Abstract
Aim
Term and preterm neonates are at high risk for serious adverse drug reactions (ADRs).
Methods
A descriptive study of reports registered in the French pharmacovigilance database from 1986 to 2012 were obtained. All reports concerning neonates (≤1 month of life) with direct drug exposure were retrieved. Characteristics of the reports, including reported ADR(s), drug(s) and the causality assessment using the French causality assessment method, were described.
Results
A total of 1688 reports were analyzed and more than half of them were classified as serious (n = 995). Median age at ADR occurrence was 9 days. Overall, 3127 ADRs were described in these reports in relation to 2238 suspect/interacting drugs. The most commonly reported system organ classes (SOCs) were injury, poisoning and procedural complications (16%), general disorders and administration site conditions (12.5%) and blood and lymphatic system disorders (12%). In the majority of ADRs reported (73%), infants fully recovered and less than 4% of neonates deceased as a consequence of the reported ADR. One out of five ADRs was associated with drug administration errors. Therapeutic classes commonly incriminated were anti‐infectives, nervous system and alimentary tract drugs. Substances most frequently related to serious ADRs were zidovudine, ibuprofen and nevirapine. Among the 10 most frequently encountered drug−ADR pairs, two substances were mainly implicated, zidovudine in haematological adverse reactions and phytomenadione in maladministrations.
Conclusions
Anti‐infective drugs, mainly antiretroviral therapy, account for the majority of ADRs reported in neonates. The specific issue of drug maladministration and medication errors remains to be addressed in neonates.
Keywords: adverse drug reactions, neonates, pharmacovigilance, spontaneous reporting
What is Already Known about this Subject
Previous pharmacovigilance studies reported that in children approximately one ADR notification out of four concerns infants under the age of 1 year.
No study has focused on describing ADRs following direct drug exposure in neonates more in‐depth.
What this Study Adds
The majority of neonatal ADR reports were classified as serious and 3.7% of neonates deceased as a consequence of an ADR.
Anti‐infective medicines, mainly antiretroviral therapy, account for the large majority of reported ADRs and ADR fatalities.
Vitamins such as phytomenadione also account for a large number of ADR reports mainly associated witho drug administration errors without clinical consequences.
Introduction
There are many reasons why neonates may face a higher risk for adverse drug reactions (ADRs) than other age groups. Their organ immaturity and the rapid developmental changes that occur after birth may impact on both drug pharmacokinetics and pharmacodynamics 1. Sick neonates often require treatment with a substantial number of drugs because of the complexity and seriousness of their conditions and most prescribed drugs have never been specifically evaluated in this neonatal population. Indeed, the percentage of patients receiving at least one unlicensed or ‘off‐label’ drug ranges from 80% to 100% in neonatology units 2, 3, 4.
Spontaneous reporting systems are an important source of information on drug safety and detection of safety signals. This is essential for products that have not undergone a marketing authorization process, the most commonly encountered situation in paediatric practice. Previous pharmacovigilance (PV) studies reported that in children approximately one ADR notification out of four concerns infants under the age of 1 year 5, 6, 7 but few studies specifically described reports in neonates 8, 9, 10. In these studies assessing ADRs after in utero and direct exposure, congenital disorders and reactions related to pregnancy were predominant. Currently, no study has focused on describing ADRs following direct drug exposure in neonates more in‐depth.
Consequently, the objective of this study was to describe the nature of all ADRs and drug/ADR pairs reported in neonates following direct drug exposure and included in the French pharmacovigilance database.
Methods
The French pharmacovigilance database
This is a descriptive study of reports registered in the French pharmacovigilance database (FPVDB). This national database was set up in 1985–1986 to allow online input of spontaneous ADR reports from a network of 31 regional PV centres. Reporting of serious and/or unexpected ADRs has been compulsory for physicians, dentists and midwifes since 1984 and for pharmacists since 1995. Also, nurses (since 1995) and patients, families and associations (since 2011) can contribute on a voluntary basis. PV reports include ADRs following drug use in usual conditions but also medication errors and overdoses.
Spontaneous ADR reports are sent to the regional PV centres mainly by means of a standardized form. Each report is validated and a drug causality assessment is performed by qualified personnel before anonymous storage in the national database.
Database search
For the present study, reports contained in the FPVDB were extracted from January 1 1986 to November 30 2012. To focus on the neonatal population, the database was searched for all notifications with a reported age at the occurrence of the ADR of equal to or less than 30 days, 1 month or 4 weeks. To avoid neonatal ADRs secondary to maternal drug intake, reports classified as related to ‘pregnancy’ or ‘breastfeeding’ were discarded.
Data collection
Data were recorded in four relational tables: ‘report’, ‘drugs’, ‘ADRs’ and ‘medical history’ tables. Thus, a single report can be linked to one or more drugs, ADRs and previous medical history. The ‘report’ table contains information about 1) the notification: date of initial reporting, date of last follow‐up, regional PV centre of registration, type of the reporter, type of the reporter's practice, global seriousness of the ADR report and criteria for seriousness 11 and type of the ADR and 2) the patient: date of birth, reported age at ADR occurrence, gender, date and cause of death when applicable. Patient information was completed by data from the ‘medical history’ table.
For each reported drug, information from the ‘drugs’ table was retrieved: active substance, brand name, dates of beginning of treatment and drug withdrawal, drug indication, frequency and route of administration, role of the drug (suspect, concomitant, interacting) and causality assessment according to the French causality assessment method 12. This is an algorithmic method that combines semiological and chronological criteria to give an ‘intrinsic score’ (0: excluded, 1: doubtful, 2: possible, 3: probable, 4: certain) and then adds bibliographic data from standardized sources to give an ‘extrinsic score’ (0: never described before, 1: not described, 2: not well known, 3: well known). The ‘ADRs’ table includes information on each reported ADR: date of occurrence, date of resolution if applicable, type of the ADR and outcome of the patient.
All medical history, causes of death, drug indications and type of ADRs are coded according to the Medical Dictionary for Regulatory Activities (MedDRA), version 15.1 (http://www.meddra.org/). This dictionary is built as a five‐level scale comprising 26 system organ classes (SOC) divided into high level group terms (HLGT), high level terms (HLT), preferred terms (PT) and finally lowest level terms (LLT). The term often used to describe an ADR in spontaneous reporting systems is the PT because it corresponds to a single medical concept. Anatomical therapeutic chemical (ATC) codes for drugs were computed as the FPVDB does not include this information. Recoding to the ATC coding system was possible for 97.6%, not applicable for 0.5% and not possible for 2.1% of reported drugs. Throughout the paper, the word ‘drug’ will denote the ATC substance level or else as mentioned. Medical history of neonates was classified based on PTs to three major groups: prematurity, infectious disease and genetic and/or congenital abnormality. We excluded from analysis 26 reports (1.5%) where time between occurrence of the reported ADR and date of reporting to the FPVDB had a negative value or a value of more than 10 years. Time between the beginning of drug administration and occurrence of the reported ADR was assessed only for 767 drugs as from the remaining information on dates was missing.
Data analysis
A primary descriptive analysis was performed on the entire dataset between 1986 and 2012. However, during the calendar year 2001, an over‐reporting of ADRs in neonates was observed mainly due to the retrospective reporting of ADRs in relation to antiretroviral therapy. Therefore, a secondary descriptive analysis was performed excluding ADRs reports from that specific year. Results of the primary and secondary analysis were compared.
Descriptive data include absolute numbers (percentages) for categorical variables and medians [1st quartile (Q1)3rd quartile (Q3)] for continuous variables or else as mentioned. To apprehend the impact of impaired medical condition on adverse drug outcomes, differences in mortality between neonates with and those without medical history were tested using the chi‐square test. For the description of drug‐ADR pairs, we only retained suspect and interacting drugs. Analysis was performed with SAS version 9.2 software (SAS Inc, Cary, North Carolina, USA.).
Results
Characteristics of ADR reports
From 1986 to 2012, a total of 2019 notifications were retrieved from the FPVDB. After discarding cases of maternal exposure to drugs during pregnancy and duplicate reports, notifications for 1688 neonates were analyzed. More than half of these reports (995, 59%) were rated as serious. Trends over time in the number and characteristics of notified ADRs are reported in Figure 1 and Table 1, respectively. Taking into account the number of neonates in France (http://www.insee.fr), 778.468 and 792.996 in 1986 and 2011 respectively, the notification rate increased from 0.03 to 0.17 per 1000 neonates. Median age at occurrence of ADR was 9 days and a slight predominance of male neonates was noted (male : female ratio: 1.3). A medical history was specified for 240 (14%) neonates. Among those, 111 presented a preterm birth (46%), 26 an infection (11%) and 50 a genetic and/or congenital abnormality (21%). The median number of reports per regional PV centre was 36 (20.5–48.5). However, two centres accounted for 37% of all reports (320 and 297, respectively) assessed over the study period, whereas the total number of reports for the remaining centres ranged between 5 and 102.
Figure 1.
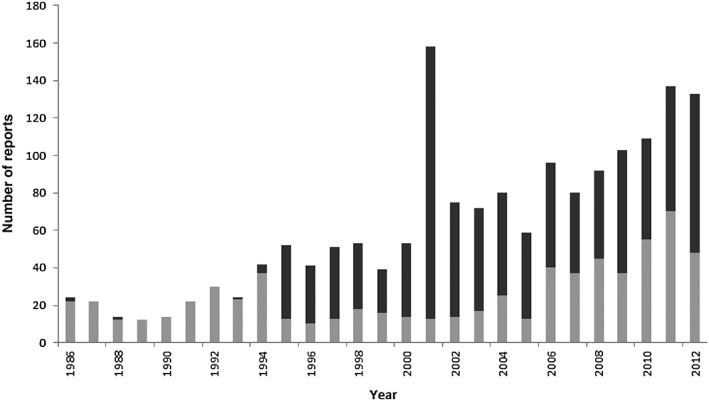
Trends over time in the number of ADR reports in neonates.  number of reports with non‐serious ADRs,
number of reports with non‐serious ADRs,  number of reports with serious ADRs
number of reports with serious ADRs
Table 1.
Characteristics of reports of adverse drug reactions (ADRs) in neonates
| Characteristics of reports (n = 1688) | Median (Q1 ‐ Q3) or number (%) | |
|---|---|---|
| n | % | |
| Gender | ||
| Male | 922 | 55 |
| Female | 735 | 44 |
| Note Reported | 32 | 2 |
| Age (days) | 9 (2‐21) | |
| Serious ADR | ||
| Yes | 995 | 59 |
| Death | 88 | 5 |
| Life‐threatening | 166 | 10 |
| Hospitalization (initial or prolonged) | 632 | 37 |
| Disability | 10 | 1 |
| Other clinically relevant conditions | 99 | 6 |
| No | 693 | 41 |
| Type of reported ADR | ||
| Adverse drug reaction | 1331 | 79 |
| Medical errors‐overdoses | 347 | 20.4 |
| Drug interaction | 10 | 0.6 |
| Type or reporter | ||
| General practitioner | 84 | 5 |
| Medical specialist | 1302 | 77 |
| Pharmacist | 137 | 8 |
| Nurse | 29 | 1.7 |
| Dentist | 2 | 0.1 |
| Other health professional | 37 | 2.2 |
| Non‐health professional | 56 | 3.3 |
| Unknown | 41 | 2.4 |
| Type of practice | ||
| University hospital | 964 | 57 |
| Other hospital | 307 | 18 |
| Private clinic | 8 | 0.5 |
| Private practice | 286 | 17 |
| Other | 31 | 2 |
| Unknown | 92 | 5.5 |
Overall, 88 neonates deceased at a median age of 14 days 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 of life, after median of 1 day (0–9 days) from first reported ADR occurrence and with a predominance of male neonates (ratio 1.5). Approximately 72% (n = 63) of these deaths were related to a reported ADR (not ADR related deaths = 17, missing information = 8). The majority of fatal ADRs were reported by medical specialists (n = 76, 92%). Fatal outcomes were more frequent in neonates with medical history (20/240, 8.3%) than in neonates without (68/1455, 4.7%, P value = 0.02).
Type of ADRs reported
A total of 3127 ADRs were reported (median of one ADR per report [1–3)]) which corresponded to 479 distinct PT codes. Median time between date of reporting and date of occurrence of the ADR was 21 days (6–103). The most commonly reported system organ class was injury, poisoning and procedural complications (n = 492, 16%), followed by general disorders and administration site conditions (n = 391, 12.5%), blood and lymphatic system disorders (n = 373, 12%), gastrointestinal disorders (n = 255, 8.2%), investigations (n = 251, 8%) and nervous system disorders (n = 245, 8%). The other system organ classes were involved less commonly (<8%) (Figure 2). Distribution of fatal and non‐fatal ADRs according to the different system organ classes is shown in Figure 2 and the 10 most commonly reported ADRs are given in Table 2. Regarding outcomes, neonates fully recovered without sequelae in 73%, recovered with sequelae in 6%, were recovering in 2%, deceased in 4% (ADR‐related 97; not‐ADR related 24; undetermined origin 12) and outcome was unknown/not yet established in 15% of ADRs.
Figure 2.
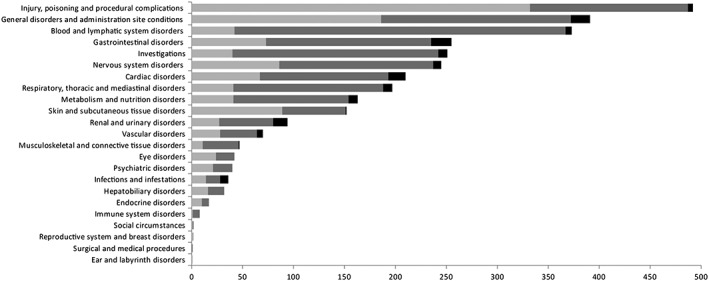
Types of reported ADRs in neonates according to MedDRA system organ class (SOC).  no serious ADRs,
no serious ADRs,  serious, non fatal ADRs,
serious, non fatal ADRs,  serious, fatal ADRs
serious, fatal ADRs
Table 2.
Ten most frequently reported ADRs according to the preferred terms (PTs) of MedDRA coding system
| All ADRs reported†† (n = 3127) | ADRs with serious outcome (n = 1969) | ADRs with fatal outcome (n = 133) | |||
|---|---|---|---|---|---|
| Number | Number | Number | |||
| Medication error | 148 | Neutropenia | 120 | Death† | 11 |
| Neutropenia | 128 | Anaemia | 86 | Renal failure acute | 8 |
| Anaemia | 91 | Blood lactic acid increased | 66 | Sepsis neonatal | 6 |
| No adverse event * | 82 | Bradycardia | 46 | Intestinal perforation | 5 |
| Blood lactic acid increased | 66 | Anaemia macrocytic | 43 | Bradycardia | 4 |
| Bradycardia | 62 | Malaise | 39 | Enterocolitis | 4 |
| Incorrect drug administration rate | 56 | Hypertriglyceridaemia | 33 | Blood creatinine increased | 3 |
| Accidental overdose | 51 | Medication error | 32 | Cardiac failure | 3 |
| Malaise | 47 | Accidental overdose | 31 | Hyperkalaemia | 3 |
| Wrong drug administered | 47 | Hypotonia | 31 | Hypotension | 3 |
| Incorrect dose administered | 47 | ||||
These ADRs correspond to errors of drug administration without clinical consequences
Reaction was coded as such with no further details
In this column 11 ADRs are presented because the last threeADRs were reported in an equal number of reports.
Of note, 14% (437/3127) of the reported ADRs were coded as maladministrations (n = 189, incorrect drug administration rate, wrong drug administered, incorrect drug dosage form administered, etc.) or medication errors (n = 182; drug dispensing errors, drug prescribing errors, etc.) or overdoses (n = 66). Thirty percent of these ADRs (n = 135/437) were serious and they resulted in three neonatal deaths. Also, 3% (82/3127) of the reported ADRs were coded as ‘no adverse event’ or ‘adverse effect absent’ and referred to errors of drug administration without clinical consequences.
Medicinal drugs reported
A total of 2797 drugs were reported (median of one drug per report 1, 2) which corresponded to 446 distinct ATC drug codes (5th level codes, n = 417; 3th or 4th level codes, n = 29; not applicable/missing, n = 3). Median time between the beginning of drug administration and the occurrence of the reported ADR was 7 days (1–21 days). A total of 2238 (80%) drugs was classified as suspect or interacting drugs and 559 (20%) as concomitant. Reported drugs by anatomical main group and role (suspect/interacting or concomitant) in the occurrence of adverse reactions are given in Figure 3. There was no particular trend over time with respect to the main therapeutic classes or the specific drugs involved (data not shown) except for anti‐infectives, mainly antiretroviral molecules, that accounted for the majority of drugs reported in the FPVDB in 2001 (58% of all ATC codes reported that year).
Figure 3.
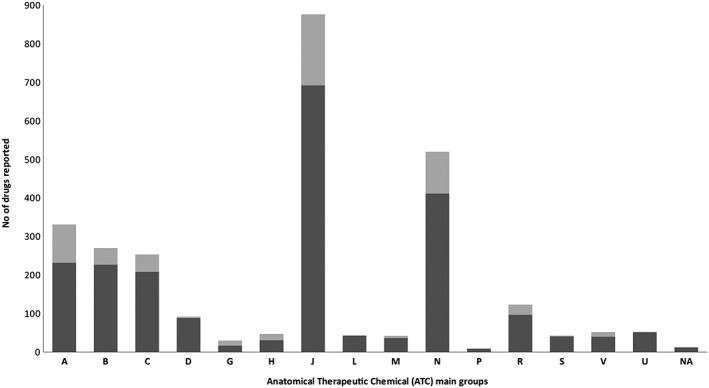
Reported drugs by anatomical main group and role (suspect/interacting or concomitant) in the occurrence of adverse reactions (A) alimentary tract and metabolism, (B) blood and blood forming organs, (C) cardiovascular system, (D) dermatologicals, (G) genito‐urinary system and sex hormones, (H) systemic hormonal preparations, excluding sex hormones and insulins, (J) anti‐infectives for systemic use, (L)antineoplastic and immunomodulating agents, (M) musculo‐skeletal system, (N) nervous system, (P) antiparasitic products, insecticides and repellents, (R) respiratory system, (S) sensory organs, (V) various, (U) unknown (ATC code could not be attributed because the commercial name or active substance of the drug were missing) and (NA) not applicable (no ATC codes attributed to these substances).  suspect/interacting drugs,
suspect/interacting drugs,  concomittant drugs
concomittant drugs
For 90% of the suspect (n = 2215) and interacting (n = 23) drugs the route of administration was reported: 7% topical, 43% enteral, 39% parenteral and <1% other routes. The most commonly reported drugs (>1% of suspect or interacting drugs) are given in Table 3.
Table 3.
Most frequently reported suspect or interacting drugs (n = 2238)
| Drug | Number | % of suspect or interacting drugs (n = 2238) | ATC code |
|---|---|---|---|
| Zidovudine | 217 | 9.7 | J05AF01 |
| Phytomenadione | 106 | 4.7 | B02BA01 |
| Ibuprofen | 73 | 3.2 | C01EB16 |
| Chlorhexidine | 41 | 1.8 | D08AC02 |
| Vancomycine | 38 | 1.7 | J01XA01 |
| Caffeine | 36 | 1.6 | N06 BC01 |
| Ergocalciferol | 35 | 1.6 | A11CC01 |
| Cefotaxime | 35 | 1.6 | J01DD01 |
| Nevirapine | 33 | 1.5 | J05AG01 |
| Combinations of vitamins | 31 | 1.4 | A11JA |
| Lamivudine | 31 | 1.4 | J05AF05 |
| BCG vaccine | 31 | 1.4 | L03AX03 |
| Amikacin | 29 | 1.3 | J01GB06 |
| Amoxicillin | 28 | 1.2 | J01CA04 |
| Stavudine | 27 | 1.2 | J05AF04 |
| Paracetamol | 27 | 1.2 | N02BE01 |
| Midazolam | 27 | 1.2 | N05CD08 |
| Combinations of solutions for parenteral nutrition | 25 | 1.1 | B05BA10 |
| Morphine | 25 | 1.1 | N02AA01 |
| Fentanyl | 23 | 1.0 | N01AH01 |
All other drugs accounted for less than 1% of the reported suspect or interacting drugs.
ATC recoding not possible: n = 51
Use of a total of 1423 suspect/interacting drugs were mentioned in reports classified as serious (n = 996) and the most frequently reported drugs were zidovudine (n = 195, 14%), ibuprofen (n = 65, 5%) and nevirapine (n = 33, 2%). Use of a total of 166 suspect/interacting drugs were mentioned in reports with a fatal outcome (n = 88). Again, three drugs were mainly reported: ibuprofen (n = 19, 11%), zidovudine (n = 7, 4%) and electrolyte solutions (n = 5, 3%).
Analysis of drug–ADR pairs
Overall, 4106 drug–ADR pairs were reported in neonates from 1986 to 2012. The 10 most frequent drug‐ADR pairs are given in Table 4. Two drugs are implicated in eight out of these 10 pairs, zidovudine and phytomenadione (vitamin K1).
Table 4.
Most frequently reported drugs and MedDRA high‐level terms (HLT) pairs (suspect and interacting drugs only)
| Adverse drug reaction | Drug | ATC | Number of reports | % of the reports (n = 1688)* |
|---|---|---|---|---|
| Anaemias | Zidovudine | J05AF01 | 113 | 6.7 |
| Neutropenias | Zidovudine | J05AF01 | 89 | 5.3 |
| Medication errors | Phytomenadione | B02BA01 | 84 | 5.0 |
| Maladministration | Phytomenadione | B02BA01 | 71 | 4.2 |
| Blood gas and acid base analyses | Zidovudine | J05AF01 | 51 | 3.0 |
| Adverse effect absent | Phytomenadione | B02BA01 | 39 | 2.3 |
| Neutropenias | Nevirapine | J05AG01 | 29 | 1.7 |
| Renal failure and impairment | Ibuprofen | C01EB16 | 24 | 1.4 |
| Thrombocytoses | Zidovudine | J05AF01 | 24 | 1.4 |
| Liver function analyses | Zidovudine | J05AF01 | 23 | 1.4 |
Because individual reports may contain more than one active substance – HLT pair, the sum of all percents would be >100%
Intrinsic causality was ‘doubtful’ for 1437 (35%) of drug–ADR pairs, ‘possible’ for 1327 (32%), ‘probable’ for 845 (21%), ‘certain’ for 297 (7%) and not attributed for 200 drug–ADR pairs (5%). Drug–ADR pairs that were given a score of ‘certain’ were mainly phytomenadione (vitamin K1) with maladministration or medication error or no adverse effect (n = 118) and fluconazole with maladministration (n = 8). The drug–ADR pair was ‘never described before’ for 90 (2%), ‘not described’ for 459 (11%), ‘not well known’ for 437 (11%), ‘well known’ for 2103 (51%) and not attributed for 1017 (25%). The substance most frequently implicated in pairs ‘never described before’ was chlorhexidine for ADRs such as erythema, inflammation or non‐therapeutic responses (n = 10).
The three most frequently reported substances by MedDRA SOCs are given in Table 5.
Table 5.
The three most frequently reported drugs per type of ADRs according to MedDRA system organ class (SOCs) (suspect and interacting drugs only)
| SOCs | Total n | Drug | n | Drug | n | Drug | n |
|---|---|---|---|---|---|---|---|
| Blood and lymphatic system disorders | 587 | Zidovudine | 239 | Nevirapine | 62 | Lamivudine | 48 |
| Injury, poisoning and procedural complications | 527 | Phytomenadione | 173 | Chlorhexidine | 31 | Zidovudine | 22 |
| General disorders and administration site conditions | 478 | Phytomenadione | 50 | Chlorhexidine | 24 | Solutions for parenteral nutrition | 17 |
| Investigations | 346 | Zidovudine | 107 | Stavudine | 24 | Nevirapine | 24 |
| Nervous system disorders | 326 | Zidovudine | 19 | Midazolam | 15 | Ergocalciferol | 11 |
| Gastrointestinal disorders | 321 | Ibuprofen | 55 | Zidovudine | 54 | Ergocalciferol | 20 |
| Cardiac disorders | 279 | Diphemanil methylsulfate | 17 | Caffeine | 17 | Combinations of vitamins | 15 |
| Respiratory, thoracic and mediastinal disorders | 268 | Ergocalciferol | 22 | Carbocisteine | 13 | Combinations of vitamins | 11 |
| Metabolism and nutrition disorders | 224 | Zidovudine | 33 | Stavudine | 16 | Nevirapine | 16 |
| Skin and subcutaneous tissue disorders | 190 | Chlorhexidine | 9 | Amoxicillin | 9 | Cefotaxime | 9 |
| Renal and urinary disorders | 136 | Ibuprofen | 24 | Amikacin | 9 | Fentanyl | 9 |
| Vascular disorders | 96 | Midazolam | 6 | Doxapram | 6 | Labetalol | 5 |
| Musculoskeletal and connective tissue disorders | 72 | Sufentanil | 5 | Potassium clorazepate | 5 | Diazepam | 3 |
| Eye disorder | 56 | Oxytetracycline | 12 | Zidovudine | Chlorhexidine | 5 | |
| Hepatobiliary disorders | 54 | Methylthioninium chloride | 4 | Ceftriaxone | 3 | Paracetamol | 3 |
| Infections and infestations | 52 | BCG vaccine | 16 | i.v. solution additives | 6 | Amino acids IV solutions | 3 |
| Psychiatric disorders | 51 | Caffeine | 5 | Zidovudine | 4 | Phytomenadione | 3 |
Remaining SOCs account for less than 50/4106 drug‐ADR pairs
In ADRs coded as maladministration, medication errors or overdose (n = 464 pairs), the most frequently reported drugs were phytomenadione (n = 164, 35%), chlorhexidine (n = 31, 7%), paracetamol (n = 15, 3%), ergocalciferol (n = 14, 3%) and gentamycin (n = 13, 3%). Three neonatal deaths in this category of ADRs were related to digoxin (n = 2) and lopinavir/ritonavir (n = 1) overdoses.
Secondary analysis
The results of the analysis excluding 158 reports (year 2001) were overall comparable with those of the full dataset with regard to the characteristics of the reports, types and frequency of ADRs, therapeutic drug classes and frequency of suspect/interacting drugs reported (data not shown). However, some differences were noted in the nature of the drug–ADR pairs reported. Notably, the association zidovudine–thrombocytoses, zidovudine–blood gas and acid base analyses and nevirapine–neutropenias were less frequent compared with the full dataset analysis. On the contrary, the following associations: ibuprofen intestinal ulcers and perforation, chlorhexidine maladministration, chlorhexidine medical errors, BCG vaccine infections and zidovudine colitis became more frequent (details are given in Appendix).
Discussion
We provide a unique overview of PV reports after direct drug exposure in neonates using national data over a period of 26 years. The number of neonatal reports is steadily increasing parallel to the global increase in PV reporting observed over the years worldwide 9, 10, 13. Although few PV studies have been published in children, all show that a substantial number of ADRs occurs in the first years of life 5, 6, 7, 8, 14, 15, 16, 17, 18. Yet, reporting rates in our study were relatively low compared with previously published data with estimations as high as 0.5% in children 6, 19. Possible explanations comprise either a marginal drug exposure or a more frequent under‐reporting in neonates compared witho older age groups. Most reports concerned male neonates and occurred in the first days of life. Births and hospitalizations in the neonatal intensive care unit (NICU) of male neonates generally exceed those of female ones 20, 21. In our study, fatal outcomes were also more frequent in males, further pointing towards an effect of the gender on the susceptibility to sickness and by extension to ADRs early in life 9, 10.
As sick neonates are mainly cared for in specialized units, the majority of reports were issued by medical specialists from university hospitals. However, the paucity of ADRs reports provided by nurses is striking. Nurses are key caregivers in NICUs and thus their role in neonatal ADR identification and reporting should be enhanced. Conversely, non‐health professionals were very recently endorsed to report in France which explains the paucity of reports provided by them.
Reports were addressed mainly to two out of the 31 regional PV centres. Although clinicians are expected to report to the PV centre of their region, some may forward reports to centres that have a specific expertise in paediatric PV and thereby seek expert guidance. Another explanation may be the exhaustive ADR reporting in the context of a research study. In fact, a substantial number of reports in 2001 were associated with the retrospective assessment of ADRs related to antiretroviral therapy in a single PV centre. This centre has a recognized expertise in antiretroviral drug safety in children 22 and actively participates in a national prospective epidemiological study on the risk and prevention of mother‐to‐child HIV transmission 23.
Neonatal ADRs were mainly classified as serious (59%) compared with previous PV studies with any paediatric age (13%–37%) 6, 8, 19, 24. However, the seriousness was essentially related to hospitalizations (admission or prolonged stay) rather than the occurrence of disability or fatalities. Despite the physiological vulnerability of neonates, fatalities were less frequent in our study (3.7%) compared with data reported in older children (5.6%–7%)8, 15. Even though reassuring, this finding may actually reflect the difficulty of neonatologists in diagnosing serious ADRs in the context of multiple comorbidities such as extreme prematurity. In line with previous studies on medication use in NICUs 20, the high number of drugs reported should also be highlighted especially since many are not licensed for use in neonates 25.
Yet, the importance of our description lies in the fact that it enables comparison with the reaction–drug patterns observed in other age groups to identify safety issues specific to neonates. First, we observed a clear predominance of ADRs related to the use of anti‐infectives. Antiretrovirals have already been incriminated in the occurrence of serious and fatal ADRs in neonates mainly through in utero exposure 9, 10. Our study confirms that direct exposure in early life entails the same risks namely that of zidovudine, which is the recommended first line antiretroviral to prevent mother‐to‐child HIV transmission in France 26. Antibiotics were also frequently reported in various neonatal ADRs. Although, anti‐infectives are prescribed in NICUs to treat or prevent severe, life‐threatening conditions, their toxicity profile should urge neonatologists to evaluate systematically the risk–benefit ratio of their use or alternatively, to define adequate conditions of exposure and monitoring.
Second, one out of five ADRs was reported in terms of incorrect drug administration under the ‘injury, poisoning and procedural complications’ SOC. Drug formulations are often not adapted to the youngest,.Subsequently administration and dosage errors are frequent in NICUs 27, 28, 29. On the one hand, these errors occurred in the hospital setting and led to serious consequences as in the case of electrolyte solutions 30. On the other hand, a significant number of errors occurred in the outpatient environment related to the administration of vitamins or antiseptic preparations such as chlorhexidine. In France, prescription of oral phytomenadione is common in exclusively breastfed babies and errors are frequent as some parents tend to give it once daily instead of once weekly 31. These ADRs led to mild adverse outcomes or had no clinical consequences. However, they draw attention to the difficulties that parents might face when administering a drug to their newborn and, therefore, the importance of appropriate education and training. In France, two ‘dear doctor’ letters were issued to limit administration errors for phytomenadione in 2008 31 and for ergocalciferol in 2011 32.
Third, our data show that intake of vitamins or solutions of parenteral nutrition such as amino acids, was not devoid of risk. Their use was related to a variety of ADRs, including infections and cardiac disorders and this should warrant their cautious use and administration in neonates especially in preterms. Likewise, use of nervous system drugs such as caffeine, midazolam or morphine was frequently incriminated. These drugs are part of the essential bulk of NICU medications although there is limited evidence on their adequate use which probably exposes neonates to substantial safety risks.
Finally, toxicity of ibuprofen, in particular renal and gastrointestinal, in neonates has been clearly illustrated. The number, type and severity of ibuprofen‐related ADRs described in our study are not predominant in older children 9. The main clinical indication of ibuprofen in neonates is the closure of patent ductus arteriosus and, currently, there is no validated therapeutic alternative 33. In this context, the only possible improvement in terms of safety would be to optimize ibuprofen dosing schemes through pharmacokinetic/pharmacodynamic modelling but very few studies have undertaken this task 34.
Our results should be viewed in light of the known limitations of spontaneous reporting databases 35. Important information such as the term of birth or medical history may not have been exhaustively reported because this information often lies in free‐text fields and is not captured when analyzing standard fields of generic PV forms. Several data were often missing such as date of treatment initiation and/or discontinuation or drug dose and frequency of administration, which may be related to incomplete reporting but also errors during data capture and transfer. Yet, compared with previous paediatric PV studies 5, rates of missing data were very low in the FPVDB, only 2.1% for drugs and 0.01% for ADRs.
Notwithstanding these limitations, spontaneous reporting databases are currently the most valuable source of drug safety information in neonates 30, 36, 37. Development of a paediatric PV expertise and interactions with specialised pharmacologists in PV centres should be further encouraged in order to address the need to increase physicians' and nurses' awareness and to train them to diagnose and report better ADRs in neonates. To enhance further collection of informative reporting, another proposition would be the elaboration of a specific neonatal PV report comprising fields of information which are currently absent in the generic PV form but are essential to the drug–event causality assessment with respect to neonatal specificities.
In conclusion, this is a unique overview of neonatal ADRs after direct drug exposure. Reporting of ADRs in neonates is increasing. The majority of reports are classified as serious and a subsequent proportion is associated with drug administration errors. Anti‐infective medicines, mainly antiretroviral therapy, account for the majority of reported ADRs and ADR fatalities. Although based on national data, our results inform clinicians and stakeholders about the safety of drug use in neonates and the challenges of neonatal pharmacovigilance.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
The French Medicines Agency (ANSM) provided the data and approved the conduct of the study but was not involved in data analysis and elaboration of this publication. The sole responsibility for the content of this publication lies with the authors.
This analysis of the French pharmacovigilance database was made possible thanks to the work of the 31 regional pharmacovigilance centres and the French Medicines Agency (ANSM). The authors acknowledge the support and assistance of Professor Elisabeth Autret‐Leca, Department of Clinical Pharmacology Regional Centre of Pharmacovigilance of Tours and Mrs Geneviève Brizion, Centre de Documentation Médico‐Pharmaceutique, AGEPS.
Funding
No sources of funding were used to assist in the preparation of this study and the drafting of the article.
Contributors
FK designed the study, conducted data analysis and interpretation of results. FB‐S, APJ‐B and EJ‐A contributed to data analysis and interpretation. The manuscript was initially drafted by FK and was critically reviewed and subsequently approved by each co‐author in its final form.
Most frequently reported drugs and MedDRA high‐level terms (HLT) pairs (suspect and interacting drugs only) after exclusion of calendar year 2001
| Adverse drug reaction | Drug | ATC | Number of reports | % of the reports (n = 1688)* |
|---|---|---|---|---|
| Medication errors NEC | Phytomenadione | B02BA01 | 84 | 5.0 |
| Maladministration | Phytomenadione | B02BA01 | 71 | 4.2 |
| Anaemias NEC | Zidovudine | J05AF01 | 60 | 3.6 |
| Adverse effect absent | Phytomenadione | B02BA01 | 39 | 2.3 |
| Neutropenias | Zidovudine | J05AF01 | 31 | 1.8 |
| Renal failure and impairment | Ibuprofen | C01EB16 | 24 | 1.4 |
| Intestinal ulcers and perforation NEC | Ibuprofen | C01EB16 | 20 | 1.2 |
| Maladministration | Chlorhexidine | D08AC02 | 17 | 1.0 |
| Infections NEC | BCG vaccine | L03AX03 | 16 | 0.9 |
| Colitis (excluding infective) | Zidovudine | J05AF01 | 15 | 0.9 |
Because individual reports may contain more than one active substance – HLT pair, the sum of all percents would be >100%
NEC not elsewhere classified.
Kaguelidou, F. , Beau‐Salinas, F. , Jonville‐Bera, A. P. , and Jacqz‐Aigrain, E. (2016) Neonatal adverse drug reactions: an analysis of reports to the French pharmacovigilance database. Br J Clin Pharmacol, 82: 1058–1068. doi: 10.1111/bcp.13034.
References
- 1. Kearns GL, Abdel‐Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology‐‐drug disposition, action, and therapy in infants and children. N Engl J Med 2003; 349: 1157–67. [DOI] [PubMed] [Google Scholar]
- 2. Conroy S, McIntyre J, Choonara I. Unlicensed and off label drug use in neonates. Arch Dis Child 1999; 80: F142–F144 ; discussion F4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. 't Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Anker JN. A survey of the use of off‐label and unlicensed drugs in a Dutch children's hospital. Pediatrics 2001; 108: 1089–93. [DOI] [PubMed] [Google Scholar]
- 4. Lass J, Kaar R, Jogi K, Varendi H, Metsvaht T, Lutsar I. Drug utilisation pattern and off‐label use of medicines in Estonian neonatal units. Eur J Clin Pharmacol 2011; 67: 1263–71. [DOI] [PubMed] [Google Scholar]
- 5. Aagaard L, Weber CB, Hansen EH. Adverse drug reactions in the paediatric population in Denmark: a retrospective analysis of reports made to the Danish Medicines Agency from 1998 to 2007. Drug Saf 2010; 33: 327–339 .Epub 2010/03/20 [DOI] [PubMed] [Google Scholar]
- 6. Kimland E, Rane A, Ufer M, Panagiotidis G. Paediatric adverse drug reactions reported in Sweden from 1987 to 2001. Pharmacoepidemiol Drug Saf 2005; 14: 493–9. [DOI] [PubMed] [Google Scholar]
- 7. Moore TJ, Weiss SR, Kaplan S, Blaisdell CJ. Reported adverse drug events in infants and children under 2 years of age. Pediatrics 2002; 110: e53. [DOI] [PubMed] [Google Scholar]
- 8. Aldea A, Garcia Sanchez‐Colomer M, Fernandez Quintana E, Garcia SM. Paediatric adverse drug reactions reported to the Spanish Pharmacovigilance System from 2004 to 2009. Eur J Clin Pharmacol 2012; 68: 1329–38. [DOI] [PubMed] [Google Scholar]
- 9. de Bie S, Ferrajolo C, Straus SM, Verhamme KM, Bonhoeffer J, Wong IC, et al. Pediatric Drug Safety Surveillance in FDA‐AERS: A Description of Adverse Events from GRiP Project. PLoS One 2015; 10: e0130399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Star K, Noren GN, Nordin K, Edwards IR. Suspected adverse drug reactions reported for children worldwide: an exploratory study using VigiBase. Drug Saf 2011; 34: 415–28. [DOI] [PubMed] [Google Scholar]
- 11. EMA . 1995. Note for Guidance on clinical Safety Data Management: Definitions and standards for expedited reporting [online]. (CPMP/ICH/377/95) Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002749.pdf (accessed 16 April 2015).
- 12. Begaud B, Evreux JC, Jouglard J, Lagier G. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 1985; 40: 111–8 . Imputabilite des effets inattendus ou toxiques des medicaments. Actualisation de la methode utilisee en France. [PubMed] [Google Scholar]
- 13. Thiessard F, Roux E, Miremont‐Salame G, Fourrier‐Reglat A, Haramburu F, Tubert‐Bitter P, et al. Trends in spontaneous adverse drug reaction reports to the French pharmacovigilance system (1986‐2001). Drug Saf 2005; 28: 731–40. [DOI] [PubMed] [Google Scholar]
- 14. Barzaga Arencibia Z, Lopez Leyva A, Mejias Pena Y, Gonzalez Reyes AR, Fernandez Manzano E, Choonara I. Pharmacovigilance in children in Camaguey Province, Cuba. Eur J Clin Pharmacol 2012; 68: 1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carleton BC, Smith MA, Gelin MN, Heathcote SC. Paediatric adverse drug reaction reporting: understanding and future directions. Can J Clin Pharmacol 2007; 14: e45‐57. [PubMed]
- 16. Hawcutt DB, Mainie P, Riordan A, Smyth RL, Pirmohamed M. Reported paediatric adverse drug reactions in the UK 2000‐2009. Br J Clin Pharmacol 2012; 73: 437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morales‐Olivas FJ, Martinez‐Mir I, Ferrer JM, Rubio E, Palop V. Adverse drug reactions in children reported by means of the yellow card in Spain. J Clin Epidemiol 2000; 53: 1076–80. [DOI] [PubMed] [Google Scholar]
- 18. Ufer M, Kimland E, Bergman U. Adverse drug reactions and off‐label prescribing for paediatric outpatients: a one‐year survey of spontaneous reports in Sweden. Pharmacoepidemiol Drug Saf 2004; 13: 147–52. [DOI] [PubMed] [Google Scholar]
- 19. Aagaard L, Christensen A, Hansen EH. Information about adverse drug reactions reported in children: a qualitative review of empirical studies. Br J Clin Pharmacol 2010; 70: 481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 2006; 117: 1979–87. [DOI] [PubMed] [Google Scholar]
- 21. Jacobs A. Different sex ratios at birth in Europe and North America. Does it matter? BMJ 2002; 325: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet 1999; 354: 1084–89. [DOI] [PubMed] [Google Scholar]
- 23. Barret B, Tardieu M, Rustin P, Lacroix C, Chabrol B, Desguerre I, et al. Persistent mitochondrial dysfunction in HIV‐1‐exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS 2003; 17: 1769–85. [DOI] [PubMed] [Google Scholar]
- 24. Wallerstedt SM, Brunlof G, Sundstrom A. Rates of spontaneous reports of adverse drug reactions for drugs reported in children: a cross‐sectional study with data from the Swedish adverse drug reaction database and the Swedish Prescribed Drug Register. Drug Saf 2011; 34: 669–82. [DOI] [PubMed] [Google Scholar]
- 25. Kimland E, Odlind V. Off‐label drug use in pediatric patients. Clin Pharmacol Ther 2012; 91: 796–801. [DOI] [PubMed] [Google Scholar]
- 26. Prise en charge médicale des personnes vivant avec le VIH. Recommandations du groupe d'experts. Rapport, 2013. [online]. Available from: http://social‐sante.gouv.fr/ministere/documentation‐et‐publications‐officielles/rapports/sante/article/rapport‐2013‐sur‐la‐prise‐en‐charge‐medicale‐des‐personnes‐vivant‐avec‐le‐vih (accessed 16 May 2016).
- 27. Ligi I, Arnaud F, Jouve E, Tardieu S, Sambuc R, Simeoni U. Iatrogenic events in admitted neonates: a prospective cohort study. Lancet 2008; 371: 404–10. [DOI] [PubMed] [Google Scholar]
- 28. Chedoe I, Molendijk HA, Dittrich ST, Jansman FG, Harting JW, Brouwers JR, et al. Incidence and nature of medication errors in neonatal intensive care with strategies to improve safety: a review of the current literature. Drug Saf 2007; 30: 503–13. [DOI] [PubMed] [Google Scholar]
- 29. Wong IC, Ghaleb MA, Franklin BD, Barber N. Incidence and nature of dosing errors in paediatric medications: a systematic review. Drug Saf 2004; 27: 661–70. [DOI] [PubMed] [Google Scholar]
- 30. Star K, Edwards IR. Pharmacovigilance for children's sake. Drug Saf 2014; 37: 91–8. [DOI] [PubMed] [Google Scholar]
- 31. Information importante sur le bon usage de la spécialité VITAMINE K1 ROCHE 2 mg/0,2 ml NOURRISSONS, solution buvable et injectable ‐ Lettre aux professionnels de santé. ANSM (30/06/2008) [online]. Available at: http://ansm.sante.fr/S‐informer/Informations‐de‐securite‐Lettres‐aux‐professionnels‐de‐sante/Information‐importante‐sur‐le‐bon‐usage‐de‐la‐specialite‐VITAMINE‐K1‐ROCHE‐2‐mg‐0‐2‐ml‐NOURRISSONS‐solution‐buvable‐et‐injectable/%28language%29/fre‐FR (accessed 19 February 2016).
- 32. Uvestérol Vitaminé ADEC et Uvestérol D 1500UI/mL: rappel des recommandations d'administration ‐ Lettre aux professionnels de santé. ANSM (18/03/2011) [online]. Available at: http://ansm.sante.fr/S‐informer/Informations‐de‐securite‐Lettres‐aux‐professionnels‐de‐sante/Uvesterol‐Vitamine‐ADEC‐et‐Uvesterol‐D‐1500UI‐mL‐rappel‐des‐recommandations‐d‐administration‐Lettre‐aux‐professionnels‐de‐sante/%28language%29/fre‐FR (accessed 19 February 2016).
- 33. DeMauro SB, Wright CJ. Acetaminophen: a possible alternative to ibuprofen in patent ductus arteriosus closure. J Pediatr 2014; 165: 209–10. [DOI] [PubMed] [Google Scholar]
- 34. Hirt D, Van Overmeire B, Treluyer JM, Langhendries JP, Marguglio A, Eisinger MJ, et al. An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study. Br J Clin Pharmacol 2008; 65: 629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dal Pan GJ, Lindquist M, Gelperin K. Postmarketing Spontaneous Pharmacovigilance Reporting Systems In: Pharmacoepidemiology, 5th edn, eds Strom BL, Kimmel SE, Hennessy S. Oxford, UK: Wiley‐Blackwell, 2012. [Google Scholar]
- 36. Fabiano V, Mameli C, Zuccotti GV. Adverse drug reactions in newborns, infants and toddlers: pediatric pharmacovigilance between present and future. Expert Opin Drug Saf 2012; 11: 95–105. [DOI] [PubMed] [Google Scholar]
- 37. Hawcutt DB, O'Connor O, Turner MA. Adverse drug reactions in neonates: could we be documenting more? Expert Rev Clin Pharmacol 2014; 7: 807–20. [DOI] [PubMed] [Google Scholar]


