Abstract
In the treatment of cancer patients, cisplatin (CDDP) exhibits serious cardiac and renal toxicities, while classical combinations related to CDDP are unable to solve these problems and may result in worse prognosis. Alternately, this study covalently conjugated 6-mercaptopurine (6MP) onto the surface of mercapto-modified mesoporous silica nanoparticles (MSNS) to form MSNS-6MP and loaded CDDP into the holes on the surface of MSNS-6MP to form MSNS-6MP/CDDP, a tumor-targeting nano-releasing regime for CDDP and 6MP specifically. In the S180 mouse model, the anti-tumor activity and overall survival of MSNS-6MP/CDDP (50 mg·kg−1·day−1, corresponding to 1 mg·kg−1·day−1 of 6MP and 5 mg·kg−1·day−1 of CDDP) were significantly higher than those of CDDP alone (5 mg·kg−1·day−1) or CDDP (5 mg·kg−1·day−1) plus 6MP (1 mg·kg−1·day−1). The assays of serum alanine aminotransferase, aspartate aminotransferase and creatinine, as well as the images of myocardium and kidney histology, support that MSNS-6MP/CDDP is able to completely eliminate liver, kidney and heart toxicities induced by CDDP alone or CDDP plus 6MP.
Keywords: 6-mercaptopurine, cisplatin, mesoporous silica nanoparticles, cancer therapy, nanomedicine
Introduction
As one of the first-line chemotherapeutic drugs, cisplatin (CDDP) is of generally clinical importance in treating testicular, ovarian, head and neck, cervical, bladder and small-cell lung cancers.1–9 However, the dose-limiting toxicities and the protein binding property of CDDP lead to deactivation, low efficacy and severe side effects. In respect of the toxicity profiles, nephrotoxicity is a major side effect in 20%–30% of patients receiving high-dose CDDP,10,11 and testicular cancer patients treated by CDDP experience a decrease in the number of morphologically abnormal spermatozoa.12,13 In respect of the toxic outcome, cardiac arrhythmias, paroxysmal supraventricular tachycardia, atrial fibrillation and bradycardia are correlated with CDDP therapy,14 while severe peripheral neurotoxicity often impairs the quality of life of cancer patients receiving CDDP.15–17 In respect of the side effects, myelosuppression is still correlated with the use of CDDP.18–20 Clinically, hydration is able to reduce CDDP-induced nephrotoxicity, but it cannot mitigate the side effects and toxicities.21,22 To reduce CDDP therapy-related side effects, some combination regimens are practiced, such as combination of CDDP with palonosetron, aprepitant and dexamethasone in the emetic prophylaxis.23 To reduce CDDP therapy-related toxicities, various combination regimens are also practiced, such as combination of CDDP with gemcitabine to reduce myelotoxicities and thrombocytopenia for patients with advanced non-small-cell lung cancer and recurrent cervical cancer.24,25 The anti-tumor efficacy and tolerance remain to be the clinical issues of CDDP therapy, and some combination regimens are extensively practiced to improve the treatment status of various patients, such as combination of CDDP with vinorelbine,26 irinotecan,27 sunitinib and gemcitabine,28 as well as with gemcitabine and bevacizumab,29 for patients with advanced large-cell neuroendocrine carcinoma of the lung, for patients with advanced urothelial carcinoma, for patients with synchronous primary lung cancer and pulmonary metastatic colorectal cancer and for patients with taxane pre-treated non-small-cell lung cancer, respectively. To generally improve the survival, quality of life and tolerability of cancer patients, CDDP is also combined with necitumumab and gemcitabine.30 Regimens, objects and results of these CDDP combinations are summarized in Table 1. As seen, in respect of the effects of the combination regimens on CDDP therapy-related side effects, toxicities, therapeutic efficacy, survival, quality of life and tolerability, few of these clinical trials gave positive results.
Table 1.
Some interesting clinical combinations and the outcomes of CDDP-based therapy
| Regimens | Object | Results |
|---|---|---|
| CDDP plus palonosetron, aprepitant and dexamethasone23 | Prevention of CDDP-induced nausea and vomiting in patients with breast cancer | Relatively small sample size, been not randomized, without control arm and the role of aprepitant |
| CDDP plus gemcitabine24,25 | Reducing myelotoxicities and thrombocytopenia for patients with advanced non-small-cell lung cancer and recurrent cervical cancer | Cumulative myelosuppression led to treatment delays of gemcitabine and CDDP |
| CDDP plus vinorelbine26 | Assess the efficacy and tolerance in non-small-cell lung cancer patients pre-treated with taxane-based regimen | A second-line active regimen with an acceptable toxicity profile and a reasonable survival |
| CDDP plus irinotecan27 | Lengthening survival period of patients with advanced large-cell neuroendocrine carcinoma of the lung | Response rate and overall survival period for the patients were inferior, and small numbers of patients were a major limitation |
| CDDP plus sunitinib and gemcitabine28 | Improving outcomes of patients in advanced urothelial carcinoma | Poorly tolerated, did not improve outcomes and treatment delivery was limited by myelotoxicity |
| CDDP plus gemcitabine and bevacizumab29 | Reporting a case with concomitant primary lung cancer and metastatic pulmonary colorectal cancer | Modest effects; lack of large randomized controlled trials; only a salvage therapy after the failure of 5-FU, oxaliplatin or irinotecan therapies |
| CDDP plus necitumumab and gemcitabine30 | Characterizing quality of life and tolerability of patients with stage IV squamous non-small-cell lung cancer | Particularly benefits patients with more severe baseline symptoms or lower quality of life |
Abbreviations: CDDP, cisplatin; 5-FU, 5-fluorouracil.
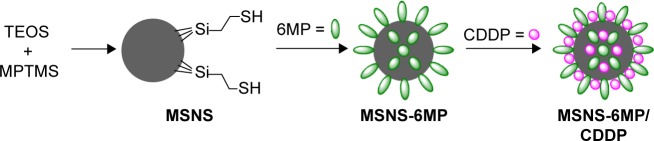
In this context, this study prepared mesoporous silica nanoparticles having mercapto groups-modified surface (MSNS) covalently linked 6-mercaptopurine (6MP), a key drug for treating acute lymphoblastic leukemia,31 with the surface mercapto groups of MSNS to form MSNS-6MP, loaded CDDP into the surface pores of MSNS-6MP to provide MSNS-6MP/CDDP (Figure 1), imaged the nanofeature, tested the release of 6MP and CDDP, evaluated the anti-tumor activities and estimated the safety.
Figure 1.
Preparation of MSNS-6MP/CDDP.
Abbreviations: MSNS, SH surfaced mesoporous silica nanoparticles; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP; 6MP, 6-mercaptopurine; CDDP, cisplatin; TEOS, tetraethylorthosilicate; MPTMS, tetraethoxy-silane-3-(trimethoxysilypropane)-1-thiol.
Considering that mesoporous silica nanoparticles have ordered structure,32,33 large surface area, big volume pores of narrow size distribution, good chemical and/or thermal stability, excellent biocompatibility and in vivo biodegradability, and thereby could be used in drug delivery and controlled release,34,35 MSNS-6MP/CDDP should be superior to the combination regimens mentioned earlier in improving CDDP therapy-related side effects, toxicities, therapeutic efficacy, survival, quality of life and tolerability.
Materials and methods
Preparing MSNS and MSNS-6MP
MSNS was prepared by following the literature.34 Then, MSNS was covalently modified with 6MP. In brief, to a solution of 275 mg (1.08 mmol) of I2 in 10 mL of anhydrous dimethyl sulfoxide (DMSO), a solution of 68 mg (0.21 mmol) of 6MP in 2 mL of anhydrous DMSO was added to form the dimer of 6MP, into which 1.00 g of MSNS was added and the suspension was stirred at room temperature for 8 h under strict exclusion of light to form MSNS-6MP. After centrifugation, the precipitates were successively washed with DMSO and anhydrous ethanol until no 6MP could be detected in the supernatant on an ultraviolet (UV) spectrophotometer (Shimadazu UV-2550 spectrophotometer, at 322 nm), and the precipitates were dried in vacuum for 24 h. The content of covalently conjugated 6MP was also identified with UV absorption spectroscopy, and the % loading of 6MP onto the nanoparticles was 2%.
Preparing MSNS-6MP/CDDP
To a solution of 20 mg of CDDP in 5 mL of normal saline (NS), 300 mg of MSNS-6MP was added. The suspension was ultrasonically oscillated at room temperature for 30 min, then stirred for another 8 h at 37°C and filtrated. The filtrate was washed with NS to perform the loading procedure. This loading procedure was repeated three times. The gravimetric analysis showed that 1 g of MSNS-6MP/CDDP contained 100 mg of CDDP.
Measuring differential scanning calorimeter (DSC) spectra
DSC spectra of MSNS, 6MP, CDDP, 6MP plus CDDP, MSNS-6MP or MSNS-6MP/CDDP were recorded (Netzcsh, 204F1). A sample of 1 mg of MSNS, 6MP, CDDP, 6MP plus CDDP, MSNS-6MP or MSNS-6MP/CDDP was added to the aluminum crucible and analyzed at 20°C–450°C.
Measuring transmission electron microscope (TEM) images
The nanostructures of MSNS, MSNS-6MP or MSNS-6MP/CDDP were imaged on a TEM (JSM-6360 LV; JEOL, Tokyo, Japan). Onto a formvar-coated copper grid, the solution of 0.5 mg of MSNS, MSNS-6MP or MSNS-6MP/CDDP in 1.0 mL of alcohol was dripped, and to enhance the removal of water, a drop of anhydrous ethanol was added. First, the grid was allowed to dry in air thoroughly and was then kept at 37°C for 24 h. The copper grids were viewed under TEM, and the nano-images were recorded from counting over 100 species in randomly selected regions on the copper grids. All TEM measurements were performed in triplicate grids, which were operated at 80 kV electron beam accelerating voltage. TEM images were recorded on an imaging plate (Gatan Bioscan Camera model 1792) with 20 eV energy windows at 6,000–400,000× and were enlarged digitally.
Measuring scanning electron microscope (SEM) images
The feature and diameter of the lyophilized powders from solutions of MSNS, MSNS-6MP and MSNS-6MP/CDDP in ultrapure water of pH 7.0 were examined by SEM (JEM-1230, JEOL, 50 kV), and were attached onto a copper plate with double-sided tape (Euromedex, Souffelweyersheim, France). On a JEOL JFC-1600 Auto Fine Coater, the copper plates were coated with 20 nm gold–palladium. At 15 kV, 30 mA and 200 mTorr (argon), the coater was operated for 60 s. By examining >100 particles in randomly selected regions on the SEM alloy, the feature and diameter distributions of the nanoparticles were visualized. Each measurement was performed with triplicate copper plates. The images were recorded on an imaging plate of Gatan Bioscan Camera Model 1792 (Gatan, Inc.) with 20 eV energy windows and at 100–10,000× digitally enlarged.
Measuring zeta potentials
At 25°C, the surface zeta potential of the nanospecies of MSNS, MSNS-6MP or MSNS-6MP/CDDP in ultrapure water was measured on a Zeta PlusPotential Analyzer (ZetaPlus S/N 21394; Brookhaven Instruments Corporation) with a BIC Zeta Potential Analyzer. The measurement of zeta potential of each sample was repeated for three runs, and the data were automatically calculated using the software from the electrophoretic mobility based on the formula of Smoluchowski.36
Measuring the release of 6MP from MSNS-6MP
The release of 6MP from 20.0 mg of MSNS-6MP was tested in 50 mL of phosphate buffered solution (PBS, pH 7.4, 37°C) with glutathione (GSH, 0.065 mM)/dithiolthreitol (DTT, 0.052 mM) by UV methods (Supplementary materials and Figure S1). To avoid the effect of external diffusion constraints on the release rate, a constant rotation speed (120 rpm) was maintained during the test.
Measuring the release of CDDP from MSNS-6MP/CDDP
The release of CDDP from 20.0 mg of MSNS-6MP/CDDP was tested in 50 mL of PBS (37°C, pH 5.5, 6.5 and 7.4) (Supplementary materials and Figure S2). To alleviate the limitation of the release rate by external diffusion constraints, a constant rotation speed (120 rpm) was maintained during the test.
In vivo tumor growth assay
Male ICR mice (6-week-old, 18–22 g) were commercially provided by the Animal Department of Capital Medical University (Beijing Laboratory Animal Center, Beijing, People’s Republic of China) and kept in an air-conditioned room. The care and handling of the mice were based on the approval of Committee of Institutional Authority for Laboratory Animal Care of Capital Medical University, and the Universiy Guideline of Animals Welfare. S180 cells were commercially provided by Vital River Laboratory Animal Technology Co., Ltd, Beijing, China, and xenografted subcutaneously in male ICR mice. The mice were kept in sterile isolated cages at 22°C–25°C and provided food and water freely. The growth of the tumor was monitored by measuring the tumor’s perpendicular diameter with a caliper. The volume of the tumor was calculated by using an equation; that is, volume = length × width2/2 cm3. When the average volume of the tumors reached 0.3–0.7 cm3, S180 tumor-xenografted mice were randomly divided into groups (each 12 mice) of sodium carboxyl methyl cellulose (CMCNa; 5‰, 10 mL·kg−1·day−1), CDDP (5 mg·kg−1·day−1) plus 6MP (1 mg·kg−1·day−1), MSNS-6MP (50 mg·kg−1·day−1) and MSNS-6MP/CDDP (50 mg·kg−1·day−1). The mice were intraperitoneally injected with CMCNa, CDDP, CDDP plus 6MP, MSNS-6MP or MSNS-6MP/CDDP for 7 consecutive days; the mice were bred for an additional 6 days, anesthetized with ether and then sacrificed to measure the survival, tumor volume, tumor weight and various parameters.
Acute toxicity assays
Male ICR mice (22±2 g) were kept in cages (25°C±1°C, 12 h light/dark cycle) with 50%–60% relative humidity. One day after adaptation, mice were randomly divided into three groups (ten mice, each) and intraperitoneally administered with CMCNa, CDDP, CDDP plus 6MP or MSNS-6MP/CDDP. Six days after the administration, the living status of the mice was monitored every day. On the 13th day, the mice were anesthetized with ether and sacrificed. Blood samples were collected by excising the eyeballs from mice and were centrifuged at 4°C (3,000× g) for 10 min to collect the serum. Serum levels of alanine transaminase (ALT), aspartate aminotransferase (AST) and creatinine (Cr) were determined according to the guidance of the commercial kits (Nanjing Jiancheng Co., Ltd, Nanjing, People’s Republic of China; n=5).
Histopathological examination
A small portion of heart and left kidney tissue were fixed in 10% solution of neutral buffered formalin and embedded in paraffin. Sections (5 μm in thickness) were cut, stained with hematoxylin and eosin (H&E) dyes and examined on a Nikon Eclipse E600 microscope (Nikon Corporation, Tokyo, Japan) at 100× magnification.
Results and discussion
Preparation of MSNS-6MP and MSNS-6MP/CDDP
As depicted in Figure 1, the reaction of tetraethylorthosilicate and tetraethoxy-silane-3-(trimethoxysilypropane)-1-thiol resulted in MSNS; that is, the surface of MSN was modified by mercapto groups. The coupling of MSNS and 1,2-di(purin-6-yl)-disulfane resulted in MSNS-6MP; that is, 6MP covalently modified MSNS. UV spectra showed that 1 g of MSNS-6MP coupled 20 mg of 6MP. The repeated absorption encouraged pushed CDDP to be loaded into the holes of MSNS-6MP and provided MSNS-6MP/CDDP. Gravimetric analysis showed that 1 g of MSNS-6MP/CDDP contained 100 mg of CDDP.
Characterization of MSNS-6MP/CDDP
The structure of MSNS-6MP/CDDP was defined by DSC (Netzsch, Germany) spectra. Figure 2 indicates that the DSC spectrum of MSNS is characterized by a peak at ~383°C, the DSC spectrum of 6MP is characterized by two peaks at ~170°C and ~370°C, the DSC spectrum of MSNS + 6MP is characterized by two peaks at ~170°C and ~376°C, the DSC spectrum of CDDP is characterized by a peak at ~340°C, the DSC spectrum of MSNS + CDDP is characterized by a peak at ~356°C and the DSC spectrum of MSNS + CDDP + 6MP is characterized by four peaks at ~154°C, ~361°C, ~353°C and ~380°C. On the other hand, however, the DSC specof 6MP covalently modified MSNS (MSNS-6MP) and CDDP-loaded MSNS-6MP (MSNS-6MP/CDDP) have no characterized peaks of both 6MP and CDDP. This means that the covalent modification of 6MP by SH surfaced silica nanoparticles and the loading of CDDP into nanoscaled holes on the surface of MSNS-6MP lead to the complete integration of MSNS, 6MP and CDDP and support the success of the preparation of MSNS-6MP and MSNS-6MP/CDDP.
Figure 2.
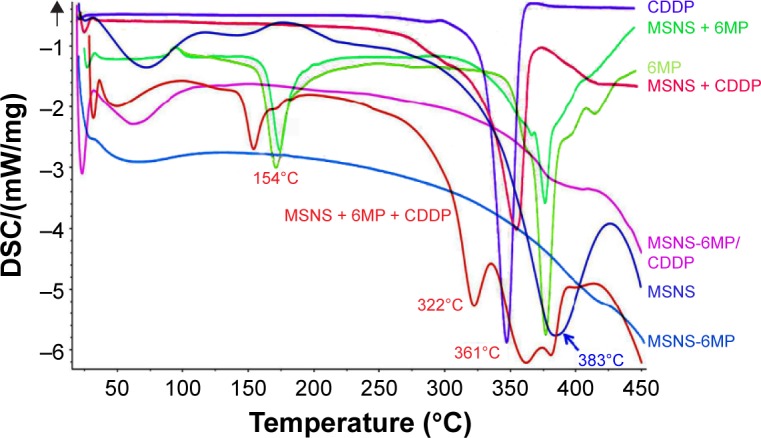
DSC spectra of MSNS, CDDP, 6MP, MSNS + CDDP, MSNS + 6MP, MSNS + CDDP + 6MP and MSNS-6MP/CDDP.
Abbreviations: DSC, differential scanning calorimeter; MSNS, SH surfaced mesoporous silica nanoparticles; CDDP, cisplatin; 6MP, 6-mercaptopurine; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
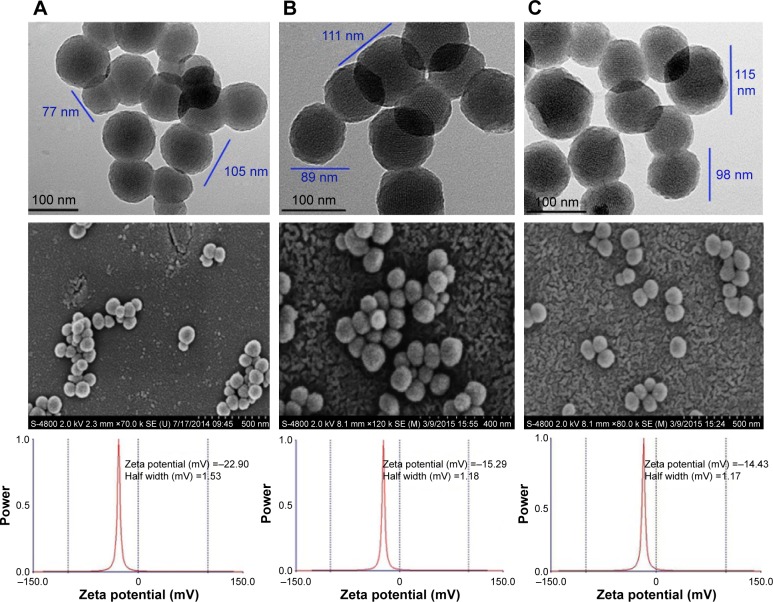
TEM image of MSNS, MSNS-6MP and MSNS-6MP/CDDP
The size, shape and surface of the nanoparticles of MSNS, MSNS-6MP and MSNS-6MP/CDDP were explored with TEM (JEOL-200CX) and are shown in Figure 3. The image indicates that the diameter of the nanoparticles of MSNS ranges from 77 nm to 105 nm, and on the surface, there are numerous holes of ~2.0 nm in diameter (Figure 3A). The images also indicate that the diameter of the nanoparticles of MSNS-6MP ranges from 89 nm to 111 nm, and on the surface, there are also numerous holes of ~1.5 nm in diameter (Figure 3B). The images still indicate that the diameter of the nanoparticles of MSNS-6MP/CDDP ranges from 98 nm to 115 nm, and on the surface, there are again numerous holes of ~1.3 nm in diameter (Figure 3C). The change of the diameter reflects that the covalent modification of 6MP leads to the increase of the size of the nanoparticles and the load of CDDP leads to the decrease of the diameter of the surface holes of the nanoparticles. In general, the nanoparticles of 77–115 nm in diameter are suitable for cell uptake, ~1.5 nm diameter of the surface holes of the nanoparticles of MSNS-6MP benefit loading CDDP and ~1.3 nm diameter of the surface holes of the nanoparticles of MSNS-6MP/CDDP benefit releasing CDDP.44,45
Figure 3.
TEM images of (A) MSNS, (B) MSNS-6MP and (C) MSNS-6MP/CDDP.
Note: Concentration of MSNS, MSNS-6MP and MSNS-6MP/CDDP is 0.5 mg/mL.
Abbreviations: TEM, transmission electron microscope; MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; CDDP, cisplatin; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
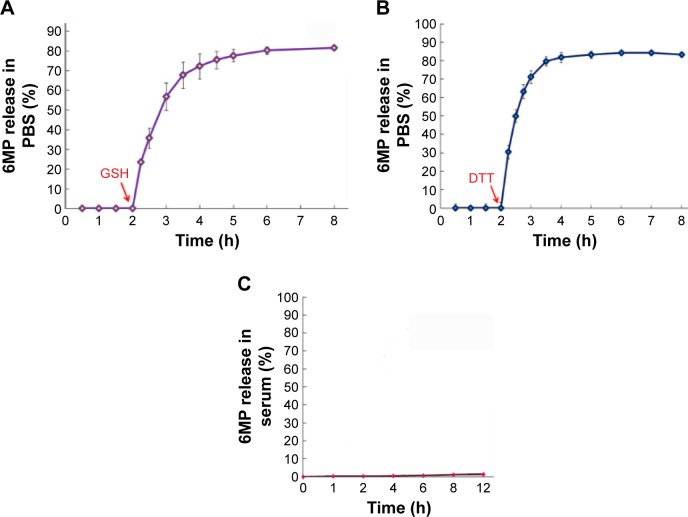
MSNS-6MP releasing 6MP
The release of 6MP from 10.0 mg of MSNS-6MP was carried out in 30.0 mL of PBS (pH 7.4, 37°C) with 0.065 mM of GSH as the reducer. The accumulative release was performed by sampling the solution of MSNS-6MP in PBS, and the curve is shown in Figure 4A. As seen, in the absence of GSH, MSNS-6MP did not release 6MP at 0.5, 1, 1.5 and 2 h points. With the addition of GSH, the 6MP rapidly accumulates and at 8 h point reaches the maximal value. This observation suggests that inside cytosol, but not outside cells, 6MP is optionally released from MSNS-6MP because the level of GSH inside cytosol is two to three orders higher than that of GSH outside cells. The release of 6MP from 10.0 mg of MSNS-6MP was also carried out in 30.0 mL PBS (pH 7.4, 37°C) with 0.052 mM of DTT as the reducer. The cumulative release was performed by sampling the solution, and the curve is shown in Figure 4B. As seen, in the absence of DTT, MSNS-6MP did not release 6MP at 0.5, 1, 1.5 and 2 h points. With the addition of DTT, 6MP rapidly accumulates and at 8 h point reaches the maximal value. This observation suggests that inside cytosol, but not outside cells, 6MP is optionally released from MSNS-6MP because the level of DTT inside cytosol is two to three orders higher than that of DTT outside cells.46–48
Figure 4.
Accumulative release of 6MP from MSNS-6MP.
Notes: (A) GSH promoted accumulative release profiles of 6MP from MSNS-6MP in pH 7.4 PBS; (B) DTT promoted accumulative release profiles of 6MP from MSNS-6MP in pH 7.4 PBS; (C) the accumulative release profiles of 6MP from MSNS-6MP in rat serum.
Abbreviations: 6MP, 6-mercaptopurine; MSNS, SH surfaced mesoporous silica nanoparticles; GSH, glutathione; PBS, phosphate buffered saline; DTT, dithiolthreitol; MSNS-6MP, 6MP surfaced MSNS.
The release profile of 6MP from MSNS-6MP was further carried out in rat serum, and the curve is shown in Figure 4C. As seen, in rat serum, MSNS-6MP released almost no 6MP at all time points. This observation suggests that in blood circulation, MSNS-6MP can be delivered without releasing 6MP, thereby making it possible to completely enter the tumoral tissue.
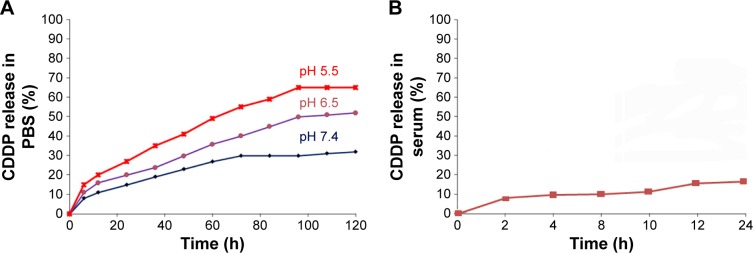
MSNS-6MP/CDDP releasing CDDP
A 120 h release profile of CDDP from 20.0 mg of MSNS-6MP/CDDP was carried out in 50.0 mL of PBS (pH 7.4, 6.5 and 5.5) at 37°C by sampling the solution at 5 h intervals, and the curves are shown in Figure 5A. In the environments of pH 7.4, 6.5 and 5.5, the accumulation of CDDP time-dependently increases; that is, MSNS-6MP/CDDP time-dependently releases CDDP. Besides, with the increase of the pH value, the accumulation of CDDP generally decreases; pH 5.5 (the environment pH of tumor tissue) is the most favorable pH for MSNS-6MP/CDDP releasing CDDP, while pH 7.4 (the environment pH of blood and healthy tissue) is the most unfavorable pH for MSNS-6MP/CDDP releasing CDDP. These observations suggest that MSNS-6MP/CDDP releases CDDP in a tumor-targeting manner. The release curves also show that the maximal accumulations of CDDP occur at 100–120 h, suggesting that the release of CDDP from MSNS-6MP/CDDP is in a prolonged model. The release profile of CDDP from 200 μL of 5 mg/mL MSNS-6MP/CDDP was also carried out in 200 μL of rat serum at 37°C by sampling the serum at 2 h intervals, and the curve is shown in Figure 5B. As seen, MSNS-6MP/CDDP has been incubated in rat serum for 24 h; the release of CDDP was <20%. This emphasizes the stability of MSNS-6MP/CDDP in blood circulation and benefits the delivery of MSNS-6MP/CDDP toward tumoral tissue.49,50
Figure 5.
The release profile of CDDP from MSNS-6MP/CDDP.
Notes: (A) Release profile of CDDP from MSNS-6MP/CDDP at pH 5.5, 6.5 and 7.4; (B) release profile of CDDP from MSNS-6MP/CDDP in rat serum.
Abbreviations: CDDP, cisplatin; MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP; PBS, phosphate buffered saline.
In vivo therapies of MSNS-6MP/CDDP for S180 mice
The in vivo anti-tumor activity of MSNS-6MP/CDDP was evaluated in S180 mouse model. In brief, S180 cells were xenografted subcutaneously in male ICR mice kept in sterile isolated cages at 22°C–25°C. Tumor size was measured by using a caliper and calculated based on volume = length × width2/2 cm3. Seven days after the xenograft of S180 cells, the size of the tumor reached ~0.5 cm3 and the mice were randomly divided into five groups of 12 mice each. The mice were intraperitoneally injected with CMCNa (5‰, 10 mL·kg−1·day−1), a suspension of CDDP (5 mg·kg−1·day−1) and 6MP (1 mg·kg−1·day−1) in CMCNa, or a suspension of MSNS-6MP (50 mg·kg−1·day−1) in CMCNa, or a suspension of MSNS-6MP/CDDP (50 mg·kg−1·day−1) in CMCNa, for 7 consecutive days. The mice were bred for an additional 6 days and then given ether anesthesia to be sacrificed for the following investigations.
Overall survival of S180 mice treated by MSNS-6MP/CDDP
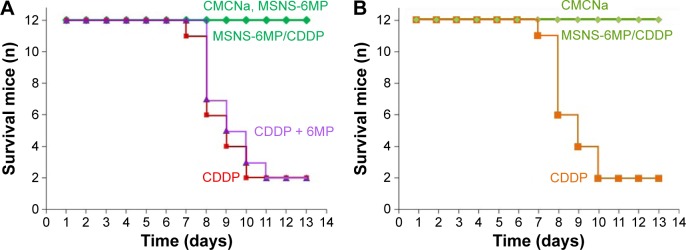
The systematic toxicity of CDDP therapy can be reflected with the overall survival. Figure 6 indicates that the overall survival of 13-day therapy of CMCNa, MSNS-6MP and MSNS-6MP/CDDP is up to 100%, while the overall survival rates of 8-day therapies of CDDP alone and CDDP plus 6MP are 50% and 42%, respectively, and the overall survival of 13-day therapies of them is 17% only. Distinct overall survival suggests that MSNS-6MP/CDDP can effectively eliminate the systematic toxicity of CDDP therapy and the combination therapy of CDDP with 6MP.
Figure 6.
Survival numbers of S180 mice and healthy ICR mice.
Notes: (A) Overall survival rates of S180 mice during 8-day and 13-day therapies of CMCNa, CDDP alone, CDDP plus 6MP, MSNS-6MP and MSNS-6MP/CDDP; (B) overall survival rates of healthy ICR mice during 13-day therapies of CDDP alone and MSNS-6MP/CDDP.
Abbreviations: CMCNa, sodium carboxyl methyl cellulose; CDDP, cisplatin; 6MP, 6-mercaptopurine; MSNS, SH surfaced mesoporous silica nanoparticles; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
To explore whether the longer survival for mice treated with MSNS-6MP/CDDP, but not CDDP, is a result of the reduced systemic toxicity of MSNS-6MP/CDDP, the survival rates of health ICR mice receiving CDDP and MSNS-6MP/CDDP for 13 days were examined and are shown in Figure 6B. As seen, during 13 days of administration, a total of 9 of 12 mice receiving CDDP died, while none of the 12 mice receiving MSNS-6MP/CDDP died. This comparison shows that the longer survival for mice treated with MSNS-6MP/CDDP, but not with CDDP (Figure 6A), is due to the reduced systemic toxicity rather than the enhanced therapeutic efficacy of MSNS-6MP/CDDP.
Tumor size of S180 mice treated by MSNS-6MP/CDDP
The therapeutic efficacy was reflected with the tumor size of the treated S180 mice. Figure 7 indicates the tumor volumes of the mice treated with CMCNa (5‰, 10 mL·kg−1·day−1), CDDP alone (5 mg·kg−1·day−1), CDDP (5 mg·kg−1·day−1) plus 6MP (1 mg·kg−1·day−1), MSNS-6MP (50 mg·kg−1·day−1) and MSNS-6MP/CDDP (50 mg·kg−1·day−1, corresponding to 1 mg·kg−1·day−1 of 6MP and 5 mg·kg−1·day−1 of CDDP) on the 7th day at the given dose. The tumor sizes indicate that, except CMCNa, 7 days after the given dosage, CDDP alone, CDDP plus 6MP, MSNS-6MP and MSNS-6MP/CDDP significantly inhibit the growth of the tumor. Since the overall survival rates of 8-day therapies of CDDP alone and CDDP plus 6MP were only 50% and 42%, respectively, the tumor sizes of their 13-day treatments were unavailable. On the 13th day, however, the tumor size of the mice treated with CMCNa is significantly larger than that of the mice treated with MSNS-6MP. This means that at the given dose, MSNS-6MP effectively slows the growth of the tumor. Besides, on the 13th day, the tumor size of the mice treated with MSNS-6MP is significantly larger than that of the mice treated with MSNS-6MP/CDDP. This means that at the same dose of 6MP, the anti-tumor activity of MSNS-6MP/CDDP is significantly higher than that of MSNS-6MP, suggesting the loading of CDDP significantly enhances the anti-tumor activity of 6MP and does not shorten the overall survival of the treated mice.
Figure 7.
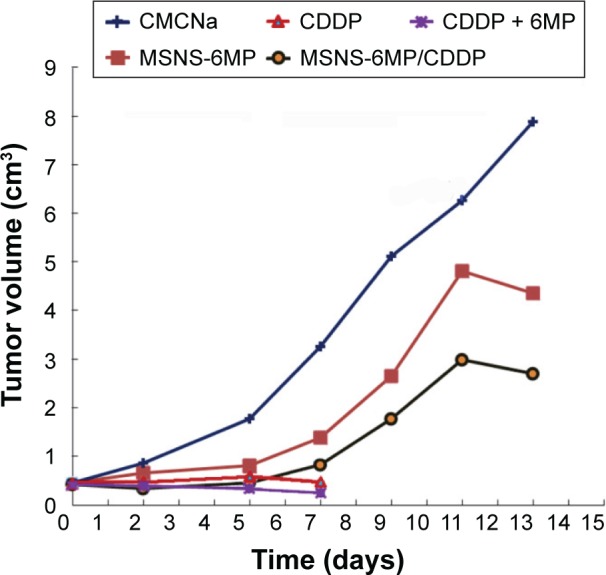
Tumor volumes of the treated mice.
Notes: CMCNa, CDDP plus 6MP, MSNS-6MP and MSNS-6MP/CDDP; because the overall survival rates of 8-day therapies of CDDP alone and CDDP plus 6MP were only 50% and 42%, respectively, the tumor sizes of 13-day treatments of them were unavailable; n=12.
Abbreviations: CMCNa, sodium carboxyl methyl cellulose; CDDP, cisplatin; 6MP, 6-mercaptopurine; MSNS, SH surfaced mesoporous silica nanoparticles; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
Tumor weight of S180 mice treated by MSNS-6MP/CDDP
The therapeutic efficacy was also reflected with the tumor weight of S180 mice treated by CMCNa (5‰, 10 mL·kg−1·day−1), CDDP alone (5 mg·kg−1·day−1), CDDP (5 mg·kg−1·day−1) plus 6MP (1 mg·kg−1·day−1), MSNS-6MP (50 mg·kg−1·day−1) and MSNS-6MP/CDDP (50 mg·kg−1·day−1, corresponding to 1 mg·kg−1·day−1 of 6MP and 5 mg·kg−1·day−1 of CDDP). Since the overall survival rates of 8-day therapies of CDDP alone and CDDP plus 6MP were only 50% and 42%, respectively, the tumor weights of 13-day treatments of them were unavailable. Figure 8 indicates that on the 13th day, the tumor weight of the mice treated with CMCNa is significantly higher than that of the mice treated with MSNS-6MP; thus, MSNS-6MP effectively inhibits the growth of the tumor. On the 13th day, the tumor weight of the mice treated with MSNS-6MP is significantly higher than that of the mice treated with MSNS-6MP/CDDP; thus, the loading of CDDP significantly enhances the anti-tumor activity of 6MP and does not shorten the overall survival of the treated mice.
Figure 8.
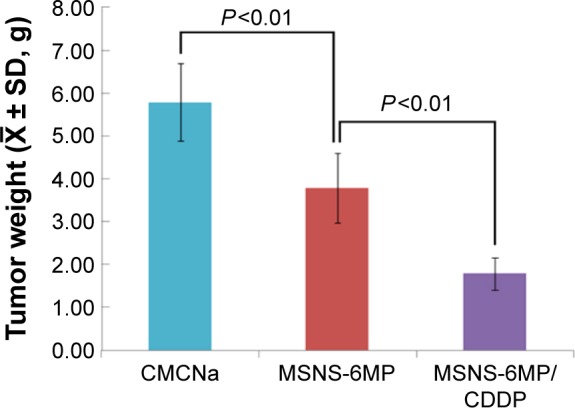
Tumor weights of S180 mice treated with CMCNa, MSNS-6MP and MSNS-6MP/CDDP, n=12.
Abbreviations: CMCNa, sodium carboxyl methyl cellulose; MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; CDDP, cisplatin; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
Body weight and ratios of organ weight/body weight of S180 mice treated by MSNS-6MP/CDDP
Table 2 represents the body weight and the ratio of organ weight/body weight of S180 mice treated by CDDP (5 mg·kg−1·day−1) plus 6MP (1 mg·kg−1·day−1) and MSNS-6MP/CDDP (50 mg·kg−1·day−1, corresponding to 1 mg·kg−1·day−1 of 6MP and 5 mg·kg−1·day−1 of CDDP). As seen, the body weight and the ratios of spleen, heart, liver, kidney and brain weight/body weight of S180 mice treated by 6MP plus CDDP are significantly different from those of the mice treated by CMCNa or MSNS-6MP/CDDP, while the body weight and the ratios of spleen, heart, liver, kidney and brain weight/body weight of S180 mice treated by CMCNa are at the same levels as those of S180 mice treated by MSNS-6MP/CDDP. This suggests that even treating for 6, 7 and 8 days, the combination of 6MP and CDDP still seriously slows the growth of S180 mice and damages the spleen, liver, kidney and brain of S180 mice. In contrast, 13-day treatment of MSNS-6MP/CDDP only slightly slows the growth of S180 mice and does not damage the spleen, heart, liver, kidney and brain of S180 mice.
Table 2.
Body weight (mean ± SD, g) and ratio of organ weight/body weight (mean ± SD, %) of S180 mice treated by 6MP plus CDDP and MSNS-6MP/CDDP
| Agents | Body weight | Heart/body | Liver/body | Spleen/body | Brain/body | Kidney/body |
|---|---|---|---|---|---|---|
| CMCNa | 41.85±1.79 | 0.41±0.06 | 6.96±0.61 | 1.03±0.27 | 0.79±0.22 | 0.50±0.07 |
| 6MP plus CDDP | 22.23±2.70a | 0.50±0.07a | 5.23±0.87a | 0.19±0.08a | 1.39±0.18a | 0.85±0.18a |
| MSNS-6MP/CDDP | 38.14±2.63 | 0.42±0.08 | 7.15±0.61 | 0.90±0.16 | 0.65±0.13 | 0.56±0.08 |
Notes:
Compared to CMCNa, P<0.01; seven, one and two S180 mice treated by 6MP plus CDDP died on the 8th day, 9th day and 10th day, respectively, and the bodies and organs were immediately weighed after death; n=12.
Abbreviations: 6MP, 6-mercaptopurine; CDDP, cisplatin; MSNS, SH surfaced mesoporous silica nanoparticles; CMCNa, sodium carboxyl methyl cellulose.
Liver and kidney toxicity of S180 mice treated by MSNS-6MP/CDDP
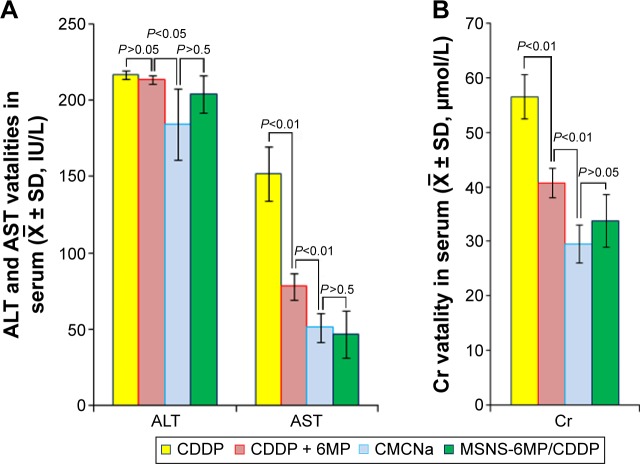
The superiority of MSNS-6MP/CDDP (50 mg·kg−1·day−1, corresponding to 1 mg·kg−1·day−1 of 6MP and 5 mg·kg−1·day−1 of CDDP) over CDDP (5 mg·kg−1·day−1) alone and CDDP (5 mg·kg−1·day−1) plus 6MP (1 mg·kg−1·day−1) is further explained by the serum ALT, AST and Cr of the treated S180 mice. Since 8-day treatments of CDDP alone and CDDP plus 6MP caused most S180 mice to die, the levels of serum ALT, AST and Cr on the 13th day were unavailable. Figure 9A indicates that on the 13th day, the levels of serum ALT and AST of the S180 mice treated by CMCNa are equal to those of the S180 mice treated by MSNS-6MP/CDDP and significantly lower than those of the S180 mice treated by CDDP alone and 6MP plus CDDP. Figure 9B indicates that on the 13th day, the level of serum Cr of the S180 mice treated by CMCNa is equal to that of the S180 mice treated by MSNS-6MP/CDDP and significantly lower than those of the S180 mice treated by CDDP alone and 6MP plus CDDP. Serum levels of ALT and AST reflect liver toxicity, and serum level of Cr reflects kidney toxicity. Serum levels of ALT, AST and Cr of S180 mice treated with CMCNa are not significantly different from those of S180 mice treated with MSNS-6MP/CDDP, implying that MSNS-6MP/CDDP therapy does not injure liver and kidney; that is, in contrast to CDDP and CDDP plus 6MP, CDDP-loaded MSNS-6MP can completely limit liver and kidney toxicities of CDDP.
Figure 9.
Serum ALT, AST and Cr levels of the mice treated with CMCNa, CDDP, CDDP plus 6MP and MSNS-6MP/CDDP.
Note: (A) serum ALT and AST of the S180 mice treated by CMCNa, CDDP plus 6MP, CDDP and MSNS-6MP/CDDP; (B) serum Cr of the S180 mice treated by CMCNa, CDDP plus 6MP, CDDP and MSNS-6MP/CDDP.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, creatinine; CMCNa, sodium carboxyl methyl cellulose; CDDP, cisplatin; 6MP, 6-mercaptopurine; MSNS, SH surfaced mesoporous silica nanoparticles; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP.
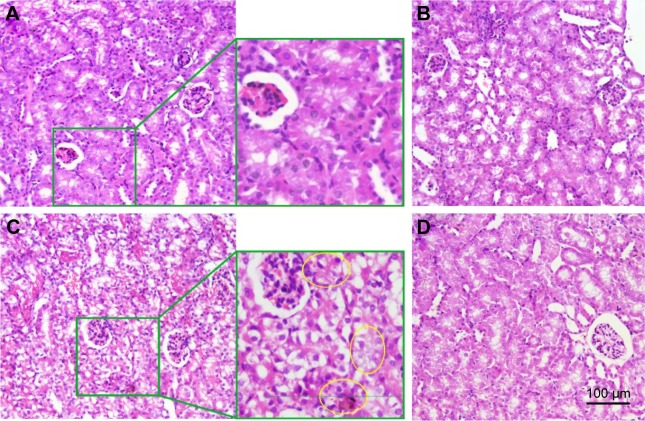
The superiority of MSNS-6MP/CDDP (50 mg·kg−1·day−1, corresponding to 1 mg·kg−1·day−1 of 6MP and 5 mg·kg−1·day−1 of CDDP) over CDDP (5 mg·kg−1·day−1) plus 6MP (1 mg·kg−1· day−1) is explained by the left kidney histology of the treated S180 mice. Figure 10C indicates that the histomorphological image of the left kidney section of the mice treated by 6MP plus CDDP shows damage to the tubular epithelium, the formation of intratubular cast and tubular dilatation. In contrast, the histology images of the left kidney sections of the mice treated by CMCNa, MSNS and MSNS-6MP/CDDP give no such histomorphological changes (Figure 10A, B and D), ensuring MSNS-6MP/CDDP therapy completely limits kidney apoptosis incidence induced by CDDP at the histology level.
Figure 10.
Effect of CMCNa, MSNS, 6MP plus CDDP and MSNS-6MP/CDDP on the left kidney histology.
Notes: (A) Left kidney histology of S180 mice treated by CMCNa; (B) left kidney histology of S180 mice treated by MSNS (50 mg·kg−1·day−1); (C) left kidney histology of S180 mice treated by 6MP (1 mg·kg−1·day−1) plus CDDP (5 mg·kg−1·day−1), and the area labelled by yellow circles show damage to the tubular epithelium, the formation of intratubular cast and tubular dilatation; (D) left kidney histology of S180 mice treated by MSNS-6MP/CDDP (50 mg·kg−1·day−1, corresponding to 1 mg·kg−1·day−1 of 6MP and 5 mg·kg−1·day−1 of CDDP); H&E stain, original magnification 40×, n=5.
Abbreviations: CMCNa, sodium carboxyl methyl cellulose; MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; CDDP, cisplatin; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP; H&E, hematoxylin and eosin.
Heart toxicity of S180 mice treated by MSNS-6MP/CDDP
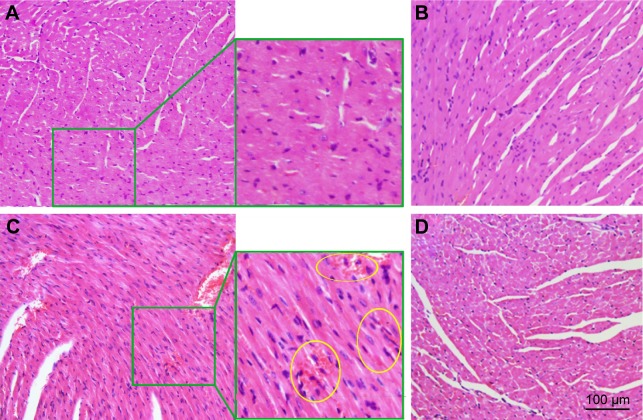
To examine the heart toxicity of MSNS-6MP/CDDP, the H&E stain of the heart sections of the mice treated by MSNS-6MP/CDDP (50 mg·kg−1·day−1, corresponding to 1 mg·kg−1·day−1 of 6MP and 5 mg·kg−1·day−1 of CDDP), CDDP (5 mg·kg−1·day−1) plus 6MP (1 mg·kg−1·day−1) and MSNS was performed. Figure 11C shows histomorphologic changes of the heart section of the mice treated by 6MP plus CDDP, which images edematous and hemorrhagic myocardium with expanded interstitium (making with yellow rings), visualizes hyperchromatic, polymorphic and irregularly localized nuclei and discloses eosinophilia in the cytoplasm of necrosis areas. In contrast, the histology images of the heart sections of the mice treated by CMCNa, MSNS and MSNS-6MP/CDDP do not give such abnormal myocardium histology (Figure 11A, B and D), ensuring MSNS-6MP/CDDP therapy completely limits heart damage induced by CDDP.
Figure 11.
Effect of CMCNa, MSNS, 6MP plus CDDP and MSNS-6MP/CDDP on mouse myocardium histology.
Notes: (A) Effect of CMCNa on mouse myocardium histology; (B) effect of MSNS on mouse myocardium histology; (C) effect of 6MP plus CDDP on mouse myocardium histology, and the area labelled by yellow circles show damage to the tubular epithelium, the (D) effect of MSNS-6MP/CDDP on mouse myocardium histology, and the edematous and hemorrhagic myocardium with expanded interstitium, visualizes hyperchromatic, polymorphic and irregularly localized nuclei and discloses eosinophilia in the cytoplasm of necrosis area were labelled by yellow circles; H&E stain, original magnification 40×, n=5.
Abbreviations: CMCNa, sodium carboxyl methyl cellulose; MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; CDDP, cisplatin; MSNS-6MP, 6MP covalently modified MSNS; MSNS-6MP/CDDP, CDDP-loaded MSNS-6MP; H&E, hematoxylin and eosin.
Distribution of Pt in S180 mice treated by MSNS-6MP/CDDP and CDDP
To explore the Pt distributions (Figure 12) of MSNS-6MP/CDDP (50 mg·kg−1·day−1, corresponding to 1 mg·kg−1·day−1 of 6MP and 5 mg·kg−1·day−1 of CDDP) and CDDP (5 mg·kg−1·day−1) treated mice, Pt concentrations were determined in blood, urine, feces, organs and tumor tissue using an ICP-OES instrument (Pt, 214.423 nm; ICP-710ES; Varian, USA). Blood, urine, feces, organs and tumor tissue samples were diluted with 10 mL of solutions (HNO3:30% H2O2 in aqueous solution =2:1) and digested in a microwave digester (Mars-Xpress; CEM, USA).
Figure 12.
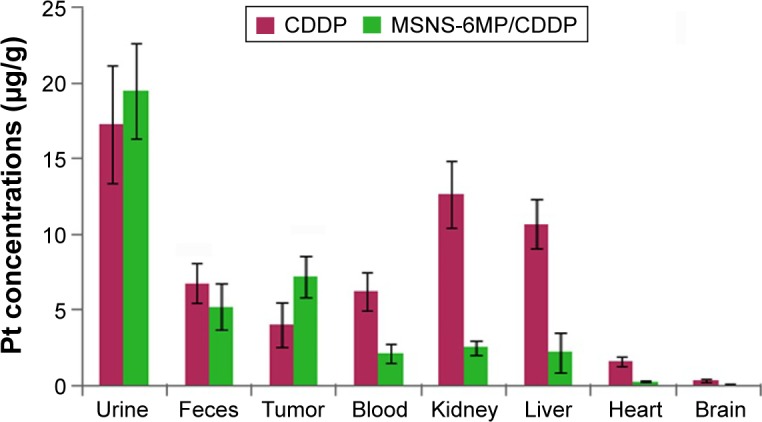
Distribution of Pt in S180 mice treated by MSNS-6MP/CDDP and CDDP (μg/g).
Notes: For CDDP group, n=2, the survival mice; for MSNS-6MP/CDDP group, n=7.
Abbreviations: MSNS, SH surfaced mesoporous silica nanoparticles; 6MP, 6-mercaptopurine; CDDP, cisplatin.
Conclusion
CDDP is a first-line chemotherapeutic drug for treating testicular, ovarian, head and neck, cervical, bladder and small-cell lung cancers. To eliminate the dose-related toxicities, various combination therapies were clinically and experimentally practiced, but the systematic toxicity and overall survival remain undesirable. In contrast to the known combination strategies, this study covalently modified the mercapto groups on the surface of MSNS to form MSNS-6MP, and CDDP was loaded into the surface holes of MSNS-6MP to form MSNS-6MP/CDDP, a tumor-targeting nanomedicine for releasing 6MP and CDDP. The superiority of MSNS-6MP/CDDP to classical combination of 6MP plus CDDP is reflected by the in vivo anti-tumor activity and overall survival rates of MSNS-6MP/CDDP, which were significantly higher than the classical combination of 6MP plus CDDP, as well as by MSNS-6MP/CDDP therapy, completely eliminating the serious renal and cardiac toxicities induced by CDDP. Thus, MSNS-6MP/CDDP alters the combination strategy of 6MP plus CDDP in a safe and effective manner, consequently providing an interesting nanomedicine.
Supplementary materials
Measuring the release of CDDP from MSNS-6MP/CDDP
The release of CDDP from 20.0 mg of MSNS-6MP/CDDP was tested in 50 mL of PBS (37°C, pH 5.5, 6.5 and 7.4). To alleviate the limitation of the release rate by external diffusion constraints, a constant rotation speed (120 rpm) was maintained during the test. Collect all of the sample solution and 1.0 mL aliquots of the liquid samples which were diluted to 10 mL of solutions (HNO3: 30% H2O2 in aqueous solution =2:1) and digested in a microwave digester (Mars-Xpress, CEM, USA). Triplicate digestions were conducted for each sample. An ICP-710ES (Varian, USA) inductively coupled plasma optical emission spectrophotometer (ICP-OES) was used to analyze the elements in digested samples. Levels of Platinum (Pt) in digested samples were determined using the ICP conditions: 214.423 nm, 0–50 mg/mL, the regression equation is y =389.89x −228.3, R2=0.9996.
MSNS-6MP/CDDP releasing CDDP in rat serum
Male Sprague Dawley rat plasma was centrifuged twice (4°C, 4,000 rpm, 10 min) to get the rat serum. The release profile of CDDP from 200 μL of 5 mg/mL MSNS-6MP/CDDP was carried out in 200 μL of rat serum at 37°C by sampling at 0, 1, 2, 4, 6, 8, 12 and 24 h, respectively. A total of 10.00 mg of MSNS-MP/CDDP was dissolved in 1,000 μL of pH 7.4 phosphate buffered solution to prepare the stock solution (10 mg/mL); then 1,000 μL of stock solution was added in 1,000 μL of rat serum in a 37°C water bath, shaking at 120 rpm and sampled at 0, 1, 2, 4, 6, 8, 12 and 24 h. The samples were centrifuged at 12,000 rpm, 10 min, and 200 μL supernatant was collected and diluted five times by adding pH 7.4 PBS for measurement, and 200 μL of new rat serum was added into the remaining sample solution to keep the whole volume at 1,000 μL. ICP-OES was used to measure Pt concentration following the same procedure of the section “Measuring the release of CDDP from MSNS-6MP/CDDP”.
ALT, AST and Cr assay
Rat blood samples were added in a tube to 3.8% sodium citrate solution as the anticoagulant, then centrifuged (4°C, 4,000 rpm, 10 min), and the supernatant was used to measure serum levels of ALT, AST and Cr according to the guidance of the commercial kits (Nanjing Jiancheng Co., Ltd, Nanjing, People’s Republic of China, n=5).
Statistics
All experiments were performed at least six times. All data were represented as the average ± SD. A paired two-sample Student’s t-test was used for statistical analysis. A P-value of <0.05 was considered to be statistically significant, and a P-value <0.01 was considered very significant.
Standard curve of 6MP (4–20 mg/mL).
Abbreviation: 6MP, 6-mercaptopurine.
Standard curve of platinum (0–50 mg/mL).
Acknowledgments
This work was supported by the Beijing Area Major Laboratory of Peptide and Small Molecular Drugs, PHR (IHLB), TJSHG201310025008, the NSFC (21171120, 81172930, 81270046 and 81373265), Beijing Natural Science Foundation (7132020 and 7132032), Beijing Nova Programme xx2013039, 863 programme 2015AA020902 and Project for Science and Technology Development, Beijing Commission of Education (SQKM201210025007) and The Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality. We thank Engineering Research Center of Endogenous Prophylactic of Ministry of Education of China for technical support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 2.Bai MY, Liu SZ. A simple and general method for preparing antibody-PEG-PLGA sub-micron particles using electrospray technique: an in vitro study of targeted delivery of cisplatin to ovarian cancer cells. Colloids Surf B Biointerfaces. 2014;117:346–353. doi: 10.1016/j.colsurfb.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 3.Tseng YY, Wang YC, Su CH, et al. Concurrent delivery of carmustine, irinotecan, and cisplatin to the cerebral cavity using biodegradable nanofibers: in vitro and in vivo studies. Colloids Surf B Biointerfaces. 2015;134:254–261. doi: 10.1016/j.colsurfb.2015.06.055. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Zhang YL, Han YZ, et al. Characterization and cytotoxicity studies of DPPC: M2+ novel delivery system for cisplatin thermo sensitivity liposome with improving loading efficiency. Colloids Surf B Biointerfaces. 2015;131:12–20. doi: 10.1016/j.colsurfb.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Messori L, Merlino A. Cisplatin binding to proteins: a structural perspective. Coord Chem Rev. 2016;315:67–89. [Google Scholar]
- 6.Ho GY, Woodward N, Coward JIG. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Crit Rev Oncol Hematol. 2016;102:37–46. doi: 10.1016/j.critrevonc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Fennell DA, Summers Y, Cadranel J, et al. Cisplatin in the modern era: the backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat Rev. 2016;44:42–50. doi: 10.1016/j.ctrv.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Petrelli F, De Stefani A, Raspagliesi F, Lorusso D, Barni S. Radiotherapy with concurrent cisplatin-based doublet or weekly cisplatin for cervical cancer: a systematic review and meta-analysis. Gynecol Oncol. 2014;134:166–171. doi: 10.1016/j.ygyno.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 9.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334(2):115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 11.Nojiri T, Hosoda H, Kimura T, et al. Protective effects of ghrelin on cisplatin-induced nephrotoxicity in mice. Peptides. 2016;82:85–91. doi: 10.1016/j.peptides.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Jahnukainen K, Ehmcke J, Hou M, Schlatt S. Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab. 2011;25(2):287–302. doi: 10.1016/j.beem.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Aminsharifi A, Hekmati P, Noorafshan A, et al. Scrotal cooling to protect against cisplatin-induced spermatogenesis toxicity: preliminary outcome of an experimental controlled trial. Urology. 2016;91:90–98. doi: 10.1016/j.urology.2015.12.062. [DOI] [PubMed] [Google Scholar]
- 14.Kounis NG, Cervellin G, Lippi G. Cisplatin-induced bradycardia: cardiac toxicity or cardiac hypersensitivity and Kounis syndrome? Int J Cardiol. 2016;202:817–818. doi: 10.1016/j.ijcard.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Carozzi VA, Chiorazzi A, Canta A, et al. Chemotherapy-induced peripheral neurotoxicity in immune-deficient mice: new useful ready-to-use animal models. Exp Neurol. 2015;264:92–102. doi: 10.1016/j.expneurol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Sharawy N, Rashed L, Youakim MF. Evaluation of multi-neuroprotective effects of erythropoietin usingcisplatin induced peripheral neurotoxicity model. Exp Toxicol Pathol. 2015;67(4):315–322. doi: 10.1016/j.etp.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Mendonc LM, da Silva Machado C, Teixeira CCC, de Freitas LAP, de Lourdes Pires Bianchi M, Antunes LMG. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology. 2013;34:205–211. doi: 10.1016/j.neuro.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe K, Inai S, Jinnouchi K, Baba S, Yagi T. Expression of caspase-activated deoxyribo-nuclease (CAD) and caspase 3 (CPP32) in the cochlea of cisplatin (CDDP)-treated guinea pigs. Auris Nasus Larynx. 2003;30(3):219–225. doi: 10.1016/s0385-8146(03)00049-x. [DOI] [PubMed] [Google Scholar]
- 19.Buffoni L, Dongiovanni D, Barone C, et al. Fractionated dose of cisplatin (CDDP) and vinorelbine (VNB) chemotherapy for elderly patients with advanced non-small cell lung cancer: phase II trial. Lung Cancer. 2006;54(3):353–357. doi: 10.1016/j.lungcan.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Martoni AA, Melotti B, Sperandi F, et al. Hybrid (intravenous and oral) administration of vinorelbine plus cisplatinum followed by oral vinorelbine as first-line therapy of advanced non-small cell lung cancer: a phase II study. Lung Cancer. 2008;60(3):387–392. doi: 10.1016/j.lungcan.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Sun K, Ni L, Wang X, Wang D, Zhang J. Sodium selenosulfate at an innocuous dose markedly prevents cisplatin-induced gastrointestinal toxicity. Toxicol Appl Pharmacol. 2012;258(3):376–383. doi: 10.1016/j.taap.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Fan KY, Gogineni H, Zaboli D, et al. Comparison of acute toxicities in two primary chemoradiation regimens in the treatment of advanced head and neck squamous cell carcinoma. Ann Surg Oncol. 2012;19(6):1980–1987. doi: 10.1245/s10434-012-2219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang C-K, Wu C-E, Liaw C-C. Combination of palonosetron, aprepitant, and dexamethasone as primary antiemetic prophylaxis for cisplatin-based chemotherapy. Biomed J. 2016;39(1):60–66. doi: 10.1016/j.bj.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortesi E, Ramponi S, Corona M, et al. Decreased myelotoxicity of gemcitabine and cisplatin in advanced non-small cell lung cancer (NSCLC) with cisplatin infusion on day 15. Lung Cancer. 2001;31(2–3):271–276. doi: 10.1016/s0169-5002(00)00198-7. [DOI] [PubMed] [Google Scholar]
- 25.Matulonis UA, Campos S, Duska L, et al. Gynecologic Cancer Program of Dana Farber-Harvard Cancer Center Phase I/II dose finding study of combination cisplatin and gemcitabine in patients with recurrent cervical cancer. Gynecol Oncol. 2006;103(1):160–164. doi: 10.1016/j.ygyno.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Makrantonakis P, Ziotopoulos P, Agelidou A, et al. Vinorelbine and cisplatin combination in pretreated patients with advanced non-small cell lung cancer pretreated with a taxane-based regimen: a multicenter phase II study. Lung Cancer. 2006;53(1):85–90. doi: 10.1016/j.lungcan.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Niho S, Kenmotsu H, Sekine I, et al. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: a multicenter phase II study. J Thorac Oncol. 2013;8(7):980–984. doi: 10.1097/JTO.0b013e31828f6989. [DOI] [PubMed] [Google Scholar]
- 28.Geldart T, Chester J, Casbard A, et al. SUCCINCT: an open-label, single-arm, non-randomised, phase 2 trial of gemcitabine and cisplatin chemotherapy in combination with sunitinib as first-line treatment for patients with advanced urothelial carcinoma. Eur Urol. 2015;67(4):599–602. doi: 10.1016/j.eururo.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H-M, Pan C-C, Tsai C-M, Hsu W-H, Yang S-H, Yen C-C. Concomitant primary lung cancer and metastatic pulmonary colorectal cancer that responded to gemcitabine/cisplatin/bevacizumab combination therapy. J Cancer Res Pract. 2015;2:76–82. [Google Scholar]
- 30.Reck M, Socinski MA, Luft A, et al. The effect of necitumumab in combination with gemcitabine plus cisplatin on tolerability and on quality of life: results from the phase 3 SQUIRE trial. J Thorac Oncol. 2016;11(6):808–818. doi: 10.1016/j.jtho.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 31.de Beaumais Tiphaine A, Hjalgrim LL, Nersting J, et al. Evaluation of a pediatric liquid formulation to improve 6-mercaptopurine therapy in children. Eur J Pharm Sci. 2016;83:1–7. doi: 10.1016/j.ejps.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Yildirim A, Turkaydin M, Garipcanc B, Bayindir M. Cytotoxicity of multifunctional surfactant containing capped mesoporous silica nanoparticles. RSC Adv. 2016;6:32060. [Google Scholar]
- 33.Ma TY, Liu L, Yuan ZY. Direct synthesis of ordered mesoporous carbons. Chem Soc Rev. 2013;42(9):3977–4003. doi: 10.1039/c2cs35301f. [DOI] [PubMed] [Google Scholar]
- 34.Slowing II, Vivero-Escoto JL, Wu CW, Lin VS. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60(11):1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Ambrogio MW, Thomas CR, Zhao YL, Zink JI, Stoddart JF. Mechanized silica nanoparticles: a new frontier in theranostic nanomedicine. Acc Chem Res. 2011;44(10):903–913. doi: 10.1021/ar200018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standard curve of 6MP (4–20 mg/mL).
Abbreviation: 6MP, 6-mercaptopurine.
Standard curve of platinum (0–50 mg/mL).