Abstract
Background
The objective of this study was to determine the rate and pattern of nodal recurrence in patients who underwent a therapeutic, lateral neck dissection (LND) for papillary thyroid cancer (PTC) with clinically evident cervical metastases and to determine if there was any correlation between the extent of initial dissection and the rate and pattern of neck recurrence.
Methods
A total of 3,664 patients with PTC treated between 1986 and 2010 at Memorial Sloan Kettering Cancer Center were identified from our institutional database. Tumor factors, patient demographics, extent of initial LND, and adjuvant therapy were recorded. Patterns of recurrent lateral neck metastases by level involvement were recorded and outcomes calculated using the Kaplan-Meier method.
Results
A total of 484 patients had an LND for cervical metastases; 364 (75%) had a comprehensive LND (CLND) and 120 (25%) had a selective neck dissection (SND). The median duration of follow-up was 63.5 months. As expected, patients with CLND had a greater number of nodes removed as well as a greater number of positive nodes (P < .001). There was no difference in overall lateral neck recurrence-free status (CLND 94.4% vs SND 89.4%, P = .158), but in the dissected neck, the ipsilateral lateral neck recurrence-free status was superior in the CLND patients (97.7% vs 89.4%, P < .001).
Conclusion
Patients with clinically evident neck metastases from PTC managed by CLND have lesser rates of recurrence in the dissected neck compared with patients managed by SND. SND should only be done in highly selected cases with small volume disease.
In Patients With Well-Differentiated Papillary Thyroid Cancer (PTC), metastases to the cervical lymph nodes are common. The majority of these are clinically occult, metastatic nodes, which are thought to be of no prognostic significance in the majority of patients.1,2 In contrast, clinically evident neck metastases, especially in the lateral neck, are of greater clinical importance, because these patients are at increased risk of additional regional nodes and distant disease. This is especially true in patients >45 years of age.3,4 In patients with clinically evident lateral neck disease, optimum management of the lateral neck is, therefore, of great importance.
There has been much debate in the literature as to the extent of dissection of the lateral neck nodes when clinically evident neck metastases are present. The most commonly involved levels of the lateral neck with metastases are levels III and IV.5 These levels are included in all lateral neck dissections (LNDs) carried out for metastatic PTC. Nodal metastases to level I occur rarely, and therefore, dissection of this level is generally not indicated.6 In contrast, metastases to level II and level V are not infrequent. Operations that have been recommended range from a modified radical neck dissection type 3, removing lymph nodes in levels II–V, to the super-selective nodal dissections, which remove only the compartments demonstrating gross nodal disease. There is no published evidence that one procedure is better than another, although it is well known that “berrypicking” procedures are associated with a greater incidence of regional recurrence.7
The decision on the extent of neck dissection is based on volume of disease and levels involved as well as surgeon and patient preference. At our institution, patients with gross lateral neck nodal disease are managed with a comprehensive lateral dissection of lymph nodes from levels II–V. Rarely, patients with lesser volume disease localized to levels III or IV may be managed with a selective neck dissection (SND). The objectives of our study were to answer 3 questions. First, which patients are selected to have comprehensive LND (CLND) versus SND? Second, what is the neck recurrence rate and pattern of nodal recurrence in patients who have had CLND and SND? Lastly, do patients who undergo CLND have less recurrence in the ipsilateral neck compared with those who have an SND?
METHODS
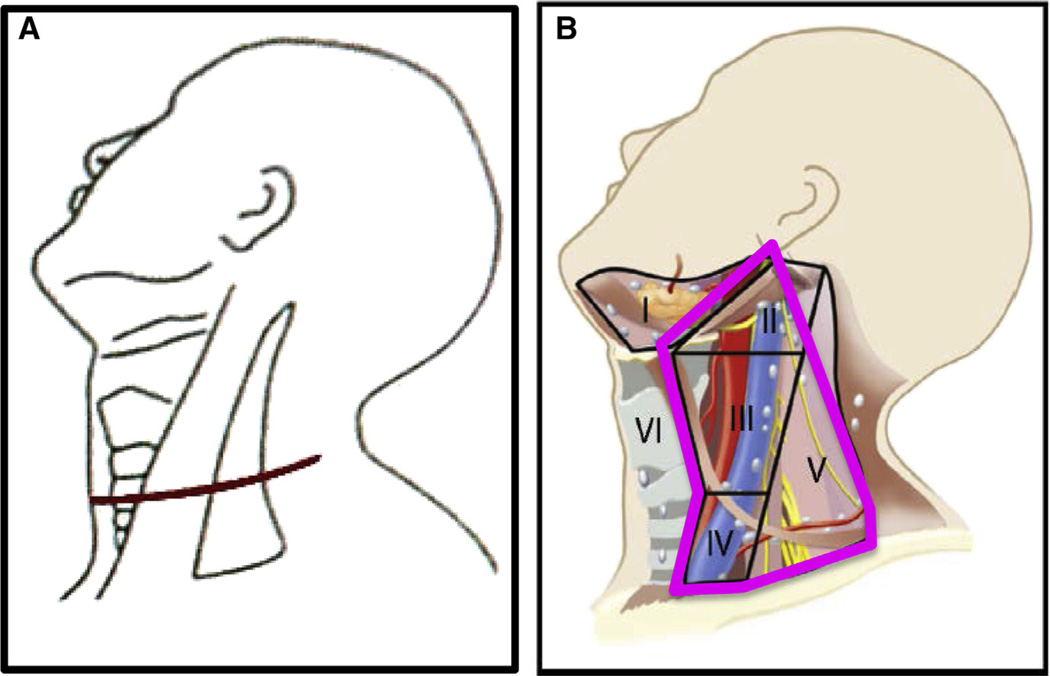
After Institutional Review Board approval, patients who had a therapeutic LND were identified from an institutional database of 3,664 patients with thyroid cancer treated between 1986 and 2010 at Memorial Sloan Kettering Cancer Center. Patients who had nonpapillary histology, M1 disease at presentation, and those who were considered to have unresectable disease were excluded, leaving 484 patients available for analysis. LND was categorized into comprehensive (CLND), which includes levels II–V, and selective (SND), which removes only the nodal basins with macroscopic disease with or without the addition of other at-risk levels. In CLND for thyroid cancer, we remove levels II–V preserving the sternocleidomastoid muscle, internal jugular vein, and accessory nerve. In level II, level IIA is dissected. Level IIB is dissected if there are suspicious lymph nodes in level IIA. In level V, only level VB (infra-accessory nerve) is dissected; it has been reported widely that metastases to level VA are rare.8 In SND, we dissect levels III and IV routinely but will dissect level II if clinically suspicious nodes are found at operation. The neck dissections were done in conjunction with thyroidectomy. The incision used for both SND and CLND were skin crease incisions in the mid neck extending laterally. The incision used for CLND extends more posteriorly to the level of the trapezius muscle. Both incisions follow natural skin creases (Fig 1).
Fig 1.
Skin incision (A) and levels dissected (B) in CLND for thyroid cancer. (Color version of figure is available online.)
Data collected included patient demographics and operative details such as the extent of thyroidectomy and the extent of LND. Histopathologic details recorded included pathologic T stage, N stage, and presence of extracapsular extension. TNM/International Union Against Cancer stage as defined in the American Joint Committee on Cancer Staging Manual 2010 was used.9 N status was defined as pN0 if nodes were removed and found to be benign on pathology. pN1a was defined as pathologically positive metastatic nodes removed from the central compartment (level VI), and pN1b was defined as pathologically positive metastatic nodes removed from the lateral neck (levels I–V) or from superior mediastinum (level VII). The postoperative treatment details recorded were use of radioactive iodine (RAI) or external beam radiotherapy.
The neck was staged by clinical examination supplemented with imaging and cytology. Preoperative ultrasonography (US) is effective for identifying metastatic nodes in the lateral neck. Since the year 2000, the sensitivity of this imaging modality has improved considerably, and we have increased our use of the modality. In addition, in patients with bulky neck disease, we supplement our examination with preoperative contrast-enhanced computed tomography (CT).
The number and level of positive lymph nodes in the neck dissection specimen were recorded from pathology reports. In patients who had neck recurrence, the patterns of recurrent lateral neck metastases by level involvement were recorded. Recurrence was detected by physical examination, radiologic imaging (I-131 whole body uptake scan, US, CT, and positron emission tomography), and/or measurements of serum thyroglobulin. Fine-needle aspiration biopsy of metastatic nodes was performed to confirm recurrent thyroid cancer in all patients with regional recurrence. The time from the initial thyroidectomy and neck dissection until a documented recurrence and subsequent treatment was determined for all patients.
Statistical analysis was carried out using SPSS (version 21, IBM Corporation, Armonk, NY). Clinical and pathologic variables were compared between CLND and SND groups using the χ2 analysis. Recurrence outcomes were analyzed using the Kaplan-Meier method. The overall lateral neck recurrence-free probability (Overall LNRFP) and the ipsilateral dissected neck recurrence free probability (Ipsilateral LNRFP) was determined for the CLND and SND groups using the Kaplan-Meier method and compared using the log-rank test.
RESULTS
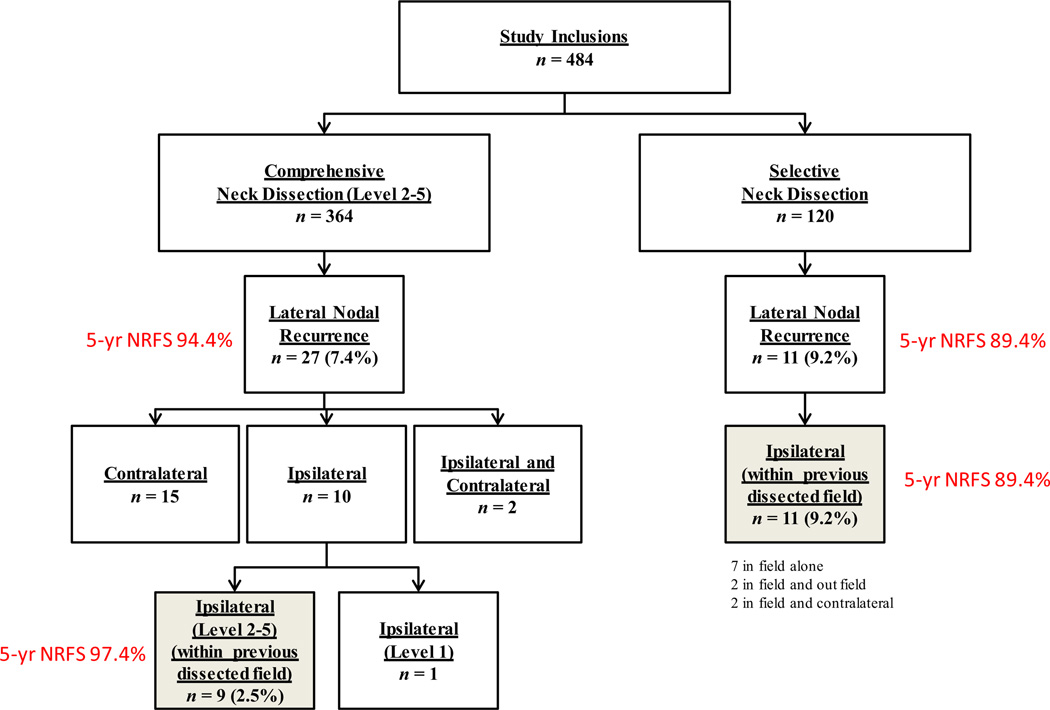
Of the 484 patients, 281 (58%) patients were female, and 203 (42%) were male; 275 (56.8%) were >45 years old; 432 (89.3%) patients underwent total thyroidectomy; 364 (75%) patients had a CLND; and 120 (25%) patients had an SND (Fig 2).
Fig 2.
Flowchart showing pattern of recurrence and recurrence rates in CLND and SND patients. NRFS, Nodal recurrence free survival.
Comparison of clinical and pathologic characteristics of CLND and SND patients
The 2 groups were similar with respect to age, sex, and initial thyroidectomy performed (Table). There was no difference between the 2 groups regarding adjuvant RAI administration or postoperative radiation therapy (P > .3 each). With regard to tumor characteristics, there was no difference between the 2 groups for pT stage; 222 patients in the CLND group (62%) had T3 or T4 tumors versus 73 (62%) patients in the SND group (P = .34). As expected, the CLND group had more advanced nodal disease with 96% of patients having pN1b disease versus 78% in the SND group (P = .002). The CLND group had a greater number of nodes removed in the specimen as well as a greater number of positive nodes (ie, Nodal burden) than the SND group did (P < .001).
Table.
Comparison of patient, tumor, and treatment characteristics in patients managed with CLND and SND
| All patients | CLND patients | SND patients | |||||
| Variable | n | % | n | % | n | % | P value |
| Age (y) | |||||||
| ≤45 | 275 | 56.8 | 214 | 58.8 | 61 | 50.8 | .127 |
| >45 | 209 | 43.2 | 150 | 41.2 | 59 | 49.2 | |
| Sex | |||||||
| Female | 281 | 58.1 | 209 | 57.4 | 72 | 60.0 | .619 |
| Male | 203 | 41.9 | 155 | 42.6 | 48 | 40.0 | |
| Operation | |||||||
| Less than total thyroid | 52 | 10.7 | 42 | 11.5 | 10 | 8.3 | .325 |
| Total thyroidectomy | 432 | 89.3 | 322 | 88.5 | 110 | 91.7 | |
| T stage | |||||||
| pT1 | 146 | 30.4 | 114 | 31.6 | 32 | 26.9 | .335 |
| pT2 | 39 | 8.1 | 25 | 6.9 | 14 | 11.8 | |
| pT3 | 228 | 47.5 | 176 | 48.8 | 52 | 43.7 | |
| pT4 | 67 | 14.0 | 46 | 12.7 | 21 | 17.6 | |
| N stage | |||||||
| pN0 | 23 | 4.8 | 11 | 3.0 | 12 | 10.0 | .002 |
| pN1a | 20 | 4.1 | 5 | 1.4 | 15 | 12.5 | |
| pN1b | 441 | 91.1 | 348 | 95.6 | 93 | 77.5 | |
| ECS | |||||||
| No | 154 | 59.7 | 120 | 58.5 | 34 | 64.2 | .458 |
| Yes | 104 | 40.3 | 85 | 41.5 | 19 | 35.8 | |
| PORT | |||||||
| No | 475 | 98.1 | 356 | 97.8 | 119 | 99.2 | .463 |
| Yes | 9 | 1.9 | 8 | 2.2 | 1 | 0.8 | |
| RAI | |||||||
| No | 120 | 24.8 | 86 | 23.6 | 34 | 28.3 | .3 |
| Yes | 364 | 75.2 | 278 | 76.4 | 86 | 71.7 | |
| Variable | Mean | SD | Mean | SD | Mean | SD | P value |
| Number of positive nodes | 5.2 | 5.3 | 5.7 | 5.4 | 3.6 | 4.5 | <.001 |
| Total number of nodes | 32.2 | 19.4 | 35.2 | 19.4 | 22.1 | 15.6 | <.001 |
| Duration of follow-up | 91 | 73.9 | 94.3 | 73.1 | 81.1 | 75.6 | .098 |
CLND, Comprehensive lateral neck dissection; ECS, extracapsular extension; PORT, postoperative radiation therapy; SND, selective neck dissection; RAI, radioactive iodine.
Overall neck recurrence rate and pattern of nodal recurrence in patients who had CLND and SND
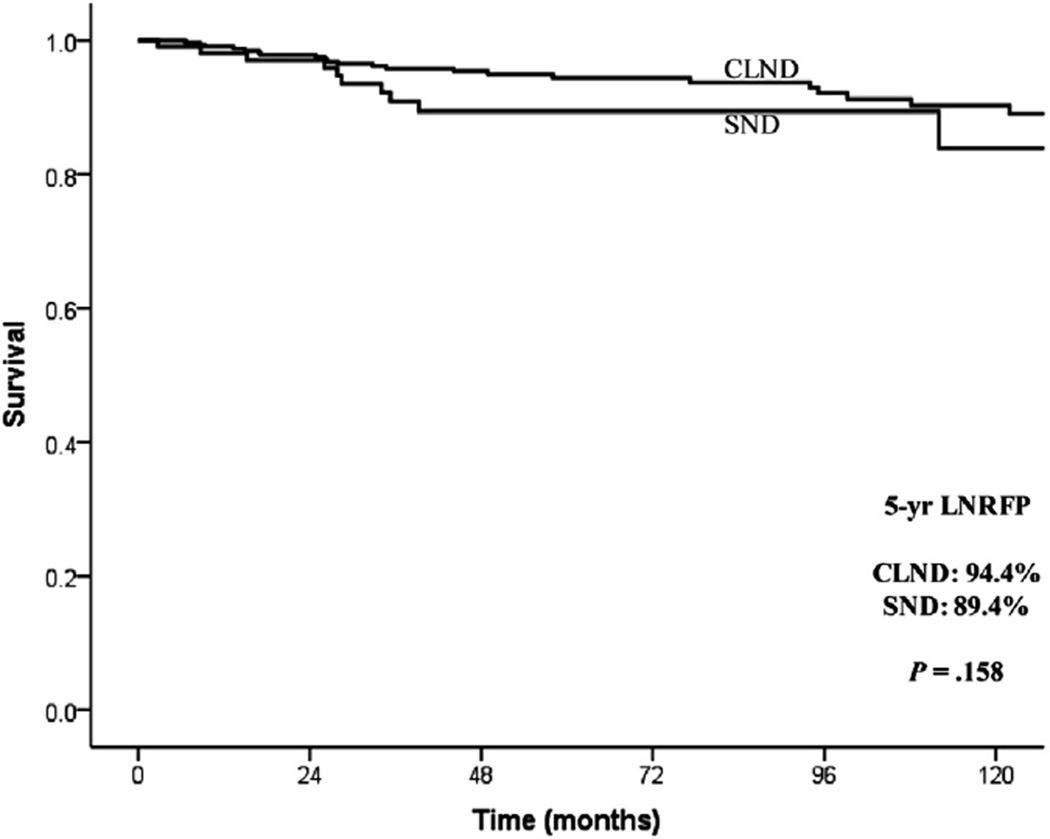
The median duration of follow-up was 63.5 months. Overall, 38 (8.0%) patients developed a lateral neck recurrence: 27 of 364 (7.4%) in the CLND group and 11 of 120 (9.2%) in the SND group (Fig 2). In CLND patients, 9 recurrences occurred in the ipsilateral dissected neck (9 of 364 = 2.5%), 1 in ipsilateral level 1 (0.2%), 15 (4.1%) in the contralateral neck, and 2 (0.5%) in both ipsilateral and contralateral neck. In SND patients, all recurrences (11 of 120 = 9.2%) were in the ipsilateral dissected neck. Seven patients recurred within the previously dissected nodal levels only. Two patients recurred within the previous dissected nodal levels as well as in ipsilateral levels that had not been dissected previously. Two patients recurred in both the ipsilateral (dissected and undissected neck levels) and contralateral neck. Kaplan-Meier analysis demonstrated no difference in overall LNRFP between the 2 groups (CLND 94.4% vs 89.4%; P = .158) (Fig 3).
Fig 3.
Kaplan-Meier plot showing the 5-year overall lateral neck recurrence-free probability (ipsilateral and contralateral) in CLND and SND patients.
Ipsilateral (dissected neck field) LNRFP for CLND and SND groups
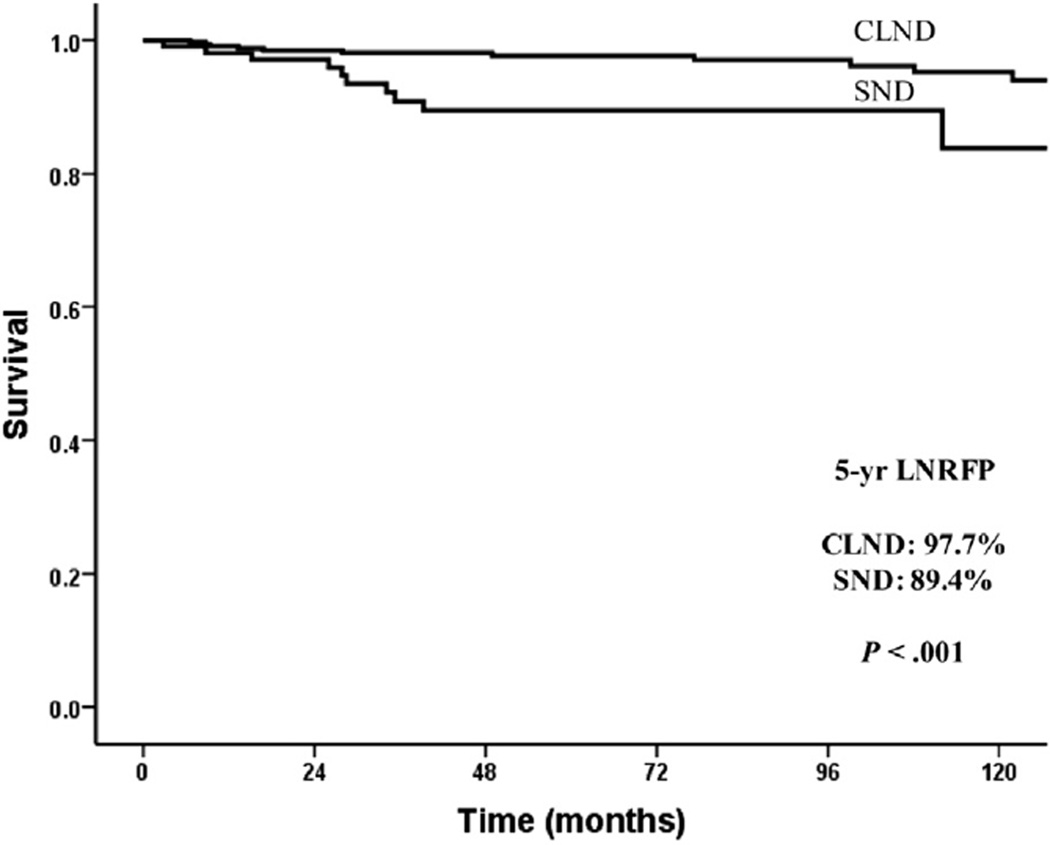
Although there was no difference in overall neck recurrence rates, patients who underwent SND were more likely to recur in the ipsilateral dissected neck compared with the CLND patients (Fig 2 shaded boxes: 9.2% vs 3%; P = .001). Kaplan-Meier analysis demonstrated a difference in ipsilateral (dissected neck) LNRFP between the 2 groups (CLND 97.7% vs 89.4%; P < .001) (Fig 4).
Fig 4.
Kaplan-Meier plot showing the 5-year ipsilateral (dissected neck field), lateral neck, recurrence-free probability in CLND and SND patients.
Complications associated with neck dissection for CLND and SND
Complications associated with neck dissection, ie, chyle leak, hematoma, shoulder dysfunction, and hypoglossal nerve or marginal mandibular nerve injury, are shown in the Supplementary Figure. Shoulder dysfunction complications were more frequent in patients having CLND (P = .019); however, these were temporary in duration, and only 1 patient had long-term shoulder dysfunction. There were no differences in the rate of chyle leak, hematoma, and hypoglossal or marginal mandibular nerve injury between groups.
DISCUSSION
Clinically evident lateral neck disease in patients with differentiated thyroid cancer is clinically important because these patients are at increased risk of developing additional regional and distant disease. This possibility is especially true in patients who are >45 years of age. A recent report from our institution demonstrated that patients with PTC who are ≥45 years old with N1b disease at presentation have poorer, disease-specific survival as well as distant recurrence-free survival compared with patients who have pN0/Nx or N1a disease.4 Optimum management of the lateral neck is, therefore, of great importance, especially in the older patient. Because there has been much debate as to the extent of dissection of the lateral neck in patients with clinically evident lateral neck disease, we sought to answer 3 questions. First, which patients are selected to have a CLND versus an SND? Second, what is the neck recurrence rate and pattern of nodal recurrence in patients who had CLND and SND? Finally, do patients who undergo CLND have less recurrence in the ipsilateral neck compared with those who have an SND?
Our data support our practice of offering CLND to patients with more extensive and bulky nodal disease (Table) with more N1b disease. Those patients with a lesser volume of neck disease and disease limited to 1 or 2 levels were chosen to have a more selective approach. With regard to question 2, we found that in these highly selected patients who had a limited neck dissection, all recurrences were within the previously dissected neck levels (2 patients also had contralateral neck recurrence). This finding was in contrast to patients managed by CLND, where more than half of the patients developed contralateral neck recurrence, which would be consistent with the CLND cohort’s more advanced neck disease, including metastasized neck nodes and a greater volume and number of levels compared with patients with SND. For question 3, we report that the overall neck recurrence rates between the 2 groups were similar. It is important, however, to observe that control in the ipsilateral dissected neck was superior in the CLND patients compared with the SND patients, despite the fact that the CLND patients had a greater volume of neck disease; this finding would be consistent with the greater efficacy of a CLND compared to an SND.
In our series, there were 27 recurrences in the CLND patients (7.4%) and 11 recurrences in the SND patients (9.2%), with an overall recurrence rate of 38 (7.8%). Published data analyzing specific disease outcomes in patients with PTC who undergo therapeutic LNDs at the time of primary thyroidectomy are limited. The literature suggests recurrence that develops in the cervical lymph nodes ranges from 5.4–13% after the initial thyroidectomy.10 A study by O’Neill et al11 demonstrated a 13% neck nodal recurrence rate after total thyroidectomy, central neck dissection, and compartment-oriented nodal dissection of involved lateral lymph node compartments (n = 116). Nine of their 15 recurrences occurred within the previously dissected ipsilateral lateral neck, and 3 patients recurred in the contralateral neck, totaling a lateral neck recurrence rate of 10.3%.
Kim et al12 reviewed 126 patients who underwent therapeutic LND with total thyroidectomy and bilateral central neck dissection concomitantly for PTC at the National Cancer Center in Korea. Recurrence occurred in 22 patients (17.5%), with 1 patient (0.8%) dying of brain metastasis. Excluding 2 of those recurrences who recurred with distant disease only and another 4 patients who recurred in the central neck only, they had 16 lateral neck recurrences (including contralateral lateral neck recurrences) for a lateral neck recurrence rate of 12.7%; 12 patients recurred in the previously dissected ipsilateral lateral neck. Lastly, Forest et al13 reported an 11.8% recurrence rate in 34 patients who underwent central and LND with the initial management of their PTC.
Although our data suggest that the ipsilateral neck recurrence rate is less in patients who had CLND (3%) compared with SND (9%), one may argue that the rates of recurrence for SND are still acceptable and may potentially avoid any complications that may occur from a more comprehensive neck dissection. It is widely accepted that complications, such as chyle leak, hematoma, and shoulder dysfunction resulting from damage either to the accessory nerve or to the nerve supply to the posterior triangle neck musculature, are more common after CLND.14–18 In our own series, we did not observe any statistically significant differences in complication rates for chyle leak, hematoma, and hypoglossal or marginal nerve injury. Shoulder dysfunction complications were more frequent in patients undergoing a CLND (P = .019), but shoulder dysfunctions were temporary in duration, and only 1 patient had long-term shoulder dysfunction.
We do, however, acknowledge that a retrospective chart review for functional complications in shoulder movement is highly prone to underreporting. It is important to note that we were only able to achieve a recurrence rate of 9% in SND patients by being highly selective and only carrying out this operation in patients with small volume lymph nodes limited to 1 or 2 neck levels. One would expect recurrence rates to be greater if SND was done in multilevel bulky neck nodes scenarios.
As is the nature of retrospective studies, our data have limitations associated with retrospective data collection. Collected data are dependent on the accuracy of chart review as well as pathology review. It is well recognized that the nodal yield reported by pathologists can vary widely between pathologists and is highly dependent on the investment of time and detail to which neck dissection specimens are scrutinized. Our institution has many years of experience in the management of thyroid cancer, and as such, the variation of nodal yield from pathologic analysis is limited by such quality control. Second, one can never fully account for the selection bias that takes place in the decision to carry out an LND and the extent of such a dissection, but our philosophy of treatment has remained uniform; patients with bulky lateral neck disease tend to have more comprehensive neck dissection.
We were stringent in choosing patients for SND, limiting our selection to patients with low volume of disease restricted to 1 or 2 neck levels. As well as selection bias, we recognize that there is also possible misclassification bias within our own data set. For example, some surgeons may remove part of level V, such as the anterior portion, yet these dissections may be labeled as an SND of levels II–IV. In addition, some surgeons may remove level V incompletely through a medial approach and code this as a CLND (levels II–V).
Furthermore, our data may underestimate the extent of neck recurrence. Regular US and serial monitoring of serum thyroglobulin has only become routine practice in the last decade. Many of the patients included in this data set were managed before this time and as such were followed with clinical examination alone. For this reason, small, nonpalpable recurrent neck disease may not have been identified in those early years, which would lead to an underestimation of the rate of subclinical neck recurrence that we are reporting.
In conclusion, despite having more aggressive disease, patients with clinically evident lateral neck disease in PTC managed by CLND have comparable overall rates of neck recurrence to patients with SND. Importantly, we report less rates of ipsilateral dissected neck recurrence in the CLND cohort compared with patients managed by SND (3% vs 9%, P <.05) in keeping with the more comprehensive nature of these types of neck dissection. As such, we recommend that SND should be reserved only for highly selected patients who have low volume disease localized to 1 or 2 specific nodal compartments.
Supplementary Material
Acknowledgments
Supported in part through NIH/NCI Cancer Center Support Grants P30 CA008748.
Footnotes
The authors have no disclosures.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.surg.2016.02.005.
REFERENCES
- 1.Randolph GW, Duh Q, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22:1144–1152. doi: 10.1089/thy.2012.0043. [DOI] [PubMed] [Google Scholar]
- 2.Bozec A, Dassonville O, Chamorey E, Poissonnet G, Sudaka A, Peyrottes I, et al. Clinical impact of cervical lymph node involvement and central neck dissection in patients with papillary thyroid cancer: a retrospective analysis of 368 cases. Eur Arch Otorhinolaryngol. 2011;268:1205–1212. doi: 10.1007/s00405-011-1639-2. [DOI] [PubMed] [Google Scholar]
- 3.Hughes CJ, Shaha AR, Shah JP, Loree TR. Impact of lymph node metastasis in differentiated carcinoma of the thyroid: a matched-pair analysis. Head Neck. 1996;18:127–132. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<127::AID-HED3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Nixon IJ, Wang LY, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery. 2014;156:137–146. doi: 10.1016/j.surg.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Machens A, Hinze R, Thomusch O, Dralle H. Patterns of nodal metastasis for primary and reoperative thyroid cancer. World J Surg. 2002;26:22–28. doi: 10.1007/s00268-001-0176-3. [DOI] [PubMed] [Google Scholar]
- 6.Kupferman ME, Patterson M, Mandel SJ, Livolsi V, Weber RS. Patterns of lateral neck metastasis in papillary thyroid cancer. Arch Otolarnygol Head Neck Surg. 2004;130:857–860. doi: 10.1001/archotol.130.7.857. [DOI] [PubMed] [Google Scholar]
- 7.Caron NR, Clark OH. Papillary thyroid cancer: surgical management of lymph node metastases. Curr Treat Options Oncol. 2005;6:311–322. doi: 10.1007/s11864-005-0035-9. [DOI] [PubMed] [Google Scholar]
- 8.Khafif A, Medina JE, Robbins KT, Silver CE, Weber RS, Rinaldo A, et al. Level V in therapeutic neck dissections for papillary thyroid carcinoma. Head Neck. 2013;35:605–607. doi: 10.1002/hed.21952. [DOI] [PubMed] [Google Scholar]
- 9.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th. New York: Springer-Verlag; 2010. [Google Scholar]
- 10.Grant CS, Hay ID, Gough IR, Bergstralh EJ, Goellner JR, McConahey WM. Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery. 1988;104:954–962. [PubMed] [Google Scholar]
- 11.O’Neill CJ, Coorough N, Lee JC, Clements J, Delbridge LW, Sippel R, et al. Disease outcomes and nodal recurrence in patients with papillary thyroid cancer and lateral neck nodal metastases. ANZ J Surg. 2014;84:240–244. doi: 10.1111/ans.12045. [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Park SY, Lee YJ, Lee EK, Kim SK, Kim TH, et al. Risk factors for recurrence after therapeutic lateral neck dissection for primary papillary thyroid cancer. Ann Surg Oncol. 2014;21:1884–1890. doi: 10.1245/s10434-014-3507-y. [DOI] [PubMed] [Google Scholar]
- 13.Forest VI, Clark JR, Ebrahimi A, Cho EA, Sneddon L, Gao K, et al. Central compartment dissection in thyroid papillary carcinoma. Ann Surg. 2011;253:123–130. doi: 10.1097/SLA.0b013e3181fc9644. [DOI] [PubMed] [Google Scholar]
- 14.Kupferman ME, Patterson DM, Mandel SJ, LiVolsi V, Weber RS. Safety of modified radical neck dissection for differentiated thyroid carcinoma. Laryngoscope. 2004;114:403–406. doi: 10.1097/00005537-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Terrell JE, Welsh DE, Bradford CR, Chepeha DB, Esclamado RM, Hogikyan ND, et al. Pain, quality of life, and spinal accessory nerve status after neck dissection. Laryngoscope. 2000;110:620–626. doi: 10.1097/00005537-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Inoue H, Nibu K, Saito M, Otsuki N, Ishida H, Onitsuka T, et al. Quality of life after neck dissection. Arch Otolaryngol Head Neck Surg. 2006;132:662–666. doi: 10.1001/archotol.132.6.662. [DOI] [PubMed] [Google Scholar]
- 17.van Wilgen CP, Dijkstra PU, van der Laan BF, Plukker JT, Roodenburg JL. Shoulder complaints after neck dissection; is the spinal accessory nerve involved? Br J Oral Maxillofac Surg. 2003;41:7–11. doi: 10.1016/s0266-4356(02)00288-7. [DOI] [PubMed] [Google Scholar]
- 18.Cappiello J, Piazza C, Giudice M, De Maria G, Nicolai P. Shoulder disability after different selective neck dissections (levels II–IV versus levels II–V): a comparative study. Laryngoscope. 2005;115:259–263. doi: 10.1097/01.mlg.0000154729.31281.da. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.