Abstract
Aims
This study aimed to evaluate the impact of esomeprazole on the pharmacokinetics of sonidegib.
Methods
This Phase I study evaluated the impact of the proton pump inhibitor (PPI) esomeprazole on the oral absorption and pharmacokinetics (PKs) of a single dose of sonidegib under fasted conditions. A total of 42 healthy subjects were enrolled to receive either sonidegib alone (200 mg single dose) or sonidegib in combination with esomeprazole (40 mg pre‐treatment 5 days and combination were given on day 6). Primary PK parameters assessed in the study were area under the concentration‐time curve (AUC) from 0–14 days and 0–7 days and maximum observed plasma concentration (C max).
Results
The plasma exposure (AUC0‐14d, AUC0‐7d and C max) of a single 200 mg oral dose of sonidegib was decreased by 32–38% when sonidegib was co‐administered with esomeprazole compared with sonidegib alone, with no apparent change in elimination slope and t max. Baseline gastric pH was similar between the two arms.
Conclusions
These results suggest a modest reduction in the extent of sonidegib absorption by esomeprazole. There was no obvious metabolic drug–drug interaction between the two agents. Both sonidegib and esomeprazole were well tolerated in the study population.
Keywords: basal cell carcinoma; drug–drug interaction; Hh pathway, esomeprazole; pH‐dependent solubility; sonidegib
What is Already Known about this Subject
Sonidegib is a newly approved drug in various regions globally for treating locally advanced basal cell carcinoma which cannot be treated with curative surgery or radiation therapy.
Drugs having pH‐dependent solubility may have drug–drug interaction with gastric pH agents (e.g., PPI, H2 blocker) when in combination.
In vitro, sonidegib follows pH‐dependent solubility, with lower solubility at higher pH.
Gastric pH agents are commonly used in cancer patients.
What this Study Adds
This study investigates the effect of a proton pump inhibitor on the oral absorption and pharmacokinetics of sonidegib.
The exposure changes observed when sonidegib is given in combination with esomeprazole are not considered to be clinically relevant.
Introduction
Sonidegib (LDE225; Odomzo™) is a potent, selective and orally bioavailable inhibitor of Smoothened (SMO), part of the Hedgehog (Hh) signalling pathway. It has pH‐dependent aqueous solubility in vitro, with lower solubility at higher pH. Aberrant activation of the Hh pathway is associated with the pathogenesis of certain cancers, including basal cell carcinoma (BCC) 1. Mutations leading to loss of function in the Patched (PTCH) gene and activating mutations in the Smoothened gene have been identified in patients with this disease 2. Sonidegib selectively binds to SMO, leading to suppression of the Hh signalling pathway and the inhibition of tumour cell growth. Sonidegib has recently been approved in various countries for treating locally advanced BCC which cannot be treated with curative surgery or radiation therapy, and is approved for metastatic BCC in certain regions 3, 4.
The solubility of sonidegib is pH dependent (lower at higher pH levels 5.), and it is classified as a BCS (Biopharmaceutical Classification System) class II drug. The extent of absorption for sonidegib is low and estimated to be less than 10% 3, 4. Proton pump inhibitors (PPI) are commonly prescribed drugs used in gastro‐oesophageal reflux disease (GORD) and peptic ulcer disease and often prescribed to cancer patients 6, 7. This group of agents suppresses gastric acid secretion by specific inhibition of the H+/K+‐ATPase pump in gastric parietal cells 8, thereby increasing gastric pH for a sustained time, and thus may alter the bioavailability of drugs with pH‐dependent solubility when used in combination.
Sonidegib is primarily metabolized by CYP3A in the liver. The major metabolite is pharmacologically inactive. Sonidegib is highly bound to human plasma proteins in vitro and the binding is concentration independent. Pharmacokinetic (PK) data from previous studies have shown that the median t max occurred at 2–4 h after a single dose, and 2–13 h after repeated doses. Pharmacokinetic exposure of sonidegib is approximately linear from dose 100–400 mg. The estimated elimination half‐life t 1/2 of sonidegib in healthy subjects is approximately 10 days after a single dose, while for patients it is estimated to be 28.3 days. Steady state is reached in approximately 4 months and the accumulation ratio of sonidegib based on once daily dosing was estimated to be 19.4‐fold in patients 3.
Esomeprazole, a proton pump inhibitor, is the S‐isomer of the racemic compound omeprazole. Esomeprazole is an optical isomer of omeprazole and is subject to less first‐pass metabolism and lower plasma clearance than the parent compound, offering potentially higher systemic bioavailability 9. Esomeprazole is known to have a strong gastric acid suppression effect and lacks the potential to interact directly with sonidegib, for example through CYP3A4 metabolic interaction 10. In addition, esomeprazole has a good safety and tolerability profile, and was found to be well tolerated in healthy volunteers 11.
This study aimed to evaluate the impact of esomeprazole on the pharmacokinetics of sonidegib.
Subjects and methods
Subjects
Subjects eligible for inclusion in the study included healthy males or healthy sterile or postmenopausal females between 18 and 55 years of age, with body mass index (BMI) of 18–29.9 kg m−2, and body weight ≥ 50 kg. Males who were sexually active agreed to use a condom during intercourse for the duration of the study and for 6 months afterwards. Subjects had to have no clinically significant abnormalities as determined by past medical history, physical examination, vital signs, electrocardiogram (ECG), and clinical laboratory tests, sufficient hepatic and renal function, and a baseline gastric stomach pH of less than 4 under fasted conditions (confirmed by an intranasal pH probe). None of the subjects took antacids, anticholinergics, H2‐antagonists or PPIs within 72 h prior to dosing or during the course of the study except the study drug. Subjects had not taken any prescription drug within 4 weeks or within 10 elimination‐half‐lives prior to dosing (whichever was longer) or over‐the‐counter medication (except paracetamol) within 72 h prior to dosing. Subjects taking acetaminophen on a daily basis for more than two consecutive days were not enrolled. Subjects who enrolled did not have impairment of gastrointestinal (GI) function or GI disease (e.g. ulcerative disease, uncontrolled nausea, vomiting, diarrhoea, malabsorption syndrome, small bowel resection). Subjects had to have abstained from alcohol 48 h prior to dosing and for the entire study duration. Subjects were non‐smokers. The study protocol was reviewed by the Independent Ethics Committee. All subjects provided signed written informed consent.
Study design
This was a single‐centre, Phase I, randomized, open‐label, two‐arm parallel study to evaluate the effect of esomeprazole on the relative bioavailability of a single oral dose of sonidegib in healthy volunteers. The secondary objective was to assess the safety and tolerability of sonidegib with or without esomeprazole in healthy volunteers. Approximately 40 healthy subjects were to be randomized into the study to obtain at least 20 evaluable subjects per treatment arm. The sample size n = 20 per arm comes from a precision‐based approach with the half‐width of 90% CI for test‐reference comparison on the log scale extending 0.368 from the observed difference in means for the primary PK parameters.
Subjects were randomized 1:1 into two parallel arms (Figure 1), the control arm and the esomeprazole arm. A 200 mg single dose of sonidegib capsule was used in this study as this is the approved clinical dose for patients with advanced basal cell carcinoma. Esomeprazole 40 mg once daily was selected for this study as this is the maximum dose marketed for clinical use and is a commonly used dose in drug–drug interaction studies. There was a screening period of up to 28 d and subjects remained in the study centre from Day −1 to Day 8 (Arm 1) or to Day 13 (Arm 2). Sonidegib (200 mg capsule) was supplied by Novartis. Esomeprazole capsules (40 mg) were obtained commercially by the study site.
Figure 1.
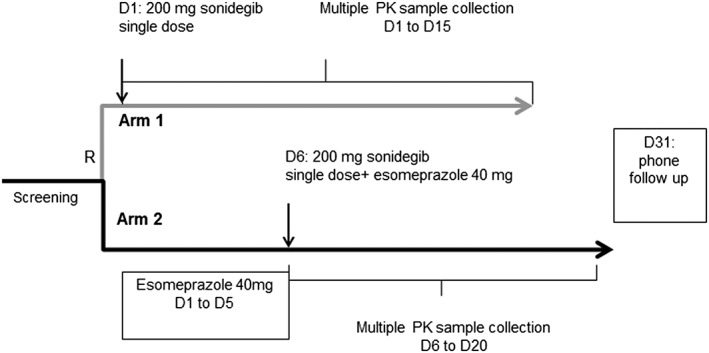
Study design. R: randomization; D: day
The subjects in this study were randomized into:
Arm 1: Subjects were fasted for at least 10 h overnight prior to sonidegib dose on Day 1 and also fasted for an additional 4 h after sonidegib dose. Subjects received a single 200 mg capsule to be swallowed whole for the sonidegib dose.
Arm 2: From Day 1 to Day 5, subjects received esomeprazole under fasted conditions. Subjects were fasted for at least 10 h overnight before each dose of esomeprazole and 1 h after esomeprazole dosing. On Day 6, subjects were fasted for 10 h overnight before the dose of esomeprazole, then fasted for 2 h prior to sonidegib dose. After the sonidegib dose, subjects were fasted for an additional 4 h.
Water was freely available, except for 1 h before and after drug administration (i.e., esomeprazole and sonidegib). All meals taken (e.g., evening snack, meal time, estimated percentage consumed) were recorded.
After randomization, serial PK blood samples for sonidegib were collected from Day 1 (for Arm 1) or from Day 6 (for Arm 2) and up to 14 d at the following time points: 0 (pre‐dose), 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 48, 72, 96, 120, 144, 168, 216, 264, and 336 h post sonidegib dose. Blood samples (3 ml) were collected from the forearm of each subject and placed in tubes containing potassium ethylenediaminetetraacetic acid (K3‐EDTA). Blood samples were centrifuged at 4°C for 10 min at 2000 g to separate the plasma. Immediately after centrifugation, the plasma was transferred into a 1.8 ml Nunc Cryobank 2D coded tube and frozen immediately over dry ice or in a freezer at −70°C. Samples were stored at or below −70°C before analysis.
A parallel arm design was used for this study instead of a cross‐over design due to the long half‐life of sonidegib and the time needed for wash‐out. In order to achieve esomeprazole steady state and maintain an elevated gastric pH, esomeprazole was given for 5 d prior to the concurrent administration with sonidegib. On Day 6, sonidegib was administered 2 h after esomeprazole for maximum gastric acid suppression effect at the time of sonidegib dissolution. In addition, the 14 d designated as the PK collection duration was considered adequate to gather the PK information needed (i.e. absorption phase) to compare the two treatment arms.
The minimum criteria for a subject to be considered as evaluable were the following: subjects have received 200 mg of sonidegib and fasted for at least 10 h (Arm 1) or 12 h (Arm 2) overnight before sonidegib dose and for 4 h after sonidegib dose; received all scheduled doses of esomeprazole (Arm 2) and fasted for at least 10 h overnight before esomeprazole dose and for 1 h after esomeprazole dose (Day 1 to 5) or fasted 4 h after sonidegib dosing (6 h after esomeprazole dose) (Day 6); have not vomited within 4 h of sonidegib or esomeprazole administration; and did not discontinue within 24 h of sonidegib dosing.
The end of study evaluation was conducted on Day 15 or Day 20 for Arms 1 and 2, respectively. A safety follow‐up was conducted 30 days after the last dose administration.
Safety assessment
Safety was monitored by assessing vital signs, physical examination, ECG, haematology, blood chemistry, coagulation and urinalysis at site visits. Adverse events (AEs) were documented at every visit. All clinical analysis was performed by the local laboratory. Additional laboratory tests could be performed at the investigator's discretion for safety measures in the event of an AE.
Sonidegib bioanalytical methods
Plasma sonidegib concentrations were measured at a Novartis designated laboratory (SGS Cephac, France) using a validated high‐performance liquid chromatography/tandem mass spectrometry (HPLC/MS–MS). The analytical range was 0.5–1000 ng ml−1.
Plasma samples were prepared using a protein precipitation extraction procedure, and sonidegib concentrations were determined using a validated liquid chromatography/tandem mass spectrometry (LC/MS–MS) assay using an API 4000 triple quadrupole mass spectrometer (AB Sciex, Ontario, Canada) equipped with a turbo ionSpray interface. Sample extracts were analysed using gradient reverse‐phase chromatography with Ascentis Express C8, 50 × 2.1 mm ID, 2.7‐μm particles (Ref. 53831‐U) (Supelco, Bellefonte, Pennsylvania, USA). The mobile phase gradient consisting of water/0.1% formic acid solution and acetonitrile containing 0.1% of formic acid solution pumped through the column at a flow rate of 500 μl min−1. Positive‐ion multiple reaction monitoring (MRM) with a labelled internal control and a lower limit of quantitation of 0.5 ng ml−1 of plasma was used for detection. The MRM transition monitored for sonidegib, and the labelled internal standard was m/z 486.2 to 428.3 and 490.2 to 432.2, respectively. The LC/MS–MS chromatograms of all analysed baseline samples showed no interfering peaks, demonstrating selectivity of the method. Intra‐day and inter‐day precision as represented by the coefficient of variation and accuracy as represented by the mean bias were within 20%.
Pharmacokinetic analysis
The PK parameters for sonidegib were determined by non‐compartmental methods using Phoenix WinNonlin (version 6.2, Pharsight, Mountain View, CA). The PK analyses used the actual dose received and actual elapsed time from dosing. The C max and t max were taken from the observed values and AUC was calculated using the linear trapezoidal rule.
The primary PK parameters assessed were maximum observed plasma concentration after drug administration [ng ml−1] (C max), area under the concentration–time curve (AUC) from time zero to 14 days [ng h ml−1] (AUC0‐14d), and AUC from time zero to 7 days [ng h ml−1] (AUC0‐7d). The secondary PK parameters assessed were AUC from time zero to 3 days [ng h ml−1] (AUC0‐3d) and the time to reach C max [h], (t max). Due to the long half‐life of sonidegib, other PK parameters (e.g. AUCinf (area under curve from time zero to infinity), CL/F (apparent oral clearance), Vz/F (apparent volume of distribution), and t 1/2 (elimination half‐life)) were not part of the analysis.
Statistical analysis
For the primary statistical analysis, a formal analysis was performed on the pharmacokinetic analysis set (PAS) for the primary PK parameters to assess the effect of esomeprazole on the PKs of sonidegib. The PAS included subjects who met all the evaluability criteria described in the methods section and who provided at least one primary PK parameter (i.e. C max, AUC0‐14d or AUC0‐7d). A linear model was fitted to the log‐transformed PK parameters including treatment as a fixed effect. Sonidegib alone was considered as reference treatment and sonidegib after treatment with esomeprazole was considered as test treatment.
The point estimate of the treatment difference and the corresponding 90% confidence interval (CI) was calculated and anti‐logged to obtain the point estimate and 90% CI for the geometric mean ratio of test versus reference on the original scale.
Results
Subject demographics
A total of 42 subjects were enrolled in the study (21 subjects in each of the two treatment arms). One subject in the sonidegib + esomeprazole arm withdrew consent and discontinued from the study, and 41 subjects completed the study. Baseline demographic characteristics were well balanced between the two arms. The median age of the subjects was 49 years (range 21–54 years), median body weight was 74.7 kg and median body mass index (BMI) was 24.6 kg m−2. All subjects were Caucasian. Thirty‐one subjects (74%) were males. Five (11.9%) female subjects were post‐menopausal and six (14.3%) were of childbearing age but sterile.
Subject exposure
All subjects in the sonidegib arm received a single oral dose of 200 mg sonidegib on Day 1. All subjects in the sonidegib + esomeprazole arm received 40 mg esomeprazole daily from Day 1 to Day 6 and one single dose of 200 mg sonidegib on Day 6; except one subject who received a single oral dose of 40 mg esomeprazole on Day 1 and then discontinued the study.
Pharmacokinetics of sonidegib
A total of 40 out of 42 subjects (21 in the sonidegib alone arm and 19 in the sonidegib + esomeprazole arm) were included in the PK analysis. Two subjects in the sonidegib + esomeprazole arm were excluded from the analysis because one subject discontinued the study early and one subject could not be confirmed as meeting the fasting criteria (i.e. at least 10 h) before dosing on one of the dosing days.
Concentration–time profiles of sonidegib
The mean (with SD) concentration–time profiles of sonidegib, with and without esomeprazole, are shown in Figure 2(A) (0–14 d), and Figure 2(B) (0–48 h). The concentration–time profiles show that the sonidegib plasma exposures are slightly lower when sonidegib was co‐administered with esomeprazole compared with sonidegib alone. The elimination slope up to 14 d appears to be similar between the two arms.
Figure 2.
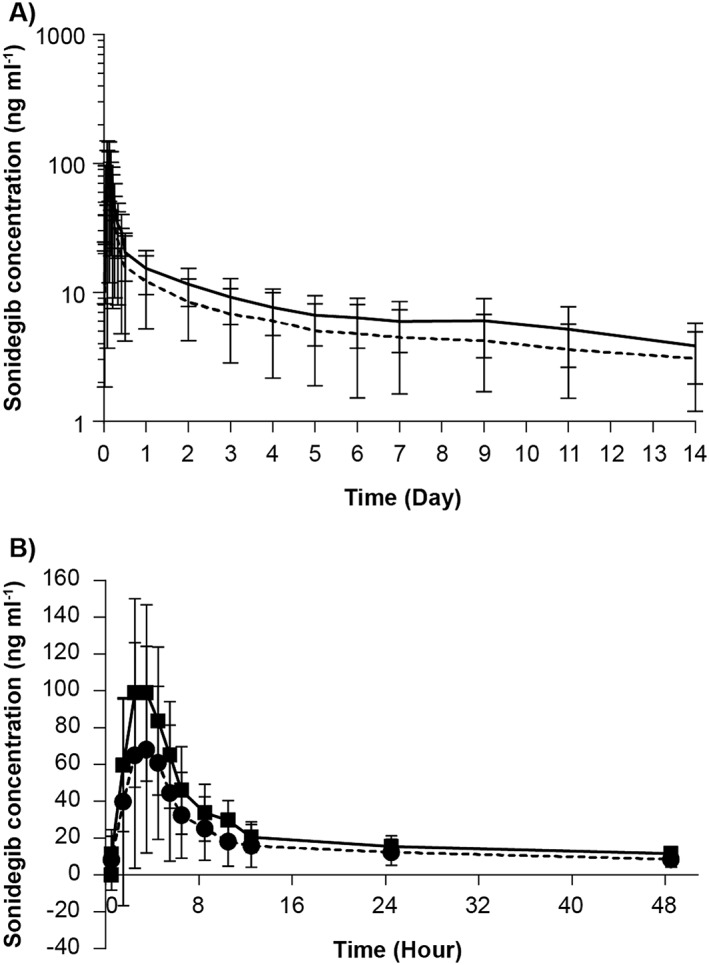
(A) Arithmetic mean (SD) plasma concentration–time profiles (0–14 days) for sonidegib, by treatment (semi‐logarithmic view); arithmetic mean (sonidegib), arithmetic mean (sonidegib+esomeprazole). (B) Arithmetic mean (SD) plasma concentration–time profiles (0–48 h) for sonidegib, by treatment; sonidegib, sonidegib+esomeprazole
PK parameters
The sonidegib PK parameters are summarized in Table 1. Lower exposures (AUC0‐14d, AUC0‐7d and C max) of sonidegib were seen when sonidegib was co‐administered with esomeprazole compared with sonidegib alone. There was a 32% decrease in sonidegib exposure (i.e. AUC0‐7d and AUC0‐14d) when sonidegib was co‐administered with esomeprazole compared with sonidegib alone, as indicated by geometric mean ratios and 90% CIs for AUC0‐14d and AUC0‐7d of 0.681 (90% CI: 0.523, 0.889) and 0.677 (90% CI: 0.520, 0.881), respectively. The geometric mean ratio and 90% CI for C max was 0.616 (90% CI: 0.434, 0.875), indicating a 38% decrease in sonidegib C max when co‐administered with esomeprazole compared with sonidegib alone (Table 1). The individual subject values from both arms and geometric means of sonidegib primary PK parameters are further plotted in Figure 3.
Table 1.
Summary of PK parameters for sonidegib, by treatment groups
| Sonidegib n = 21 | Sonidegib + esomeprazole n = 19 | Geo‐mean ratio (Sonidegib with esomeprazole/ Sonidegib alone) (90% CI) | |
|---|---|---|---|
| Primary parameters | |||
| AUC 0‐14d (ng h ml −1 ) | |||
| Mean (SD) | 2960 (1140) | 2180 (1320) | |
| Geo‐mean (% CV) | 2740 (44.3) | 1860 (61.8) | 0.681 (0.523–0.889) |
| AUC0‐7d (ng h ml −1 ) | |||
| Mean (SD) | 2080 (744) | 1540 (954) | |
| Geo‐mean (% CV) | 1940 (41.9) | 1310 (63.2) | 0.677 (0.520–0.881) |
| C max ( ng ml −1 ) | |||
| Mean (SD) | 112 (50.1) | 81.6 (63.5) | 0.616 (0.434–0.875) |
| Geo‐mean (% CV) | 100 (54.7) | 61.7 (92.6) | |
| Secondary parameters | |||
| AUC0‐3d (ng h ml−1) | |||
| Mean (SD) | 1400 (496) | 1020 (640) | |
| Geo‐mean (% CV) | 1310 (40.2) | 869 (63.7) | |
| t max (h) | |||
| Median (min; max) | 3.0 (1.00; 5.00) | 3.0 (1.00; 4.98) | |
CI, confidence interval; CV% geo‐mean, sqrt (exp (variance for log transformed data)‐1)*100; n, number of subjects with non‐missing values; SD, standard deviation; %CV, coefficient of variation (%) = SD/mean*100.
Figure 3.
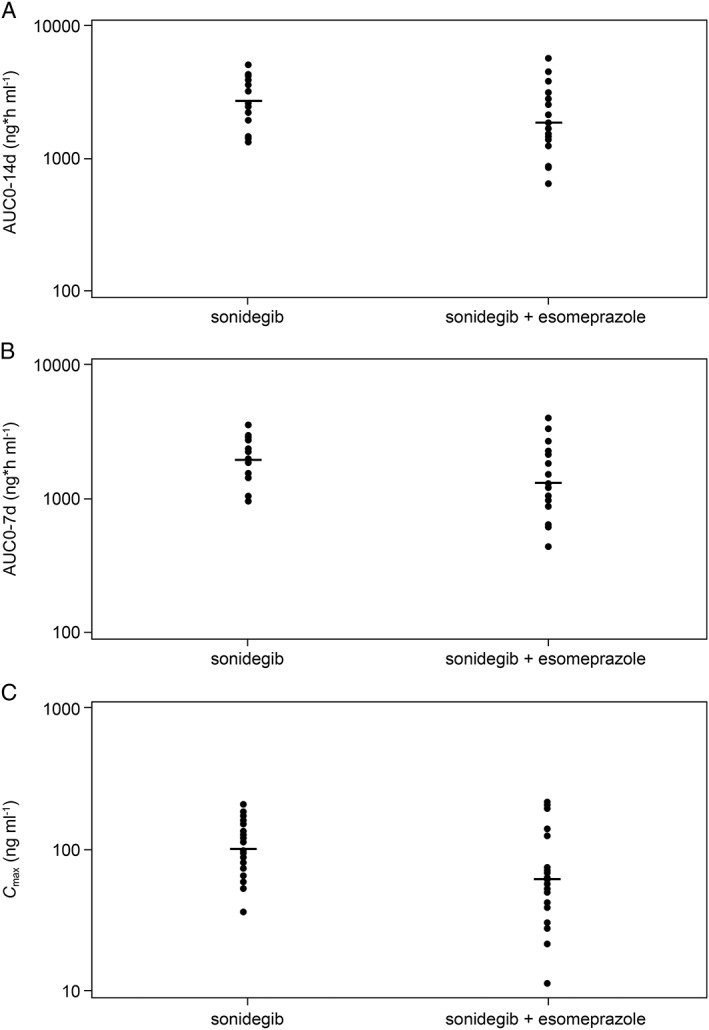
Individual subject values and geometric means of sonidegib primary PK parameters. (A) Individual values and geometric mean for AUC0‐14d; (B) Individual values and geometric mean for AUC0‐7d; (C) Individual values and geometric mean for C max. individual PK parameter, geometric mean
When co‐administered with esomeprazole, the inter‐subject variability (geo‐mean coefficient of variation [CV], %) for AUC0‐14d, AUC0‐7d, and C max were larger than those observed when sonidegib was administered alone (i.e. 62–93% with sonidegib + esomeprazole vs. 42–55% with sonidegib alone).
The secondary PK parameter (AUC0‐3d) followed a similar trend as that obtained with the primary PK parameters. Co‐administration of sonidegib with esomeprazole resulted in lower exposures (geometric mean 869 vs. 1310 ng h ml−1) and higher variability compared with administration of sonidegib alone. The median t max of sonidegib was similar for the two treatment arms (3.0 h; range: 1.0–5.0).
Overall, the plasma exposure of a 200 mg oral dose of sonidegib was decreased by 32–38% when sonidegib was co‐administered with esomeprazole. The variability of sonidegib PK was higher when co‐administered with esomeprazole. The terminal phase of the profile appeared unchanged, indicating that the clearance of sonidegib remained constant. Esomeprazole co‐administration did not change t max of sonidegib.
Safety and tolerability
Both sonidegib and esomeprazole were well tolerated in the study population. There were no deaths, serious adverse events (SAEs) or other significant AEs reported in the study. Overall, 11 subjects [five (23.8%) in the sonidegib arm and six (28.6%) in the sonidegib + esomeprazole arm] experienced at least one AE. The most commonly reported AEs included headache (9.5%), nasopharyngitis (7.1%) and decreased appetite (4.8%) (Table 2). All the reported AEs were grade 1–2 in severity and all except one resolved without any treatment. One subject in the sonidegib arm experienced grade 2 headache (two episodes) and was treated with paracetamol. Three subjects (14.3%) in the sonidegib arm reported AEs which were suspected by the investigator to be related to the study drug (fatigue [one subject], and headache and decreased appetite [two subjects each]).
Table 2.
Adverse events
| Sonidegib n = 21 n (%) | Sonidegib + esomeprazole n = 21 n (%) | All subjects n = 42 n (%) | |
|---|---|---|---|
| Any adverse event | 5 (23.8) | 6 (28.6) | 11 (26.2) |
| Gastrointestinal disorders | 0 | 4 (19.0) | 4 (9.5) |
| Abdominal distension | 0 | 1 (4.8) | 1 (2.4) |
| Abdominal pain upper | 0 | 1 (4.8) | 1 (2.4) |
| Diarrhoea | 0 | 1 (4.8) | 1 (2.4) |
| Flatulence | 0 | 1 (4.8) | 1 (2.4) |
| Regurgitation | 0 | 1 (4.8) | 1 (2.4) |
| Fatigue | 1 (4.8) | 0 | 1 (2.4) |
| Nasopharyngitis | 1 (4.8) | 2 (9.5) | 3 (7.1) |
| Decreased appetite | 2 (9.5) | 0 | 2 (4.8) |
| Headache | 3 (14.3) | 1 (4.8) | 4 (9.5) |
There were no clinically relevant changes from baseline in clinical laboratory values, vital signs or ECG values. There were no clinically significant changes from baseline for primary haematology parameters, including blood cell counts and coagulation profiles. Sixteen subjects had blood pressure and pulse rate values outside the normal range. No subject had any clinically significant ECG abnormalities, and among subjects who had changes in QT interval, none were considered clinically significant. Overall, none of the abnormal values or changes were considered to be clinically significant and none were considered to be AEs by the investigator.
Gastric pH at screening ranged from 1.4 to 2.6 for the sonidegib arm and from 1.3 to 3.6 for the sonidegib + esomeprazole arm. Post‐treatment gastric pH was not measured.
Discussion
Sonidegib (LDE225, Odomzo™) is a weak base, and an orally administered drug. Sonidegib has pH‐dependent aqueous solubility, with lower solubility at higher pH (i.e. pH > 4.5). Drugs such as PPIs that inhibit gastric acid secretion to elevate the gastric pH may have an impact on the solubility of sonidegib and change its bioavailability. Many other cancer therapy medications which have pH‐dependent solubility have also been investigated to determine the effect of gastric pH elevating agents on their bioavailability, and for some of them (e.g., dasatinib, erlotinib, gefitinib), there are profound changes in their exposure 6. The primary objective of this study was to determine the effect of esomeprazole (a PPI) on the pharmacokinetics of a single oral dose of sonidegib in healthy subjects.
The plasma exposure (AUC0‐14d, AUC0‐7d and C max) of a 200 mg oral dose of sonidegib was decreased by 32–38% when co‐administered with esomeprazole compared with sonidegib alone. Other PK parameters (e.g. AUCinf, CL/F, t 1/2, etc.) are not part of the analysis given the long half‐life of sonidegib; however, the expected change in AUCinf should be similar to those observed for AUC0‐7d and AUC0‐14d. Even though PPIs have been shown to significantly reduce gastric motility and delay gastric emptying in human subjects 12, 13, no change in t max for sonidegib was observed in this study when administered with esomeprazole. When co‐administered with esomeprazole, the inter‐subject variability was larger than that observed when sonidegib was administered alone (62–93% with sonidegib + esomeprazole vs. 42–55% with sonidegib alone). The increased variability in the sonidegib + esomeprazole arm could be due to the lower solubility of sonidegib as a result of the change in gastric pH.
Co‐medications which elevated gastric pH were allowed in the sonidegib Phase II efficacy pivotal study (BOLT), and approximately 30% of patients took such agents 14. The subgroup analysis in BOLT demonstrated consistent objective response rates in patients taking sonidegib with or without concomitant gastric pH elevating agents. Consistent with this current study, population PK analysis, which included PK data from BOLT, estimated the concomitant administration of a PPI or histamine (H)‐2‐receptor antagonist decreases the geometric mean sonidegib steady‐state AUC0‐24 h by 34% 3. When testing the effect of gastric pH agents on bioavailability, PPI decreased bioavailability by 31% and no effect was noted from histamine‐2‐receptor antagonists. Considering the large variability of sonidegib exposures (i.e. at steady state in patients, geo‐mean CV% for C min is 64%) and the variability observed for sonidegib with esomeprazole in this study, the extent of the decrease (~30%) still falls within the range of clinically relevant exposure. Overall, the extent of the decrease observed in this study is not thought to be clinically significant.
Newer PPIs, including esomeprazole, offer several advantages over older agents 15, 16, in that they achieve more rapid, profound and sustained inhibition of acid secretion. Esomeprazole at 40 mg once daily has been shown to maintain an intragastric pH > 4 for a significantly longer duration 12, 17. Studies compared different PPIs (including 20 mg rabeprazole, 40 mg esomeprazole, 20 mg omeprazole, 30 mg lansoprazole, 40 mg pantoprazole) for their gastric acid inhibition following 5 days of administration 18, 19, 20. At the end of 5 days, intragastric pH greater than 4 was maintained longer with esomeprazole, and more patients had a pH greater than 4 for more than 12 h.
Sonidegib (a single 200 mg oral dose) was generally safe and well tolerated by healthy subjects enrolled in this study. No deaths, SAEs, grade 3–4 AEs, or significant laboratory abnormalities were reported in the study. The gastrointestinal AEs reported in the sonidegib + esomeprazole arm (abdominal distension, abdominal pain, diarrhoea, flatulence and regurgitation) are typical of both the sonidegib and esomeprazole safety profiles.
Conclusion
Since it is common for cancer patients to take gastric pH‐elevating agents, it would be difficult to simply restrict these agents in practice for drugs with pH‐dependent solubility. Therefore it is important to properly evaluate the potential interaction and the clinical relevance of co‐administration with gastric pH‐elevating agents, preferably starting with PPIs which provide more sustained and deeper pH‐elevating effects than other gastric pH agents (e.g., histamine‐2‐receptor antagonists or antacids). Different impacts on the sonidegib exposure or PK profile might be expected if other less potent proton pump inhibitors or pH‐elevating agents are used concurrently with sonidegib.
The results from this study suggested a modest reduction in the extent of sonidegib absorption by esomeprazole. Such an effect is unlikely to have a clinically significant impact on sonidegib therapy. No new safety concerns were identified in this study, and the safety profile observed was consistent with the known safety profile of sonidegib.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. KG is a contract employee at Novartis Pharmaceuticals Corporation.
The study was supported by Novartis Pharmaceutical Corporation. The authors thank the research staff members at PAREXEL International GmbH for their assistance in the conduct of the clinical study. Statistical analysis was performed by PRA International, Reading, UK. Editorial support in the preparation of the manuscript was provided by Tina Patrick, Novartis Ireland Ltd.
Zhou, J. , Quinlan, M. , Glenn, K. , Boss, H. , Picard, F. , Castro, H. , and Sellami, D. (2016) Effect of esomeprazole, a proton pump inhibitor on the pharmacokinetics of sonidegib in healthy volunteers. Br J Clin Pharmacol, 82: 1022–1029. doi: 10.1111/bcp.13038.
References
- 1. Low JA, de Sauvage FJ. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol 2010; 28: 5321–26. [DOI] [PubMed] [Google Scholar]
- 2. Otsuka A, Levesque MP, Dummer R, Kabashima K. Hedgehog signaling in basal cell carcinoma. J Dermatol Sci 2015; 78: 95–100. [DOI] [PubMed] [Google Scholar]
- 3. Novartis . ODOMZO® (sonidegib) capsules, for oral use: US Prescribing Information. Available at http://www.accessdatafdagov/2015 (last accessed 27 July 2015).
- 4. Novartis Pharmaceuticals Australia Pty Ltd . ODOMZO sonidegib diphosphate 200 mg hard capsule blister pack. Public Summary for ARTG entry. Available at https://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=226544&agid=(PrintDetailsPublic)&actionid=1 (last accessed 28 June 2016).
- 5. FDA Burst Email to ASCPT Members. Available at http://www.ascpt.org/Knowledge-Center/FDA-Bursts (last accessed 15 August 2015).
- 6. Lind T, Cederberg C, Ekenved G, Haglund U, Olbe L. Effect of omeprazole – a gastric proton pump inhibitor – on pentagastrin stimulated acid secretion in man. Gut 1983; 24: 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH‐dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther 2012; 92: 203–13. [DOI] [PubMed] [Google Scholar]
- 8. AstraZeneca . 2011. Esomeprazole [NEXIUM(TM)] full prescribing information.
- 9. Richter JE, Kahrilas PJ, Johanson J, Maton P, Breiter JR, Hwang C, et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol 2001; 96: 656–65. [DOI] [PubMed] [Google Scholar]
- 10. Andersson T, Hassan‐Alin M, Hasselgren G, Rohss K. Drug interaction studies with esomeprazole, the (S)‐isomer of omeprazole. Clin Pharmacokinet 2001; 40: 523–37. [DOI] [PubMed] [Google Scholar]
- 11. Wilder‐Smith CH, Rohss K, Nilsson‐Pieschl C, Junghard O, Nyman L. Esomeprazole 40 mg provides improved intragastric acid control as compared with lansoprazole 30 mg and rabeprazole 20 mg in healthy volunteers. Digestion 2003; 68: 184–88. [DOI] [PubMed] [Google Scholar]
- 12. Tougas G, Earnest DL, Chen Y, Vanderkoy C, Rojavin M. Omeprazole delays gastric emptying in healthy volunteers: an effect prevented by tegaserod. Aliment Pharmacol Ther 2005; 22: 59–65. [DOI] [PubMed] [Google Scholar]
- 13. Parkman HP, Urbain JL, Knight LC, Brown KL, Trate DM, Miller MA, et al. Effect of gastric acid suppressants on human gastric motility. Gut 1998; 42: 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double‐blind Phase 2 trial. Lancet Oncol 2015; 16: 716–28. [DOI] [PubMed] [Google Scholar]
- 15. Robinson M. New‐generation proton pump inhibitors: overcoming the limitations of early‐generation agents. Eur J Gastroenterol Hepatol 2001; 13 (Suppl 1): S43–7. [PubMed] [Google Scholar]
- 16. Rohss K, Lind T, Wilder‐Smith C. Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro‐oesophageal reflux symptoms. Eur J Clin Pharmacol 2004; 60: 531–9. [DOI] [PubMed] [Google Scholar]
- 17. Yin OQ, Gallagher N, Fischer D, Demirhan E, Zhou W, Golor G, et al. Effect of the proton pump inhibitor esomeprazole on the oral absorption and pharmacokinetics of nilotinib. J Clin Pharmacol 2010; 50: 960–7. [DOI] [PubMed] [Google Scholar]
- 18. Miner P Jr, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five‐way crossover study. Am J Gastroenterol 2003; 98: 2616–20. [DOI] [PubMed] [Google Scholar]
- 19. Kalaitzakis E, Bjornsson E. A review of esomeprazole in the treatment of gastroesophageal reflux disease (GERD). J Ther Clin Risk Manag 2007; 3: 653–63. [PMC free article] [PubMed] [Google Scholar]
- 20. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep 2008; 10: 528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


