based on their status as correlates of vaccine-mediated protection, this comparative study offers data to begin to consolidate the body of work that points toward extra-neutralizing antibody activity as important to protection.
Although other studies have also aimed to compare different assays of Fc receptor function, ours is the first to report systematic evaluation of multiple assays of Fc receptor-mediated anti-HIV-1 antibody effector function, based on a common set of active and control samples tested in a blinded fashion. ADCC, ADCVI and phagocytosis functions measured by seven different assays in four laboratories were determined for purified IgG samples from HIV elite controllers, treated and untreated HIV-infected subjects, as well as VAX004 vaccine and placebo recipients. Antibody activity was generally lower among the vaccine recipients than among HIV-infected subjects. Despite previous reports describing enhanced antibody effector function among subjects exhibiting viral control or classified as long-term non-progressors (8), we did not observe statistically enhanced antibody activity among elite controllers in any assay, consistent with a recent report (49). Because our study utilized purified IgG rather than dilute serum or plasma, it is possible that other serum factors may contribute to the enhanced antibody activity observed in viral suppressors in previous studies. Alternatively, this specific cohort, or responses to the BaL isolate may differ from those evaluated previously in a way that impacted study outcome. Lastly, because this was not a prospective study based on randomized subjects, differences, or lack thereof, in antibody activity observed may be due to factors other than infection status.
Based on evaluation of polyclonal control samples, all seven assays appeared to reasonably discriminate the negative from the positive controls, although the Virion Phagocytosis assay rendered the smallest dynamic range of responses. Differences in activity between samples from HIV-infected subject groups and that observed in healthy uninfected subjects (VAX004 placebo group) were most pronounced in antibody functions captured by the BVADCC, GTL ADCC, and RFADCC assays. Such discrimination was also observed between VAX004 vaccine recipients relative to placebo samples and is an indication that these assays possess superior signal to noise resolution, or that these activities were better elicited among vaccinees and HIV-infected subjects than the other functions evaluated. Given the ability of the LUC ADCC, ADCVI, and ADCP assays to measure activity among our control samples and in some cases correlate with efficacy outcomes in other studies (1, 2, 12, 17), the differences in clustering observed here provide support for utilization of these assays in characterizating a broad spectrum of humoral responses to vaccination.
A major purpose of this study was to identify functional assays that captured unique biological activities. To this end, we observed that while a subset of ADCC assays were grouped in the principal component analysis, assay readouts measuring other Fc functions were only moderately or weakly correlated with each other, suggesting that multiple assays are needed to measure the full spectrum of distinct antibody functions in HIV vaccine studies aimed at identifying correlates of protection against HIV infection.
Interestingly, the coordination of different antibody functions based on their correlations appeared to be highest among elite controllers and weaker among other infected subject groups and VAX004 vaccine recipients. Reduced functional coordination was also observed in a previous study of a similar vaccine regimen conducted in a different population (VAX003) (2), suggesting that this pattern may be vaccine-specific and population-independent. A more coordinated and polyfunctional antibody response was observed among vaccinees in the RV144 trial (2), which demonstrated a moderate level of efficacy against HIV infection. Taken together, these observations suggest that more orchestrated antibody responses may contribute to maintenance of lower levels of HIV viremia for HIV in infected subjects and a reduction in risk of HIV infection in vaccinated subjects, whereas the lack of coordinated antibody activity may be one possible reason for the lack of significant efficacy of the VAX004.
Complex associations were observed between FcγR affinity-dependent b12 mutants and antibody activity. Consistent with previous reports, ADCC and ADCP (22, 39) assays exhibited differentiation among Fc variants, where affinity-enhanced mutants were generally associated with increased antibody activity, and affinity-compromised mutants with reduced activity. However, strong activity patterns proportional to FcγR binding affinity were not observed in the ADCVI and BVADCC assays. While FcγR2a affinity did not seem to be associated with virion phagocytosis activity, the set of FcγR3a-affinity-enhanced mutants demonstrated reduced phagocytosis activity, and FcγR3a-affinity-compromised mutants demonstrated enhanced phagocytic activity. This relationship may indicate a role for competition between FcγR receptors in the response to an opsonized virus or infected cells, and points toward the potential importance of relative affinity differences between different receptors. Such relative differences have most frequently been described as the ratio between binding affinity to activating (A) versus inhibitory (I) receptors, or the A/I ratio (50), associated with a resulting difference in intracellular signaling via inhibitory or activating motifs (51). Our results are consistent with apparent competition among activating receptors.
In some cases, FcγR2a and FcγR3a affinity mutants appeared to exhibit the opposite effect as might have been expected. For example, in the BVADCC assay high affinity mutants demonstrated lower activity than their low affinity counterparts. Interestingly, the BVADCC assay is known to exhibit a striking prozone effect, in which higher concentrations of antibody often lead to lower activity within a certain concentration range. The high affinity of these variants may have driven differential prozone effects across the panel.
Additionally, the use of monoclonal Abs recognizing individual epitopes also exposes potential differences in the effect of epitope presentation or availability across assays. Among assays that utilize different infected cell populations, epitopes may be differentially present or prevalent. Beyond this consideration, several of the assays utilize gp120-coated target cells, for which the CD4 binding site recognized by b12 is likely to be occluded. In fact, activity of this monoclonal antibody was not observed in the RFADCC assay. Overall, the diversity of relationships between FcγR affinity and antibody activity observed for the b12 Fc domain point mutant panel further support and extend our general observations regarding the complementary nature of many of the assays.
This study provides an extensive comparative data set for different FcγR-mediated assays evaluated with a common set of samples. Diverse FcγR-mediated antibody activity at different levels was detected by assays using different effector cell types via different mechanisms. Principal component analysis and correlation analyses identified assays that provided the most distinct measures of antibody effector function. The b12 point mutant panel further supported and extended differentiation between assays in terms of their sensitivity to epitope and Fc affinity for FcγR. The relative activity of the polyclonal control HIVIG and HIVIG-C samples was also different among assays. While HIVIG possessed higher activity than HIVIG-C in the BVADCC, GTL ADCC, and LUC ADCC assays, no difference was observed in the RFADCC, ADCVI, or Phagocytosis assays, and the polyclonal control activity profiles were reversed in the ADCP assay. Furthermore, evaluation of multiple functional assessments in combination enabled determination of relative differences in the extent of functional coordination between subject groups.
Collectively, these data identify and support the utilization of a complementary set of functional assays in the evaluation of humoral responses to HIV vaccines. Continued consideration of the benefit that extra-neutralizing properties may provide in vivo, and which cell-based assays may best identify antibody types and responses that are associated with protection or therapeutic benefit may also be key to advancing prophylactic and therapeutic antibodies clinically (reviewed in (52)). Identification of orthogonal functional assays described by this data set may provide a basis for future vaccine studies in defining meaningful functional antibody activities as potential correlates of protection against HIV infection, and deployment of these functional assessments will facilitate identification of functionally unique humoral responses in future HIV vaccine research efforts.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Rick Koup of the NIH VRC and the Collaboration for AIDS Vaccine Discovery Comprehensive CTVIMC for PBMC, Dr. Christina Ochsenbauer of the University of Alabama at Birmigham for LucR BaL reporter virus used in the LUC ADCC assay, Global Solutions for Infectious Disease for samples from the VAX004 trial, the Ragon Institute of MGH, MIT, and Harvard for cohort samples, Dr. Dennis Burton of The Scripps Research Institute for the panel of b12 Fc mutants, and Dr. Jeff Lifson, NCI, for AT-2 inactivated BaL virions. The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: Catalog #3957, HIVIG from NABI and NHLBI. HIVIG-C was prepared by using pooled purified IgG from clade C infected subjects from S. African blood bank samples donated by Dr. Lynn Morris of the National Institute for Communicable Diseases. The authors thank Lindsay Carpp for editorial assistance in the revision of the manuscript.
Footnotes
These studies were supported by the Collaboration for AIDS Vaccine Discovery (OPP1032144: Comprehensive Antibody Vaccine Immune Monitoring Consortium to D.C.M. G.F., D.F., K.G., H.G.; OPP1032817 to G.A., C.B.-K., K.G.D., H.D.C., M.E.A.; OPP1032317 to Y. H., B.B., L.H.; OPP1033109 to G.K.L), NIH 1R01AI102691 to M.E.A., 5R01Al080289-03 to G.A., R01AI090656 to D.N.F., U01-AI067854 to J.C.K., and R01AI087181 to G.K.L.; and facilities of the Virology, and Genetic Sequencing cores of the UAB Center for AIDS Research (P30-AI-27767).
References
- 1.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Science translational medicine. 2014;6:228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 3.Li SS, Gilbert PB, Tomaras GD, Kijak G, Ferrari G, Thomas R, Pyo CW, Zolla-Pazner S, Montefiori D, Liao HX, Nabel G, Pinter A, Evans DT, Gottardo R, Dai JY, Janes H, Morris D, Fong Y, Edlefsen PT, Li F, Frahm N, Alpert MD, Prentice H, Rerks-Ngarm S, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Robb ML, O’Connell RJ, Haynes BF, Michael NL, Kim JH, McElrath MJ, Geraghty DE. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. The Journal of clinical investigation. 2014;124:3879–3890. doi: 10.1172/JCI75539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. Journal of virology. 2012;86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forthal DN, Landucci G, Keenan B. Relationship between antibody-dependent cellular cytotoxicity, plasma HIV type 1 RNA, and CD4+ lymphocyte count. AIDS research and human retroviruses. 2001;17:553–561. doi: 10.1089/08892220151126661. [DOI] [PubMed] [Google Scholar]
- 8.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- 9.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS pathogens. 2012;8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell host & microbe. 2015;17:500–506. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, Ahmad A. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. Journal of clinical immunology. 2001;21:227–233. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 12.Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178:6596–6603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 13.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 14.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florese RH, Van Rompay KK, Aldrich K, Forthal DN, Landucci G, Mahalanabis M, Haigwood N, Venzon D, Kalyanaraman VS, Marthas ML, Robert-Guroff M. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol. 2006;177:4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 16.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng’ang’a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. HIV-1 vaccines. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349:320–324. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, Dugast AS, Alter G, Ferguson M, Li W, Earl PL, Moss B, Giorgi EE, Szinger JJ, Eller LA, Billings EA, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Pau MG, Schuitemaker H, Robb ML, Kim JH, Korber BT, Michael NL. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155:531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis GK. Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology. 2014;142:46–57. doi: 10.1111/imm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackerman ME, Alter G. Opportunities to exploit non-neutralizing HIV-specific antibody activity. Current HIV research. 2013;11:365–377. doi: 10.2174/1570162x113116660058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, Baker B, Trocha A, Rosenberg R, Mackey E, Ueda P, Lu Z, Cohen D, Wrin T, Petropoulos CJ, Rosenberg ES, Walker BD. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. The Journal of infectious diseases. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 21.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF H. I. V. V. S. G. rgp. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. The Journal of infectious diseases. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 22.Moldt B, Schultz N, Dunlop DC, Alpert MD, Harvey JD, Evans DT, Poignard P, Hessell AJ, Burton DR. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcgamma receptors to define the role of effector functions in protection against HIV. Journal of virology. 2011;85:10572–10581. doi: 10.1128/JVI.05541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imbach P, Barandun S, d’Apuzzo V, Baumgartner C, Hirt A, Morell A, Rossi E, Schoni M, Vest M, Wagner HP. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1:1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 24.Cummins LM, Weinhold KJ, Matthews TJ, Langlois AJ, Perno CF, Condie RM, Allain JP. Preparation and characterization of an intravenous solution of IgG from human immunodeficiency virus-seropositive donors. Blood. 1991;77:1111–1117. [PubMed] [Google Scholar]
- 25.Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, Alter G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcgammaR2a and FcgammaR2b. Journal of virology. 2013;87:5468–5476. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambor A, Garcia A, Berrong M, Pickeral J, Brown S, Rountree W, Sanchez A, Pollara J, Frahm N, Keinonen S, Kijak GH, Roederer M, Levine G, D’Souza MP, Jaimes M, Koup R, Denny T, Cox J, Ferrari G. Establishment and maintenance of a PBMC repository for functional cellular studies in support of clinical vaccine trials. Journal of immunological methods. 2014;409:107–116. doi: 10.1016/j.jim.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2011;79:603–612. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, Whitesides JF, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, O’Connell RJ, Kim JH, Michael NL, Montefiori DC, Tomaras GD, Liao HX, Haynes BF, Ferrari G. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. Journal of virology. 2014;88:7715–7726. doi: 10.1128/JVI.00156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Roman VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, Robert-Guroff M. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. Journal of immunological methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, Arthur LO, Henderson LE, Lifson JD. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. Journal of virology. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chertova E, Bess JW, Jr, Crise BJ, Sowder IR, Schaden TM, Hilburn JM, Hoxie JA, Benveniste RE, Lifson JD, Henderson LE, Arthur LO. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. Journal of virology. 2002;76:5315–5325. doi: 10.1128/JVI.76.11.5315-5325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chertova E, Crise BJ, Morcock DR, Bess JW, Jr, Henderson LE, Lifson JD. Sites, mechanism of action and lack of reversibility of primate lentivirus inactivation by preferential covalent modification of virion internal proteins. Current molecular medicine. 2003;3:265–272. doi: 10.2174/1566524033479889. [DOI] [PubMed] [Google Scholar]
- 34.Mengistu M, Ray K, Lewis GK, DeVico AL. Antigenic properties of the human immunodeficiency virus envelope glycoprotein gp120 on virions bound to target cells. PLoS pathogens. 2015;11:e1004772. doi: 10.1371/journal.ppat.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis GK, Guan Y, Kamin-Lewis R, Sajadi M, Pazgier M, Devico AL. Epitope target structures of Fc-mediated effector function during HIV-1 acquisition. Current opinion in HIV and AIDS. 2014;9:263–270. doi: 10.1097/COH.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, Lovo E, Wu X, Robinson JE, Seaman MS, Fouts TR, Gallo RC, DeVico AL, Lewis GK. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E69–78. doi: 10.1073/pnas.1217609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. Journal of virology. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, Dugast AS, Parren PW, Nimmerjahn F, Evans DT, Alter G, Forthal DN, Schmitz JE, Iida S, Poignard P, Watkins DI, Hessell AJ, Burton DR. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. Journal of virology. 2012;86:6189–6196. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. Journal of immunological methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Team, R. C. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- 41.Murtagh F. A Survey of Recent Advances in Hiearchical Clustering Algorithms. The Computer Journal. 1983;26:354–359. [Google Scholar]
- 42.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning : data mining, inference, and prediction. Springer; New York: 2009. [Google Scholar]
- 43.Pornillos O, Ganser-Pornillos BK. HIV-1 Virion Structure. In: Hope TJ, Stevenson M, Richman D, editors. Encyclopedia of AIDS. Springer; New York: 2014. pp. 1–6. [Google Scholar]
- 44.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wren L, Parsons MS, Isitman G, Center RJ, Kelleher AD, Stratov I, Bernard NF, Kent SJ. Influence of cytokines on HIV-specific antibody-dependent cellular cytotoxicity activation profile of natural killer cells. PloS one. 2012;7:e38580. doi: 10.1371/journal.pone.0038580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. The Journal of clinical investigation. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wren LH, Chung AW, Isitman G, Kelleher AD, Parsons MS, Amin J, Cooper DA, Stratov I, Navis M, Kent SJ A. s. c. investigators. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology. 2013;138:116–123. doi: 10.1111/imm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J, Kent SJ, Stratov I. Activation of NK cells by ADCC antibodies and HIV disease progression. Journal of acquired immune deficiency syndromes. 2011;58:127–131. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, Suscovich TJ, Alter G. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS pathogens. 2016;12:e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 51.Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. The Journal of experimental medicine. 1999;189:179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boesch AW, Alter G, Ackerman ME. Prospects for engineering HIV-specific antibodies for enhanced effector function and half-life. Current opinion in HIV and AIDS. 2015;10:160–169. doi: 10.1097/COH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.