SUMMARY
Acute myelogenous leukemia (AML) is an aggressive disease associated with drug resistance and relapse. To improve therapeutic strategies, it is critical to better understand the mechanisms that underlie AML progression. Here we show that the integrin binding glycoprotein CD98 plays a central role in AML. CD98 promotes AML propagation and lethality by driving engagement of leukemia cells with their microenvironment and maintaining leukemic stem cells. Further, delivery of a humanized anti-CD98 antibody blocks growth of patient-derived AML, highlighting the importance of this pathway in human disease. These findings indicate that microenvironmental interactions are key regulators of AML and that disrupting these signals with inhibitors such as CD98-antibodies may be a valuable therapeutic approach for adults and children with this disease.
Graphical abstract
INTRODUCTION
The microenvironment that surrounds cancer cells can play a critical regulatory role in malignant growth and expansion. Early studies in breast cancer using both 3D cultures and in vivo approaches identified a role for stromal elements such as fibroblasts, vascular cells and the extracellular matrix in sustaining tumor growth and dissemination, in part by activating TGF-β, SDF1-α/CXCR4 signaling pathways, and through the release of metalloproteinases (Bissell and Radisky, 2001; Orimo et al., 2005; Petersen et al., 1992). Further, epithelial interactions with extracellular matrix components were subsequently shown to be necessary for effective dissemination and metastasis of breast cancers (Desgrosellier et al., 2009; Desgrosellier and Cheresh, 2010), and to contribute to chemoresistance (Nakasone et al., 2012). Recent studies have challenged the dogma that the microenvironment is always supportive of oncogenesis by showing that targeted depletion of stromal cells can enhance pancreatic cancer growth (Lee et al., 2014; Ozdemir et al., 2014; Rhim et al., 2014). Although leukemic cells experience less anchorage than solid tumor cells, and are often thought to not be as spatially restricted, they do grow and reside within the bone marrow surrounded by a large network of microenvironmental cells. However, the specific molecular cues that drive the engagement of leukemia cells with the microenvironment and their role in sustaining and promoting oncogenesis remain poorly understood.
To address these key questions we have focused on CD98, a molecule that amplifies adhesive signals induced by a variety of extracellular matrix components through interactions with integrins (Fenczik et al., 1997; Feral et al., 2005), and plays an important regulatory role in assembly of a fibronectin matrix (Feral et al., 2007). Because of its role in mediating signals from multiple integrins, blocking CD98 can impair a broad spectrum of adhesive signals, and be a powerful approach to disrupting interactions of cancer cells with their microenvironment. Structurally, CD98 is a transmembrane protein complex that consists of a single pass heavy chain (CD98hc encoded by SLC3A2) disulfide linked to a multipass light chain (consisting of amino acid transporters such as LAT1). The heavy chain of CD98 promotes adhesive signals in part by binding to multiple β1 and β3-integrins (Cantor et al., 2008; Zent et al., 2000) and thereby increasing cell spreading, migration, survival and growth. The association of CD98 heavy chain with any one of six light chains also regulates essential amino acid transport, which can contribute to cell survival (Imai et al., 2010; Rosilio et al., 2014). In the immune system, modulating CD98 function through blocking antibodies has implicated CD98 in B and T cell proliferation and activation (Haynes et al., 1981). In addition, conditional deletion of CD98 has been shown to inhibit clonal proliferation of T cells in response to antigens and prevent establishment of autoimmune disease (Cantor et al., 2011), as well as abrogate the ability of B cells to respond to mitogens, leading to defects in plasma cell formation (Cantor et al., 2009).
Because of its role in clonal expansion, CD98 has also been studied in cancer. CD98 is highly expressed in many solid tumors, and its expression has been associated with poor prognosis (reviewed in (Cantor and Ginsberg, 2012). The functional relevance of CD98 has primarily been tested in context of solid cancer cell lines. For example, ectopic expression of CD98 can transform epithelial cell lines like CHO (Henderson et al., 2004) and NIH3T3 (Hara et al., 1999) and promote their anchorage-independent growth and tumor formation in immunocompromised mice, while CD98 inhibitory antibodies can block the proliferation of bladder cancer cells lines (Yagita et al., 1986). Further, the inhibition of a CD98 light chain (LAT1) can affect proliferation of breast and lung cancer cell lines by impairing amino acid transport (Imai et al., 2010; Shennan and Thomson, 2008). The development of a conditional Slc3a2 (referred to as Cd98hc) allele (Feral et al., 2007) has allowed genetic analysis of the role of CD98. Cd98hc deletion can prevent the formation of embryonic stem cell teratomas (Feral et al., 2005) and squamous cell carcinomas (Estrach et al., 2014) and heterozygous loss of Cd98hc reduces intestinal adenoma formation (Nguyen et al., 2011). However, the role of CD98 in hematologic malignancies and the relative contribution of adhesive signaling and amino acid transport functions to cancer have not been examined. To address this, we tested whether CD98 controls the establishment and propagation of primary leukemia cells within their native microenvironment by using both a genetic approach and the delivery of a newly developed therapeutic in context of de novo AML.
RESULTS
CD98 in normal hematopoiesis
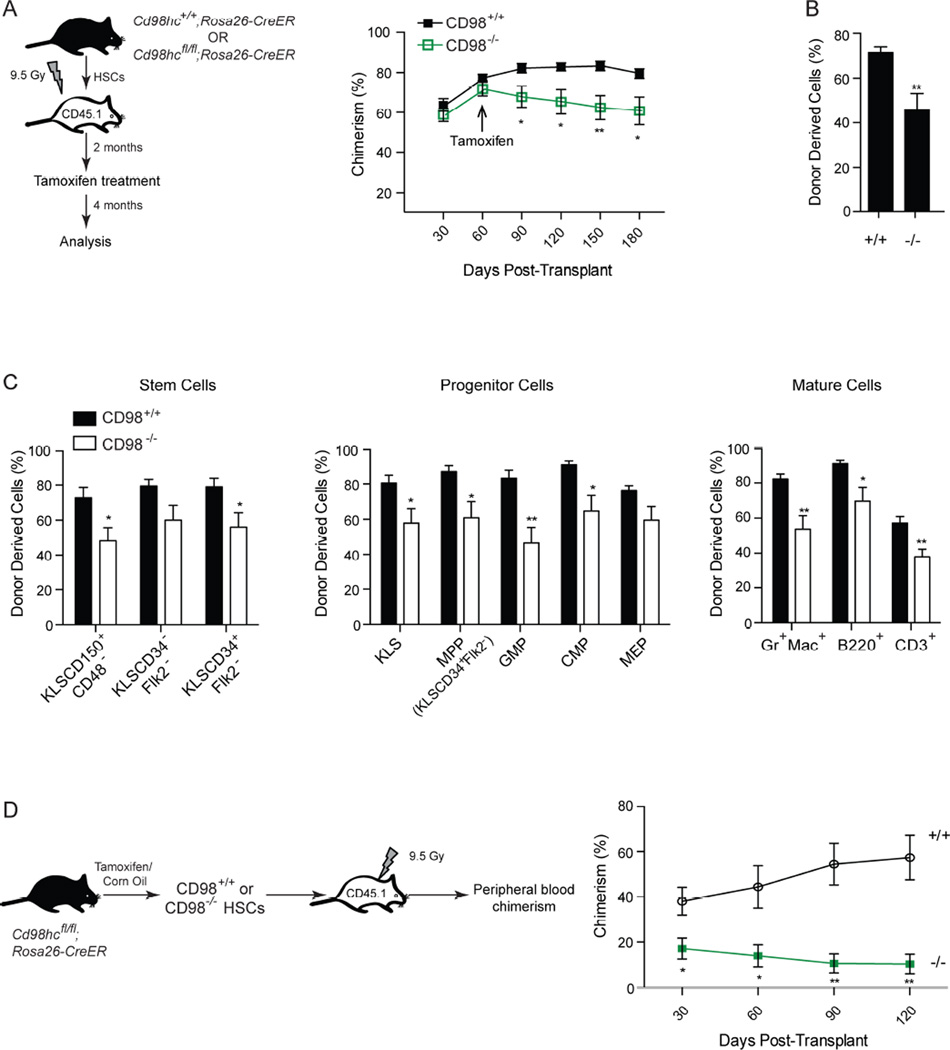
Given that CD98 was expressed in all hematopoietic lineages (Figures S1A, B), we tested its function using by conditionally deleting Cd98hc using the Rosa26-CreERT2 model. Cre-mediated recombination leads to the excision of exons 1 and 2, which encode the transmembrane and cytoplasmic regions of the CD98 heavy chain, thus resulting in complete loss of gene expression (Feral et al., 2007). The Cd98hcfl/fl;Rosa26-CreER+/+ mice (denoted hereafter as Cd98hcfl/fl) allowed temporal control over CD98 expression and enabled efficient loss of CD98 after tamoxifen delivery (Figure S1C).
Conditional deletion of Cd98hc (Figures S1C, D; denoted as CD98+/+ or CD98−/− post-treatment) did not adversely affect bone marrow cellularity (Figure S1E) or HSC numbers (cKit+Lin−Sca1+CD150+CD48− or KLSCD34+/−Flk2−; Figure 1A). Some changes such as an increase in KLSCD150+CD48− cells (Figure 1A), a reduction in multipotent myeloid progenitors (Figures 1B, S1F), and an increase in differentiated B and T cells (Figure 1C), were noted and could be due to alterations in differentiation and/or proliferation. To identify the cell intrinsic impact of eliminating CD98 expression, we transplanted Cd98hcfl/fl or Cd98hc+/+ HSCs into wild-type recipients. Deletion following stable reconstitution two months after transplant had no effect on apoptosis (Figure S1G) and led to a minor 1.5 fold decrease in chimerism (Figures 2A–C). However transplantation of HSCs lacking CD98 showed a functional dependence on this signal in context of rapid proliferative need (Figure S1H): thus, while CD98+/+ chimerism increased from 38% to 57%, CD98−/− chimerism dropped from 17% to 10% (Figure 2D). These suggest that while CD98 loss may not affect the numbers of established HSCs and progenitors, it can impact their proliferative and regenerative capacity.
Figure 1. Generation and analysis of conditional CD98 deficient mice.
(A) Cd98hc+/+;Rosa26-CreER (denoted as CD98+/+) and Cd98hcfl/fl;Rosa26-CreER (denoted as CD98−/−) mice were treated with tamoxifen and analyzed 3 days later (n=5 for each cohort). Representative FACS plots (gated on KLS cells), average stem cell frequencies and absolute numbers of stem cells and progenitor cells in CD98+/+ and CD98−/− mice are shown.
(B) Representative FACS plots show myeloid and erythroid progenitor frequency in CD98+/+ and CD98−/− mice (gated on Lin−IL7R−cKit+Sca− cells). The average frequencies and numbers of progenitors (MPP: KLSCD34+Flk2+; GMP: Lin−IL7Ra−Kit+Sca1−CD34+CD16/32+; CMP: Lin−IL7Ra−Kit+Sca1−CD34+CD16/32−; MEP: Lin−IL7Ra−Kit+Sca1−CD34−CD16/32−) are shown.
(C) Average frequencies and numbers of differentiated cells in CD98+/+ and CD98−/− mice.
Error bars represent ±SEM, *p<0.05, **p<0.01, ***p<0.001, Unpaired t test.
See also Figure S1
Figure 2. Effect of CD98 loss on HSC function.
(A) KLSCD34−Flk2− cells from Cd98hc+/+;Rosa26-CreER (denoted as CD98+/+) or Cd98hcfl/fl;Rosa26-CreER (denoted as CD98−/−) mice were transplanted and tamoxifen delivered after 2 months (schematic, left). Hematopoietic stem cells were subsequently analyzed at 4 months after transplant (n=9 per cohort). Average donor chimerism in the peripheral blood prior to and after tamoxifen treatment is shown (right).
(B) Average donor chimerism in the bone marrow of transplanted mice 4 months after tamoxifen delivery.
(C) Average frequency of HSCs, progenitors and differentiated cells in the bone marrow of mice transplanted with CD98+/+ and CD98−/− HSCs (n=9 for each cohort).
(D) HSCs from tamoxifen or corn oil treated Cd98hcfl/fl;Rosa26-CreER mice were transplanted (schematic, left), and peripheral blood chimerism analyzed (right, n=5–6 for per cohort).
Error bars represent ±SEM, *p<0.05, **p<0.01, Mann-Whitney U Test
CD98 in de novo AML initiation and maintenance
We tested the importance of CD98 in leukemogenesis using an MLL-driven model of de novo AML, a highly drug resistant disease in adults and children. Because MLL driven AML often presents with NRAS mutations, we used MLL-AF9 and NRASG12V oncogenes to establish myeloid leukemia with Cd98hcfl/fl cells (Figures 3A, S2A–C). CD98 loss led to a marked increase in survival (55%) relative to controls (0%) (Figures 3A, S2D, E), indicating that CD98 is important for MLL-leukemia initiation. To determine if continued AML propagation requires CD98, we assessed the capacity of sorted cKit+ leukemia cells to propagate disease (Figure 3B). Tamoxifen induced CD98 loss led to a dramatic reduction in the number of leukemic colonies formed in vitro (Figure 3C) and significantly increased the median survival of mice transplanted with cKit+ AML cells from 21.5 days to 33 days (Figure 3D). These data indicate that abrogating CD98 expression reduced morbidity from established disease. Serial transplantation of CD98−/− cKit+ leukemia cells not only impaired self-renewal but also led to a striking improvement in survival (0% for CD98+/+ vs. 46% for CD98−/−) (Figure 3D, S2F). Finally, because patients generally present with full-blown disease, we modeled a more clinically relevant setting by eliminating CD98 expression after AML establishment (Figure 3E). Loss of CD98 in this context led to a significant increase in survival (0% for CD98+/+ vs. 66% for CD98−/−, Figure 3E). CD98 appeared to be broadly required by non-MLL AML as well, with its loss improving survival dramatically in disease driven by AML-ETO9a and NRASG12V (Figure 3F). These data demonstrated a requirement for CD98 in the initiation, self-renewal and propagation of de novo AML, and provided clear genetic evidence that targeting CD98 may be of therapeutic value.
Figure 3. CD98 loss impairs AML growth and propagation.
(A) KLS cells from Cd98hcfl/fl;;Rosa26-CreER mice were retrovirally transduced with MLL-AF9-IRES-NGFR and NRASG12V-IRES-YFP and transplanted to establish disease (schematic, left). The start of treatment is indicated by an arrow on each survival curve (right, n=8–9 for each cohort, data combined from two independent experiments).
(B–D) cKit+ cells from established Cd98hcfl/fl;Rosa26-CreER leukemia (schematic in B) were isolated and cultured in vitro in the presence of tamoxifen or vehicle, and colony formation assessed (C). Cells were transplanted, 2° recipients treated with tamoxifen or vehicle and survival monitored (n=8 for vehicle and n=11 for tamoxifen treatment, data combined from two independent experiments). The cKit+ CD98+/+ or CD98−/− leukemia cells from 2° mice were also transplanted into 3° recipients to monitor in vivo self renewal capacity (n=13 for each cohort, data combined from two independent experiments) (D).
(E) cKit+ Cd98hcfl/fl;Rosa26-CreER AML cells were transplanted, recipients treated with tamoxifen or vehicle from day 8–13 (schematic, left) and survival monitored (right, n=8 for control and n=9 for tamoxifen treatment, data combined from two independent experiments).
(F) KLS cells from Cd98hcfl/fl;Rosa26-CreER mice were isolated, infected with AML-ETO9a and NRAS oncogenes, and transplanted. Mice were subsequently treated with tamoxifen or corn oil, starting 8 days after transplant (n=9 for vehicle and n=8 for tamoxifen treatment, data combined from two independent experiments).
Survival curves depict log rank test p values. Error bars represent ±SEM.
See also Figure S2.
CD98 loss impairs survival and depletes leukemia stem cells
To determine the cellular basis of CD98’s effect, we analyzed the impact of CD98 loss on leukemia proliferation, apoptosis and differentiation. Loss of CD98 led to a modest decline in proliferation (Figures 4A, B), but triggered a dramatic rise in apoptosis, suggesting that CD98 is critical for leukemia cell survival (Figures 4C, D). Gene expression analysis of candidate apoptosis-related genes showed that the loss of CD98 led to decreased expression of anti-apoptotic genes such as Bcl2L1 and Akt1 and increased expression of pro-apoptosis genes (e.g. Casp1; Figure 4E). To determine if these play a functional role downstream of CD98, we tested if their re-expression could rescue colony-forming ability of CD98−/− AML cells. While Bcl2L alone was able to partially rescue the colony formation of CD98−/− cells to ~18% of control, the addition of activated Myr-AKT increased colony forming ability to 50% (Figure S3A).
Figure 4. Loss of CD98 triggers apoptosis and depletion of AML stem cells.
(A, B) cKit+ Cd98hcfl/fl;Rosa26-CreER AML cells were transplanted and treated with tamoxifen or corn oil. 18 days after transplant, mice were injected with BrdU and cells analyzed for incorporation 22 hr later. Representative FACS plots show BrdU and 7AAD staining of MLL-AF9+NRAS+ leukemia cells from CD98+/+ and CD98−/− mice (A). Average frequency of cells in distinct phases of the cell cycle (n=2–3 per cohort) (B).
(C, D) Representative FACS plots (C) and graph (D) show analysis of early and late apoptosis in MLL-AF9+NRAS+ leukemia cells 18–21 days post-transplant (n=3 per cohort).
(E) Expression of apoptosis associated genes in leukemia cells from representative wild-type and CD98−/− leukemia. Data shown are from triplicate of one sample per cohort. Similar results were obtained from three independent samples per cohort.
(F) Mean fluorescence intensity of CD98 expression in control unstained, cKit+ and cKit− MLL-AF9+NRAS+ leukemia cells (n=4).
(G) Representative FACS plots of cKit expression in CD98+/+ and CD98−/− leukemia cells. Average frequency of cKit+ leukemia stem cells in secondary and tertiary MLL leukemia is shown (n=3–5 per cohort for secondary leukemia and n=3 per cohort for tertiary leukemia).
(H) Expression of MLL-leukemia stem cell genes in sorted cKit+ cells from representative wild-type and CD98−/− leukemia. Data shown are from triplicate of one sample per cohort. Similar results were obtained from three independent experiments.
Error bars represent ±SEM, *p<0.05, **p<0.01, ***p<0.001, Unpaired t test.
See also Figure S3.
We also analyzed leukemia stem cells (LSCs), marked in this model as cKit+ or Gr1−/lowcKit+ (Shi et al., 2013; Somervaille and Cleary, 2006; Zuber et al., 2011). CD98 loss clearly affected the frequency of LSCs, consistent with enriched CD98 expression in this population (Figures 4F and S3B). Leukemias arising from CD98−/− cells showed a marked depletion of both cKit+ (Figure 4G) and Gr1locKit+ stem cells (Figures S3C–G). Consistent with this, CD98−/− AML stem cells expressed significantly lower levels of MLL-leukemia stem cell associated genes (Somervaille et al., 2009) (Figure 4H). These collectively indicate that CD98 is important for promoting survival of leukemia cells and for maintaining leukemia stem cells in MLL-driven AML.
CD98 mediated integrin-signaling is required for leukemia growth
Since CD98 can regulate both integrin-mediated signaling and amino acid transport, we tested the importance of each function for leukemia progression by reconstituting the two functions individually in CD98−/− cells using chimeric human CD98-CD69 proteins (Figures 5A and S4A). These chimeric proteins separated CD98 functions by replacing either the integrin binding cytoplasmic and transmembrane domain or the extracellular amino-acid transport domain of CD98 with parts of CD69, another type II transmembrane protein (Fenczik et al., 2001; Feral et al., 2005). Loss of colony forming ability could be rescued almost completely by the full length human CD98 heavy chain (as a control) as well as partially by the chimera that restored integrin-binding and signaling (Figure 5B), indicating that downstream activation of integrin signaling is critical for leukemia growth. In contrast, the chimera that only restored amino acid transport failed to rescue the defect. Interestingly, rescue was most efficient when the chimera that preserves integrin binding was co-delivered with the chimera that restores amino acid transport. These data suggest that integrin binding function of CD98 is critically required for leukemic growth and that the amino acid transport function can synergize with integrin binding to fully restore normal function of leukemia stem cells. We should note that CD98 loss affected cells grown on semi-soft substrates such as methylcellulose or in vivo, but not when plated in liquid culture (Figures 3C, D, 5B and data not shown). This is consistent with previous work that CD98 mediated signaling is required for proliferation and survival on soft substrates but not stiff substrates (Estrach et al., 2014), possibly because there is enough tension on contact with stiff substrates (like plastic) that CD98 is not needed to amplify integrin signaling.
Figure 5. AML is dependent on CD98 mediated integrin signaling.
(A) Human CD98-CD69 chimeric constructs designed to specifically reconstitute either the amino acid transport or the integrin signaling function of CD98.
(B) Average number of colonies formed by Cd98hcfl/fl cKit+ cells transduced with human CD98 chimeric proteins and grown in the presence of ethanol (vehicle) or tamoxifen (to delete Cd98hc), n=3.
(C) Representative images of calvarial bone marrow (magenta) of mice transplanted with CD98+/+ and CD98−/− AML cells (green). Arrowheads indicate blood vessels (marked by VE-cadherin in blue), and arrow points to a leukemia cell adhering to vessel. Scale bar: 25 µm
(D) Average frequency of CD98+/+ and CD98−/− leukemia cells in contact with blood vessels (distance <0.5µm) for 25 min or longer (stable/long-term) or for less than 10 min (transient/short-term) (n=12 movies for each group, from 4–5 mice per cohort. Data were compiled from two independent experiments).
(E) Representative pictomicrographs show CD98+/+ and CD98−/− leukemia cells (indicated by arrows) adhering to HUVECs. Scale bar: 25 µm
(F) Average frequency of CD98+/+ and CD98−/− cKit+ cells adhering to HUVECs (n=3 from a representative experiment; similar results were obtained from two independent experiments).
(G) Impact of anti-VCAM1 antibody on adhesion of CD98+/+ and CD98−/− cKit+ cells to HUVECs (n=3).
(H) Representative FACS plots shows cKit expression in wild-type leukemia cells after co-culture with HUVECs in the presence or absence of anti-VCAM1. Average frequency of cKit+ cells at the indicated time points is quantified (n=3 for each condition).
(I) Flow cytometric plots showing frequency of Annexin+cKit+ AML cells. Average frequency of Annexin+cKit+ cells at the indicated time points is quantified (n=3 for each condition).
Error bars represent ±SEM, *p<0.05, **p<0.01, ***p<0.001, Unpaired t test.
As integrin-dependent adhesion mediates leukemia cell interactions with their microenvironment, we tested if eliminating CD98 expression affected the dynamic in vivo associations of leukemic cells with their niche by real-time imaging. We first tested if wild-type leukemia cells were capable of forming long term interactions with blood vessels over time and found that about 25% of cells in the marrow space remained in close contact with vessels for 25 min or longer. The loss of CD98 reduced long-term interactions by two-fold (Figures 5C, D and Movies S1 and S2), indicating that CD98 enables stable interactions of leukemia cell with their niche in vivo.
To define whether the interactions of leukemia cells with endothelium are physiologically important for maintaining stem cell properties, we used HUVEC cells as a surrogate. Consistent with in vivo imaging analysis, the ability of CD98−/− cKit+ leukemia cells to adhere to HUVECs was reduced by ~40% (Figures 5E, F). To interfere with adhesion and not other signals regulated by CD98, we used anti-VCAM-1 to block integrin-mediated adhesion. Endothelial cells express the integrin ligand VCAM-1 (Swerlick et al., 1992), and AML cells express the integrin α4/ β1 (or VLA-4) (Matsunaga et al., 2003), providing a rationale for testing if adhesion of cKit+ leukemia cells could be blocked by VCAM-1 inhibition, and thus mimic the effect of CD98 loss on adhesion. While blocking VCAM-1 reduced adherence of wild-type cKit+ cells by two-fold, it had no impact on the attachment of CD98-deficient cKit+ cells, confirming that CD98 is required for VCAM-1/VLA-4 mediated interaction of leukemic cells with blood vessels (Figures 5G, S4B, C). Importantly, blocking adhesion led to a 2.5-fold loss of cKit+ leukemia cells (Figure 5H), predominantly due to apoptosis (Figure 5I). These data indicate that CD98-integrin mediated interactions with endothelial cells are needed for survival and maintenance of leukemia stem cells.
CD98 is important for human AML propagation
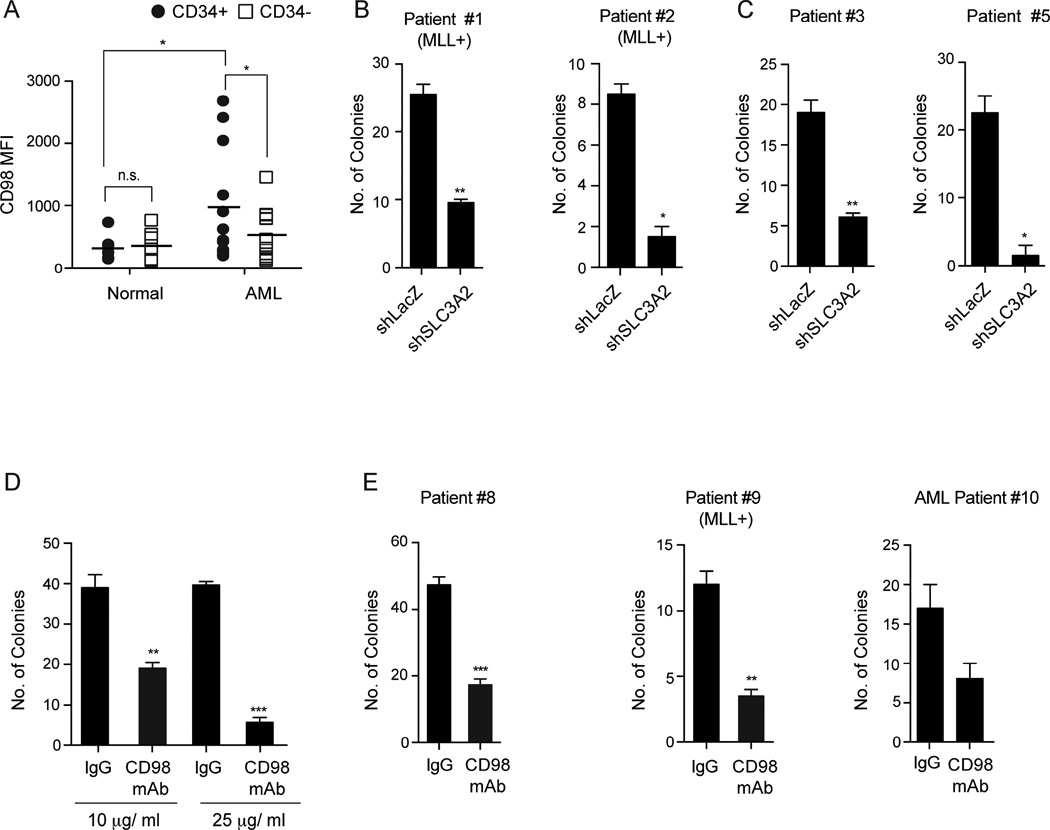
Analysis of normal and leukemia stem cells revealed that while CD98 was expressed at the cell surface on normal CD34+ HSPCs, its expression was three-fold higher in AML CD34+ cells (Figure 6A). CD98 expression was also 1.8-fold higher in the CD34+ AML stem cells compared to CD34− cells, consistent with enriched expression in murine leukemia stem cells (Figures 4F,G, S3B). Functionally, shRNA delivery in primary patient-derived AML cells as well as cell lines, led to a consistent, albeit inefficient, reduction of CD98 expression, affecting both adhesion to endothelial cell lines (Figures S5A, B), and colony formation by 2.7 to 15-fold (Figures 6B, C, S5C–E). Importantly, CD98 inhibition affected colony formation of human AMLs driven by both MLL-translocations (Figures 6B) or by other mutations (Figures 6C, S5D and Table S1). Most importantly, CD98 knockdown led to reduced leukemia burden across several primary patient-derived xenografts (Figures S5F, G).
Figure 6. CD98 inhibition impairs human AML growth in vitro.
(A) Mean fluorescence intensity of CD98 in CD34+ and CD34− normal human cells (n=7) and in AML (n=12).
(B, C) Human MLL+ AML (B) and non-MLL AML (C) samples were transduced with lentiviral shRNAs targeting LacZ (control) or human SLC3A2 (CD98hc). Infected cells were sorted and plated in methylcellulose. Average numbers of colonies formed are shown (n=2–3 for each group).
(D) Primary human AML cells (Patient #6) were seeded in methylcellulose in the presence of varying concentrations of control IgG or CD98 mAb. Average colony formation at each concentration was scored to determine optimal dose (n=3 for each condition).
(E) Three independent AML patient samples were grown in methylcellulose in the presence or absence of CD98 mAb (25 µg/ml). Average numbers of colonies formed are shown (n=2–3 for each group).
Error bars represent ±SEM, * p<0.05, **p<0.01, ***p<0.001, Unpaired t test.
As CD98 is a cell-surface molecule, we tested the impact of antibody mediated targeting in the context of primary AML in vitro and in vivo. To this end we used a humanized CD98 antibody, IGN523 (Hayes et al., 2014). Treatment with IGN523 antibody (denoted as CD98 mAb) significantly impacted the adhesion of AML cells to endothelial cells (Figure S5H). Further, six primary patient samples showed a ~2 to 7 fold decrease in colony forming ability when treated with the antibody (Figures 6D, E, S5I, J). This suggests that inhibition of CD98 via a deliverable could be as effective as genetic silencing of CD98 in blocking the growth of human AML.
Finally we tested the impact of CD98 inhibition on in vivo propagation of human AML. Immunodeficient mice were transplanted with patient derived leukemia cells and treated with CD98 mAb (18-2A, non-humanized clone) or control IgG starting one day after transplant. This resulted in a complete depletion of leukemia engraftment in antibody-treated mice 4 weeks after transplantation (Figures 7A–C and Table S2), indicating that CD98 expression and function is required for human AML establishment in vivo. To determine if the antibody can have an impact on established disease, we treated mice with detectable chimerism in the peripheral blood; mice were distributed into two cohorts with more of the high chimerism mice assigned to the CD98 antibody treatment group in order to test the ability of CD98 inhibition to control high burden disease (Figures 7D–F). While chimerism increased in control treated animals, CD98 antibody delivery dramatically reduced disease burden in the peripheral blood by ~33–100% (Avg. 66% reduction, Figure 7G). Additionally CD98 antibody delivery reduced disease burden by ~12–98% in the bone marrow of treated mice (Avg. 67% reduction, Figure 7G). Importantly, CD98 antibody delivery had no detectable effect on the colony forming ability of normal human CD34+ HSPCs in vitro (Figures S6A) or their ability to engraft irradiated recipients in vivo (Figures S6B, C), paralleling data from the CD98-deficient mouse models. The fact that the antibody may have a more marked effect on human leukemia cells identifies a potential therapeutic window, and indicates that the CD98 mAb may be effective in the clinic.
Figure 7. Loss of CD98 impairs human AML in vivo.
(A–C) NSG mice transplanted with cells from four primary AML patient samples were treated with either control IgG or CD98 mAb (15 mg/kg) once a week, starting one day after transplant (schematic in A). Representative FACS plots show human cell chimerism in the peripheral blood (B). Chimerism in individual xenograft recipients at 4 weeks in peripheral blood (PB) and bone marrow (BM). Xenografts include 2 mice/cohort for AML sample #12 (red); 1 mouse/cohort for #13 (green); 2–3 mice/ cohort for #16 (blue); and 3 mice/ cohort for #17 (black) (C).
(D–G) NSG mice were transplanted with cells from four primary AML patient samples: 2–3 mice/ cohort for #7 (black); 2 mice/cohort for #14 (red); 1 mouse/ cohort for #15 (green); and 3 mice/cohort for #18 (blue). Disease was allowed to establish until there was detectable chimerism in peripheral blood (schematic in D). Mice were then treated with either control IgG or CD98 mAb (15 mg/kg) once a week for three weeks. (E) Representative FACS plots show human cell chimerism in peripheral blood before and after treatment. (F) Leukemia cell chimerism in peripheral blood or bone marrow of individual xenograft recipients. (G) Fold change in leukemia cell chimerism in peripheral blood or bone marrow following delivery of control or CD98 mAb, shown relative to peripheral blood chimerism measured at treatment initiation.
**p<0.01, ***p<0.001, Mann-Whitney U Test.
DISCUSSION
Our work here focuses on defining the molecular signals that mediate microenvironmental influences on leukemia growth. To address this we generated a conditional knockout model of CD98, a surface molecule critical for integrating adhesive signals from the environment. While CD98 loss did not significantly affect the normal hematopoietic pool, it had a dramatic impact on the development and propagation of MLL-driven de novo AML. The impact of eliminating CD98 expression could be attributed, at least in part, to defects in localization and adhesion dynamics with endothelial cells, elements critical for sustaining leukemia stem cell survival (see Model, Figure 8). We corroborated our genetic data by testing an inhibitory humanized CD98 antibody, which effectively blocked the growth of primary AML samples in vitro and in patient-derived xenografts. Collectively, these data provide strong genetic evidence for the requirement of CD98 in leukemia development, and suggest that use of therapeutic anti-CD98 antibodies in AML could be beneficial for both adult and pediatric AML.
Figure 8. Functional role of CD98 in AML.
Model depicting the role of CD98 in wild-type AML and the effect of its deletion on disease progression.
Analysis of CD98−/− mice provided definitive genetic insight into the role of CD98 in normal hematopoiesis. While CD98 deficiency had only a minor impact on the normal adult hematopoietic system in vivo, the CD98−/− HSCs exhibited a markedly reduced ability to form colonies and reconstitute hematopoiesis in a transplant setting. This suggests that while CD98 is not critical for maintaining an established pool of hematopoietic cells, it is needed to drive extensive HSC expansion. These findings are consistent with previous data that while CD98 is not required for homeostatic proliferation and maintenance of B and T cells, it is required for their rapid antigen-driven clonal expansion (Cantor et al., 2009; Cantor et al., 2011). Thus, the fact that inhibition of CD98 did not impact the established hematopoietic compartment suggests that the therapeutic use of CD98 antibody in context of leukemia should be further explored.
The inability of standard chemotherapies to trigger a durable remission in AML has led to concerted efforts to identify regulatory events that allow continued propagation of disease, and may serve as effective targets for therapy. These efforts have predominantly centered around intrinsic signals such as β-catenin (Wang et al., 2010), PI3K (Doepfner et al., 2007; Xu et al., 2003), NFκB (Guzman et al., 2001), MAPK (Milella et al., 2001) and Msi2 (Kwon et al., 2015; Park et al., 2015). However, the specific molecules that integrate signal reception from the microenvironment remain relatively unexplored. In this context, our data identifies CD98 as a key molecular player in mediating the influence of the bone marrow niche on AML initiation and progression. The finding that CD98 works in part by enabling MLL-AF9 leukemia cells to establish long-term interactions with blood vessels in vivo reveals a remarkable parallel with the fact that endothelial cells have been implicated in the survival and proliferation of normal stem cells (Ding et al., 2012; Kunisaki et al., 2013). This also complements prior work on interactions of MLL-AF9 transduced progenitors with osteoblasts (Lane et al., 2011), and provides a distinct view of the role, and molecular basis, of the interactions of leukemia stem cells with endothelial cells. Consistent with adhesion being a key mechanism of action for sustaining leukemia cells, we find that active blockade of leukemia stem cell adhesion to endothelial cells can deplete stem cells and thus impair cancer progression. As CD98 heavy chain can bind to the cytoplasmic tails of both integrins β1 and β3, its loss likely results in decreased VCAM-1/ VLA-4 (integrin α4β1) signaling. The identification of CD98 as a key molecular driver of adhesive events important in leukemogenesis contributes to an emerging view that adhesive signals, such as those mediated by CXCR4 (Sison et al., 2013; Tavor et al., 2004), CD44 (Jin et al., 2006), Tspan3 (Kwon et al., 2015) and FAKs (Despeaux et al., 2012; Tyner et al., 2008), play a crucial role in controlling distinct aspects of cancer (Desgrosellier and Cheresh, 2010; Konopleva and Jordan, 2011; Seguin et al., 2015). This work also indicates that other proteins that bind and amplify integrin signals, such as Talin and Kindlin (Calderwood et al., 2013), should be studied as potential regulators of myeloid leukemia initiation and maintenance.
Our study with CD98, which binds to integrin β1, provides an important genetic complement to prior work showing that inhibition of VLA-4 mediated adhesive interactions with fibronectin can prevent chemoresistance in AML (Jacamo et al., 2014; Matsunaga et al., 2003). The fact that integrin β3, which is also upstream of CD98, can reduce MLL-driven leukemia, (Miller et al., 2013), suggests that activity of several adhesive molecules are integrated through CD98 to collectively mediate and control initiation and progression of AML in vivo. Although the integrin-binding function is critical for the observed effect of CD98 on AML growth, we should note that more complete rescue of CD98−/− defects is only observed following restoration of the amino acid transport as well as integrin signaling functions. Thus, it is likely that the combined inhibition of integrin-binding and amino acid transport activity of CD98 are necessary to fully resolve human disease progression in vivo, and thus targeting CD98 may be more powerful than solely targeting upstream adhesive signals.
Because CD98 is particularly well-suited for antibody-mediated therapy, an anti-CD98 antibody (IGN523) has recently been developed (Hayes et al., 2014). Both genetic deletion and mAb mediated blockade of CD98 suggest that CD98 inhibition impairs adhesion and survival of leukemia cells. We should note that previous work (Hayes et al., 2014) indicates that IGN523, which reduces CD98 expression, can also increase lysosomal membrane permeability, reduce amino acid transport and trigger antibody-dependent cellular cytotoxicity (ADCC). Thus, anti-CD98 mAb could act through multiple mechanisms that include defects in cell adhesion to the microenvironment, increased apoptosis and ADCC. While the CD98 mAb has been shown to delay growth of AML cell lines and patient-derived lung cancer in xenografts, whether CD98 is needed for growth of primary patient samples at physiological sites such as the bone marrow and peripheral blood had remained unknown. The CD98 conditional knockout mouse models reported here provide important genetic evidence for a role of CD98 in AML; further, the finding that blocking CD98 with either shRNAs or humanized antibody strongly affects the growth of human leukemia both in vitro and at physiologic sites in patient derived xenografts strongly supports the use of CD98-directed therapy in myeloid leukemia. Since the models we used were predominantly driven by MLL rearrangements - translocations found in more than two-thirds of all infant leukemias, and associated with a particularly poor prognosis (Krivtsov and Armstrong, 2007) - our data also suggest that CD98 inhibition should be considered for targeting both adult and pediatric leukemia.
EXPERIMENTAL PROCEDURES
See supplemental information for more extensive methods.
Generation of experimental mice
The conditional knockout mice (Cd98hcfl/fl, also Slc3a2-loxP)(Feral et al., 2007) were mated with Rosa26-CreERT2 mice (Strain: B6;129-Gt(ROSA)26Sortm1(cre/Esr1)Tyj) mice to generate the Cd98hcfl/fl;Rosa26-CreER mice. B6-CD45.1 (Strain: B6.SJL-PtprcaPepcb/BoyJ) mice were used as transplant recipients. All mice were 6–16 weeks of age. Mice were bred and maintained in the animal care facilities at the University of California San Diego. All animal experiments were performed according to protocols approved by the University of California San Diego Institutional Animal Care and Use Committee.
In vivo transplantation assays
For bone marrow transplants, 500 LT-HSCs (KLSCD150+CD48− or KLSFlk2−CD34−) isolated from bone marrow of mice expressing CD45.2 were transplanted into lethally irradiated (9.5 Gy) congenic recipient mice (expressing CD45.1) along with 2 × 105 Sca1-depleted bone marrow rescue cells. Peripheral blood of recipient mice was collected every 4 weeks for 4–6 months after transplant. Where indicated, adult mice were administered tamoxifen (Sigma) in corn oil (20 mg/ml) daily by i.p. injections (150 µg per gram of body weight), for five consecutive days.
Generation and analysis of leukemia models
Bone marrow KLS cells were sorted from Cd98hcfl/fl;Rosa26-CreER+/+ mice and cultured overnight in RPMI media (Life Technologies) supplemented with 20% fetal bovine serum, 50µM 2-mercaptoethanol, stem cell factor (SCF, 100 ng/ml, R&D Systems), IL3 and IL6 (10 ng/ml, R&D Systems). Cells were retrovirally infected with MSCV-MLL-AF9-IRES-tNGFR and MSCV-NRASG12V-IRES-YFP. Cells were harvested 48 hours after infection and retro-orbitally transplanted into cohorts of sublethally irradiated (6Gy) B6-CD45.1 mice. For secondary transplants, cells from primary transplanted mice were sorted for cKit+ MLL-AF9-IRES-tNGFR+/MSCV-NRAS-IRES-YFP+ cells and 2,000 to 4,000 cells transplanted per mouse. Where indicated, adult mice were administered tamoxifen (Sigma) in corn oil (20 mg/ml) daily by i.p. injections (150 µg per gram of body weight), for five consecutive days. Recipients were maintained on antibiotic water (sulfamethoxazole and trimethoprim) and evaluated daily for signs of morbidity, weight loss, failure to groom, and splenomegaly. Pre-morbid animals were sacrificed and relevant tissues harvested and analyzed by flow cytometry. For homing assays, lethally irradiated recipients were transplanted with 106 CD98+/+ or CD98−/− MLL-AF9-IRES-tNGFR+/MSCV-NRAS-IRES-YFP+ cells, and bone marrow analyzed 16 hr post-transplant. Apoptosis assays were performed by staining cells with AnnexinV and 7AAD (eBioscience). Analysis of in vivo BrdU incorporation was performed using the APC BrdU Flow Kit (BD Pharmingen) after a single i.p. injection of BrdU (2 mg at 10 mg/ml).
Human leukemia samples and cell lines
18 patient samples were obtained from Singapore General Hospital, the Duke Adult Bone Marrow Transplant Clinic, the Fred Hutchinson Cancer Research Center and UC San Diego Moores Cancer Center from institutional review board (IRB)-approved protocols with written informed consent in accordance with the Declaration of Helsinki. Leukemia cells were cultured in Iscoves modified Dulbecco medium (IMDM) with 10% FBS, 100 IU/ml penicillin and 100 µg/ml streptomycin, 55 µM 2-mercaptoethanol and supplemented with 100 ng/ml of SCF and TPO (R&D Systems). The human AML cell lines THP1 and M-V-411 (ATCC) were maintained in RPMI with 10% FBS, 100 IU/ml penicillin and 100 µg/ml streptomycin. For colony forming assays with shRNAs, primary leukemia cells were transduced with lentiviral shRNA and RFP+ cells were sorted 48 hr post-infection and plated in complete methylcellulose medium (H4434 StemCell Technologies). Colony numbers were counted 10–14 days after plating. All experiments were conducted with two independent hairpin shRNAs targeting SLC3A2 that more effectively knock down expression in AML cells. For in vivo experiments, NSG mice (Strain: NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) were sub-lethally irradiated (2.5 Gy) and retro-orbitally transplanted with primary human AML cells.
In vitro adhesion assays
For in vitro adhesion assays, 5000 cKit+ AML cells from CD98+/+ or CD98−/− leukemia were plated on confluent HUVEC cells (Life Technologies) activated with 10 ng/ml human TNFα (eBioscience) in RPMI with 20% fetal bovine serum (FBS), 100 IU/ml penicillin and 100 µg/ml streptomycin and 55 µM 2-mercaptoethanol in a 96-well plate, and incubated at 37°C for 2 hr. To determine the effect of loss of adhesion on cKit expression and apoptosis, 30 µg/ml anti-VCAM1 blocking antibody (R&D Systems) or control IgG (Sigma) were added along with the leukemia cells where indicated. The wells were washed 6–7 times to remove unattached cells and images taken on an inverted microscope (Leica). Cells were trypsinized and analyzed on FACSAria III (Becton Dickinson) to obtain an absolute count of adherent leukemia cells/well.
In vivo imaging
Imaging was done as described earlier (Fox et al., 2016; Koechlein et al., 2016; Kwon et al., 2015; Zimdahl et al., 2014). Briefly, Actin-dsRed NOD SCID mice (Strain: NOD.Cg-Prkdcscid Tg(CAG-DsRed* MST)1Nagy/KupwJ) were transplanted with CD98+/+ (denoted as +/+) and CD98−/− (denoted as −/−) MLL leukemia cells and imaged 7–10 days post-transplant. VE-Cadherin conjugated to AlexaFluor 647 (eBiosciences) was administered at a concentration of 10ug diluted in 100ul, 15 minutes prior to imaging. Images were acquired by the Leica LAS AF 2.7.3 software with TCS SP5 upright DM600 CFS Leica confocal system using an HCX APO L 20×/1.00 W Leica Plan Apochromat objective. Images were continuously captured in 1024 × 1024 format (approximately 7 seconds per scan) for up to 1 hour. Images were analyzed using the Leica AF 2.7.3 software.
Supplementary Material
SIGNIFICANCE.
Little is known about how adhesive signals from the microenvironment control leukemia growth. In this study we show that CD98, which controls both adhesion and amino acid transport, plays a critical role in AML. CD98 deletion in a mouse model of AML impaired tumor initiation and propagation and increased survival. In vivo imaging revealed that CD98−/− leukemia cells had fewer stable interactions with the endothelium, and restoring CD98’s adhesive function could rescue CD98−/− defects. Finally, CD98 blocking antibodies effectively impaired the growth of human AML cells in vitro and in xenografts. These findings identify CD98 as a mediator of adhesive signaling in AML, and provide key genetic evidence for the clinical utility of targeting CD98 in pediatric and adult AML.
Highlights.
Development and analysis of conditional CD98−/− model in the hematopoietic system
CD98 loss impairs propagation of established AML in mouse models of disease
Antibody mediated CD98 blockade impairs primary human AML growth
CD98 mediated adhesion to vasculature promotes leukemia stem cell maintenance
In Brief.
Bajaj et al. demonstrate the importance of CD98-mediated adhesion for survival of acute myeloid leukemia (AML) and show that genetic deletion of CD98 in mice or antibody blockade of CD98 in patient-derived xenografts blocks AML growth.
Acknowledgments
We are grateful to Annette Luo for technical support, Marcie Kritzik for help with manuscript preparation, and Sanford Shattil for advice on the project. We would like to thank Scott Armstrong, Scott Lowe and Michael Cleary for providing MLL-AF9, AML1-ETO9a and other plasmids. J.B. received a postdoctoral fellowship from the National Cancer Center; T.K. received a postdoctoral fellowship from the Japanese Society for the Promotion of Science; N.K.L. received support from T32 GM007752 and a National Research Service Award F31 CA206416; J.N.A. received a post-doctoral fellowship from the Arthritis Foundation; J.M.C. is funded in part by a Melanoma Research Alliance Young Investigator Award; and T.R. was supported by a Lymphoma and Leukemia Society Scholar Award. This work was also supported by NIH grants HL078784 and HL117807 awarded to M.H.G. and HL097767, DK099335, DP1 CA174422 and R35 CA197699 awarded to T.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.B. designed and performed experiments on human and murine leukemia progression, provided analysis of mAb impact, imaging, and mechanistic studies, and helped write the paper; T.K. designed and performed experiments on normal hematopoiesis and leukemia progression in mouse models. N.K.L., H.Y.K., J.N.A. and J.M.C. provided experimental data and help. D.R., C.C., V.G.O., E.H.B. and E.D.B. provided primary leukemia patient samples and experimental advice. E.H.vH. provided the CD98 antibody and experimental advice. M.H.G and T.R. conceived of the project, planned and guided the research and wrote the paper.
REFERENCES
- Bissell MJ, Radisky D. Putting tumours in context. Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J, Browne CD, Ruppert R, Feral CC, Fassler R, Rickert RC, Ginsberg MH. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat. Immunol. 2009;10:412–419. doi: 10.1038/ni.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J, Slepak M, Ege N, Chang JT, Ginsberg MH. Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity. J. Immunol. 2011;187:851–860. doi: 10.4049/jimmunol.1100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J. Cell Sci. 2012;125:1373–1382. doi: 10.1242/jcs.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JM, Ginsberg MH, Rose DM. Integrin-associated proteins as potential therapeutic targets. Immunol. Rev. 2008;223:236–251. doi: 10.1111/j.1600-065X.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat. Med. 2009;15:1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despeaux M, Chicanne G, Rouer E, De Toni-Costes F, Bertrand J, Mansat-De Mas V, Vergnolle N, Eaves C, Payrastre B, Girault JA, et al. Focal adhesion kinase splice variants maintain primitive acute myeloid leukemia cells through altered Wnt signaling. Stem Cells. 2012;30:1597–1610. doi: 10.1002/stem.1157. [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia. 2007;21:1921–1930. doi: 10.1038/sj.leu.2404813. [DOI] [PubMed] [Google Scholar]
- Estrach S, Lee SA, Boulter E, Pisano S, Errante A, Tissot FS, Cailleteau L, Pons C, Ginsberg MH, Feral CC. CD98hc (SLC3A2) loss protects against ras-driven tumorigenesis by modulating integrin-mediated mechanotransduction. Cancer Res. 2014;74:6878–6889. doi: 10.1158/0008-5472.CAN-14-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81–85. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- Fenczik CA, Zent R, Dellos M, Calderwood DA, Satriano J, Kelly C, Ginsberg MH. Distinct domains of CD98hc regulate integrins and amino acid transport. J. Biol. Chem. 2001;276:8746–8752. doi: 10.1074/jbc.M011239200. [DOI] [PubMed] [Google Scholar]
- Feral CC, Nishiya N, Fenczik CA, Stuhlmann H, Slepak M, Ginsberg MH. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. U. S. A. 2005;102:355–360. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feral CC, Zijlstra A, Tkachenko E, Prager G, Gardel ML, Slepak M, Ginsberg MH. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J. Cell Biol. 2007;178:701–711. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RG, Lytle NK, Jaquish DV, Park FD, Ito T, Bajaj J, Koechlein CS, Zimdahl B, Yano M, Kopp JL, et al. Image-based detection and targeting of therapy resistance in pancreatic adenocarcinoma. Nature. 2016;534:407–411. doi: 10.1038/nature17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem. Biophys. Res. Commun. 1999;262:720–725. doi: 10.1006/bbrc.1999.1051. [DOI] [PubMed] [Google Scholar]
- Hayes GM, Chinn L, Cantor JM, Cairns B, Levashova Z, Tran H, Velilla T, Duey D, Lippincott J, Zachwieja J, et al. Antitumor activity of an anti-CD98 antibody. Int. J. Cancer. 2014 doi: 10.1002/ijc.29415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Hemler ME, Mann DL, Eisenbarth GS, Shelhamer J, Mostowski HS, Thomas CA, Strominger JL, Fauci AS. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J. Immunol. 1981;126:1409–1414. [PubMed] [Google Scholar]
- Henderson NC, Collis EA, Mackinnon AC, Simpson KJ, Haslett C, Zent R, Ginsberg M, Sethi T. CD98hc (SLC3A2) interaction with beta 1 integrins is required for transformation. J. Biol. Chem. 2004;279:54731–54741. doi: 10.1074/jbc.M408700200. [DOI] [PubMed] [Google Scholar]
- Imai H, Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, Sunaga N, Ishizuka T, Nagamori S, Promchan K, et al. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30:4819–4828. [PubMed] [Google Scholar]
- Jacamo R, Chen Y, Wang Z, Ma W, Zhang M, Spaeth EL, Wang Y, Battula VL, Mak PY, Schallmoser K, et al. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-kappaB mediates chemoresistance. Blood. 2014;123:2691–2702. doi: 10.1182/blood-2013-06-511527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- Koechlein CS, Harris JR, Lee TK, Weeks J, Fox RG, Zimdahl B, Ito T, Blevins A, Jung SH, Chute JP, et al. High-resolution imaging and computational analysis of haematopoietic cell dynamics in vivo. Nat. Commun. 2016;7:12169. doi: 10.1038/ncomms12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J. Clin. Oncol. 2011;29:591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat. Rev. Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HY, Bajaj J, Ito T, Blevins A, Konuma T, Weeks J, Lytle NK, Koechlein CS, Rizzieri D, Chuah C, et al. Tetraspanin 3 Is Required for the Development and Propagation of Acute Myelogenous Leukemia. Cell Stem Cell. 2015;17:152–164. doi: 10.1016/j.stem.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SW, Wang YJ, Lo Celso C, Ragu C, Bullinger L, Sykes SM, Ferraro F, Shterental S, Lin CP, Gilliland DG, et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011;118:2849–2856. doi: 10.1182/blood-2011-03-345165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc. Natl. Acad. Sci. U. S A. 2014;111:E3091–E3100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, Akiyama T, Kuroda H, Kawano Y, Kobune M, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat. Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D, Konopleva M, Zhao S, Estey E, Andreeff M. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J. Clin. Invest. 2001;108:851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PG, Al-Shahrour F, Hartwell KA, Chu LP, Jaras M, Puram RV, Puissant A, Callahan KP, Ashton J, McConkey ME, et al. In Vivo RNAi Screening Identifies a Leukemia-Specific Dependence on Integrin Beta 3 Signaling. Cancer Cell. 2013;24:45–58. doi: 10.1016/j.ccr.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, Fein M, Rasch MG, Tan YX, Qiu J, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HT, Dalmasso G, Torkvist L, Halfvarson J, Yan Y, Laroui H, Shmerling D, Tallone T, D'Amato M, Sitaraman SV, et al. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J. Clin. Invest. 2011;121:1733–1747. doi: 10.1172/JCI44631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Gonen M, Vu L, Minuesa G, Tivnan P, Barlowe TS, Taggart J, Lu Y, Deering RP, Hacohen N, et al. Musashi2 sustains the mixed-lineage leukemia-driven stem cell regulatory program. J. Clin. Invest. 2015;125:1286–1298. doi: 10.1172/JCI78440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosilio C, Nebout M, Imbert V, Griessinger E, Neffati Z, Benadiba J, Hagenbeek T, Spits H, Reverso J, Ambrosetti D, et al. L-type amino-acid transporter 1 (LAT1): a therapeutic target supporting growth and survival of T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia. Leukemia. 2014 doi: 10.1038/leu.2014.338. [DOI] [PubMed] [Google Scholar]
- Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25:234–240. doi: 10.1016/j.tcb.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shennan DB, Thomson J. Inhibition of system L (LAT1/CD98hc) reduces the growth of cultured human breast cancer cells. Oncol. Rep. 2008;20:885–889. [PubMed] [Google Scholar]
- Shi J, Wang E, Zuber J, Rappaport A, Taylor M, Johns C, Lowe SW, Vakoc CR. The Polycomb complex PRC2 supports aberrant self-renewal in a mouse model of MLL-AF9;Nras(G12D) acute myeloid leukemia. Oncogene. 2013;32:930–938. doi: 10.1038/onc.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sison EA, Rau RE, McIntyre E, Li L, Small D, Brown P. MLL-rearranged acute lymphoblastic leukaemia stem cell interactions with bone marrow stroma promote survival and therapeutic resistance that can be overcome with CXCR4 antagonism. Br. J. Haematol. 2013;160:785–797. doi: 10.1111/bjh.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Somervaille TC, Matheny CJ, Spencer GJ, Iwasaki M, Rinn JL, Witten DM, Chang HY, Shurtleff SA, Downing JR, Cleary ML. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell. 2009;4:129–140. doi: 10.1016/j.stem.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerlick RA, Lee KH, Li LJ, Sepp NT, Caughman SW, Lawley TJ. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J. Immunol. 1992;149:698–705. [PubMed] [Google Scholar]
- Tavor S, Petit I, Porozov S, Avigdor A, Dar A, Leider-Trejo L, Shemtov N, Deutsch V, Naparstek E, Nagler A, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004;64:2817–2824. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- Tyner JW, Walters DK, Willis SG, Luttropp M, Oost J, Loriaux M, Erickson H, Corbin AS, O'Hare T, Heinrich MC, et al. RNAi screening of the tyrosine kinome identifies therapeutic targets in acute myeloid leukemia. Blood. 2008;111:2238–2245. doi: 10.1182/blood-2007-06-097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- Yagita H, Masuko T, Hashimoto Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation-associated cell surface antigen system in rats and humans. Cancer Res. 1986;46:1478–1484. [PubMed] [Google Scholar]
- Zent R, Fenczik CA, Calderwood DA, Liu S, Dellos M, Ginsberg MH. Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. J. Biol. Chem. 2000;275:5059–5064. doi: 10.1074/jbc.275.7.5059. [DOI] [PubMed] [Google Scholar]
- Zimdahl B, Ito T, Blevins A, Bajaj J, Konuma T, Weeks J, Koechlein CS, Kwon HY, Arami O, Rizzieri D, et al. Lis1 regulates asymmetric division in hematopoietic stem cells and in leukemia. Nat. Genet. 2014;46:245–252. doi: 10.1038/ng.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.