Abstract
Kidney cancer is not a single disease but is made up of a number of different types of cancer classified by histology that are disparate in presentation, clinical course, and genetic basis. Studies of families with inherited renal cell carcinoma (RCC) have provided the basis for our understanding of the causative genes and altered metabolic pathways in renal cancer with different histologies. Von Hippel-Lindau disease was the first renal cancer disorder with a defined genetic basis. Over the next two decades, the genes responsible for a number of other inherited renal cancer syndromes including hereditary papillary renal carcinoma, Birt-Hogg-Dubé syndrome, hereditary leiomyomatosis and renal cell carcinoma, and succinate dehydrogenase–associated renal cancer were identified. Recently, renal cell carcinoma has been confirmed as part of the clinical phenotype in individuals from families with BAP1-associated tumor predisposition syndrome and MiTF-associated cancer syndrome. Here we summarize the clinical characteristics of and causative genes for these and other inherited RCC syndromes, the pathways that are dysregulated when the inherited genes are mutated, and recommended clinical management of patients with these inherited renal cancer syndromes.
Keywords: Von Hippel-Lindau, Hereditary papillary renal carcinoma, Birt-Hogg-Dubé syndrome, Hereditary leiomyomatosis and renal cell carcinoma, Kidney neoplasms, Inherited renal cancer syndromes
1. Overview: Hereditary forms of kidney cancer
An estimated 62,700 new cases of kidney cancer will be diagnosed in the United States in 2016, resulting in 14,200 deaths and a steadily increasing incidence over the last decade [1]. It is important for the clinician to recognize whether a patient has an inherited form of kidney cancer or sporadic renal tumors because this will impact patient management. Early onset (<40 years of age), family history of renal cancer, and bilateral or multifocal renal tumor presentation are suggestive of an inherited predisposition. Presence of a particular group of manifestations in a patient or family may suggest a specific inherited renal cancer syndrome, and it is helpful for the clinician to be aware of the major inherited renal cancer syndromes, their manifestations, the causative genes and implicated pathways, patient management practices and available therapeutic options (Table 1, Fig. 1).
Table 1.
Inherited renal cancer syndromes.
| Syndrome | Chromosome location | Predisposing gene | Renal tumor histology | Recommended surgical management | Potential therapeutic targets |
|---|---|---|---|---|---|
| Von Hippel-Lindau disease (VHL) | 3p25 | VHL | Clear cell | Active surveillance <3 cm; surgical excision ≥3 cm | HIF-VEGF pathway |
| Hereditary papillary renal carcinoma (HPRC) | 7q31 | MET | Type 1 papillary | Active surveillance <3 cm; surgical excision ≥3 cm | Met kinase |
| Birt-Hogg-Dubé syndrome (BHD) | 17p11.2 | FLCN | Chromophobe, hybrid oncocytic, clear cell, oncocytoma | Active surveillance <3 cm; surgical excision ≥3 cm | mTOR pathway |
| Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) | 1q42–43 | FH | Type 2 papillary | Wide margin surgical excision | HIF-VEGF pathway; antioxidant response pathway; reductive carboxylation pathway |
| Succinate dehydrogenase-deficient renal cancer (SDH-RCC) | 1p36.13 1q23.3 11q23.1 |
SDHB SDHC SDHD |
Clear cell, chromophobe, oncocytic neoplasm | Surgical excision | HIF-VEGF pathway; reductive carboxylation pathway |
| Tuberous sclerosis complex (TSC) | 9q34 16p13.3 |
TSC1 TSC2 |
Angiomyolipoma, RCC, variable | AML, embolization; RCC, surgical excision | mTOR pathway |
| BAP1 tumor predisposition syndrome | 3p21.2 | BAP1 | Clear cell, can be high grade | Surgical excision | TBD |
| MiTF-associated cancer syndrome | 3p14.1-p12.3 | MiTF | ND | TBD | TBD |
ND, not determined; TBD, to be determined; RCC, renal cell carcinoma.
Fig. 1.
Hereditary kidney cancer. Kidney cancer is not a single entity but made up of a number of different types of cancer, each with a distinct histology, caused by a different gene, with a different clinical course, and responding differently to therapy. Germline von Hippel-Lindau (VHL) gene mutations cause von Hippel-Lindau disease and clear cell kidney tumors. Germline MET oncogene mutations predispose to hereditary papillary renal carcinoma with type 1 papillary tumors. Germline mutations in the folliculin (FLCN) gene are inherited in patients with Birt-Hogg-Dubé syndrome who present with hybrid oncocytic tumors, chromophobe renal tumors and benign oncocytomas. Germline fumarate hydratase (FH) gene mutations in patients with hereditary leiomyomatosis and renal cell carcinoma predispose affected individuals to develop renal tumors with papillary type 2 histology. Germline mutations in the genes encoding subunits of succinate dehydrogenase, SDHB/SDHC/SDHD, predispose to renal tumors with an oncocytic phenotype in SDH-deficient RCC patients. Patients with tuberous sclerosis complex inherit germline mutations in tuberous sclerosis complex 1 or 2 (TSC1, TSC2) genes and are at risk to develop angiomyolipomas in the kidney and, occasionally, renal tumors. Adapted and reprinted with permission [97].
2. Von Hippel-Lindau: Clear cell renal carcinoma
2.1. Clinical manifestations
Von Hippel-Lindau (VHL) is an autosomal dominant inherited multisystem disorder in which affected individuals are at risk for the development of clear cell kidney tumors and cysts, adrenal gland tumors (pheochromocytomas), pancreatic cysts and islet cell tumors, hemangioblastomas of the central nervous system (CNS) and retina, endolymphatic sac tumors of the inner ear, and epididymal cystadenomas (Fig. 2). Kidney cancer develops in 25%–45% of VHL patients and is uniformly clear cell, bilateral, and multifocal. VHL-related kidney cancer tends to occur in the 2nd to 4th decades of life with a penetrance of 70% by the age of 60 [2]. It has been estimated that up to 600 tumors and as many as 1100 cysts may develop in a single kidney of a VHL patient [3].
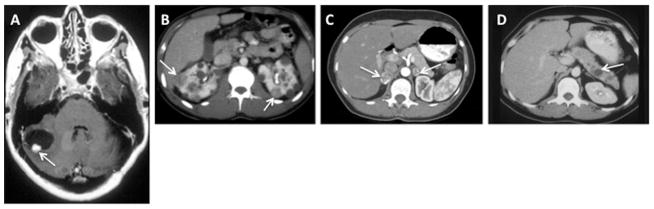
Fig. 2.
Clinical manifestations of von Hippel-Lindau disease. (A) Contrast-enhanced MRI of a cerebellar hemangioblastoma (arrow) with an associated cyst (adjacent dark area) in a 40-year-old VHL patient. (B) Bilateral multifocal renal tumors (arrows) and multiple cysts in a 22-year-old VHL patient. (C) Bilateral pheochromocytomas (arrows) in the adrenal glands of a 29-year-old VHL patient. (D) Pancreatic neuroendocrine tumor (arrow) in the pancreas of a VHL patient. Adapted and reprinted with permission [98].
2.2. Patient management
A multidisciplinary approach is recommended to manage VHL patients to monitor the development of the benign and malignant lesions associated with this disorder. At-risk individuals undergo lifelong surveillance for kidney tumors, most often with either computed tomography (CT) or magnetic resonance imaging (MRI). Since these individuals are at risk to develop multiple tumors that may require repeat surgeries during their lifetime, nephron-sparing surgery is recommended when possible to preserve kidney function. It is recommended that VHL patients be managed by active surveillance and undergo surgical intervention when the largest tumor reaches 3 cm in diameter to potentially reduce risk for metastatic disease while minimizing the number of repeat surgeries and preserving renal function [4].
2.3. Genetics of VHL disease: VHL gene
Genetic linkage analysis was performed in families with VHL in order to identify the VHL locus at chromosome 3p25 [5]. Germline VHL mutations are detected in nearly 100% of families with more than 420 unique mutations of all types reported worldwide [6] and genotype–phenotype correlations have emerged. The majority of families that develop RCC and CNS hemangioblastomas without pheochromocytomas, classified as type 1, have truncating mutations or intragenic deletions. Type 2 VHL families have predominantly missense mutations and present with pheochromocytomas either without (type 2A) or with (type 2B) RCC and hemangio-blastomas (types 2A and 2B) [2]. VHL is a classic tumor-suppressor gene in which both copies of the gene must be inactivated for tumor development.
2.4. VHL gene mutations in sporadic clear cell renal carcinoma
VHL mutations with subsequent inactivation of the wild-type VHL allele are also found in a high percentage of tumors from patients with sporadic clear cell RCC [7]. Nickerson and colleagues detected VHL mutation or hypermethylation in 92% of sporadic clear cell RCCs [8].
2.5. Consequence of VHL gene mutation: upregulation of hypoxia-inducible factor and its target genes
The VHL protein (pVHL) is part of an E3 ubiquitin ligase multi-protein complex, including elongins B and C [9,10], cullin-2 [11], and Rbx1 [12] that targets proteins for ubiquitin-mediated degradation by the proteasome. pVHL functions as the substrate recognition site for the hypoxia-inducible factor alpha (HIFα) family of transcription factors [13]. During normoxia, HIFα becomes hydroxylated on critical prolines by HIF prolyl hydroxylase (PHD), enabling HIFα binding to pVHL and degradation by the E3 ligase complex [14,15]. PHD requires cofactors α-ketoglutarate, ascorbate, iron and molecular oxygen to function. Under hypoxia, HIFα is not hydroxylated by PHD and, therefore, not targeted by pVHL. Either reduced cellular oxygen levels or VHL mutations that abrogate elongin C or HIFα binding lead to HIFα accumulation, driving expression of HIFα transcriptional targets that support neo-angiogenesis [erythropoietin (EPO), vascular endothelial growth factor (VEGF)], cell proliferation [platelet-derived growth factor (PDGFβ), transforming growth factor (TGF-α)], and glucose metabolism [glucose transporter 1 (GLUT 1)] [16].
2.6. Targeted therapy for VHL disease and clear cell RCC
Targeting the HIF pathway has been an approach to treating advanced VHL-associated and sporadic clear cell RCC. There are a number of drugs approved by the US Food and Drug Administration (FDA) for metastatic renal carcinoma: five drugs that target the HIF-VEGF pathway, including sunitinib and sorafenib (VEGFR2/3, PDGFRβ), bevacizumab (VEGF), pazopanib (VEGFR1/2/3, PDGFRβ, c-kit), and axitinib (VEGFR 1/2/3); and two drugs that target the mechanistic target of rapamycin (mTOR) pathway, everolimus and temsirolimus. Most patients, however, do not achieve complete response and will eventually progress on treatment [17].
3. Hereditary papillary renal carcinoma: Type 1 papillary renal carcinoma
3.1. Clinical manifestations
Hereditary papillary renal carcinoma (HPRC), another autosomal dominant inherited renal cancer syndrome, is characterized by the development of bilateral, multifocal papillary type 1 renal tumors [18]. It was estimated that more than 3,000 microscopic papillary tumors (“incipient” lesions) [19] may develop in apparently normal renal parenchyma within a single HPRC kidney representing multiple independent early events [20]. HPRC is rare, with fewer than 35 families reported worldwide [21]. Renal tumors develop most often in the 6th and 7th decades of life [18,22], although early-onset families have been reported [21]. HPRC displays nearly complete penetrance by age 80 [22].
3.2. Patient management
Hereditary papillary renal tumors may be difficult to image due to their hypovascularity [23] and can often be confused with cysts necessitating taking tumor measurements with and without contrast enhancement. Active surveillance with MRI or CT rather than ultrasound is recommended for at-risk HPRC family members [24]. Tumors tend to be slow-growing and patients are often managed by surgical intervention when the largest tumor dimension reaches 3 cm, using nephron-sparing surgery where possible to maintain maximum renal function [4].
3.3. Genetics of HPRC: MET gene
Linkage analysis in HPRC families localized the disease locus to chromosome 7q31, and mutations in the MET proto-oncogene were identified in the germline of affected family members [25]. HPRC-associated MET mutations are missense (amino acid substitution), located in the intracellular tyrosine kinase domain, and predicted to constitutively activate the Met kinase [22,25,26].
3.4. Consequence of MET gene mutation: constitutive activation of Met
The MET proto-oncogene encodes the receptor for hepatocyte growth factor/scatter factor (HGF/SF). HGF binding to Met causes autophosphorylation of critical tyrosines in the Met kinase domain resulting in recruitment of intracellular effectors triggering a signal cascade that drives programs supporting cell growth, motility, migration, differentiation, and branching morphogenesis [27]. The germline MET mutations in HPRC are predicted to destabilize the inactive (or stabilize the active) Met kinase conformation supporting ligand-independent constitutive kinase activation [28], and have shown oncogenic potential in both in vitro and in vivo models [29]. Papillary type 1 renal tumors are characterized by trisomy of chromosome 7 [30], and nonrandom duplication of the chromosome 7 bearing the mutant MET allele has been demonstrated in HPRC tumors [31], which may give kidney tumor cells a growth advantage.
Although MET mutations have been identified in fewer than 15% of sporadic papillary renal carcinoma [26,32], Met amplification may provide one mechanism by which sporadic papillary RCC develops [32].
3.5. Met-targeted therapy for HPRC
The discovery that activating MET mutations are responsible for HPRC led to assessment of therapeutic agents that target the Met kinase. A multicenter phase 2 study of foretinib, an oral agent that targets Met, VEGFR2, RON and AXL, in patients with HPRC and sporadic papillary RCC was recently completed. The overall response rate was 13.5% with median progression-free survival of 9.3 months. The presence of a germline MET mutation was found to be highly predictive of response. Among patients with MET germline mutations, 5 of 10 (50%) responded with a reduction in largest tumor dimension ranging from 30%–60% compared to a 9% response rate (5 of 57) among patients with wild-type MET [33]. A phase 2 study to evaluate the efficacy of INC280, a selective Met kinase inhibitor, in hereditary and sporadic papillary RCC patients, is currently in the recruitment phase (clinicaltrials.gov; NCT02019693).
4. Birt-Hogg-Dubé syndrome: Hybrid oncocytic and chromophobe renal carcinoma
4.1. Clinical manifestations
Individuals with the autosomal dominant inherited disorder Birt-Hogg-Dubé (BHD) syndrome are at risk for developing benign cutaneous hair follicle tumors (fibrofolliculomas), multiple lung cysts, and spontaneous pneumothorax (Fig. 3) [34,35]. More than 80% of BHD-affected individuals develop fibrofolliculomas or lung cysts, and 27%–30% will experience at least one pneumothorax episode [35,36]. Approximately one third of BHD-affected individuals develop renal tumors that are usually bilateral and multifocal [35–38] with variable histologies including hybrid oncocytic tumors containing features of both chromophobe RCC and oncocytoma (50%), chromophobe RCC (34%), clear cell RCC (9%), and benign oncocytomas (5%) [39]. Renal “oncocytosis”, defined as microscopic oncocytic tumors scattered in the grossly normal renal parenchyma, are characteristic of BHD-associated tumors [39].
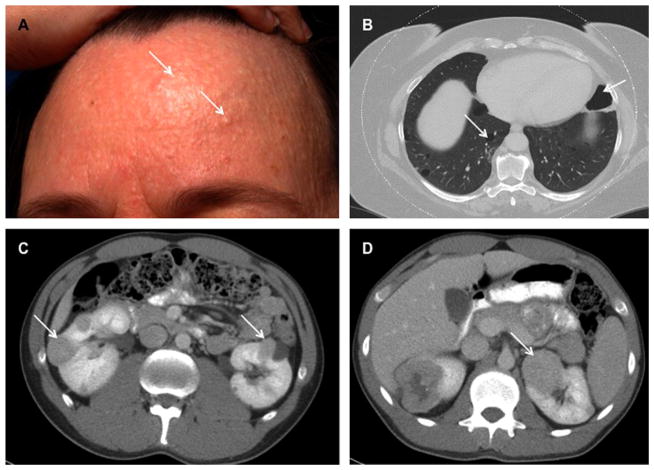
Fig. 3.
Clinical manifestations of Birt-Hogg-Dubé syndrome. (A) Multiple fibrofolliculomas on the forehead of a BHD patient (arrows). (B) Chest CT scan of a BHD patient showing bilateral multiple pulmonary cysts (arrows) that can lead to spontaneous pneumothorax. (C,D) Abdominal CT scans demonstrating bilateral multifocal renal tumors in BHD patients (arrows). Adapted and reprinted with permission [54].
4.2. Patient management
BHD is phenotypically variable within families and among different families with the same germline genetic alteration. Patients at risk for BHD are evaluated by a dermatologist for fibrofolliculomas and may undergo both thoracic and abdominal imaging to screen for lung cysts, evidence of pneumothorax, and for presence of renal tumors. Surveillance by annual or biannual CT or MRI is recommended starting at the age of 20 [40,41]. As with VHL and HPRC, current recommendations for managing BHD renal tumors include active surveillance until the largest diameter reaches 3 cm, at which time surgical intervention is recommended. Nephron-sparing surgery to preserve normal renal function is recommended, since BHD patients are at risk to develop multiple tumors and may undergo repeated surgeries during their lifetime [40].
4.3. Genetics of BHD syndrome: FLCN gene
The disease locus for BHD was mapped to chromosome 17p by genetic linkage analysis in BHD families, and germline mutations in a novel gene folliculin (FLCN) were identified in BHD-affected individuals [42]. The majority of the 149 unique FLCN mutations reported in BHD families are protein truncating and predicted to be inactivating, although FLCN missense mutations have been reported [43]. The overall mutation detection rate approaches 85% [36,38]. Loss or mutation of the wild-type FLCN allele in 70% of BHD-associated renal tumors [44] and tumor development in mice injected with FLCN-deficient human kidney cells [45] support a role for FLCN as a tumor-suppressor.
4.4. Consequence of FLCN gene mutation: modulation of mTOR activity
In experiments to elucidate FLCN function, a novel interacting protein, folliculin interacting protein 1 (FNIP1), was identified [46] and, subsequently, a second folliculin interacting partner, FNIP2, was discovered by bioinformatics searches, which had 49% identity to FNIP1 [47]. Both FNIP1 and FNIP2 interact with the carboxy-terminus of FLCN, and also with 5′-AMP–activated protein kinase (AMPK) [46,47], an important cellular energy-sensor and negative regulator of mTOR, which controls protein synthesis and cell growth [48]. In some Flcn-deficient mouse kidneys and human FLCN-null renal tumors, mTORC1 [49,50] and mTORC2 [51] were activated, suggesting a possible target for therapeutic intervention. Other in vivo data suggest that FLCN deficiency results in mTOR inactivation [52,53]. Multiple lines of evidence support potential roles for FLCN in a number of cellular processes and metabolic pathways including TFE3 transcriptional activation, regulation of PGC-1α expression and mitochondrial biogenesis, membrane trafficking, TGF-β signaling, autophagy, cell–cell adhesion and cell polarity [54]. Research efforts in multiple laboratories are focused on determining which of these critical pathways/processes, when dysregulated by loss of FLCN, results in BHD-associated kidney cancer.
4.5. Targeting the FLCN pathway
Currently there are no therapeutic agents with proven efficacy for treating BHD-associated renal tumors. In a single report, the mTOR inhibitor everolimus provided a longer time to progression when used as a second-line treatment against metastatic papillary RCC in a BHD-affected patient who failed to respond to other anti-VEGF systemic therapies [55]. The finding that mTOR was activated in some FLCN-deficient in vivo and in vitro models provided the basis for a phase 2 study to evaluate the efficacy of everolimus in BHD patients and patients with advanced sporadic chromophobe RCC (clinicaltrials.gov; NCT02504892).
5. Hereditary leiomyomatosis and renal cell carcinoma: Type 2 papillary renal carcinoma
5.1. Clinical manifestations
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) is an autosomal dominant inherited cancer disorder that predisposes to cutaneous leiomyomas, multiple uterine leiomyomas (fibroids), and an increased risk for type 2 papillary renal tumors (Fig. 4). Cutaneous leiomyomas, small flesh-colored nodules usually on the trunk or extremities that often exhibit pain and paresthesias, are the most common manifestations occurring in 76%–100% of HLRCC patients [56,57]. Uterine leiomyomas develop at an early age in most female HLRCC patients, cause pain and menorrhagia, and often necessitate hysterectomy before the age of 40 [58]. Renal tumors, which can be early onset, may develop in up to 18% of HLRCC patients [56,57,59]. Tumors are often unilateral and solitary but can be bilateral and multifocal, are highly aggressive, and can metastasize even when small [60]. Histologically, HLRCC tumors are characterized by large nuclei with prominent orangiophilic nucleoli and perinucleolar clearing, and a unique type 2 papillary architecture, but can also be tubular, solid, or mixed [61].
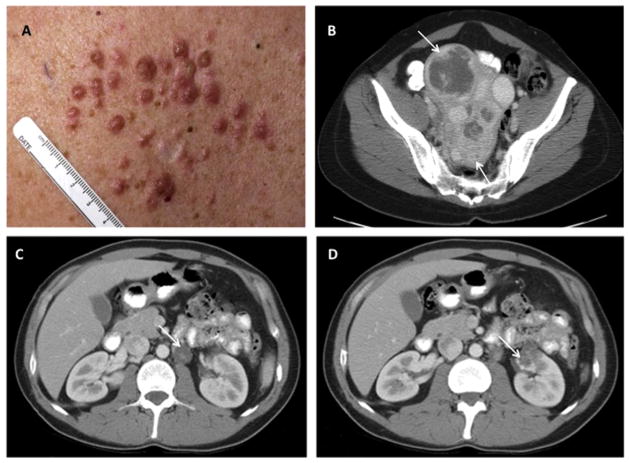
Fig. 4.
Clinical manifestations of hereditary leiomyomatosis and renal cell carcinoma. (A) Multiple cutaneous leiomyomas in an HLRCC patient. (B) CT image showing multiple large uterine leiomyomas (arrows) that occur in HLRCC. (C) Para-aortic nodal disease (arrow) and (D) renal tumor (arrow) in patients with HLRCC. Adapted and reprinted with permission [60].
5.2. Patient management
HLRCC patients are managed with a multidiscipline approach for early detection of cutaneous and uterine leiomyomas and kidney tumors. In contrast to recommended management of patients with VHL, HPRC, and BHD, renal masses in HLRCC patients are not managed by active surveillance. HLRCC-associated renal tumors may be aggressive with rapid growth rates; advanced disease at presentation with nodal involvement or distant metastases can occur even when tumors are small [60]. Consequently, at-risk individuals are screened by annual abdominal MRI. Screening begins early in at-risk children, since tumors have been detected as early as 11 years of age [62]. Nephron-sparing surgical excision is often recommended with open procedures and wide surgical margins. Enucleative resection and ablative procedures are not recommended for patients with HLRCC, and avoidance of intraoperative tumor spillage is a high priority.
5.3. Genetics of HLRCC: FH gene
Launonen et al [63] described the association of cutaneous and uterine leiomyomas with renal tumors in two kindreds, and mapped the HLRCC locus to chromosome 1q42-44. Subsequently, germline mutations in the fumarate hydratase (FH) gene were identified in HLRCC kindreds [64]. More than 130 unique FH mutations of all types have been reported [65] with a mutation detection rate approaching 90% [56,58]. FH encodes the Krebs cycle enzyme fumarate hydratase that converts fumarate to malate and is located in the mitochondrial matrix. Fumarate hydratase enzyme activity is reduced in lymphoblastoid and fibroblast cell lines established from HLRCC patients [66,67], and was lower in cell lines with FH missense mutations than in cells carrying FH protein truncating mutations [58,64,66]. Since FH is a homotetrameric protein, it has been proposed that the FH missense mutant proteins would abrogate formation of almost all homotetramers, thereby having a more severe effect on fumarate hydratase activity than protein truncating mutations [59]. The FH gene acts as a tumor suppressor based on the identification of LOH or “second hit” somatic mutations in the wild-type FH allele in syndromic uterine and cutaneous leiomyomas [63,64,66] and HLRCC-associated renal tumors [66,68].
5.4. Consequence of FH gene mutation: activation of HIF and antioxidant response pathways
Loss of fumarate hydratase activity as a result of germline FH mutation and LOH at chromosome 1q42 leads to accumulation of fumarate in the cytoplasm of renal cells and tissues of HLRCC patients resulting in aberrant activation of two pathways: (a) HIFα pathway and (b) Nrf2 antioxidant response pathway. Excess fumarate competitively inhibits prolyl hydroxylase (PDH), resulting in HIFα stabilization as seen during hypoxia or when VHL is mutated. The subsequent upregulation of HIFα transcriptional targets supports neo-vascularization and elevated glucose uptake resulting in a metabolic switch to aerobic glycolysis (“Warburg effect”) for energy production, which contributes to the aggressive phenotype of HLRCC-associated renal tumors [68–70]. In addition, since FH-deficient tumors have an altered Krebs cycle that does not generate acetyl CoA for lipogenesis, cells have adapted to utilizing glutamine as a carbon source for fatty acid production by isocitrate dehydrogenase (IDH)-dependent reductive carboxylation of α–ketoglutarate, and generation of acetyl-CoA by ATP-citrate lyase cleavage of citrate [71].
Fumarate is an electrophile and can spontaneously react with sulfhydryl groups in cysteines in a number of proteins, a process known as succination [72]. One of the proteins succinated by fumarate is Kelch-like ECH-associated protein 1 (KEAP1), the substrate recognition component of a cullin 3 (CUL3)-based E3 ubiquitin ligase complex that targets nuclear factor erythroid 2-related factor 2 (Nrf2) for proteasomal degradation [73,74]. Nrf2 facilitates a cellular adaptive response to electrophilic and oxidative stress by transcriptionally upregulating its target genes through anti-oxidant response elements located in their promoters. KEAP1, in complex with CUL3, binds to Nrf2, targeting it for ubiquitin-mediated degradation [75]. Fumarate can act as an oncometabolite using its electrophilic properties to succinate specific cysteines in KEAP1, which results in a conformational change that inhibits KEAP1 binding to Nrf2, thereby permitting Nrf2 accumulation and activation of the antioxidant response pathway [73,74]. Targeting HIF and its targets, components of the glutamine reductive carboxylation pathway, or the components of the antioxidant response pathway may be potential therapeutic options for HLRCC.
5.5. Targeted therapy for HLRCC involving HIF and antioxidant response pathways
Therapeutic targeting of FH-deficient renal tumors in HLRCC patients may be approached in several ways. First, elevated fumarate inhibits PHD, thereby stabilizing HIFα and driving expression of target genes VEGF and GLUT1 that can be targeted by anti-angiogenic therapies. A phase 2 study of bevacizumab and erlotinib in patients with advanced HLRCC-associated type 2 papillary RCC and sporadic papillary RCC is currently in progress (clinicaltrials.gov; NCT01130519).
Second, drug screening identified the tyrosine kinase inhibitor vandetanib as a potent inhibitor of cell growth in an HLRCC-derived kidney cancer cell line, and stable reintroduction of wild-type FH abrogated its cytotoxicity [76]. Evaluation of FH-deficient in vitro and in vivo models revealed that the nonreceptor tyrosine kinase ABL1 was activated in FH-deficient kidney tumors. ABL1 upregulated aerobic glycolysis through the mTOR/HIFα pathway and promoted nuclear localization and activation of Nrf2, the master regulator of the antioxidant response pathway that allows tumor cells to tolerate the oxidative stress caused by fumarate accumulation [76]. Vandetanib was shown to be an effective inhibitor of ABL1 phosphorylation, glucose transporter GLUT1 upregulation, and lactate secretion (measure of aerobic glycolysis) in FH-deficient kidney cancer cells. Treatment with vandetanib resulted in regression of a murine xenograft derived from an FH-deficient kidney tumor cell line [76]. On the basis of these findings, a phase 1/2 study of the effect of vandetanib in combination with metformin is currently in progress in patients with HLRCC or advanced sporadic papillary RCC (clinicaltrials.gov, NCT02495103).
6. Succinate dehydrogenase-deficient kidney cancer
6.1. Mutations in another Krebs cycle enzyme predispose to RCC
Succinate dehydrogenase–deficient kidney cancer (SDH-RCC) is characterized by bilateral, multifocal early onset (<40 years of age) renal tumors that are inherited in an autosomal dominant manner and can be found in the setting of inherited head and neck paragangliomas (PGLs) and adrenal or extra-adrenal pheochromocytomas (PCCs). Vanharanta et al reported PGL/PCC and RCC in two families with early age of renal tumor onset (<30 years) and variable histologies who harbored germline mutations in the SDHB gene encoding subunit B of the Krebs cycle enzyme succinate dehydrogenase [77]. Three families with germline SDHB mutations and RCC as the only manifestation were subsequently reported [78]. Ricketts et al have described 11 families with germline SDHB mutations of various types and clinical manifestations that included only renal tumors (45.5%), or renal tumors and PGL/PCC (54.5%) [79]. The renal tumors were solid or mixed solid/cystic lesions, but most shared common “oncocytic neoplastic” features and were characterized by early age of onset (average 33 years) and the presence of metastases in 1/3 of cases. Two families with RCC and germline SDH subunit C (SDHC) mutations have been reported [79,80], as well as one family with RCC and a germline SDH subunit D (SDHD) mutation [79].
6.2. Patient management
Annual abdominal imaging of at-risk SDHB/C/D mutation carriers by MRI or CT is recommended for early detection of renal tumors. Given the paucity of SDH-RCC, the clinical experience managing these patients is limited. However, considering the early age of onset and tendency of these tumors to potentially metastasize even when small, it is recommended that surgical excision of tumors be performed with nephron-sparing procedures promptly when tumors are detected following the management approach for HLRCC [79].
6.3. Consequence of SDHB/C/D gene mutation: activation of the HIF pathway
SDH is a multi-subunit enzyme that functions as Complex II in the inner mitochondrial membrane and converts succinate to fumarate generating reducing equivalents to drive the electron transport chain for energy production. Mutations in any of the subunits will interfere with proper complex assembly and result in succinate accumulation that will in turn block PHD by product inhibition, thereby stabilizing HIFα and its transcriptional targets [81]. Studies with an SDH-deficient renal tumor cell line derived from a patient with a germline SDHB mutation have shown that, as in HLRCC, oxidative phosphorylation was severely compromised, and SDH activity was abrogated, causing increased levels of intra-cellular succinate that resulted in elevated HIF1α protein levels presumably through succinate-mediated competitive inhibition of PHD [82]. Metabolic profiling demonstrated that, in the absence of mitochondrial respiration, these tumor cells underwent a metabolic shift to aerobic glycolysis to generate ATP, and displayed a dependence on reductive carboxylation of α–ketoglutarate derived from glutamine [82].
7. Tuberous sclerosis complex
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder that presents with a variety of manifestations in multiple tissues including severe neurologic disorders due to the presence of cerebral cortical tubers, facial angiofibromas, lymphangioleio-myomatosis (LAM) of the lung, and renal angiomyolipomas (AML). TSC patients develop renal carcinoma at a frequency similar to that of the general population (2%–3%) but with an earlier age of onset [83]. Germline mutations in the TSC1 gene on chromosome 9q34 that encodes hamartin or the TSC2 gene on chromosome 16p13 encoding tuberin are responsible for this multisystem disorder [84,85]. TSC1/TSC2 form a protein complex that negatively regulates the mTOR axis and mutations in either gene can abrogate the function of the complex leading to mTOR activation [83]. mTOR inhibitors including rapamycin analogs have been used to treat angiomyolipomas and lymphangioleiomyomatosis in TSC patients. In a phase 3 clinical trial of TSC patients with AMLs treated with everolimus, the response rate (50% reduction in AML volume from base line and no progression) was 42% compared to placebo (0%) with 80% of patients demonstrating at least 30% reduction in AML volume relative to the placebo group [86]. Inhibition of mTOR with everolimus may provide an alternative treatment for TSC patients for whom surgical intervention may otherwise not be an option.
8. BAP1-associated tumor predisposition syndrome
BRCA1-associated protein-1 (BAP1), a tumor-suppressor gene that encodes a nuclear deubiquitinase, part of the polycomb group repressive deubiquitinase complex that is involved in cell cycle progression and chromatin modification, has been found to be both somatically and germline inactivated in uveal melanoma and malignant mesothelioma [87]. Recently, BAP1 was reported to be mutated in up to 14% of sporadic clear cell RCCs and is associated with more aggressive tumors and poor patient prognosis [88,89]. Two reports have described BAP1 mutations in the germline of individuals with early-onset, bilateral and multifocal clear cell RCCs that cosegregate with RCC in those families. BAP1-associated tumors displayed LOH at chromosome 3p where BAP1 is located and loss of BAP1 protein staining by immunohistochemistry [90,91]. Additional reports of uveal melanoma, cutaneous melanoma, and malignant mesothelioma families with germline BAP1 mutations and a family history of RCC have been summarized by Rai et al [92]. Testing for BAP1 mutations should be considered in patients with early-onset, clear cell RCC, family history of RCC, and/or one of the BAP1-associated malignancies who test negative for mutations in genes known to be associated with inherited RCC syndromes. Annual or biannual abdominal screening of at-risk individuals will enable early detection and monitoring of these potentially aggressive kidney tumors.
9. MiTF-associated cancer syndrome
Microphthalmia-associated transcription factor (MiTF), one member of the MiTF family of transcription factors, plays a critical role in melanocyte homeostasis, and deregulation of MiTF is associated with melanoma disease characterizing it as a melanoma oncogene [93]. A germline missense variant of MITF (c.952G→A; p.E318K) has been identified at higher frequency in patients with family history of cutaneous malignant melanoma or primary multiple melanomas relative to healthy controls [94,95]. Interestingly, epidemiological studies have noted a phenotypic association of melanoma with renal cancer, and sequencing of MITF revealed a higher frequency of the germline MITF p.E318K variant in patients with either RCC or RCC and melanoma [94–96]. Individuals that inherited the missense variant had a greater than fivefold increased risk of developing melanoma, RCC, or both compared to non-carriers [94]. MiTF transcriptionally upregulates HIF-1α, which is known to drive renal tumorigenesis, and MiTF transcriptional activity is suppressed by SUMOylation, a post-translational modification, at a SUMO consensus site involving the E318 codon. However, negative regulation of MiTF by SUMOylation was severely impaired in the MiTF p.E318K variant, thereby deregulating MiTF and promoting transcriptional activation of HIF-1α [94]. Although further research is necessary, the evidence strongly supports a role for the MiTF p.E318K variant as a medium-penetrance, germline mutation that predisposes to melanoma and RCC and potentially other cancers as well.
10. Conclusion
Eleven renal cancer predisposing genes—VHL, MET, FLCN, FH, SDHB/C/D, TSC1, TSC2, BAP1, and MiTF—have now been identified through studies of families with the inherited renal cancer syndromes VHL disease, HPRC, BHD syndrome, HLRCC, SDH-RCC, TSC, BAP1 tumor predisposition syndrome, and MiTF-associated cancer syndrome. Valuable insight into the genetic basis of kidney cancer has been gained from familial kidney cancer studies. The identification of pathways dysregulated as a result of germline mutations in these genes has laid the foundation for developing more effective targeted therapies for patients with inherited renal cancer syndromes that may also be promising for treatment of sporadic forms of kidney cancer.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project has been funded in part with Federal funds from the Frederick National Laboratory for Cancer Research, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Footnotes
Conflicts of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Nordstrom-O’Brien M, van der Luijt RB, van Rooijen E, et al. Genetic analysis of von Hippel-Lindau disease. Hum Mutat. 2010;31:521–37. doi: 10.1002/humu.21219. [DOI] [PubMed] [Google Scholar]
- 3.Walther MM, Lubensky IA, Venzon D, Zbar B, Linehan WM. Prevalence of microscopic lesions in grossly normal renal parenchyma from patients with von Hippel-Lindau disease, sporadic renal cell carcinoma and no renal disease: clinical implications. J Urol. 1995;154:2010–4. [PubMed] [Google Scholar]
- 4.Walther MM, Choyke PL, Glenn G, et al. Renal cancer in families with hereditary renal cancer: prospective analysis of a tumor size threshold for renal parenchymal sparing surgery. J Urol. 1999;161:1475–9. doi: 10.1016/s0022-5347(05)68930-6. [DOI] [PubMed] [Google Scholar]
- 5.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–20. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 6.Béroud C, Joly D, Gallou C, Staroz F, Orfanelli MT, Junien C. Software and database for the analysis of mutations in the VHL gene. Nucleic Acids Res. 1998;26:256–8. doi: 10.1093/nar/26.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 8.Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14:4726–34. doi: 10.1158/1078-0432.CCR-07-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan DR, Pause A, Burgess WH, et al. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995;269:1402–6. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- 10.Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG., Jr Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269:1444–6. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 11.Pause A, Lee S, Worrell RA, et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci U S A. 1997;94:2156–61. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamura T, Koepp DM, Conrad MN, et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284:657–61. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 14.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 15.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 16.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtney KD, Choueiri TK. Updates on novel therapies for metastatic renal cell carcinoma. Ther Adv Med Oncol. 2010;2:209–19. doi: 10.1177/1758834010361470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zbar B, Tory K, Merino M, et al. Hereditary papillary renal cell carcinoma. J Urol. 1994;151:561–6. doi: 10.1016/s0022-5347(17)35015-2. [DOI] [PubMed] [Google Scholar]
- 19.Lubensky IA, Schmidt L, Zhuang Z, et al. Hereditary and sporadic papillary renal carcinomas with c-met mutations share a distinct morphological phenotype. Am J Pathol. 1999;155:517–26. doi: 10.1016/S0002-9440(10)65147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ornstein DK, Lubensky IA, Venzon D, Zbar B, Linehan WM, Walther MM. Prevalence of microscopic tumors in normal appearing renal parenchyma of patients with hereditary papillary renal cancer. J Urol. 2000;163:431–3. [PubMed] [Google Scholar]
- 21.Schmidt LS, Nickerson ML, Angeloni D, et al. Early onset hereditary papillary renal carcinoma: germline missense mutations in the tyrosine kinase domain of the met proto-oncogene. J Urol. 2004;172(4 Pt 1):1256–61. doi: 10.1097/01.ju.0000139583.63354.e0. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt L, Junker K, Weirich G, et al. Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene. Cancer Res. 1998;58(8):1719–22. [PubMed] [Google Scholar]
- 23.Choyke PL, Glenn GM, Walther MM, Zbar B, Linehan WM. Hereditary renal cancers. Radiology. 2003;226:33–46. doi: 10.1148/radiol.2261011296. [DOI] [PubMed] [Google Scholar]
- 24.Barrisford GW, Singer EA, Rosner IL, Linehan WM, Bratslavsky G. Familial renal cancer: molecular genetics and surgical management. Int J Surg Oncol. 2011;2011:658767. doi: 10.1155/2011/658767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt LS, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt L, Junker K, Nakaigawa N, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–50. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 27.Dharmawardana PG, Giubellino A, Bottaro DP. Hereditary papillary renal carcinoma type I. Curr Mol Med. 2004;4(8):855–68. doi: 10.2174/1566524043359674. [DOI] [PubMed] [Google Scholar]
- 28.Schiering N, Knapp S, Marconi M, et al. Crystal structure of the tyrosine kinase domain of the hepatocyte growth factor receptor c-Met and its complex with the microbial alkaloid K-252a. Proc Natl Acad Sci U S A. 2003;100:12654–9. doi: 10.1073/pnas.1734128100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeffers M, Schmidt LS, Nakaigawa N, et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci U SA. 1997;94:11445–50. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovacs G. Molecular differential pathology of renal cell tumours. Histopathology. 1993;22:1–8. doi: 10.1111/j.1365-2559.1993.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhuang Z, Park WS, Pack S, et al. Trisomy 7-harbouring non-random duplication of the mutant MET allele in hereditary papillary renal carcinomas. Nat Genet. 1998;20:66–9. doi: 10.1038/1727. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Research Network. Linehan WM, Spellman PT, et al. Comprehensive molecular characterization of papillary renal cell carcinoma. N Engl J Med. 2016;374:135–45. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choueiri TK, Vaishampayan U, Rosenberg JE, et al. PhaseII and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol. 2013;31:181–6. doi: 10.1200/JCO.2012.43.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–7. [PubMed] [Google Scholar]
- 35.Zbar B, Alvord WG, Glenn GM, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. [PubMed] [Google Scholar]
- 36.Schmidt LS, Nickerson ML, Warren MB, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 2005;76:1023–33. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toro JR, Glenn G, Duray P, et al. Birt-Hogg-Dubé syndrome: a novel marker of kidney neoplasia. Arch Dermatol. 1999;135:1195–202. doi: 10.1001/archderm.135.10.1195. [DOI] [PubMed] [Google Scholar]
- 38.Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321–31. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavlovich CP, Walther MM, Eyler RA, et al. Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2002;26:1542–52. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Stamatakis L1, Metwalli AR, Middelton LA, Marston Linehan W. Diagnosis and management of BHD-associated kidney cancer. Fam Cancer. 2013;12:397–402. doi: 10.1007/s10689-013-9657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menko FH, van Steensel MA, Giraud S, et al. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 42.Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2:157–64. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 43.Lim DH, Rehal PK, Nahorski MS, et al. A new locus-specific database (LSDB) for mutations in the folliculin (FLCN) gene. Hum Mutat. 2010;31:E1043–51. doi: 10.1002/humu.21130. [DOI] [PubMed] [Google Scholar]
- 44.Vocke CD, Yang Y, Pavlovich CP, et al. High frequency of somatic frameshift BHD gene mutations in Birt-Hogg-Dubé-associated renal tumors. J Natl Cancer Inst. 2005;97:931–5. doi: 10.1093/jnci/dji154. [DOI] [PubMed] [Google Scholar]
- 45.Hong SB, Oh H, Valera VA, et al. Tumor suppressor FLCN inhibits tumori-genesis of a FLCN-null renal cancer cell line and regulates expression of key molecules in TGF-beta signaling. Mol Cancer. 2010;9:160. doi: 10.1186/1476-4598-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baba M, Hong SB, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552–7. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasumi H, Baba M, Hong SB, et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415:60–7. doi: 10.1016/j.gene.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mihaylova MM1, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baba M, Furihata M, Hong SB, et al. Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyper-proliferation, and polycystic kidneys. J Natl Cancer Inst. 2008;100:140–54. doi: 10.1093/jnci/djm288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Futami K, Petillo D, et al. Deficiency of FLCN in mouse kidney led to development of polycystic kidneys and renal neoplasia. PLoS One. 2008;3:e3581. doi: 10.1371/journal.pone.0003581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasumi Y, Baba M, Ajima R, et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc Natl Acad Sci U S A. 2009;106:18722–7. doi: 10.1073/pnas.0908853106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudon V, Sabourin S, Dydensborg AB, et al. Renal tumour suppressor function of the Birt-Hogg-Dubé syndrome gene product folliculin. J Med Genet. 2010;47:182–9. doi: 10.1136/jmg.2009.072009. [DOI] [PubMed] [Google Scholar]
- 53.Hartman TR, Nicolas E, Klein-Szanto A, et al. The role of the Birt-Hogg-Dubé protein in mTOR activation and renal tumorigenesis. Oncogene. 2009;28:1594–604. doi: 10.1038/onc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt LS, Linehan WM. Molecular genetics and clinical features of Birt-Hogg-Dubé syndrome. Nat Rev Urol. 2015;12:558–69. doi: 10.1038/nrurol.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura M, Yao M, Sano F, et al. A case of metastatic renal cell carcinoma associated with Birt-Hogg-Dubé syndrome treated with molecular-targeting agents [Japanese] Hinyokika Kiyo. 2013;59:503–6. [PubMed] [Google Scholar]
- 56.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smit DL, Mensenkamp AR, Badeloe S, et al. Hereditary leiomyomatosis and renal cell cancer in families referred for fumarate hydratase germline mutation analysis. Clin Genet. 2011;79:49–59. doi: 10.1111/j.1399-0004.2010.01486.x. [DOI] [PubMed] [Google Scholar]
- 58.Wei MH, Toure O, Glenn GM, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43:18–27. doi: 10.1136/jmg.2005.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gardie B, Remenieras A, Kattygnarath D, et al. Novel FH mutations in families with hereditary leiomyomatosis and renal cell cancer (HLRCC) and patients with isolated type 2 papillary renal cell carcinoma. J Med Genet. 2011;48:226–34. doi: 10.1136/jmg.2010.085068. [DOI] [PubMed] [Google Scholar]
- 60.Grubb RL, 3rd, Franks ME, Toro J, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007;177:2074–80. doi: 10.1016/j.juro.2007.01.155. [DOI] [PubMed] [Google Scholar]
- 61.Merino MJ, Torres-Cabala C, Pinto PA, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578–85. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 62.Alrashdi I, Levine S, Paterson J, et al. Hereditary leiomyomatosis and renal cell carcinoma: very early diagnosis of renal cancer in a paediatric patient. Fam Cancer. 2010;9:239–43. doi: 10.1007/s10689-009-9306-0. [DOI] [PubMed] [Google Scholar]
- 63.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–92. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–10. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 65.Bayley JP, Launonen V, Tomlinson IP. The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency. BMC Med Genet. 2008;9:20. doi: 10.1186/1471-2350-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alam NA, Rowan AJ, Wortham NC, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12:1241–52. doi: 10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- 67.Pithukpakorn M, Wei MH, Toure O, et al. Fumarate hydratase enzyme activity in lymphoblastoid cells and fibroblasts of individuals in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43:755–62. doi: 10.1136/jmg.2006.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 69.Pollard PJ, Briere JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–9. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 70.Tong WH, Sourbier C, Kovtunovych G, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–27. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mullen AR, Wheaton WW, Jin ES, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481:385–8. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alderson NL, Wang Y, Blatnik M, et al. S-(2-succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys. 2006;450:1–8. doi: 10.1016/j.abb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Adam J, Hatipoglu E, O’Flaherty L, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–37. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ooi A, Wong JC, Petillo D, et al. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell. 2011;20:511–23. doi: 10.1016/j.ccr.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 75.Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–9. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sourbier C, Ricketts CJ, Matsumoto S, et al. Targeting ABL1-mediated oxidative stress adaptation in fumarate hydratase-deficient cancer. Cancer Cell. 2014;26:840–50. doi: 10.1016/j.ccell.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vanharanta S, Buchta M, McWhinney SR, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74:153–9. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ricketts C, Woodward ER, Killick P, et al. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst. 2008;100:1260–2. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 79.Ricketts CJ, Shuch B, Vocke CD, et al. Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer. J Urol. 2012;188:2063–71. doi: 10.1016/j.juro.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malinoc A, Sullivan M, Wiech T, et al. Biallelic inactivation of the SDHC gene in renal carcinoma associated with paraganglioma syndrome type 3. Endocr Relat Cancer. 2012;19:283. doi: 10.1530/ERC-11-0324. [DOI] [PubMed] [Google Scholar]
- 81.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 82.Saxena N, Maio N, Crooks DR, et al. SDHB-deficient cancers: the role of mutations that impair iron sulfur cluster delivery. J Natl Cancer Inst. 2016;108(1) doi: 10.1093/jnci/djv287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Eng J Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 84.European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–15. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 85.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–8. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 86.Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–24. doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]
- 87.Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer. 2013;13:153–9. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pena-Llopis S, Vega-Rubın-de-Celis S, Liao A, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–9. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–9. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Popova T, Hebert L, Jacquemin V, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013;92:974–80. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farley MN, Schmidt LS, Mester JL, et al. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res. 2013;11:1061–71. doi: 10.1158/1541-7786.MCR-13-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rai K, Pilarski R, Cebulla CM, Abdel-Rahman MH. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet. 2016;89:285–94. doi: 10.1111/cge.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bressac-de-Paillerets B, Lesueur F, Bertolotto C. A germline oncogenic MITF mutation and tumor susceptibility. Eur J Cell Biol. 2014;93:71–5. doi: 10.1016/j.ejcb.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 94.Bertolotto C, Lesueur F, Giuliano S, et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature. 2011;480:94–8. doi: 10.1038/nature10539. [DOI] [PubMed] [Google Scholar]
- 95.Yokoyama S, Woods SL, Boyle GM, et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;480:99–103. doi: 10.1038/nature10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghiorzo P, Pastorino L, Queirolo P, et al. Prevalence of the E318K MITF germline mutation in Italian melanoma patients: associations with histological subtypes and family cancer history. Pigment Cell Melanoma Res. 2013;26:259–62. doi: 10.1111/pcmr.12047. [DOI] [PubMed] [Google Scholar]
- 97.Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163–72. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 98.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–67. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]