Abstract
Aims
Stabilization of anticoagulation control is seminal to reducing the risk of adverse effects of vitamin K antagonists. Reliable information on how ageing influences this is lacking. We set out to assess the true age‐related changes in anticoagulation control, how gender and patient setting influence this, and the possible implications of these for patient outcomes and management.
Methods
In atrial fibrillation (AF) patients of a unified anticoagulant service monitoring patients in general practice or hospital‐based clinics and housebound patients at home, international normalized ratio (INR) and warfarin dose data between 2000 and 2013 were extracted via the DAWN dosing program. Anticoagulation control was assessed by calculating percentage time spent within target INR (TTR).
Results
A total of 2094 AF patients [938 (44.8%) in general practice (GP) and 531 (25.4%) in hospital (H)‐based clinics and 625 (29.8%) through the domiciliary service (D)] were evaluated. The frequency of warfarin dose changes and INR monitoring events declined until about age 67, then increased as patients got older. The TTR according to age was significantly lower and the probability of having a TTR ≤65% according to age was higher for D than for H and GP, and females had a greater probability of having a TTR ≤65% than age‐matched males.
Conclusion
Identification of factors underlying poorer anticoagulation control in older housebound patients and the introduction of effective modifications to improve the clinical effectiveness of anticoagulation in such patients is needed.
Keywords: ageing, anticoagulation, atrial fibrillation, stroke, TTR, warfarin
What is Already Known about this Subject
Cross‐sectional studies suggest that older age and male gender are associated with better stability of anticoagulation with vitamin K antagonists, and domiciliary monitored patients have poorer control than clinic attenders.
What this Study Adds
Anticoagulation control shows a biphasic relationship with age, peaking at 67 years, is poorer in females than in males, and patients monitored at home due to dependency and immobility than those attending clinics.
Exploration of modifiable factors affecting anticoagulation control in heterogeneous populations is warranted to optimize outcomes.
Introduction
Time in therapeutic INR range (TTR) 1 is an important quality measure of anticoagulation control with vitamin K antagonists (VKAs). Optimizing anticoagulation control is important as TTR correlates inversely with bleeding and thromboembolic complications 2, 3.
In an earlier cross‐sectional study we established that housebound AF patients requiring domiciliary monitoring of INR due to a high level of dependency and immobility have poorer anticoagulation control than those attending hospital‐ or GP‐based clinics 4. Anticoagulation control was also poorest in the oldest patients, which may explain their higher risk of warfarin‐related complications than the more independent patients who attend clinic for monitoring 5. Cross‐sectional studies, however, are limited by the design, providing only a snapshot of the outcome and the characteristics associated with it at a specific point in time and, as such, it is impossible to infer causality. Only a longitudinal study design can identify true age‐related changes in anticoagulation control, and their possible implications for treatment outcomes. We therefore set out to investigate the impact of ageing, longitudinally, on anticoagulant control in patients with atrial fibrillation on warfarin therapy, and the extent to which gender and different patient settings of monitoring influence this. In patients being managed in a standard way by staff of the unified Newcastle upon Tyne Hospitals Trust Anticoagulation Monitoring Service, for whom dosing and testing is guided by the DAWN computer dosing program (version 6.10, Milnthorpe, Cumbria, UK) 6, we audited INR and dosage data for mobile patients who attended either the hospital‐ or GP‐based clinic based on personal preference, and for patients housebound by physical dependency or limited mobility who were monitored through the domiciliary service, whereby trained staff visited them at their place of residence for venous INR checks.
Methods
This study involved the audit of anonymized anticoagulant control data held within the secondary healthcare Trust providing and managing the monitoring service and as such it was deemed not to require prior institutional board approval. Inclusion criteria were to have AF with a target INR of 2.0–3.0 and to have been on warfarin for at least 5 years, after excluding a six‐month initial stabilization period. Only patients anticoagulated for stroke and systemic embolism prevention in AF were selected in order to reduce bias related to different indications or target INR ranges.
As part of the unified service, all patients prior to commencement of therapy received a standard 2–3 hour education session led by either a doctor or trained nurse at their local general practice, according to the UK National Patient Safety Agency (NPSA) educational material 7. Patients were taught about atrial fibrillation and the clinical benefits and risks of anticoagulation. Information was given about the pharmacology of warfarin, and factors that affect the INR, particularly adherence, drug interactions and diet. Written information and a modified educational session were delivered to patients entering the domiciliary service. For clinic‐attending patients, at each monitoring visit, potential reasons for any deviation outside the target range is discussed, and education about these and the importance of good adherence stressed where appropriate. For domiciliary patients, this is done when the result from the venous INR sample is available either by phone or at the next monitoring visit.
Data mining was facilitated by DAWN which allowed extraction of information on individual patients, including a DAWN coded patient ID (in order to preserve patient anonymity for data analysis), age, sex, indication for anticoagulation therapy, target INR range, date commencing warfarin treatment and warfarin starting dose, duration of warfarin therapy, mean yearly warfarin dose, yearly number of INR monitoring events and warfarin dose changes. Information on co‐morbidity and concurrent therapy was not available. Between 2000 and 2013, based on the study inclusion criteria, 1490 AF patients starting warfarin therapy accessing hospital and general practice clinic and 627 patients using the domiciliary service were identified. Of these patients 23 were identified as having switched setting during the course of warfarin therapy with ten switching from hospital to domiciliary, seven from hospital to GP, five from GP to hospital and one from GP to domiciliary monitoring. The 23 patients were subsequently omitted from further analysis. TTR and time spent below and above the therapeutic INR range were established using the linear extrapolation method of Rosendaal et al. 1. Time in therapeutic range is the estimated total percentage of time that the INR is within a predetermined therapeutic range which, for AF patients, is between 2.0 and 3.0, with time above and below being estimated as total percentage time above an INR of 3.0 and below an INR of 2.0 respectively.
The INR at both hospital and GP clinics was determined using the KC1 capillary technique according to the manufacturer's instruction (Trinity Biotech, Bray, Eire). For home monitored patients venous INR was determined by Instrument Laboratories (their machine and reagents) IL (UK) Ltd, Warrington, Cheshire. Internal quality check was performed daily and external quality assurance undertaken monthly through National External Quality Assessment Service (NEQAS), Sheffield, UK.
Statistical analysis
The mean of the individual variables was determined according to each year of age. On examination of the data it was clear that INR control was less good in the youngest and oldest patients. We decided to fit a quadratic model with age to the means to describe this effect in a fairly simple way and to examine how the covariates (setting and gender) affected this model. The numbers of warfarin dose changes were transformed into their square roots to approach normality. Quadratic (curvilinear) and linear regression models were used to examine the effect of age for all the variables tested. Weighted analysis was used in all statistical calculations because of the large variation in sample sizes across age groups. Individual observations were also analysed using random effects to take account of the longitudinal nature of the data. This form of analysis was more complex due to the non‐normal nature of the data. The same conclusions were obtained and so the analysis of means is presented here, as the interpretation of the analysis is much more straightforward. Data were analysed using Minitab statistical software (version 17).
Results
Data on 2094 AF patients [938 (44.8%) in general practice (GP) and 531 (25.4%) in hospital (H)‐based clinics and 625 (29.8%) through the domiciliary service (D); altogether 891 (43%) females, and 1203 (57%) males] on warfarin therapy for 5–14 years, extracted from DAWN software were analysed, which constituted a total of 16 604 patient‐years of INR monitoring. Demographic data according to patients' gender, age and setting are shown in Table 1. As over 99% of the study population were white Caucasians, no separate analysis by race or ethnicity was possible. The number of patients for every year of age for the whole population, patients monitored in clinic (hospital and GP) and those monitored at home are shown in Figure 1.
Table 1.
Patients' demographic data
| Home | GP and hospital | Total | |
|---|---|---|---|
| Male N (%) | 259 (22) | 944 (78) | 1203 |
| * Age mean (SD) | 74 (7) | 67 (9) | |
| Female N (%) | 336 (41) | 525 (59) | 891 |
| * Age mean (SD) | 76 (8) | 70 (9) | |
| Total N (%) | 625 (30) | 1469 (70) | 2094 |
Age at 6 months after starting warfarin therapy.
Figure 1.
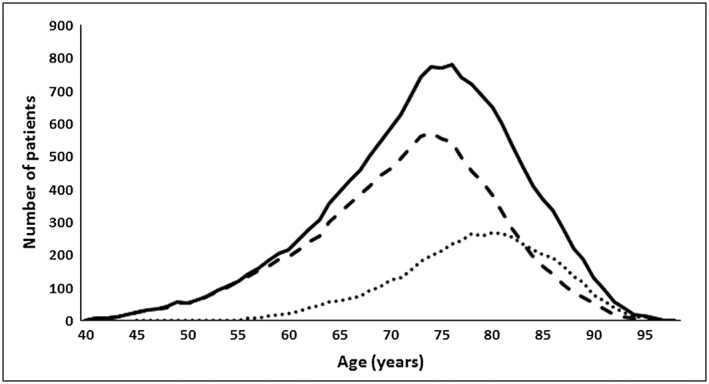
Number of patients by age.  All patients,
All patients,  Clinic‐based patients,
Clinic‐based patients, 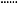 Home‐bound patients
Home‐bound patients
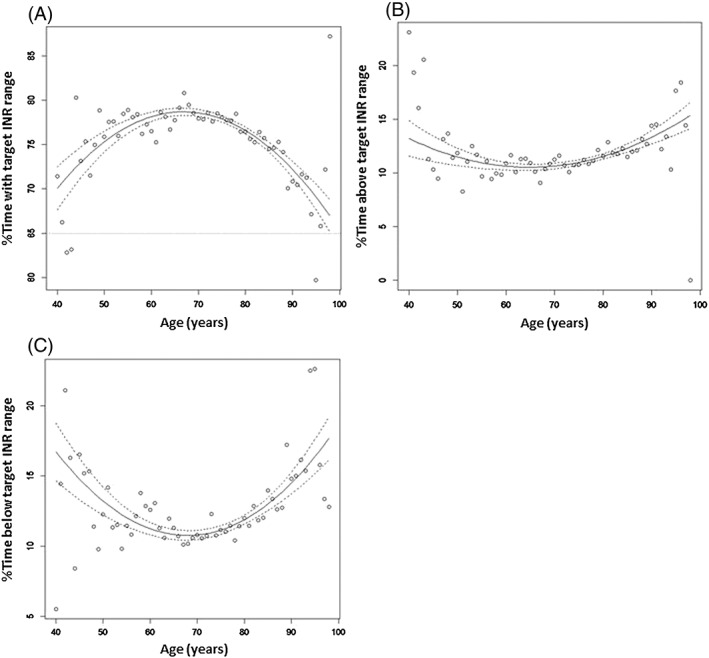
There was a significant relationship between TTR, time spent above and below target INR range and age for the whole population (P < 0.0001 in each case; quadratic regression analysis) as shown in Figure 2. For the whole patient population, age accounted for 70%, 48% and 53% of the variability in mean TTR, time spent above and below target INR range, respectively. TTR showed a biphasic relationship with age, increasing to about 67 years of age, declining thereafter. Unsurprisingly, time spent above and below the target INR range showed the opposite pattern (Figure 2). This relationship held when considering patient setting; thus a significant relationship was found between mean TTR, time spent above and below therapeutic range INR range and age for both home‐monitored patients (P < 0.001, R 2 = 0.52; P = 0.001, R 2 = 0.24; P < 0.001, R 2 = 0.36, respectively) and clinic‐monitored patients (P < 0.001, R 2 = 0.36; P = 0.009, R 2 = 0.16; P = 0.001, R 2 = 0.21, respectively) (Figure 3). Only a small proportion of the domiciliary monitored patients were in 24‐hour care with no difference noted in their TTR compared with the cohort as a whole.
Figure 2.
(a) TTR, (b) time above therapeutic INR range and (c) time below therapeutic INR range for the whole patient population. The solid lines are the fitted curves and the dashed lines are 95% confidence limits based on the observed sample sizes
Figure 3.
Time spent within target INR range for (a) home‐based and (b) clinic‐based patients; time spent above target INR range for (c) home‐based and (d) clinic‐based patients; time spent below target INR range for (e) home‐based and (f) clinic‐based patients. The solid lines are the fitted curves and the dashed lines are 95% confidence limits based on the observed sample sizes
Mean TTR (determined by age) was significantly lower (P < 0.001) and the mean time spent below therapeutic INR range significantly higher (P < 0.05), for patients monitored at home than for patients monitored at hospital or in general practice clinics (Figure 3). There was no significant difference in mean time spent above target INR range between patients monitored at home and those monitored in clinic.
In females the mean TTR was marginally lower [by 1.3% (P < 0.001)] and the mean time below range marginally higher [by 1.0% (P < 0.001)] than those in males (Figure 4). Sex had no significant effect on mean time spent above target INR range.
Figure 4.
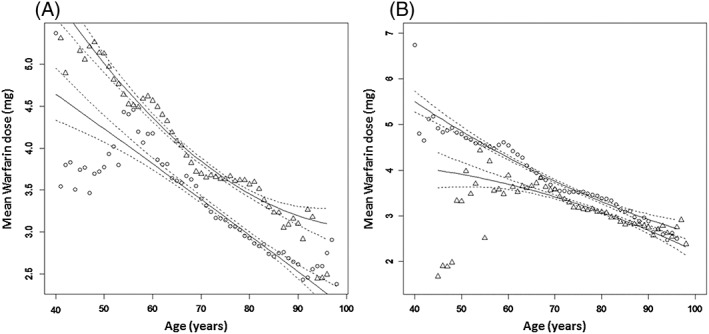
(a) Mean warfarin dose in females (circles) and males (triangles) with lines of best fit (solid lines) and 95% confidence limits (dashed lines); (b) Mean warfarin dose in clinic‐monitored (circles) and home‐monitored patients (triangles) with lines of best fit (solid lines) and 95% confidence limits (dashed lines)
For the whole patient population both the number of dose changes (as mean square root) (P < 0.0001, R 2 = 0.65) and the mean number of INR monitoring events (P = 0.0001, R 2 = 0.44; quadratic regression) were significantly related to age. The number of warfarin dose changes and INR monitoring events were shown to decline until about age 67 years and then increase as patients got older. Similar findings were also noted for both home‐monitored patients (square root of number of warfarin dose changes: P < 0.001, R 2 = 0.40; mean number of INR monitoring events: P < 0.001, R 2 = 0.31) and clinic‐monitored patients (square root of number of dose changes: P < 0.001, R 2 = 0.31; square root of the number of INR monitoring event: P = 0.005, R 2 = 0.17). The home‐bound patients had a higher number of warfarin dose changes and INR monitoring events compared to clinic‐monitored patients for all ages, with the difference increasing with increasing age.
There was a strong and highly significant negative relationship between mean TTR and both the mean number of warfarin dose changes and INR monitoring events (determined as the square root) (P < 0.0001, R 2 = 0.70 and P < 0.0001, R 2 = 0.61, respectively; linear regression). Although the general pattern did not differ between the sexes, females had a higher age‐adjusted frequency of dose changes and monitoring events [by 0.15 for the square root of the number of warfarin dose changes (P < 0.001) and by 1.1 for the square root of the number of monitoring events (P < 0.001)]. Age accounted for 97% of the variability in mean warfarin dose requirement, which fell with increasing age (P < 0.0001; quadratic regression). Females required significantly lower warfarin doses (P < 0.001) than their male counterparts, reflecting a smaller body weight (Figure 4a). Similarly, home monitored patients needed significantly lower warfarin doses compared to clinic monitored patients. However, the size of the difference in warfarin dose requirements between the two groups fell with increasing age (Figure 4b).
A logistic regression analysis was deployed to evaluate the probability of having a TTR of ≤65% according to age. A quadratic regression model was found to best fit the data (P < 0.001). According to the model, home‐monitored patients had a higher probability of having a TTR ≤65% (P < 0.001), compared to clinic‐monitored patients (Figure 5a), as did females compared to age‐matched males (P < 0.001) (Figure 5b).
Figure 5.
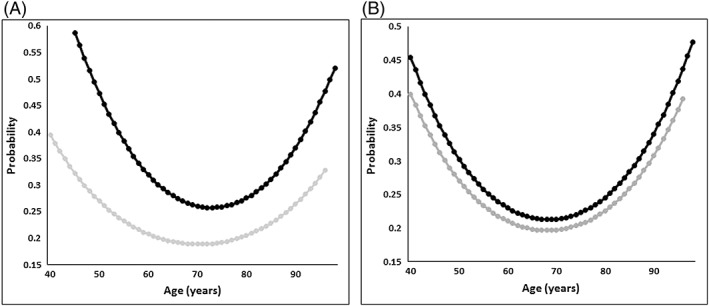
Probability of TTR ≤65% by (a) patient setting ( Home‐bound patients,
Home‐bound patients,  Clinic‐based patients) and (b) sex (
Clinic‐based patients) and (b) sex ( Males,
Males,  Females)
Females)
Discussion
In spite of evidence to support use of oral anticoagulants to prevent stroke and systemic embolism in patients with AF 8, 9, 10 and the high prevalence of AF of >5% in patients over 70 year olds and >10% in patients over 85 year olds, many patients do not get optimal prophylaxis 11, 12, partly because of concerns about the risk of bleeding. Benefit from VKA therapy is greatest for patients remaining within their target therapeutic range; bleeding risk is increased when INR exceeds target range and thromboembolic events increase when INR is below target range. Stable anticoagulation in patients receiving VKA is influenced by anticoagulant service provision, with patients in Europe and the UK having better INR control than those managed in North America 13 and patients managed by individual clinicians having poorer control than cohorts managed in anticoagulant clinics 13. The anticoagulated patients in our area were well managed with a median TTR value of 78.6% at 69 years and 75.8% at 74 years, compatible with values reported in Sweden which has a similar anticoagulant management system to the UK 14.
A comprehensive examination of patient‐level characteristics predicting TTR in a veteran population reported that older age was associated with better TTR, although the population studied differed considerably from ours, as it had an upper age band of ≥75, lower average TTR of 61%, a higher mental and physical health burden and only 2.7% were female 15. Through our large dataset which allowed a detailed examination of ageing effect, we found that the likelihood of having a TTR ≤65% increases over the age of 70 years and that not only is anticoagulation control better overall in clinic attending than in domiciliary‐monitored patients, but that in the latter, anticoagulation control declines more rapidly with age. This is in spite of more warfarin dose changes and INR monitoring events, and a greater age‐related increase in these in the home‐monitored patients compared to clinic‐monitored patients, in an attempt to improve control. This indicates the importance of the need for ongoing evaluation of patients once a decision to anticoagulate with a VKA is made.
Similar to previous reports 16, 17, 18, we found that patients at a younger age (below 60 years) had poorer anticoagulation control compared to older patients. Possible explanations include poor adherence, social factors such as employment and alcohol use, and clinical factors as additional clinical morbidities will have been required to justify anticoagulation given their age.
The 1.3% lower age‐adjusted TTR of women, in spite of more dose changes and monitoring events, is consistent with other previous studies reporting on the predictors of anticoagulation control 15, 16. Poor and erratic dietary vitamin K intake in women has been suggested as one possible explanation for their poorer anticoagulation 17. The greater proportion of women than men in our domiciliary‐monitored group (59% vs. 41%) compared to the clinic‐monitored group (36% vs. 64%) may be one factor contributing to the difference in TTR between them.
Although identifying modifiable factors contributing to anticoagulation control is required for improving care of patients receiving VKAs, the evidence base is very limited. Whilst taking more than 16 medications and four or more hospitalizations are associated with erratic patterns of INR control, predictors like cancer and dementia are associated with directional poor control 19. In our study domiciliary monitoring and female gender were associated with more time spent below target range, which could be due to patient factors, especially poor adherence. Whilst dosing decisions were computer driven, and adhered to in the vast majority of monitoring events, staff could override these based on clinical circumstances which might also have potentially influenced outcomes.
In a French study of patients over 80 years old, in rehabilitation or institutionalized care, poorer anticoagulation control was associated with being in hospital, antibiotic use and falls 20. The conclusions from that study – that frequent falls may be a marker of frailty and sacropenia, and antibiotic use a marker of acute illness which may result in deterioration in chronic co‐morbidities and changes in drug use, leading to INR instability – may also be relevant to the steeper decline in anticoagulation control observed in our domiciliary group. Whilst our ambulatory patients who attend clinics are a less dependent population, in our previous work in a cross‐section of this population 4 we noted no significant difference in either the number of drugs taken (five cardiovascular medications including warfarin), or the number of chronic co‐morbidities (two cardiovascular diseases including AF plus one other), between the groups, perhaps because warfarin is discontinued for more dependent patients as the risk/benefit balance changes. This suggests that the poorer stability of anticoagulation noted in the domiciliary‐monitored patients in both our cross‐sectional and longitudinal study of similar populations is the result of diverse factors which may include dietary intake of vitamin K, alcohol intake, weight differences, female gender, cognitive impairment, adherence, social support, attitudes and frailty, and not simply co‐morbidities and concurrent use of drugs. Disease and drug therapy, dietary variations and barriers to adherence which include lower cognitive function, poorer physical function, living alone and a higher perceived illness burden, require elucidation as any influence might also extend to outcomes with the use of direct oral anticoagulants (DOACs) 21. The licensing of DOACs presents clinicians with a choice of oral anticoagulants, both for newly diagnosed patients and for those who are currently taking VKAs. The latter is particularly relevant if anticoagulation control is poor as cost‐effectiveness of DOACs compared to VKAs is highly dependent on anticoagulant control 22. Our results indicate that, for some patients as they age, maintaining TTR becomes more difficult, particularly for people who cannot attend a monitoring clinic, which raises the question as to whether a DOAC would be a better option for them as DOACs offer benefit in terms of risk reduction of stroke, largely because of reduced incidence of haemorrhagic stroke and a greater relative risk reduction in major bleeding, when centre‐based TTR in VKA‐treated patients is <66% 23.
It could be argued that the present study was limited by its retrospective nature. However, a retrospective design study for investigating stability of anticoagulation control is appropriate given the longitudinal nature of the investigation in a large cohort of AF patients, all of whom were anticoagulated with warfarin through a unified monitoring service using the same method of dosing. Further, any selection and observational biases were minimized given that information on INR values and warfarin doses for individual patients was obtained directly from electronic clinic records through the DAWN program, within the confines of the study inclusion criteria. The effects of co‐morbidity and concurrent therapy were not assessed, because data on these covariates were not available through the DAWN program 6, nor were any potential contributions from variances in patient education at initiation of warfarin and at monitoring visits which were inevitable between the clinic and domiciliary monitored groups. Nonetheless, the primary aim of the present study was to examine whether age in the context of patient setting per se influences anticoagulation control with warfarin rather than identifying the factors which contribute to the variance in anticoagulant control and we were able to achieve this in this large cohort studied for up to 14 years. This information is relevant as warfarin remains a cost‐effective option for anticoagulation, with informed decision making between this and a DOAC being appropriately made by patient and clinician based on the patient's clinical features and preferences.
We have demonstrated that there are both inter‐ and intra‐individual differences in anticoagulation control achieved with warfarin, influenced by age, gender and physical dependency. In view of the poorer stability of anticoagulation control in older, home‐bound patients, the importance of reviewing patients' anticoagulant management at least annually is confirmed. Exploration of factors affecting anticoagulation control with warfarin, and whether such factors might also affect response to DOACs, is warranted. Characterization of the modifiable factors which contribute to these observations and how to optimize these has the potential to improve outcomes for anticoagulated patients.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosur e.pdf (available on request from the corresponding author). FK had support from Daiichi Sankyo UK for the submitted work. PK had previously received support from Daiichi Sankyo UK and Bayer UK for work unrelated to the submitted work. This study was supported by an unrestricted educational grant from Daiichi Sankyo UK (grant no: RES/0247/7536). Daiichi Sankyo did not have any role in the interpretation of the data, or in the writing of the manuscript.
We would like to thank the IT staff at both DAWN and our Trust for their help with accessing and extracting data from the electronic database held regionally.
Contributors
All authors contributed to the study design. SA refined the database. Both SA and PA undertook statistical analysis of the data. All the authors contributed to the writing and reviewing of the manuscript.
Abohelaika, S. , Wynne, H. , Avery, P. , Robinson, B. , Kesteven, P. , and Kamali, F. (2016) Impact of age on long‐term anticoagulation and how gender and monitoring setting affect it: implications for decision making and patient management. Br J Clin Pharmacol, 82: 1076–1083. doi: 10.1111/bcp.13046.
References
- 1. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993; 69: 236–239. [PubMed] [Google Scholar]
- 2. Wan Y, Heneghan C, Perera R, Roberts N, Hollowell J, Glasziou P, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes 2008; 1: 84–91. [DOI] [PubMed] [Google Scholar]
- 3. Nieuwlaat R, Connolly BJ, Hubers LM, Cuddy SM, Eikelboom JW, Yusuf S, et al. Quality of individual INR control and the risk of stroke and bleeding events in atrial fibrillation patients: a nested case control analysis of the ACTIVE W study. Thromb Res 2012; 129: 715–719. [DOI] [PubMed] [Google Scholar]
- 4. Abohelaika S, Kamali F, Avery P, Robinson B, Kesteven P, Wynne H. Anticoagulation control and cost of monitoring of older patients on chronic warfarin therapy in three settings in North East England. Age Ageing 2014; 43: 708–711. [DOI] [PubMed] [Google Scholar]
- 5. Goudie BM, Donnan PT, Fairfield G, Al‐Agilly SS, Cachia PG. Dependency rather than old age increases the risk of warfarin‐related bleeding. Br J Gen Pract 2004; 54: 690–692. [PMC free article] [PubMed] [Google Scholar]
- 6. 4S Information Systems Ltd TS, Milnthorpe, Cumbria, LA7 7QJ.
- 7. National Health Services (NHS) . Actions that can make oral anticoagulant therapy safer: Information for patients and carers. London: NHS, 2007. [Google Scholar]
- 8. Cowan C, Healicon R, Robson I, Long WR, Barrett J, Fay M, et al. The use of anticoagulants in the management of atrial fibrillation among general practices in England. Heart 2013; 99: 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Royal College of Physicians . Sentinel Stroke National Audit Programme (SSNAP). London: Royal College of Physicians, 2013. [Google Scholar]
- 10. Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GYH, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370: 493–503. [DOI] [PubMed] [Google Scholar]
- 11. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One 2013; 8: e63479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events – European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014; 16: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mearns ES, White CM, Kohn CG, Hawthorne J, Song JS, Meng J, et al. Quality of vitamin K antagonist control and outcomes in atrial fibrillation patients: a meta‐analysis and meta‐regression. Thromb J 2014; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet 2010; 376: 975–983. [DOI] [PubMed] [Google Scholar]
- 15. Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Patient characteristics associated with oral anticoagulation control: results of the Veterans AffaiRs Study to Improve Anticoagulation (VARIA). J Thromb Haemost 2010; 8: 2182–2191. [DOI] [PubMed] [Google Scholar]
- 16. Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Risk‐adjusted percent time in therapeutic range as a quality indicator for outpatient oral anticoagulation: results of the Veterans Affairs Study to Improve Anticoagulation (VARIA). Circ Cardiovasc Qual Outcomes 2011; 4: 22–29. [DOI] [PubMed] [Google Scholar]
- 17. Arbring K, Uppugunduri S, Lindahl TL. Comparison of prothrombin time (INR) results and main characteristics of patients on warfarin treatment in primary health care centers and anticoagulation clinics. BMC Health Serv Res 2013; 13: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe‐TT(2)R(2) score. Chest 2013; 144: 1555–1563. [DOI] [PubMed] [Google Scholar]
- 19. Razouki Z, Ozonoff A, Zhao S, Rose AJ. Pathways to poor anticoagulation control. J Thromb Haemost 2014; 12: 628–634. [DOI] [PubMed] [Google Scholar]
- 20. Plichart M, Berrut G, Maubourguet N, Jeandel C, Emeriau JP, Ankri J, et al. Use of vitamin K antagonist therapy in geriatrics: a French national survey from the French Society of Geriatrics and Gerontology (SFGG). Drugs Aging 2013; 30: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 21. Mueller S, Pfannkuche M, Breithardt G, Bauersachs R, Maywald U, Kohlmann T, et al. The quality of oral anticoagulation in general practice in patients with atrial fibrillation. Eur J Intern Med 2014; 25: 247–254. [DOI] [PubMed] [Google Scholar]
- 22. Amin A, Deitelzweig S, Jing Y, Makenbaeva D, Wiederkehr D, Lin J, et al. Estimation of the impact of warfarin's time‐in‐therapeutic range on stroke and major bleeding rates and its influence on the medical cost avoidance associated with novel oral anticoagulant use –learnings from ARISTOTLE, ROCKET‐AF, and RE‐LY trials. J Thromb Thrombolysis 2014; 38: 150–159. [DOI] [PubMed] [Google Scholar]
- 23. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]


