Abstract
Aim
Subjects with increasing age are more sensitive to the effects of the anti‐muscarinic agent scopolamine, which is used (among other indications) to induce temporary cognitive dysfunction in early phase drug studies with cognition enhancing compounds. The enhanced sensitivity has always been attributed to incipient cholinergic neuronal dysfunction, as a part of the normal aging process. The aim of the study was to correlate age‐dependent pharmacodynamic neuro‐physiologic effects of scopolamine after correcting for differences in individual exposure.
Methods
We applied a pharmacokinetic and pharmacodynamic modelling approach to describe individual exposure and neurocognitive effects of intravenous scopolamine administration in healthy subjects.
Results
A two‐compartment linear kinetics model best described the plasma concentrations of scopolamine. The estimated scopolamine population mean apparent central and peripheral volume of distribution was 2.66 ± 1.050 l and 62.10 ± 10.100 l, respectively and the clearance was 1.09 ± 0.096 l min−1. Age was not related to a decrease of performance in the tests following scopolamine administration in older subjects. Only the saccadic peak velocity showed a positive correlation between age and sensitivity to scopolamine. Age was, however, correlated at baseline with an estimated slower reaction time while performing the cognitive tests and to higher global δ and frontal θ frequency bands measured with the surface EEG.
Conclusions
Most of the differences in response to scopolamine administration between young and older subjects could be explained by pharmacokinetic differences (lower clearance) and not to an enhanced sensitivity when corrected for exposure levels.
Keywords: ageing, cholinergic dysfunction, healthy subjects, NONMEM, pharmacokinetic–pharmacodynamic modelling, scopolamine
What is Already Known About this Subject
It has been thought that healthy older adults are more sensitive to scopolamine effects when compared to younger subjects.
This difference has been hypothesized as a consequence of cholinergic dysfunction as a part of the cognitive decline that takes place during the normal ageing process.
What this Study Adds
A higher sensitivity in cognitive functions to scopolamine in healthy older subjects as a normal ageing process is unlikely.
The hypothesized increased sensitivity to scopolamine among older subjects is, however, largely due to differences in pharmacokinetics, with older subjects having a lower clearance.
Introduction
The involvement of the cholinergic system in the aetiology of geriatric cognitive dysfunction was already suggested in the early 1980s 1. At that time, evidence from different sources using mostly semi‐parametric statistical methods, suggested that cholinergic neuronal dysfunction played a major role in the brains of healthy older subjects, subjects with mild cognitive impairment (MCI) and to a greater extent in those of patients with dementia 2. Research in older subjects shows that cognitive abilities such as encoding new memories of episodes or facts, working memory (the simultaneous short‐term maintenance and manipulation of information involving executive processes) and processing speed, seem to be the most affected by ageing 3. Recently, the age‐related decrease in specific areas of the brain in healthy subjects was quantified prospectively with MRI imaging. Acetylcholine‐rich regions in the brain including the hippocampus, the entorhinal cortex, the inferior temporal cortex and the prefrontal white matter, decreased with age. The hippocampus was the only structure that showed a non‐linear increased rate of shrinkage with increasing age, providing evidence of an age‐related decline in hippocampal volume in subjects with increasing age and indirectly suggesting a decline in hippocampal neurons, an area rich in cholinergic neurons 4.
The cholinergic hypothesis was further strengthened by evidence that the severity of cognitive deficits appeared to be related to the extent of cholinergic impairment 5, and to the observation that anti‐cholinergic compounds induce cognitive dysfunction 6, 7, 8. The most commonly used cholinergic challenge utilizes scopolamine as a selective competitive muscarinic antagonist. Scopolamine has a high affinity for all five muscarinic receptor subtypes (M1–M5) and a negligible affinity for histaminergic and dopaminergic receptors 9. Intravenous administration of scopolamine leads to a reduction in attention, arousal, verbal and non‐verbal working memory and episodic memory. The reduction in cognitive functioning was observed in both healthy young and healthy older subjects, but appeared to be more severe in healthy older subjects. A significant difference between young adults compared to older subjects was observed in several cognitive tests evaluating short‐term verbal and numeric working memory 10, 11, 12, attention 10, acquisition 10, visuo‐spatial praxis 13 and episodic memory 12. Additionally, some authors have suggested that scopolamine use in clinical practice in older patients might induce a higher risk of cognitive impairment when compared to younger subjects 13, 14. It is hypothesized that this difference between young adults and older subjects receiving scopolamine is due to incipient cholinergic neuronal dysfunction in the healthy older subjects and hence an enhanced sensitivity to anti‐cholinergic drug effects 15, 16. Consequently, a lower scopolamine dose in the older subjects will result in similar effects compared to younger subjects. However, none of these studies measured age‐related difference in plasma scopolamine concentrations.
In the current paper, a nonlinear mixed effects (NLME) approach was used to quantitatively correlate the pharmacodynamic neurophysiologic effects of scopolamine as an indirect measure of muscarinic brain activity after correcting for differences in exposure (pharmacokinetics). This allows the identification of pharmacokinetic (PK) and pharmacodynamic (PD) differences at population and individual level, and their relationships with covariates like age. NLME methods are a useful tool when differences in exposure explain a different measurable effect, while biological differences, such as inter‐subject and intra‐subject random variability, are also taken into consideration and may be explained by covariates (e.g. body weight, height, BMI, age, etc.).
Methods
Study population
A total of 135 healthy subjects participated in four separate clinical studies performed at the Centre of Human Drug Research (Leiden, the Netherlands). Subjects' demographics can be found in Table 1. A medical ethics committee approved the four different study protocols. After giving written informed consent, all subjects were medically screened prior to study participation. Exclusion criteria included the use of agents or drugs known to influence CNS performance (including smoking and drug or alcohol abuse), consuming more than five cups of caffeine‐containing drinks per day and evidence of relevant medical abnormalities. Older subjects were required to score 28 points (or higher) on the mini‐mental state examination 17 (MMSE) at screening.
Table 1.
Subject demographics
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | All | |
|---|---|---|---|---|---|
| Subjects (n) | 43 | 43 | 13 | 36* | 135 |
| Age (years) | 29.3 (18–55) | 27.7 (18–55) | 25.4 (19–36) | 69.0 (65–78) | 39.0 (18–78) |
| Weight (kg) | 78.6 ± 7.54 | 78.6 ± 7.74 | 81.1 ± 9.76 | 77.8 ± 11.82 | 78.6 ± 9.08 |
| BMI ( kg m −2 ) | 23.6 ± 2.30 | 23.3 ± 2.69 | 24.6 ± 2.82 | 25.9 ± 2.51 | 24.2 ± 2.73 |
| Scopolamine dose (mg) | 0.5 | 0.5 | 0.5 | 0.3 | NA |
Mean ± standard deviation. Age is presented as mean (minimum – maximum).
13 female subjects. NA: not applicable.
Study design
Data for this analysis were obtained from all four studies. In the first two trials the effects of two different glycinergic compounds were studied using a scopolamine challenge model 18, 19, the third one was a study in which the effects of scopolamine on cognitive functions were compared with those of mecamylamine (a nicotinic acetylcholine receptor antagonist). In the fourth study, the effects of an α7 nicotinic acetylcholine receptor agonist were examined in older subjects using a scopolamine challenge without a placebo control. Only the data from the occasions where scopolamine, scopolamine in combination with placebo or only placebo (comparison placebo group), was administered were included for analysis.
An intravenous dose of 0.5 mg of scopolamine hydrobromide was administered to subjects under 65 years of age and an intravenous dose of 0.3 mg to the older subjects group (≥ 65 years), according to previous recommendations 13, 20, 21. In all four studies, scopolamine was administered as an intravenous infusion over 15 min. Plasma scopolamine concentrations were determined using a validated, selective and sensitive liquid LC–MS/MS method with a lower limit of quantification of 10 pg ml−1. Across the study sample analytical runs, the accuracy (expressed as the percentage of bias) of scopolamine QC samples ranged from 2.0 to 3.3% and assay precision (expressed as the percentage of CV) ranged from 2.5 to 4.0%.
A battery of CNS tests (neurophysiological, psychomotor and cognitive tests, subjective drug effects) was performed to quantify the pharmacodynamics effects of scopolamine, which provided information on the effects of scopolamine on different functional CNS domains. All pharmacodynamic tests were performed in a quiet room with ambient illumination with only one subject in the same room with a research assistant per session. All subjects were thoroughly trained and familiarized with the psychometric tests within 14 days preceding study start to minimize learning effects during the study. Each baseline or pre‐dose assessment was performed twice at the beginning of each occasion. The lack of placebo arm in the older subjects group was handled by using the PD baseline measurements to estimate the scopolamine effects. A combination of tests evaluating both neurophysiological and cognitive variables on which an effect was previously reported 7 were analysed.
N‐back test
Subjects were asked to remember and correlate a sequence of letters presented in a random order, thereby allowing evaluation of (short‐term) working memory. This test evaluates working memory 22. Performance was expressed as the percentage of correct answers on the 0‐, 1‐ and 2‐back paradigms, and as reaction time on the 0‐back paradigm. N‐back ratio data was analysed as count data and therefore the data was logit‐transformed.
Adaptive tracker test
The test evaluates attention and executive skills as visuo‐motor coordination 23, 24. Subjects were asked to use a joystick to keep a randomly moving target on the screen inside a circle. The percentage accuracy was recorded.
Saccadic eye movements test
This is one of the most sensitive tests for sedation 24. Subjects were requested to look at a randomly moving target on the computer screen. With the help of two electrodes, the horizontal peak velocity eye movements and inaccuracy were measured.
Electro‐encephalogram
Four cranial superficial electrodes (Fz, Cz, Pz, Oz) were placed following the 10–20 system and subjects were asked to close their eyes. Fast Fourier transformed absolute power (μV) was calculated from the raw measurements in the α [7.5–11.5 Hz], β [11.5–30 Hz], δ [0.5–3.5 Hz] and θ [3.5–7.5 Hz] frequency ranges in two bipolar leads: Fz‐Cz and Pz‐Oz.
Software
Pharmacokinetics and pharmacodynamics modelling analyses were performed using nonlinear mixed‐effect (NLME) modelling in NONMEM v7.2 and v7.3 25 (ICON Development Solution, Hanover, MD). The database and all graphs were created using R v2.13.1 26 (R Foundation for Statistical Computing, Vienna, Austria).
Model development and evaluation
Plasma scopolamine concentration‐time dependent data was analysed using a consecutive NLME modelling approach; once the best pharmacokinetic model was obtained, the individual PK parameter estimates were fixed to develop the pharmacodynamic models. The first order conditional estimation method with interaction (FOCE‐I) was used for all data except for the N‐back ratio data where a Laplacian method for count data was also compared to FOCE‐I. Several compartment models were explored for the pharmacokinetic model before inter‐individual variability (IIV) was tested for PK parameters, as were additive, proportional or combined error models. Body weight, height and age were tested as potential covariates for parameters on which IIV could be identified.
For the PD endpoints, indirect E MAX model structures were preferred to assess the age‐related sensitivity to scopolamine, in terms of EC 50 and E MAX. Delay compartments were taken into consideration for the pharmacodynamics models. Once the structural model was defined, IIV was tested in each parameter estimate, as were additive, proportional or combined error models. Correlations between post‐hoc Bayesian estimates and between post‐hoc Bayesian estimates and potential covariates were explored using Pearson's correlation coefficients (r 2). A coefficient of determination ≥ 0.4 was considered relevant and taken forward in testing of the omega block structures and covariate analysis (body weight, age and height). Age was tested as a covariate in all models regardless of correlation. Competing models were compared based on their goodness of fit (GOF) plots, improvement in OFV, plausibility of parameter estimates, residual error, parameter precision (in terms of residual standard error, RSE), shrinkage and parameter distribution. A decrease in the OFV of 3.84 units (P < 0.05) was considered statistically significant. GOF plots included population and individual predictions vs. observations, time‐ and observations‐dependent conditional weighted residuals (with interaction) and IIV distribution graphs. Visual predictive checks (VPCs) were obtained by simulating 1000 subjects, using the population parameter estimates and the full variance–covariance matrix. Covariates were sampled from the observed population distribution (assuming a normal distribution, with resampling).
Results
Model development – Plasma scopolamine concentrations
Pharmacokinetic model graphical representation and parameter estimates can be found in Figure 1 and Table 2, respectively, and the model equations and GOF plots can be found in the Supplemental Materials. A two‐compartment linear model structure proved superior to a one‐compartment linear plasma scopolamine pharmacokinetic model (∆OFV = −1474 points). Inter‐individual variability could be identified on the central (V c) and peripheral (V p) volumes of distribution and clearance. As displayed in Equation A.1 in the Supplemental Materials section, age and body weight were identified as covariates on clearance (∆OFV = −29 and −38 points, respectively). Body weight was also identified as a covariate on peripheral volume of distribution (∆OFV = −13 points; Equation A.2).
Figure 1.
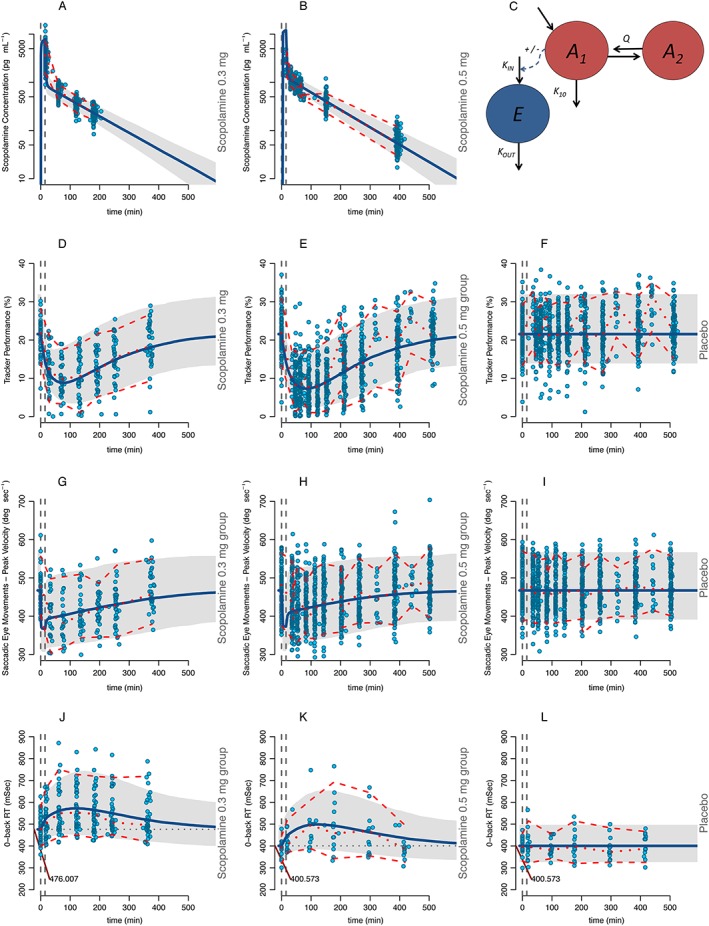
Scopolamine pharmacokinetics and pharmacodynamic effects. The column on the left represents the scopolamine 0.3 mg group, the middle column the scopolamine 0.5 mg group and the column on the right the placebo group. Figures 1A and 1B represent the plasma scopolamine concentrations. Figure 1C is the schematic representation of the model where the red circles represent the central (A1) and peripheral (A2) PK compartments and the blue circle (E) the effect (PD) compartment. Figures 1D, 1E and 1F display the adaptive tracker test, Figures 1G, 1H and 1I the peak velocity of the saccadic eye movements test, Figures 1J, 1K and 1L the Reaction Time of the 0‐Back test. The continuous blue line represents the model population predicted values per group and the grey area represents the 95% prediction interval. Circles represent the observations. The middle red discontinuous line represents the median of the observations with the upper and lower 95% confidence intervals. Dotted, vertical grey lines represent the start and stop times of the scopolamine infusion. The differences in baseline‐estimated values are indicated by a red line and the population value (when applicable). Simulations were performed with 1000 subjects
Table 2.
Population estimates for pharmacokinetic and pharmacodynamic models for scopolamine
| CL [α] ( l min −1 ) | IIV (shrinkage) | V c (l) | IIV (shrinkage) | V p [β] (l) | IIV (shrinkage) | Q ( min −1 ) | CAC | CWC | CWV | Error (σ 2 ) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Scopolamine pharmacokinetics | 1.09 ± 0.096* , † | 10.3% (9.7) | 2.66 ± 1.050 | 74.1% (49.3) | 62.10 ± 10.100† | 9.5% (25.6) | 1.01 ± 0.247 | −0.12 ± 0.019 | 0.56 ± 0.097 | 0.38 ± 0.120 | 0.045 (prop) |
| EC 50 [ε] (pg ml−1) | IIV (shrinkage) | E MAX (%) | IIV (shrinkage) | K IN [λ] | IIV (shrinkage) | K OUT | CAB or CAE | γ‡ | OC | Error (σ2) | |
| 0‐back RT (msec) | 1300 ± 363 | 99.8% (33.2) | 0.837 ± 0.118 | NA | 3.42 ± 0.361* | 10.5% (8.13) | 0.00974 ± 0.00099 | 229 ± 40.9 | NA | NA | 0.00999 (prop) |
| Saccadic inaccuracy (%) | 55.1 ± 29.8 | 13.7% (36.9)¶ | 0.388 ± 0.079 | 49.9% (36.2)¶ | 22.8 ± 0.731 | 13.2% (21.86) | 3.33 ± 0.0398 | NA | NA | −0.06 ± 0.157 | 0.0493 (prop) |
| Saccadic peak velocity (deg sec−1) | 2530 ± 213* | 277.0% (20.9) | 0.232 ± 0.00113 | NA | 1280 ± 21 | 9.9% (3.1) | 2.73 ± 0.0468 | 34.0 ± 1.39 | NA | NA | 768.0 (prop) |
| Adaptive tracker (%) | 386 ± 22.6 | 85.5% (10.50) | 1 | NA | 0.636 ± 0.0413 | 22.4% (4.1) | 0.0294 ± 0.0018 | NA | 1.10 ± 0.063 | NA | 8.64 (add) |
| EEG alpha Fz‐Cz (μV) | 2.55 ± 1.06 | 1530.0% (48.4) | 0.232 ± 0.0258 | 95.4% (14.9) | 0.206 ± 0.0344 | 46.6% (1.1) | 0.0699 ± 0.0113 | NA | NA | NA | 0.0256 (prop) |
| EEG alpha Pz‐Oz (μV) | 14.7 ± 0.168 | 1309.3% (27.9) | 0.443 ± 0.00389 | 37.6% (18.0) | 3.05 ± 0.135 | 57.9% (2.1) | 0.553 ± 0.0055 | NA | NA | NA | 0.0429 (prop) |
| EEG delta Fz‐Cz (μV) | 469 ± 70.8 | 154.5% (35.5)¶ | 0.419 ± 0.0515 | 325.4% (19.2)¶ | 0.0354 ± 0.00369* | 28.9% (2.1)¶ | 0.0166 ± 0.00161 | 161 ± 30 | NA | 1.410 ± 0.722 (EC 50 vs. E MAX); −0.153 ± 0.0494 (E MAX vs. k in) | 0.0338 (prop) |
| EEG delta Pz‐Oz (μV) | 1230 ± 368 | 1166.2% (29.6) | 0.537 ± 0.0601 | 55.8% (48.0) | 0.726 ± 0.186* | 30.3% (1.7) | 0.348 ± 0.084 | 210 ± 63.7 | NA | NA | 0.0304 (prop) |
| EEG theta Fz‐Cz (μV) | 4110 ± 1410 | 2162.7% (31.1) | 0.594 ± 0.106 | NA | 0.863 ± 0.0422* | 28.4% (1.8) | 0.594 ± 0.106 | 142 ± 24.7 | NA | NA | 0.0297 (prop) |
| EEG theta Pz‐Oz (μV) | 8240 ± 6270 | 1778.6% (27.9) | 1.15 ± 0.179 | NA | 13 ± 8.79 | 33.4% (0.5) | 6.08 ± 4.23 | NA | NA | NA | 0.0379 (prop) |
| EC 50 (pg ml−1) | K IN | IIV (shrinkage) | K OUT | E MAX (%) | IIV (shrinkage) | E 0 | IIV (shrinkage) | NA | OC | Error (σ2) | |
| 0‐back ratio (% correct answers) ** | 2140 ± 1720 | 0.0354 ± 0.00244§ | NA | 0.036 ± 0.0061 | 1 | 631.3% (42.1)¶ | 3.77 ± 0.274 | 37.8% (20.1)¶ | NA | 0.40 ± 0.141 | 0.0039 (add) |
| 1‐back ratio (% correct answers) ** | 0.624 ± 0.0053 | 1.10 ± 0.0175 | 578.5% (47.3) | 221 ± 4.35 | 0.961 ± 0.00511 | 121.6% (30.0) | 3.33 ± 0.0293 | NA | NA | NA | 0.0059 (add) |
| 2‐back ratio (% correct answers) ** | 212 ± 72.4 | 0.54 ± 0.103 | NA | 0.317 ± 0.0704 | 1.09 ± 0.248 | 109.8% (31.9)¶ | 2.84 ± 0.164 | 35.9% (21.5)¶ | NA | 0.23 ± 0.107 | 0.0085 (add) |
Parameters are reported as population estimate ± standard error. IIV: Inter‐individual variability expressed as coefficient of variation and shrinkage in parentheses. Baseline is calculated as the ratio of K in and K out.
Age used as a covariate (ε and λ in Equation B.1 of the Supplemental Materials section).
Weight used as covariate.
Exponent in EMAX (γ).
Four buffer compartments.
Omega block structure.
Parameters reported as natural log odds. Prop: proportional error. CAC: coefficient relating age and clearance. CWC: coefficient relating age and weight. CWV: coefficient relating apparent peripheral volume of distribution and weight. CAB: coefficient relating age and K IN. CAE: coefficient relating age and EC 50. OC: correlation between Omegas. E 0: baseline. Add: additive error. NA: not applicable. Please refer to the Supplemental Materials section for the model equations and the parameter‐covariate relationships.
Model development – Scopolamine effects
The scopolamine effect on the β frequency in the EEG (in both Fz‐Cz and Pz‐Oz leads) was negligible and therefore the parameter was excluded from the analysis. An indirect E MAX model accurately described scopolamine's effect on the other pharmacodynamics tests in the CNS test battery.
Reaction time in the 0‐back paradigm of the N‐back test
Older subjects had a slower (prolonged) reaction time at baseline when compared to young subjects while performing the 0‐back paradigm of the N‐back test (average per group 402 ms for young vs. 476 ms for older subjects). Following scopolamine administration, the reaction time of the 0‐Back paradigm increased significantly from baseline. Adding IIV to E MAX provided a non‐significant decrease in the OFV (3 points) and therefore was abandoned. Age was identified as covariate for the baseline (k in) estimated value (Figure 2), which provided a significant decrease in the OFV (24 points) and a better fit.
Figure 2.
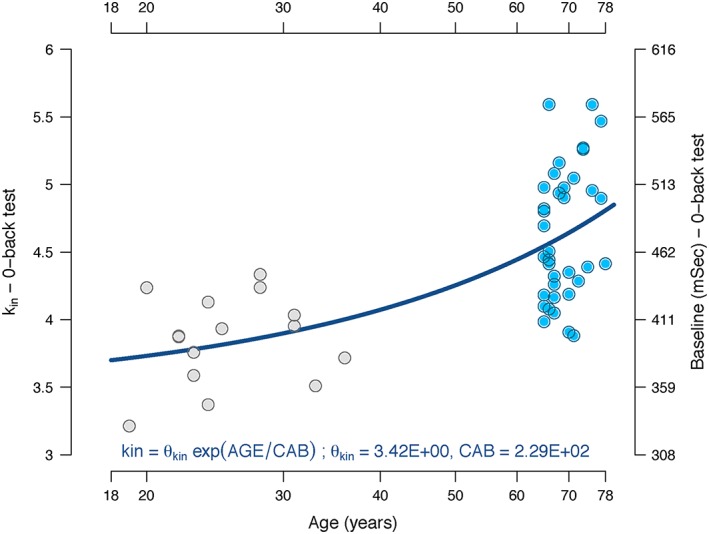
Relationship between k IN, baseline and age in the reaction time of the 0‐back test. The estimated k IN value is represented by the blue (younger adults) and grey (older adults) circles plotted against age. The right column represents the baseline value in milliseconds. The continuous line represents the function with the population values for k IN
Saccadic eye movements (SEM) test – inaccuracy
Scopolamine increased the saccadic inaccuracy in the SEM test. IIV was identified for k in (baseline), EC 50 and E MAX. An omega block structure between EC 50 and E MAX was tested. This did not reduce the OFV but provided a reasonable shrinkage reduction from 90.3 to 36.9% and improved the GOF of the model, and was therefore kept in the model.
Saccadic eye movements (SEM) test – saccadic peak velocity
Scopolamine decreased the saccadic peak velocity in the SEM test. IIV was identified for baseline (k in) and EC 50. IIV on E MAX did not significantly improve the model fit. Age was identified as covariate for EC 50, which decreased the OFV (25 points), improved the GOF, and the IIV on the parameter decreased (CV = 3.5 vs. 2.8). Figure 3 provides a visual representation of the correlation between age and estimated EC 50 values. This was the only model from the battery of tests where age was correlated with the estimated EC 50 (Equation B.1), indicating a higher sensitivity to scopolamine with increasing age.
Figure 3.
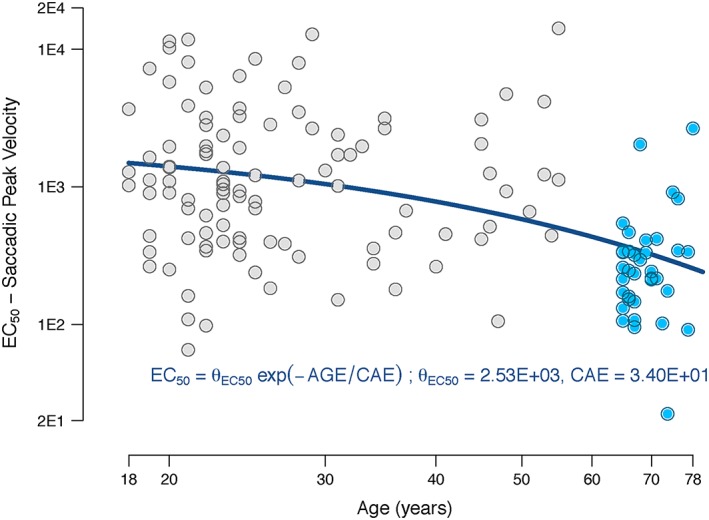
Relationship between EC 50 and age in the saccadic eye movements peak velocity. The estimated EC 50 value is represented by the blue (younger adults) and grey (older adults) circles plotted against age. The continuous line represents the function with the population values for EC 50
Adaptive tracker test (percentage of accuracy)
Shortly after scopolamine was administered, an abrupt decrease in the performance in the adaptive tracker test was observed. In previous models where E MAX was estimated, the result was consistently 1 (100% decrease in performance). Fixing E MAX at 1 resulted in a reduction of the parameter uncertainty, improved the model's stability and resulted in no change in the OFV or GOF. Therefore E MAX was fixed at 1. The addition of an exponent in the E MAX function (γ = 1.1 ± 0.063), even though this value is near to 1 and has a low variability, improved the model fit and decreased the OFV by approximately 7 points and therefore was accepted. IIV was identified for k in and EC 50.
Electro‐encephalogram
Scopolamine induced a decrease in the absolute power in the α frequency band in both Fz‐Cz and Pz‐Oz leads. On the other hand, scopolamine increased the absolute power in both δ and θ frequency bands (with a greater effect on the δ frequency band) in the EEG of the analysed bipolar leads (Fz‐Cz and Pz‐Oz). Addition of E MAX IIV resulted in a significant decrease of the OFV of 196 points in the α Fz‐Cz EEG and 22 points in the α Pz‐Oz models and was therefore accepted. Addition of age as covariate in any of the parameters of the α models (both Fz‐Cz and Pz‐Oz) resulted in an increase in OFV and a worse fit and therefore was abandoned. Addition of E MAX IIV resulted in a significant decrease in the OFV of 29 points in the δ Fz‐Cz EEG and 38 points in the δ Pz‐Oz models and therefore was accepted. Age could be identified as covariate for the baseline (k in) estimates in the δ frequency band in Fz‐Cz (Equation B.2; average of the older 1.360 μV and the younger adults group 1.745 μV) and Pz‐Oz (older 1.468 μV vs. younger adults 1.776 μV). Once incorporated, the OFV decreased 68 points in the Fz‐Cz and 12 points in the Pz‐Oz model. In the δ Fz‐Cz model, IIV could be identified for EC 50 and E MAX and K in. An omega block structure between EC 50 and E MAX and between EC 50 and K in was tested. This resulted in a model improvement (∆OFV = −36 and −29 points respectively). In the θ frequency band in the Fz‐Cz lead (but not in the Pz‐Oz lead), IIV tested for E MAX did not significantly improve any of the models. Age was used as covariate for the baseline measurements (older group average 1.535 μV vs. younger adults 2.024 μV).
Percentage of correct answers in the 0‐, 1‐ and 2‐back paradigms of the N‐back test
Subjects scored at baseline (pre‐dose measurements) on average 97.75% for the 0‐back, 96.54% for the 1‐back and 94.48% for the 2‐back test. Following scopolamine administration, the number of correct answers decreased significantly compared to placebo. In all models (0‐, 1‐ and 2‐back) IIV was identified on E MAX, however IIV at EC 50 could not be identified. For the 0‐ and 2‐back models, IIV was identified at baseline (E 0) and an omega block (variance–covariance structure) between E MAX and E 0 was required. In the 1‐back model IIV was identified at k in and E MAX, but could not be identified in E 0. Only for the 0‐back test was the addition of transit compartments to create a delay for the effect required (∆OFV = −80 points). E MAX was fixed to 1 as this reduced the parameter uncertainty and improved the model's stability while providing only a marginal increase in the OFV (a maximum of +10 points). No covariates could be identified in any of the estimated model parameters.
Model evaluation
In general, for the pharmacokinetic, adaptive tracker, EEG (α, θ and δ frequencies of the Fz‐Cz and Pz‐Oz leads) and saccadic eye movement (peak velocity and inaccuracy) tests, the GOF plots indicate that the central and individual trend of the data is well described, and that no bias occurs over time or observations (Supplemental Materials). The RSEs are relatively small, indicating acceptable accuracy of the parameter estimates, with acceptable shrinkage (Table 2). The VPCs indicate that the variability for these parameters is well described as 95% of the data lie within the 95% prediction interval (Figure 1). However, for N‐back ratios, even though the VPC graphs indicate that the observations are contained within the 95% prediction interval and the GOF plots show a good description of the central and individual trends of the data, the estimated variability (appreciated by the 95% prediction interval in the VPC graphs shown in Figure 1) is considerable, probably related to the great inter‐ and intra‐individual variability observed in the individual observations during the test where a substantial IIV is estimated to describe outliers; this was also reflected in the high estimated shrinkage in the E MAX (42.1%) of the 0‐back and the K in (47.3%) of the 1‐back ratio models. Similarly, the shrinkage values for the α frequency EC 50 (48.4%) in the Fz‐Cz lead and δ frequency E MAX (48.0%) in the Pz‐Oz lead were considerable; probably related to the high inter‐individual variability of the test and the modest effect of scopolamine on the EEG. Finally, the central volume of distribution V c in the PK model showed a high shrinkage (49.3%).
Discussion
Our results show that age‐related increase in scopolamine exposure (pharmacokinetics), rather than previously hypothesized ‘higher sensitivity’ of older subjects, explains enhanced age‐related anti‐cholinergic drug effects.
The developed structural model is in agreement with the two‐exponential equation describing bi‐phasic pharmacokinetics (central and peripheral volume of distribution) as reported in previous literature using nonlinear regression (noncompartmental analysis) and NLME methods 7, 27, 28, 29. Also, the relatively large apparent volume of distribution, mainly of the peripheral compartment, is in agreement with the weak basic nonpolar alkaloid high lipophilicity physicochemical properties of scopolamine. Body weight and age were not earlier reported as covariates associated with plasma scopolamine pharmacokinetics. Body weight was directly related and age was inversely related to the total clearance. Scopolamine is hardly detected unchanged in urine (~2.6%), and based on in vitro and interaction studies in humans, scopolamine seems to be mainly eliminated hepatically, with CYP3A4 as the responsible elimination pathway 27. Increasing age has been correlated with a diminished blood flow to the liver and a reduced liver volume and functional capacity, explaining reduced clearance with increase in age 30, 31. Medication was controlled in the current studies, however use of concomitant CYP3A inhibitors, thereby further decreasing clearance, could explain the higher incidence of side effects in other studies in older subjects after scopolamine administration, especially if scopolamine exposure was not taken into consideration.
The neurophysiologic eye movement parameter saccadic peak velocity was the only test where an age‐related augmented sensitivity to scopolamine was found, a finding not earlier reported. Age was correlated with a decline in the EC 50 in the peak velocity of the horizontal saccadic eye movements test with a decrease mainly observed above ~45 years. Pons and midbrain, both located in the brainstem, are particularly rich in muscarinic acetylcholine receptors 32 which are an important binding site for anti‐muscarinic compounds as demonstrated in vivo with imaging techniques that measured enrichment of radiolabelled scopolamine in these brain areas 33. The horizontal saccadic eye movements test evaluates the coordinated action of brain areas that involve the occipital lobe for acquisition and brainstem structures such as the pons and midbrain for execution, explaining the high sensitivity of this test in detecting scopolamine effects.
Previous studies have reported significant differences in sensitivity of older subjects compared to younger subjects after scopolamine administration, only while carrying out cognitive tests involved in short‐term acquisition or verbal and numeric working memory 10, 11, 12 or visuo‐spatial praxis 13. The N‐back test was the most sensitive experiment performed in our battery of memory tests specifically evaluating verbal acquisition, processing and execution. Even though in all three paradigms (0‐, 1‐ and 2‐back) a small percentage of the subjects in the older subjects group scored lower even when receiving a lower dose compared to the younger group, the great majority did not have a larger decrease in performance. The above‐mentioned outliers in the older subjects group scored consistently poorer in the three tests as reflected in the individual E MAX estimates graphed per age (Figure 4). A larger number of older subjects scored lower than the younger group where subjects with an estimated E MAX above the sample's upper 95% CI were above 65 years, even when receiving a lower dose; at the population level there was no significant difference between the higher decrease in the score or baseline and age. This might provide evidence that, while in general older subjects perform similarly to younger adults after scopolamine administration in tests evaluating verbal working memory and execution, the number of subjects who perform worse during the test increases with a higher age; suggesting that although age as such is not correlated with a worse performance during scopolamine administration, the number of subjects with a higher sensitivity to the anti‐cholinergic compound increases with age. Future research should be directed to longitudinally study whether these subjects are prone to develop mild cognitive impairment.
Figure 4.
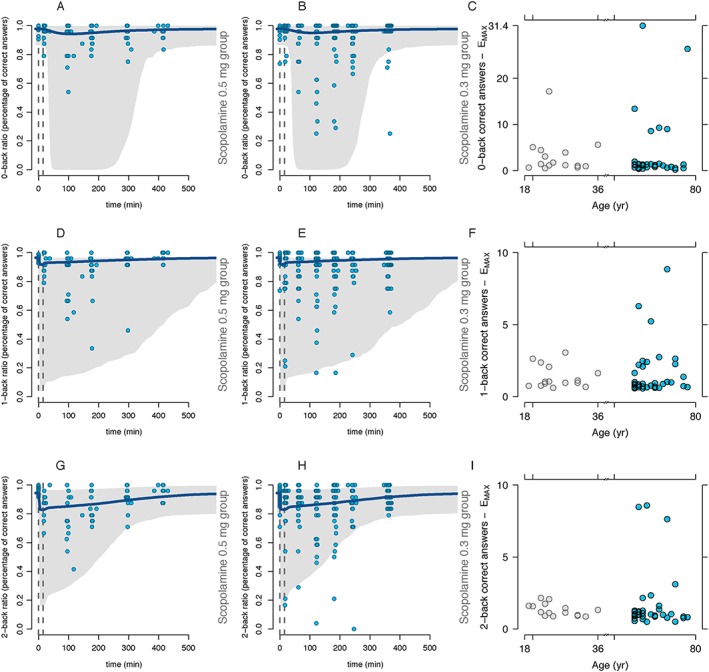
Scopolamine pharmacodynamic effects in the N‐back (number of correct answers). The column on the left represents the scopolamine 0.3 mg group (Figures 4A, 4D and 4G), the middle column the scopolamine 0.5 mg group (Figures 4B, 4E and 4H) and the column on the right the estimated E MAX (Figure 4C, 4F and 4I). The first row represents the 0‐back, the second row the 1‐back and the last row the 2‐back percentage of correct answers in the paradigm. The continuous blue line represents the model population predicted values per group and the grey area represents the 95% prediction interval. Circles represent the actual observations. Dotted, vertical gray lines represent the start and stop times of the scopolamine infusion. Simulations were performed with 1000 subjects
Contrary to previous literature, we suggest that scopolamine administration does not lead to an increase in sensitivity of cognitive function tests (e.g. decrease in performance) in healthy older subjects compared to healthy young subjects. This finding suggests that increased sensitivity to scopolamine in healthy older subjects may not be due to incipient cholinergic neuronal degeneration in the majority of older subjects. Healthy subjects of advanced age perform similarly to younger subjects after scopolamine administration in tests evaluating verbal working memory and execution. This finding seems to be in line with previous experiments that could not demonstrate a decline in the availability of nicotinic acetylcholine receptors with increasing age in healthy human older subjects with a MMSE score above 27 points, a similar population in the current study 34. Prescription of scopolamine in healthy older subjects should in general be safe and well tolerated. However, caution is advised when co‐administered with CYP3A4 inhibitors or in subjects of low weight, as the apparent peripheral volume of distribution was influenced by total body weight. Further studies should be performed in subjects over 78 years of age and in subjects with cognitive impairment.
Regarding the reaction time measured during the N‐back test at baseline, older subjects were slower in responding than younger subjects. Nevertheless once scopolamine was administered, the increase in the reaction time was similar in both groups. According to the estimations in our model, on the average population estimate, a subject would increase his/her reaction time by a rate of 4.46% every 10 years (Figure 2), similar to a decrease previously reported 35.
Scopolamine effects on the resting state EEG performed with eyes closed included a decrease in α and an increase in δ and θ frequencies of both Fz‐Cz and Pz‐Oz leads. In addition, no difference in sensitivity was found between older and younger subjects after scopolamine administration. These findings are consistent with previous literature; intramuscular administration of 0.25 mg of scopolamine in subjects between 24 and 31 years produced a significant increase in absolute δ power and in relative δ and θ power in subjects with eyes closed, mainly over central and parieto‐occipital regions, and a decrease in absolute and relative α power, widespread but mainly over frontal regions 36. Higher intramuscular doses of scopolamine (0.5 and 0.75 mg) induced a dose‐related increase of relative power in low and high frequencies and a decrease in the α range in the posterior leads 37. A previously published PK‐PD model that described the EEG effects of an intravenous and intramuscular administration of 0.5 mg of scopolamine reported a decrease in the α power over the frontal, central and occipital brain areas compared to placebo, explained by a sigmoidal E MAX model. Even though nonsignificant, the appreciable changes in the δ, θ and β frequencies were previously reported 28.
In the present study, an age related difference in the EEG baseline was detected in the low frequency ranges (δ and θ) but not in the α frequency. Older subjects had a decreased absolute power in the δ frequency and θ frequency. These findings are consistent with reported literature where an age‐dependent decrease in EEG low frequency activity was identified 38.
Due to teratogenicity risks related to other studied drugs rather than scopolamine, no women were allowed to participate in the first three studies. However, no sex differences are to be expected based on neuroimaging morphology 4 and longitudinal assessments in cognitive function 39.
This study provides evidence that plasma scopolamine concentrations in healthy older subjects are influenced not only by body weight but also by age, and once this difference in exposure is corrected for, most age‐related increases in anticholinergic sensitivity disappear. The peak velocity of the saccadic eye movements was the only test where sensitivity increased with age.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare to have no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We would like to thank the study staff, Ria Kroon, Anna Uit den Boogaard, Esther Davidse, Sanderein Snel and Danielle Mulder for their hard work helping collect the data.
Supporting information
Figure S1 Age distribution among subjects
Supporting info item
Supporting info item
Alvarez‐Jimenez, R. , Groeneveld, G. J. , van Gerven, J. M. A. , Goulooze, S. C. , Baakman, A. C. , Hay, J. L. , and Stevens, J. (2016) Model‐based exposure‐response analysis to quantify age related differences in the response to scopolamine in healthy subjects. Br J Clin Pharmacol, 82: 1011–1021. doi: 10.1111/bcp.13031.
References
- 1. Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982; 217: 408–414. [DOI] [PubMed] [Google Scholar]
- 2. Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science 1983; 219: 1184–1190. [DOI] [PubMed] [Google Scholar]
- 3. Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci 2004; 5: 87–96. [DOI] [PubMed] [Google Scholar]
- 4. Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 2005; 15: 1676–1689. [DOI] [PubMed] [Google Scholar]
- 5. Mufson EJ, Ma SY, Cochran EJ, Bennett DA, Beckett LA, Jaffar S, et al. Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer's disease. J Comp Neurol 2000; 427: 19–30. [DOI] [PubMed] [Google Scholar]
- 6. Broks P, Preston GC, Traub M, Poppleton P, Ward C, Stahl SM. Modelling dementia: effects of scopolamine on memory and attention. Neuropsychologia 1988; 26: 685–700. [DOI] [PubMed] [Google Scholar]
- 7. Liem‐Moolenaar M, de Boer P, Timmers M, Schoemaker RC, van Hasselt JGC, Schmidt S, et al. Pharmacokinetic‐pharmacodynamic relationships of central nervous system effects of scopolamine in healthy subjects. Br J Clin Pharmacol 2011; 71: 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newhouse PA, Potter A, Corwin J, Lenox R. Modeling the nicotinic receptor loss in dementia using the nicotinic antagonist mecamylamine: effects on human cognitive functioning. Drug Dev Res 1994; 31: 71–79. [Google Scholar]
- 9. Ali‐Melkkilä T, Kanto J, Iisalo E. Pharmacokinetics and related pharmacodynamics of anticholinergic drugs. Acta Anaesthesiol Scand 1993; 37: 633–642. [DOI] [PubMed] [Google Scholar]
- 10. Zemishlany Z, Thorne AE. Anticholinergic challenge and cognitive functions: a comparison between young and elderly normal subjects. Isr J Psychiatry Relat Sci 1991; 28: 32–41. [PubMed] [Google Scholar]
- 11. Ray PG, Meador KJ, Loring DW, Zamrini EW, Yang XH, Buccafusco JJ. Central anticholinergic hypersensitivity in aging. J Geriatr Psychiatry Neurol 1992; 5: 72–77. [DOI] [PubMed] [Google Scholar]
- 12. Molchan SE, Martinez RA, Hill JL, Weingartner HJ, Thompson K, Vitiello B, et al. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Res Brain Res Rev 1992; 17: 215–226. [DOI] [PubMed] [Google Scholar]
- 13. Flicker C, Ferris SH, Serby M. Hypersensitivity to scopolamine in the elderly. Psychopharmacology (Berl) 1992; 107: 437–441. [DOI] [PubMed] [Google Scholar]
- 14. Seo SW, Suh MK, Chin J, Na DL. Mental confusion associated with scopolamine patch in elderly with mild cognitive impairment (MCI). Arch Gerontol Geriatr 2009; 49: 204–207. [DOI] [PubMed] [Google Scholar]
- 15. Terry AV, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer's disease‐related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther 2003; 306: 821–827. [DOI] [PubMed] [Google Scholar]
- 16. Dumas JA, Newhouse PA. The cholinergic hypothesis of cognitive aging revisited again: cholinergic functional compensation. Pharmacol Biochem Behav 2011; 99: 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Folstein MF, Folstein SE, McHugh PR. ‘Mini‐mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 18. Liem‐Moolenaar M, Zoethout RWM, de Boer P, Schmidt M, de Kam ML, Cohen AF, et al. The effects of the glycine reuptake inhibitor R213129 on the central nervous system and on scopolamine‐induced impairments in psychomotor and cognitive function in healthy subjects. J Psychopharmacol 2010; 24: 1671–1679. [DOI] [PubMed] [Google Scholar]
- 19. Liem‐Moolenaar M, Zoethout RWM, de Boer P, Schmidt M, de Kam ML, Cohen AF, et al. The effects of a glycine reuptake inhibitor R231857 on the central nervous system and on scopolamine‐induced impairments in cognitive and psychomotor function in healthy subjects. J Psychopharmacol 2010; 24: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 20. Snyder PJ, Bednar MM, Cromer JR, Maruff P. Reversal of scopolamine‐induced deficits with a single dose of donepezil, an acetylcholinesterase inhibitor. Alzheimers Dement 2005; 1: 126–135. [DOI] [PubMed] [Google Scholar]
- 21. Thomas E, Snyder PJ, Pietrzak RH, Jackson CE, Bednar M, Maruff P. Specific impairments in visuospatial working and short‐term memory following low‐dose scopolamine challenge in healthy older adults. Neuropsychologia 2008; 46: 2476–2484. [DOI] [PubMed] [Google Scholar]
- 22. Lim H‐K, Juh R, Pae C‐U, Lee B‐T, Yoo S‐S, Ryu S‐H, et al. Altered verbal working memory process in patients with Alzheimer's disease: an fMRI investigation. Neuropsychobiology 2008; 57: 181–187. [DOI] [PubMed] [Google Scholar]
- 23. Borland RG, Nicholson AN. Visual motor co‐ordination and dynamic visual acuity. Br J Clin Pharmacol 1984; 18 (Suppl 1): 69S–72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Steveninck AL, Schoemaker HC, Pieters MS, Kroon R, Breimer DD, Cohen AF. A comparison of the sensitivities of adaptive tracking, eye movement analysis and visual analog lines to the effects of incremental doses of temazepam in healthy volunteers. Clin Pharmacol Ther 1991; 50: 172–180. [DOI] [PubMed] [Google Scholar]
- 25. Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM User's Guides. Ellicott City, MD: Icon Development Solutions, 2009. [Google Scholar]
- 26. R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2013. .Available from: http://www.r‐project.org/ [Google Scholar]
- 27. Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit 2005; 27: 655–665. [DOI] [PubMed] [Google Scholar]
- 28. Ebert U, Grossmann M, Oertel R, Gramatté T, Kirch W. Pharmacokinetic‐pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol 2001; 41: 51–60. [DOI] [PubMed] [Google Scholar]
- 29. Putcha L, Tietze KJ, Bourne DWA, Parise CM, Hunter RP, Cintrón NM. Bioavailability of intranasal scopolamine in normal subjects. J Pharm Sci 1996; 85: 899–902. [DOI] [PubMed] [Google Scholar]
- 30. Woodhouse KW, James OF. Hepatic drug metabolism and ageing. Br Med Bull 1990; 46: 22–35. [DOI] [PubMed] [Google Scholar]
- 31. Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 1989; 9: 297–301. [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi RM, Palkovits M, Hruska RE, Rothschild R, Yamamura HI. Regional distribution of muscarinic cholinergic receptors in rat brain. Brain Res 1978; 154: 13–23. [DOI] [PubMed] [Google Scholar]
- 33. Frey KA, Koeppe RA, Mulholland GK, Jewett D, Hichwa R, Ehrenkaufer RLE, et al. In vivo muscarinic cholinergic receptor imaging in human brain with [C‐11] scopolamine and positron emission tomography. J Cereb Blood Flow Metab 1992; 12: 147–154. [DOI] [PubMed] [Google Scholar]
- 34. Ellis JR, Nathan PJ, Villemagne VL, Mulligan RS, Ellis KA, Tochon‐Danguy HJ, et al. The relationship between nicotinic receptors and cognitive functioning in healthy aging: an in vivo positron emission tomography (PET) study with 2‐[(18)F]fluoro‐A‐85380. Synapse 2009; 63: 752–763. [DOI] [PubMed] [Google Scholar]
- 35. Ratcliff R, Thapar A, McKoon G. The effects of aging on reaction time in a signal detection task. Psychol Aging 2001; 16: 323–341. [PubMed] [Google Scholar]
- 36. Kikuchi M, Wada Y, Nanbu Y, Nakajima A, Tachibana H, Takeda T, et al. EEG changes following scopolamine administration in healthy subjects. Neuropsychobiology 1999; 39: 219–226. [DOI] [PubMed] [Google Scholar]
- 37. Sannita WG, Maggi L, Rosadini G. Effects of scopolamine (0.25‐0.75 mg i.m.) on the quantitative EEG and the neuropsychological status of healthy volunteers. Neuropsychobiology 1987; 17: 199–205. [DOI] [PubMed] [Google Scholar]
- 38. Leirer VM, Wienbruch C, Kolassa S, Schlee W, Elbert T, Kolassa IT. Changes in cortical slow wave activity in healthy aging. Brain Imaging Behav 2011; 5: 222–228. [DOI] [PubMed] [Google Scholar]
- 39. Aartsen MJ, Martin M, Zimprich D. Gender differences in level and change in cognitive functioning: results from the longitudinal aging study Amsterdam. Gerontology 2004; 50: 35–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Age distribution among subjects
Supporting info item
Supporting info item


