Abstract
Cancer cells devote the majority of their energy consumption to ribosome biogenesis, and pre-ribosomal RNA transcription accounts for 30–50% of all transcriptional activity. This aberrantly elevated biological activity is an attractive target for cancer therapeutic intervention if approaches can be developed to circumvent the development of side effects in normal cells. TIF-IA is a transcription factor that connects RNA polymerase I with the UBF/SL-1 complex to initiate the transcription of pre-ribosomal RNA. Its function is conserved in eukaryotes from yeast to mammals, and its activity is promoted by the phosphorylation of various oncogenic kinases in cancer cells. The depletion of TIF-IA induces cell death in lung cancer cells and mouse embryonic fibroblasts but not in several other normal tissue types evaluated in knock-out studies. Furthermore, the nuclear accumulation of TIF-IA under UTP down-regulated conditions requires the activity of LKB1 kinase, and LKB1-inactivated cancer cells are susceptible to cell death under such stress conditions. Therefore, TIF-IA may be a unique target to suppress ribosome biogenesis without significantly impacting the survival of normal tissues.
Keywords: TIF-IA, Transcription factor, Pre-ribosomal RNA synthesis, RNA polymerase I, Oncogenic kinases, Targeted Therapy
1. Introduction
Hyperactivation of ribosomal RNA (rRNA) transcription is an important molecular alteration in cancer cells. The nucleolus is the nuclear subdomain in which RNA polymerase I (RNA Pol I) transcription and the assembly of ribosomal subunits occurs in eukaryotic cells. The abnormal nucleolus has been used as a marker for aggressive cancer for over 100 years [1], and more recent studies have linked dysregulated nucleolar morphology with the hyperactivation of rRNA transcription [2]. It has been estimated that approximately 80% of cancer cell energy consumption is used for rRNA biogenesis [3]. The synthesis of pre-rRNA, the first event in this process, is efficiently regulated mostly through reversible modification of RNA Pol I transcription factors [2]. 30–50% of RNA transcription in cancer cells is for pre-rRNA synthesis [4], and accelerated rRNA synthesis is one of the most important molecular alterations in cancer cells [5].
Pre-rRNA synthesis follows three essential steps: (i) the initial binding of the upstream binding factor (UBF) to the ribosomal DNA (rDNA) promoter leads to recruitment of the SL-1 (a.k.a. TIF-IB), (ii) the resultant UBF/SL-1 complex facilitates binding of an initiation-competent subpopulation of RNA Pol I, which is associated with TIF-IA, and (iii) TIF-IA mediates the interaction between RNA Pol I and SL-1, to form the pre-initiation complex at the rDNA promoter (Figure 1). The regulation of rRNA synthesis involves many transcription factors and has been reviewed extensively [2, 6, 7]. This review focuses on TIF-IA, which is the rate-limiting factor in the initiation of prerRNA transcription and is activated by various oncogenic kinases in human cancers.
Figure 1.
Essential steps in the initiation of pre-rRNA synthesis
2. The TIF-IA gene: RRN3
2.1. The discovery of TIF-IA protein and the cloning of RRN3
In a human cell system, the combination of three well-purified factors, UBF, SL-1/TIF-IB, and RNA Pol I, was initially found to be sufficient to support the correct initiation of rDNA transcription in vitro with a suitable DNA template [8]. However, some suspected the involvement of other factors which may co-purify with these factors. This led to the discovery of TIF-IA protein, which is strongly associated with RNA Pol I [9]. The yeast homolog of TIF-IA gene is called RRN3, which was cloned from Saccharomyces cerevisiae in 1996 [10]. Human TIF-IA gene was cloned in 2000 [11, 12]. Human TIF-IA protein is 21% identical and 43% similar to the yeast protein. The RNA Pol I transcription initiation complex in yeast is quite different from that in the mammalian system, and consists of four transcription factors, upstream activation factor (UAF), core factor (CF), TATA binding protein (TBP), and Rrn3p [4, 13, 14]. A transcription factor corresponding to UAF has not been found in the mammalian system [15]. However, human TIF-IA protein is capable of rescuing a yeast strain with a RRN3 disruption, indicating TIF-IA-mediated regulation of ribosomal RNA synthesis is conserved among eukaryotes [11].
2.2. Analysis of RRN3 in human cancers
The original human TIF-IA gene, RRN3, locates on the short arm of chromosome 16 (16p13.11), and it contains 28 exons. An untranscribed pseudogene is found on chromosome 2p22.2, which may complicate the genetic analysis or manipulation of RRN3 locus but should not affect expression based analysis such as RNAseq. However, there are two other duplicates of RRN3 that are actively transcribed but unable to generate the TIF-IA protein [16]. The first duplicate copies the original gene to 16p11.2. It loses the last exon and also contains a 3,517 base pair (bp) interstitial deletion which includes exons 11 and 12. This locus was duplicated again to 16p12.2. Again, this second duplication is also incomplete which results in the loss of the last three exons. These duplication events were found in the chimpanzee genome but not in the rhesus genome. Other vertebrate genomes and non-vertebrate organisms only have a single copy of the RRN3 gene, indicating that these duplication events occurred after the human-chimp-rhesus split but before the human-chimp split [16]. The presence of these duplicates in human cells poses a challenge in the mutational analysis of the RRN3 locus in human tumors (see below), and an obstacle in the manipulation of the RRN3 locus, such as using CRISPR to introduce a specific mutation into the TIF-IA protein.
The RRN3 locus is not frequently altered on a DNA level in human cancers, and it is unlikely to be a driver-gene for human cancers. Of all the studies available in the cBioportal database, only two of them report significant genetic alterations in RRN3. In a neuroendocrine prostate cancer study by Trento/Cornell/Broad, 17 (15.9%) out of 107 cases contain RRN3 amplifications. In a breast cancer patient xenograft study, RRN3 was found to be amplified in 6 (20.7%) out of 29 samples [17]. Of the 77 somatic RRN3 mutations collected in all tumors analyzed by The Cancer Genome Atlas (TCGA), 8 (11%) are nonsense or frame-shift mutations. Interestingly, one third of these missense mutations occur either at codon 9 or codon 11. All 12 of the codon 11 mutations contain a proline to serine mutation, and most of the codon 9 mutations (12/13) contain arginine to cysteine mutation. The recurrent nature of these mutations suggested that such alterations may promote the function of TIF-IA as an oncogene [18]. However, a closer analysis indicated that the RNN3 duplicate on 16p12.2 contains both alterations, and these missense mutations are not likely to be real genetic alterations of the original RRN3 locus in human tumors.
3. The regulation of TIF-IA protein expression
3.1. Transcriptional regulation of RRN3
TIF-IA appears to be ubiquitously expressed in all tissues [12], and its expression can be altered by environmental stresses. For example, amino acid withdrawal decreased TIF-IA expression in HeLa cells after 6 hours [19], and AICAR treatment can induced TIF-IA protein expression in 24 hours [20]. However, not much is known about the transcriptional regulation of RRN3. Chromatin immunoprecipitation (ChIP) studies using genomic tiling arrays (ChIP-chip) indicated that the yeast RRN3 locus is an in vivo binding target of Met4 and Met32 [21], suggesting that Met4 and Met32 directly bind to this locus to regulate its transcription. A microarray RNA expression analysis indicated that the deletion of SFP1 leads to lower levels of RRN3 expression and a lower level of total RNA content [22], implying that SFP1 is a transcription factor that is required for the adequate transcription of RRN3. The most comprehensive study in this area was done with the transcription factor MYC (product of c-Myc oncogene). MYC stimulates RNA Pol II-dependent transcription of a cohort of factors associated with RNA Pol I transcription [23, 24]. MYC also binds to the rDNA promoter region and activates RNA Pol I transcription through remodeling rDNA chromatin structure [25]. RRN3 was found to be a direct transcriptional target of c-MYC in the regulation of ribosomal RNA transcription during granulocyte differentiation [24]. Based on these observations, RNA Pol I has been proposed as a therapeutic target for MYC-driven cancers [26].
3.2. The degradation of TIF-IA
The half-life of a FLAG-tagged TIF-IA is approximately 3 hours in U-2OS cells, and it is degraded by the proteasome [27]. Mdm2 (mouse double-minute 2) is the E3 ubiquitin ligase for TIF-IA, and the N-terminal 94 amino acids of TIF-IA are required for its ubiquitination. The half-life of TIF-IA can be prolonged by the overexpression of oncogenic kinase AKT [28].
4. Protein structure features of TIF-IA
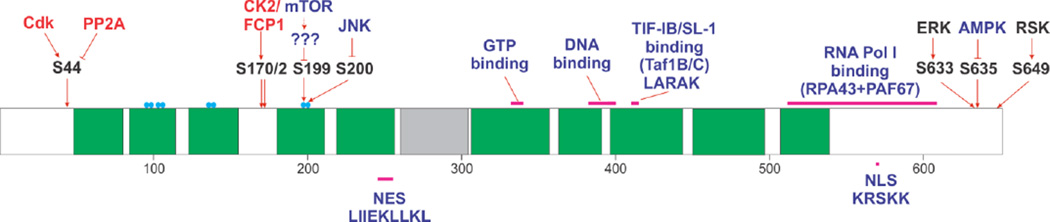
The crystal structure of yeast TIF-IA protein, Rrn3, has been resolved at 2.8 Å resolution [29]. The recombinant Rrn3 protein forms a homodimer, but it binds RNA Pol I as a monomer. This protein contains 10 HEAT (Huntingtin, elongation factor 3 (EF3), protein phosphatase 2A (PP2A), and the yeast kinase TOR1) repeats and an acidic loop which resides between HEAT repeat 5 and 6 (Figure 2). There are eight serine residues, clustered in four pairs (S101/102, S109/110, S145/146, and S185/186) on the surface of Rrn3, forming a serine patch. Rrn3 S145D mutant does not bind RNA Pol I, and the S185D mutant only binds weakly, indicating that this serine patch in involved in RNA Pol I binding. These positions correspond to S97/S98, L102/T103, S138/A139, and S199/T200 in human TIF-IA protein, thus only the first set still retains both serine residues (Figure 1). S199/T200 are target sites for various kinases that regulate TIF-IA protein function (discussed in more detail later), which is consistent with crystal structure studies. Lysine cross-linking analysis indicates the K558 of Rrn3 interacts of A190 and AC40 subunit of RNA Pol I. The acidic loop of Rrn3 (residues 253–322) cannot be resolved by crystal structure analysis. However, the deletion of this acidic loop does not cause a growth phenotype in yeast [29].
Figure. 2. Structure features of TIF-IA.
Green box: HEAT motif, grey box: acidic loop, blue circle: serine patch, NES: nuclear export signal, and NLS: nuclear leading signal.
5. TIF-IA as a transcription factor
5.1. TIF-IA and RNA Pol I interaction
In yeast, total TIF-IA protein in cell lysates is more abundant than UAF or CF, but only a small fraction is associated with RNA Pol I [30–32], and the recruitment of RNA Pol I to the promoter is largely Rrn3-dependent [15]. Approximately 10% of catalytically active RNA Pol I is associated with TIF-IA. In both the yeast and mammalian system, there are reports indicating that RNA Pol I can be recruited to a template without TIF-IA, but such complex cannot initiate transcription, because the formation of the first phosphodiester bond requires TIF-IA [9, 33–35]. At the ribosomal gene promoter, TIF-IA was found to interact with two subunits of RNA Pol I, RPA43 and PAF67, through its C-terminal region (AAs 512–609) [36]. The RPA49/RPA34 dimer of RNA Pol I was found to regulate and initiate recruitment and subsequent release of TIF-IA during transcription [37]. TIF-IA functions stoichiometrically in rDNA transcription, and its ability to associate with RNA Pol I is lost during the transcription reaction. The released TIF-IA can associate with another preinitiation complex. Hence, mammalian TIF-IA becomes the limiting factor as the transcription reaction proceeds [38].
Topoisomerase 2 alpha (Top2α) is also present in the RNA Pol I/TIF-IA complex [39]. It directly interacts with TIF-IA through its C-terminal regions, and its recruitment to the rDNA promoter is TIF-IB/SL-1 dependent, which facilitates the assembly of RNA Pol I pre-initiation complex. Like TIF-IA, Top2α dissociates from RNA PoI I following transcription initiation [40].
Actin and myosin I are unexpected players in the TIF-IA/RNA Pol I interaction. In a systematic analysis of protein complexes in the yeast proteome, yeast RRN3 was found to be in a protein complex containing actin (ACT1) using ERG13 as an entry point [41]. The interaction between TIF-IA and actin was confirmed in a biochemical analysis of RNA Pol I transcription complex in vitro [42]. TIF-IA was found to bind to nuclear myosin I (NMI), while actin binds to RNA Pol I. Neither actin nor NMI binds to UBF or TIF-IB/SL-1. Furthermore, the binding of actin to RNA Pol I does not require TIF-IA, but the phosphorylation of TIF-IA at Ser649 is necessary for TIF-IA/NMI to associate with RNA Pol I/actin to initiate rRNA transcription. Therefore, nuclear actin and myosin I are both required for rRNA synthesis.
A related discovery is the 2012 finding that filamin A (FLNA) is a nucleolar protein capable of inhibiting rRNA transcription through its actin-binding domain by suppressing the recruitment of RNA Pol I to the rDNA promoter [43]. A splice variant of TIF-IA was found in normal and leukemia cells [44]. This variant lacks exon 6 of the TIF-IA gene, thus the protein product, TIF-90, contain an in-frame 30 amino acid deletion. Interestingly, TIF-90 strongly interacts with RNA Pol I, whereas full-length TIF-IA does so to a lesser extent. FLNA also interacts with TIF-90 to inhibit rRNA synthesis [44].
The amount of TIF-IA that is associated with RNA Pol I can also be decreased by the depletion of amino acid in the media, and re-addition of amino acid not only restores this association but also partially rescues RRN3 occupancy at the rDNA promoter [19].
5.2. TIF-IA and SL-1 interaction
During the initiation of pre-ribosomal RNA transcription, TIF-IA binds SL-1/ TIF-IB but not UBF complex, thus it forms a bridge between UBF/SL-1 and RNA Pol I, and it is essential for the recruitment of Pol I by TIF-IB/SL1 to the rDNA promoter [31]. TIF-IA was found to interact with Taf1B and Taf1C subunits of TIF-IB/SL-1 through a short, conserved motif (LARAK, AAs411-415) [31, 36]. Similarly, Rrn3 was found to interact with Rrn6 of the CF complex in yeast. [45]. The interaction between TIF-IA and TIF-IB/ SL-1 can be disrupted by various phosphorylation changes on TIF-IA (Table 1, and below)
Table 1.
Phosphorylation sites on TIF-IA
| Phosphorylation Sites |
Flanking Seq | Consensus | Kinase/ Phosphatase |
Function | Interaction with Pol I or SL-1 |
Stress | Reference |
|---|---|---|---|---|---|---|---|
| Ser44 | FNSPPRKTV | [S/T]PX[K/R] | CDK | activate | no change | [52] | |
| PP2A | inactivate | no change | Rapamycin | [52] | |||
| Ser170/172 | DVSDSDDED | SxxE/D | CK2 | activate |
disrupts RNA Pol I interaction |
AKT as upstream event |
[53] |
| FCP1 | activate |
enhance RNA Pol I interaction |
[53] | ||||
| Ser199 | IARYVPSTP | unknown | unknown | inactivate | disrupts RNA Pol I and TIF-IB/SL-1 interaction |
Rapamycin | [28] |
| Thr200 | VPSTP | VPxTP | JNK | inactivate | disrupts RNA Pol I and TIF-IB/SL-1 interaction |
[52] | |
| Ser633 | FDTHF- RSPSSSVGSPPVLYMQP- SPL |
ERK | activate | serum stimulation |
[54]. | ||
| Ser635 | DTHFRSPSSSVGSPPVLY | LRRVxSxxNL | AMPK | inactivate | disrupt SL-1 interaction |
glucose free | [56] |
| MKKSxSxxDV | AICAR | [57] | |||||
| IxHRxSxxEI | |||||||
| Ser649 | FDTHF- RSPSSSVGSPPVLYMQP- SPL |
R/LxRxxS/T | RSK | activate | phosphorylation of S649 by RSK is a pre-requisite for S633 by ERK |
serum stimulation |
[56] |
5.3. TIF-IA as a DNA binding protein and a GTPase
In addition to forming a bridge between TIF-IB/SL-1 and RNA Pol I, a recent study also indicated that TIF-IA binds DNA using a domain that is similar to heat shock transcription factor 2. Deletion of this region, which comprises amino acids 382–400 (FLEHLWKKLQD.PSNPAIIR), abolishes the DNA binding ability of TIF-IA [46]. Interestingly, the deletion mutation is still capable of interacting with RPA43 of RNA Pol I and TIF-IB/SL-1, but it cannot complement the rrn3-ts mutant. Hence, the DNA binding ability of TIF-IA is also essential for transcription by RNA Pol I.
TIF-IA also contains a consensus GTP binding motif at amino acids 333–340 (GXXXX-GKS/T340), and mutation of threonine 340 to asparagine abolishes its interaction with GTP-sepharose. TIF-IA-GTP binds ErbB3-binding protein 1 (Ebp1), and in T cells, the presence of Ebp1 is important for the retention of TIF-IA at the site of rRNA synthesis in the nucleolus [47]. Therefore, the binding of TIF-IA to GTP may also be important in its regulation of ribosomal RNA synthesis.
6. The regulation of TIF-IA function by oncogenic kinases
6.1. The phosphorylation of Ser44 by cyclin-dependent kinase
Various oncogenic alterations have been associated with increased rRNA synthesis [48], and the phosphorylation of TIF-IA by oncogenic kinases has been evaluated. TIF-IA protein was found to be predominantly phosphorylated in vivo when it is not bound to RNA Pol I [49]. However, a subsequent study indicated that the phosphorylation of Rrn3 regulates rDNA transcription [50]. A proteomic study in 2002 identified the phosphorylation of TIF-IA on Ser44, Ser170 and Ser172 [51]. Ser44 has the flanking sequence of FNSPPRKTV, which contains a consensus target, [S/T]PX[K/R], for cyclin-dependent kinase (Cdk). The phosphorylation of Ser44 is required for TIF-IA activity as demonstrated by the failure of the S44A mutant to enhance the transcription of RNA Pol I reporter. Interestingly, this mutant maintains its interaction with either RNA Pol I or TIF-IB/SL-1 [52]. Hence, this phosphorylation event does not disrupt the interaction of TIF-IA with other pre-rRNA transcription machinery. Rapamycin treatment leads to the rapid dephosphorylation of Ser44, and protein phosphatase 2A (PP2A) has been shown to remove the phosphorylation of this residue under these conditions [52].
6.2. The phosphorylation and dephosphorylation of Ser170 and 172
The phosphorylation and dephosphorylation of TIF-IA at these two serine residues occur during each round of transcription and are required for multiple rounds of RNA Pol I transcription [53]. Ser170 and 172 reside in the sequence DVSDSDDED, which contains the consensus target (SXXE/D) for casein kinase II (CK2). Casein kinase IIα (CK2α)-mediated phosphorylation at these sites leads to the release of TIF-IA from RNA Pol I after transcription initiation, thus most free TIF-IA molecules are phosphorylated at these two residues, while those associated with RNA Pol I are unphosphorylated at these sites [53]. TFIIF-associating C-terminal domain phosphatase 1 (FCP1) has been shown to be able to dephosphorylate TIF-IA at these two sites to enhance the interaction between TIF-IA and RNA Pol I. In leukemia cells, activated AKT was found to enhance TIF-IA-mediated rRNA synthesis by stabilizing TIF-IA and altering TIF-IA phosphorylation at Ser170 and Ser172 through CK2α [28].
6.3. JNK2 and mTOR target Ser199 and Thr200, respectively
Ser199 and Thr200 correspond to one of the four consecutive serine residues that make up the serine patch described in yeast, and they are also regulated by upstream kinases. These sites reside in a VPSTP sequence which contains a p38/JNK target site (VPxTP). JNK2 (c-Jun N-terminal kinase 2) has been shown to directly phosphorylate TIF-IA at Thr200 in vitro, and a phospho-mimetic T200D mutant failed to activate transcription. This phosphorylation event disrupted the interaction of TIF-IA with RNA Pol I and TIF-IB/SL-1, and caused translocation of TIF-IA from the nucleolus into the nucleoplasm [54].
The phosphorylation level of Ser199 is reduced after rapamycin treatment. While the S199A mutant has similar activity as the wild type TIF-IA, the S199D mutant was reported to be not active because it failed to associate with RNA Pol I and TIF-IB/SL-1 [52]. Rapamycin inhibits the kinase activity of mTOR (mammalian target of rapamycin), yet neither mTOR nor its downstream target S6K1 phosphorylates nor co-purifies with TIF-IA. Hence, the identity of the upstream kinase Ser199 is still unknown [52].
6.4. The C-terminus of TIF-IA protein is phosphorylated by ERK2, RSK and AMPK
Compared to yeast Rrn3p, TIF-IA in humans contains an extra 34 amino acids, which plays an important regulatory function. Ser649 is phosphorylated by RSK2, and its phosphorylation is a prerequisite for Ser633 phosphorylation by ERK2. These two residues are phosphorylated after serum stimulation, and the phosphorylation of both residues is required to activate TIF-IA function, which enables a rapid increase in pre-rRNA synthesis in response to growth factor stimulation [55, 56]
The withdrawal of glucose from the cell culture media activates AMPK, which phosphorylates TIF-IA directly on Ser635. This phosphorylation event impairs the interaction of TIF-IA with TIF-IB/SL1, thus disrupting the formation of the transcription initiation complex [57]. AMPK is negative regulator of mTOR [58, 59], but this study indicated that AMPK directly regulates TIF-IA. Interestingly, the extent of AMPK activation is also important in this setting. 2-deoxyglucose (2-DG) is a glycolytic inhibitor, which is commonly used to block glycolysis and activate AMPK [60–63]. While 10 mM 2-DG treatment leads to the loss of TIF-IA in the rDNA promoter, 2 mM treatment does not even though such treatment also activates AMPK and suppresses pre-rRNA synthesis. Activated AMPK can also suppress pre-rRNA synthesis through KDM2A-dependent reduction of pre-rRNA synthesis [64].
6.5. Oncogenic kinases that indirectly regulate TIF-IA function
Earlier studies indicated that the mTOR regulates rRNA synthesis [65, 66]. Rapamycin treatment was found to suppress the activity of ribosomal protein S6 kinase 1 (S6K1), which led to the dephosphorylation of UBF and a reduction in UBF/SL-1 interaction [67]. Inhibition of S6K1 also blocks amino acid-driven RNA Pol I elongation but does not affect transcription initiation [19]. The effect of rapamycin on TIF-IA is interesting because it causes hyperphosphorylation at Ser199 and hypophosphorylation at Ser44. As described above, both of these events lead to the inactivation of TIF-IA. Because rapamycin inhibits mTOR, these findings suggest that mTOR kinase indirectly regulates TIF-IA phosphorylation to enhance the formation of the Pol I pre-initiation complex at the rDNA promoter [52].
Rapamycin treatment was also reported to cause a decrease in the amount of Rrn3-RNA Pol I complex in yeast [15]. A more recent study in yeast indicated that TOR inactivation causes proteasome-dependent Rrn3p reduction in initiation competent Pol I–Rrn3p complexes, and degradation of Rrn3, and that deletion of the N-terminal 17 amino acids of the yeast Rrn3 renders this protein more stable upon TOR inactivation [68].
Another oncogenic kinase that indirectly regulates TIF-IA function is AKT. AKT promotes rRNA synthesis and synergizes with MYC to stimulate ribosome biogenesis [69]. In leukemia cells, activated AKT was found to enhance TIF-IA-mediated rRNA synthesis by altering TIF-IA phosphorylation at Ser170 and Ser172 through CK2α. Furthermore, activated AKT also inhibits the ubiquitination of TIF-IA to extend the half-life of this protein [28]. AKT was also found to promote rRNA synthesis by preventing the interaction between splicing isoform of TIF-IA, TIF-90, and FLNA cleavage product in leukemia cells [44].
7. Cellular localization and transport of TIF-IA
TIF-IA has been found in the cytoplasm, nucleoplasm and nucleolus, and its cellular localization can be altered by various environmental stresses. In HeLa, NIH373 and MEF cells, anisomycin or H2O2 treatment releases TIF-IA from the nucleolus to the nucleoplasm through their activation of JNK2 kinase [54]. Rapamycin treatment of HeLa cells causes a significant increase in cytoplasmic TIF-IA [52]. However, not all stress-induced TIF-IA inactivation leads to alterations in TIF-IA cellular location. Heat shock treatment has been recently shown to inactivate TIF-IA but did not displace TIF-IA from nucleoli [70]. AICAR treatment results in the depletion of the intracellular pyrimidine pool [71, 72], which leads to the transfer of TIF-IA from the cytoplasm to the nucleus within 4 hours [20]. Interestingly, the tumor suppressor LKB1 appears to play an essential role in the process but does not mediate its effect through its well-known downstream target AMPK [20].
TIF-IA contains a nuclear location signal (aa 568–572, KRSKK), and a nuclear export signal (aa 246–256, LIIEKLLKL). The dynamic distribution of TIF-IA under various stress conditions in the cytosol, nucleus, and nucleolus was determined in a 2009 study using a GFP-TIF-IA system in HeLa cells [73]. At steady state, 48% of total TIF-IA is found in the cytoplasm, while 45% and 7% resides in the nucleoplasm and nucleoli, respectively. The concentration of TIF-IA, however, is 3-fold higher in the nucleus than cytoplasm, and 23-fold higher in the nucleolus. Because the molecular weight of TIF-IA is approximately 75 KDa, it is too large to move across the nuclear pore complex by simple diffusion, thus, TIF-IA is likely to be actively transported into the nucleus by Ran/importin machinery. The free pool of TIF-IA available in the cytoplasm may facilitate the response of TIF-IA to environmental changes. Little is known about the export of TIF-IA from the nucleus to the cytoplasm, except that it is not altered by an inhibitor of exportin1, leptomycin B [73].
8. The effects of TIF-IA deletion
RNAi depletion of TIF-IA induced PARP and caspase-3 cleavage in NSCLC cells [20]. Deletion of exon 12–14 of the TIF-IA gene in mice leads to embryonic lethality at or before day 9.5. TIF-IA−/− embryos have no detectable level of pre-rRNA, and they display growth and developmental retardation. Cre-mediated depletion of TIF-IA in MEF cells leads to disruption of nucleoli and cell cycle arrest. In addition, it promotes p53-mediated apoptosis [74]. The depletion of TIF-IA in adult hippocampal neurons, however, has no impact on the survival of neural cells [75]. Hence, apoptosis induced by TIF-IA depletion is cell-type dependent.
Knockout of the TIF-IA gene in medium spiny neurons (MSNs) has no immediate phenotype after birth, but the depletion of TIF-IA in MSNs causes inhibition of pre-rRNA synthesis, loss of mature rRNA, and perturbation of nucleolar structure, which eventually leads to striatal neurodegeneration and motor deficits after 40 weeks [76]. The level of p53 was also increased after TIF-IA depletion in MSNs, but the knockout of p53 in addition to TIF-IA did not attenuate the effects of nucleolar stress on neuronal survival. Hence, even though p53 expression increases after TIF-IA deletion in various cell types, its ultimate cellular effect is also cell-type dependent.
Tissue-specific knockout of TIF-IA in insulin producing β-cells induces an efficient and specific β-cell ablation [77]. Interestingly, the β-cell mass can be spontaneously regenerated in adult mice following the progressive loss of 95% of β-cells.
9. The role of TIF-IA in cancer therapy
9.1. Resistance to 5-FU based therapy
5-Fluorouracil (5-FU) inhibits thymidylate synthetase, thus preventing the de novo conversion of dUMP to dTMP. The best known therapeutic use of 5-FU is based on its effect on DNA because the inhibition of thymidylate synthetase leads to thymine-less death [78]. However, many studies indicated that uridine, but not thymidine, rescues the cytotoxic and apoptotic effects of 5-FU, suggesting that RNA-based effects also contribute significantly to the efficacy of 5-FU [79–82], although the underlying mechanism remains elusive. In yeast, the pseudouridylation activity of Cbf5p causes 5-FU toxicity in rrp6-d mutant. Interesting, RRN3 was found to genetically interact with Cbf5p as a suppressor [83], and RRN3 suppresses the 5-FU hypersensitivity of a cbf5 mutant with impaired 35S pre-rRNA transcription [84]. If this phenomenon can be observed in human cancer cells, TIF-IA may also play a role in developing resistance against 5-FU based chemotherapy.
9.2. Suppression of rRNA synthesis for cancer therapy
The nucleolus is an emerging therapeutic target for cancer treatment [2, 7, 48, 85]. Three FDA-approved anti-cancer agents have been shown to suppress rRNA synthesis: (i) temsirolimus/everolimus (mTOR inhibitors) inhibit RNA Pol I transcription [86, 87], (ii) topotecan/irinotecan modulate early rRNA processing [88], and (iii) 5-FU impairs late rRNA processing [89]. It is possible that some of the efficacies of these reagents are due to their effects on rRNA generation. There are also extensive efforts in developing agents that specifically targets RNA Pol I-mediated transcription, some of which are in phase I or phase II clinical trial. For example, CX-3543 disrupts Pol I transcription elongation [90], CX-5461 inhibits Pol I initiation [91], and ellipticine impairs SL-1 rDNA promoter binding [92].
A promising finding of these studies is that normal cell can tolerate reduction of pre-rRNA synthesis while cancer cells cannot, thus prone to cell death. In A375 melanoma and MIA PaCa-2 pancreatic cancer cell lines, CX-5461 treatment leads to autophage-induced cell death, but normal cells are capable of tolerate this treatment [91]. CX-5461 also selectively kills B-cell lymphoma cells but spares wild-type cells of the same lineage [93]. A similar phenomenon was observed while targeting the TIF-IA/RNA Pol I interaction. A highly conserved region of 22 amino acids in RPA43 interacts with TIF-IA. A peptide containing this sequence is capable of inhibiting rRNA transcription both in vitro and in vivo. In normal cells, this peptide reversibly inhibits cell proliferation, but it causes cell death in tumor cells through both apoptosis and necrosis [94]. Ribosomal DNA genes contain high frequency of GC-rich sequence, which can be targeted by a lead compound BMH21. This molecule causes proteasome-dependent degradation of the large catalytic subunit of RNA Pol I, and suppresses the growth of A375 and HCT116 xenografts without causing changes in mice weight or organ histology[95]. Therefore, even though rRNA synthesis is an essential housekeeping process in normal cells, targeting rRNA synthesis is still a viable approach because with the proper therapeutic dosing, it is possible to preferentially induce cell death in cancer cells.
9.3. Targeting defects in TIF-IA nuclear import
It is also possible to develop therapeutic strategy that can discriminate between highly proliferating normal cells and cancer cells through TIF-IA. One potential approach to specifically target pre-rRNA synthesis in a subset of cancer cells relies on the finding that downregulation of intracellular uridine only suppresses pre-rRNA synthesis in LKB1-mutant cells, which eventually leads to cell death [20]. In LKB1-wild type cells, LKB1 kinase activity actively promotes TIF-IA nuclear import to maintain TIF-IA mediated pre-rRNA synthesis and prevent cell death under such stress conditions. This discovery suggests the potential of developing a novel treatment concept to specifically eliminate LKB1-mutant tumor cells using an existing clinical reagent (Leflunomide) that inhibits the de novo synthesis of UMP and preferentially induces cell death in LKB1-mutant cells.
The current cancer treatment paradigm is to inhibit biological pathways that are hyperactive in cancer cells with pharmaceutical agents. While these approaches have proven successful in the clinic, they share two common problems. First, the targeted proteins or pathways are likely to play important physiological roles in some normal tissues, and their inhibition thus leads to toxic side effects. Second, cancer cells have defective DNA damage/repair checkpoint(s) which make them genetically heterogeneous, and systemic therapy creates an environment for the selection of cancer cells with mutated target proteins that no longer interact with the drug. A therapeutic approach based on the defective nuclear import of TIF-IA in response to UTP downregulation has at least two advantages. First, this defect is only observed in cells without LKB1 kinase function, LKB1 is ubiquitously expressed in all tissue types, and therapeutic approaches against LKB1-null cells should not have toxic effects on normal tissues (i.e. fewer side effects). Second, the genetic codes for LKB1 are lost in LKB1-null cancer cells by biallelic loss, and are unlikely to be restored by genetic instability.
10. Conclusions
TIF-IA is an essential mediator of pre-rRNA synthesis by RNA Pol I. Its function is conserved from yeast to mammals and its activity is promoted in cancer cells through various oncogenic kinases either directly or indirectly to support the demand of ribosome biogenesis. Even though ribosomal RNA synthesis is a house-keeping function, emerging evidence indicates that normal cells can tolerate the suppression of pre-rRNA synthesis while cancer cells cannot. Furthermore, it is possible to specific target LKB1-inactivated cancers that lacks the proper TIF-IA nuclear importation signal under uridine-depleted conditions. These discoveries make TIF-IA a novel and attractive target for cancer therapy.
Acknowledgments
We would like to thank Dr. Anthea Hammond for editing this manuscript. WZ is an Anise McDaniel Brock Scholar, a Georgia Cancer Coalition Distinguished Cancer Scholars, and an American Cancer Society Research Scholar. This work was supported in part by R01-CA140571, P01-CA116676, R01-CA194027, and P30CA138292.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest related to this manuscript.
References
- 1.Pianese G, Teuscher R. Beitrag zur Histologie und Aetiologie des Carcinoms. Histologische und experimentelle Untersuchungen. Jena: G. Fischer, Place Published; 1896. [Google Scholar]
- 2.Drygin D, Rice WG, Grummt I. The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu. Rev. Pharmacol. Toxicol. 2010;50:131–156. doi: 10.1146/annurev.pharmtox.010909.105844. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988–2996. doi: 10.1038/sj.onc.1202751. [DOI] [PubMed] [Google Scholar]
- 4.Reeder RH. Regulation of RNA polymerase I transcription in yeast and vertebrates. Prog. Nucleic Acid Res. Mol. Biol. 1999;62:293–327. doi: 10.1016/s0079-6603(08)60511-5. [DOI] [PubMed] [Google Scholar]
- 5.Williamson D, Lu YJ, Fang C, Pritchard-Jones K, Shipley J. Nascent pre-rRNA overexpression correlates with an adverse prognosis in alveolar rhabdomyosarcoma. Genes Chromosomes Cancer. 2006;45:839–845. doi: 10.1002/gcc.20347. [DOI] [PubMed] [Google Scholar]
- 6.Grummt I. Life on a planet of its own: regulation of RNA polymerase I transcription in the nucleolus. Genes Dev. 2003;17:1691–1702. doi: 10.1101/gad.1098503R. [DOI] [PubMed] [Google Scholar]
- 7.Hein N, Hannan KM, George AJ, Sanij E, Hannan RD. The nucleolus: an emerging target for cancer therapy. Trends Mol Med. 2013;19:643–654. doi: 10.1016/j.molmed.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Zawel L, Kumar KP, Reinberg D. Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev. 1995;9:1479–1490. doi: 10.1101/gad.9.12.1479. [DOI] [PubMed] [Google Scholar]
- 9.Schnapp A, Schnapp G, Erny B, Grummt I. Function of the growth-regulated transcription initiation factor TIF-IA in initiation complex formation at the murine ribosomal gene promoter. Mol. Cell. Biol. 1993;13:6723–6732. doi: 10.1128/mcb.13.11.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto RT, Nogi Y, Dodd JA, Nomura M. RRN3 gene of Saccharomyces cerevisiae encodes an essential RNA polymerase I transcription factor which interacts with the polymerase independently of DNA template. EMBO J. 1996;15:3964–3973. [PMC free article] [PubMed] [Google Scholar]
- 11.Moorefield B, Greene EA, Reeder RH. RNA polymerase I transcription factor Rrn3 is functionally conserved between yeast and human. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4724–4729. doi: 10.1073/pnas.080063997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodem J, Dobreva G, Hoffmann-Rohrer U, Iben S, Zentgraf H, Delius H, Vingron M, Grummt I. TIF-IA, the factor mediating growth-dependent control of ribosomal RNA synthesis, is the mammalian homolog of yeast Rrn3p. EMBO Rep. 2000;1:171–175. doi: 10.1093/embo-reports/kvd032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.M N. Transcription factors used by Saccharomyces cerevisiae RNA polymerase I and the mechanism of initiation. Springer-Verlag and R. G. Landes Company, Place Published; 1998. [Google Scholar]
- 14.M N. Ribosomal RNA gene, RNA polymerases, nucleolar structures and synthesis of rRNA in the yeast Saccharomyces cerevisiae. Cold Spring Harbor, Place Published; 2001. [DOI] [PubMed] [Google Scholar]
- 15.Claypool JA, French SL, Johzuka K, Eliason K, Vu L, Dodd JA, Beyer AL, Nomura M. Tor pathway regulates Rrn3p-dependent recruitment of yeast RNA polymerase I to the promoter but does not participate in alteration of the number of active genes. Mol. Biol. Cell. 2004;15:946–956. doi: 10.1091/mbc.E03-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amit M, Sela N, Keren H, Melamed Z, Muler I, Shomron N, Izraeli S, Ast G. Biased exonization of transposed elements in duplicated genes: A lesson from the TIF-IA gene. BMC Mol. Biol. 2007;8:109. doi: 10.1186/1471-2199-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eirew P, Steif A, Khattra J, Ha G, Yap D, Farahani H, Gelmon K, Chia S, Mar C, Wan A, Laks E, Biele J, Shumansky K, Rosner J, McPherson A, Nielsen C, Roth AJ, Lefebvre C, Bashashati A, de Souza C, Siu C, Aniba R, Brimhall J, Oloumi A, Osako T, Bruna A, Sandoval JL, Algara T, Greenwood W, Leung K, Cheng H, Xue H, Wang Y, Lin D, Mungall AJ, Moore R, Zhao Y, Lorette J, Nguyen L, Huntsman D, Eaves CJ, Hansen C, Marra MA, Caldas C, Shah SP, Aparicio S. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 2015;518:422–426. doi: 10.1038/nature13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang J, Kusnadi EP, Ogden AJ, Hicks RJ, Bammert L, Kutay U, Hung S, Sanij E, Hannan RD, Hannan KM, Pearson RB. Amino acid-dependent signaling via S6K1 and MYC is essential for regulation of rDNA transcription. Oncotarget. 2016 doi: 10.18632/oncotarget.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Jin R, Liu X, Huang H, Wilkinson SC, Zhong D, Khuri FR, Fu H, Marcus A, He Y, Zhou W. LKB1 promotes cell survival by modulating TIF-IA-mediated pre-ribosomal RNA synthesis under uridine downregulated conditions. Oncotarget. 2015 doi: 10.18632/oncotarget.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrillo E, Ben-Ari G, Wildenhain J, Tyers M, Grammentz D, Lee TA. Characterizing the roles of Met31 and Met32 in coordinating Met4-activated transcription in the absence of Met30. Mol. Biol. Cell. 2012;23:1928–1942. doi: 10.1091/mbc.E11-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cipollina C, van den Brink J, Daran-Lapujade P, Pronk JT, Porro D, de Winde JH. Saccharomyces cerevisiae SFP1: at the crossroads of central metabolism and ribosome biogenesis. Microbiology. 2008;154:1686–1699. doi: 10.1099/mic.0.2008/017392-0. [DOI] [PubMed] [Google Scholar]
- 23.Poortinga G, Hannan KM, Snelling H, Walkley CR, Jenkins A, Sharkey K, Wall M, Brandenburger Y, Palatsides M, Pearson RB, McArthur GA, Hannan RD. MAD1 and c-MYC regulate UBF and rDNA transcription during granulocyte differentiation. EMBO J. 2004;23:3325–3335. doi: 10.1038/sj.emboj.7600335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poortinga G, Wall M, Sanij E, Siwicki K, Ellul J, Brown D, Holloway TP, Hannan RD, McArthur GA. c-MYC coordinately regulates ribosomal gene chromatin remodeling and Pol I availability during granulocyte differentiation. Nucleic Acids Res. 2011;39:3267–3281. doi: 10.1093/nar/gkq1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandori C, Gomez-Roman N, Felton-Edkins ZA, Ngouenet C, Galloway DA, Eisenman RN, White RJ. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat Cell Biol. 2005;7:311–318. doi: 10.1038/ncb1224. [DOI] [PubMed] [Google Scholar]
- 26.Poortinga G, Quinn LM, Hannan RD. Targeting RNA polymerase I to treat MYC-driven cancer. Oncogene. 2015;34:403–412. doi: 10.1038/onc.2014.13. [DOI] [PubMed] [Google Scholar]
- 27.Fatyol K, Grummt I. Proteasomal ATPases are associated with rDNA: the ubiquitin proteasome system plays a direct role in RNA polymerase I transcription. Biochim. Biophys. Acta. 2008;1779:850–859. doi: 10.1016/j.bbagrm.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen le XT, Mitchell BS. Akt activation enhances ribosomal RNA synthesis through casein kinase II and TIF-IA. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20681–20686. doi: 10.1073/pnas.1313097110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blattner C, Jennebach S, Herzog F, Mayer A, Cheung AC, Witte G, Lorenzen K, Hopfner KP, Heck AJ, Aebersold R, Cramer P. Molecular basis of Rrn3-regulated RNA polymerase I initiation and cell growth. Genes Dev. 2011;25:2093–2105. doi: 10.1101/gad.17363311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milkereit P, Tschochner H. A specialized form of RNA polymerase I, essential for initiation and growth-dependent regulation of rRNA synthesis, is disrupted during transcription. EMBO J. 1998;17:3692–3703. doi: 10.1093/emboj/17.13.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller G, Panov KI, Friedrich JK, Trinkle-Mulcahy L, Lamond AI, Zomerdijk JC. hRRN3 is essential in the SL1-mediated recruitment of RNA Polymerase I to rRNA gene promoters. EMBO J. 2001;20:1373–1382. doi: 10.1093/emboj/20.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bier M, Fath S, Tschochner H. The composition of the RNA polymerase I transcription machinery switches from initiation to elongation mode. FEBS Lett. 2004;564:41–46. doi: 10.1016/S0014-5793(04)00311-4. [DOI] [PubMed] [Google Scholar]
- 33.Aprikian P, Moorefield B, Reeder RH. New model for the yeast RNA polymerase I transcription cycle. Mol. Cell. Biol. 2001;21:4847–4855. doi: 10.1128/MCB.21.15.4847-4855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavanaugh AH, Evans A, Rothblum LI. Mammalian Rrn3 is required for the formation of a transcription competent preinitiation complex containing RNA polymerase I. Gene Expr. 2008;14:131–147. [PMC free article] [PubMed] [Google Scholar]
- 35.Schnapp A, Grummt I. Transcription complex formation at the mouse rDNA promoter involves the stepwise association of four transcription factors and RNA polymerase I. J. Biol., Chem. 1991;266:24588–24595. [PubMed] [Google Scholar]
- 36.Yuan X, Zhao J, Zentgraf H, Hoffmann-Rohrer U, Grummt I. Multiple interactions between RNA polymerase I, TIF-IA and TAF(I) subunits regulate preinitiation complex assembly at the ribosomal gene promoter. EMBO Rep. 2002;3:1082–1087. doi: 10.1093/embo-reports/kvf212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beckouet F, Labarre-Mariotte S, Albert B, Imazawa Y, Werner M, Gadal O, Nogi Y, Thuriaux P. Two RNA polymerase I subunits control the binding and release of Rrn3 during transcription. Mol. Cell. Biol. 2008;28:1596–1605. doi: 10.1128/MCB.01464-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirschler-Laszkiewicz I, Cavanaugh AH, Mirza A, Lun M, Hu Q, Smink T, Rothblum LI. Rrn3 becomes inactivated in the process of ribosomal DNA transcription. J. Biol. Chem. 2003;278:18953–18959. doi: 10.1074/jbc.M301093200. [DOI] [PubMed] [Google Scholar]
- 39.Panova TB, Panov KI, Russell J, Zomerdijk JC. Casein kinase 2 associates with initiation-competent RNA polymerase I and has multiple roles in ribosomal DNA transcription. Mol. Cell. Biol. 2006;26:5957–5968. doi: 10.1128/MCB.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray S, Panova T, Miller G, Volkov A, Porter AC, Russell J, Panov KI, Zomerdijk JC. Topoisomerase IIalpha promotes activation of RNA polymerase I transcription by facilitating pre-initiation complex formation. Nature communications. 2013;4:1598. doi: 10.1038/ncomms2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, Bauer A, Schultz J, Rick JM, Michon AM, Cruciat CM, Remor M, Hofert C, Schelder M, Brajenovic M, Ruffner H, Merino A, Klein K, Hudak M, Dickson D, Rudi T, Gnau V, Bauch A, Bastuck S, Huhse B, Leutwein C, Heurtier MA, Copley RR, Edelmann A, Querfurth E, Rybin V, Drewes G, Raida M, Bouwmeester T, Bork P, Seraphin B, Kuster B, Neubauer G, Superti-Furga G. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- 42.Philimonenko VV, Zhao J, Iben S, Dingova H, Kysela K, Kahle M, Zentgraf H, Hofmann WA, de Lanerolle P, Hozak P, Grummt I. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat Cell Biol. 2004;6:1165–1172. doi: 10.1038/ncb1190. [DOI] [PubMed] [Google Scholar]
- 43.Deng W, Lopez-Camacho C, Tang JY, Mendoza-Villanueva D, Maya-Mendoza A, Jackson DA, Shore P. Cytoskeletal protein filamin A is a nucleolar protein that suppresses ribosomal RNA gene transcription. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1524–1529. doi: 10.1073/pnas.1107879109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen le XT, Chan SM, Ngo TD, Raval A, Kim KK, Majeti R, Mitchell BS. Interaction of TIF-90 and filamin A in the regulation of rRNA synthesis in leukemic cells. Blood. 2014;124:579–589. doi: 10.1182/blood-2013-12-544726. [DOI] [PubMed] [Google Scholar]
- 45.Peyroche G, Milkereit P, Bischler N, Tschochner H, Schultz P, Sentenac A, Carles C, Riva M. The recruitment of RNA polymerase I on rDNA is mediated by the interaction of the A43 subunit with Rrn3. EMBO J. 2000;19:5473–5482. doi: 10.1093/emboj/19.20.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepanchick A, Zhi H, Cavanaugh AH, Rothblum K, Schneider DA, Rothblum LI. DNA binding by the ribosomal DNA transcription factor rrn3 is essential for ribosomal DNA transcription. J. Biol. Chem. 2013;288:9135–9144. doi: 10.1074/jbc.M112.444265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen le XT, Lee Y, Urbani L, Utz PJ, Hamburger AW, Sunwoo JB, Mitchell BS. Regulation of ribosomal RNA synthesis in T cells: requirement for GTP and Ebp1. Blood. 2015;125:2519–2529. doi: 10.1182/blood-2014-12-616433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quin JE, Devlin JR, Cameron D, Hannan KM, Pearson RB, Hannan RD. Targeting the nucleolus for cancer intervention. Biochim. Biophys. Acta. 2014;1842:802–816. doi: 10.1016/j.bbadis.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Fath S, Milkereit P, Peyroche G, Riva M, Carles C, Tschochner H. Differential roles of phosphorylation in the formation of transcriptional active RNA polymerase I. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14334–14339. doi: 10.1073/pnas.231181398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavanaugh AH, Hirschler-Laszkiewicz I, Hu Q, Dundr M, Smink T, Misteli T, Rothblum LI. Rrn3 phosphorylation is a regulatory checkpoint for ribosome biogenesis. J. Biol. Chem. 2002;277:27423–27432. doi: 10.1074/jbc.M201232200. [DOI] [PubMed] [Google Scholar]
- 51.Schlosser A, Bodem J, Bossemeyer D, Grummt I, Lehmann WD. Identification of protein phosphorylation sites by combination of elastase digestion, immobilized metal affinity chromatography, and quadrupole-time of flight tandem mass spectrometry. Proteomics. 2002;2:911–918. doi: 10.1002/1615-9861(200207)2:7<911::AID-PROT911>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 52.Mayer C, Zhao J, Yuan X, Grummt I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 2004;18:423–434. doi: 10.1101/gad.285504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bierhoff H, Dundr M, Michels AA, Grummt I. Phosphorylation by casein kinase 2 facilitates rRNA gene transcription by promoting dissociation of TIF-IA from elongating RNA polymerase I. Mol. Cell. Biol. 2008;28:4988–4998. doi: 10.1128/MCB.00492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayer C, Bierhoff H, Grummt I. The nucleolus as a stress sensor: JNK2 inactivates the transcription factor TIF-IA and down-regulates rRNA synthesis. Genes Dev. 2005;19:933–941. doi: 10.1101/gad.333205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefanovsky VY, Pelletier G, Hannan R, Gagnon-Kugler T, Rothblum LI, Moss T. An immediate response of ribosomal transcription to growth factor stimulation in mammals is mediated by ERK phosphorylation of UBF. Mol. Cell. 2001;8:1063–1073. doi: 10.1016/s1097-2765(01)00384-7. [DOI] [PubMed] [Google Scholar]
- 56.Zhao J, Yuan X, Frödin M, Grummt I. ERK-dependent phosphorylation of the transcription initiation factor TIF-IA is required for RNA polymerase I transcription and cell growth. Mol. Cell. 2003;11:405–413. doi: 10.1016/s1097-2765(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 57.Hoppe S, Bierhoff H, Cado I, Weber A, Tiebe M, Grummt I, Voit R. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17781–17786. doi: 10.1073/pnas.0909873106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 59.Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 60.Karczmar GS, Arbeit JM, Toy BJ, Speder A, Weiner MW. Selective depletion of tumor ATP by 2-deoxyglucose and insulin, detected by 31P magnetic resonance spectroscopy. Cancer Res. 1992;52:71–76. [PubMed] [Google Scholar]
- 61.Kang HT, Hwang ES. 2-Deoxyglucose: an anticancer and antiviral therapeutic, but not any more a low glucose mimetic. Life Sci. 2006;78:1392–1399. doi: 10.1016/j.lfs.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Zhong D, Guo L, de Aguirre I, Liu X, Lamb N, Sun SY, Gal AA, Vertino PM, Zhou W. LKB1 mutation in large cell carcinoma of the lung. Lung Cancer. 2006;53:285–294. doi: 10.1016/j.lungcan.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 63.Zhong D, Liu X, Schafer-Hales K, Marcus AI, Khuri FR, Sun SY, Zhou W. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther. 2008;7:809–817. doi: 10.1158/1535-7163.MCT-07-0559. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka Y, Yano H, Ogasawara S, Yoshioka S, Imamura H, Okamoto K, Tsuneoka M. Mild Glucose Starvation Induces KDM2A-Mediated H3K36me2 Demethylation through AMPK To Reduce rRNA Transcription and Cell Proliferation. Mol. Cell. Biol. 2015;35:4170–4184. doi: 10.1128/MCB.00579-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaragoza D, Ghavidel A, Heitman J, Schultz MC. Rapamycin induces the G0 program of transcriptional repression in yeast by interfering with the TOR signaling pathway. Mol. Cell. Biol. 1998;18:4463–4470. doi: 10.1128/mcb.18.8.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Powers T, Walter P. Regulation of ribosome biogenesis by the rapamycinsensitive TOR-signaling pathway in Saccharomyces cerevisiae. Mol. Biol. Cell. 1999;10:987–1000. doi: 10.1091/mbc.10.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L, Moss T, Poortinga G, McArthur GA, Pearson RB, Hannan RD. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol. Cell. Biol. 2003;23:8862–8877. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Philippi A, Steinbauer R, Reiter A, Fath S, Leger-Silvestre I, Milkereit P, Griesenbeck J, Tschochner H. TOR-dependent reduction in the expression level of Rrn3p lowers the activity of the yeast RNA Pol I machinery, but does not account for the strong inhibition of rRNA production. Nucleic Acids Res. 2010;38:5315–5326. doi: 10.1093/nar/gkq264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan JC, Hannan KM, Riddell K, Ng PY, Peck A, Lee RS, Hung S, Astle MV, Bywater M, Wall M, Poortinga G, Jastrzebski K, Sheppard KE, Hemmings BA, Hall MN, Johnstone RW, McArthur GA, Hannan RD, Pearson RB. AKT promotes rRNA synthesis and cooperates with c-MYC to stimulate ribosome biogenesis in cancer. Sci Signal. 2011;4:ra56. doi: 10.1126/scisignal.2001754. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Z, Dammert MA, Hoppe S, Bierhoff H, Grummt I. Heat shock represses rRNA synthesis by inactivation of TIF-IA and lncRNA-dependent changes in nucleosome positioning. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sabina RL, Patterson D, Holmes EW. 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J. Biol. Chem. 1985;260:6107–6114. [PubMed] [Google Scholar]
- 72.Bardeleben C, Sharma S, Reeve JR, Bassilian S, Frost P, Hoang B, Shi Y, Lichtenstein A. Metabolomics identifies pyrimidine starvation as the mechanism of 5-aminoimidazole-4-carboxamide-1-beta-riboside (AICAr) induced apoptosis in multiple myeloma cells. Mol Cancer Ther. 2013;12:1310–1321. doi: 10.1158/1535-7163.MCT-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szymański J, Mayer C, Hoffmann-Rohrer U, Kalla C, Grummt I, Weiss M. Dynamic subcellular partitioning of the nucleolar transcription factor TIF-IA under ribotoxic stress. Biochim. Biophys. Acta. 2009;1793:1191–1198. doi: 10.1016/j.bbamcr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Parlato R, Kreiner G, Erdmann G, Rieker C, Stotz S, Savenkova E, Berger S, Grummt I, Schütz G. Activation of an endogenous suicide response after perturbation of rRNA synthesis leads to neurodegeneration in mice. J. Neurosci. 2008;28:12759–12764. doi: 10.1523/JNEUROSCI.2439-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kiryk A, Sowodniok K, Kreiner G, Rodriguez-Parkitna J, Sonmez A, Gorkiewicz T, Bierhoff H, Wawrzyniak M, Janusz AK, Liss B, Konopka W, Schutz G, Kaczmarek L, Parlato R. Impaired rRNA synthesis triggers homeostatic responses in hippocampal neurons. Front. Cell. Neurosci. 2013;7:207. doi: 10.3389/fncel.2013.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kreiner G, Bierhoff H, Armentano M, Rodriguez-Parkitna J, Sowodniok K, Naranjo JR, Bonfanti L, Liss B, Schutz G, Grummt I, Parlato R. A neuroprotective phase precedes striatal degeneration upon nucleolar stress. Cell Death Differ. 2013;20:1455–1464. doi: 10.1038/cdd.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shamsi F, Parlato R, Collombat P, Mansouri A. A genetic mouse model for progressive ablation and regeneration of insulin producing beta-cells. Cell Cycle. 2014;13:3948–3957. doi: 10.4161/15384101.2014.952176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 79.Linke WA, Ivemeyer M, Olivieri N, Kolmerer B, Ruegg JC, Labeit S. Towards a molecular understanding of the elasticity of titin. J. Mol. Biol. 1996;261:62–71. doi: 10.1006/jmbi.1996.0441. [DOI] [PubMed] [Google Scholar]
- 80.Pritchard JK, Schaeffer SW. Polymorphism and divergence at a Drosophila pseudogene locus. Genetics. 1997;147:199–208. doi: 10.1093/genetics/147.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoskins J, Scott Butler J. Evidence for distinct DNA- and RNA-based mechanisms of 5-fluorouracil cytotoxicity in Saccharomyces cerevisiae. Yeast. 2007;24:861–870. doi: 10.1002/yea.1516. [DOI] [PubMed] [Google Scholar]
- 83.Cadwell C, Yoon HJ, Zebarjadian Y, Carbon J. The yeast nucleolar protein Cbf5p is involved in rRNA biosynthesis and interacts genetically with the RNA polymerase I transcription factor RRN3. Mol. Cell. Biol. 1997;17:6175–6183. doi: 10.1128/mcb.17.10.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoskins J, Butler JS. RNA-based 5-fluorouracil toxicity requires the pseudouridylation activity of Cbf5p. Genetics. 2008;179:323–330. doi: 10.1534/genetics.107.082727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pickard AJ, Bierbach U. The cell's nucleolus: an emerging target for chemotherapeutic intervention. Chem Med Chem. 2013;8:1441–1449. doi: 10.1002/cmdc.201300262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahajan PB. Modulation of transcription of rRNA genes by rapamycin. Int. J. Immunopharmacol. 1994;16:711–721. doi: 10.1016/0192-0561(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 87.Devlin JR, Hannan KM, Hein N, Cullinane C, Kusnadi E, Ng PY, George AJ, Shortt J, Bywater MJ, Poortinga G, Sanij E, Kang J, Drygin D, O'Brien S, Johnstone RW, McArthur GA, Hannan RD, Pearson RB. Combination Therapy Targeting Ribosome Biogenesis and mRNA Translation Synergistically Extends Survival in MYC-Driven Lymphoma. Cancer Discov. 2016;6:59–70. doi: 10.1158/2159-8290.CD-14-0673. [DOI] [PubMed] [Google Scholar]
- 88.Burger K, Muhl B, Harasim T, Rohrmoser M, Malamoussi A, Orban M, Kellner M, Gruber-Eber A, Kremmer E, Holzel M, Eick D. Chemotherapeutic drugs inhibit ribosome biogenesis at various levels. J. Biol. Chem. 2010;285:12416–12425. doi: 10.1074/jbc.M109.074211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghoshal K, Jacob ST. An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem. Pharmacol. 1997;53:1569–1575. doi: 10.1016/s0006-2952(97)00040-3. [DOI] [PubMed] [Google Scholar]
- 90.Drygin D, Siddiqui-Jain A, O'Brien S, Schwaebe M, Lin A, Bliesath J, Ho CB, Proffitt C, Trent K, Whitten JP, Lim JK, Von Hoff D, Anderes K, Rice WG. Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Cancer Res. 2009;69:7653–7661. doi: 10.1158/0008-5472.CAN-09-1304. [DOI] [PubMed] [Google Scholar]
- 91.Drygin D, Lin A, Bliesath J, Ho CB, O'Brien SE, Proffitt C, Omori M, Haddach M, Schwaebe MK, Siddiqui-Jain A, Streiner N, Quin JE, Sanij E, Bywater MJ, Hannan RD, Ryckman D, Anderes K, Rice WG. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011;71:1418–1430. doi: 10.1158/0008-5472.CAN-10-1728. [DOI] [PubMed] [Google Scholar]
- 92.Andrews WJ, Panova T, Normand C, Gadal O, Tikhonova IG, Panov KI. Old drug, new target: ellipticines selectively inhibit RNA polymerase I transcription. J. Biol. Chem. 2013;288:4567–4582. doi: 10.1074/jbc.M112.411611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C, Wall M, Cluse L, Drygin D, Anderes K, Huser N, Proffitt C, Bliesath J, Haddach M, Schwaebe MK, Ryckman DM, Rice WG, Schmitt C, Lowe SW, Johnstone RW, Pearson RB, McArthur GA, Hannan RD. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22:51–65. doi: 10.1016/j.ccr.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rothblum K, Hu Q, Penrod Y, Rothblum LI. Selective inhibition of rDNA transcription by a small-molecule peptide that targets the interface between RNA polymerase I and Rrn3. Mol Cancer Res. 2014;12:1586–1596. doi: 10.1158/1541-7786.MCR-14-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peltonen K, Colis L, Liu H, Trivedi R, Moubarek MS, Moore HM, Bai B, Rudek MA, Bieberich CJ, Laiho M. A targeting modality for destruction of RNA polymerase I that possesses anticancer activity. Cancer Cell. 2014;25:77–90. doi: 10.1016/j.ccr.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]