Abstract
Background
Osteoarthritis (OA) is characterized by cartilage degradation in the affected joints. Pomegranate fruit extract (PFE) inhibits cartilage degradation in vitro. Here we determined whether oral consumption of PFE inhibits disease progression in rabbits with surgically-induced OA.
Methods
OA was surgically induced in the tibiofemoral joints of adult NZW rabbits. In one group animals were fed PFE in water for 8 weeks post-surgery. In the second group, animals were fed PFE for 2 weeks before surgery andfor 8 weeks post-surgery.Histological assessment and scoring of the cartilage was per OARSI guidelines. Gene expression and MMPs activity were determined using qRT-PCR and fluorometric assay, respectively. IL-1β, MMP-13, IL-6, PGE2 and COL2A1 levels in synovial fluid/plasma/culture mediawere quantified using ELISA. Expression of active Caspase-3 and PARP p85 was determined by immunohistochemistry. Effect of PFE and inhibitors of MMP-13, MAPK and NF-κB was studied in IL-1β-stimulated rabbit articular chondrocytes.
Results
Safranin-O-staining and chondrocyte cluster formation was significantly reduced in the ACLT+PFE fed groups. Expression of MMP-3, MMP-9 and MMP-13 mRNAwas higher in the cartilage of rabbits given water alone but was significantly lower in the animals fed PFE. PFE-fed rabbits had lowerIL-6, MMP-13 and PGE2 levels in the synovial fluid and plasma respectively and showed higher expression of ACAN and COL2A1 mRNA. Significantly higher numbers of chondrocytes were positive for markers of apoptosisin the joints of rabbits with OA given water only compared to rabbits in the PFE-fed groups. PFE pretreatment significantly reduced IL-1β induced IL-6 and MMPs expression in rabbit articular chondrocytes. These effects were also mimicked using MMP-13, MAPK and NF-κB inhibitors in IL-1β-stimulated rabbit chondrocytes. In an in vitro activity assay, PFE blocked the activity of MMP-13.Like MAPK and NF-κB inhibitors, PFE was also effective in inhibiting IL-1β-induced PGE2 production in rabbit chondrocytes. PFE also reversed the inhibitory effect of IL-1β on COL2A1 mRNA and protein expression in IL-1β-stimulated rabbit chondrocytes.
Conclusion
Our data highlighted the chondroprotective effects of PFE oral consumption in a model of post-traumatic OA and suggests that PFE derived compounds may have potential value in the management of OA.
Keywords: Osteoarthritis, ACLT, Rabbit, Pomegranate, MMPs, PGE2
1. Introduction
Osteoarthritis (OA) is the most common form of arthritis worldwide and a major cause of morbidity, physical limitation, disability and socioeconomic burden [1-3]. Although cartilage matrix breakdown is the major characteristic of OA, the joint pathology also involves synovial membrane and subchondral bone changes [4]. OA is believed to be a consequence of mechanical and biochemical events that result in an imbalance between the synthesis and degradation of articular cartilage matrix consisting of proteoglycans (PGs), collagens (type II, IX, XI, X and others) and water [4-6]. Evidence now firmly supports the view that inflammation not only contributes to the development of symptoms but also plays a role in the progression of structural damage including cartilage degradation [7-9]. Excessive production of cartilage degrading enzymes such as the aggrecanases (ADAMTS-4 and −5) and matrix metalloproteinases (MMPs), which are the key player in degradation of ACAN and collagen type II (COL2A1) has been shown in OA joints [10-12]. Studies have correlated increased chondrocytes apoptosis with OA severity in humans and animal models [13-15]. Moreover, cell death positively correlates with matrix degradation and calcification [16, 17]. In this regard, increased caspase-3 expression has been found to correlate with reduced cell density in human OA cartilage[18], while inhibition of apoptosis using caspase inhibitors reduced the severity of cartilage lesions in experimental OA [19]. Currently, goals of OA management include controlling pain, maintaining and improving the range of movement and stability of affected joints, and limiting functional impairment [20, 21]. Because of the association of severe adverse events with the use of NSAIDs, at least one third of the older adults use some form of complementary and alternative medicine (CAM) for pain [22, 23].
Pomegranate (Punicagranatum L., Punicaceae) is an ancient fruit that has not changed much throughout human history. The fruit itself is a rich source of two types of polyphenolic compounds: anthocyanins (such as delphinidin, cyanidin, and pelargonidin), which give the fruit and juice its red color, and hydrolyzable tannins (such as punicalin, pedunculagin, punicalagin, gallagic, and ellagic acid), which account for 92% of the antioxidant activity of the whole fruit [24, 25]. Studies have also shown that the antioxidant capacity of pomegranate juice is three times that of the popular antioxidant-containing beverages such as red wine and green tea, presumably due to the presence of hydrolyzable tannins in the rind, along with anthocyanins and ellagic acid derivatives [25-27]. In a comparative analysis, anthocyanins from pomegranate were also shown to possess higher antioxidant activity than vitamin-E (a-tocopherol), ascorbic acid and 3-carotene [26]. Pomegranate extract has also been shown to protect from NSAID and ethanol-induced gastric ulceration [28]. The whole fruit extract is widely used in several traditional medicinal systems for the treatment of inflammation and pain in arthritis and other diseases [22]. The family of phytochemicals found in pomegranate fruit act together with greater potency than any of the single constituents alone[29]. Previously we have shown that a standardized Pomegranate fruit extract (PFE) inhibit the production of MMPs via blocking the activation of p38-MAPK and the transcription factor NF-κB in OA chondrocytes [30]. In other studies, we have shown that bioavailable metabolites of PFE inhibited COX-2 activity in OA chondrocytes [31], and that consumption of PFE suppressed the inflammation and joint destruction in collagen induced arthritis (CIA) mouse model [32]. We also demonstrate that pretreatment of OA chondrocytes with PFE inhibited the IL-1β-induced activation of the upstream kinase MKK3 resulting in the inhibition of p38α-MAPK isoform and the activation and DNA binding activity of the transcription factor RUNX-2 [33]. In the present study we examined the cartilage and chondroprotective effects of oral consumption of PFEin vivo using the rabbit model of post traumatic OA in which the disease is induced through Anterior Cruciate Ligament Transection (ACLT) [34, 35]. Our results demonstrate that in rabbits that orallyconsumed PFE, there was less cartilage damage and fewer apoptotic chondrocytes in the joints with surgically-induced OA. Expression ofIL-6, MMPs and PGE2 was also reduced while the expression of ACAN and COL2A1 was high in the OA joints of rabbits fed PFE. Inhibition of IL-1β-induced IL-6 and MMPs expression, MMP-13 activity and PGE2 production with an increase in COL2A1 level was observed by PFE pretreatment in rabbit articular chondrocytes. These data suggests that consumption of PFE may be chondroprotective and may be used as an adjunct therapy for the management of OA.
2. Materials and Methods
2.1. Anterior Cruciate Ligament Transection (ACLT) Surgery to induce OA in Rabbits
The model used to study the impact of PFE consumption on the development of OA was the rabbit ACLT model as this model produces a reliable and reproducible degradation of articular cartilage [36]. All animal studies were approved by the IACUC of the Case Western Reserve University (CWRU), Cleveland, OH (protocol no. 2009-0209) and the IACUC of the Northeast Ohio Medical University (Protocol #13-002). Animal surgeries and euthanasia were carried out using the facilities of the Animal Resource Center of the CWRU. We used 27adult male New Zealand White rabbits (8 months old)for this study. They were divided into 3 experimental groups of 6 rabbits/group and 3 groups of 3 rabbits/group for sham surgery as follows:
Group-1. Six Rabbits with ACLT surgery maintained on plain water, pre- and post-surgery
Group-2. Three Rabbits with Sham surgery maintained on plain water, pre- and post-surgery
Group-3. Six Rabbits with ACLT surgery maintained on plain water pre-surgery and then on PFE (34mg/Kg/day) for 8 weeks post-surgery
Group-4. Three Rabbits with Sham surgery maintained on plain water pre-surgery and then on PFE (34mg/Kg/day) for 8 weeks post-surgery
Group-5. Six Rabbits with ACLT surgery maintained on PFE (34mg/Kg/Day)for two weeks pre-surgery and then on PFE (34mg/Kg/day) for 8 weeks post-surgery
Group-6. Three Rabbits with Sham surgery maintained on PFE (34mg/Kg/Day)for two weeks pre-surgery and then on PFE (34mg/Kg/day) for 8 weeks post-surgery
The dose of PFE (34/mg/Kg body weight) was chosen based on our previous study where PFE at the tested dose (34/mg/Kg) was effective in delaying the onset and incidence of CIA in mice and these mice also showed less cartilage damage[32]. An analgesic/anesthetic technique was employed prior to surgery in which Ketamine HCl (35 mg/kg; 500 mg/ml) and 2 ml of an 8% solution of Xylazine (5mg/ml) was administered intramuscularly followed by local infiltration at surgery site with 2% lidocaine. During surgery rabbits were maintained on 2.5-5% of Isoflurane and oxygen via inhalation route.The contralateral knee was employed as the non-operated control. Left knee of each anesthetized rabbit was shaved, prepped, and draped in a sterile fenestrated drape. ACLT was performed in left knees using a medial parapatellar approach as previously described [36]. In the sham surgery group (3 rabbits/group), the surgery site in right knee was exposed, the anterior crucial ligament was touched with a scalpel blade, but the ligament was not transected.After surgery, the animals were allowed unrestrained cage activities while they were monitored for infections and other post-surgical complications.
Plasma and synovial fluid was collected from the rabbits under anesthesia initially (day 0) and at the termination of the study. Briefly, 1 ml of synovial fluid was aspirated using an 18G needle after injecting 0.5 ml of physiological saline into the joint and flexing the knee 10 times. The extracted fluid was then diluted in 1 ml of 2X PBS and centrifuged at 13,000 rpm for 15 min to remove the cells and tissue debris. The supernatants were stored in 0.5 ml aliquots at −20°C until use.
2.2. Preparation of pomegranate fruit extract (PFE) and treatment regimen
Pomegranate fruit was procured from local grocery stores and the whole fruit's ethanoic extract was prepared essentially as previously described [37, 38]. Fresh PFE solution was prepared daily by dissolving the required concentration (34 mg/kg/day) in drinking water and given to rabbits ad libitum.
2.3. Macroscopic and histological assessment of cartilage damage
Harvested rabbit knees were kept in PBS containing protease inhibitors (2mM Na2-EDTA, 1mM PMSF, 5mM Benz-HCL, 10 mM NEM) for 1 h and then the femoral condyles were stained with India ink solution. After a quick wash in sterile water to remove excess ink, the condyles were photographed and assessed for gross changes in the articular cartilage (three knees per experimental group and 2 knees per sham surgery group) performed for each harvested knee: medial femoral condyle, lateral femoral condyle, medial tibial plateau, and lateral tibial plateau. Joints stained with India ink were used for biochemical analyses (below).
For histological analyses, knees not stained with India ink were used and excised femoral and tibial cartilage was fixed in 10% neutral buffered formalin and decalcified using 10% EDTA, dehydrated and embedded in paraffin and 5μm sections were cut. Representative sections were stained for proteoglycans with Safranin-O and counterstained with Fast Green. Histologic assessment was performed on the femorotibial joints of rabbits in the experimental and sham surgery groups as previously described [39]. Three histologic sections from each site were evaluated for histological/histochemical assessment as per OARSI guidelines (Table 1).
Table 1.
Effect of PFE administration on the histological assessment of articular cartilage changes in the Post-Traumatic Osteoarthritis model was performed as per OARSI guidelines [39] and the criteria for histological assessment are shown in the table.
|
Parameter
|
| Safranin O-fast green staining |
| 0 = uniform staining throughout articular cartilage |
| 1 = loss of staining in superficial zone of hyaline cartilage <50% the length of the condyle or plateau |
| 2 = loss of staining in superficial zone of hyaline cartilage ≥50% the length of the condyle or plateau |
| 3 = loss of staining in the upper 2/3's of hyaline cartilage <50% the length of the condyle or plateau |
| 4 = loss of staining in the in the upper 2/3's hyaline cartilage ≥50% the length of the condyle or plateau |
| 5 = loss of staining in all the hyaline cartilage <50% the length of the condyle or plateau |
| 6 = loss of staining in all the hyaline cartilage ≥50% the length of the condyle or plateau |
| Structure |
| 0 = normal |
| 1 = surface irregularities |
| 2 = fissures in <50% surface |
| 3 = fissures in ≥50% surface |
| 4 = erosion 1/3 hyaline cartilage <50% surface |
| 5 = erosion 1/3 hyaline cartilage ≥50% surface |
| 6 = erosion 2/3 hyaline cartilage <50% surface |
| 7 = erosion 2/3 hyaline cartilage ≥50% surface |
| 8 = Full depth erosion hyaline cartilage <50% surface |
| 9 = Full depth erosion hyaline cartilage ≥50% surface |
| 10 = Full depth erosion hyaline and calcified cartilage to the subchondral bone <50% surface |
| 11 = Full depth erosion hyaline and calcified cartilage to the subchondral bone ≥50% surface |
| Chondrocyte density |
| 0 = no decrease in cells |
| 1 = focal decrease in cells |
| 2 = multifocal decrease in cells |
| 3 = multifocal confluent decrease in cells |
| 4 = Diffuse decrease in cells |
| Cluster formation |
| 0 = normal |
| 1 = <4 clusters |
| 2 = ≥4 but <8 clusters |
| 3 = ≥8 clusters |
Source: Laverty et al., 2010 [39]
2.4. Immuno-histochemical evaluation
To detect the expression of Caspase-3 and PARP p85 cleavage specific antibodies were used. Immunohistochemistry was carried out using R.T.U. Vectastain® Universal ABC kit (Vector Laboratories, Burlingame, CA). Sections were deparaffinized and rehydrated and antigen retrieval was performed by heating slides for 10 min at 95°C in 0.01M sodium citrate in Decloaking chamber (Biocare Medical, Concord, CA). Endogenous peroxide was blocked by treating the sections with 0.1% H2O2 (Sigma-Aldrich, St Louis, MO) in water for 5 min. After wash with 1xPBS, non-specific binding was blocked by incubation with normal horse serum provided in the kit. The sections were incubated with diluted primary Caspase-3 (MP Biomedicals, Inc., CA, USA), and PARP (MP Biomedicals, Inc., CA, USA) at 4°C overnight. The negative control sections were incubated with IgG isotype control. Thereafter, sections were treated sequentially with biotinylated secondary antibody and streptavidin-peroxidase conjugate, and then were developed using DAB substrate kit for peroxide (Vector Laboratories). The sections were counterstained with Safranin O-Fast Green stains.
2.5. Rabbit articular chondrocytes culture and treatment
Chondrocytes were prepared by the enzymatic digestion of rabbit tibia and femoral cartilage as previously described [30]. Rabbit chondrocytes were pretreated with PFE (50 or 100 μg/ml) or PD98059 (Cayman Chemicals, Ann Arbbor, MI) (30μM; MAPK kinase inhibitor) or BAY11-7082 (Cayman Chemicals, Ann Arbor, MI)(10μM; NF-κB inhibitor) for 2h followed by IL-1β (10ng/ml) treatment for 24h.
2.6. Gene expression analyses
Cartilage pieces were grounded to a fine powder in liquid nitrogen using Freezer mill (SPEX, Metuchen, NJ) and then processed to purify the total RNA using a commercially available kit (Qiagen, Chatsworth, CA). For some studies RNA was prepared from treated rabbit articular chondrocytes. Genomic DNA-free total RNA (500ng) was reverse-transcribed using QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer's protocol. Expression of MMP-1, MMP-3, MMP-9, MMP-13, IL-6, ACAN, and COL2A1 mRNAs was quantified using the TaqMan Assays and a StepOne Plus system (Life Technologies, Carlsbad, CA). Expression level of β-actin mRNA was used as a normalizing control. A threshold cycle (Ct) was observed in exponential phase of amplification and quantification of relative expression levels was determined by ΔΔCt method. The value of each control sample was set at 1 and was used to calculate the relative change in the target mRNA expression.
2.7. MMP-13 activity assays
Effect of PFE on the MMPs activity was determined using purified MMP-13 protein (Anaspec, San Jose, CA, USA) with PFE (50 and 100 μg/ml) in a cell free system. WAY170523 (Santa Cruz Biotechnology, CA, USA) (20 nM; MMP-13 inhibitor) was used as a positive control to selectively inhibit MMP-13 activity. MMP-13 activity was also measured in the culture media from the PFE (100 μg/ml) treated and IL-1β-stimulated rabbit chondrocytes using SensoLyte ™ Fluorimetric MMP-13 activity assay kit essentially as per manufacturer's instruction (AnaSpec, San Jose, CA). Purified MMP-13 (10 ng/ml) or culture supernatant protein were first activated with Aminophenyl mercuric acetate (APMA) (1 mM)) at 37°C for 40 min. The activated MMP-13 was incubated with PFE (50-150 μg/ml) or WAY 170523 (20 nM) for 15 min at 37°C. To initiate the enzymatic reaction, the above reaction mix or activated culture supernatant was incubated with MMP-13 FRET substrate for 60 min at 37°C and fluorescence signal was recorded at Ex/Em=490 nm/520 nm using the Synergy H1 multi-mode plate reader (Bio-Tek Instruments Inc., Winooski, VT, USA).
2.8. Enzyme-linked immunosorbent assays (ELISA)
Measurement of MMP-13, Interleukin (IL)-1β, IL-6, prostaglandin-E2 (PGE2)and COL2A1 production in plasma/synovial fluid from the joints or culture media was done by a Rabbit Type-II Collagen-specific ELISA according to the instructions of the manufacturer (MyBioSource, San Diego CA). Plates were read using Synergy HT micro plate reader (Biotek.
2.9. Statistical analysis
Results are presented as Mean ± SE. Comparisons were performed using GraphPad prism 6.0 software package (using up-paired two tailed t-test) and p<0.05 was considered significant. Data was also analyzed for Gaussiandistribution of variables. In case of non-Gaussian distribution Kruskal-Wallis test was used.All experiments were performed using chondrocytes or cartilage from indicated number of animals as stated in the figures legends.
3. Results
3.1. Effect of PFE administration on macroscopic and histological parameters
Postmortem inspection of rabbit knees revealed that ACLT was complete in all rabbits included in the study. In the right femorotibial joints subjected to sham surgery the articular cartilage macroscopic grades were normal or scarcely perceptible ink uptake and no cartilage erosions were observed in any of the joint compartments (Supplementary Figure 1) while the induced OA lesions (erosion of articular cartilage) were focal in joints in which ACLT was performed. In animals that were fed PFE either pre- and post-ACLT or post-ACLT, disease severity grades of macroscopic articular cartilage lesions in joint compartments were lower when the grades were compared with the animals given water only pre- or post-ACLT (Supplementary Figure 1). Representative histologic sections (Figure-1) from these samples were also analyzed and scored. Rabbits in groups that were fed PFE pre-& post sham surgery or post sham surgery revealed no significant histological changes in the articular cartilage. We observed a significant loss of Safranin-O-staining with chondrocyte clustering in animals given water onlyand subjected to ACLT (Figure 1A, B and H; P<0.01). Loss of Safranin-O-staining was accompanied by cleft formation in the joints of some rabbitsin this group but did not extend into the middle or deep zones of the cartilage (Figure 1B). Interestingly, a significantly lower mean score for the loss of Safranin-O-staining (P<0.05), structural changes (P<0.01), and cluster formation (P<0.05) was observed in the joints of rabbits fed PFE pre- and post-ACLT surgery compared to the animals in the groups subjected to ACLT but given water only (Figure 1H). There was a trend toward higher chondrocyte density in the animals fed PFE pre- and post-ACLT compared to groups subjected to ACLT but given water only but the difference was not significant (Figure 1H; P>0.05). Chondrocyte cluster formation (cloning) was only seen in ACLT joints, never in Sham joints. When all histologic parameters were combined from each site to obtain a total histological score, statistically significant differences were detected between ACLT group given water only and those in the ACLT+ pre-&-post PFE and ACLT+post PFE group (Figure 1G; P<0.01).
Figure 1.

Histological analysis of cartilage protective effects of pomegranate fruit extract (PFE) against cartilage degradation in ACLT rabbit model. A-F, Representative Safranin O-fast green stained histologic sections are shown. A, Normal (Sham) articular cartilage with intact surface in vehicle-administrated rabbit. B, ACLT knee after 8 week of surgery showing severe loss of Safranin-O-staining, cleft in articular cartilage and cluster formation. C-D, Rabbits were fed with PFE 35/mg/Kg/day for 8 weeks in Sham or ACLT surgery groups. E-F, Rabbits were fed with PFE for 2 weeks prior to surgery followed by 8 weeks post ACLT surgery group or Sham group. G and H, Histological scores after 8 weeks of surgery. Total histological score and scores for histological parameters with comparison between groups are shown. Values are shown as Mean ± SE.*P<0.05 **P<0.01
3.2. Effect of PFE administration on the MMPs expression in the joints of ACL-transected rabbits
The cartilage matrix componentsACAN and COL2A1 both are degraded by various MMPs [40, 41]. To determine whether consumption of PFE can modulate MMPs expression in OA joints in vivo, gene expression of MMP-3, −9 and −13 was determined by TaqMan assays using RNA prepared directly from the cartilage obtained from different groups employed in this study.We observed a significantly higher mRNA expression of MMP-3 (~562%; P<0.01), MMP-9 (~1880%; P<0.01), and MMP-13 (~277%; P<0.01) in the joints of animals in the ACLT group compared to Sham surgery group (Figure 2). A significant decrease in MMP-3 (~72-80%; P<0.05) and MMP-9 (~87-92%; P<0.05) expression was observed in the joints ofrabbits that were fed PFE pre- and post ACLT or 8 weeks post ACLT surgery compared to the rabbits subjected to ACLT surgery but were given water only (Figure A and B). Expression of MMP-13 mRNA was significantly reduced (~81%; P<0.01) in rabbit joints in both the groups that were fed PFE (Figure 2C).
Figure 2.

Effects of pomegranate fruit extract (PFE) on mRNA expression of matrix metalloproteinases (MMP)-3, −9 and −13, in the joint cartilage of ACLT rabbit model. Knee joints were harvested at the termination of the study. A-C, RNA was prepared from rabbit knee cartilage (n=4 each group) and expression levels of MMP-3, MMP-9 and MMP-13 were determined by quantitative Real-Time PCR using TaqMan assay. Gene expression of β-actin was used as normalizing control. Each experiment was performed in duplicate. Values are shown as Mean ± SE.*P<0.05 **P<0.01
3.3. Effect of PFE on cartilage aggrecan (ACAN) and type II collagen (COL2A1) gene expression
Articular cartilage is primarily composed of COL2A1 and proteoglycans of which ACAN is the most abundant [42]. We determined the expression levels of ACAN and COL2A1mRNAs in the cartilage isolated from rabbit joints using qRT-PCR. The mRNA expression of ACAN (~60%; P<0.01) and COL2A1 (~50%; P<0.01) was significantly decreased in the cartilage of rabbits subjected to ACLT surgery compared with the expression level in the joints of rabbits in the Sham group (Figure 3). However, expression of both aggrecan (~192-272%; P<0.05) and COL2A1 (~172-254%; P<0.01) was significantly increased in the joint cartilage of rabbits in both the PFE-fed groups compared to the rabbits given water only after ACLT surgery (Figure 3A and B).
Figure 3.
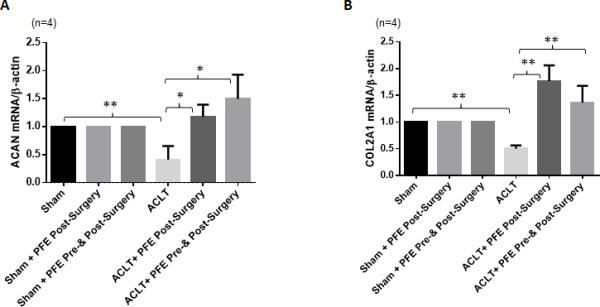
Effects of pomegranate fruit extract (PFE) on messenger RNA expression of aggrecan (ACAN) and collagen type II (COL2A1) in the joint cartilage of ACLT rabbit model. Knee joints were harvested at the termination of the study (8 week post-surgery). A and B, RNA was prepared from rabbit knee cartilage (n=4 each group) and expression levels of ACAN and COL2A1 were determined by quantitative Real-Time PCR using TaqMan assay. Gene expression of β-actin was used as normalizing control. Each experiment was performed in duplicate. Values are shown as Mean ± SE.*P<0.05 **P<0.01
3.4. Effects of PFE on the IL-1β, IL-6, MMP-13 and PGE2 levels in plasma and synovial fluid of rabbits
Both PGE2 and MMP-13 are associated with pain, disease severity and pathogenesis of OA [11, 43]. When levels of circulating MMP-13 and PGE2 were examined in the plasma and in the joint microenvironment (synovial fluid) of the study animals, plasma PGE2 levels were not significantly different between groups (Figure 4A; P>0.05). However, PGE2 levels were significantly higher (~269%; P<0.01)in the synovial fluid of rabbits given water only after ACLT and compared to the levels detected in the synovial fluid obtained from the joints of rabbits in the sham surgery group (Figure 4B). Importantly, a significant decrease in the PGE2 level was observed in the synovial fluid of rabbits in both the groups of rabbits given PFE either before and after (~48%; P<0.01) or after the ACLT surgery (~71%; P<0.01) when compared to the levels found in the synovial fluid of rabbits subjected to ACLT surgery but given water only (Figure 4B). PFE administration significantly inhibited the circulating plasma MMP-13 levels in rabbits in both the groups that were fed PFE but the effect was more pronounced in the group that was fed PFE both pre- and post-ACLT surgery (Figure 4C). The synovial fluid MMP-13 level was significantly higher in the joints of rabbits in the water fed animals subjected to ACLT surgery (~71%; P<0.05) compared to the levels detected in the synovial fluid of rabbits in the PFE-fed groups (Figure 4D; P<0.05). In the present study, a significant increase in the synovial fluid IL-1β levels was observed in ACLT alone group compared to sham group (Figure 5A). However, no significant change in the IL-1β-levels in rabbit synovial fluid was observed after PFE administration in both pre- and post-ACLT or post-ACLT groups (Figure 5A). IL-1β levels in plasma were barely detectable and were not significantly different between the groups (data not shown). We also observed an increase in serum and synovial fluid IL-6 levels in ACLT group which were significantly inhibited by PFE administration in both ACLT+pre-&-post PFE and ACLT+post PFE groups (Figure 5B and C).
Figure 4.
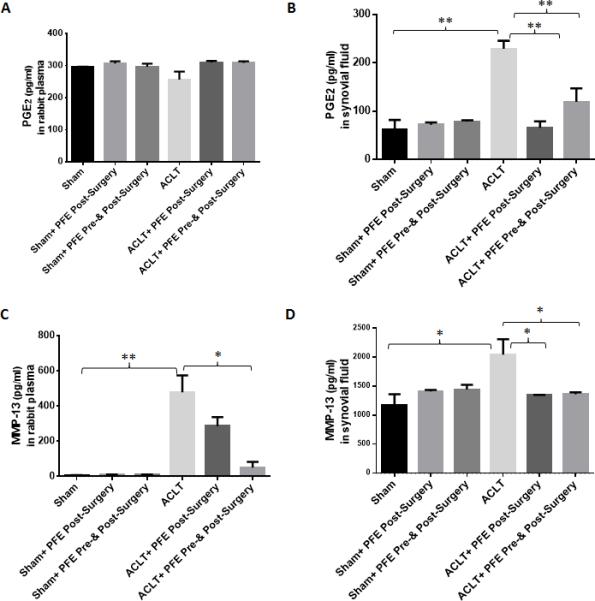
Effects of pomegranate fruit extract (PFE) on the production of Prostaglandin-E2(PGE2) and matrix metalloproteinases-13 (MMP-13) in the plasma and synovial fluid of ACLT rabbit model. Knee joints were harvested after 8 week of ACLT surgery. A-D, Plasma and synovial fluids from different groups (Sham n=3;ACLT n=6;Sham+Post-Surgery PFE n=3;ACLT+ Post-Surgery PFE n=6, Sham+ Pre-&Post-Surgery PFEn=3;ACLT+ Post-Surgery PFE n=6) were analyzed for A and B, PGE2 and C and D, MMP-13 production using ELISA according to the manufacturer instruction. Each experiment was performed in duplicate. Values are shown as Mean ± SE.*P<0.05 **P<0.01
Figure 5.
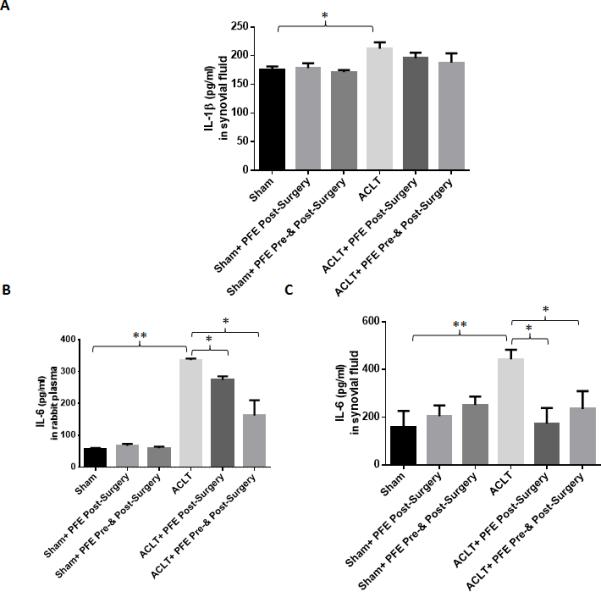
Effects of pomegranate fruit extract (PFE) on the production of IL-1β and IL-6 in the plasma and synovial fluid of ACLT rabbit model. Knee joints were harvested after 8 week of ACLT surgery. A-C, Plasma and synovial fluids from different groups (Sham n=3;ACLT n=6;Sham+Post-Surgery PFE n=3;ACLT+ Post-Surgery PFE n=6, Sham+ Pre-&Post-Surgery PFEn=3;ACLT+ Post-Surgery PFE n=6) were analyzed for A, IL-1β and B and C, IL-6 production using ELISA according to the manufacturer instruction. Each experiment was performed in duplicate. Values are shown as Mean ± SE.*P<0.05 **P<0.01
3.5. Expression of Caspase-3 and cleaved p85 fragment of poly (ADP-ribose) polymerase (PARP-p85) in rabbit joints
To investigate the effect of PFE in this post-traumatic OA model, joint cartilage sections were analyzed for the presence of activated caspase-3 (a key regulator of most apoptosis pathway) and for cleavedPARP, a feature and marker of apoptosis. Immunohistochemical analysis revealed the presence of PARP p85 and Caspase 3 in ACLT cartilage compared to Sham joint cartilage (Figure 6). Figure 6 shows that 8 week after ACLT, expression of PARP p85 and Caspase 3 in rabbit cartilage from ACLT group showed greater intensity then contralateral sham control (Figure 6A, B, G and H). In the PFE administered ACLT groups, analysis of the cartilage showed a reduced number of cells with active caspase-3 and PARP p85 (Figure 6 D, F, J and L) compared to their respective controls (Figure 6 C, E, and I and K). These results support indicate that caspase-mediated apoptosis pathway contributing to cartilage pathology is effectively modulated by PFE administration in rabbit joints with surgically-induced OA.
Figure 6.
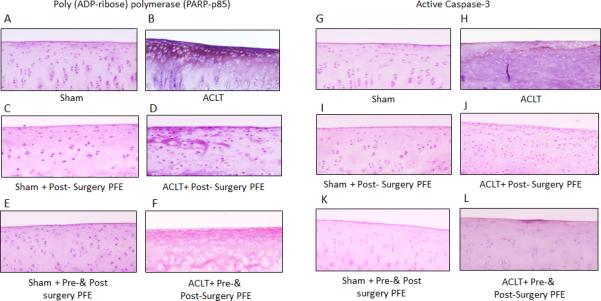
Immunohistochemistry for the p85 fragment of poly(ADP-ribose) polymerase (PARP-p85) and active caspase-3 in knee cartilage of ACLT rabbits administered pomegranate fruit extract (PFE). Knee joints were harvested from (A and G) Sham, (B and H) ACLT, (C and I) Sham+ Post-Surgery PFE, (D and J) ACLT+ Post- Surgery PFE, (E and K) Sham+ Pre-&Post-Surgery PFEand (F and L) ACLT+ Post-Surgery PFE. (A-F), Sections were stained with antibody to the p85 cleaved form of PARP or with the antibody ofactive form of (G-L) caspase-3. (Original magnification X 10)
3.6. Effect of PFE pre-treatment on the IL-6 and MMPs expression and MMP-13 activity in IL-1β-stimulated rabbit articular chondrocytes
To verify ACLT model findings where PFE oral administration inhibited IL-6 and MMPs expression, we used rabbit articular chondrocytes.The expression of MMP-1, −3, and -13 was increased by several-fold in chondrocytes stimulated with IL-1β. In agreement with our in vivo findings, PFE pretreatment significantly inhibited the IL-1β-induced expression of MMP-1 (~98%; P<0.01), MMP-3 (~98%; P<0.01) and MMP-13 (~95%; P<0.01) (Figure 7A-C). Pretreatment with PFE also inhibited the IL-1β-induced IL-6 (~30.6%;P<0.05) expression compared with that detected in rabbit chondrocytes stimulated with IL-1β alone (Figure 7D). Moreover, when effect of PFE treatment on the IL-1β-induced activity of MMP-13 was determined in rabbit articular chondrocytes using fluorescence based assay, a significant decrease in the MMP-13 activity was observed by PFE pretreatment compared to IL-1β alone treatment (~63.6%; P<0.01) (Figure 7E). A similar decrease in MMP-13 activity was also found in cell free assay system, where purified MMP-13 was used with PFE (~88%; P<0.01) or commercially available MMP-13 inhibitor (WAY170-523; ~56%; P<0.01) (Figure 7F).
Figure 7.
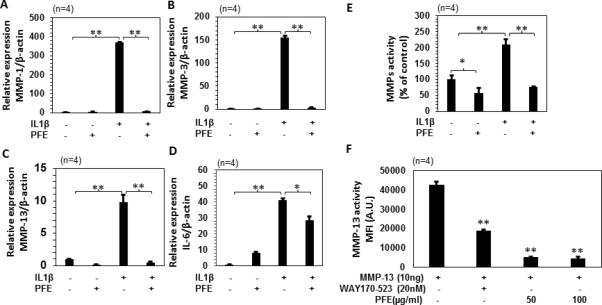
Effects of pomegranate fruit extract (PFE) on mRNA expression of IL-6, MMPs expression and MMP-13 activity in IL-1β-stimulated rabbit articular chondrocytes. A-D, Rabbits articular chondrocytes (n=4) were pretreated with PFE (100μg/ml) for 2 h followed by IL-1β (10 ng/ml) stimulation for 24 h. Gene expression of MMP-1, MMP-3, MMP-13 and IL-6 was determined by quantitative Real-Time PCR using TaqMan assay. Gene expression of β-actin was used as normalizing control. Each experiment was performed in duplicate. E, MMP-13 activity in the culture media from rabbit articular chondrocytes (n=4) was determined by fluorogenic peptide assay using commercially available kit. Rabbit chondrocytes were pretreated with PFE for 2 h and then stimulated with IL-1β for 24 h. F, MMP-13 activity was determined using a purified MMP-13 protein with PFE (50 and 100 μg/ml) in a cell free system and WAY170-523 (20 nM; MMP-13 inhibitor) was used as a positive control. Values are shown as Mean ± SE. *P<0.05 **P<0.01
3.7. Effect of MAPK and NF-κB inhibitors on the expression of MMPs, IL-6 and PGE2 production in IL-1β-stimulated rabbit chondrocytes
As we observed inhibition of MMPs and IL-6 by PFE treatment in IL-1β-induced rabbit articular chondrocytes. Next we studied the role of MAPK and NF-κβ pathways in the IL-1β-induced MMPs, IL-6 and PGE2 production in rabbit articular chondrocytes (Figure 8). Rabbit articular chondrocytes were pretreated with MAPK inhibitor (PD98059) or NF-κB (BAY11-7082) or PFE for 2h and then stimulated with IL-1β for 24h. Pretreatment with PD98059 or BAY11-7082 significantly inhibited IL-1β-induced MMP-1(~78% and ~94% respectively; P<0.01), MMP-3(~68%and ~99% respectively; P<0.01) and MMP-13 (~89% and ~94% respectively; P<0.01) production in rabbit articular chondrocytes (Figure 8 A-C). A decrease in the IL-1β-induced IL-6 (~76%and ~99% respectively; P<0.01) and PGE2(~38% and 66% respectively) production was also observed in rabbit chondrocytes by PD98059 or BAY11-7082 pretreatment (Figure 8 D and E). Expression of PGE2(~56%; P<0.01) was also significantly downregulated by pretreatment with PFE treatment compared with chondrocytes treated with IL-1β alone(~56%;P<0.01) (Figure 8E).
Figure 8.
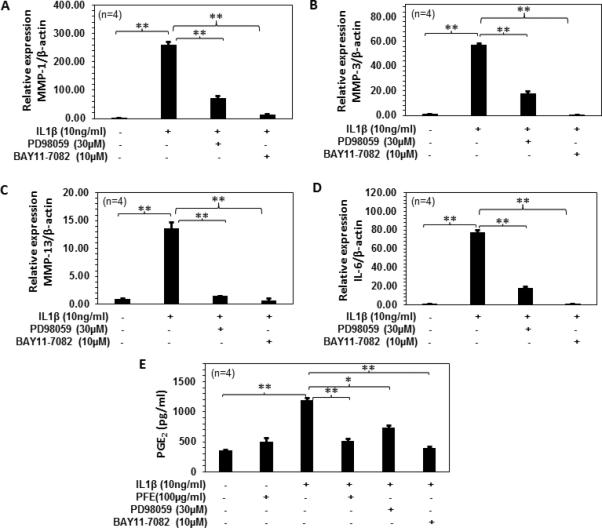
Role of MAPK and NF-κB pathways in the IL-1β-induced MMPs, IL-6 expression and PGE2 production in rabbit articular chondrocytes.A-D, Rabbits articular chondrocytes (n=4) were pretreated with PD98059 (30 μM) or BAY11-7082 (10μM) for 2 h followed by IL-1β (10 ng/ml) stimulation for 24 h. Gene expression of MMP-1, MMP-3, MMP-13 and IL-6 was determined by quantitative Real-Time PCR using TaqMan assay. Gene expression of β-actin was used as normalizing control. Each experiment was performed in duplicate. E, Rabbit chondrocytes (n=4) were pretreated with PFE (100 μg/ml) or PD98059 (30 μM) or BAY11-7082 (10μM) for 2 h and then stimulated with IL-1β for 24 h. Changes in PGE2 production were determined using ELISA. Values are shown as Mean ± SE. *P<0.05 **P<0.01
3.8. Effect of PFE pre-treatment on the COL2A1 mRNA and protein level in IL-1β-stimulated rabbit chondrocytes
In the present study, a significantly higher COL2A1 mRNA expression was observed in the articular cartilage of ACLT rabbits after PFE oral administration (Figure 3A). As MMP-13 specifically acts on the protein accumulation of COL2A1 in cell culture medium by its enzymatic digestion, we verified in vivo results and studied the effect of PFE on both COL2A1 mRNA expression and release in culture medium in IL-1β-stimulated rabbit articular chondrocytes (Figure 9). We found a significant decrease in COL2A1 mRNA expression (~49%; P<0.05) and significant increase in COL2A1 levels (~56%; P<0.01) in culture medium treated with IL-1β (10 ng/ml, 24 h) after, However pretreatment with PFE significantly upregulated COL2A1 mRNA expression and induced a significant decrease in release of COL2A1 levels in culture supernatant in IL-1β-stimulated rabbit articular chondrocytes indicating that PFE treatment inhibits the IL-1β mediated degradation of COL2A1 at both mRNA and protein levels (Figure 9A and B).
Figure 9.
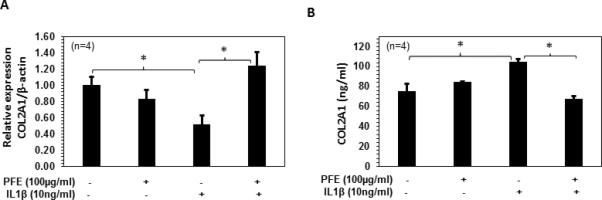
Effects of pomegranate fruit extract (PFE) on the COL2A1 mRNA and protein expression in IL-1β-stimulated rabbit articular chondrocytes. A and B, Rabbits articular chondrocytes (n=4) were pretreated with PFE (100μg/ml) for 2 h followed by IL-1β (10 ng/ml) stimulation for 24 h. COL2A1 mRNA and protein levels in the culture media was determined by quantitative Real-Time PCR and ELISA respectively. Values are shown as Mean ± SE. *P<0.05 **P<0.01
4. Discussion
The current study was carried out to determine the effect of oral administration of PFE on cartilage degeneration in rabbit model of post-traumatic OA. In this OA model, oral administration of PFE was markedlyeffective against joint destruction. The histological findings were informative with respect to the effects of PFE on OA cartilage structural changes. In fact, rabbits in both the groups that were fed PFE exhibited a significant decrease in the markers of cartilage matrix damage, such as loss of Safranin-O-staining,structural changes and cloningindicatinginhibition ofmatrix degradation compared to the water fed group. Overall, oral administration of PFE resulted in a significant reduction of histological scores of OA in both the PFE-fed groups (Figure 1G). These findings are in accordance with those of Shukla et al., where consumption of PFE was shown to suppress the destruction of bone and cartilage in mice with inflammatory arthritis [32]. Consistent with the results shown here are the findings of chondroprotective effect of Pomegranate juice consumption in monoiodoacetate-induced OA in a mouse model [44]. The structural and biochemical homeostasis of OA is destroyed by chondrocyte apoptosis which is largely modulated by caspase-3 [45]. Caspases exist as latent precursors, which, when activated, initiate the death program by destroying key components of the cellular infrastructure and activating factors that mediate damage to the cells. Increased caspase-3 expression and cleavage of PARP p85, markers of apoptosis, has been noted to correlate with reduced cell density in human OA cartilage [18], while inhibition of apoptosis using caspase inhibitors reduced the severity of cartilage lesions in experimental OA [19]. In accordance with this, the chondroprotective effect of PFE observed in the present study was the significantly reduced number of chondrocytes positive for active caspase 3 and the cleaved PARP p85 in rabbits that were fed PFE.
Many proteases have been shown to play a major role in catabolism of OA cartilage. For instance, MMP-3 is capable of cleaving the ACAN core protein as well as COL2A1 in cartilage [12]] and MMP-13 is of particular importance because it is found elevated in OA and cleave COL2A1, the major component of the cartilage matrix, more efficiently[12]. In ACLT dog model of OA, inhibition of the synthesis of MMPs by treatment with Pioglitazone (a peroxisome proliferator-activated receptor-γ agonist) [46] and Licofelone (a dual inhibitor of 5-lipoxygenase and cyclooxygenase)[47], has been found to be associated with a reduction in the development of cartilage lesions. These enzymes, by cleaving the triple helix of COL2A1 and core protein of aggrecan respectively, induce irreversible damage to the cartilage matrix structure. Thereby, they can modify the biophysical properties of cartilage and reduce its resilience to the abnormal biomechanical forces present in traumatized joints. In the present study, oral administration of PFE reduced the mRNA levels of MMP-3 and MMP-13 in the cartilage. When these findings were verified using rabbit articular chondrocytes, PFE pretreatment showed similar inhibition of the MMPs post-IL-1β-stimulation. Moreover,we have previously shown thatthe treatment with PFE inhibits the IL-1β-induced expression of MMP-1, MMP-3 and MMP-13 expression in human OA chondrocytes, which was mediated by inhibiting the activation of p38- and JNK-MAPK pathway, thereby reducing the available pool of activated c-Jun and activating transcription factor 2 [30]. In the present study plasma MMP-13 levels were significantly inhibited in ACLT+ pre-&-post PFE groups compared to ACLT alone group. Studies suggest that drugs that can reduce MMP-13 synthesis in cartilage and bone and prevent subchondral bone resorption could exert a disease modifying effect in OA patients [47, 48]. On a similar note a recent study suggested a protective role of pomegranate juice in reducing serum MMP-13 levels, WOMAC (Western Ontario and McMaster Universities Osteoarthritis index) total score and stiffness score, and physical function in knee OA patients after 6 week intervention[49]. Although, IL-1β levels were weakly increased in the synovial fluid of ACLT alone group compared to sham group, a clear increase in plasma and synovia fluid IL-6 level was observed in ACLT animals showing a systemic and local response of OA process, which was absent in sham animals. Furthermore, a significantly less IL-6 levels were detected in both plasma and synovial fluid of PFE fed animals.In the present study, PFE pretreatment also showed an inhibition of IL-6 expression in IL-1β-stimulated rabbit articular chondrocytes. In the present study, we also found that PFE oral administration prevented the loss of cartilage matrix and increased the mRNA expression of ACAN and COL2A1 in the cartilage (Figure 3). Following the addition of IL-1β to the rabbit chondrocytes culture, a decrease in COL2A1 production along with an increase in MMP-13 activity was observed. However, PFE treatment reversed this effect by increasing COL2A1 levels and inhibiting MMP-13 activity in rabbit articular chondrocytes. That the protective effect was mediated through the inhibition of MMP-13 activity also gets strength from the in vitro activity assay data where exogenously added PFE suppressed the enzyme activity. We have previously shown the inhibitory effects of PFE on IL-1β-induced proteoglycan breakdown in cartilage explants in vitro[30]. Prodelphinidin a condensed polymeric tannin found in pomegranate has been shown to induce proteoglycan and COL2A1 synthesis in human chondrocytes[50].
PGE2, a product of arachidonic acid metabolism,plays a key role in the progression of cartilage destruction in OA and has also been implicated in the remodeling of cartilage and bone in OA[43]. Oral consumption of PFE decreased the level of PGE2 in the synovial fluid of rabbits with surgically-induced OA compared to the rabbits subjected to ACLT and given water alone. A decrease in PGE2 production by PFE was also observed in IL-1β-stimulated rabbit articular chondrocytes. Previous studies demonstrated that PFE constituents become bioavailable after repeated oral administration and suppress COX-2 activity in vitro[51-53]. Results reported here suggest that oral administration of PFE inhibits the expression and production pathologic inflammatory molecules in vivo that have been implicated in OA pathogenesis.Activation of MAPKs and NF-κB is intimately associated with the increased expression of critical mediators of inflammation involved in OA pathogenesis, including the expression of MMPs, IL-6 and PGE2[54]. Inhibition of MAPKs and NF-κB pathways resulted in the inhibition of IL-1β-induced MMPs, IL-6 and PGE2 production in rabbit chondrocytes. We have previously shown that inhibition of IL-6 and IL-8 expression in PMACI (phorbol- 12-myristate 13-acetate plus calcium ionophore A23187)-stimulated KU812 cells by PFE was mediated by inhibition of NF-κB activity and the activation of c-Jun-N-terminal kinases (JNKs) and the extracellular regulated kinase (ERK)–MAPK pathways[38]. We have also shown that PFE inhibited the IL-1β-induced activation of MKK3 and the p38α-MAPK isoform and DNA binding activity of the runt-related transcription factor 2 (RUNX-2) in human OA chondrocytes [33]. Taken together with our currentfindings these dataprovide a deeper mechanistic insight onanti-inflammatory and cartilage protective effects of PFE consumption in vivo.
The present study has limitations largely imposed by the study design. One such limitation is the duration of the design (8 week). A longer study would provide more information on the potential effect of PFE against the long-term development of OA. Moreover, study design involved prophylactic use of the drug and conclusions drawn might have been influenced by the treatment schedule. A further study in which therapeutic administration is employed would be informative and complementary to the present one.Overall, our data indicate that oral consumption of PFE has anti-inflammatory and chondroprotective effects in an animal model of post-traumatic OA by enhancing the expression of matrix components ACAN, COL2A1, and by suppressing the expression of catabolic mediators (IL-6, MMPs and PGE2). Therefore, consumption of PFE may be of value as adjunct therapy or in approaches designed to prevent the onset of OA with no potential adverse events.
Supplementary Material
Acknowledgments
This work was supported in part by grants from National Institute of Health grants (RO1 AT-003267; RO1-AT-005520, R21-AT504615) and funds from the Northeast Ohio Medical University.
Footnotes
Authors’ contributions
TMH conceived the study. NA and TMH were responsible for its design and coordination of the data acquisition and analyses, interpretation of the data, manuscript preparation and statistical analyses. NA performed the ACL-transection surgeries. NMK set up the rabbit chondrocyte cultures, performed the MMPs activity assay and ELISA, data acquisition and interpretation. OA performed the PGE2 ELISA and data acquisition and interpretation. All authors approved the final version of the manuscript.
Competing interests
The authors declare that they have no conflict of interest.
References
- 1.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi: 10.1016/S0140-6736(11)60243-2. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014. 73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg WB. Pathophysiology of osteoarthritis. Joint Bone Spine. 2000;67(6):555–556. doi: 10.1016/s1297-319x(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 5.Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100(1):93–106. doi: 10.1172/JCI119526. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billinghurst RC, Wu W, Ionescu M, Reiner A, Dahlberg L, Chen J, et al. Comparison of the degradation of type II collagen and proteoglycan in nasal and articular cartilages induced by interleukin-1 and the selective inhibition of type II collagen cleavage by collagenase. Arthritis Rheum. 2000;43(3):664–672. doi: 10.1002/1529-0131(200003)43:3<664::AID-ANR24>3.0.CO;2-D. doi: 10.1002/1529-0131(200003)43:3<664::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.Samuels J, Krasnokutsky S, Abramson SB. Osteoarthritis: a tale of three tissues. Bull NYU Hosp Jt Dis. 2008;66(3):244–250. doi: 10.1016/j.bone.2012.02.012. [PubMed] [Google Scholar]
- 8.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature reviews Rheumatology. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 10.Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9(6):539–552. doi: 10.1053/joca.2001.0427. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. doi: 10.1186/ar4133. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 13.Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146(1):75–85. [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27(2):455–462. [PubMed] [Google Scholar]
- 15.Mistry D, Oue Y, Chambers MG, Kayser MV, Mason RM. Chondrocyte death during murine osteoarthritis. Osteoarthritis Cartilage. 2004;12(2):131–141. doi: 10.1016/j.joca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Lo MY, Kim HT. Chondrocyte apoptosis induced by collagen degradation: inhibition by caspase inhibitors and IGF-1. J Orthop Res. 2004;22(1):140–144. doi: 10.1016/S0736-0266(03)00117-7. doi:10.1016/S0736-0266(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto S, Ochs RL, Rosen F, Quach J, McCabe G, Solan J, et al. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc Natl Acad Sci U S A. 1998;95(6):3094–3099. doi: 10.1073/pnas.95.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharif M, Whitehouse A, Sharman P, Perry M, Adams M. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis Rheum. 2004;50(2):507–515. doi: 10.1002/art.20020. doi: 10.1002/art.20020. [DOI] [PubMed] [Google Scholar]
- 19.D'Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54(6):1814–1821. doi: 10.1002/art.21874. doi:10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 20.Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(8):S230–235. [PubMed] [Google Scholar]
- 21.Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43(9):1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. doi:10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Akhtar N, Haqqi TM. Current nutraceuticals in the management of osteoarthritis: a review. Ther Adv Musculoskelet Dis. 2012;4(3):181–207. doi: 10.1177/1759720X11436238. doi: 10.1177/1759720X11436238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Dube CE, Eaton CB, McAlindon TE, Lapane KL. Longitudinal use of complementary and alternative medicine among older adults with radiographic knee osteoarthritis. Clin Ther. 2013;35(11):1690–1702. doi: 10.1016/j.clinthera.2013.09.022. doi: 10.1016/j.clinthera.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102(41):14813–14818. doi: 10.1073/pnas.0505870102. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48(10):4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 26.Seeram NP, Nair MG. Inhibition of lipid peroxidation and structure-activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J Agric Food Chem. 2002;50(19):5308–5312. doi: 10.1021/jf025671q. [DOI] [PubMed] [Google Scholar]
- 27.Sreekumar S, Sithul H, Muraleedharan P, Azeez JM, Sreeharshan S. Pomegranate fruit as a rich source of biologically active compounds. Biomed Res Int. 2014;2014:686921. doi: 10.1155/2014/686921. doi: 10.1155/2014/686921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajaikumar KB, Asheef M, Babu BH, Padikkala J. The inhibition of gastric mucosal injury by Punicagranatum L. (pomegranate) methanolic extract. J Ethnopharmacol. 2005;96(1-2):171–176. doi: 10.1016/j.jep.2004.09.007. doi: 10.1016/j.jep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16(6):360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM. Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J Nutr. 2005;135(9):2096–2102. doi: 10.1093/jn/135.9.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Bioavailable constituents/metabolites of pomegranate (Punica granatum L) preferentially inhibit COX2 activity ex vivo and IL-1beta-induced PGE2 production in human chondrocytes in vitro. J Inflamm (Lond) 2008;5:9. doi: 10.1186/1476-9255-5-9. doi: 10.1186/1476-9255-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition. 2008;24(7-8):733–743. doi: 10.1016/j.nut.2008.03.013. doi: 10.1016/j.nut.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasheed Z, Akhtar N, Haqqi TM. Pomegranate extract inhibits the interleukin-1beta- induced activation of MKK-3, p38alpha-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res Ther. 2010;12(5):R195. doi: 10.1186/ar3166. doi: 10.1186/ar3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vignon E, Arlot M, Hartmann D, Moyen B, Ville G. Hypertrophic repair of articular cartilage in experimental osteoarthrosis. Ann Rheum Dis. 1983;42(1):82–88. doi: 10.1136/ard.42.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro F, Glimcher MJ. Induction of osteoarthrosis in the rabbit knee joint. Clin Orthop Relat Res. 1980;(147):287–295. [PubMed] [Google Scholar]
- 36.Badlani N, Inoue A, Healey R, Coutts R, Amiel D. The protective effect of OP-1 on articular cartilage in the development of osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):600–606. doi: 10.1016/j.joca.2007.09.009. doi: 10.1016/j.joca.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Tzulker R, Glazer I, Bar-Ilan I, Holland D, Aviram M, Amir R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J Agric Food Chem. 2007;55(23):9559–9570. doi: 10.1021/jf071413n. doi: 10.1021/jf071413n. [DOI] [PubMed] [Google Scholar]
- 38.Rasheed Z, Akhtar N, Anbazhagan AN, Ramamurthy S, Shukla M, Haqqi TM. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-kappaB in human KU812 cells. J Inflamm (Lond) 2009;6:1. doi: 10.1186/1476-9255-6-1. doi: 10.1186/1476-9255-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage. 2010;18(3):S53–65. doi: 10.1016/j.joca.2010.05.029. doi: 10.1016/j.joca.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Fosang AJ, Last K, Maciewicz RA. Aggrecan is degraded by matrix metalloproteinases in human arthritis. Evidence that matrix metalloproteinase and aggrecanase activities can be independent. J Clin Invest. 1996;98(10):2292–2299. doi: 10.1172/JCI119040. doi: 10.1172/JCI119040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sondergaard BC, Henriksen K, Wulf H, Oestergaard S, Schurigt U, Brauer R, et al. Relative contribution of matrix metalloprotease and cysteine protease activities to cytokine-stimulated articular cartilage degradation. Osteoarthritis Cartilage. 2006;14(8):738–748. doi: 10.1016/j.joca.2006.01.016. doi: 10.1016/j.joca.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12(1):19–32. doi: 10.1038/sj.cr.7290106. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 43.Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, et al. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46(7):1789–1803. doi: 10.1002/art.10356. doi: 10.1002/art.10356. [DOI] [PubMed] [Google Scholar]
- 44.Hadipour-Jahromy M, Mozaffari-Kermani R. Chondroprotective effects of pomegranate juice on monoiodoacetate-induced osteoarthritis of the knee joint of mice. Phytother Res. 2010;24(2):182–185. doi: 10.1002/ptr.2880. doi: 10.1002/ptr.2880. [DOI] [PubMed] [Google Scholar]
- 45.Green DR, Llambi F. Cell Death Signaling. Cold Spring Harb Perspect Biol. 2015;7(12) doi: 10.1101/cshperspect.a006080. doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boileau C, Martel-Pelletier J, Fahmi H, Mineau F, Boily M, Pelletier JP. The peroxisome proliferator-activated receptor gamma agonist pioglitazone reduces the development of cartilage lesions in an experimental dog model of osteoarthritis: in vivo protective effects mediated through the inhibition of key signaling and catabolic pathways. Arthritis Rheum. 2007;56(7):2288–2298. doi: 10.1002/art.22726. [DOI] [PubMed] [Google Scholar]
- 47.Pelletier JP, Boileau C, Boily M, Brunet J, Mineau F, Geng C, et al. The protective effect of licofelone on experimental osteoarthritis is correlated with the downregulation of gene expression and protein synthesis of several major cartilage catabolic factors: MMP-13, cathepsin K and aggrecanases. Arthritis Res Ther. 2005;7(5):R1091–1102. doi: 10.1186/ar1788. doi: 10.1186/ar1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelletier JP, Boileau C, Brunet J, Boily M, Lajeunesse D, Reboul P, et al. The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone. 2004;34(3):527–538. doi: 10.1016/j.bone.2003.11.021. doi: 10.1016/j.bone.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 49.Ghoochani N, Karandish M, Mowla K, Haghighizadeh MH, Jalali MT. The effect of pomegranate juice on clinical signs, matrix metalloproteinases and antioxidant status in patients with knee osteoarthritis. J Sci Food Agric. 2016 doi: 10.1002/jsfa.7647. doi: 10.1002/jsfa.7647. [DOI] [PubMed] [Google Scholar]
- 50.Garbacki N, Angenot L, Bassleer C, Damas J, Tits M. Effects of prodelphinidins isolated from Ribes nigrum on chondrocyte metabolism and COX activity. Naunyn Schmiedebergs Arch Pharmacol. 2002;365(6):434–441. doi: 10.1007/s00210-002-0553-y. doi: 10.1007/s00210-002-0553-y. [DOI] [PubMed] [Google Scholar]
- 51.Cerda B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. 2003;42(1):18–28. doi: 10.1007/s00394-003-0396-4. doi: 10.1007/s00394-003-0396-4. [DOI] [PubMed] [Google Scholar]
- 52.Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta. 2004;348(1-2):63–68. doi: 10.1016/j.cccn.2004.04.029. doi: 10.1016/j.cccn.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 53.Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136(10):2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 54.Firestein GS, Manning AM. Signal transduction and transcription factors in rheumatic disease. Arthritis Rheum. 1999;42(4):609–621. doi: 10.1002/1529-0131(199904)42:4<609::AID-ANR3>3.0.CO;2-I. doi: 10.1002/1529-0131(199904)42:4<609::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


