Abstract
Background Respiratory viral infections remain an underrecognized cause of morbidity and mortality among preterm infants in the neonatal intensive care unit (NICU).
Case Report An eight day old, 650 gram birth weight, 23 weeks' gestational age female developed “culture-negative” sepsis manifested by respiratory deterioration, hypoxia, leukocytosis, and thrombocytopenia. She was diagnosed with pneumonia and hepatitis due to adenovirus HAdV-D (H29F9) by polymerase chain reaction (PCR) testing, but died at the age of 18 days despite treatment with cidofovir and immune globulin intravenous.
Conclusion As the ability to diagnosis respiratory viral infections in the NICU has improved greatly with the use of PCR testing, the impact and contribution of these viruses to neonatal disease is now being recognized and the notion of “culture-negative” sepsis needs reassessment. The diagnosis of these infections in high risk infants is important not only for etiologic and epidemiologic reasons but ultimately for informing antimicrobial stewardship efforts.
Keywords: adenovirus, preterm infant, respiratory virus, neonatal intensive care unit
The role of respiratory viruses in disease causation among infants in the neonatal intensive care unit (NICU) is only being defined recently as molecular methodologies such as polymerase chain reaction (PCR) are implemented for their optimal diagnosis.1 2 Before PCR technology, disseminated adenoviral infection was difficult to diagnose promptly as diagnostic tests lacked sensitivity. We present the case of an extremely low-birth-weight (birth weight < 1,000 g) infant who died secondary to disseminated adenoviral infection diagnosed late in the course of disease. Our objective is to alert neonatologists and other health care professionals to consider respiratory viruses as an important cause of “culture-negative sepsis.”
Case
A 650-g female neonate was born vaginally at 23 weeks' gestation to a 27-year-old, gravida 3, para 2 African American mother who presented to the emergency department with abdominal pain, rupture of fetal membranes, and foul-smelling, bloody vaginal discharge. The mother had not received prenatal care, and she reported having up to six alcoholic drinks daily. She denied recent illness or fever, and received one dose of betamethasone, two doses of intravenous aqueous penicillin G for group B streptococcal prophylaxis, and intravenous magnesium sulfate for fetal neuroprotection. Testing for hepatitis B surface antigen, Neisseria gonorrhoeae, and Chlamydia trachomatis was negative, but testing for human immunodeficiency virus (HIV) antibodies was not performed. Apgar scores were 5 and 8 at 1 and 5 minutes, respectively. The neonate was intubated in the delivery room for apnea, received endotracheal surfactant, and admitted to the NICU for further management.
The neonate's growth parameters were appropriate for gestational age (weight, 650 g, 56%; length, 31 cm, 73%; frontal-occipital circumference, 21.5 cm, 70%) and the physical examination was notable only for postaxial polydactyly of both hands and right foot. On the first day of age, she was extubated to nasal continuous positive airway pressure (CPAP) with Fio 2 < 25%. She received prophylactic indomethacin therapy for 3 days, as well as caffeine and fluconazole prophylaxis. Ampicillin and gentamicin were discontinued when blood culture from birth was sterile at 48 hours. She remained on nasal CPAP and tolerated trophic feeds of maternal or donor human milk, and had a normal cranial ultrasound at 1 week of age. HIV-antibody testing was negative. Newborn screening for metabolic disorders and severe combined immunodeficiency was normal.
On the eighth day of age, the neonate developed hyperglycemia and leukocytosis with white blood cell (WBC) count of 72.00 × 109/L (neutrophils, 0.61; bands, 0.16; lymphocytes, 0.04; metamyelocytes, 0.08; and monocytes, 0.1), with an immature (I)/total (T) neutrophil ratio of 0.3 and platelet count of 133.00 × 109/L. Blood and urine cultures were obtained, and vancomycin and gentamicin therapies were initiated but discontinued after 48 hours of sterile culture results. On the 11th day of age, she developed an increased number of apneic episodes necessitating endotracheal intubation and ventilatory support. Chest radiograph showed haziness in both lung fields suggestive of edema or microatelectasis, with one focal area of opacity in the right costophrenic sulcus (Fig. 1A). A second sepsis evaluation (blood and urine cultures) was performed, and vancomycin and gentamicin treatment were initiated. WBC count was 59.60 × 109/L with an I:T ratio of 0.44 (neutrophils, 0.46; bands, 0.31; lymphocytes, 0.12; metamyelocytes, 0.06; and monocytes, 0.06) and platelet count of 94.00 × 109/L. A lumber puncture was performed the next day; cerebrospinal fluid had no red blood cells, one WBC, glucose of 6.49 mmol/L, and protein content of 1,400 mg/L. The blood and cerebrospinal fluid cultures were sterile, and the urine culture obtained by catheterization yielded 4 × 106 CFU/L of Staphylococcus epidermidis and treatment with vancomycin was continued for possible urinary tract infection (UTI). The respiratory status continued to deteriorate and on the 14th day of age, she developed severe hypoxemia, hypercapnia despite maximal ventilatory support, and hypotension requiring dopamine therapy. Chest radiograph revealed multilobar opacification with air bronchograms in the right lung and streaky opacities in the left lung (Fig. 1B) without pleural effusion by chest ultrasonography. Another blood culture was obtained, and piperacillin–tazobactam was added to the antibiotic regimen. At 15 days of age, the alanine aminotransferase (ALT) was 0.70 µkat/L, aspartate aminotransferase (AST) was 3.22 µkat/L, and gamma-glutamyltransferase was 18.70 µkat/L.
Fig. 1.
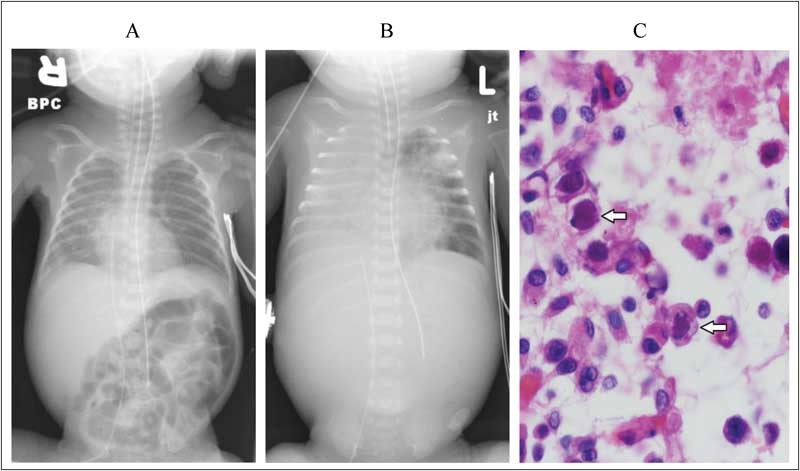
Chest radiograph, (A) 11 days of age and (B) 14 days of age, showing the progression of lung disease from haziness and focal opacity at the right costophrenic angle to complete opacification and air bronchograms of the right lung and streaky infiltrates in the left lung, and (C) lung tissue from autopsy showing severe necrotizing pneumonitis with many cells having round eosinophilic intranuclear inclusions (arrows).
On the 16th day of age, consultation with a pediatric infectious specialist was obtained. Urine for cytomegalovirus DNA by PCR testing was negative, but nasopharyngeal respiratory viral PCR test (Nationwide Children's Hospital Virology Laboratory, Columbus, OH) was positive for adenovirus.3 4 Adenoviral DNA PCR testing on blood was positive, and scavenged blood from 12 days of age was also positive for adenovirus DNA (Table 1). The mother denied any respiratory illness, but reported having red eyes when the neonate became ill.
Table 1. Results of semiquantitative adenovirus real-time PCR testing (Nationwide Children's Hospital, Columbus, OH) that targets a conserved region of the adenovirus hexon gene.
| Age at specimen collection (d) | Source | Cycle threshold | Estimated viral load (copies/mL) |
|---|---|---|---|
| 12 | Whole blood | 25.5 | 3,888,155 |
| 16 | Nasopharynxa | 17 | 79,000,000 |
| 16 | Whole blood | 26.5 | 2,129,145 |
| 17 | Plasma | 22.8 | 2,570,000 |
Abbreviation: PCR, polymerase chain reaction.
Note: The cycle threshold inversely correlates with viral burden.
Culture of specimen positive for adenovirus.
The clinical condition continued to deteriorate with multiorgan failure manifested by oliguria, elevated creatinine (112.85 µmol/L), blood urea nitrogen (27.13 mmol/L), ALT of 0.85 µkat/L, AST of 6.60 µkat/L, and hypotension treated with dopamine, epinephrine, and hydrocortisone. On the 17th day of age, she received intravenous cidofovir (1 mg/kg) and immune globulin intravenous (IGIV; 500 mg/kg) for disseminated adenovirus disease. She developed bluish abdominal discoloration with a gasless bowel pattern on abdominal radiograph, but ultrasonography did not show pneumatosis or ascites. Cranial ultrasonography remained normal, but because of the poor and rapidly deteriorating clinical condition, the mother requested withdrawal of support and the neonate died at 18 days of age.
An autopsy was performed that demonstrated disseminated adenoviral infection with severe necrotizing pneumonitis of both lungs which contained numerous immunopositive cells with viral cytopathic effect seen by immunohistochemical staining (Fig. 1C). In addition, there was also viral cytopathic effect in the trachea, larynx, esophagus, stomach, and pancreatic ductal mucosa; few small necrotic parenchymal lesions in the liver; and extensive necrosis of the spleen. The brain had recent intraventricular, periventricular, and cerebellar hemorrhages. Placental histopathology did not detect viral inclusions by hematoxylin and eosin or specific adenovirus immunohistochemical staining.
The adenovirus PCR-positive nasopharyngeal specimen was cultured with isolation of a human adenovirus (HAdV) of species D. Using PCR amplification and sequencing of the hexon and fiber genes for molecular typing,5 the strain had a HAdV-D29-like hexon gene and a HAdV-D9-like fiber gene (GenBank KU230354 and KU230355). Both amplicons exhibited sequences identical to those obtained for the recently described genotype HAdV-D56.6
Discussion
Despite advances in the diagnosis of respiratory viral infections, there remains a large knowledge gap among health care professionals in the NICU with respect to their occurrence, testing, and possible treatment options. We present the case of an extremely preterm neonate who received antimicrobial therapy for “culture-negative” sepsis and “low colony count UTI” but subsequently was diagnosed with disseminated adenoviral infection after 8 days of progressive disease and clinical deterioration that resulted in death. First reported as a cause of neonatal pneumonia in 1939,7 adenovirus is known to cause severe disseminated infection in neonates with a mortality rate as high as 68%.8 9 Lack of prompt recognition and treatment likely contributes to this high mortality.
Respiratory viral infections in the NICU remain underrecognized as PCR testing for these viruses has not been available routinely in the general hospitals that house NICUs. Using PCR technology, the incidence of respiratory viruses among infants evaluated for possible late-onset sepsis in the NICU has been 8 to 10%.1 2 Moreover, in a year, prospective surveillance study of respiratory viral infections detected by PCR testing of 50 preterm infants < 33 weeks' gestation, 26 infants (52%) tested positive for a respiratory virus at least once during their NICU birth hospitalization, although none had adenovirus.10 Detection of a respiratory virus was associated with longer length of stay, prolonged ventilator support, a diagnosis of bronchopulmonary dysplasia, and more clinical deterioration episodes, highlighting, as in our case, the importance of timely diagnosis of these infections.
We report the youngest gestational age neonate with adenoviral infection in the current literature. This 23 week neonate was on nasal CPAP at the time of the clinical deterioration, and the chest radiograph had an unusual unilateral multilobar pulmonary infiltrate that was not considered to be of viral origin, further underscoring the difficulties in diagnosis based on radiographic appearance. The lack of positive bacterial cultures in the face of clinical deterioration despite antibiotic therapy should prompt clinicians to investigate the possibility of a viral infection.
The adenovirus strain that was isolated from nasopharyngeal secretions in our patient exhibited hexon and fiber genes identical to those described for genotype HAdV-D56.6 The latter was first identified in a 10-day-old term neonate who died from adenoviral disease and was the source of transmission to health care providers.6 11 HAdV-D56 is also a known cause of epidemic keratoconjunctivitis,12 13 suggesting that our patient may have acquired the infection from the mother who had a red eye for a few days before the neonate's illness.
Unfortunately, there is little clinical experience and evidence for successful treatment of neonates with antiviral medications such as ribavirin14 or cidofovir9 for adenoviral infection. Such studies are urgently needed. Ronchi et al9 reported a 17-day-old term neonate with disseminated adenoviral disease and pneumonia who was treated successfully for 9 days with cidofovir (1 mg/kg/dose IV once a day; Monday, Wednesday, Friday), probenecid (250 mg by mouth daily), and IGIV (500 mg/kg IV once).
In conclusion, respiratory viruses such as adenovirus are introduced into NICUs worldwide, causing severe disease and even outbreaks. Diagnosis of respiratory viral infections is now possible and readily available, and effort to diagnose these infections in high-risk infants is important for etiologic and epidemiologic reasons as well as to inform antimicrobial stewardship efforts. As these infections are identified, the notion of culture-negative sepsis may need reassessment. Finally, knowing the full impact and contribution of these viruses to neonatal disease may spur research efforts aimed at developing effective antiviral therapies for this population.
Footnotes
Financial Disclosure and Conflict of Interest None of the authors has any financial disclosure or conflict of interest in regard to the article submitted. Funding No honorarium, grant, or other payment was given to anyone to produce this article.
References
- 1.Kidszun A, Hansmann A, Winter J. et al. Detection of respiratory viral infections in neonates treated for suspicion of nosocomial bacterial sepsis: a feasibility study. Pediatr Infect Dis J. 2014;33(1):102–104. doi: 10.1097/INF.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 2.Ronchi A, Michelow I C, Chapin K C. et al. Viral respiratory tract infections in the neonatal intensive care unit: the VIRIoN-I study. J Pediatr. 2014;165(4):690–696. doi: 10.1016/j.jpeds.2014.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaggi P, Kajon A E, Mejias A, Ramilo O, Leber A. Human adenovirus infection in Kawasaki disease: a confounding bystander? Clin Infect Dis. 2013;56(1):58–64. doi: 10.1093/cid/cis807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song E, Kajon A E, Wang H. et al. Clinical and virologic characteristics may aid distinction of acute adenovirus disease from Kawasaki disease with incidental adenovirus detection. J Pediatr. 2016;170:325–330. doi: 10.1016/j.jpeds.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Kajon A E, Lamson D, Shudt M. et al. Identification of a novel intertypic recombinant species D human adenovirus in a pediatric stem cell transplant recipient. J Clin Virol. 2014;61(4):496–502. doi: 10.1016/j.jcv.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson C M, Singh G, Henquell C. et al. Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology. 2011;409(2):141–147. doi: 10.1016/j.virol.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodpasture E W, Auerbach S H, Swanson H S, Cotter E F. Virus pneumonia of infants secondary to epidemic infections. Am J Dis Child. 1939;57:997–1011. [Google Scholar]
- 8.Lynch J P III, Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med. 2011;32(4):494–511. doi: 10.1055/s-0031-1283287. [DOI] [PubMed] [Google Scholar]
- 9.Ronchi A Doern C Brock E Pugni L Sánchez P J Neonatal adenoviral infection: a seventeen year experience and review of the literature J Pediatr 20141643529–35..e1, 4 [DOI] [PubMed] [Google Scholar]
- 10.Bennett N J, Tabarani C M, Bartholoma N M. et al. Unrecognized viral respiratory tract infections in premature infants during their birth hospitalization: a prospective surveillance study in two neonatal intensive care units. J Pediatr. 2012;161(5):814–818. doi: 10.1016/j.jpeds.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henquell C, Boeuf B, Mirand A. et al. Fatal adenovirus infection in a neonate and transmission to health-care workers. J Clin Virol. 2009;45(4):345–348. doi: 10.1016/j.jcv.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto M, Okafuji T, Okafuji T. et al. Isolation of an intertypic recombinant human adenovirus (candidate type 56) from the pharyngeal swab of a patient with pharyngoconjunctival fever. Jpn J Infect Dis. 2012;65(5):457–459. doi: 10.7883/yoken.65.457. [DOI] [PubMed] [Google Scholar]
- 13.Huang G, Yao W, Yu W. et al. Outbreak of epidemic keratoconjunctivitis caused by human adenovirus type 56, China, 2012. PLoS One. 2014;9(10):e110781. doi: 10.1371/journal.pone.0110781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavin P J, Katz B Z. Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics. 2002;110(1 Pt 1):e9. doi: 10.1542/peds.110.1.e9. [DOI] [PubMed] [Google Scholar]


