Abstract
Air breathing was critical to the terrestrial radiation and evolution of tetrapods and arose in fish. The vertebrate lung originated from a progenitor structure present in primitive boney fish. The origin of the neural substrates, which are sensitive to metabolically produced CO2 and which rhythmically activate respiratory muscles to match lung ventilation to metabolic demand, is enigmatic. We have found that a distinct periodic centrally generated rhythm, described as “cough” and occurring in lamprey in vivo and in vitro, is modulated by central sensitivity to CO2. This suggests that elements critical for the evolution of breathing in tetrapods, were present in the most basal vertebrate ancestors prior to the evolution of the lung. We propose that the evolution of breathing in all vertebrates occurred through exaptations derived from these critical basal elements.
Keywords: Lamprey, Breathing, CO2, Evolution, Exaptation, Cough
One Sentence Summary
lamprey “cough” is produced by a central rhythm generator sensitive to CO2, an element critical for tetrapod breathing, which may have evolved through exaptation from this basal vertebrate characteristic.
1. Introduction
Air breathing in tetrapods is achieved via lungs, which likely arose from gas-filled bladders functioning for gas exchange and/or buoyancy control in primitive air-breathing fish prior to the radiation of ray-finned (Actinopterygi) and lobe-finned (Sarcopterygi) fishes (Perry et al., 2001; Remmers et al., 2001; Wilson et al., 2000). Air breathing in tetrapods, however, requires more than just a lung. Also required are a “breath”, output from a brainstem central rhythm generator (CRG) activating respiratory muscles to ventilate the lung, and populations of brainstem chemoreceptors sensitive to CO2/pH that modulate CRG activity. Together with the lung this system produces breathing matched to metabolic demand. The CO2/pH-modulated air-breathing CRG (CRGAB) is anatomically and functionally distinct from the CRG producing gill ventilation, which is not modulated by CO2/pH (Milsom, 2010; Wilson et al., 2002). The origin of the requisite CO2/pH-modulated CRGAB is unknown, but may have preceded the evolution of the lung (Perry et al., 2001).
Lamprey are cartilaginous and jawless fish reminiscent of the basal vertebrate lineage. The larval (ammocoete) stage is considered among the most “primitive” living vertebrates, resembling non-vertebrate chordates. Ammocoetes are microphagous suspension feeders that form burrows in soft sediment. Water flow is generated by continuous rhythmic ventilation of the pharyngeal pouch, which acquires nutrients but also satisfies metabolic gas exchange requirements. O2 diffuses from water across the surface areas of exchange epithelia including the pharynx and gills (Hsia et al., 2013; Mallatt, 1981; Rovainen, 1996), and metabolically produced CO2 easily diffuses across all body surfaces and dissipates into surrounding water. Brainstems isolated from lamprey exhibit rhythmic discharge on cranial nerves that innervate ventilatory muscles. This discharge results from a central rhythm generator for pharyngeal ventilation (CRGP) (Cinelli et al., 2013; Gariépy et al., 2012; Martel et al., 2007; Rovainen, 1996). An additional pattern of periodic activity occurs, characterized as a “slow rhythm” or “cough” (Martel et al., 2007; Rovainen, 1996; Rovainen, 1977). These patterns of putative pharyngeal ventilation and “cough” result from anatomically distinct CRGs and resemble patterns of activity present in isolated brainstems of larval amphibians, which represent the products of distinct CRGs for gill ventilation and a functionally and anatomically distinct tetrapod CRG for air breathing (CRGAB) (Martel et al., 2007; Missaghi et al., 2013; Wilson et al. 2002). In amphibians and all higher amniotes, the CRGAB is responsive to CO2/pH-sensitive central chemoreceptors that modulate ventilation to meet metabolic demand. The origin of the vertebrate CRGAB is unknown and the presence of central CO2/pH-sensitive chemoreceptors prior to the amphibians is controversial (Milsom, 2010; Wilson et al., 2000). We propose that the lamprey “slow rhythm” or “cough” CRG is the progenitor to the tetrapod CRGAB and test the hypothesis that this progenitor will be modulated by CO2/pH.
We predict the existence, in the basal vertebrate, of a CO2/pH-modulated CRG distinct from that producing gill ventilation. Subsequent evolution of a lung provided the substrates that, through exaptation (Gould and Vrba, 1982) or functional retasking, resulted in the complex combination of lung, chemoreceptor and CO2/pH-modulated CRGAB. This new system was then capable of producing and regulating air breathing to meet metabolic demand for ventilation critical for subsequent evolution of amphibians, the transition to terrestrial habitats and further evolution of reptiles, birds, and mammals. Here we show that a CO2/pH-modulated CRG, distinct from that producing gill ventilation occurs in lamprey, a jawless, lungless and exclusively water-breathing “primitive” fish representative of the basal ancestor common to all vertebrates. We propose this CO2/pH-modulated CRG represents a critical progenitor characteristic, present in basal vertebrates epochs prior to evolution of the lung.
2. Methods
2.1 Animals
Animal use was done in accordance with the guidelines of the “Guide for the Care and Use of Laboratory Animals” of the National Institutes of Health and were approved by the University of Alaska Fairbanks Institutional Animal Care and Use Committee. Animal collection was approved by the State of Alaska Department of Fish and Game. Larval (ammocoete, 7–15 cm, 1–5 g) lamprey (Lampetra camtschatica or L. alaskense; Tilesius) were collected through sediment sifting or electroshock, from natural populations occurring in shallow, slow-moving fresh-water streams in interior Alaska. Captive animals were housed at 12 °C in 20-liter aquaria containing 3.125 g salt (Instant Ocean) per L deionized water. Ammocoetes were fed dry yeast three times weekly. Filters were used to maintain water quality and remove excess food. Aquaria were continuously aerated.
2.2 Isolated-Brainstem Preparation
Procedures to isolate the brainstem and spinal cord en bloc from ammocoetes, and record from whole nerves associated with ventilation, were slightly modified from those we use to isolate and record from similar tissues derived from larval amphibians (Davies et al., 2009; Taylor and Brundage, 2013; Taylor et al., 2003). Ammocoetes were anesthetized using tricaine methanesulfonate (MS222, Sigma-Aldrich; 0.3 g/L in deionized water buffered with 2.4 g/L NaHCO3) until unresponsive to a tail pinch. Anesthetized animals were transected caudal to the branchial pores and the ventral half of the head was removed so the cranium rested on a dissection tray. Subsequent dissection occurred with tissues constantly irrigated with an artificial cerebrospinal fluid (aCSF) equilibrated with 1% CO2, balance O2 (Davies et al., 2009; Martel et al., 2007). The aCSF comprised (in mM) NaCl (130), KCl (2.1), CaCl2 (2.6), MgCl2 (1.8), HEPES (4), D-glucose (4), and NaHCO3 (1) buffered to pH 7.4 with NaOH. The dorsal cranium was removed to expose the brain and spinal cord. Each brain was transected at the optic lobes, the spinal cord was cut approximately 5 mm caudal to obex, and meninges and choroid plexus were removed from regions of the 3rd and 4th ventricles. Remaining cranial and spinal nerves were cut and the decerebrated brainstem was removed en bloc and transferred to an acrylic superfusion recording chamber supplied with aCSF equilibrated with 1.5% CO2 balance O2. Cranial nerves V and X (CN V/X) were drawn into glass suction electrodes. Whole-nerve signals were amplified (bipolar recording of whole nerve relative to recording chamber) and filtered (first stage 100×, 10 Hz low, 1 kHz high, DAM 50, World Precision Instruments (Sarasota, FL, USA); 2nd stage 1000×, 100 Hz low, 1 kHz high, Model 1700 DAC Amplifier, A-M Systems, Carlsborg, WA, USA), and recorded using a computer analogue-to-digital data acquisition system (Powerlab, AD Instruments, Colorado Springs, CO, USA). After dissection, tissues were allowed at least 40 min to recover. During the experiment, cranial nerve discharge was recorded for 30 min during superfusion with normocapnic aCSF (equilibrated with 1.5% CO2, balance O2), followed by 10 min hypercapnia (5% CO2, balance O2), and at least 30 min of subsequent normocapnia. All experiments were conducted at constant temperatures approximating animal chamber temperature 10–15 °C.
2.3 Control Experiments
Each experiment was concluded with tissues exposed to normocapnia subsequent to hypercapnia. Hypercapnic “cough” frequency was reduced during subsequent normocapnia, and was no difference from that during initial normocapnia. Separate trials were conducted during which brainstems were maintained under constant normocapnia. In no case was “cough” frequency observed to increase with time independent of hypercapnic exposure.
2.4 Data Analysis
Periods for analysis (10 min), for each experiment, were extracted from original data records representing the final period of initial normocapnia, the period of hypercapnia and normocapnia subsequent to hypercapnia. Neurograms were scored for rhythmic patterns of burst activity previously characterized as fictive ventilation of the pharynx (a “fast rhythm”) and a distinct periodic burst pattern characterized as a “slow rhythm” or “cough” (Martel et al., 2007; Rovainen, 1996; Rovainen, 1977). The total number of “coughs” occurring during each period was determined and treatment means were statistically assessed.
2.5 Statistical Analysis
Data were analyzed using repeated measures analysis of variance (RM-ANOVA) comparing “cough” frequency in each preparation during normocapnia and hypercapnia. Where significance was found, RM-ANOVAs were followed by post-hoc multiple comparison using the Holm-Sidak method.
2.6 Data Exclusion
The described dataset is derived from 5 in vitro preparations treated with identical protocols. Additional individual replicates had been done but were excluded. One replicate was excluded as it was inadvertently exposed to 2% CO2 during “normocapnia” prior to the 5% hypercapnia (rather than 1.5%); three replicates were excluded because tissues received a different treatment followed by a prolonged recovery prior to the hypercapnic exposure trial; one replicate was excluded because experiments were conducted in a different recording apparatus using recirculated solutions (Wilson et al., 1999). None of these differences would be expected to confound the experiment and, were these trials included, we would still find a statistically significant increase in “cough” frequency with hypercapnia (N = 10; P = 0.007; NC vs. HC P=0.011, t=3.371; HC vs. RNC, P=0.018, t =2.943, 1-way RMANOVA). Fictive pharyngeal ventilation was relatively regular and appeared consistent within each preparation. However, the amplitude was variable and at times individual bursts were difficult to distinguish from background. The occurrence of “cough” appeared to influence subsequent pharyngeal ventilation. The variability and potential complexity of this pattern prevented detailed quantification and, as the focus of the investigation was on the “cough” pattern, pharyngeal ventilation is not considered beyond cursory observation that there were no consistent frequency changes with hypercapnic exposure.
2.7 Whole-Animal Plethysmography
Twelve roughly equal sized (1.0–2 g) ammocoetes were individually identified and placed into aerated individual 1-liter aquaria that were visually isolated from one another and maintained at 12 °C.
For plethysmography, animals were placed in a transparent acrylic tube (1 cm i.d., 30 cm long) attached at one end to a customized resistance pneumotachograph and open at the other end. The pneumotachograph was fabricated from two short (7–10 mm) cylinders constructed from thick-walled, 5 mm i.d. acrylic tube, each fitted with a 1 cm length of 18 gauge steel tube to access the inner diameter through the tube wall. The two cylinders were joined in series with cyanoacrylate adhesive, in opposition to a mesh resistance barrier (300 µm nylon net) separating the two. Two poles of a water-filled differential pressure transducer (Validyne DP-45; 2 cm H2O pressure diaphragm; Validyne Engineering, Northridge CA, USA) were connected across the resistance with water-filled polyethylene tubing. Prior to experiments, the pneumotachograph was attached to the transparent tube with hot glue. In this configuration, water flowing across the resistance produced a differential pressure, measured by the transducer. The pneumotachograph was not calibrated for absolute flow measurements, but accurately reflected relative flow patterns. The magnitude of the differential pressure was proportional to water flow, with polarity indicative of inward or outward direction of flow (Fig 2).
Fig 2. In vivo experiments.
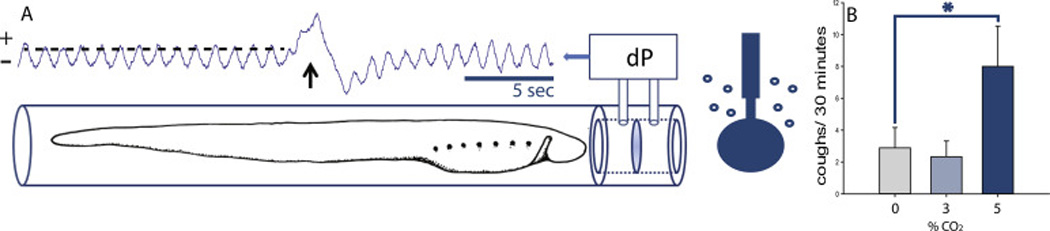
Intact ammocoetes were placed in a clear tube (A). In vivo pharyngeal ventilation and “cough” were recorded by measuring the differential pressure (dP) produced by water flowing across a fine mesh resistance ahead of the animal. Water flow patterns were distinct between the two behaviours. During pharyngeal ventilation, water flow is cyclic and unidirectional. Movement of a velum in the anterior pharynx draws water across the mesh resistance while loading this cavity, and propels it caudally through a branchial basket and lateral branchial pores (Martel et al., 2007; Rovainen, 1977), and out the open distal end of the tube. Exposing ammocoetes to elevated aquatic CO2 stimulated a formerly infrequent and distinct water flow pattern of tidal “cough” (arrow); a seemingly normal loading of the pharyngeal pouch followed by forceful anterior expulsion of water from the pouch in a reverse direction across the mesh resistance. The frequency of cough (B) was determined during randomized 30-minute periods when water was equilibrated with either 0, 3, or 5% CO2 (balance air). “Cough” frequency was enhanced by hypercapnia (P=0.034, F=4.215, 1-way RMANOVA; Holm-Sidak MCP, 0 vs. 3% P=0.6, t=0.535, n=9; 0 vs. 5% P=0.030, t=2.714, n=9).
Ammocoetes were positioned close to the pneumotachograph and often spontaneously placed the vestibular openings to their pharynx within the pneumotachograph itself, greatly enhancing measurement resolution.
The tube was placed in a partially filled, temperature-controlled aquarium (13 °C ± 1 °C) aerated from a controlled gas supply that allowed mixing of CO2 and air. Aeration was achieved using a spherical aluminum gas diffuser stone placed approximately 2 cm beyond the pneumotachograph. Ammocoetes acclimated for 20 min prior to experimentation, with aeration by air (0% CO2). Experiments consisted of a 30-min period of observation when the aquarium continued to be aerated with either air, 3% or 5% CO2 mixtures (balance air). Gas mixture composition was confirmed by sampling mixed gas through a CO2 analyzer (Capnomac; Datex Instrument Corporation, Helsinki Finland). Aquatic pH was not measured. Gas treatments in a given experiment were randomized, and each individual was exposed to each gas treatment by repeating experiments on subsequent days. Only one treatment was administered to an individual on a single day.
The pressure transducer output was demodulated and amplified (CD15; Validyne Engineering, Northridge CA, USA), and recorded using a computer analogue-to-digital data acquisition system (Powerlab, AD Instruments, Colorado Springs, CO, USA). Ammocoetes were simultaneously visually monitored within transparent tubes from below the glass aquarium using a USB digital microscope (MicroXplore PC200, Orion Telescopes, Watsonville CA, USA). Continuous digital video records were acquired and synchronized with pressure recordings. The experimental apparatus was illuminated from above, allowing body movements and ventilation to be resolved from the video record and matched to patterns of pressure generation.
2.8 Data Analysis
Data records were de-identified with respect to subject and experimental treatment, so that data analysis was blinded. Pressure recordings and video records were scored for patterns representative of pharyngeal ventilation or cough. The total number of coughs per 30-min experimental period was determined for each trial. Data were then re-identified, tabulated and statistically assessed.
2.9 Statistical Analysis
Data were analyzed using repeated measures analysis of variance (RM-ANOVA), comparing “cough” frequency in each individual subject between trials with exposure to normocapnia and hypercapnia. Where significant difference was found, RM-ANOVAs were followed by Holm-Sidak post-hoc multiple comparison analysis.
2.10 Interpretation
Ventilatory water flows were interpreted from pneumotachograph pressure fluctuations measured at the anterior of the animal. Pressure measurements during pharyngeal pouch ventilation matched expected patterns, as ammocoete ventilation is unidirectional with water being drawn past the pneumotachograph resistance into the mouth, passing through the branchial pores (Mallatt, 1981; Rovainen, 1996) and flowing caudally through the unrestricted distal end of the tube. In ammocoetes, “inspiratory” unidirectional water flow is primarily generated by movement of an internal vellum drawing water into the mouth and pharynx, back through the branchial basket and out through the branchial pores, augmented at times by compression of the branchial basket (Mallatt, 1981; Rovainen, 1996; Rovainen and Schieber, 1975). Movement of the vellum and branchial basket were easily observable in the transilluminated animal within the transparent tube.
We did not attempt to quantify ventilatory volumes as the amplitude of the pressure deflection during rhythmic pharyngeal ventilation varied not only with apparent ventilatory effort but also with the position of the ammocoete with respect to the pneumotachograph. Pressure signals were synchronized with visible movements of the vellum, as expected by the vellum acting as the primary pump (Mallatt, 1981; Rovainen, 1996). At times movements of the vellum were coordinated with branchial basket compression. Subjectively, pressures measured during rhythmic tidal pharyngeal ventilation were relatively uniform. Pressures generated during rhythmic pharyngeal ventilation were short, monophasic and negative at the internal face of the resistance with respect to the outer face (Fig 2A upper trace). This pressure profile is consistent with a negative pressure generated in the pharyngeal pouch by the velum drawing a volume of water into the tube across the resistance. Supplemental compression of the branchial basket to augment stroke volume through the branchial pores would not influence recorded pressure at the anterior of the animal.
Mechanical changes associated with periodic “cough” were not quantified although, subjectively, “cough” was always observed in association with large branchial basket compression. Presumably, vellar movements were also altered during “cough”. Pressures measured during periodic “cough” suggested a seemingly normal inspiratory loading followed by a larger amplitude and slightly longer duration pressure pulse, positive at the internal face of the resistance with respect to the outer face (Fig 2A upper trace). This profile is consistent with a positive pressure generated in the pharyngeal pouch and/or branchial basket forcing a volume of water anteriorly from the vestibular opening and out across the resistance. Although not quantified, the area of the positive pressure deflection associated with the “cough” was noticeably greater than that of the negative pressure typical of unidirectional tidal pharyngeal ventilation. This would suggest that the stroke volume of pharyngeal ventilation (presumably approximating pharyngeal pouch volume) is exceeded during the “cough”, and may indicate contributions from compression of the branchial basket cavity to the volume of the “cough”. As water flows were only measured at the anterior of the animal, measurements do not show whether or not water was also expelled through the branchial pores during “cough”.
With respect to the example pressure tracings (Fig 2A) the large negative pressure deflection subsequent to the illustrated “cough” may represent post-“cough” expansion of the branchial cavity contributing additional volume to the subsequent inspiration, or possible slight caudal movement of the animal acting as a piston and drawing water into the tube. Apparent differences in pressure deflections associated with pharyngeal stroke volume before and after a “cough” were not quantified.
2.11 Data Exclusion
In order to remove possible confounding influence of mechanosensory input influencing cough (Martel et al., 2007; Rovainen, 1977), trials in which debris was visible within or proximal to the pharyngeal basket, regardless of cough expression, were excluded from the data set. In such cases, data records were re-identified and trials were repeated with data then de-identified and analyzed blind. Data from two additional trials were excluded for high cough frequencies, characteristic of mechanically induced cough (Martel et al., 2007; Rovainen, 1977), although the source of this stimulation was not identified.
3. Results
Ammocoete brainstems were isolated and activity was recorded from cranial nerves, which innervate structures generating rhythmic pharyngeal ventilation (Fig 1A). With normocapnia, cranial nerve discharge exhibited regular and rhythmic burst patterns representative of pharyngeal pouch ventilation, with irregular, periodic and distinct “cough” (Fig 1B) (Hsia et al., 2013; Martel et al., 2007; Rovainen, 1996; Rovainen, 1977). Isolated brainstems were exposed to normocapnia (NC; 1.5% CO2) and hypercapnia (HC; 5% CO2). Burst patterns were analyzed for event frequency in 10-minute periods immediately prior to and after the gas change, and with recovery following a return to NC (RNC; Fig 1C). Elevating CO2 increased the frequency of “cough” (event/10 min; N = 5; P = 0.02; NC vs. HC P=0.031, t=3.32; HC vs. RNC, P=0.037, t =2.937, 1-way RMANOVA).
Fig 1. In vitro experiments.
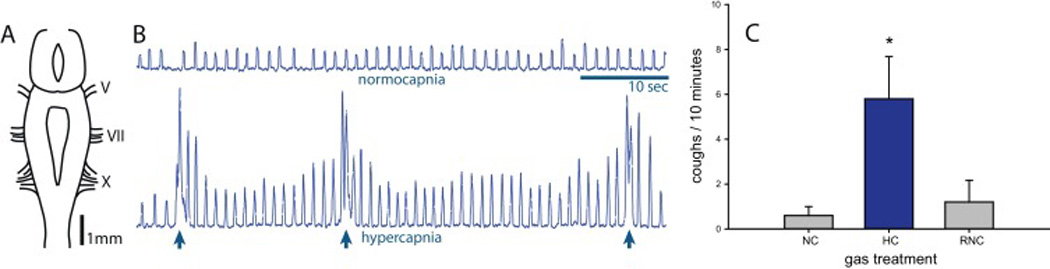
Ammocoete brainstems were isolated and exposed to normocapnia (1.5% CO2) and hypercapnia (5% CO2) to investigate the “cough” CRG response. Activity was recorded from intact cranial nerve roots of the in vitro brainstem, which normally innervate structures generating rhythmic pharyngeal ventilation (A; CN V, VII and X illustrated). With normocapnia, cranial nerve discharge (CN VII shown) exhibited regular and rhythmic burst patterns representative of pharyngeal pouch ventilation (top), with irregular, periodic and distinct “cough” (bottom; Hsia et al., 2013; Martel et al., 2007; Rovainen, 1977) indicated by arrows (B). Data were analyzed for event frequency in 10-minute periods immediately prior to (NC) and after (HC) the gas change, and with recovery following a return to NC (RNC; C). Elevating CO2 increased the frequency of “cough” (n = 5; P = 0.02; NC vs. HC P=0.031, t=3.32; HC vs. RNC, P=0.037, t =2.937, 1-way RMANOVA).
Intact ammocoetes (in vivo) were placed in a clear tube, and patterns of pharyngeal ventilation were recorded by measuring the differential pressure produced by water flowing across a fine mesh resistance ahead of the animal (Fig 2A). Water flow patterns generated by intact lamprey ammocoetes in vivo, matched previous characterizations of rhythmic unidirectional pharyngeal ventilation; loading the anterior pharyngeal pouch through caudal movement of a velum, and expulsion of water caudally through lateral branchial pores (Mallatt, 1981; Rovainen, 1996). During pharyngeal ventilation, water flow was cyclic and unidirectional.
Exposing ammocoetes to elevated aquatic CO2 had no apparent effect on pharyngeal ventilation, but stimulated a formerly infrequent and distinct water flow pattern of tidal “cough” (Fig 2 B); a seemingly normal loading of the pharyngeal pouch was followed by forceful anterior expulsion of water from the pouch in a reverse direction across the mesh resistance. The reconfiguration of active elements resulting in this different flow pattern is unknown. The frequency of “cough” was determined during randomized 30-minute periods when water was equilibrated with 0, 3, or 5% CO2 (balance air). “Cough” frequency was enhanced by hypercapnia (event/30 min; P=0.034, F=4.215, 1-way RMANOVA; Holm-Sidak MCP, 0 vs 3% P=0.6, t=0.535, n=9; 0 vs 5% P=0.030, t=2.714, n=9). Elevated aquatic CO2 also agitated ammocoetes and induced large body movements and swimming.
4. Discussion
Our results indicate that the anatomically distinct CRG for the slow rhythm/“cough” in larval lamprey is modulated by CO2/pH. Furthermore we show that “cough” is stimulated in vivo in response to aquatic hypercapnia and that animals become agitated under these conditions. Observations identify central CO2/pH chemosensitivity in vitro and complimentary responsiveness of the intact animal.
Small filter feeding lamprey ammocoetes generate water currents by continuous rhythmic pharyngeal pouch ventilation to supply nutrients and, in so doing, satisfy gas exchange requirements as O2 and CO2 diffuse between water and large surface areas of exchange epithelia including the pharynx and gills (Hsia et al., 2013). It may seem curious that a CO2/pH-modulated CRG would occur in an organism where gas exchange needs are generally satisfied by water currents produced during feeding, and in an aquatic environment that normally poses no limitation to CO2 excretion. Consider, however, that lamprey ammocoetes commonly inhabit narrow tube burrows dug in soft sediments. Substrate can occlude burrow entrances and limit water flow. Metabolically produced CO2, which normally dissipates into water, would build up in an occluded burrow. Thus, a CO2/pH-modulated CRG that induces “cough” would be adaptive. Forceful “coughs” and agitation of the whole animal would contribute to maintaining a patent opening and/or prompt the animal to evacuate a collapsing burrow. Ammocoete ventilation is modulated by aquatic O2 availability and stimulated by hypoxia (Rovainen 1996). Aquatic hypoxia is common and it may be that despite ventilatory sensitivity to hypoxia, CO2-mediated initiation of cough provides for an alarm response to burrow occlusion with better sensitivity than would a response initiated by hypoxia alone.
The presence, in the most basal vertebrate, of a CO2/pH-modulated CRG distinct from that producing rhythmic pharyngeal ventilation provides insight into the evolution of ventilatory control and the conservation of basic control mechanisms across vertebrate lineages. Our findings support the following hypothetical model wherein mechanisms common and critical to vertebrate breathing arose through exaptation (Gould and Vrba, 1982) from those present in the basal vertebrate ancestor (Fig 3).
Fig 3. Evolution of Air-Breathing Central Rhythm Generator.
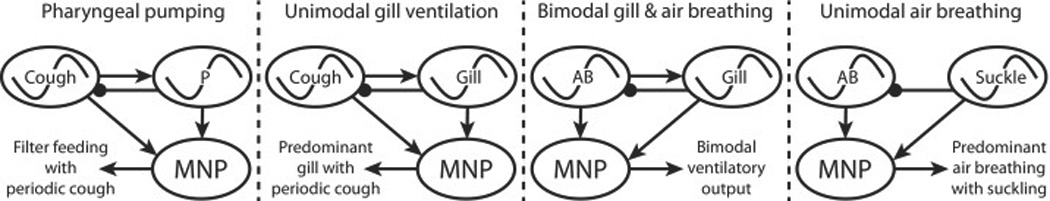
Schematic illustration of the speculative evolution of ventilatory rhythm control in vertebrates: basic elements critical for ventilatory control and producing phasic pharyngeal basket ventilation and periodic “cough” were present in a basal, common vertebrate ancestor. These elements transition during vertebrate evolution through a process of exaptation or functional retasking. Mechanisms generating rhythmic pharyngeal ventilation are exapted to produce gill ventilation, buccal cavity ventilation, gular fluttering and suckling. Distinct mechanisms producing “cough” are sensitive to CO2 and are exapted to produce air breathing. In this scheme, as described in the text, pharyngeal pumping in the basal vertebrate resulted from the pool of pump muscle motor neurons (MNP) being activated by relatively continuous activity from a central rhythm generator (CRG, ~) generating a pharyngeal ventilatory rhythm and, periodically by a distinct CO2/pH-modulated CRGcough producing water flows to clear debris from filter feeding areas and habitat. A first exaptation occurred with the evolution of unimodal gill ventilation when the pharyngeal pump was retasked to generate rhythmic gill ventilation (CRGP becomes the CRGgill) and the CRGcough was retained. A second exaptation facilitated bimodal gill and air breathing; a retasked CO2/pH modulated CRGcough functions as the CRG for air-breathing (CRGAB), while the CRGgill is retained for gill ventilation. Unimodal air breathing continued to be generated by the CO2/pH modulated CRGAB in reptiles, birds and mammals, while the CRGgill is retasked to produce periodic rhythmic gular fluttering and suckling.
Represented by the lamprey ammocoete, basal vertebrates possessed two distinct CRGs. One CRG generated rhythmic ventilation of the pharynx and branchial basket and produced a water current for filter feeding that also satisfied gas exchange needs (CRGP) (Hsia et al., 2013). A second and distinct CO2/pH-modulated CRGcough produced water flows to clear debris from filter feeding areas, aided in maintaining patent exchange surfaces and chambers in the animal’s habitat, and responded when chamber water exchange was restricted. The evolution of jaws in gnathostomes separated feeding and gas exchange (Mallatt, 1996). A first exaptation occurred when the rhythmic CRGP-driven pharyngeal pump for filter feeding was retasked generating rhythmic continuous gill ventilation for gas exchange (CRGP becomes the CRGgill). The progenitor CRG for “cough” was retained to produce forceful water currents functioning to clear delicate gill structures. Bimodal ventilation with the evolution of the lung in primitive fish (and represented by extant lunged fish and larval amphibians) involved continued recruitment of the CRGgill for gill ventilation, along with a second exaptation; retasking the CRGcough to function as the CRG for air-breathing (CRGAB). The existing CO2/pH modulation of the CRGcough/CRGAB allowed periodic lung ventilation to respond to requirements imposed by metabolically produced CO2. The CRGAB retained this function in adult amphibians, while the CRGgill generates rhythmic ventilation of the buccal cavity contributing to ventilation, olfaction and vocalization despite the resorption of gills. Air breathing in reptiles, birds and mammals derives from the CO2/pH-modulated CRGAB, producing periodic air breathing in ectothermic tetrapods with increasing frequency as metabolic demand increases. The high metabolism of endotherms dictates that the CRGAB is continuously active. In “higher” amniotes a third exaptation retasked the CRGgill to produce the high frequency, although periodic, rhythms underlying gular fluttering in reptiles and birds, and suckling in mammals.
We propose that the motor pattern produced by the ancestral CRGcough facilitated exaptation to air breathing. Fish are commonly reported to “cough”, presumably as a result of the distinct CRGcough (Ballintijn, 1985; Ballintijn and Jüch, 1984; Ballintijn and Punt, 1985;; Burleson and Smith, 2001), and there is evidence that “cough” in fish can be modulated by acidity (Bishop and McIntosh, 1981; Hargis, 1976; Lunn et al., 1976; Nevitt 1991; Rose-Janes and Playle, 2001; Ross et al. 2001; Satchell and Maddalena, 1972). Our model predicts that exaptation retasked the CRGcough with the evolution of air breathing in tetrapods, making absent the ancestral mechanism of cough. It is apparent that amphibians and reptiles do not “cough” (presumably as these groups lack a CRGcough). The irritant receptor mediated activity known as “cough” in mammals (Brooks, 2011; Canning, 2008) is a newly derived and distinct reflex seemingly mechanistically unrelated to the ancestral CRGcough.
5. Concluding Remarks
Our results illustrate the presence of a central CO2/pH-sensitive chemoreceptor in lamprey, consonant with such presence in the common ancestor to all vertebrates. Lamprey possess a CO2/pH-sensitive CRG, the presence of which in basal vertebrates may have provided the substrate critical for the evolution of vertebrate air breathing.
Supplementary Material
Highlights.
Lamprey “cough” is produced by a central rhythm generator sensitive to CO2.
These data suggests that these elements critical for the evolution of breathing in tetrapods, were present in the most basal vertebrate ancestors prior to the evolution of the lung.
We propose that the evolution of breathing in all vertebrates occurred through exaptations derived from these critical basal elements.
Acknowledgments
Funding: This work was funded by the National Institutes of Health [2U54NS041069 to MBH and BET] and the National Science Foundation [IOS 1022442 to BET].
We thank Dr. Trent Sutton and Dr. Andrew Seitz for assistance in acquiring research animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Study design, MBH, MH, BET; Experimentation MBH, MH; Data analysis and interpretation MBH, MH; Manuscript preparation MBH, MH, BET.
Author Competing Interests: No competing interests declared.
References
- Ballintijn CM. The respiratory function of gill filament muscles in the carp. Respir Physiol. 1985;60(1):59–74. doi: 10.1016/0034-5687(85)90039-8. [DOI] [PubMed] [Google Scholar]
- Ballintijn CM, Punt GJ. Gill arch movements and the function of the dorsal gill arch muscles in the carp. Respir Physiol. 1985;60(1):39–57. doi: 10.1016/0034-5687(85)90038-6. [DOI] [PubMed] [Google Scholar]
- Ballintijn CM, Jüch PJ. Interaction of respiration with coughing, feeding, vision and oculomotor control in fish. Brain Behav Evol. 1984;25(2–3):99–108. doi: 10.1159/000118855. [DOI] [PubMed] [Google Scholar]
- Bishop WE, McIntosh AW. Acute lethality and effects of sublethal cadmium exposure on ventilation frequency and cough rate of bluegill (Lepomis macrochirus) Arch Environ Contam Toxicol. 1981;10(5):519–530. doi: 10.1007/BF01054876. [DOI] [PubMed] [Google Scholar]
- Brook SM. Perspective on the human cough reflex Cough. 2011;7:10. doi: 10.1186/1745-9974-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleson ML, Smith RL. Central nervous control of gill filament muscles in channel catfish. Respir Physiol. 2001;126(2):103–112. doi: 10.1016/s0034-5687(01)00215-8. [DOI] [PubMed] [Google Scholar]
- Canning BJ. The cough reflex in animals: relevance to human cough research. Lung. 2008;186(Suppl 1):S23–S28. doi: 10.1007/s00408-007-9054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli E, Robertson B, Mutolo D, Grillner S, Pantaleo T, Bongianni F. Neuronal mechanisms of respiratory pattern generation are evolutionary conserved. J Neurosci. 2013;33(21):9104–9112. doi: 10.1523/JNEUROSCI.0299-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BL, Brundage CM, Harris MB, Taylor BE. Lung respiratory rhythm and pattern generation in the bullfrog: role of neurokinin-1 and mu-opioid receptors. J Comp Physiol B. 2009;179(5):579–592. doi: 10.1007/s00360-009-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy JF, Missaghi K, Chartré S, Robert M, Auclair F, Dubuc R. Bilateral connectivity in the brainstem respiratory networks of lampreys. J Comp Neurol. 2012;520(7):1442–1456. doi: 10.1002/cne.22804. [DOI] [PubMed] [Google Scholar]
- Hargis JR. Ventilation and metabolic rate of young rainbow trout (Salmo gairdneri) exposed to sublethal environmental pH. J Exp Zool. 1976;196(1):39–44. doi: 10.1002/jez.1401960105. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Schmitz A, Lambertz M, Perry SF, Maina JN. Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr Physiol. 2013;3(2):849–915. doi: 10.1002/cphy.c120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn CR, Toews DP, Pree DJ. Effects of three pesticides on respiration, coughing, and heart rates of rainbow trout (Salmo gairdneri Richardson) Can J Zool. 1976;54(2):214–219. doi: 10.1139/z76-023. [DOI] [PubMed] [Google Scholar]
- Mallatt J. Ventilation and the origin of jawed vertebrates: a new mouth. Zool J. Linnean Soc. 1996;117:329–404. [Google Scholar]
- Mallatt J. The suspension feeding mechanism of the larval lamprey petromyzon marinus. J. Zool. Lond. 1981;194:103–142. [Google Scholar]
- Martel B, Guimond JC, Gariépy JF, Gravel J, Auclair F, Kolta A, Lund JP, Dubuc R. Respiratory rhythms generated in the lamprey rhombencephalon. Neuroscience. 2007;148(1):279–293. doi: 10.1016/j.neuroscience.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Missaghi K, Auclair F, Dubuc R. Neuroscience Meeting Planner. San Diego CA: Society for Neuroscience; 2013. On the organization and location of the generator for the slow respiratory rhythm in lampreys. 782.22. Online. [Google Scholar]
- Milsom WK. The phylogeny of central chemoreception. Respir Physiol Neurobiol. 2010;173:195–200. doi: 10.1016/j.resp.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Nevitt GA. Do fish sniff? A new mechanism of olfactory sampling in pleuronectid flounders. J Exp Biol. 1991;157:1–18. doi: 10.1242/jeb.157.1.1. [DOI] [PubMed] [Google Scholar]
- Perry SF, Wilson RJ, Straus C, Harris MB, Remmers JE. Which came first, the lung or the breath? Comp Biochem Physiol A Mol Integr Physiol. 2001;129:37–47. doi: 10.1016/s1095-6433(01)00304-x. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Torgerson C, Harris M, Perry SF, Vasilakos K, Wilson RJ. Evolution of central respiratory chemoreception: a new twist on an old story. Respir Physiol. 2001;129(1–2):211–217. doi: 10.1016/s0034-5687(01)00291-2. [DOI] [PubMed] [Google Scholar]
- Rose-Janes NG, Playle RC. Protection by two complexing agents, thiosulphate and dissolved organic matter, against the physiological effects of silver nitrate to rainbow trout (Oncorhynchus mykiss) in ion-poor water. Aquat Toxicol. 2000;51(1):1–18. doi: 10.1016/s0166-445x(00)00103-x. [DOI] [PubMed] [Google Scholar]
- Ross RM, Krise WF, Redell LA, Bennett RM. Effects of dissolved carbon dioxide on the physiology and behavior of fish in artificial streams. Environ Toxicol. 2001;16(1):84–95. [PubMed] [Google Scholar]
- Rovainen CM. Feeding and breathing in lampreys. Brain Behav Evol. 1996;48(5):297–305. doi: 10.1159/000113208. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Neural control of ventilation in the lamprey. Fed Proc. 1977;36(10):2386–2389. [PubMed] [Google Scholar]
- Rovainen CM, Schieber MH. Ventilation of larval lampreys. J. Comp. Physiol. 1975;104:185–203. [Google Scholar]
- Satchell GH, Maddalena DJ. The cough or expulsion reflex in the Port Jackson shark, Heterodontus portusjacksoni. Comp Biochem Physiol A Comp Physiol. 1972;41(1):49–62. doi: 10.1016/0300-9629(72)90033-3. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Brundage CM. Chronic, but not acute, ethanol exposure impairs central hypercapnic ventilatory drive in bullfrog tadpoles. Respir Physiol Neurobiol. 2013;185(3):533–542. doi: 10.1016/j.resp.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Harris MB, Leiter JC, Gdovin MJ. Ontogeny of central CO2 chemoreception: chemosensitivity in the ventral medulla of developing bullfrogs. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1461–R1472. doi: 10.1152/ajpregu.00256.2003. [DOI] [PubMed] [Google Scholar]
- Wilson RJA, Taylor BE, Harris MB. Evolution of vertebrate respiratory neural control. In: Adelman G, Smith BH, editors. Encyclopedia of Neuroscience. 4th. Elsevier Ltd; 2009. pp. 67–75. [Google Scholar]
- Wilson RJ, Vasilakos K, Harris MB, Straus C, Remmers JE. Evidence that ventilatory rhythmogenesis in the frog involves two distinct neuronal oscillators. J Physiol (Lond) 2002;540:557–570. doi: 10.1113/jphysiol.2001.013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJA, Harris MB, Remmers JE, Perry SF. Evolution of air-breathing and central CO2/H+ sensitivity: New insights from an old fish. J Exp Biol. 2000;203:3505–3512. doi: 10.1242/jeb.203.22.3505. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Straus C, Remmers JE. Efficacy of a low volume recirculating superfusion chamber for long term administration of expensive drugs and dyes. J Neurosci Methods. 1999;87(2):175–184. doi: 10.1016/s0165-0270(99)00005-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


