Abstract
Hippocampal neurons exhibit spatially localized firing patterns that, at the population level, represent a particular environment or context. Many studies have examined how hippocampal neurons switch from an existing representation to a new one when the environment is changed, a process referred to as remapping. New representations were commonly thought to emerge rapidly, within a few minutes and then remain remarkably stable thereafter. However, a number of recent studies suggest that hippocampal representations may be more fluid than previously thought and most of the previous studies only required that subjects switch from a familiar environment to a novel one. In the present study, we examined the concurrent development of two distinct hippocampal representations by exposing rats to two distinct environmental contexts in an ABAB pattern and we recorded neuronal activity for eight daily training sessions. Hippocampal neurons exhibited normal place fields with typical firing properties during the initial exposure to each context on the first day. However, when the rats were returned to the original context after having spent 15 minutes in the second context, many of the neurons fired in new locations (i.e. they remapped) as if the rat had encountered a new environment. By the third day, the representations had stabilized and were highly consistent across visits to the same context. These results suggest that when subjects concurrently encode multiple contexts, hippocampal representations may require repeated experiences to fully stabilize.
Keywords: Learning, Memory, Remapping, Navigation, Place Cell
INTRODUCTION
Pyramidal neurons in the hippocampus reliably exhibit spatially localized firing patterns (i.e. place fields, O'Keefe and Dostrovsky, 1971) as rats explore an environment and hippocampal place cells are among the most extensively studied phenomena in neuroscience (for reviews see Best et al., 2001; Muller, 1996; Shapiro, 2001). Place cell firing is influenced by a variety of environmental features including proximal and distal cues (e.g., Renaudineau et al., 2007; Shapiro et al., 1997), the geometric configuration of the environment (e.g., Jeffery and Anderson, 2003; Wills et al., 2005) and various background cues such as the color and ambient odor of the environment (e.g., Anderson and Jeffery, 2003). However, place cell firing is also sensitive to non-spatial features of the situation, including the subject's motivational state (Kennedy and Shapiro, 2009), the behavioral task demands (Markus et al., 1995; Smith and Mizumori, 2006b), strategies (Yeshenko et al., 2004) and expectations (Skaggs and McNaughton, 1998), suggesting that hippocampal firing patterns are best thought of as a representation of the spatial context rather than a strictly spatial map (Smith, 2008)
Context plays critical role in memory. Learned items become associated with the learning context and these contextual associations are important for interference free retrieval of the memories that belong to the context (e.g., Smith and Bulkin, 2014; Smith and Mizumori, 2006a). The hippocampus is involved in a variety of contextual learning tasks (Kim and Fanselow, 1992; Liu et al., 2012; Phillips and LeDoux, 1992) and hippocampal neurons form unique firing patterns for each context a subject encounters, commonly referred to as remapping (e.g., Alme et al., 2014; Frank et al., 2004; Leutgeb et al., 2005a; Muller and Kubie, 1987). Moreover, the hippocampus is specifically needed in order to use contextual information in order to prevent interference (Butterly et al., 2012). We have argued that when subjects return to a familiar context, re-expression of the hippocampal context representation triggers the retrieval of the relevant memories, thereby preventing interference caused by intrusions of memories that belong to other contexts (Smith and Bulkin, 2014; see also, Colgin et al., 2008; Hirsh, 1974; Nadel et al., 1985). Thus, the quality of hippocampal representations is critical for memory.
Many studies have investigated hippocampal remapping, so there is a detailed body of knowledge about how hippocampal neurons switch from one representational state to another (Frank et al., 2004; Jezek et al., 2011; Leutgeb et al., 2005a; Muller and Kubie, 1987; Wills et al., 2005). There are also a number of accounts of how hippocampal representations emerge when subjects explore a new environment. Several studies have suggested that spatial representations emerge quickly and stabilize over the course of a few minutes (Bostock et al., 1991; Frank et al., 2006; Hill, 1978). Others suggest that the stabilization of new place fields may be slower (Cacucci et al., 2007), remapping can proceed slowly under some circumstances (e.g., Lever et al., 2002; Shapiro et al., 1997; Smith and Mizumori, 2006b), and CA1 representations form more rapidly than those in CA3 (Leutgeb et al., 2004). Classic studies suggest that once formed, hippocampal representations are remarkably stable (Muller et al., 1987; Thompson and Best, 1990). However, recent studies suggest that these representations may not be as stable as previously thought (e.g., Mankin et al., 2012; Manns et al., 2007; Ziv et al., 2013). In most of these studies subjects only learn about a single context. However, hippocampal representations may be particularly important for resolving interference (Bulkin et al., 2016; Butterly et al., 2012; Colgin et al., 2008), which can be a problem in concurrent learning situations. In the present study, we examined the concurrent development of two distinct hippocampal representations by exposing rats to two distinct environments in an ABAB pattern.
METHODS
Subjects and Microdrive Implantation
The subjects were 4 adult male Long Evans rats (Charles River Laboratories, Wilmington, MA) weighing 300–350 g at the time of surgery. Two rats were implanted with microdrives containing 12 independently moveable tetrodes (Harlan 12 Drive, Neuralynx Inc., Bozeman, MT) positioned unilaterally in the CA1 region of the hippocampus of the right hemisphere (−3.5mm [AP], =2.5mm [LAT] from bregma). The other two rats were implanted with a custom built microdrive (MacDonald et al., 2011) containing 16 independently moveable tetrodes, which targeted the CA1 region bilaterally (4.0 mm posterior and 2.5 mm lateral from bregma). Coordinates were derived from the atlas of Paxinos and Watson (1998). For both drives, tetrodes consisted of four strands of 12.7 mm platinum/iridium (90/10%) wire (California Fine Wire, Grover Beach, CA) that were platinum-plated to reduce impedance to between 120 and 200 kOhms at 1 kHz. The rats were given an antibiotic (5 mg/kg Baytril) and an analgesic (5 mg/kg ketoprofen) prior to surgery. All procedures complied with guidelines established by the Cornell University Animal Care and Use Committee.
Following recovery, the rats were placed on a restricted feeding regimen (~85% of free feeding weight) and trained to forage for chocolate sprinkles in a cylindrical apparatus. This apparatus was distinct from the two contexts used in the experiment and served as a place to lower electrodes and check records. Tetrodes were gradually lowered, at a rate of approximately 40μm per day over 2-4 weeks, until a majority of them reached the CA1 layer. The amplitude and phase of theta waves, the amplitude and sign of sharp-wave events, and the presence of complex spiking cells were used to determine when the electrodes were localized within CA1. The experiment began once isolatable single units were obtained with spike waveforms that matched those of pyramidal neurons. After each of the daily recording sessions, one quarter to one half of the tetrodes were lowered (~15–30μm) in order to maximize the cell population for the following day.
Two to four days prior to the start of the experiment, the rats were given two acclimation sessions in each of the contexts so that neophobia would not inhibit foraging on the first day of testing. For each session, the rats were connected to the recording system, placed in one of the two contexts, and allowed to forage for chocolate sprinkles for 15 min. This was followed by a 5 min ITI period spent in an opaque plastic cylinder (30 cm diameter, 65 cm height), and then 15 min of foraging in the other context. These acclimation sessions were similar to the subsequent recording sessions in all respects, except that the rats were only given one trial in each context and did not repeatedly visit the contexts.
General Training Procedures and Neuronal Recording
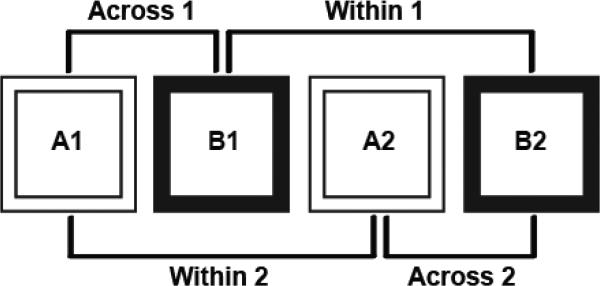
During the recording sessions the rats foraged for chocolate sprinkles in two distinct contexts (PVC boxes measuring 100cm × 100cm × 50cm deep) that differed in the following ways: box color (white or black), surrounding color (white curtains with the black box and black with the white box), floor surface (smooth PVC or soft plastic mat), background masking noise (pink or white noise), background odor (mint or vanilla), and position of researcher dispersing the sprinkles (north or east side of the box). Recordings were obtained during 4 15 min trials each day in an ABAB format (Fig. 1), except for the first rat which was trained in an ABA format. The presentation order of the black and white contexts was counterbalanced across rats and days. In between trials, the rat was placed in an opaque plastic cylinder (30 cm diameter, 65 cm height) for a 5 min ITI period while the contexts were changed.
Figure 1.
Schematic representation of the context presentations. Rats were exposed to two different contexts (black and white boxes with varying background colors, odors and masking noises, see text) in an ABAB format. The order of the presentation of contexts was counterbalanced across days. Comparisons were made between repeated visits within the same context (A1-A2 and B1-B2) as well as across the two different contexts (e.g., A1-B1 and A2-B2).
Data Collection and Analysis
Neuronal spike and video data were collected throughout the task with the Cheetah Digital Data Acquisition System (Neuralynx Inc., Bozeman, MT). The rat's position was monitored by digitized video (sampled at 30 Hz) of LEDs attached to the rat's head. Signals from the electrodes were filtered at 600 Hz and 6 kHz, and all waveforms exceeding a user-defined threshold were stored along with the time of occurrence for offline analysis. Standard spike sorting techniques were used to sort multiple unit records into single units (Spikesort 3D, Neuralynx). Waveform features used for sorting included spike amplitude, spike width, waveform principle components, and waveform area.
A total of 656 neurons were recorded. However, because instability in the recordings could cause spurious observations of remapping across the recording trials of a session, we took several steps to prevent or exclude unstable records. First, we never recorded when any electrode had been lowered within the last ~22 hrs. Second, during spike sorting the experimenter rated each cluster on a scale of 1-3 as follows: 1 = completely isolated, large amplitude spike, with little or no overlap with other clusters, with no evidence of drift in a plot of spike amplitude over the recording session; 2 = well-isolated, medium to large amplitude spike, with some overlap but no evidence of drift; 3 = incompletely isolated spikes and/or evidence of drift. For all of the reported analysis, we limited the data to clusters with a score of 1 or 2. As a result, we excluded 146 neurons and retained the remaining 510 neurons as our final data set. However, as an additional check, we analyzed a subset of the highest quality records (i.e. clusters with a rating of 1, N=251 neurons). The pattern of results from the reduced data set were similar to the full data set for all of the major findings. The number of neurons recorded from each subject and recording day are shown in Table 1.
Table 1.
The table shows the number of neurons that were recorded for each rat across the 8 days of training.
| Rat ID | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1412 | 13 | 11 | 12 | 16 | 12 | 13 | 17 | 12 | 106 |
| 1413 | 8 | 6 | 17 | 10 | 18 | 32 | 24 | 17 | 132 |
| 1784 | 18 | 16 | 22 | 24 | 19 | 31 | 12 | 11 | 153 |
| 1792 | 19 | 16 | 15 | 12 | 19 | 14 | 13 | 11 | 119 |
| Total | 58 | 49 | 66 | 62 | 68 | 90 | 66 | 51 | 510 |
The data were analyzed to determine whether the recorded neurons exhibited spatial firing in one or both of the contexts using custom programs written in Matlab (MathWorks, Natick, MA). The floors of the recording chambers were divided into ~3.5 × 3.5 cm square pixels, and the firing rate of each neuron was determined by dividing the total number of spikes in each pixel by the time spent in the pixel. Spatial firing rate maps were smoothed by convolution with a 5×5 pixel Gaussian kernel with unity sum. After smoothing, place fields were defined as any set of 6 or more contiguous pixels where the neuron fired with a rate at least half of the maximum firing rate for that neuron. Spatial bins that contained less than one second of occupancy were discarded. Putative interneurons (average firing rate of greater than 5 Hz or a ‘place field’ occupying greater than 50% of the recording chamber) were excluded from the analysis.
To analyze the similarity of spatial firing patterns in different trials, pixel-by-pixel pairwise correlations (Pearson's r) were computed between the firing rate maps generated for each neuron. For each neuron, spatial correlations were separately calculated for the repeated visits to the same context (Fig. 1, A1-A2 and B1-B2) and for the visits to different contexts (A1-B1 and A2-B2). These values were then averaged to produce a single within-context correlation score and a single across-context correlation score for each neuron. For the rat that was given only three trials (ABA), we used A1-A2 for the within-context correlation and we averaged together A1-B1 and B1-A2 correlations as the across-context correlation. The spatial correlations included all of the pixels visited in the two conditions, including those with firing rates of zero. In addition to spatial correlations, various other measures were used to compare spatial firing across the trials within each session. These included trial by trial firing rates, a rate remapping index (computed as the difference between the maximum and minimum firing rates divided by the summed firing rates, Leutgeb et al., 2005b), and spatial information content (Markus et al., 1994). Because neurons with low firing rates can produce spuriously high spatial information estimates, we identified significant values using the method established by Markus et al (1994). 500 surrogate data sets were created by randomly offsetting spike trains from position data (5-100 seconds) and spatial information content was calculated for each, the significance of the original data was estimated using the distribution of shuffled data. Only information scores more than 1.96 standard deviations above the mean of the distribution (i.e. p<.05) were included for analysis.
Histology
After the completion of the experiment, the rats were deeply anesthetized and transcardially perfused with paraformaldehyde. The brains were removed, sectioned into 40-μm coronal slices, mounted on slides and stained with cresyl violet in order to verify the placement of electrodes in the CA1 region of the hippocampus.
RESULTS
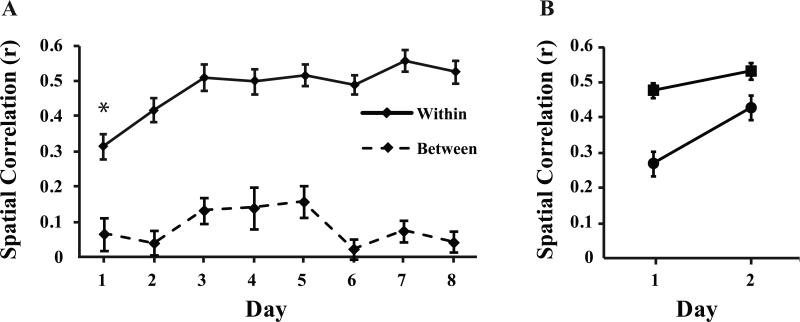
The goals of our analysis were 1) to determine how rapidly distinct and stable spatial representations form for each of the contexts and 2) to identify the source of any initial instability in the representations. We examined how distinct representations were by measuring remapping across exposures to different contexts and we examined the stability of new representations by comparing firing patterns across repeated visits to the same context (see Fig. 1 and Methods). We then submitted the average within- and between-context correlations to a repeated measures ANOVA with correlation type (within- and between-contexts) as the within subjects factor and recording day as the between subjects factor (8 days, with largely different populations of neurons recorded in each). Results showed a significant main effect of spatial correlation type (F[1,502] = 959.103, p < 0.001), a main effect of recording day (F[7,502] = 3.152, p < 0.001) and an interaction of the correlation type and recording day variables (F[7,502] = 4.154, p < 0.01). Not surprisingly, the representations were more similar across repeated visits to the same context than across visits to different contexts throughout the recording days (Fig. 2A, compare the solid and dashed lines). However, the within-context correlations changed with learning. Correlations were relatively low on Day 1, increased somewhat on Day 2 and significantly increased on Day 3, remaining high thereafter (Tukey LSD comparing Day 1 and subsequent days, Day 2 p = 0.188, Day 3-8 all p < 0.05). The low correlations were seen in both of the within-context correlation scores (A1-A2 and B1-B2), which did not differ from each other (F[1,390] = .163, p = 0.69, Fig. S1). Thus, the individual context representations were initially somewhat unstable and only became more stable after repeated experience. This result stands in contrast to previous reports that in new environments place fields become stable over the course of a few minutes (Bostock et al., 1991; Frank et al., 2006; Hill, 1978). Examples of spatial firing patterns from early and late recording sessions are shown in figures 3 and 4. Data from all 510 neurons are shown in supplementary figure S2.
Figure 2.
In plot A, Average spatial correlation scores are shown for repeated visits to the same context (Within, solid line) and across visits to different context (Between, dashed line). The correlation scores (Pearson's r) are shown for neurons recorded during each of the eight recording sessions (Day). The (*) indicates that the day 1 within-context correlation score was significantly different from days 3-8. Plot B illustrates the average within-trial correlations comparing the first and second halves of a single visit to each context (squares) and across-trial correlations computed with equivalent amounts of data across different visits to the same context (circles, see text), indicating that representations were stable within a visit but shifted across visits to each context.
Figure 3.
Examples of spatial firing plots for the different context exposures for 12 different neurons from the first recording session. The scale bar indicates range of firing rates (Hz) for each neuron. Many neurons exhibited remapping across visits to the same context. Data from all 510 neurons of the study are shown in supplementary figure S2.
Figure 4.
Examples of spatial firing plots for the various context exposures for 12 different neurons from the final two recording sessions. The scale bar indicates range in firing rates (Hz) for each neuron. Spatial firing was far more stable than during the initial recording days.
Recent studies have shown that hippocampal representations can slowly change with the passage of time (Mankin et al., 2012; Manns et al., 2007). In order to determine whether similar but more rapid time-dependent change might have caused the low initial within-context correlations, we broke the 15 min trials into two 7.5 min trial halves and examined them. The firing patterns were well correlated within each trial (i.e. comparison of the first and second half of each trial, Fig. 2B). Indeed, these correlations were comparable to the within-context correlations seen after several recording sessions. In contrast, correlations computed with equivalent amounts of data across different visits to the same context (i.e. 2nd half of A1 correlated with 1st half of A2 and 2nd half of B1 correlated with 1st half of B2) showed reduced correlations similar to those obtained with the full trials. These results suggest that representations did not change very much within a given visit to one of the contexts, but instead remapping occurred in between repeated visits to the same context, after having visited a different context.
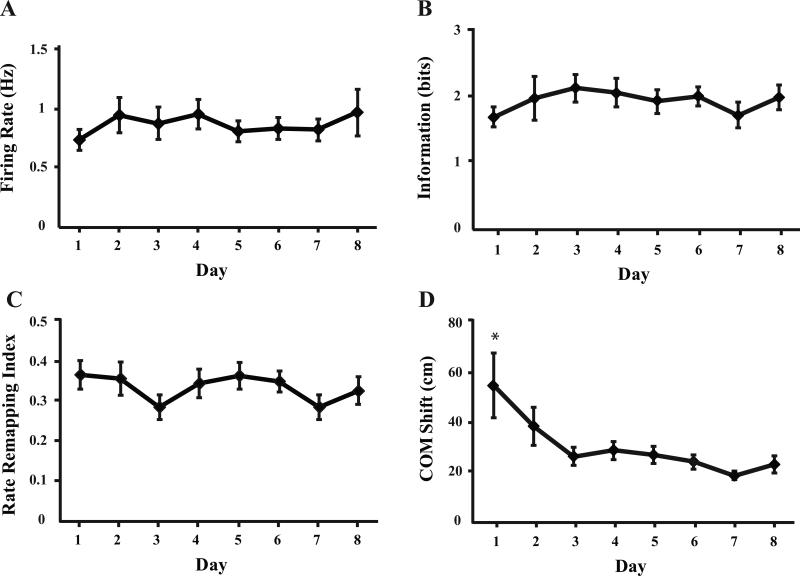
One possible explanation for the reduced stability of representations during the first two days is that the general quality of the place fields may have been poor initially but improved with experience (Cacucci et al., 2007). To assess this possibility, we examined several place field characteristics. The percentage of neurons that exhibited a place field in one or both contexts did not change across days (χ2(7) = 11.423, p = 0.121), indicating that the neurons were capable of exhibiting spatially localized firing during the initial training days. Similarly, the average place field size (F[7,441] = 0.366, p = 0.922) and the specificity of place field firing (in field to out of field firing rate ratio (F[7,441] = 1.068, p = 0.383) did not change across days. The average firing rate of the neurons also did not change across training days (F[7,509] = 0.375, p = 0.896 Fig. 5A). Finally, the spatial information content of those neurons that showed some spatial sensitivity (i.e. a z-score > 1.96; see Methods) did not become more informative about the location of the animal across days (F[7,485] = 0.592, p = 0.762, Fig. 5B). Together, these analyses suggest that the initial instability of hippocampal representations did not result from poor quality or incompletely-formed spatial representations.
Figure 5.
Plot A illustrates the average firing rates for the eight recording sessions (all neurons and contexts). Plot B illustrates the average information content (all neurons and contexts). Plot C illustrates the average rate remapping scores reflecting changes in firing rate across repeated visits to the same context (A1-A2 and B1-B2). Plot D illustrates the average shift in the center of mass (COM) of the place fields across repeated visits to the same context. Only the COM shift changed significantly across the recording sessions (* indicates a significant difference from all other days of training).
If the basic characteristics of the place fields did not change with experience, then the initial instability of the representations was likely due to spontaneous remapping across repeated visits to the same context. Hippocampal neurons can undergo remapping in several ways: place fields can be present in one condition and absent in another, they can shift locations, or they can maintain the same location but show a change in firing rate (i.e. rate remapping). We examined each of these possibilities. The amount of rate remapping seen across repeated visits to the same context did not change significantly across days (F[7,509] = 1.209, p = 0.409, see Methods Fig. 5C) and the percentage of neurons that exhibited a place field during one visit to a given context but not the other visit also did not change significantly across days (χ2(7) = 3.749, p = 0.253). However, we did find evidence that place fields spontaneously shifted locations between the first and second visits to each context. We computed the change in the center of mass (COM, Leutgeb et al., 2005b; Mehta et al., 1997) for those neurons that had place fields in both visits to each context and found that these values changed significantly with experience (F[7,305] = 4.511, p < 0.001, Fig. 5D). Post hoc tests revealed that the shift in place field location was significantly larger on Day 1 than on all other days (Tukey LSD, all p < 0.05). These results suggest that the primary cause of the initial instability of hippocampal representations was that neurons with place fields shifted their preferred firing location across repeated visits to the same context.
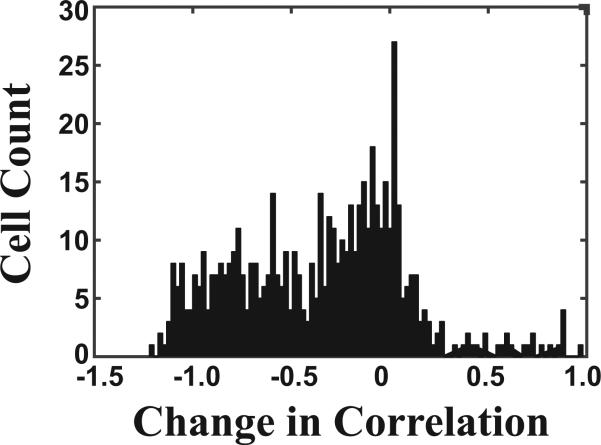
These shifts in place field were not caused by rotations of the spatial representations, which could occur if the rats became confused about their orientation within the testing room. To examine this, we rotated the firing rate maps 90, 180 or 270 degrees for the second visit to each context (A2 and B2) and re-computed the spatial correlation between the first and second visits. The changes in the spatial correlation for each neuron and rotation are shown in Figure 6. Although the correlations improved in a small number of cases, most correlations were unchanged or reduced as a result of the rotations.
Figure 6.
Distribution of the change in spatial correlations for repeated visits to the same context resulting from rotation of one of the firing rate maps. Data are shown for all neurons and all rotations (90, 180 or 270 degrees) from the first recording day. Most correlations were similar to the original non-rotated values or were reduced (i.e. values near zero and to the left of zero).
Behavioral factors such as running speed and differences in the time spent in each area of the apparatus (e.g. due to thigmotaxis) can influence neural firing and could have contributed to the apparent initial instability of the place fields during the initial recording sessions. We hoped to minimize the contribution of these factors by acclimating the rats to the contexts before beginning the recordings (see Methods) and there were no obvious differences in the rats’ behavior during the initial recording sessions. Running speed did not differ across the four trials within a session (F[3,21] = 0.880, p = 0.458) or the eight days of training (F[7,21] = 1.275, p = 0.310) and there was no interaction of the trial number and training day variables (F[7,21] = 1.031, p = 0.448). The average running speeds for each rat are shown in supplementary figure S3. We assessed the possible contribution of the areas visited in two ways. First, we created a reduced data set that excluded any pixels that were only visited briefly during any of the trials (i.e <2 sec of occupancy) and re-computed the ANOVA. The same pattern of reduced within-context correlations during the initial sessions was present (interaction of the correlation type and recording day, F[7,457] = 2.144, p < 0.05). As a second test, we also re-ran our analysis of the COM shifts but we limited the data to those place fields that had at least 10 passes through the region in all trials. The results were the same. The COM shift differed across days (F[7,275] = 5.445, p < 0.001) and post hoc tests revealed that the shift in place field location was significantly larger on Day 1 than on all other days (Tukey LSD, all p < 0.05). These results suggest that differences in the rats’ behavior were not likely to have contributed to our results.
DISCUSSION
Previous research has shown that hippocampal neurons develop place fields rapidly, within the first few minutes of exploring a new environment (Bostock et al., 1991; Frank et al., 2006; Hill, 1978), and that they can remain stable for months thereafter (Muller et al., 1987; Thompson and Best, 1990). In these studies, subjects learned about a single new context. Our data show that it may take several sessions for stable representations to emerge when subjects are exposed to multiple contexts in succession. Notably, it was not the case that the hippocampus generated poor quality representations during the early exposures or that there was insufficient time to develop fully formed representations. By all measures, the place fields were normal from the outset. Instead, the representations underwent remapping across repeated visits to the same context. This remapping was not as extensive as that seen between visits to different contexts. Nevertheless, the within-context correlations were significantly reduced during the initial session compared to later sessions.
We did not record from rats that were repeatedly exposed to a single-context, so we cannot rule out the possibility that similar results would have occurred under those conditions. However, we found that the representations were quite stable within a trial (see Fig 2B), suggesting that the initial instability was not simply an accelerated form of the continuously changing representations that have been reported over longer time periods (Mankin et al., 2012; Ziv et al., 2013). Instead, many neurons exhibited remapping upon the second visit to the context after spending an intervening trial in a different context. These results suggest that the hippocampus generates normal representations quite rapidly but they do not become stable immediately and can apparently be disrupted by exposure to another new context, perhaps due to interference (Bulkin et al., 2016; Butterly et al., 2012; Colgin et al., 2008). Consistent with this interpretation, although the rats were acclimated to each context twice (see Methods) and spatial representations presumably formed during these visits, the initial recording sessions were the first time the rats were given repeated visits to the same context with intervening visits to a different context all on the same day.
The present results join other studies that have found that hippocampal representations can emerge slowly under some conditions. For example, Shapiro and colleagues (1997) performed a series of cue rotation experiments with a radial maze task. During the initial rotation trials place fields were most likely to rotate with the distal cues, but with experience entirely new representations emerged (i.e. remapping that was not simply a rotation of the place fields). In a similar result, a change in the color of a large cue card led to the slow emergence of a new representation (Bostock et al., 1991) and exposure to differently shaped environments placed within the same room framework led to the slow emergence of distinct representations over the course of several days (Lever et al, 2002). In mice, place fields may become more specific and reliable over the course of several days, even in a single environment (Cacucci et al., 2007). A study from our laboratory (Smith and Mizumori, 2006b) found that training rats to discriminate two different behaviorally defined contexts (i.e. the same environment but with different task rules) led to the development of two distinct hippocampal representations over the course of several days of training as the rats learned to discriminate the two conditions. It may be the case that some experience is needed for rats to learn that there are two distinct sets of conditions before the hippocampus can develop distinct representations for them. This seems particularly likely in the Smith et al (2006) study where the changing task demands may not have been apparent to the rat at the outset of training.
Few studies have systematically examined the simultaneous development of two or more hippocampal representations of distinct environments. A recent study found that CA3 neurons can readily form unique representations of at least eleven different environments (Alme et al., 2014). However, the subjects did not repeatedly visit the environments so we do not know how stable those representations were. Leutgeb and colleagues (Leutgeb et al., 2006) performed an experiment that was similar to the present study, in which they recorded CA1 and CA3 neurons over the course of two days while rats explored two novel contexts. Interestingly, they found evidence of rate remapping in CA3 during repeated visits to the same context and this was greater on the first day than the second, suggesting some initial instability in the representation. However, they did not find large scale changes in place field locations during repeated visits to the same context, either in CA3 or CA1. The apparent discrepancy between the Leutgeb (2006) study and the present results may be due to methodological differences. First, they presented the two different boxes in an ABBA sequence which could be less disruptive than the ABAB procedure of the present study (e.g. if the initial instability reflects a failure of interference reduction mechanisms, Butterly et al., 2012; Colgin et al., 2008). More importantly, they tested the rats in two different colored boxes which occupied the same location within the recording room, so the distal cues remained constant. This procedure is known to produce rate remapping, but typically does not produce large shifts in place field location or the disappearance of the place field altogether (i.e. global remapping), even for visits to different colored boxes (Leutgeb et al., 2005b). In the present study, the two boxes occupied the same location within the recording room, resulting in some distal cues remaining constant (e.g. the tether and overhead camera). However, the background environment was quite different between the two contexts, including the addition of curtains, as well as different ambient odors and background masking noise. Thus, our context manipulation was probably more like a full change in the recording room, which induces large scale global remapping (Leutgeb et al., 2005b). Taken together, the Leutgeb et al (2006) study and the present results suggest that manipulations that induce rate remapping across different contexts will also induce rate remapping across visits to the same context during initial learning and, similarly, manipulations that induce global remapping will induce the same kind of large scale remapping across visits to the same context on the first day.
The functional consequences of this initial instability are not entirely clear. It is possible that during the initial sessions, the rats simply do not recognize the second visit to each context as familiar. To the extent that hippocampal representations support context recognition, our results suggest that the rats treat the repeated visits as novel experiences. This interpretation suggests a failure of the memory system, in which initially unstable representations lead to the erroneous labeling of a familiar experience as a novel one. Similar findings of spontaneous remapping upon revisiting a context in subjects given protein synthesis inhibitors (Kentros et al., 1998) are consistent with the idea that this is an encoding or consolidation failure. However, it is also plausible that this ‘instability’ is advantageous. We have argued that hippocampal context representations are important, in part, because the output of these complex activity patterns could be used to automatically trigger the retrieval of relevant memories whenever the subject revisits a familiar context (Smith and Bulkin, 2014). Since a given response (e.g. search for food) can easily be associated with many contexts, the default tendency may be for the hippocampus to generate a new representation, and stable context representations may only emerge after some amount of experience confirms that the situation is stable. This process may be extended by exposing the rats to alternating contexts during the initial stages of learning.
These results join a growing body of research indicating that hippocampal representations are much more fluid than previously thought. Several studies have shown that spontaneous remapping is an ongoing process that occurs in the background and may be a useful mechanism for differentiating similar experiences that happen at different times, which is a key component of episodic memory (Alme et al., 2014; Mankin et al., 2012; Manns et al., 2007). Consistent with this, a recent study tracked the activity of hundreds of CA1 neurons for 45 days using calcium imaging in freely moving mice and found that only 15% of the initially active neurons were still part of the representation 30 days later and many new neurons had been recruited (Ziv et al., 2013). More recently, a similar study showed that simultaneously recorded representations of two distinct environments co-evolved over time (Rubin et al, 2015). Similarly, Tayler et al (Tayler et al., 2013) used an activity dependent form of green fluorescent protein to label active neurons in the hippocampus during contextual fear conditioning and retrieval. They found that only 40% of the initially active neurons were reactivated during a retention test 2 weeks later even though the fear memory remained strong. These findings are consistent with the idea that hippocampal representations are constructed from neural ensembles that undergo a slow but continuous process of formation and dissolution. Rather than forming in response to a particular sensory experience, these ensembles appear to form spontaneously and are only subsequently ‘assigned’ to represent experiences (Dragoi and Tonegawa, 2013). The findings described above suggest that these ensembles continue to systematically change even after the initial learning experience. Additional studies with appropriate behavioral assays of memory will be needed to determine whether the initial instability of hippocampal representations seen in the present study reflect a memory failure or an adaptive process for associating behaviors with their appropriate context.
Supplementary Material
Acknowledgments
Grant sponsor:_NIH__; Grant number:_MH083809 to DMS.
REFERENCES
- Alme CB, Miao C, Jezek K, Treves A, Moser EI, Moser MB. Place cells in the hippocampus: eleven maps for eleven rooms. Proc Natl Acad Sci U S A. 2014;111(52):18428–35. doi: 10.1073/pnas.1421056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23(26):8827–35. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best PJ, White AM, Minai A. Spatial processing in the brain: the activity of hippocampal place cells. Annu Rev Neurosci. 2001;24:459–86. doi: 10.1146/annurev.neuro.24.1.459. [DOI] [PubMed] [Google Scholar]
- Bostock E, Muller RU, Kubie JL. Experience-dependent modifications of hippocampal place cell firing. Hippocampus. 1991;1(2):193–205. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- Bulkin DA, Law LM, Smith DM. Placing memories in context: Hippocampal representations promote retrieval of appropriate memories. Hippocampus. 2016 doi: 10.1002/hipo.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterly DA, Petroccione MA, Smith DM. Hippocampal context processing is critical for interference free recall of odor memories in rats Hippocampus. Hippocampus. 2012;22(4):906–913. doi: 10.1002/hipo.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacucci F, Wills TJ, Lever C, Giese KP, O'Keefe J. Experience-dependent increase in CA1 place cell spatial information, but not spatial reproducibility, is dependent on the autophosphorylation of the alpha-isoform of the calcium/calmodulin-dependent protein kinase II. J Neurosci. 2007;27(29):7854–9. doi: 10.1523/JNEUROSCI.1704-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31(9):469–77. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Development of schemas revealed by prior experience and NMDA receptor knock-out. Elife. 2013;2:e01326. doi: 10.7554/eLife.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Stanley GB. Hippocampal and cortical place cell plasticity: implications for episodic memory. Hippocampus. 2006;16(9):775–84. doi: 10.1002/hipo.20200. [DOI] [PubMed] [Google Scholar]
- Frank LM, Stanley GB, Brown EN. Hippocampal plasticity across multiple days of exposure to novel environments. J Neurosci. 2004;24(35):7681–9. doi: 10.1523/JNEUROSCI.1958-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AJ. First occurrence of hippocampal spatial firing in a new environment. Exp Neurol. 1978;62(2):282–97. doi: 10.1016/0014-4886(78)90058-4. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behav Biol. 1974;12(4):421–44. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Anderson MI. Dissociation of the geometric and contextual influences on place cells. Hippocampus. 2003;13(7):868–72. doi: 10.1002/hipo.10162. [DOI] [PubMed] [Google Scholar]
- Jezek K, Henriksen EJ, Treves A, Moser EI, Moser MB. Theta-paced flickering between place-cell maps in the hippocampus. Nature. 2011;478(7368):246–9. doi: 10.1038/nature10439. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc Natl Acad Sci U S A. 2009;106(26):10805–10. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science. 1998;280(5372):2121–6. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser MB, Moser EI. Progressive transformation of hippocampal neuronal representations in “morphed” environments. Neuron. 2005a;48(2):345–58. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005b;309(5734):619–23. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Moser EI, Moser MB. Fast rate coding in hippocampal CA3 cell ensembles. Hippocampus. 2006;16(9):765–74. doi: 10.1002/hipo.20201. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–8. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O'Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416(6876):90–4. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484(7394):381–5. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–49. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK. Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci U S A. 2012;109(47):19462–7. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56(3):530–40. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus EJ, Barnes CA, McNaughton BL, Gladden VL, Skaggs WE. Spatial information content and reliability of hippocampal CA1 neurons: effects of visual input. Hippocampus. 1994;4(4):410–21. doi: 10.1002/hipo.450040404. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15(11):7079–94. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MR, Barnes CA, McNaughton BL. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc Natl Acad Sci U S A. 1997;94(16):8918–21. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R. A quarter of a century of place cells. Neuron. 1996;17(5):813–22. doi: 10.1016/s0896-6273(00)80214-7. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7(7):1951–68. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB., Jr. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7(7):1935–50. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Willner J, Kurz EM. Cognitive maps and environmental context. In: Balsam P, Tomie A, editors. Context and Learning. Erlbaum; Hillsdale, NJ: 1985. pp. 385–406. [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–5. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Renaudineau S, Poucet B, Save E. Flexible use of proximal objects and distal cues by hippocampal place cells. Hippocampus. 2007;17(5):381–95. doi: 10.1002/hipo.20277. [DOI] [PubMed] [Google Scholar]
- Shapiro M. Plasticity, hippocampal place cells, and cognitive maps. Arch Neurol. 2001;58(6):874–81. doi: 10.1001/archneur.58.6.874. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Tanila H, Eichenbaum H. Cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus. 1997;7(6):624–42. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Spatial firing properties of hippocampal CA1 populations in an environment containing two visually identical regions. J Neurosci. 1998;18(20):8455–66. doi: 10.1523/JNEUROSCI.18-20-08455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM. The hippocampus, context processing and episodic memory. Handbook of Episodic Memory. 2008;18:465–481. [Google Scholar]
- Smith DM, Bulkin DA. The form and function of hippocampal context representations. Neurosci Biobehav Rev. 2014;40:52–61. doi: 10.1016/j.neubiorev.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006a;16(9):716–29. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Learning-related development of context-specific neuronal responses to places and events: the hippocampal role in context processing. J Neurosci. 2006b;26(12):3154–63. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23(2):99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res. 1990;509(2):299–308. doi: 10.1016/0006-8993(90)90555-p. [DOI] [PubMed] [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N, O'Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308(5723):873–6. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshenko O, Guazzelli A, Mizumori SJ. Context-dependent reorganization of spatial and movement representations by simultaneously recorded hippocampal and striatal neurons during performance of allocentric and egocentric tasks. Behav Neurosci. 2004;118(4):751–69. doi: 10.1037/0735-7044.118.4.751. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16(3):264–6. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.