Abstract
Recent studies of sensorimotor processing have benefited from decision-making paradigms that emphasize the selection of appropriate movements. Selecting when to make those responses, or action timing, is important as well. Although the cerebellum is commonly viewed as a controller of movement dynamics, its role in action timing is also firmly supported. Several lines of research have now extended this idea. Anatomical findings have revealed connections between the cerebellum and broader timing circuits, neurophysiological results have suggested mechanisms for timing within its microcircuitry, and theoretical work has indicated how temporal signals are processed through it and decoded by its targets. These developments are inspiring renewed studies of the role of the cerebellar loops in action timing.
Introduction
Research on the cerebellar control of movement has focused on within-movement dynamics, characterizing how the cerebellum adjusts the degree and timing of muscle activations needed to achieve spatial accuracy. In addition to fine, muscle-level control, the cerebellum also contributes to inter-movement timing, or deciding when to move [1, 2]. This effector-level control – which we will call action timing – relies on distributed brain circuits involving the basal ganglia and cerebral cortex in addition to the cerebellum [3–12]. A challenge for behavioral neuroscience is to reconcile the evidence from across the brain to reveal the underlying networks for action timing and the cerebellum’s participation in them. A good start is to review what is known about the flow of temporal information in cortical and subcortical circuits. We will evaluate the position of the cerebellum in timing circuits and compare signals related to the timing of behavior in the inputs and outputs of the cerebellum. We limit the scope of this review to processes involved in the initiation of single movements, although action timing of multiple movements in parallel (e.g. hand-eye or bimanual coordination) or serially (e.g. coordination of tapping in timed sequences) is also of interest.
The cerebellum is part of a larger timing network
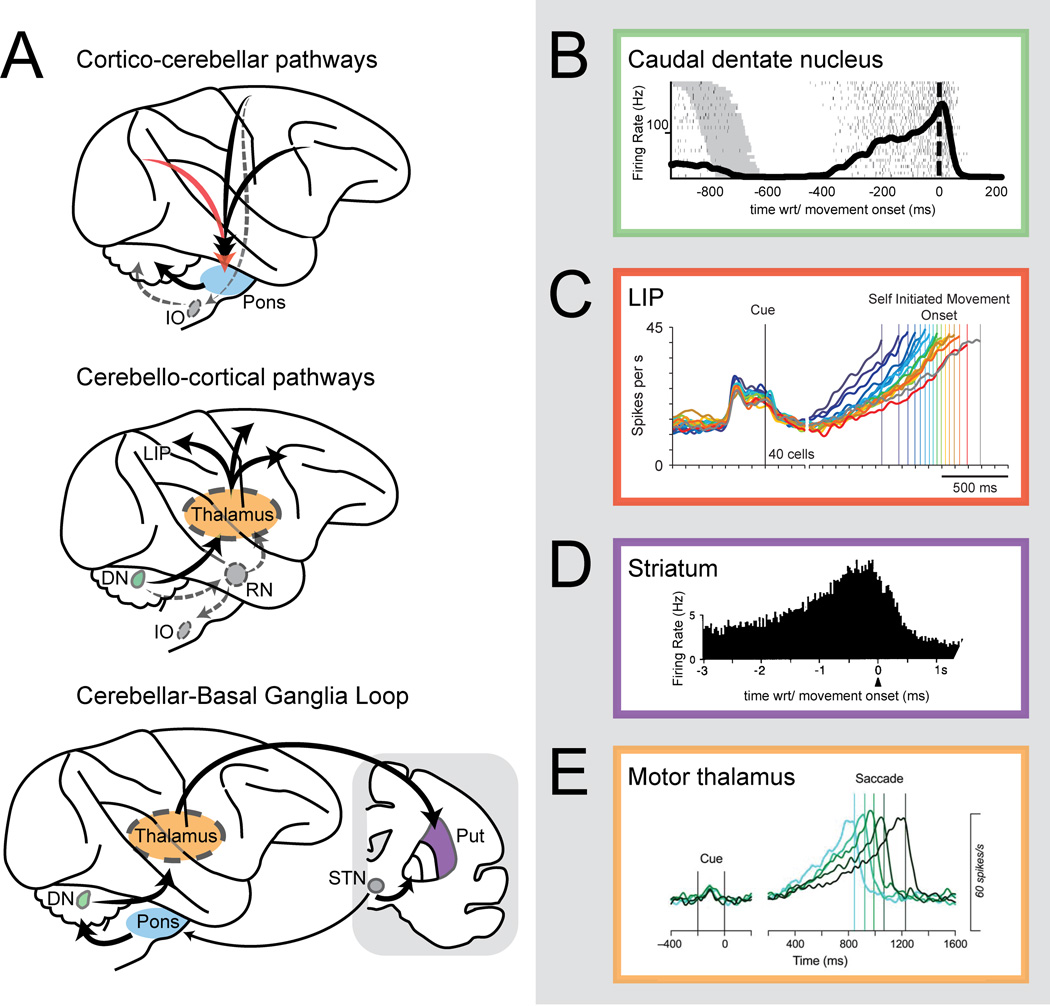
The schematic in Fig. 1A gives a basic overview of connectivity between the cortex, basal ganglia, and cerebellum. Major cerebellar afferents derive from brainstem nuclei that receive outflow (often collaterals of descending projections) from extended cerebral cortical regions and the basal ganglia. The basal ganglia and cerebrum, in turn, receive afferent input from thalamic structures targeted by cerebellar nuclei [13, 14, 15●]. A recent study from our laboratory [16●] demonstrated a strong correlation between oculomotor timing and neural activity in one of the nuclei, the dentate nucleus (DN). In monkeys trained to initiate saccadic eye movements to a visual target after an uncued, learned interval, a large proportion of DN neurons produced smooth ramps of activity up to and peaking at the initiation of the self-timed movement (Fig. 1B). While this was the first quantified report of such activity in the cerebellum, comparable ramps have been found in nearly every other brain area where similar tasks have been studied, including parietal cortex [17] (for example, Fig. 1C), motor cortex [18, 19], premotor cortex [20], supplementary motor cortex [21], pre-supplementary motor cortex [21], and prefrontal cortex [24]. Responses of neurons recorded at the input stages of the basal ganglia (specifically, regions of the striatum) [22, 23], also show similar responses (Fig. 1D). Finally thalamic nuclei that receive feedback projections from the cerebellum, basal ganglia or cerebral cortex exhibit ramping responses as well [24] (Fig. 1E). Work in computational neuroscience has added to these findings, showing that timing-related ramping activity can arise from dynamics embodied by recurrent excitatory connections in the cerebral cortex [25]. Other models show how different sorts of temporal representation might arise in the cerebellum [26, 27], or basal ganglia [28, 29●].
Figure 1.
Circuits and signals for action timing. (A) Schematic view of the circuits connected to the cerebellum for action timing. IO, inferior olive; DN, dentate nucleus; RN, red nucleus; Put., putamen; STN, subthalamic nucleus. Long-lead activity that ramps up before self-timed movements is found throughout those circuits, including (B) the caudal DN [16●], (C) the lateral intraparietal area (LIP) [17], (D) the striatum [23] and (E) the motor thalamus [24]. Figure from [17] adapted by permission from Macmillan Publishers Ltd: http://www.nature.com/neuro/, Nature Neuroscience, copyright 2006. Figures from [23] and [24] adapted with kind permission from Springer Science and Business Media and the Society for Neuroscience, respectively.
One begins to sense a chicken-and-egg problem when attempting to explain cerebellar contributions to action timing. If upstream timing representations are transmitted to the cerebellum, deficits in action timing associated with cerebellar lesions [1, 30, 31] could be simply a function of inaccurate decoding by cerebellar circuits. Inappropriate temporal signals would then carry forward into behavior via projections from the cerebellar nuclei to downstream motor structures in the cerebral cortex, the brainstem and spinal cord. Alternatively, the cerebellum may help generate temporal signals, distributing them to recipient motor circuits (Fig. 1A). Moreover, both cerebral cortical and cerebellar circuits seem capable of refining temporal signals, to the extent that in vivo and in vitro studies demonstrate that they can generate temporally precise outputs given temporally imprecise inputs [32, 33]. Similar evidence is lacking for basal ganglia circuits, although computational models implicate them in related forms of information processing for the generation of timing signals [28, 29●]. In addition, aforementioned reports of ramping-like activity in regions of motor thalamus that receive input from the cortex, cerebellum or basal ganglia, suggest that this information is transmitted, with little discernible difference, between all of these structures.
In the next sections, we present outstanding issues that we believe must be addressed to grasp the specific contributions of cerebellar circuits to action timing. We posit that understanding the signals passed between structures in carefully controlled behavioral paradigms can answer many of these questions, based on the success of this approach in answering earlier questions in cerebellar motor control.
How are timing signals emanating from the cerebellum decoded?
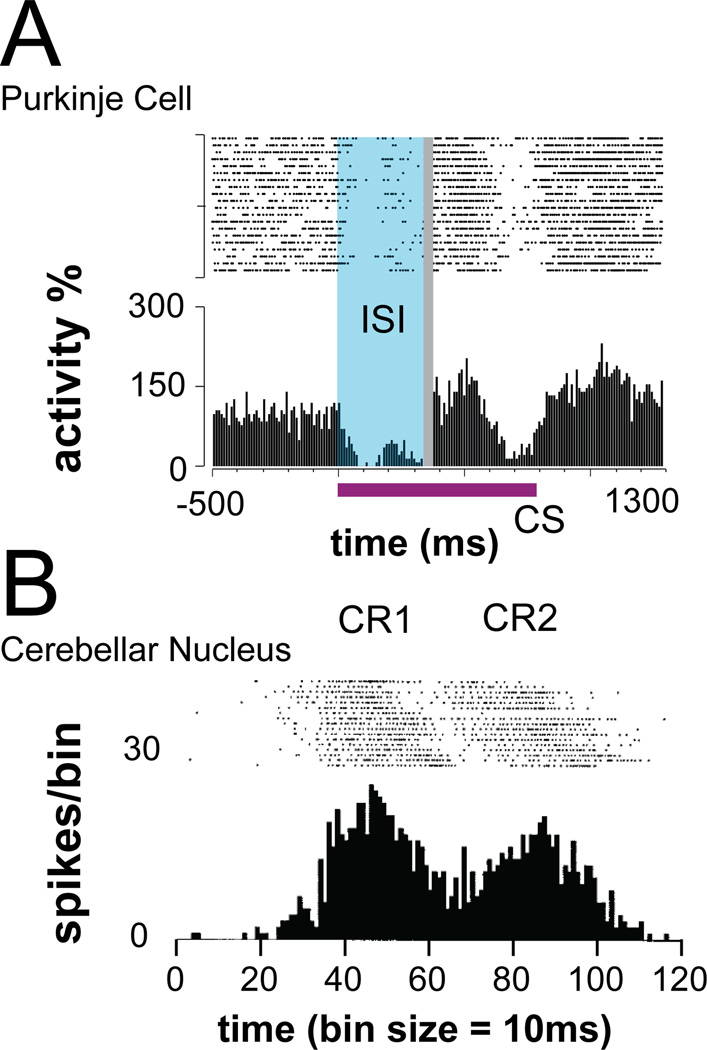
It is well accepted that cerebellar output is necessary for well-timed movements, based on myriad cerebellar lesion, degeneration, and inactivation studies [1, 2, 31, 34–37]. But at the level of information processing, how is cerebellar activity used? To begin answering this question, we need to know which cerebellar output pathways are influencing action timing and what signals are sent along these pathways. By describing the temporal representations conveyed to the motor periphery, we can investigate how they might be decoded. To illustrate this point, consider cerebellar studies on eyeblink conditioning. Pharmacological inactivation experiments showed that cerebellar circuits, in tandem with brainstem circuits, are necessary for learning to execute conditioned eyeblink responses that are timed to the predicted occurrence of air puffs [38, 39]. Notably, recording studies have shown that during eyeblink conditioning, pauses in Purkinje cells responses promote bursts of activity in deep cerebellar nuclear neurons (Fig. 2), which drive the conditioned eyeblink response via the red nucleus [40–44]. These pauses (for Purkinje cells) and bursts (cerebellar nuclear neurons) are modified via conditioning to generate the temporal structure of the eyeblink response [45], while convergent motor inputs to downstream brainstem circuitry regulates other movement parameters [44].
Figure 2.
Timing signals in cerebellar microcircuitry. Raster plots and response histogram are illustrated for (A) a Purkinje cell following a paired electrical conditional stimulus–unconditional stimulus protocol [62], and (B) an anterior interpositus nucleus neuron following eyeblink conditioning [42]. Activity patterns associated with the conditioned response (CR) include well-timed pauses for Purkinje cells and well-timed bursts for nuclear neurons. ISI, interstimulus interval; CS, conditional stimulus. Figures from [62] and [42] adapted with kind permission from the Proceedings of the National Academy of Sciences and the American Physiological Association, respectively.
Similar pharmacological studies – or more sophisticated genetically-based pathway manipulations [39, 46, 47] – are needed to clarify whether there is similar cerebellar involvement in interval timing, perceptual timing, and the timing of multiple movements in sequences. Future studies will also need to consider the signals passed along cerebellar output pathways to see how temporal information is communicated to downstream areas. Such data are required to guide our understanding of how this information is decoded by target structures. As an example, consider our study of the DN (Fig. 1B). The timing-related ramping activity we recorded in DN may be conveyed, at least in part, through projections to the intermediate layers of the superior colliculus, an area responsible for triggering eye movements [48]. If so, does the superior colliculus use incoming ramping activity as a timing signal to trigger eye movements? How might it convert this activity into suitably-timed saccade-related bursts? A solution could potentially be derived from a model framework developed for decision-related ramping activity [49]. Questions like these are likely to arise for all efferent pathways along which timing related information leaves the cerebellum. A fruitful avenue of research, in this regard, is to develop circuit-specific decoding models that are capable of extracting timing related information from ramps (i.e. accelerating firing rates) or other temporal representations. Just as we have models of how elements of the timing circuit generate temporally precise outputs, we need models for how each element of the timing circuit can readout temporally precise inputs.
What does the cerebellum do with the information it receives?
Understanding the input to the cerebellum is another important piece of the puzzle. There is essentially no data on signals entering the cerebellum during self-timed movements. A few related studies are promising, however. In the case of trace eyeblink conditioning, at the level of mossy fibers, the combination of persistent activity related to the memory of conditioned stimulus and phasic activity triggered by its onset may allow the cerebellum to learn the temporal representation necessary to execute timed eyeblink responses [33, 50, 51●]. Previously mentioned models suggest how these mossy fiber inputs can be converted into the temporally precise representations required to drive timed eyelid movements, via dynamics in the granule cell layer of the cerebellum alongside plasticity at the granule cell-Purkinje cell synapse [27]. Transposing those findings to our own work on self-timing, we may expect that inputs to the cerebellar cortex consist of similar combinations of persistent and phasic activity produced by associative cortices. These might then be transformed, by the cerebellum, into ramping output via the same or similar computations in granule cell layer microcircuitry [27, 52●●].
In the case of trace eyeblink conditioning, the cerebellum exploits inherent temporal dynamics (e.g. persistent activity) from cerebral cortical inputs to calculate when to move. Structures antecedent to the cerebellum may perform similar roles. One example is the inferior olive, which provides the second category of cerebellar afferents, climbing fibers, that may convey a readout of elapsed time based on sensory information [53, 54]. More generally, as mentioned above, many cortical and subcortical structures that innervate the cerebellum contain timing related information. It would not be surprising to find many examples of timing-related information at the input to the cerebellum. Interpreting such signals would be difficult, however, given that the cerebellum is reciprocally connected with these other structures through polysynaptic and heavily collateralized pathways. Indeed, the output of the cerebellum targets multiple structures, some of which in an apparent closed-loop manner (Fig. 1A). This leads to a situation where the cerebellum receives, via the brainstem, a mixture of sensory and motor inputs that includes refined cerebellar signals. To identify non-cerebellar contributions, we therefore need to understand the effect of cerebellar feedback on its targets. One way we can achieve this is by experimentally interrupting feedback loops, as has been done for eyeblink conditioning [51●]. This could help determine if the cerebellum is truly receiving and utilizing timing-related input signals.
Is the cerebellum involved in specific types of motor timing?
The cerebellum need not be required for timing all types of movements. A classic example comes from cerebellar patients with impaired action timing in the sub-second time range, but not for longer durations [55]. Also, cerebellar lesions may result in selective timing impairment of discrete movements, as opposed to continuous movements [56]. Finally, temporal aspects of movement dynamics may be preserved in cerebellar ataxia [57]. Unfortunately, while many studies of motor timing have separately examined rhythmic tapping or interval timing abnormalities in patients with disorders or lesions of the cerebellum, frontal cortex, or basal ganglia, only a handful of studies have tried to compare behavioral responses in the same paradigm [55, 58, 59, 60●●, 61]. The same is true in the context of animal physiology, where very few studies have attempted to study responses across brain areas using a single paradigm. Millisecond level self-timing of responses has been studied in the context of eye movements in the cerebellum and central thalamus, as mentioned before, but in few other places. Arm movements typically have been studied in animals using longer duration time intervals, complicating interpretation of similarities between structures. These examples suggest that there is a clear need to hone in on specific, uniformly implemented paradigms in future research. Studies in this realm can take a cue from studies on eyeblink conditioning, which has used one paradigm to unravel the networks which underlie the timing of trace conditioning [38].
Conclusions
Our current understanding of the action-timing network is summarized in Figure 3. Many questions are now on the horizon for investigations concerning cerebellar contributions to action timing. Below, we have attempted to explicitly state some of the salient physiological and computational problems that have been highlighted in this review. To researchers in timing, we hope they will be seen as interesting avenues of research to pursue in the coming years. To non-timing aficionados, we believe they reveal how timing provides a window into how cerebellar circuits contribute to motor control in general.
Figure 3.
Schematic representation of the distributed circuit for action timing. The thalamus is omitted for conciseness. Color codes represent the multiple signal streams that flow through the cerebral cortex, basal ganglia and cerebellum. Generalized recurrent connectivity (input arrows) implies that all structures (rectangles) read out signals and operate at multiple time scales. Their specific contributions (output arrows), however, impact separate levels of action timing.
Open questions
How do signals along cortico-pontine or subthalamo-pontine pathways code for the self-timing of movements, if at all?
Is timing-related information present at the mossy fiber input to the cerebellum?
How do circuits decode ramping activity? Can these decoding computations be instantiated in biologically realistic models for each module of the motor control circuitry?
How do neural responses in the cerebral cortex, cerebellum, and basal ganglia compare during the performance of self-timed vs. rhythmic tapping?
How do neural responses in the cerebral cortex, basal ganglia and cerebellum compare when tested on movements requiring supra-second vs millisecond timing?
Action timing deficits result from cerebellar damage, but their causes are unclear.
The cerebellum is interlinked with many structures that convey timing information.
One timing signal, ramping activity, is particularly common throughout the network.
Parsing the cerebellar role in action timing thus requires a network-wide approach.
Recent input-output analyses of the cerebellum are clarifying its role in timing.
Acknowledgments
Supported by the National Eye Institute, R21EY022788 to M.A.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Nothing is declared.
References
- 1.Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- 2.Ivry RB, Diener HC. Impaired velocity perception in patients with lesions of the cerebellum. J Cogn Neurosci. 1991;3:355–366. doi: 10.1162/jocn.1991.3.4.355. [DOI] [PubMed] [Google Scholar]
- 3.Deecke L, Kornhuber HH, Lang W, Lang M, Schreiber H. Timing function of the frontal cortex in sequential motor and learning tasks. Hum Neurobiol. 1985;4:143–154. [PubMed] [Google Scholar]
- 4.Halsband U, Ito N, Tanji J, Freund HJ. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116:243–266. doi: 10.1093/brain/116.1.243. [DOI] [PubMed] [Google Scholar]
- 5.Jin DZ, Fujii N, Graybiel AM. Neural representation of time in cortico-basal ganglia circuits. Proc Natl Acad Sci U S A. 2009;106:19156–19161. doi: 10.1073/pnas.0909881106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kagerer FA, Wittmann M, Szelag E, Steinbüchel Nv. Cortical involvement in temporal reproduction: evidence for differential roles of the hemispheres. Neuropsychologia. 2002;40:357–366. doi: 10.1016/s0028-3932(01)00111-7. [DOI] [PubMed] [Google Scholar]
- 7.Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S. Keeping time: effects of focal frontal lesions. Neuropsychologia. 2006;44:1195–1209. doi: 10.1016/j.neuropsychologia.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Bartolo R, Prado L, Merchant H. Information processing in the primate basal ganglia during sensory-guided and internally driven rhythmic tapping. J Neurosci. 2014;34:3910–3923. doi: 10.1523/JNEUROSCI.2679-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhamala M, Pagnoni G, Wiesenfeld K, Zink CF, Martin M, Berns GS. Neural correlates of the complexity of rhythmic finger tapping. Neuroimage. 2003;20:918–926. doi: 10.1016/S1053-8119(03)00304-5. [DOI] [PubMed] [Google Scholar]
- 10.Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12:3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- 11.Nenadic I, Gaser C, Volz HP, Rammsayer T, Häger F, Sauer H. Processing of temporal information and the basal ganglia: new evidence from fMRI. Exp Brain Res. 2003;148:238–246. doi: 10.1007/s00221-002-1188-4. [DOI] [PubMed] [Google Scholar]
- 12.Jones CR, Malone TJ, Dirnberger G, Edwards M, Jahanshahi M. Basal ganglia, dopamine and temporal processing: performance on three timing tasks on and off medication in Parkinson's disease. Brain Cogn. 2008;68:30–41. doi: 10.1016/j.bandc.2008.02.121. [DOI] [PubMed] [Google Scholar]
- 13.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 14.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 15. Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–254. doi: 10.1016/j.tics.2013.03.003.. ● A review of the interconnectivity between the cerebellum, basal ganglia, and diverse areas of the cerebral cortex, from the group that contributed the most to deciphering the neural circuits binding those structures. Close and reciprocal interactions between these structures might explain why defects to any of them can elicit surprisingly similar deficits.
- 16. Ashmore RC, Sommer MA. Delay activity of saccade-related neurons in the caudal dentate nucleus of the macaque cerebellum. J Neurophysiol. 2013;109:2129–2144. doi: 10.1152/jn.00906.2011.. ● A study from our group recording in the caudal pole of the dentate nucleus, the output node of the lateral cerebellum, as monkeys performed a self-timed eye movement task. Prominent among the neuronal responses were long-lead ramping patterns that culminated in the self-timed motor response, reminiscent of similar activity in the thalamus, cerebral cortex, and basal ganglia.
- 17.Maimon G, Assad JA. A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci. 2006;9:948–955. doi: 10.1038/nn1716. [DOI] [PubMed] [Google Scholar]
- 18.Lebedev MA, O'Doherty JE, Nicolelis MA. Decoding of temporal intervals from cortical ensemble activity. J Neurophysiol. 2008;99:166–186. doi: 10.1152/jn.00734.2007. [DOI] [PubMed] [Google Scholar]
- 19.Murakami M, Vicente MI, Costa GM, Mainen ZF. Neural antecedents of self-initiated actions in secondary motor cortex. Nat Neurosci. 2014;17:1574–1582. doi: 10.1038/nn.3826. [DOI] [PubMed] [Google Scholar]
- 20.Merchant H, Pérez O, Zarco W, Gámez J. Interval tuning in the primate medial premotor cortex as a general timing mechanism. J Neurosci. 2013;33:9082–9096. doi: 10.1523/JNEUROSCI.5513-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mita A, Mushiake H, Shima K, Matsuzaka Y, Tanji J. Interval time coding by neurons in the presupplementary and supplementary motor areas. Nat Neurosci. 2009;12:502–507. doi: 10.1038/nn.2272. [DOI] [PubMed] [Google Scholar]
- 22.Lee IH, Assad JA. Putaminal activity for simple reactions or self-timed movements. J Neurophysiol. 2003;89:2528–2537. doi: 10.1152/jn.01055.2002. [DOI] [PubMed] [Google Scholar]
- 23.Schultz W, Romo R. Role of primate basal ganglia and frontal cortex in the internal generation of movements. I. Preparatory activity in the anterior striatum. Exp Brain Res. 1992;91:363–384. doi: 10.1007/BF00227834. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka M. Cognitive signals in the primate motor thalamus predict saccade timing. J Neurosci. 2007;27:12109–12118. doi: 10.1523/JNEUROSCI.1873-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buonomano DV, Laje R. Population clocks: motor timing with neural dynamics. Trends Cogn Sci. 2010;14:520–527. doi: 10.1016/j.tics.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buonomano DV, Mauk MD. Neural network model of the cerebellum: temporal discrimination and the timing of motor responses. Neural Computation. 1994;6:38–55. [Google Scholar]
- 27.Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. J Neurosci. 2000;20:5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 29. Oprisan SA, Buhusi CV. What is all the noise about in interval timing? Philos Trans R Soc Lond B Biol Sci. 2014;369:20120459. doi: 10.1098/rstb.2012.0459.. ● A theoretical analysis featuring a detailed description of the striatal beat frequency model, which may explain how the basal ganglia take oscillating inputs from the cerebral cortex and convert them to temporally precise outputs, as observed in vivo. The authors demonstrate how time-scale invariance, a important hallmark of timing behavior, emerges naturally from this computational framework.
- 30.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 31.Ivry RB. The representation of temporal information in perception and motor control. Curr Opin Neurobiol. 1996;6:851–857. doi: 10.1016/s0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- 32.Johnson HA, Goel A, Buonomano DV. Neural dynamics of in vitro cortical networks reflects experienced temporal patterns. Nat Neurosci. 2010;13:917–919. doi: 10.1038/nn.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalmbach BE, Ohyama T, Mauk MD. Temporal patterns of inputs to cerebellum necessary and sufficient for trace eyelid conditioning. J Neurophysiol. 2010;104:627–640. doi: 10.1152/jn.00169.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J Neurosci. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trouche E, Beaubaton D, Amato G, Grangetto A. Impairments and recovery of the spatial and temporal components of a visuomotor pointing movement after unilateral destruction of the dentate nucleus in the baboon. Appl Neurophysiol. 1979;42:248–254. doi: 10.1159/000102368. [DOI] [PubMed] [Google Scholar]
- 36.Trouche E, Beaubaton D. Initiation of a goal-directed movement in the monkey. Role of the cerebellar dentate nucleus. Exp Brain Res. 1980;40:311–321. doi: 10.1007/BF00237796. [DOI] [PubMed] [Google Scholar]
- 37.Beaubaton D, Trouche E. Participation of the cerebellar dentate nucleus in the control of a goal-directed movement in monkeys. Effects of reversible or permanent dentate lesion on the duration and accuracy of a pointing response. Exp Brain Res. 1982;46:127–138. doi: 10.1007/BF00238106. [DOI] [PubMed] [Google Scholar]
- 38.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- 39.Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- 40.Clark RE, Lavond DG. Neural unit activity in the trigeminal complex with interpositus or red nucleus inactivation during classical eyeblink conditioning. Behav Neurosci. 1996;110:13–21. doi: 10.1037//0735-7044.110.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Gould TJ, Steinmetz JE. Changes in rabbit cerebellar cortical and interpositus nucleus activity during acquisition, extinction, and backward classical eyelid conditioning. Neurobiol Learn Mem. 1996;65:17–34. doi: 10.1006/nlme.1996.0003. [DOI] [PubMed] [Google Scholar]
- 42.Choi JS, Moore JW. Cerebellar neuronal activity expresses the complex topography of conditioned eyeblink responses. Behav Neurosci. 2003;117:1211–1219. doi: 10.1037/0735-7044.117.6.1211. [DOI] [PubMed] [Google Scholar]
- 43.Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27:2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacheco-Calderón R, Carretero-Guillén A, Delgado-García JM, Gruart A. Red nucleus neurons actively contribute to the acquisition of classically conditioned eyelid responses in rabbits. J Neurosci. 2012;32:12129–12143. doi: 10.1523/JNEUROSCI.1782-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jirenhed DA, Hesslow G. Learning stimulus intervals--adaptive timing of conditioned purkinje cell responses. Cerebellum. 2011;10:523–535. doi: 10.1007/s12311-011-0264-3. [DOI] [PubMed] [Google Scholar]
- 46.Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, et al. Genetic dissection of the circuit for hand dexterity in primates. Nature. 2012;487:235–238. doi: 10.1038/nature11206. [DOI] [PubMed] [Google Scholar]
- 47.Heiney SA, Kim J, Augustine GJ, Medina JF. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J Neurosci. 2014;34:2321–2330. doi: 10.1523/JNEUROSCI.4547-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.May PJ1, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience. 1990;36:305–324. doi: 10.1016/0306-4522(90)90428-7. [DOI] [PubMed] [Google Scholar]
- 49.Lo CC, Wang XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci. 2006;9:956–963. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- 50.Siegel JJ, Kalmbach B, Chitwood RA, Mauk MD. Persistent activity in a cortical-to-subcortical circuit: bridging the temporal gap in trace eyelid conditioning. J Neurophysiol. 2012;107:50–64. doi: 10.1152/jn.00689.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Siegel JJ, Mauk MD. Persistent activity in prefrontal cortex during trace eyelid conditioning: dissociating responses that reflect cerebellar output from those that do not. J Neurosci. 2013;33:15272–15284. doi: 10.1523/JNEUROSCI.1238-13.2013.. ● A prime example of experiments designed to separate cerebellar and cerebral functional contributions. By combining inactivations of cerebellar nuclei with recordings in prefrontal cortex, the authors showed that persistent responses, a critical component of trace conditioning (associative learning with stimulus-free intervals), may be a unique contribution of the prefrontal cortex.
- 52. Kennedy A, Wayne G, Kaifosh P, Alviña K, Abbott LF, Sawtell NB. A temporal basis for predicting the sensory consequences of motor commands in an electric fish. Nat Neurosci. 2014;17:416–422. doi: 10.1038/nn.3650.. ●● An elegant experimental study which suggests how unipolar brush cells in the electrosensory lobe, a cerebellum-like structure in electric fish, can convert phasic inputs conveyed via mossy fiber inputs into longer lasting activity with richer temporal dynamics. This result outlines a potential mechanism by which the cerebellum may turn temporally imprecise inputs into temporally precise motor outputs.
- 53.Jacobson GA, Rokni D, Yarom Y. A model of the olivo-cerebellar system as a temporal pattern generator. Trends Neurosci. 2008;31:617–625. doi: 10.1016/j.tins.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Ashe J, Bushara K. The olivo-cerebellar system as a neural clock. Adv Exp Med Biol. 2014;829:155–165. doi: 10.1007/978-1-4939-1782-2_9. [DOI] [PubMed] [Google Scholar]
- 55.Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philos Trans R Soc Lond B Biol Sci. 2009;364:1907–1918. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- 57.Topka H, Konczak J, Schneider K, Boose A, Dichgans J. Multijoint arm movements in cerebellar ataxia: abnormal control of movement dynamics. Exp Brain Res. 1998;119:493–503. doi: 10.1007/s002210050365. [DOI] [PubMed] [Google Scholar]
- 58.Dreher JC, Grafman J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci. 2002;16:1609–1619. doi: 10.1046/j.1460-9568.2002.02212.x. [DOI] [PubMed] [Google Scholar]
- 59.Diedrichsen J, Ivry RB, Pressing J. Cerebellar and basal ganglia contributions to interval timing. In: Meck WH, editor. Functional and Neural Mechanisms of Interval Timing. CRC Press; 2003. pp. 457–483. [Google Scholar]
- 60. Claassen DO, Jones CR, Yu M, Dirnberger G, Malone T, Parkinson M, Giunti P, Kubovy M, Jahanshahi M. Deciphering the impact of cerebellar and basal ganglia dysfunction in accuracy and variability of motor timing. Neuropsychologia. 2013;51:267–274. doi: 10.1016/j.neuropsychologia.2012.09.018.. ●● An important study comparing timing deficits of patients with cerebellar/ataxic disorders and Parkinson’s disease using the same set of tasks. Notably, cerebellar and basal ganglia dysfunctions appeared to induce opposite deficits in timing accuracy at short intervals (250ms), with cerebellar patients also showing greater response variability. Such a framework may be usefully transposed to animal studies that seek to understand each structure’s respective role in motor timing.
- 61.Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 62.Johansson F, Jirenhed DA, Rasmussen A, Zucca R, Hesslow G. Memory trace and timing mechanism localized to cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 2014;111:14930–14934. doi: 10.1073/pnas.1415371111. [DOI] [PMC free article] [PubMed] [Google Scholar]