Abstract
Objective
To describe the clinical characteristics of Latin American patients with metastatic renal cell carcinoma (mRCC) who experienced a progression-free survival (PFS) for at least 15 months following treatment with sunitinib.
Patients and methods
In this retrospective analysis, mRCC patients in two institutions in Latin America received sunitinib at a starting dose of either 50 mg/day for 4 weeks followed by 2 weeks off treatment (Schedule 4/2) in repeated 6-week cycles or sunitinib 37.5 mg on a continuous daily dosing schedule. Clinical characteristics, tolerability, and PFS data were collected.
Results
Twenty-nine patients with long-term clinical benefit from sunitinib were identified between September 2005 and August 2009. Median PFS was 23 months (range: 15–54 months). Two of the 29 patients with prolonged PFS achieved a complete response and additional eleven had a partial response. Most patients were aged <60 years, had good performance status, favorable or intermediate Memorial Sloan Kettering Cancer Center prognostic risk, and disease limited to one or two sites. Dose reduction was necessary in all patients who started sunitinib at 50 mg/day administered on Schedule 4/2. Adverse events leading to dose reduction included grade 3 hand–foot syndrome, mucositis, fatigue, and hypertension. At the time of data cutoff, four patients were still receiving sunitinib treatment.
Conclusion
Extended PFS can be achieved in Latin American patients with mRCC treated with sunitinib. Although the small sample size and retrospective nature of this evaluation preclude the identification of pretreatment predictive factors contributing to this benefit, the current analysis warrants further investigation using a larger data set in this population.
Keywords: renal cell carcinoma, sunitinib, long-term benefit, Latin America
Introduction
Sunitinib malate (Sutent®; Pfizer Inc., New York, NY, USA) is an orally administered multitargeted inhibitor of vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and other receptor tyrosine kinases.1–3 Sunitinib has been approved worldwide for the treatment of advanced renal cell carcinoma (RCC) based on its superior efficacy compared with interferon-alpha (IFN-α) as a first-line therapy and its activity in patients who previously received cytokine therapy.4–8 Prospective studies of sunitinib have shown median progression-free survival (PFS) of 11 months in patients with treatment-naïve metastatic RCC (mRCC)6 and median PFS of ~8 months in the cytokine-refractory setting.4,5 However, there are patients treated with sunitinib who appear to achieve a longer PFS benefit than others. Evaluation of clinical and molecular characteristics of patients achieving long-term benefit may provide valuable information on predictive biomarkers that can be used prospectively to identify those most likely to respond to treatment. In a previous report, 34 long-term responders, defined as patients achieving durable complete response (CR) or remaining progression free for ≥18 months while receiving sunitinib, were identified from nine clinical trials, conducted at Memorial Sloan Kettering Cancer Center (MSKCC) between January 2003 and December 2008.9 In that report, factors that appeared to be favorably prognostic included a lack of bone and/or lung metastases and good MSKCC prognostic risk status.
This retrospective analysis was conducted to further describe the clinical characteristics of sunitinib-treated patients with mRCC from two institutions in Latin America, in whom PFS was at least 15 months.
Patients and methods
This was a retrospective chart review of all patients with mRCC who had PFS ≥15 months when treated with sunitinib in either of the two referral hospitals: Hospital Israelita Albert Einstein in Sao Paulo, Brazil (Cohort 1) and Alexander Fleming Institute in Buenos Aires, Argentina (Cohort 2). This retrospective chart review analysis was approved by the institutional review board/independent ethics committee at these aforementioned centers. Patients had signed the written informed consent prior to enrolling in the expanded access trial or the general consent prior to admission to the institutions for treatment.
Eligibility
Both cohorts included patients treated as part of the sunitinib open-label expanded-access study, which was set up to provide sunitinib to patients in countries where approval had not yet been granted, and to those ineligible for registration-directed trials but judged to have the potential to derive clinical benefit from treatment. It included both previously treated and treatment-naïve patients with RCC.10 The remaining patients had been treated with sunitinib (following its approval) on diagnosis of metastatic disease.
Treatment
Patients received sunitinib at a starting dose of either 50 mg/day for 4 weeks followed by 2 weeks off treatment (Schedule 4/2), in repeated 6-week cycles, or sunitinib 37.5 mg/day on a continuous daily dosing (CDD) schedule. Patients enrolled in the expanded-access study received sunitinib according to Schedule 4/2, with the option to transition to CDD (following dose reduction) on disease progression or worsening clinical condition, at the discretion of the investigator, during the scheduled treatment break.10 Those patients enrolled in the expanded-access study received sunitinib as a second-line therapy, whereas patients receiving first-line sunitinib were treated off protocol and, following approval, received sunitinib as the standard of care.
Patient assessment
Tumor response was monitored regularly (every 3–4 months) and assessed according to Response Evaluation Criteria in Solid Tumors (RECIST).11 Clinical benefit was defined as CR, partial response (PR), or disease stabilization by RECIST. PFS was defined as the period from starting sunitinib until the time of unequivocal documented disease progression. Safety and tolerability were assessed throughout the treatment (at the beginning of every 6-week cycle) using standard clinical practice monitoring and methods. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0 (and Version 4.0 when it became available).12 All patients with hypertension or a history of hypertension were evaluated and monitored by a specialized cardiologist, and medication was adjusted accordingly. All patients were followed up for at least 28 days after the last dose of sunitinib.
Statistical analysis
The Kaplan–Meier method was used to estimate PFS and overall survival. Treatment-emergent, all-causality AEs were summarized descriptively.
Results
Patient characteristics
Twenty-nine patients with long-term clinical benefit from sunitinib were identified between September 2005 and August 2009. Nine patients (the total number of patients treated with sunitinib during this time is not available) were treated at the Hospital Israelita Albert Einstein (Cohort 1) and 20 patients (of a total of 76 patients treated with sunitinib during this period) at the Alexander Fleming Institute (Cohort 2). Patient baseline characteristics are presented in Table 1. Patients had a median age of 56 years (range: 37–73 years), with an Eastern Cooperative Oncology Group performance status (ECOG PS) 0 (n=15) or 1 (n=14). Most (86%) patients were of intermediate risk according to MSKCC criteria, and 93% had clear cell histology. Approximately, 78% of the patients had two or fewer metastatic sites (mostly in the lungs, liver, or bone) and more than two-thirds (69%) of patients had received prior systemic therapy, reflecting a heavily pretreated population.
Table 1.
Patient characteristics at baseline
| Characteristics | Cohort 1 (n=9) |
Cohort 2 (n=20) |
Total (N=29) |
|---|---|---|---|
| Median (range) age, years | 52 (45–69) | 60 (37–73) | 56 (37–73) |
| Male/female, n (%) | 7/2 (78/22) | 12/8 (60/40) | 19/10 (66/34) |
| ECOG PS, n (%) | |||
| 0 | 0 | 15 (75) | 15 (52) |
| 1 | 9 (100) | 5 (25) | 14 (48) |
| Histology, n (%) | |||
| Clear cell | 8 (89) | 19 (95) | 27 (93) |
| Other | 1 (11) | 1 (5) | 2 (7) |
| Prior nephrectomy, n (%) | 8 (89) | 20 (100) | 28 (97) |
| Prior cytokine therapy, n (%) | 5a (56) | 14 (70) | 19 (66) |
| Treatment-naïve, n (%) | 3 (33) | 6 (30) | 9 (31) |
| MSKCC risk group, n (%) | |||
| Favorable | 4 (44) | 0 (0) | 4 (14) |
| Intermediate | 5 (56) | 20 (100) | 25 (86) |
| Sites of metastatic disease, n (%) | |||
| Lung | 4 (44) | 16 (80) | 20 (69) |
| Liver | 5 (56) | 1 (5) | 6 (21) |
| Bone | 2 (22) | 3 (15) | 5 (17) |
| Other | 7 (78) | 5 (25) | 12 (41) |
| History of hypertension, n (%) | 3 (33) | 4 (20) | 7 (24) |
Note:
An additional patient in Cohort 1 received prior gemcitabine without cytokine therapy.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; MSKCC, Memorial Sloan Kettering Cancer Center.
Treatment
All patients in Cohort 1 (n=9) received sunitinib on Schedule 4/2. In Cohort 2, sunitinib was given on Schedule 4/2 in 17 patients and, according to the treating clinician’s decision, on a CDD schedule in three patients (none of the patients who started CDD therapy were treated as part of the expanded-access study). The median duration of sunitinib treatment was 27.3 months (range: 15–46 months) in Cohort 1 and 20 months (range: 15–54 months) in Cohort 2. At data cutoff, treatment was ongoing in three patients and one patient from Cohorts 1 and 2, respectively.
Overall, dose reduction was necessary in 59% (17/29) of patients to maintain sunitinib therapy. All dose reductions were in patients who started on sunitinib 50 mg/day administered on Schedule 4/2. Patients on the sunitinib 37.5 mg/day CDD schedule did not require dose reduction. In Cohort 1, five (56%) patients required a dose reduction to 37.5 mg/day on Schedule 4/2. In four of these five patients, the dose reduction was needed to manage toxicity. In the fifth patient, it was due to disease progression while on the “2 weeks off” phase of the treatment. As a result, this patient was switched to a CDD schedule. In Cohort 2, the dose was reduced, due to asthenia/fatigue, to sunitinib 37.5 mg/day (Schedule 4/2) in 12 (60%) patients, and in two (10%) patients, reduced further to 25 mg/day (Schedule 4/2).
Efficacy
Two of the 29 patients with prolonged PFS achieved a CR and a further eleven had a PR. A summary of best tumor responses is presented in Table 2. Median PFS was 27.1 months (range: 15–46 months) in Cohort 1 and 20.0 months (range: 15–54 months) in Cohort 2 (Figure 1). In all 29 patients, median PFS was 23.0 months (range: 15–54 months). Median overall survival was 43 months (range: 15–74 months) in Cohort 1 (one patient was lost to follow-up) and 36 months (range: 15–71 months) in Cohort 2.
Table 2.
Best tumor response (RECIST) for total population and by cohort
| Response | Cohort 1 (n=9) |
Cohort 2 (n=20) |
Total (N=29) |
|---|---|---|---|
| Complete response (CR), n (%) | 0 | 2 (10) | 2 (7) |
| Partial response (PR), n (%) | 4 (44) | 7 (35) | 11 (38) |
| Stable disease (SD), n (%) | 5 (56) | 11 (55) | 16 (55) |
| Clinical benefit rate (CR + PR + SD), n (%) | 9 (100) | 20 (100) | 29 (100) |
Abbreviation: RECIST, Response Evaluation Criteria in Solid Tumors.
Figure 1.
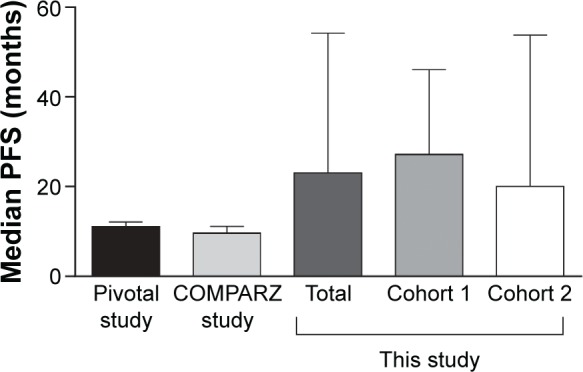
Median PFS in pivotal, COMPARZ, and the present study.
Notes: Median (range) PFS for the total population (23.0 [15–54] months), Cohort 1 (27.1 [15–46] months), and Cohort 2 (20.0 [15–54] months) in this study compared with median (95% confidence interval) PFS reported for the Pivotal study of sunitinib (11 [10–12] months)6 and the COMPARZ study (9.5 [8.3–11.1] months).13
Abbreviation: PFS, progression-free survival.
Following discontinuation of sunitinib, the majority of patients received subsequent therapy. In Cohort 1, data on subsequent therapies were available for eight of the nine patients. Subsequent therapies were sorafenib (n=3; one of whom went on to receive everolimus), everolimus (n=2; one of whom received everolimus after subsequent sorafenib), or IFN-α (n=1). Two patients remained on sunitinib at the time of data cutoff, and one patient received no other therapy. In Cohort 2, eleven of 20 patients received other therapies following discontinuation of sunitinib as follows: sorafenib (n=4), everolimus (n=3), bevacizumab (n=2), temsirolimus (n=1), and chemotherapy (n=1).
Safety
Treatment-emergent, all-causality AEs occurring in patients with prolonged PFS are presented in Table 3. The most frequently reported AEs of any grade were asthenia and/or fatigue. AEs leading to dose reduction included acute renal failure, elevated serum glutamic oxaloacetic transaminase, hand–foot syndrome (HFS; two dose reductions in one patient), mucositis, fatigue, and hypertension (all grade 3).
Table 3.
All treatment-related adverse events observed in the two cohorts
| Adverse event, n (%) | Cohort 1 (n=9)
|
Cohort 2 (n=20)
|
Total (N=29)
|
|||
|---|---|---|---|---|---|---|
| All grades | Grade 3/4 | All grades | Grade 3/4 | All grades | Grade 3/4 | |
| Asthenia and/or fatigue | 7 (78) | 1 (11) | NA | 10 (50) | NA | 11 (38) |
| Mucositis | 6 (67) | 0 | NA | 3 (15) | NA | 3 (10) |
| Diarrhea | 5 (56) | 0 | NA | 2 (10) | NA | 2 (7) |
| Nausea and/or vomiting | 7 (78) | 0 | NA | 0 | NA | 0 |
| Hand–foot syndrome | 5 (56) | 1 (11) | NA | 0 | NA | 1 (3) |
| Hypertension | 5 (56) | 1 (11) | 18 (90) | 1 (5) | 23 (79) | 2 (7) |
Note: NA, not available (some of all-grade adverse-event data were not available for Cohort 2).
Of the nine patients in Cohort 1, six developed hypertension during sunitinib therapy. Three patients had a history of hypertension prior to sunitinib treatment, but none experienced worsening of hypertension during treatment with sunitinib. Four of 20 patients in Cohort 2 had a history of hypertension and all four needed to increase their dose of antihypertensive medication due to an increase in blood pressure during sunitinib treatment.
Discussion
This retrospective analysis identified 29 patients from Latin America who had long-term clinical benefit, defined as PFS ≥15 months, with sunitinib. Overall, PFS in these patients ranged from 15 to 54 months, with a median of 23 months, and four patients remained on therapy at the time of data cutoff. These patients were typically younger than 60 years of age, with good ECOG PS, clear cell histology, favorable or intermediate MSKCC prognostic risk criteria, and disease typically limited to one or two sites. This finding was as expected, as a reduced tumor volume and better overall condition of the patient generally increase the likelihood of receiving and maintaining an appropriate dose of sunitinib, as demonstrated in the Phase III sunitinib trial by Motzer et al.6
Characteristics identified here as associated with long-term response were broadly similar to those reported by Molina et al,9 who assessed patient characteristics associated with PFS ≥18 months in patients from nine clinical trials of sunitinib (alone or in combination; n=34). In the current study, 66% of the patients experiencing long-term clinical benefit from sunitinib had lung metastases and 17% had bone metastases, compared with 53% and 15%, respectively, reported by Molina et al.9 In their study, Molina et al found a lower incidence of bone and lung metastases in the long-term responders versus the overall population and suggested that a lack of bone or lung metastases and good MSKCC risk may be predictive of long-term response. The median PFS reported here (23 months across both patient cohorts) compares favorably with the median PFS of 17.4 months (95% confidence interval 7–29.9) among the long-term responders documented by Molina et al. Not surprisingly, in the same study, median PFS among all patients was shorter (10.8 months), which is more in line with median PFS of 11 months in the pivotal Phase III study of sunitinib6 or 9.5 months with sunitinib in the COMPARZ study, where analysis was conducted based on all patients irrespective of whether they were long-term responders or not.13 These results indicate a selection bias in our study.
The current study showed long-term clinical benefit with sunitinib in Latin American patients with treatment-naïve mRCC and in those who had received previous cytokine therapy. This was consistent with the findings of Molina et al9 in which 47% of long-term responders in that analysis had received prior treatment for mRCC. However, the small number of patients reported here precludes any definitive conclusions regarding the relative magnitude of benefit derived by Latin American patients receiving sunitinib in first line versus subsequent lines of therapy.
In addition to deriving long-term clinical benefit with sunitinib, two (10%) patients in Cohort 2 experienced a CR to therapy. Complete remission is not common following tyrosine kinase inhibitor treatment for mRCC, but has been reported in some patients. A retrospective analysis of patients treated for mRCC with either sunitinib or sorafenib in France and Switzerland identified CR in 64 patients (59 of whom had received sunitinib either alone or in combination with local treatment).14 In this regard, it is worth noting of recent advances in understanding of the critical role played by T-cell-mediated immune response and immune checkpoint modulators, such as programmed death-1 ligand and its receptor, in cancer and development of resistance to antiangiogenic therapy.15,16 Nivolumab, a programmed death-1 checkpoint inhibitor, has been shown to improve survival compared with everolimus in previously treated patients with advanced RCC in a randomized, open-label Phase III trial.17 Therefore, combination therapy of sunitinib or pazopanib and immune checkpoint inhibitor with different mechanisms of action may provide further clinical benefit.18
Dose reductions were common (59%) to enable patients to remain on treatment and continue to benefit from sunitinib. A multidisciplinary approach (encompassing dermatologists, cardiologists, internal medicine practitioners, endocrinologists, medical oncologists, and specialist nurse support) is critical to ensure optimal therapy management and to maximize the benefits of treatment for patients with advanced RCC. The safety profile of sunitinib in the current study was consistent with published data and no unexpected late toxicities were identified following long-term sunitinib treatment.4,5 AEs reported by patients with long-term clinical benefit from sunitinib included a number of events currently under evaluation as potential biomarkers of sunitinib efficacy: namely, hypertension,19 asthenia/fatigue,20 and HFS.21,22 Statistically significant associations were found between these AEs and clinical outcomes in a recent retrospective multivariate analysis of pooled data from five clinical trials of sunitinib in patients with mRCC (N=770),23 wherein the results indicated that hypertension and HFS, in particular, and asthenia/fatigue to a lesser degree, were significant independent predictors of PFS and overall survival.
The limitations of the current study are the absence of comparator data for the general RCC population, the small size of the total study population, and the retrospective nature of the evaluation, all of which preclude identification of pretreatment predictive factors based on the AEs observed in patients deriving long-term benefit from sunitinib.
Conclusion
Long-term clinical benefit with sunitinib is possible in patients with advanced RCC, including those from Latin America. Several clinical biomarkers have previously been identified that may allow physicians to select appropriate therapies for individual patients and monitor patients’ response to therapy in the future. These findings are supported by preliminary data from the current study. Further efforts should be made to identify and/or confirm potential molecular biomarkers. In the meantime, appropriate management of toxicities in all patients treated with sunitinib will enable patients to remain on treatment and derive optimal benefit from the therapy.
Acknowledgments
We would like to thank all the participating patients and their families, as well as members of the research teams at all study sites. Medical writing support was provided by Christine Arris and Rachel Mason at ACUMED® (Tytherington, UK), an Ashfield Company, part of UDG Healthcare plc, and Mariko Nagashima, PhD, at Engage Scientific Solutions (Southport, CT, USA), with funding from Pfizer Inc.
Footnotes
Author contributions
All authors have made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; in drafting the article and revising it critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
O Smaletz has received honoraria from Bayer and Pfizer, research funding from Pfizer and Roche, scientific funding from Bayer, Novartis, and Pfizer, and has held a consultant or advisory role for Pfizer. The other authors report no conflicts of interest in this work.
References
- 1.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2(5):471–478. [PubMed] [Google Scholar]
- 2.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. [PubMed] [Google Scholar]
- 3.O’Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101(9):3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295(21):2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Sutent (suntinib malate) capsule [US prescribing information] [webpage on the Internet] New York, NY: Pfizer Inc; 2006. [Accessed January 7, 2016]. [updated April 2015]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=607. [Google Scholar]
- 8.Summary of product characteristics (EU SmPC): Sutent [webpage on the Internet] London: European Medicines Agency; 2006. [Accessed January 7, 2016]. [updated July 27, 2015]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000687/human_med_001069.jsp&mid=WC0b01ac058001d124. [Google Scholar]
- 9.Molina AM, Jia X, Feldman DR, et al. Long-term response to sunitinib therapy for metastatic renal cell carcinoma. Clin Genitourin Cancer. 2013;11(3):297–302. doi: 10.1016/j.clgc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10(8):757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute Cancer Therapy Evaluation Program (CTEP) [webpage on the Internet] [Accessed January 7, 2016]. [updated December 15, 2010]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 13.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 14.Albiges L, Oudard S, Negrier S, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30(5):482–487. doi: 10.1200/JCO.2011.37.2516. [DOI] [PubMed] [Google Scholar]
- 15.Liu XD, Hoang A, Zhou L, et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res. 2015;3(9):1017–1029. doi: 10.1158/2326-6066.CIR-14-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Figueroa DJ, Fay AP, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res. 2015;21(5):1071–1077. doi: 10.1158/1078-0432.CCR-14-1993. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2014;32(Suppl 15) abstr 5010. [Google Scholar]
- 19.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763–773. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis MP, Figlin RA, Hutson TE, et al. Asthenia and fatigue as potential biomarkers of sunitinib efficacy in metastatic renal cell carcinoma. Eur J Cancer. 2011;47(Suppl 1):S135. [Google Scholar]
- 21.Puzanov I, Michaelson D, Cohen D, Li S, Burnett P, Desai J. Evaluation of hand–foot syndrome (HFS) as a potential biomarker of sunitinib (SU) efficacy in patients (pts) with metastatic renal cell carcinoma (mRCC) and gastrointestinal stromal tumour (GIST) Eur J Cancer. 2011;47(Suppl 1):S182. [Google Scholar]
- 22.Michaelson MD, Cohen DP, Li S, et al. Hand–foot syndrome (HFS) as a potential biomarker of efficacy in patients (pts) with metastatic renal cell carcinoma (mRCC) treated with sunitinib (SU) J Clin Oncol. 2011;29(Suppl 7) abstr 320. [Google Scholar]
- 23.Donskov F, Michaelson MD, Puzanov I, et al. Sunitinib-associated hypertension and neutropenia as efficacy biomarkers in metastatic renal cell carcinoma patients. Br J Cancer. 2015;113(11):1571–1580. doi: 10.1038/bjc.2015.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


