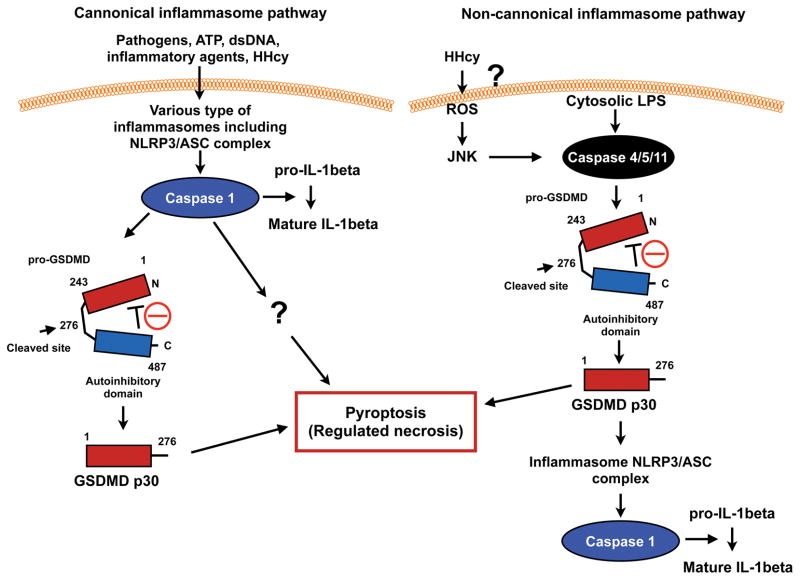
Pyroptosis is characterized by pore formation in the plasma membrane, cell swelling, and membrane disruption1, 2. These cellular events are also noted in necrosis, but not apoptosis, which is defined as a mode of “regulated or programmed necrosis”2. Jorgensen et al have defined pyroptosis by the following four criteria; (1) programmed by an inflammatory caspase activation, (2) pore formation in the plasma membrane, (3) DNA damage with TUNEL positivity at a lower intensity than apoptosis, and (4) ADP-ribose polymerase activation following the pyroptosis-mediated DNA damage3. The inflammasome is a large complex containing NOD-like receptors (NLRs), pro-caspase-1, and apoptosis-associated speck-like protein containing a CARD (ASC), which regulates both pyroptosis and the maturation and secretion of pro-inflammatory IL-1β and IL-184. A study5 from Hong Wang’s laboratory in this issue of the journal has now indicated a critical role for the caspase 1-inflammasome in regulating hyperhomocysteinemia (HHcy)-induced pyroptosis and apoptosis. In particular, they show that HHcy mediated aortic endothelial dysfunction induced by the depletion of cystathionine β-synthase was rescued by the depletion of caspase-1 and NLRP3. This suggests that the inflammasome plays a crucial role in regulating HHcy-induced endothelial dysfunction. In this editorial, we will briefly review the regulatory mechanism of caspase 1 and 4/5/11-mediated pyroptosis in modulating endothelial cell pyroptosis/apoptosis, and consequent endothelial dysfunction.
The caspase protease family has central roles in cell death and inflammation. Mammalian caspases can be divided into three groups based on their functions 6. Caspase 3, 6, and 7 are executioner caspases with a prodomain in the NH2-terminus that cleaves substrates essential for cellular homeostasis. Caspase 2, 8, 9, and 10 are initiator caspases with two DED (caspase 8 and 10) or one CARD domain (caspase 2 and 9) in its NH2-terminus region, and act as proteolitic signal transducers. Both executioner and initiator caspases contribute to apoptotic cell death. Caspase 1, 4, 5, 11, and 12 are so called “inflammatory” caspases with the CARD domain in its NH2-terminus region. Caspase 11 is present only in rodents, and its human counterparts are caspase 4 and 5 (Caspase 4/5/11). They are directly activated by cytosolic bacterial lipopolysaccharide (LPS), and play a critical role in endotoxic shock7, 8. Caspase 12 is a dominant negative regulator of caspase 19. It has recently been shown that caspase 2, 3, 8, and 10 can regulate not only programmed cell death, but also inflammatory and immune reactions to dying cells and microbial stimuli9. In contrast, caspase 9 has not yet been directly associated with inflammatory responses9.
The critical role of the caspase 1-inflammasome in regulating pyroptosis has been reported (canonical inflammasome pathway)3 (Fig. 1). However, this role was deduced from the use of caspase1-deficient mice which have been shown to also carry an inactivating passenger mutation in the caspase 11 gene, raising the question whether there is a role for caspase 11 related effects 10. In addition, a caspase 1/inflammasome-independent pyroptosis (non-cannonical inflammasome pathway) has also been proposed. Both Dixit11 and Shao’s1 labs reported the crucial role of gasdermin D (GSDMD) cleaved by caspase 1 and 4/5/11 in eliciting pyroptosis (Fig. 1). An unbiased forward genetic screen with ethyl-N-mutagenosed mice by Dixit’s lab and CRISPR-Cas9 nuclease screens of caspase 11 and caspase 1-mediated pyroptosis in mouse bone marrow macrophages by Shao’s lab link GSDMD to LPS-mediated pyroptosis. Han’s lab also detected GSDMD protein in nigericin-induced NLRP3 inflammasomes by a quantitative mass spectrometry-based analysis12. GSDMD shows cytoplasmic and membranous expression in many cell types, and lacks any obvious signal peptide or transmembrane segments1.
Figure 1. Scheme for canonical and non-canonical inflammasome signalings.
(Adapted from Kayagaki et al11 with permission from Macmillan Publishers Ltd, copyright 2015)
Caspase 1 can be activated by various stimuli including ATP, cholesterol crystals, double strand DNA, and flagellin via several different types of inflammasomes (please see other reviews3, 4). In contrast, as stated above caspase 11 in the non-canonical inflammasome has been reported to directly sense cytosolic LPS. The caspase 11 CARD binds LPS, presumably via the acidic phosphate moieties of lipid A, and cause caspase 11 CARD oligomerization and activation3, 13, 14. The depletion of GSDMD and caspase 11 inhibited cytosolic LPS-induced pyroptosis and the processing and secretion of IL-1β. Interestingly, both caspase 1 and caspase 4/5/11 directly cleave the 53-kDa inactive precursor form of GSDMD (Pro-GSDMD) to generate GSDMD p30 of the pro-pyroptotic NH2-terminus fragment. It was thought that the COOH-terminus of GSDMD (p23) inhibits the pro-pyroptotic function of GSDMD p30, and cleavage at D276↓G277 releases GSDMD p23-mediated inhibition (Fig. 1). Shi et al have reported that aa residues1-243 of GSDMD is the minimal fragment capable of triggering pyroptosis1, 11.
In the canonical pathway, caspase 1 activation induced by various types of inflammasomes triggers pyroptosis by releasing the cleaved GSDMD p30 fragment, which bears intrinsic pyroptosis-inducing activity described above1, 11. Since caspase 1-mediated pyroptosis is only delayed and not completely inhibited by the depletion of GSDMD, the existence of another caspase 1-dependent pyroptosis pathway was suggested (Fig. 1)11. Caspase 1 can also cleave caspase 3 and 7 (3/7) independently on GSDMD as described in the current study by Xi et al. However, since GSDMD cleavage occurred much earlier than Caspase 3/7 cleavage, Shi et al have suggested that caspase 3/7 cleavage plays no role in caspase 1-mediated pyroptosis, but may have some role in apoptosis. In the current study by Xi et al., caspase 3 inhibitor inhibited the R1 fraction (so called “pyroptosis” fraction) after 24 hrs of L-Hcy and/or LPS stimulation, suggesting that the R1 fraction in Fig. 3B may not be a pure “pyroptosis” fraction and may include a large number of late apoptotic and necroptotic cells. In addition, the authors showed that a caspase 9 inhibitor significantly inhibited pyroptosis in PI+/Annexin V− cells (Fig. 3A–B) raising the question of how low caspase 9 activity was detected in the “pyroptosis” fraction of PI+/Annexin V− cells. In the canonical pathway the depletion of GSDMD has no effect on caspase 1-dependent IL-1β processing, but shows some effect on IL-1β release11.
In the non-canonical pathway direct activation of caspase 4/5/11 induced by cytosolic LPS cleaves pro-GSDMD and converts it to the mature GSDMD p30 form. This is sufficient for inducing pyroptosis (Fig. 1)1. The difference between the canonical (non-LPS) and non-cannonical (cytosolic LPS dependent) pathways is that cleavage of GSDMD is required for caspase 1/IL-1β processing in the non-cannonical pathway, but not in the canonical pathway. Loss of inflammasome components such as NLRP3, ASC, or caspase 1 showed no effect on caspase 11-mediated GSDMD cleavage, suggesting that the cleavage of GSDMD is an upstream event of caspase 4/5/11-mediated inflammasome activation. In contrast, the cleavage of GSDMD is a downstream event in the canonical pathway, and GSDMD p30 is not required for initial inflammasome activation (Fig. 1)11. Of note, it remains unclear how GSDMD p30 can induce pyroptosis. Pore formation is a feature of pyroptosis, and it is suggested that GSDMD p30 may directly induce plasma membrane pore formation 12, but this needs further investigation.
In addition to GSDMD, the crucial role of pannexin 1 and purinergic receptor P2X ligand-gated ion channel (P2X7) in caspase 11-mediated pyroptosis has been reported15. Pannexin 1 is a non-selective, large-pore channel, which release nucleotide including ATP. Exogenous ATP activates P2X7, leads to pore formation, depletes cytosolic K+, and induces pyroptosis. Yang et al have reported that LPS-induced caspase 11 activation cleaves cytosolic domains of pannexin-1 and release ATP in the extracellular medium, which activates P2X7 and causes pyroptosis15. Since there were contradictory data to suggest that the depletion of pannexin-1 and P2X7 showed no effect on caspase 11-mediated events10, further investigation is necessary to resolve these discrepancies.
Because inflammasome and pyropotosis regulation are so complex it is difficult to definitively demonstrate the signaling mechanisms involved. In this current issue Xi et al have focused on caspase 1 activity in HHcy induced pyropotosis. The potential contributions of caspase 4/5/11 were not explored. Lupfer et al have reported that reactive oxygen species (ROS) increases caspase 11 expression and activation via an increase in JNK activation, and subsequently activation of the non-canonical inflammasome 16 (Fig. 1). Xi et al studied pyroptosis after 24 hrs of Hcy treatment and showed the important role of ROS induction in regulating HHcy-mediated pyroptosis. Therefore, the involvement of the non-cannonical pathway in HHcy-mediated pyroptosis cannot yet be excluded. Miao et al have described that pyroptotic cell death pathway activated cells should be annexin V positive, because pores open in the cell membrane, permiting annexin V to enter and stain the inner membrane leaflet 17. Dr. Shao’s lab has also found that pyroptosic cells are annexin V+ using anthrax lethal toxin to trigger pure caspse-1-dependent pyroptosis in mouse macrophages (personal communication from Dr. Feng Shao, National Institute of Biological Sciences, Beijing, 102206, China). Pyroptosis can occur within 6 hrs of stimulation1, but Xi et al detected pyroptosis after 24 hrs of stimulation. Therefore, it is possible that the late apoptosis and necroptotic cells are also detected in the “pyroptosis” fraction. This study relied on the fractionation of PI+/annexin V− cells, but distinguishing between pyroptosis and other forms of necrosis can be difficult by simply using this narrow definition. The development of additional reliable markers is needed to better differentiate caspase mediated cell death process such as pyroptosis from apoptosis and necrosis.
Necrosis was once recognized as an “accidental” or “physical” cell death induced by physio-chemical stress2. This view has been greatly changed, and it is now clear that necrosis can take place in a genetically and well controlled manner defined as “regulated necrosis”2. Vanden Berghe et al have defined regulated necrosis as “a genetically controlled cell death process, which leads to cellular leakage”2. Regulated necrosis is morphologically characterized by plasma membrane rupture, cytoplasmic granulation/vacuolization, and organelle and/or cellular swelling2, 18, 19. These morphological hallmarks are shared by other forms of regulated necrosis including necroptosis, parthanatos, oxytosis, ferroptosis, ETosis, NETosis, pyronecrosis and pyroptosis2. All of these processes can be characterized by unique and distinct molecular mechanisms, but also have numerous overlapping features of cell death. For example, necroptosis has been characterized by the signaling of RIPK1 (receptor-interacting protein kinase 1) and RIPK3-MLKL (mixed lineage kinase domain-like)2. Therefore, to study the pathological role of each mode of regulated necrosis it is important to define and clarify the unique signaling pathway for each form of regulated necrosis, the interplay between these different signaling pathways at the molecular level, and whether each path really has a distinct role in disease processes.
Xi et al5 have nicely shown the crucial role of caspase 1 and NLRP3 in HHcy-induced endothelial dysfunction. In addition, the possible contribution of pyroptosis in regulating HHcy-induced endothelial damage has been suggested. However, the definition of pyroptosis as PI+/Annexin V−/high caspase 1/low caspase 9 activity in cells may be an over-simplification. Determining the role of GSDMD in the process of HHcy-mediated endothelial dysfunction may also be important in clearly defining and identifying pyroptosis during this and other disease processes. This would involve exploring the distinct and unique molecular mechanisms that controls each form of regulated necrosis. It is clear that we do not know the exact contribution of various modes of regulated necrosis in endothelial dysfunction. This study has opened the door to the future study of regulated necrosis in controlling endothelial dysfunction.
Supplementary Material
Acknowledgments
Sources of Funding
The research activities of the authors are supported by grant from the National Institute of Health to Drs. Abe (HL-130193, HL-123346, HL-118462, HL-108551) and Morrell (HL124018-01A). Dr. Morrell is recipient of Established Investigator Awards of the American Heart Association (13EIA14250023).
Footnotes
Disclosures
None.
References
- 1.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of gsdmd by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 2.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Shao F. Diverse mechanisms for inflammasome sensing of cytosolic bacteria and bacterial virulence. Curr Opin Microbiol. 2016;29:37–42. doi: 10.1016/j.mib.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, Cheng X, Wang J, Qin X, Yu J, Ji Y, Yang X, Wang H. Caspase-1 inflammasome activation mediates homocysteine-induced pyrop-apoptosis in endothelial cells. Circulation Research. 2016;118 doi: 10.1161/CIRCRESAHA.116.308501. xxx-xxx [in this issue] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribe EM, Serrano-Saiz E, Akpan N, Troy CM. Mechanisms of neuronal death in disease: Defining the models and the players. Biochem J. 2008;415:165–182. doi: 10.1042/BJ20081118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic lps activates caspase-11: Implications in tlr4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical inflammasome activation by intracellular lps independent of tlr4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 9.Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases connect cell-death signaling to organismal homeostasis. Immunity. 2016;44:221–231. doi: 10.1016/j.immuni.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 11.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin d for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 12.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin d is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagar JA, Aachoui Y, Miao EA. Wildcards: Inflammatory caspases directly detect lps. Cell Res. 2015;25:149–150. doi: 10.1038/cr.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular lps. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 15.Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 requires the pannexin-1 channel and the purinergic p2x7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupfer CR, Anand PK, Liu Z, Stokes KL, Vogel P, Lamkanfi M, Kanneganti TD. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical nlrp3 inflammasome during enteric pathogen infection. PLoS Pathog. 2014;10:e1004410. doi: 10.1371/journal.ppat.1004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 19.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.