Abstract
Aims
To evaluate congenital anomaly (CA)‐medication exposure associations produced by the new EUROmediCAT signal detection system and determine which require further investigation.
Methods
Data from 15 EUROCAT registries (1995–2011) with medication exposures at the chemical substance (5th level of Anatomic Therapeutic Chemical classification) and chemical subgroup (4th level) were analysed using a 50% false detection rate. After excluding antiepileptics, antidiabetics, antiasthmatics and SSRIs/psycholeptics already under investigation, 27 associations were evaluated. If evidence for a signal persisted after data validation, a literature review was conducted for prior evidence of human teratogenicity.
Results
Thirteen out of 27 CA‐medication exposure signals, based on 389 exposed cases, passed data validation. There was some prior evidence in the literature to support six signals (gastroschisis and levonorgestrel/ethinylestradiol (OR 4.10, 95% CI 1.70–8.53; congenital heart disease/pulmonary valve stenosis and nucleoside/tide reverse transcriptase inhibitors (OR 5.01, 95% CI 1.99–14.20/OR 28.20, 95% CI 4.63–122.24); complete absence of a limb and pregnen (4) derivatives (OR 6.60, 95% CI 1.70–22.93); hypospadias and pregnadien derivatives (OR 1.40, 95% CI 1.10–1.76); hypospadias and synthetic ovulation stimulants (OR 1.89, 95% CI 1.28–2.70). Antipropulsives produced a signal for syndactyly while the literature revealed a signal for hypospadias. There was no prior evidence to support the remaining six signals involving the ordinary salt combinations, propulsives, bulk‐forming laxatives, hydrazinophthalazine derivatives, gonadotropin releasing hormone analogues and selective serotonin agonists.
Conclusion
Signals which strengthened prior evidence should be prioritized for further investigation, and independent evidence sought to confirm the remaining signals. Some chance associations are expected and confounding by indication is possible.
Keywords: congenital anomalies, drug‐induced anomalies, pharmacoepidemiology, pharmacovigilance, pregnancy, signal evaluation
What is Already Known About this Subject
There is insufficient information on the safety of the vast majority of medications when taken during pregnancy and more post‐marketing surveillance of medication safety in pregnancy is needed.
Signal detection based on spontaneous adverse effect reporting is biased and incomplete.
What this Study Adds
The EUROmediCAT database, comprising data from population‐based EUROCAT congenital anomaly registries, can be used for systematic signal detection and signal strengthening.
Our results strengthen six congenital anomaly‐medication exposure signals in the literature.
We generated seven new signals which require independent confirmation as some may be chance findings.
Introduction
Congenital anomalies (CAs), structural or functional abnormalities that are present from birth 1, are a major cause of infant mortality, childhood morbidity and long‐term disability 2. They are a diverse group of disorders of prenatal origin and can be caused by a wide range of factors such as genetics, environmental agents, medications and physical conditions 3, 4, 5. While a number of antenatal medication exposures are known to cause CAs 6, there is insufficient information on the risks and safety for the vast majority of medications 7. The critical period for most major CAs is during organogenesis, in the first trimester of pregnancy 8. It has been estimated that 22–54% of pregnancies 9, 10 are exposed to prescription medications, excluding vitamins and minerals, during this time period. As a result, the lack of information in relation to the safety of medication during human pregnancy is a serious public health problem 11.
Typically, eligibility criteria for premarketing clinical trials exclude high risk individuals such as pregnant women 12. The evaluation of medication safety in human pregnancy therefore relies on post‐marketing surveillance to detect medication safety signals 13. As defined by the World Health Organisation (WHO), a signal refers to ‘reported information on a possible causal relationship between an adverse event and a medication, the relationship being unknown or incompletely documented previously’ 14.
Signals are detected when the observed number of reports is higher than expected for a particular medication‐event combination 12, 15. Such statistical signals are frequently found because of the large number of comparisons made and do not necessarily mean that a causal association is present 12. Even strong signals can be generated by various forms of confounding 16, so once a signal is generated, signal strengthening and signal evaluation are necessary in order to reinforce or refute the potential signal 13, 16, 17. While information on true medication safety signals should not be withheld from physicians and patients, false positive signals may cause substantial harm if they limit access to safe medications 17.
Traditionally signal detection has relied on national or international spontaneous reporting systems which pool reports of adverse medication events provided by healthcare providers, consumers and medication manufacturers 15. Spontaneous report databases have a number of limitations such as under‐, over‐ and duplicate reporting, limited information on concomitant medication or comorbidities and susceptibility to bias 12, 13, 14. To overcome some of these limitations, programmes have been initiated to make use of large data pools besides spontaneous reports such as healthcare databases and disease registries 13, 18, 19. EUROmediCAT's population‐based reproductive pharmacovigilance system is based on the European Surveillance of Congenital Anomalies (EUROCAT) network. A statistical signal detection analysis was conducted using the EUROmediCAT central database to find highly statistically significant CA‐medication exposure associations 20. The aim of this paper is to describe the protocol used for evaluation of the signals produced by the EUROmediCAT statistical signal detection analysis, and to give the results of evaluation of 27 CA‐medication exposure associations to determine which should be prioritized for further investigation. We do not report here signals belonging to four medication groups which were separately investigated as part of the EUROmediCAT project: antiepileptic medications, insulin/insulin analogues, antiasthmatic medications and selective serotonin reuptake inhibitors and psycholeptics 21, 22, 23, 24.
Methods
Dataset and statistical signal detection analysis
EUROCAT registries record all cases of major CA seen, among live births, fetal deaths ≥20 weeks' gestation and termination of pregnancy for fetal anomaly (TOPFA) 25, 26, 27. Births from 15 EUROmediCAT registries across 13 countries (1995–2011) were used to create a signal detection dataset (see Supplementary Table 1 20). This included 14,950 infants with a CA, excluding genetic conditions or isolated congenital dislocation of the hip, who were exposed to a medication in the first trimester, excluding folic acid, minerals, vitamins and/or topical medication 20, coded to the Anatomic Therapeutic Chemical (ATC) classification system 28. Data on maternal medication exposures are mostly obtained from prospectively recorded maternity records 29, 30.
The signal detection methodology used has been described elsewhere 20. In brief, a case‐malformed control approach was used where cases of a specific CA subgroup 31 were compared with all other CAs in terms of exposure to each specific medication. The signal detection analysis was conducted using medications recorded at the 4th ATC level (chemical subgroup) and the 5th ATC level (chemical substance). Use of different ATC codes for the same medication and changes to ATC codes over time were taken into account. Medications with less than three exposed fetuses/babies were excluded from the analysis. Any registry with no exposures to the medication of interest, or cases of the CA of interest, were also removed from each analysis. Overall, 59 CA subgroups and 693 medication groups were tested, resulting in 40,385 analyses. In order to limit the number of false positive associations, multiple testing procedures were implemented, using a 50% false discovery rate (FDR), where the cut‐off P‐value for associations at the 5th ATC level was 0.00040 and at the 4th ATC level was 0.0011 20. As the individual medications at the 5th ATC level all contribute to the 4th ATC level group, if an association arose at both the 4th and 5th ATC levels, the 5th ATC level association was taken as the result. This analysis produced 11 CA‐medication exposure signals 20 which were from medication groups not already being investigated as part of the EUROmediCAT project 32, i.e. excluding antiepileptics, antidiabetics, antiasthmatics and SSRIs/psycholeptics.
A previous analysis of the same dataset without some of the analytic refinements reported here (such as the amalgamation of duplicate ATC codes) 33, and cut‐off P‐values for associations at the 5th and 4th ATC levels of 0.00048 and 0.0028 respectively, had identified an additional 16 signals. These original signals were included for further analysis as a comparison of the odds ratios (OR) and 95% CIs remained very similar between the original and revised analyses (Figure 1 and Table 1), and although the FDR P‐value threshold was slightly higher, it is not the sole criterion for identifying a potential association of interest. When both sets of results were combined, there were 27 CA‐medication exposure signals. Results are given combined and separately.
Figure 1.
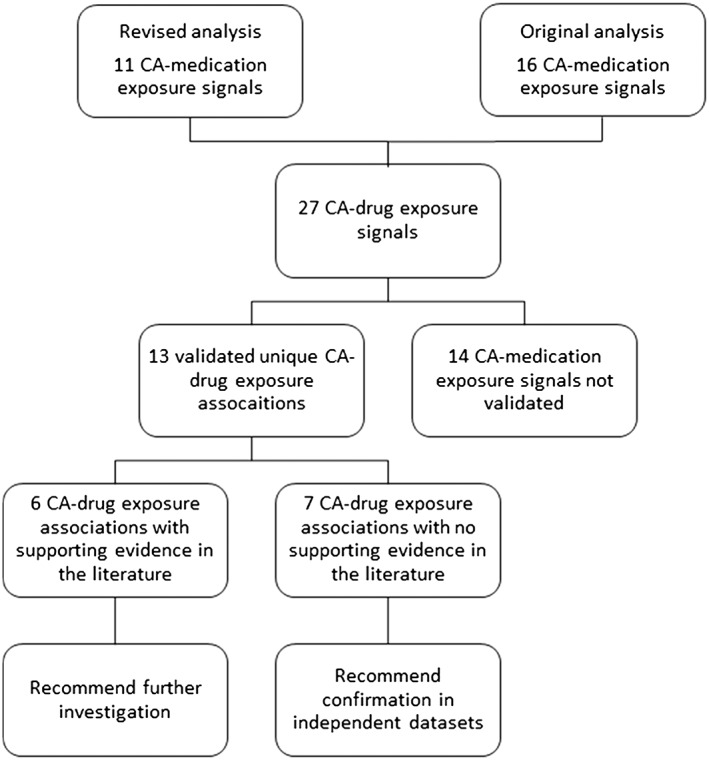
Signal evaluation flow diagram
Table 1.
Description of validated signals
| ATC code/s Medication/s and congenital anomaly | Signal OR (95% CI) | P value | Number of cases (confirmed 1st trimester exposures) | Mantel‐Haenszel adjusted * OR using validated data (95% CI) | Most prevalent concurrent medication exposures among cases (n) | Significant positive medication‐CA exposure associations not meeting FDR criteria (unvalidated † ) |
|---|---|---|---|---|---|---|
| A02AD01 Ordinary salt combinations and cleft lip with or without cleft palate | 2.38 (1.46–3.72) | 0.00036 | 23 (21) | 1.70 (1.06–2.72) | None (4), piperazine derivatives R06AE(2), other medications for peptic ulcer and gastro‐oesophageal disease A02BX (2), paracetamol N02BE01 (2), cisapride A03AF02 (1) | A02AD01 and cleft palate (OR 2.65, 95% CI 1.49–4.42)
A02AD01 and Anopthalmos/micropthalmos (OR 5.17, 95% CI 1.57–13.35) A02AB04 and polydactyly (OR 16.62, 95% CI 2.20–124.82) |
| A03FA Propulsives (metoclopramide, cisapride, domperidone, bromopride, alizapride, clebopride and itopride) and total anomalous pulmonary venous return‡ | 6.41 (1.89–17.46) | 0.0021 | 5 (5) | 10.49 (3.45–31.93) | None (10), levothyroxine sodium H03AA01 (2), omeprazole A02BC01 (1), prochlorperazine N05AB04 (1), promethazine R06AD02 (1) | None |
| A06AC Bulk‐forming laxatives (ispaghula (psylla seeds), ethulose, sterculia, linseed, methylcellulose, triticum (wheat fibre), polycarbophil calcium, ispaghula combinations, sterculia combinations and linseed combinations) and anencephalus and similar‡ | 8.98 (2.29–25.53) | 0.0015 | 4 (4) | 6.38 (2.23–18.24) | None (2), amoxicillin J01CA04 (1), follitropin alfa G03GA05 (1), chorionic gonadotrophin G03GA01 (1), levonorgestrel and ethinylestradiol G03AA07 (1) | A06AC and ventricular septal defect (OR 2.69, 95% CI 1.34–5.21)
A06AC and cleft lip with or without cleft palate (OR 3.37, 95% CI 1.16–8.10) A06AC and neural tube defects (OR 3.64, 95% CI 1.11–9.32) A06AD and club foot/talipes equinovarus (OR 2.21, 95% CI 1.09–4.09) |
| A07DA Antipropulsives (diphenoxylate, opium, loperamide, difenoxin, loperamide oxide, morphine combinations and loperamide combinations) and syndactyly‡ | 10.12 (2.42–32.05) | 0.0013 | 4 (4) | 6.41 (2.28–18.00) | None (3), nitrofurantoin J01XE01 (1) | None |
| C02DB Hydrazinophthalazine derivatives (dihydralazine, hydralazine, endralazine, cadralazine) and Atrial septal defect (ASD)‡ | 5.78 (1.39–22.81) | 0.0077 | 5 (5) | 2.78 (1.07–7.24) | None (3), methyldopa C02AB01 (2), diprophylline R03DA01 (1) | None |
| G03AB03 or G03AA07 Levonorgestrel and ethinylestradiol and gastroschisis‡ | 4.10 (1.70–8.53) | 0.0013 | 8 (8) | 2.95 (1.38–6.33)§ | None (6), trimethoprim J01EA01 (1), ibuprofen M01AE01 (1) | G03AB03 or G03AA07 and bladder exstrophy and/or epispadia (OR 7.05, 95% CI 1.36–23.2)
G03AA09 and neural tube defects (OR 4.88, 95% CI 1.23–14.18) G03AA13 and CHD (OR 6.12, 95% CI 1.16–60.46) G03AA and congenital cataract (OR 3.47, 95% CI 1.09–8.54) G03AA and anencephalus and similar (OR 2.69, 95% CI 1.05–5.76) |
| G03DA Pregnen (4) derivatives (gestonorone, medroxyprogesterone, hydroxyprogesterone and progesterone) and complete absence of a limb‡ | 6.60 (1.70–22.93) | 0.0035 | 5 (5) | 7.60 (2.34–24.67) | None (3), estradiol combinations G03CA53 (1), estradiol G03CA03 (1) | G03DA04 and ASD (OR 1.38, 95% CI 1.12–1.68)
G03DC and ASD (OR 1.79, 95% CI 1.09–2.82) G03DC and neural tube defects (OR 2.21, 95% CI 1.07–4.13) G03DC and limb reduction (OR 2.26, 95% CI 1.00–4.47) |
| G03DB Pregnadien derivatives (dydrogesterone, megestrol, medrogestone, nomegestrol, demegestone, chlormadinone, promegestone and dienogest) and hypospadias‡ | 1.40 (1.10–1.76) | 0.0036 | 91 (89) | 1.51 (1.15–1.98) | None (59), drotravine A03AD02 (7), hydroxyprogesterone G03DA03 (6), aspirin B01AC06 (4), progesterone G03DA04 (3), A03AD02 (5) | |
| G03DB and congenital heart defects (CHD) (OR 1.39, 95% CI 1.19–1.61) | ||||||
| G03GB Synthetic ovulation stimulants (cyclofemil, clomiphene and epimestrol) and hypospadias | 1.89 (1.28–2.70) | 0.00073 | 37 (36) | 1.92 (1.35–2.74) | None (22), progesterone G03DA04 (4), chorionic gonadotropin G03GA01 (4), levothyroxine sodium H03AA01 (3), labetalol C07AG01 (2) | G03GA and laterality (OR 4.92, 95% CI 1.72–11.47)
G03GA08 and ASD (OR 1.95, 95% CI 1.21–3.02) G03GA01 and congenital constriction bands/amniotic band (8.00, 95% CI 1.53–26.59) G03GA02 and neural tube defects (OR 3.12, 95% CI 1.19–6.98) G03GA04 and ventricular septal defect (OR 7.34, 95% CI 1.24–50.14) G03GA01 and bladder exstrophy and/or epispadia (OR 6.45, 95% CI 1.25–20.97) |
| L02AE Gonadotropin releasing hormone analogues (buserelin, leuprorelin, goserelin, triptorelin and histrelin) and laterality anomalies‡,¶ | 13.34 (2.52–45.08) | 0.0021 | 3 (3) | 9.09 (2.75–30.08) | follitropin alfa G03GA05 (2), chorionic gonadotrophin G03GA01 (2), urofollitropin G03GA04 (1), progesterone G03DA04 (1) | L02AE04 and severe CHD (OR 4.52, 95% CI 1.01–16.3) |
| J05AF Nucleoside and nucleotide reverse transcriptase inhibitors (zidovudine, didanosine, zalcitabine, stavudine, lamivudine, abacavir, tenofovir disoproxil, adefovir dipivoxil, emtricitabine, entecavir, telbivudine, clevudine) and congenital heart defects (CHD) | 5.01 (1.99–14.2) | 0.00012 | 18 (20)** | 2.04 (1.17–3.55)** | None (8), protease inhibitors J05AE (8), ritonavir J05AE03 (4), lopinavir and ritonavir J05AE06 (3), sulfamethoxazole and trimethoprim J01EE01 (1), combinations of sulfonamides and trimethoprim, including derivatives J01EE (1) | J05AF and severe CHD (OR 3.53, 95% CI 1.15–9.22)
J05AB11 and polydactyly (OR 7.69, 95% CI 1.38–28.46) J05AE03 and CHD (OR 5.92, 95% CI 1.05–60.21) |
| J05AF30 Combinations of nucleoside and nucleotide reverse transcriptase inhibitors and pulmonary valve stenosis | 28.2 (4.63–122.24) | 0.00039 | 3 (4)** | 5.08 (1.83–14.07)** | Nucleoside and nucleotide reverse transcriptase inhibitors J05AF (3), protease inhibitors J05AE (2), nevirapine J05AG01 (1), non‐nucleoside reverse transcriptase inhibitors J05AG (1), lopinavir and ritonavir J05AE06 (1), saquinavir J05AE01 (1) | |
| J05AF30 and ASD (OR 6.08, 95% CI 1.03–25.55) | ||||||
| N02CC Selective serotonin (5HT1) agonists (sumatriptan, naratriptan, rizatriptan, almotriptan, eletriptan and frovatriptan) and congenital constriction bands/amniotic band‡ | 12.97 (2.46–43.53) | 0.0022 | 3 (3) | 15.58 (4.44–54.62) | ispaghula A06AC01 (1), ‘other’ anti‐obesity medications A08AX (1), dalteparin B01AB04 (1), fluconazole J02AC01 (1), ibuprofen M01AE01 (1) | N02CC and encephalocele (OR 6.12, 95% CI 1.20–19.38)
N02CC and pulmonary valve atresia (OR 5.25, 95% CI 1.04–16.48) N02CC05 and ASD (OR 11.99, 95% CI 1.6–89.73) N02CC06 and club foot/talipes equinovarus (OR 8.59, 95% CI 1.33–44.29) |
More detail is provided than usual for P‐values due to the number of decimal places of relevance to the interpretation of the signal detection results.
Using validated data and adjusting for registry.
Not validated in terms of CA or medication exposure and not adjusted for registry.
Signal from original analysis not meeting revised FDR P‐value threshold.
Adjusted for maternal age category.
Laterality group includes atrial isomerism, dextrocardia, situs inversus, broncho‐pulmonary isomerism, asplenia and polysplenia.
Includes J05AX04, J05AB05, J05AB07, J05AB08, J05AB10, J05AF, J05AF01‐12, J05AF30, J05AR01‐09 and J05AR11‐13 in adjusted analysis due to changes over time in the ATC coding of Ns/NtRTIs (including in combination).
Signal validation
Initially, the exposed cases for each of the 27 CA‐medication exposure associations were validated, in terms of diagnosis, medication exposure and timing of exposure, with the local registries.
The OR based on these validated data were then adjusted for confounding by registry, i.e. where a registry may differ in both their (recorded) exposure proportion and (recorded) CA subgroup proportion in such a way as to produce artificial relationships between the exposure and outcome. Adjustment for registry was done by conducting a meta‐analysis in STATA/SE 12.1 using the fixed effect Mantel–Haenszel method 34, 35. Continuity corrections were made as per the method by Sweeting et al. 36.
With the exception of chromosomal anomalies, most CAs are not strongly associated with maternal age 37. However, gastroschisis, an abdominal wall defect, is associated with young maternal age 38 and it was necessary to adjust the gastroschisis‐medication exposure association for maternal age. This was done by stratifying the meta‐analysis by maternal age group 39, categorized as <20, 20–24, 25–29, 30–34, 35–39 and 40+.
Those CA‐medication exposure associations which persisted, when using validated data and adjusting for registry effects, were considered validated statistical signals.
Signal description
The validated statistical signals were then described in detail in terms of the signal ORs and 95% CIs, the adjusted ORs and 95% CIs using validated data, the number of exposed cases and the most prevalent concurrent medication exposures recorded among exposed cases. In addition, the statistically significant CA‐medication exposure associations which failed to meet the FDR threshold (FDR <50%) but which involved the same medication, or 3rd ATC level, exposure were also noted.
Signal literature review
A literature review was then conducted, for the validated statistical signals, by searching REPROTOX, TOXBASE, the Developmental and Reproductive Toxicology Database (DART) and PubMed. For those signals at the 5th ATC level, this involved searching initially for the specific medication and then for the 4th ATC level medication group. For signals at the 4th ATC level a literature review was conducted for both the medication group and each specific medication in the group. REPROTOX and TOXBASE were searched using the medication/group name alone. DART and PubMed were searched using the name of the medication/group combined with search terms for teratogen and CA (see Supplementary Document 1 for more detail). The reference lists of relevant articles were also searched. Cohort and case‐control studies were of particular interest but case reports/series were also noted where available as the evidence was limited for some medications. The available published evidence was categorized according to the amount of evidence to support the signal in the human literature, i.e. signal CA described in the literature, teratogenicity leading to other CA described in the literature, or no evidence of teratogenicity in the literature. When the evidence was based on case reports/series or a single case‐control or cohort study, the published evidence was noted as minimal.
Ethical approval
Ethical approval for this study was provided by the University of Ulster Nursing Research Governance Filter Committee.
Results
Signal validation
Out of the 27 original CA‐medication exposure associations 14 (seven from the original and seven from the revised analysis) were not validated as independent signals: one was a duplicate signal as more than one formulation of the medication is available (the combined contraceptive levonorgestrel and ethinylestradiol); for five CA‐medication exposure associations a proportion of the CA cases and/or first trimester medication exposures could not be verified so that the OR using validated data more than halved to less than 1.5; eight CA‐medication exposure associations were explained by confounding by registry.
This left 13 (nine from the original and four from the revised analysis) validated unique CA‐medication exposure signals related to gastrointestinal medications (n = 4), antihypertensives (n = 1), female sex hormones (n = 3), medications used in infertility treatments (n = 2), antiretrovirals (n = 2) and selective serotonin (5HT1) agonists (n = 1).
Signal description
The 13 statistical signals were based on between three and 89 confirmed CA cases with first trimester medication exposures (Table 1).
Signal literature review
Of the 13 validated signals for which a literature review was conducted, previous evidence in the literature was found for six (Table 2).
Table 2.
Results of literature review relating to 13 validated signals
| Signal | Evidence to support signal | Medication uses and literature relating to their teratogenicity in humans |
|---|---|---|
| A02AD01
Ordinary salt combinations Cleft lip with or without cleft palate |
C*, * | Ordinary salts are combinations and complexes of aluminium, calcium and magnesium compounds used as antacids. There is no evidence relating specifically to the teratogenicity of the ordinary salt combinations. One case‐control study explores the teratogenicity of combinations and complexes of aluminium, calcium and magnesium. No increase in all CAs combined among those treated with aluminium magnesium hydrocarbonate (OR 1.5, 95% CI 0.3–8.9) or aluminium magnesium hydroxide (OR 0.6, 95% CI 0.2–2.4) was reported 103. |
| A03FA
Propulsives (metoclopramide, cisapride, domperidone, bromopride, alizapride, clebopride and itopride) Total anomalous pulmonary venous return |
C | Propulsives enhance gastrointestinal motility and are used to treat nausea and vomiting. Cohort studies have found no increase in the risk of all CAs combined 90, 91, 92, 93, 94, hypospadias or orofacial clefts 95 following exposure to the propulsives. There was no evidence of an association between transposition of the great vessels, ventricular septal defect (VSD), atrial septal defect (ASD), Tetralogy of Fallot, pulmonary valve stenosis or coarctation of the aorta 96 and first trimester exposure to metoclopramide (A03FA01). A retrospective cohort study found no significant association between first trimester exposure to metoclopramide and ‘other anomalies of the circulatory system’, a group which includes total anomalous pulmonary venous return 97. However, the number of cases involved in this group was small and it is unclear what proportion, if any, had total anomalous pulmonary venous return. No studies have looked specifically at the risk of total anomalous pulmonary venous return. |
| A06AC
Bulk‐forming laxatives (ispaghula (psylla seeds), ethulose, sterculia, linseed, methylcellulose, triticum (wheat fibre), polycarbophil calcium, ispaghula combinations, sterculia combinations and linseed combinations) Anencephalus and similar anomalies |
C*, * | Bulk‐forming laxatives are used to treat constipation. The single cohort study exploring the teratogenciity of ispaghula (A06AC01) found no significant difference in the rate of all CAs combined between those who were exposed in the first trimester and those who were not 104. |
| A07DA
Antipropulsives (diphenoxylate, opium, loperamide, difenoxin, loperamide oxide, morphine combinations and loperamide combinations) Syndactyly |
B | Antipropulsives are used to treat diarrhoea. Two cohort studies explore the teratogenicity of loperamide (A07DA03) and found no increase in all CAs combined 105. An association was found between loperamide exposure and hypospadias (RR 3.2, 95% CI 1.3–6.6, n = 7) but multiple comparisons mean that this may have been due to chance 71. |
| C02DB
Hydrazinophthalazine derivatives (dihydralazine, hydralazine, endralazine, cadralazine) Atrial septal defect (ASD) |
C*, * | Hydrazinophthalazine derivatives act on arteriolar smooth muscle and are used to treat hypertension. A single case‐control study found no significant association between dihydralazine (C02DB01) exposure, before and throughout pregnancy, and all CAs combined 82. |
| G03AB03/G03AA07
Levonorgestrel and ethinylestradiol Gastroschisis |
A | Levonorgestrel and ethinylestradiol is a combined oral contraceptive containing both an oestrogen and a progestogen. Evidence specifically relating to levonorgestrel and ethinylestradiol is limited to one large case‐control study where 6/133 (4.5%) CA case and 8/129 (6.2%) non‐malformed control infants were exposed to levonorgestrel and ethinylestradiol 106. Exposure to oral contraceptives in early pregnancy does not increase the risk of all CAs combined 107, 108, neural tube defects (NTD) 109, 110, 111, CHD 108 or orofacial cleft 112. The evidence relating to gastroschisis is conflicting with some articles showing a significant association (68% of gastroschisis cases exposed vs. 26% of malformed controls 40; OR 1.8, 95% CI 1.3–2.7, n = 40 41) and others showing none 42, 43. The same is true for genital anomalies 44, 45, 113, 114, 115, 116. One case‐control study describes an increased risk of urinary tract anomalies following first trimester exposure to oral contraceptives 117. |
| G03DA
Pregnen (4) derivatives (gestonorone, medroxyprogesterone, hydroxyprogesterone and progesterone) Complete absence of a limb |
A | Pregnen (4) derivatives are progestogens, compounds with biological activity similar to progesterone, used in hormone replacement therapy, infertility and to treat menstrual problems. Cohort and case‐control studies found no significant increase in all CAs combined with any of the pregnen (4) derivatives 118, 119, 120, 121, 122, 123, 124. A cohort study found that medroxyprogesterone (G03DA02) increases the rate of CHDs, gastro‐intestinal defects, CAs of the integument, chromosome defects and all other defects. These findings may be due to chance as multiple comparisons were made and the range of defects including chromosomal defects is not plausible 125. A number of case‐control studies have found a significant association between hypospadias and both hydroxyprogesterone (G03DA03) and progesterone (G03DA04) 46, 126, 127. However, other studies have found no association 44, 47, 128 and recall bias is a concern 48. In the 1960s and 1970s a number of studies were published linking ‘sex hormones’ with an increased incidence of nongenital congenital malformations such as CHDs and limb reduction defects 49, 50, 51, 52, 53, 54, 55. However, the evidence supporting the link between progestogens and contraceptive agents with nongenital malformations was contradictory, poor methodologically and the study material lacked uniformity 121, 129, 130. By 1993 the controversy surrounding this issue meant that there had been 20 review articles written on this subject, none of which concluded that sex hormones produced nongenital organ teratogenesis 56, 57. |
| G03DB
Pregnadien derivatives (dydrogesterone, megestrol, medrogestone, nomegestrol, demegestone, chlormadinone, promegestone and dienogest) Hypospadias |
A | Pregnadien derivatives are also progestogens and are used as per the pregnadien derivatives. A review of case reports and three very small trials found no increase in all CAs combined with dydrogesterone (G03DB01) 131, 132, 133, 134. The broader medication group, the progestogens, have been associated with hypospadias 45, 47, 58 but these findings have not been consistent 44, 46, 48, 59. |
| G03GB
Synthetic ovulation stimulants (cyclofemil, clomiphene and epimestrol) Hypospadias |
A | Synthetic ovulation stimulants are used in infertility treatment. Across cohort and case‐control studies there is no evidence that exposure to clomiphene citrate (G03GB02) in the periconceptional period increases the rate of all CAs combined. There is conflicting evidence of an association with NTDs 135, 136, 137. Clomiphene has been associated with coarctation of the aorta 66, 138, anencephaly, Dandy Walker malformation, septal heart defects, muscular ventricular septal defects, esophageal atresia, cloacal exstrophy, craniosynostosis and omphalocele but multiple comparisons and small numbers of cases make these findings tentative 66. An association between periconceptional clomiphene exposure and the more severe, proximal forms of hypospadias 44, 60, 61, 62, but not all forms of hypospadias combined 63, 64, has been described. |
| L02AE
Gonadotropin‐releasing hormone analogues (GnRHa) (buserelin, leuprorelin, goserelin, triptorelin and histrelin) Laterality |
C*, * | Gonadotropin‐releasing hormone analogues are used in infertility treatment. Evidence relating to the teratogenicity of the GnRHa's is limited to case reports/series 86, 87, 88, 89. There is no evidence for a pattern of anomalies but the numbers reported are small and there is potential for reporting bias. |
| J05AF
Nucleoside and nucleotide reverse transcriptase inhibitors (Ns/NtRTIs) (zidovudine, didanosine, zalcitabine, stavudine, lamivudine, abacavir, tenofovir disoproxil, adefovir dipivoxil, emtricitabine, entecavir, telbivudine, clevudine) Congenital heart defects (CHD) |
A | The Ns/NtRTIs are used to treat HIV/AIDS and chronic hepatitis. Case‐control, cohort studies and a manufacturer maintained pregnancy registry explore the teratogenicity of individual Ns/NtRTIs and the group as a whole. There is no evidence that first trimester exposure to any of the individual Ns/NtRTIs, or the group as a whole, increases the rate of all CAs combined 139, 140, 141, 142, 143. First trimester exposure to zidovudine (J05AF01) has been found to increase the risk of CHD 143, 144, but this has not been a consistent finding 145, 146. A significant association between first trimester exposure to ARV regimes containing at least one Ns/NtRTI and CHD is reported 147. There is no evidence relating to the risk of PVS as a specific CHD. Small numbers of cases have also suggested an increased risk of central nervous system (CNS) anomalies 146 and hypospadias 148 following first trimester exposure to zidovudine and head and neck defects following first trimester exposure to didanosine (J05AF02) 143. |
| J05AF30
Combinations of nucleoside and nucleotide reverse transcriptase inhibitors Pulmonary valve stenosis (PVS) |
A | |
| N02CC
Selective serotonin agonists (sumatriptan, naratriptan, rizatriptan, almotriptan, eletriptan and frovatriptan) Congenital constriction bands or amniotic bands |
C | Selective serotonin agonists, also called triptans, are used to treat migraines. Cohort studies and a manufacturer maintained pregnancy registry explore the teratogenicity of these medications, sumatriptan (N02CC01) in particular. First trimester exposure to sumatriptan does not significantly increase the rate of all CAs combined 72, 73, 74, 75, 76, 77. Eletriptan (N02CC06) was found to significantly increase the rate of all CAs combined but this was based on 14 exposures and may have been a chance finding 78. None of the other triptans 75, 77, 79, or the triptans as a group appear to increase the rate of all CAs combined 76, 80, 81. |
A: teratogenicity leading to signal CA described in literature;
B: teratogenicity leading to other CA described in literature;
C: no evidence of teratogenicity.
Published evidence minimal
Discussion
We have found 13 CA‐medication exposure signals which require further confirmation. There was evidence in the literature, albeit conflicting at times, to support six of the 13 signals 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64. These six signals have been strengthened and should be prioritized for further evaluation. Four of these signals were related to sex hormones (gastroschisis and the contraceptive levonorgestrel/ethinylestradiol; complete absence of a limb and pregnen (4) derivatives; hypospadias and pregnadien derivatives; hypospadias and synthetic ovulation stimulants). We also had as yet unvalidated data that some other anomalies might be associated with these medications. Sex hormone‐based medications accounted for 24.8% of the medication exposures in the database 20. The other two of these signals were congenital heart defects and pulmonary valve stenosis associated with nucleoside/nucleotide reverse transcriptase inhibitors, antivirals used for HIV and chronic hepatitis. For all of these signals, the possibility of confounding by indication, or by co‐exposures, should be considered. The progestogens are used to ‘support’ pregnancies at risk of early loss. It may be that this leads to increasing survival of CA‐affected fetuses 65. Sub‐fertile women have been found to have a higher risk of having a child with a CA regardless of whether or not they receive infertility treatment 66, 67, 68, 69, and this or other co‐exposures may confound the interpretation of medication use related to subfertility or infertility 69. Those receiving antiviral treatment for HIV or hepatitis infection may have other exposures leading to an increased risk of CAs 70. However, the case‐malformed control approach used in this study will have negated this issue to some extent as the comparison group also have CAs.
The only evidence that the antipropulsive antidiarrheals may be teratogenic was a single report of an association with hypospadias 71, rather than syndactyly as in our results. These two anomalies are usually considered aetiologically unrelated.
The remaining six statistical signals did not have supporting evidence in the literature and should be confirmed in an independent dataset. For selective serotonin agonists, a number of previous studies have found no association with CA 72, 73, 74, 75, 76, 77, 78, 79, 80, 81 but these were too small to find an anomaly as rare as congenital constriction bands. Hydrazinophthalazine derivatives, antihypertensives which act on arteriolar smooth muscle, have one small previous negative study 82. Other types of antihypertensives, such as ace inhibitors, have been associated with an increased risk of CA 83 but the underlying maternal hypertension also appears to play a role in the development of CA 84. While there are concerns about assisted reproduction in general in relation to CA risk 85, and two of our other signals discussed above are medications used in assisted reproduction, there is only minimal case report evidence 86, 87, 88, 89 relating to gonadotropin releasing hormone analogues, and none of these case reports relate to laterality anomalies. Previous studies of the propulsives 90, 91, 92, 93, 94, 95, 96, 97 have been negative regarding teratogenicity and there is no evidence to support our finding. The ordinary salts and bulk‐forming laxatives are generally assumed to be safe, have low bioavailablity, do not interfere with normal physiologic salt balance and therefore were not specifically studied.
The signal detection methodology used in EUROmediCAT was based on a 50% FDR. This means that half of the associations found are expected to be chance associations, i.e. not causal. Due to this uncertainty, and the difficulties of interpretation discussed above, medication decisions should not be made based on the CA‐medication exposure signals identified but should await the results of further research.
Strengths and limitations
A strength of this study is the use of the EUROmediCAT central database. EUROmediCAT's international population‐based database contains detailed coding of all CAs 29 and includes TOPFA cases which constitute a large proportion of some CA 98. The diagnosis of CAs is standardized across the registries involved and will have ensured consistency in the diagnosis 27. There will also be much less under‐reporting and bias than in spontaneous reporting pharmacovigilance systems as all major CAs are recorded in EUROCAT, not just those which clinicians consider to be important enough or potentially linked to a medication exposure. While the EUROmediCAT database contains detailed information on medications taken during the first trimester of pregnancy, there is known underascertainment of some medications 30, 99 but while this may reduce the sensitivity of the system to detect certain teratogenic medications, it should not lead to bias due to the use of malformed controls.
It was only possible to validate the data relating to the exposed cases. This means that while the number of exposed cases may have decreased, owing to errors found in data coding, the number of exposed controls will not have changed. As a result, the data validation process could only decrease the ORs. We found evidence that other anomalies were also associated with the signal medications, but at lower levels of statistical significance which did not surpass the FDR threshold, and did not validate these data. However, data validation for the main findings is a strength of this study.
The signal detection process used did not take prior literature into account during the statistical analysis 100 but instead brought this in at the signal evaluation stage. In the EUROmediCAT analyses of antiepileptics, antidiabetics, antidepressants and antiasthmatics 21, 22, 23, 24, we first searched the literature before evaluating existing signals and detecting new signals. The signal detection process we report in this paper is intended to be used in addition to the drug class by drug class approach. It can be used to identify the most highly significant associations in the database for drug classes not otherwise undergoing analysis. We recognize that there may be many other associations in the data that did not meet the FDR threshold but which are nevertheless of potential interest. Indeed, this is shown by our evaluation of the 16 signals arising from the original signal detection analysis, which included a number of associations reported previously in the literature.
While the literature search was extensive, it is possible that relevant articles may have been missed, particularly negative evidence for a medication exposure when analysed as one of many aetiological factors in a case‐control study. We were assessing whether previous literature existed but did not conduct a meta‐analysis of the evidence to date, and this may lead to highlighting positive over negative evidence, although all evidence found is presented. It was necessary to search for each of the individual medications, rather than the broader medication group, as the 4th ATC level, chemical subgroup, was not always used in the literature or databases and returned little or no information for some of the signals. Without prior hypotheses about the mechanism of action, it can also be difficult to decide how broadly to look for related literature – for example there is a large literature on sex hormones as a class, but much less related to specific sex hormones. While positive evidence in the literature regarding risk of all CAs combined could be considered supportive, negative evidence is more difficult to interpret, as few medications increase the rate of all CAs combined, instead tending to increase the rate of specific CAs 101.
As far as possible, changes over time in the ATC codes used for particular medications were taken into account in both the signal detection analysis and the signal evaluation. It is possible, however, that some changes were missed, potentially leading to signals being missed as the exposed cases would be split across more than one ATC code in the dataset.
Although all the cases were confirmed as first trimester exposures, it is not known if these exposures actually occurred during the critical period for CA development 8. Similarly there was no information available in terms of the doses of medications taken for the majority of cases. If it was possible to identify a dose–response relationship or show exposure during the critical period for development of the specific CAs, this would provide support for a causal relationship 101. Our protocol did not include assessment of biological plausibility or possible teratogenic mechanisms 102. Although grouping of CA or of medications by potential teratogenic mechanism has been advocated 3, we found this to be of limited use as the same CAs are often related to a number of potential mechanisms, and the current imperfect knowledge of mechanisms is one of the drivers of signal detection in postmarketing surveillance.
Conclusion
A statistical signal detection analysis was conducted using the EUROmediCAT central database. Six signals had some prior supporting evidence and these should be prioritized for further investigation before being evaluated in relation to clinical decision making. A further seven CA‐medication exposure signals were found which had no prior supporting evidence and these need to be confirmed in independent datasets.
Contributors
JEG performed statistical analysis of the signals, literature review and drafted the manuscript. JML and JM performed the statistical signal detection analysis on which this article is based and advised on the interpretation of the results. ML advised on the conduct and co‐ordination of the study as well as interpretation of the results. LJB and EG advised on interpretation of the results. EG, MCA, IB, HdeW, MG, KK, BK, AL‐B, VN, AJN, MO'M, AP, DT and AW provided and verified the data. HD participated in the design, conduct and coordination of the study, advised on interpretation of the results and critically revised the manuscript. HD and LJB wrote the funding application. All authors, commented on drafts, read and approved the final manuscript.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: The study was funded by the European Commission under the 7th Framework Program (grant agreement HEALTH‐F5‐2011‐260598). AP received grants from the Institute of Clinical Physiology‐National Research Council (IFC‐CNR) during the conduct of the study for the submitted work. The institutions of HD, ML, JM, EG, MCA, IB, MG, BK, AL‐B, VN, AJN, MO'M, AP, DT and AW received funding from Glaxo Smith Kline (GSK) outside the submitted work in the previous 3 years. There are no other relationships or activities that could appear to have influenced the submitted work.
EUROCAT registries are funded as fully described in Paper 6 of Report 9 – EUROCAT Member Registries: Organization and Activities, available at http://onlinelibrary.wiley.com/doi/10.1002/bdra.20775/pdf.
We thank the people throughout Europe involved in providing and processing information, including affected families, clinicians, health professionals, medical record clerks and registry staff.
Supporting information
Table S1 EUROmediCAT signal detection dataset
Document S1 Literature review methodology
Supplementary Table 1: EUROmediCAT signal detection dataset
Supplementary document 1: Literature review methodology
Given, J. E. , Loane, M. , Luteijn, J. M. , Morris, J. K. , de Jong van den Berg, L. T. W. , Garne, E. , Addor, M. ‐C. , Barisic, I. , de Walle, H. , Gatt, M. , Klungsoyr, K. , Khoshnood, B. , Latos‐Bielenska, A. , Nelen, V. , Neville, A. J. , O'Mahony, M. , Pierini, A. , Tucker, D. , Wiesel, A. , and Dolk, H. (2016) EUROmediCAT signal detection: an evaluation of selected congenital anomaly‐medication associations. Br J Clin Pharmacol, 82: 1094–1109. doi: 10.1111/bcp.12947.
Footnotes
Chromosomal anomalies, genetic syndromes and skeletal dysplasias
References
- 1. WHO/CDC/ICBDSR . Birth Defects Surveillance: Atlas of Selected Congenital Anomalies. Geneva: World Health Organization, 2014. [Google Scholar]
- 2. EUROCAT . EUROCAT: European Surveillance of Congenital Anomalies [online]. Available at http://www.eurocat‐network.eu/ (last accessed 18 April 2016).
- 3. van Gelder MMHJ, van Rooij IALM, Miller RK, Zielhuis GA, de Jong‐van den Berg LTW, Roeleveld N. Teratogenic mechanisms of medical drugs. Hum Reprod Update 2010; 16: 378–94. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Fact sheet No. 370 Congenital anomalies [online]. Available at http://www.who.int/mediacentre/factsheets/fs370/en/ (last accessed 18 April 2016).
- 5. World Health Organization . Birth defects: report by the Secretariat [online]. Geneva: WHO, 2010. Available at http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_10‐en.pdf?ua=1(last accessed 18 April 2016).
- 6. Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med 1998; 338: 1128–37. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández‐Díaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011; 205: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Czeizel AE. Specified critical period of different congenital abnormalities: a new approach for human teratological studies. Congenit Anom (Kyoto) 2008; 48: 103–9. [DOI] [PubMed] [Google Scholar]
- 9. Daw JR, Hanley GE, Greyson DL, Morgan SG. Prescription drug use in pregnancy in developed countries: a systematic review. Pharmacoepidemiol Drug Saf 2011; 20: 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lupattelli A, Spigset O, Twigg MJ, Zagorodnikova K, Mårdby AC, Moretti ME, et al. Medication use in pregnancy: a cross‐sectional, multinational web‐based study. BMJ Open 2014; 4: e004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adam MP, Polifka JE, Friedman JM. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am J Med Genet Part C Semin Med Genet 2011; 157: 175–82. [DOI] [PubMed] [Google Scholar]
- 12. Sharrar RG, Dieck GS. Monitoring product safety in the postmarketing environment. Ther Adv Drug Saf 2013; 4: 211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suling M, Pigeot I. Signal detection and monitoring based on longitudinal healthcare data. Pharmaceutics 2012; 4: 607–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eduards IR, Biriell C. Harmonisation in pharmacovigilance. Drug Saf 1994; 10: 93–102. [DOI] [PubMed] [Google Scholar]
- 15. Hauben M, Reich L. Potential utility of data‐mining algorithms for early detection of potentially fatal/disabling adverse drug reactions: a retrospective evaluation. J Clin Pharmacol 2005; 45: 378–84. [DOI] [PubMed] [Google Scholar]
- 16. Hauben M. A brief primer on automated signal detection. Ann Pharmacother 2003; 37: 1117–23. [DOI] [PubMed] [Google Scholar]
- 17. Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf 2010; 19: 858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robert E, Vollset SE, Botto L, Lancaster PA, Merlob P, Mastroiacovo P, et al. Malformation surveillance and maternal drug exposure: the MADRE project. Int J Risk Saf Med 1994; 6: 75–118. [DOI] [PubMed] [Google Scholar]
- 19. Trifiro G, Fourrier‐Reglat A, Sturkenboom MCJM, Díaz Acedo C, Van Der Lei J. The EU‐ADR project: preliminary results and perspective. Stud Health Technol Inform 2009; 148: 43–9. [PubMed] [Google Scholar]
- 20. Luteijn JM, Morris JK, Garne E, Given J, de Jong‐van den Berg L, Addor M‐C, et al. EUROmediCAT signal detection: a systematic method for identifying potential teratogenic medication. Br J Clin Pharmacol 2016; 82: 1110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H, Wender‐Ozegowska E, Garne E, Morgan M, Loane M, Latos‐Bielenska A, et al. Use of insulin analogs in pre‐gestational diabetes and risk of congenital anomalies. Pharmacoepidemiol Drug Saf 2015; 24 (S1): 10–1. [Google Scholar]
- 22. Boyle B, Loane M, Bakker MK, Addor MC, Arriola L, Garne E, et al. Selective serotonin reuptake inhibitor (SSRI) use in first trimester pregnancy and risk of congenital anomalies: a EUROmediCAT case‐malformed control study in 12 countries. Pharmacoepidemiol Drug Saf 2015; 24 (S1): 15. [Google Scholar]
- 23. Garne E, Hansen AV, Morris J, Zaupper L, Addor M‐C, Barisic I, et al. Use of asthma medication during pregnancy and risk of specific congenital anomalies: a European case‐malformed control study. J Allergy Clin Immunol 2015; 136: 1496–502. [DOI] [PubMed] [Google Scholar]
- 24. Wang H, Loane M, Dolk H, Morris J, Garne E, De Jong‐van den Berg LTW, et al. Use of topiramate in relation to the risk of orofacial clefts. Pharmacoepidemiol Drug Saf 2015; 24 (S1): 11–2.25421570 [Google Scholar]
- 25. EUROCAT Central Registry . EUROCAT Guide 1.1. Instructions for the registration of congenital anomalies [online]. Brussels: EUROCAT, 1990. Available at http://www.eurocat‐network.eu/content/EUROCAT‐Guide‐1.1‐1990.pdf (last accessed 18 April 2016).
- 26. EUROCAT Central Registry . EUROCAT Guide 1.2. Instructions for the registration of congenital anomalies [online]. Newtownabbey: EUROCAT, 2002. Available at http://www.eurocat‐network.eu/content/EUROCAT‐Guide‐1.2.pdf (last accessed 18 April 2016).
- 27. EUROCAT Central Registry . EUROCAT Guide 1.3 and reference documents. Instructions for the registration and surveillance of congenital anomalies. Newtownabbey: EUROCAT, 2005. [Google Scholar]
- 28. WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment. Oslo: WHO Collaborating Centre for Drug Statistics Methodology, 2014. [Google Scholar]
- 29. Boyd PA, Haeusler M, Barisic I, Loane M, Garne E, Dolk H. Paper 1: The EUROCAT network‐organization and processes. Birth Defects Res Part A – Clin Mol Teratol 2011; 91: 2–15. [DOI] [PubMed] [Google Scholar]
- 30. Bakker M, de Jonge L. EUROCAT Special Report: Sources of Information on Medication Use in Pregnancy [online]. Newtownabbey: EUROCAT Central Registry, 2014. Available at http://www.eurocat‐network.eu/content/Special‐Report‐Medication‐Use‐In‐Pregnancy.pdf (last accessed 18 April 2016). [Google Scholar]
- 31. EUROCAT Central Registry . EUROCAT Guide 1.4 and Reference Documents [online]. Newtownabbey: EUROCAT, 2013. Available at http://www.eurocat‐network.eu/aboutus/datacollection/guidelinesforregistration/guide1_4 (last accessed 18 April 2016). [Google Scholar]
- 32. de Jong‐van den Berg LTW, Bakker MK, Dolk H. EUROmediCAT: European surveillance of safety of medication use in pregnancy. Pharmacoepidemiol Drug Saf 2011; 20: S46–7. [DOI] [PubMed] [Google Scholar]
- 33. EUROmediCAT . Safety of Medication Use in Pregnancy: European Conference Poznan, Poland, February 2–4, 2015 [online]. Poznan: EUROmediCAT, 2015. Available at http://euromedicat.eu/content/EUROmediCAT‐Poznan‐Conference‐Abstract‐Book.pdf (last accessed 18 April 2016).
- 34. Bradburn MJ, Deeks JJ, Berlin JA, Localio AR. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Stat Med 2007; 26: 53–77. [DOI] [PubMed] [Google Scholar]
- 35. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synth Methods 2010; 1: 97–111. [DOI] [PubMed] [Google Scholar]
- 36. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Stat Med 2004; 23: 1351–75. [DOI] [PubMed] [Google Scholar]
- 37. Loane M, Dolk H, Morris JK. Maternal age‐specific risk of non‐chromosomal anomalies. BJOG 2009; 116: 1111–9. [DOI] [PubMed] [Google Scholar]
- 38. Loane M, Dolk H, Bradbury I. Increasing prevalence of gastroschisis in Europe 1980–2002: a phenomenon restricted to younger mothers? Paediatr Perinat Epidemiol 2007; 21: 363–9. [DOI] [PubMed] [Google Scholar]
- 39. Harris RJ, Bradburn MJ, Deeks JJ, Altman DG, Harbord RM, Sterne JAC. Metan: fixed‐ and random‐effects meta‐analysis. Stata J 2008; 8: 3–28. [Google Scholar]
- 40. Drongowski RA, Smith RK, Coran AG, Klein MD. Contribution of demographic and environmental factors to the etiology of gastroschisis: a hypothesis. Fetal Diagn Ther 1991; 6: 14–27. [DOI] [PubMed] [Google Scholar]
- 41. Waller DK, Gallaway MS, Taylor LG, Ramadhani TA, Canfield MA, Scheuerle A, et al. Use of oral contraceptives in pregnancy and major structural birth defects in offspring. Epidemiology 2010; 21: 232–9. [DOI] [PubMed] [Google Scholar]
- 42. Torfs CP, Katz EA, Bateson TF, Lam PK, Curry CJ. Maternal medications and environmental exposures as risk factors for gastroschisis. Teratology 1996; 54: 84–92. [DOI] [PubMed] [Google Scholar]
- 43. Werler MM, Mitchell AA, Shapiro S. First trimester maternal medication use in relation to gastroschisis. Teratology 1992; 45: 361–7. [DOI] [PubMed] [Google Scholar]
- 44. Lind JN, Tinker SC, Broussard CS, Reefhuis J, Carmichael SL, Honein MA, et al. Maternal medication and herbal use and risk for hypospadias: data from the National Birth Defects Prevention Study, 1997–2007. Pharmacoepidemiol Drug Saf 2013; 22: 783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Rooij IALM, van der Zanden LFM, Brouwers MM, Knoers NVAM, Feitz WFJ, Roeleveld N. Risk factors for different phenotypes of hypospadias: results from a Dutch case‐control study. BJU Int 2013; 112: 121–8. [DOI] [PubMed] [Google Scholar]
- 46. Carmichael SL, Shaw GM, Laurent G, Croughan M, Olney RS, Lammer EJ. Hypospadias and maternal intake of progestins and oral contraceptives. Birth Defects Res (Part A) Clin Mol Teratol 2004; 70: 255. [Google Scholar]
- 47. Sweet RA, Schrott HG, Culp DS. Study of the incidence of hypospadias in Rochester, Minnesota, 1940–70, and a case‐control comparison of possible etiologic factors. Proceedings, Mayo Clin. 1974; 1: 52–8. [PubMed] [Google Scholar]
- 48. Kallen B, Castilla EE, Robert E, Lancaster PAL, Kringelbach M, Mutchinick O, et al. An international case‐control study on hypospadias the problem with variability and the beauty of diversity. Eur J Epidemiol 1992; 8: 256–63. [DOI] [PubMed] [Google Scholar]
- 49. Levy EP, Cohen A, Fraser FC. Hormone treatment during pregnancy and congenital heart defects. Lancet 1973; 301: –611. [DOI] [PubMed] [Google Scholar]
- 50. Nora JJ, Nora AH. Birth defects and oral contraceptives. Lancet 1973; 301: 941–2. [DOI] [PubMed] [Google Scholar]
- 51. Nora J, Nora A, Perinchief A, Ingram J, Fountain A, Peterson M. Congenital abnormalities and first‐trimester exposure to progestagen/oestrogen. Lancet 1976; 307: 313–4. [DOI] [PubMed] [Google Scholar]
- 52. Heinonen OP, Slone DS, Monson RR, Hook EB, Shapiro SS. Cardiovascular birth defects and antenatal exposure to female sex hormones. N Engl J Med 1977; 296: 67–70. [DOI] [PubMed] [Google Scholar]
- 53. Janerich DT, Dugan JM, Standfast SJ, Strite L. Congenital heart disease and prenatal exposure to exogenous sex hormones. Br Med J 1977; 1: 1058–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nora AH, Nora JJ. A syndrome of multiple congenital anomalies associated with teratogenic exposure. Arch Environ Heal An Int J 1975; 30: 17–21. [DOI] [PubMed] [Google Scholar]
- 55. Jaffe P, Liberman MM, McFadyen I, Valman HB. Incidence of congenital limb‐reduction deformities. Lancet 1975; 305: 526–7. [DOI] [PubMed] [Google Scholar]
- 56. Brent RL. Cardiovascular birth defects and prenatal exposure to female sex hormones: importance of utilizing proper epidemiological methods and teratologic principles. Teratology 1994; 49: 159–61. [DOI] [PubMed] [Google Scholar]
- 57. Brent RL. Nongenital malformations following exposure to progestational drugs: the last chapter of an erroneous allegation. Birth Defects Res A Clin Mol Teratol 2005; 73: 906–18. [DOI] [PubMed] [Google Scholar]
- 58. Källén BAJ, Martínez‐Frías ML, Castilla EE, Robert E, Lancaster PAL, Kringelbach M, et al. Hormone therapy during pregnancy and isolated hypospadias: an international case‐control study. Int J Risk Saf Med 1992; 3: 183–98. [DOI] [PubMed] [Google Scholar]
- 59. Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med 2005; 159: 957–62. [DOI] [PubMed] [Google Scholar]
- 60. Meijer WM, de Jong‐van den Berg LT, van den Berg MD, Verheij JB, de Walle HE. clomiphene and hypospadias: the necessity to investigate on a detailed level. Reprod Toxicol 2005; 3: 472–3. [Google Scholar]
- 61. Meijer WM, de Jong‐Van den Berg LTW, van den Berg MD, Verheij JBGM, de Walle HEK. Clomiphene and hypospadias on a detailed level: signal or chance? Birth Defects Res A Clin Mol Teratol 2006; 76: 249–52. [DOI] [PubMed] [Google Scholar]
- 62. Meijer W, de Walle HE, Bakkler MK, van den Berg MD, Verheij JB, de Jong‐vanden Berg LT. Association between clomiphene treatment and congenital anomalies. Pharmacoepidemiol Drug Saf 2005; 14 (Supplement 2): S74. [Google Scholar]
- 63. Sørensen HT, Pedersen L, Skriver MV, Nørgaard M, Nørgård B, Hatch EE. Use of clomifene during early pregnancy and risk of hypospadias: population based case‐control study. BMJ 2005; 330: 126–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ghassan A‐Q, Bassam A‐N, Ghazi A‐S, Eqab A‐M, Elena A‐Q. Hypospadias: does the usage of Clomiphene citrate influence the incidence? Middle East Journal of Family Medicine 2006. Available at http://www.mejfm.com/Newarchives2013/Hypospadias.htm (last accessed 18 April 2016).
- 65. UK Teratology Information Service . Exposure to oral contraceptives in pregnancy [online]. TOXBASE, 2014. Available at http://www.toxbase.org/upload/Pregnancy pdfs/Oral_Contraceptives_2014.pdf (last accessed 22 August 2014)
- 66. Reefhuis J, Honein MA, Schieve LA, Rasmussen SA. Use of clomiphene citrate and birth defects, National Birth Defects Prevention Study, 1997–2005. Hum Reprod 2011; 26: 451–7. [DOI] [PubMed] [Google Scholar]
- 67. Pinborg A, Henningsen AK, Malchau SS, Loft A. Congenital anomalies after assisted reproductive technology. Fertil Steril 2013; 99: 327–32. [DOI] [PubMed] [Google Scholar]
- 68. Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. N Engl J Med 2012; 366: 1803–13. [DOI] [PubMed] [Google Scholar]
- 69. Elizur SE, Tulandi T. Drugs in infertility and fetal safety. Fertil Steril 2008; 89: 1595–602. [DOI] [PubMed] [Google Scholar]
- 70. Kumar RM, Hughes PF, Khurranna A. Zidovudine use in pregnancy: a report on 104 cases and the occurrence of birth defects. J Acquir Immune Defic Syndr 1994; 7: 1034–9. [PubMed] [Google Scholar]
- 71. Källén B, Nilsson E, Otterblad Olausson P. Maternal use of loperamide in early pregnancy and delivery outcome. Acta Paediatr 2008; 97: 541–5. [DOI] [PubMed] [Google Scholar]
- 72. Wilton LV, Pearce GL, Martin RM, Mackay FJ, Mann RD. The outcomes of pregnancy in women exposed to newly marketed drugs in general practice in England. Br J Obstet Gynaecol 1998; 105: 882–9. [DOI] [PubMed] [Google Scholar]
- 73. Shuhaiber S, Pastuszak A, Schick B, Matsui D, Spivey G, Brochu J, et al. Pregnancy outcome following first trimester exposure to sumatriptan. Neurology 1998; 51: 581–3. [DOI] [PubMed] [Google Scholar]
- 74. Olesen C, Steffensen FH, Sørensen HT. Pregnancy outcome following prescription for sumatriptan. Headache 2000; 40: 20–4. [DOI] [PubMed] [Google Scholar]
- 75. Nezvalová‐Henriksen K, Spigset O, Nordeng H. Triptan safety during pregnancy: a Norwegian population registry study. Eur J Epidemiol 2013; 28: 759–69. [DOI] [PubMed] [Google Scholar]
- 76. Källén B, Lygner PE. Delivery outcome in women who used drugs for migraine during pregnancy with special reference to sumatriptan. Headache 2001; 41: 351–6. [DOI] [PubMed] [Google Scholar]
- 77. Ephross SA, Sinclair SM. Final results from the 16‐year sumatriptan, naratriptan, and treximet pregnancy registry. Headache 2014; 54: 1158–72. [DOI] [PubMed] [Google Scholar]
- 78. Källén B, Nilsson E, Otterblad Olausson P. Delivery outcome after maternal use of drugs for migraine: a register study in Sweden. Drug Saf 2011; 34: 691–703. [DOI] [PubMed] [Google Scholar]
- 79. Fiore M, Shields KE, Santanello N, Goldberg MR. Exposure to rizatriptan during pregnancy: post‐marketing experience up to 30 June 2004. Cephalalgia 2005; 25: 685–8. [DOI] [PubMed] [Google Scholar]
- 80. Nezvalová‐Henriksen K, Spigset O, Nordeng H. Triptan exposure during pregnancy and the risk of major congenital malformations and adverse pregnancy outcomes: results from the Norwegian Mother and Child Cohort Study. Headache 2010; 50: 563–75. [DOI] [PubMed] [Google Scholar]
- 81. Nezvalová‐Henriksen K, Spigset O, Nordeng H. Errata in ‘Triptan exposure during pregnancy and the risk of major congenital malformations and adverse pregnancy outcomes: results from the Norwegian Mother and Child Cohort Study’. Headache 2012; 52: 1319–20. [DOI] [PubMed] [Google Scholar]
- 82. Bánhidy F, Acs N, Puhó EH, Czeizel AE. Chronic hypertension with related drug treatment of pregnant women and congenital abnormalities in their offspring: a population‐based study. Hypertens Res 2011; 34: 257–63. [DOI] [PubMed] [Google Scholar]
- 83. Cooper WO, Hernandez‐Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first‐trimester exposure to ACE inhibitors. N Engl J Med 2006; 354: 2443–51. [DOI] [PubMed] [Google Scholar]
- 84. Li D‐K, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. Br Med J (Clin Res Ed) 2011; 343: d5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C. Assisted reproductive technology and birth defects: a systematic review and meta‐analysis. Hum Reprod Update 2013; 19: 330–53. [DOI] [PubMed] [Google Scholar]
- 86. Mayer A, Lunenfeld E, Wiznitzer A, Har‐Vardi I, Bentov Y, Levitas E. Increased prevalence of gestational diabetes mellitus in in vitro fertilization pregnancies inadvertently conceived during treatment with long‐acting triptorelin acetate. Fertil Steril 2005; 84: 789–92. [DOI] [PubMed] [Google Scholar]
- 87. Abu‐Heija AT, Fleming R, Yates RWS, Coutts JRT. Pregnancy outcome following exposure to gonadotrophin‐releasing hormone analogue during early pregnancy: comparisons in patients with normal or elevated luteinizing hormone. Hum Reprod 1995; 10: 3317–9. [DOI] [PubMed] [Google Scholar]
- 88. Platteau P, Gabbe M, Famelos M, Kovacs G, Healy D. Should we still advise infertile couples to use (barrier) contraception before IVF down‐regulation? Fertil Steril 2000; 74: 655–9. [DOI] [PubMed] [Google Scholar]
- 89. Elefant E, Biour B, Blumberg‐Tick J, Roux C, Thomas F. Administration of a gonadotropin‐releasing hormone agonist during pregnancy: follow‐up of 28 pregnancies exposed to triptoreline. Fertil Steril 1995; 63: 1111–3. [DOI] [PubMed] [Google Scholar]
- 90. Sørensen HT, Nielsen GL, Christensen K, Tage‐Jensen U, Ekbom A, Baron J. Birth outcome following maternal use of metoclopramide. Br J Clin Pharmacol 2000; 49: 264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Berkovitch M, Mazzota P, Greenberg R, Elbirt D, Addis A, Schuler‐Faccini L, et al. Metoclopramide for nausea and vomiting of pregnancy: a prospective multicenter international study. Am J Perinatol 2002; 19: 311–6. [DOI] [PubMed] [Google Scholar]
- 92. Choi J‐S, Han J‐Y, Ahn H‐K, Ryu H‐M, Kim M‐Y, Yang J‐H, et al. Fetal and neonatal outcomes in women taking domperidone during pregnancy. J Obstet Gynaecol 2013; 33: 160–2. [DOI] [PubMed] [Google Scholar]
- 93. Addis A, Bailey B, Lee A, Lau M, Koren G. Safety of cisapride use during pregnancy: a prospective controlled cohort study. Teratology 1997; 55: 100. [Google Scholar]
- 94. Bailey B, Addis A, Lee A, Sanghvi K, Mastroiacovo P, Mazzone T, et al. Cisapride use during human pregnancy: a prospective, controlled multicenter study. Dig Dis Sci 1997; 42: 1848–52. [DOI] [PubMed] [Google Scholar]
- 95. Anderka M, Mitchell AA, Louik C, Werler MM, Hernández‐Diaz S, Rasmussen SA. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol 2012; 94: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pasternak B, Svanström H, Mølgaard‐Nielsen D, Melbye M, Hviid A. Metoclopramide in pregnancy and risk of major congenital malformations and fetal death. J Am Med Assoc 2013; 310: 1601–11. [DOI] [PubMed] [Google Scholar]
- 97. Matok I, Gorodischer R, Koren G, Sheiner E, Wiznitzer A, Levy A. The safety of metoclopramide use in the first trimester of pregnancy. N Engl J Med 2009; 360: 2528–35. [DOI] [PubMed] [Google Scholar]
- 98. Garne E, Dolk H, Loane M, Boyd PA, on behalf of EUROCAT . EUROCAT website data on prenatal detection rates of congenital anomalies. J Med Screen 2010; 17: 97–8. [DOI] [PubMed] [Google Scholar]
- 99. de Jonge L, Garne E, Gini R, Jordan SE, Klungsoyr K, Loane M, et al. Improving information on maternal medication use by linking prescription data to congenital anomaly registers: a EUROmediCAT study. Drug Saf 2015; 38: 1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu R, Wang Q. Large‐scale combining signals from both biomedical literature and the FDA Adverse Event Reporting System (FAERS) to improve post‐marketing drug safety signal detection. BMC Bioinformatics 2014; 15: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Brent RL. The cause and prevention of human birth defects: what have we learned in the past 50 years? Congenit Anom (Kyoto) 2001; 41: 3–21. [Google Scholar]
- 102. Garlapati S, Priyanka S. Cradles of signals for pharmacovigilance process. J Pharmacovigil 2014; 3: 1–2. [Google Scholar]
- 103. Bánhidy F, Dakhlaoui A, Puhó EH, Czeizel AE. Peptic ulcer disease with related drug treatment in pregnant women and congenital abnormalities in their offspring. Congenit Anom (Kyoto) 2011; 51: 26–33. [DOI] [PubMed] [Google Scholar]
- 104. Jick H, Holmes LB, Hunter JR, Madsen S, Stergachie A. First‐trimester drug use and congenital disorders. J Am Med Assoc 1981; 246: 343–6. [PubMed] [Google Scholar]
- 105. Einarson A, Mastroiacovo P, Arnon J, Ornoy A, Addis A. Prospective, controlled, multicentre study of loperamide in pregnancy. Can J Gastroenterol 2000; 14: 1999–2001. [DOI] [PubMed] [Google Scholar]
- 106. Czeizel AE, Vass J. Teratological surveillance of oral contraceptive use in early pregnancy. Adv Contracept Deliv Syst 1996; 12: 51–9. [Google Scholar]
- 107. World Health Organization . The effect of female sex hormones on fetal development and infant health [online]. World Health Organization technical report series, 1981; 1–76. Available at http://www.ncbi.nlm.nih.gov/pubmed/6785928 (last accessed 18 April 2016). [PubMed]
- 108. Bracken MB. Oral contraception and congenital malformations in offspring: a review and meta‐analysis of the prospective studies. Obstet Gynecol 1990; 76 (3 Part 2): 552–7. [PubMed] [Google Scholar]
- 109. Kasan PN, Andrews J. Oral contraception and congenital abnormalities. Br J Obstet Gynaecol 1980; 87: 545–51. [DOI] [PubMed] [Google Scholar]
- 110. Cuckle HS, Wald NJ. Evidence against oral contraceptives as a cause of neural‐tube defects. Br J Obstet Gynaecol 1982; 89: 547–9. [DOI] [PubMed] [Google Scholar]
- 111. Shaw GM, Todoroff K, Velie EM, Lammer EJ. Maternal illness, including fever, and medication use as risk factors for neural tube defects. Teratology 1998; 57: 1–7. [DOI] [PubMed] [Google Scholar]
- 112. Hill L, Murphy M. Maternal drug histories and congenital malformations: limb reduction defects and oral clefts. J Epidemiol Community Health 1988; 42: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Raman‐Wilms L, Tseng AL, Wighardt S, Einarson TR, Koren G. Fetal genital effects of first‐trimester sex hormone exposure: a meta‐analysis. Obstet Gynecol 1995; 85: 141–9. [DOI] [PubMed] [Google Scholar]
- 114. Wogelius P, Horváth‐Puhó E, Pedersen L, Nørgaard M, Czeizel AE, Sørensen HT. Maternal use of oral contraceptives and risk of hypospadias – a population‐based case‐control study. Eur J Epidemiol 2006; 21: 777–81. [DOI] [PubMed] [Google Scholar]
- 115. Nørgaard M, Wogelius P, Pedersen L, Rothman KJ, Sørensen HT. Maternal use of oral contraceptives during early pregnancy and risk of hypospadias in male offspring. Urology 2009; 74: 583–7. [DOI] [PubMed] [Google Scholar]
- 116. Bjerkedal T, Bakketeig LS. Orientering om en undersokelse over arsaken till en registrert oking av misdannelser i urin‐og kjonnsorganer. Tidskr Nor Laegefor 1975; 95: 1436–7. [PubMed] [Google Scholar]
- 117. Li DK, Daling JR, Mueller BA, Hickok DE, Fantel AG, Weiss NS. Oral contraceptive use after conception in relation to the risk of congenital urinary tract anomalies. Teratology 1995; 51: 30–6. [DOI] [PubMed] [Google Scholar]
- 118. Varma TR, Morsman J. Evaluation of the use of prolution‐depot (hydroxyprogesterone hexanoate) in early pregnancy. Int J Gynecol Obstet 1982; 20: 13–7. [DOI] [PubMed] [Google Scholar]
- 119. Michaelis J, Michaelis H, Glück E, Koller S. Prospective study of suspected associations between certain drugs administered during early pregnancy and congenital malformations. Teratology 1983; 27: 57–64. [DOI] [PubMed] [Google Scholar]
- 120. Resseguie LJ, Hick JF, Bruen JA, Noller KL, O'Fallon WM, Kurland LT. Congenital malformations among offspring exposed in utero to progestins, Olmsted county, Minnesota, 1936–1974. Fertil Steril 1985; 43: 514–9. [DOI] [PubMed] [Google Scholar]
- 121. Katz Z, Lancet M, Skornik J, Chemke J, Mogilner BM, Klinberg M. Teratogenicity of progestogens given during the first trimester of pregnancy. Obstet Gynecol 1985; 65: 775–80. [PubMed] [Google Scholar]
- 122. Lammer EJ, Cordero JF. Exogenous sex hormone exposure and the risk for major malformations. JAMA 1986; 255: 3128–32. [PubMed] [Google Scholar]
- 123. Heinonen OP, Slone D, Shapiro S. Birth Defects and Drugs in Pregnancy. Littleton, MA: Publishing Sciences Group, 1977. [Google Scholar]
- 124. Yovich JL, Turner SR, Draper R. Medroxyprogesterone acetate therapy in early pregnancy has no apparent fetal effects. Teratology 1988; 38: 135–44. [DOI] [PubMed] [Google Scholar]
- 125. Colvin L, Slack‐Smith L, Stanley FJ, Bower C. Linking a pharmaceutical claims database with a birth defects registry to investigate birth defect rates of suspected teratogens. Pharmacoepidemiol Drug Saf 2010; 19: 1137–50. [DOI] [PubMed] [Google Scholar]
- 126. Dudás I, Gidai J, Czeizel AE. Population‐based case‐control teratogenic study of hydroxyprogesterone treatment during pregnancy. Congenit Anom (Kyoto) 2006; 46: 194–8. [DOI] [PubMed] [Google Scholar]
- 127. Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med 2005; 159: 957–62. [DOI] [PubMed] [Google Scholar]
- 128. Baeka K, Rosenwaksa Z, Poppasa DP, Palermoa GD. Does progesterone administration increase the incidence of neonatal hypospadias? Fertil Steril 2006; 86 (Supplement 2): S337. [Google Scholar]
- 129. Chez RA. Proceedings of the symposium ‘progesterone, progestins, and fetal development’. Fertil Steril 1978; 30: 16–26. [PubMed] [Google Scholar]
- 130. Rothman KJ, Fyler DC, Goldblatt A, Kreidberg MB. Exogenous hormones and other drug exposures of children with congenital heart disease. Am J Epidemiol 1979; 109: 433–9. [DOI] [PubMed] [Google Scholar]
- 131. Queisser‐Luft A. Dydrogesterone use during pregnancy: overview of birth defects reported since 1977. Early Hum Dev 2009; 85: 375–7. [DOI] [PubMed] [Google Scholar]
- 132. El‐Zibdeh MY. Dydrogesterone in the reduction of recurrent spontaneous abortion. J Steroid Biochem Mol Biol 2005; 97: 431–4. [DOI] [PubMed] [Google Scholar]
- 133. El‐Zibdeh MY, Yousef LT. Dydrogesterone support in threatened miscarriage. Maturitas 2009; 65 (Supplement 1): S43–6. [DOI] [PubMed] [Google Scholar]
- 134. Pandian RU. Dydrogesterone in threatened miscarriage: a Malaysian experience. Maturitas 2009; 65 (Suppl 1): S47–50. [DOI] [PubMed] [Google Scholar]
- 135. Greenland S, Ackerman DL. Clomiphene citrate and neural tube defects: a pooled analysis of controlled epidemiologic studies and recommendations for future studies. Fertil Steril 1995; 64: 936–41. [DOI] [PubMed] [Google Scholar]
- 136. Medveczky E, Puhó E, Czeizel EA. The use of drugs in mothers of offspring with neural‐tube defects. Pharmacoepidemiol Drug Saf 2004; 13: 443–55. [DOI] [PubMed] [Google Scholar]
- 137. Wu YW, Croen LA, Henning L, Najjar DV, Schembri M, Croughan MS. A potential association between infertility and spinal neural tube defects in offspring. Birth Defects Res A Clin Mol Teratol 2006; 76: 718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Loffredo CA, Ferencz C, Rubin JD, Correa‐Villaseinor A, Wilson PD. A comparative epidemiologic evaluation of risk factors for hypoplastic left heart syndrome, aortic stenosis, and coarctation of the aorta. Teratology 1996; 53: 115. [Google Scholar]
- 139. Joao EC, Calvet GA, Krauss MR, Hance F, Ortiz J, Ivalo SA, et al. Maternal antiretroviral use during pregnancy and infant congenital anomalies: the NISDI Perinatal Study. J Acquir Immune Defic Syndr 2010; 53: 176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Patel D, Thorne C, Fiore S, Newell M‐L. Does highly active antiretroviral therapy increase the risk of congenital abnormalities in HIV‐infected women? J Acquir Immune Defic Syndr 2005; 40: 116–8. [DOI] [PubMed] [Google Scholar]
- 141. Knapp KM, Brogly SB, Muenz DG, Spiegel HML, Conway DH, Scott GB, et al. Prevalence of congenital anomalies in infants with in utero exposure to antiretrovirals. Pediatr Infect Dis J 2012; 31: 164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Floridia M, Mastroiacovo P, Tamburrini E, Tibaldi C, Todros T, Crepaldi A, et al. Birth defects in a national cohort of pregnant women with HIV infection in Italy, 2001–2011. BJOG 2013; 120: 1466–75. [DOI] [PubMed] [Google Scholar]
- 143. Sibiude J, Mandelbrot L, Blanche S, Le Chenadec J, Boullag‐Bonnet N, Faye A, et al. Association between prenatal exposure to antiretroviral therapy and birth defects: an analysis of the French perinatal cohort study (ANRS CO1/CO11). PLoS Med 2014; 11: e1001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Brogly SB, Abzug MJ, Watts DH, Cunningham CK, Williams PL, Oleske J, et al. Birth defects among children born to human immunodeficiency virus‐infected women: pediatric AIDS clinical trials protocols 219 and 219C. Pediatr Infect Dis J 2011; 29: 721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Antiretroviral Pregnancy Registry Steering Committee . Antiretroviral Pregnancy Registry International Interim Report for 1 January 1989 through 31 July 2011, 2014.
- 146. Newschaffer CJ, Cocroft J, Anderson CE, Hauck WW, Turner BJ. Prenatal zidovudine use and congenital anomalies in a medicaid population. J Acquir Immune Defic Syndr 2000; 24: 249–56. [DOI] [PubMed] [Google Scholar]
- 147. Watts DH, Huang S, Culnane M, Kaiser KA, Mofenson L, Stanley K, et al. Birth defects among a cohort of infants born to HIV‐infected women on antiretroviral medication. J Perinat Med. 2011; 39: 163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Watts DH, Li D, Handelsman E, Tilson H, Paul M, Foca M, et al. Assessment of birth defects according to maternal therapy among infants in the Women and Infants Transmission Study. J Acquir Immune Defic Syndr 2007; 44: 299–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 EUROmediCAT signal detection dataset
Document S1 Literature review methodology
Supplementary Table 1: EUROmediCAT signal detection dataset
Supplementary document 1: Literature review methodology


