Abstract
Objective
Most studies of ketamine administered to children for procedural sedation are limited to emergency department use. The objective of this study was to describe the practice of ketamine procedural sedation outside of the operating room and identify risk factors for adverse events.
Design
Observational cohort review of data prospectively collected from 2007 to 2015 from the multicenter Pediatric Sedation Research Consortium.
Setting
Sedation services from academic, community, free-standing children’s hospitals and pediatric wards within general hospitals.
Patients
Children from birth to 21 years old or younger.
Interventions
None.
Measurements and Main Results
Describe patient characteristics, procedure type, and location of administration of ketamine procedural sedation. Analyze sedation-related adverse events and severe adverse events. Identify risk factors for adverse events using multivariable logistic regression. A total of 22,645 sedations performed using ketamine were analyzed. Median age was 60 months (range, < 1 mo to < 22 yr); 72.0% were American Society of Anesthesiologists-Physical Status less than III. The majority of sedations were performed in dedicated sedation or radiology units (64.6%). Anticholinergics, benzodiazepines, or propofol were coadministered in 19.8%, 57.9%, and 35.4%, respectively. The overall adverse event occurrence rate was 7.26% (95% CI, 6.92–7.60%), and the frequency of severe adverse events was 1.77% (95% CI, 1.60–1.94%). Procedures were not completed in 39 of 19,747 patients (0.2%). Three patients experienced cardiac arrest without death, all associated with laryngospasm.
Conclusions
This is a description of a large prospectively collected dataset of pediatric ketamine administration predominantly outside of the operating room. The overall incidence of severe adverse events was low. Risk factors associated with increased odds of adverse events were as follows: cardiac and gastrointestinal disease, lower respiratory tract infection, and the coadministration of propofol and anticholinergics.
Keywords: adverse events, ketamine, laryngospasm, pediatric procedure sedation, risk factors
There is a growing demand to provide procedural sedation (PS) for children outside of the operating room (OR). Subspecialists such as intensivists, emergency medicine physicians, anesthesiologists, and hospitalists, all provide PS for children. Ketamine has been used by emergency medicine physicians for well over 2 decades for primarily painful procedures (1–13). We used the Pediatric Sedation Research Consortium (PSRC) database to describe the administration of ketamine during PS, characterize the adverse events (AEs), and identify the risk factors associated with AEs or severe AEs (SAEs). Based on previous studies, we hypothesized that age less than 12 months or greater than 13 years, coadministration of anticholinergics or benzodiazepines, lower respiratory tract illness, and American Society of Anesthesiologists-Physical Status (ASA-PS) greater than or equal to III would be associated with an AEs (12, 14–17).
METHODS
Study Design and Data Collection
This study is an observational cohort review of prospectively collected data obtained from the multicenter PSRC database of children 21 years old and younger who received ketamine for PS from September 2007 through November 2015. PSRC data collection methodology has been reported in previous publications (18–20). The PSRC database currently receives data from 44 member institutions ranging from free-standing children’s hospitals to pediatric units within general hospitals (eTable 1, Supplemental Digital Content 1, http://links.lww.com/PCC/A284). Each member institution has the approval of the institutional review board to collect data on a standardized, secure, web-based dynamic data-entry form. Member institutions complete audits biannually to assure complete data collection and to identify systematic errors in data entry. The PSRC changed its online data collection form in November 2011, resulting in changes in the definitions of various adjunctive medications, primary diagnoses, and AEs. Dosing data are only available from September 2007 to November 2011. Only those variables common to both datasets have been used in this study.
Outcomes and AE Measures
The primary outcome measure was the incidence of AEs and SAEs associated with each PS encounter in which ketamine was administered. The secondary outcome measure was the identification of risk factors associated with increased odds of SAEs. AEs are listed and defined in eTable 2 (Supplemental Digital Content 1, http://links.lww.com/PCC/A284). The PSRC a priori defined adverse AEs by committee consensus and are adhered to by all institutions that report data to the PSRC database. SAEs were defined as any one of the following events.
Airway Obstruction
A lack of air movement in spite of respiratory effort, which may be resolved by repositioning of the patient or with a chin lift/jaw thrust maneuver.
Laryngospasm
A complete or near-complete lack of air movement despite respiratory effort and/or stridor not relieved by chin repositioning or oral/nasal airway.
Emergent Airway Intervention
This includes tracheal intubation, positive pressure ventilation, or placement of another airway device such as a nasopharyngeal tube, an oral airway, or a laryngeal mask airway because of prolonged apnea or oxygen desaturation.
Aspiration
The inhalation of secretions and/or gastric contents into the lungs due to emesis or the inability to protect one’s airway.
Cardiac Arrest
The cessation of blood circulation resulting from absent or ineffective cardiac mechanical activity. Clinically, the child is unresponsive and not breathing or only gasping and has no pulse (21).
Although multiple AEs or SAEs could occur within a single sedation encounter, the rate of AEs and SAEs was reported as the number of sedations in which at least one event occurred out of the total number of sedations. Patients could have been sedated more than one time and appear multiple times in the dataset; however, for analysis, multiple sedations on the same patient were considered independent. Multiple sedation episodes in single patients were counted because the PSRC database contains deidentified information precluding removal of a patient appearing for separate sedation encounters. This should not affect the validity of our analysis because each sedation encounter has unique risks and outcomes associated with it, and successive sedation encounters do not pose cumulative risks. Procedures defined as painful are listed in eTable 3 (Supplemental Digital Content 1, http://links.lww.com/PCC/A284).
Statistical Methods
Descriptive statistics were reported as counts and frequencies, medians and interquartile ranges, or means and CIs for patient demographics and sedation procedure characteristics. The percentages of AEs and SAEs were calculated, and 95% CI for these events have been reported. Subgroup analyses of AEs and SAEs were performed by location of sedation, procedure type, medications coadministered with ketamine, compared using chi-square tests. Multivariable logistic regression was used to identify characteristics associated with an increase in the odds of experiencing an adverse event. Resulting risk factors are presented as odds ratios with 95% CIs. Because of the potential for confounding due to patient mix, ASA status, and referral patterns across institutions, adjustment for individual center effect was not performed. Potential risk factors were identified because of their historically demonstrated association with the occurrence of AEs and included location of sedation, primary diagnosis, age, weight, sex, ASA-PS greater than or equal to III, coadministration of adjunctive medications, such as anticholinergics or benzodiazepines, total dose of ketamine greater than 5 mg/kg, performance of a painful procedure, and nil per os for solids and clear liquids. Because age and weight are highly correlated, age was used in the final model. In addition, procedure type and location are highly correlated, and location of sedation was used in the final model. All variables of interest were initially included in the model, and a modified backward elimination procedure was used to systematically remove nonsignificant variables provided that the model fit did not significantly change. Unless otherwise noted, statistical significance was assessed using a significance level of p value less than 0.05, and two-sided statistical tests are reported. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Demographics and Sedation Characteristics
Between September 2007 and November 2015, there were 22,645 sedations using ketamine reported in the PSRC database. Demographic and sedation characteristics are reported in Tables 1 and 2.
Table 1.
Demographic and Clinical Characteristics of Children Sedated With Ketamine (n = 22,645)
| Characteristics | n (%) or Median (IQR) |
|---|---|
| Age (mo) | 60 (31–120) |
| Weight (kg) | 20.2 (13.7–38.0) |
| Male | 12,824 (56.7) |
| American Society of Anesthesiologists-Physical Status (n = 22,387) |
|
| < III | 16,112 (72.0) |
| ≥ III | 6,275 (28.0) |
| NPO clear liquida (n = 20,905) < 2 hr | 144 (0.7) |
| NPO solidsa (n = 20,401) < 6 hr | 1,151 (5.3) |
| Primary diagnosis | |
| Hematology/oncology | 5,619 (24.8) |
| Infection | 3,431 (15.2) |
| Neurologic | 2,984 (13.2) |
| Orthopedics | 2,809 (12.4) |
| Gastrointestinal | 1,696 (7.5) |
| Surgical/wound management | 952 (4.2) |
| Renal | 801 (3.5) |
| Burn | 564 (2.5) |
| Respiratory-lower airway | 412 (1.8) |
| Status post trauma | 379 (1.7) |
| Metabolic/genetic (includes obesity) | 375 (1.7) |
| Dermatologic | 346 (1.5) |
| Rheumatology | 326 (1.4) |
| Cardiovascular | 270 (1.2) |
| Craniofacial abnormalities | 219 (1.0) |
| Liver disease | 194 (0.9) |
| Dental | 125 (0.6) |
| Otherb | 1,652 (7.3) |
IQR = interquartile range, NPO = nil per os.
NPO for clear liquids < 2 hr or solids < 6 hr mainly occurred in the emergency department (45%).
Other includes the following diagnoses: status post transplant, congenital conditions, respiratory-upper airway, immune compromise, and prematurity related.
Table 2.
Summary of Pediatric Procedural Sedation Characteristics Performed Using Ketamine
| Characteristics |
n (%) or Median (IQR) |
|---|---|
| Route of administrationa (n = 22,630), n (%) | |
| IV only | 20,919 (92.4) |
| IM only | 1,256 (5.6) |
| Intranasal only | 182 (0.8) |
| PO only | 167 (0.7) |
| Multiple routes | 94 (0.4) |
| Other route only | 12 (0.1) |
| Medications, n (%) | |
| Ketamine alone (no coadministered medications) |
3,934 (17.4) |
| Benzodiazepine (midazolam and lorazepam) |
13,112 (57.9) |
| Propofol | 8,012 (35.4) |
| Anticholinergics (atropine and glycopyrrolate) |
4,485 (19.8) |
| Local lidocaine | 1,453 (6.4) |
| Dexmedetomidine | 1,146 (5.1) |
| Barbiturate (pentobarbital, methohexital, and thiopental) |
71 (0.3) |
| Chloral hydrate | 7 (0.0) |
| Etomidate | 5 (0.0) |
| Doseb (mg/kg), mean (sd) | |
| IV (n = 8,725) | 1.6 (1.4) |
| IM (n = 766) | 3.5 (1.6) |
| PO (n = 102) | 5.2 (1.7) |
| IN (n = 5) | 2.1 (1.5) |
| Total average dose (n = 9,585) | 1.8 (1.6) |
| Location, n (%) | |
| Radiology/sedation unit | 14,625 (64.6) |
| Emergency department | 2,738 (12.1) |
| Speciality clinic/floor | 1,789 (7.9) |
| PICU | 1,535 (6.8) |
| Cath laboratory | 38 (0.2) |
| Dental | 37 (0.2) |
| Otherc | 1,883 (8.3) |
| Provider, n (%) | |
| Intensivist | 13,468 (59.5) |
| EM | 5,247 (23.2) |
| Anesthesiology | 788 (3.5) |
| Hospitalist | 428 (1.9) |
| Otherd | 2,714 (12.0) |
| Procedure, n (%) | |
| Radiology | 5,551 (24.5) |
| Surgical | 5,169 (22.8) |
| Hematology/oncology | 4,685 (20.7) |
| Bone/musculoskeletal | 3,278 (14.5) |
| Gastroenterology | 1,350 (6.0) |
| Neurologic | 877 (3.9) |
| Cardiac | 185 (0.8) |
| Dental | 132 (0.6) |
| Othere | 2,175 (9.6) |
| Painful proceduref | 15,826 (69.9) |
| Procedure not completeda (n = 19,747) | 39 (0.2) |
IM = intramuscular, IN = intranasal, IQR = interquartile range, PO = per os.
Indicates missing data where n is the total number of sedation encounters for which data are entered.
Dosing data are only available from 2007 to 2011 in the Pediatric Sedation Research Consortium database.
Other location includes an operating room, an endoscopy suite, and a radiation oncology unit.
Other provider includes advanced-practice registered nurses, dentists, fellows, medical technologists, nonphysician anesthetists, nurse anesthetists, oral surgeons, pediatricians, radiologists, surgeons.
Others include airway, upper endoscopy, not defined.
Painful procedures are defined in eTable 3 (Supplemental Digital Content 1, http://links.lww.com/PCC/A284).
AEs and SAEs
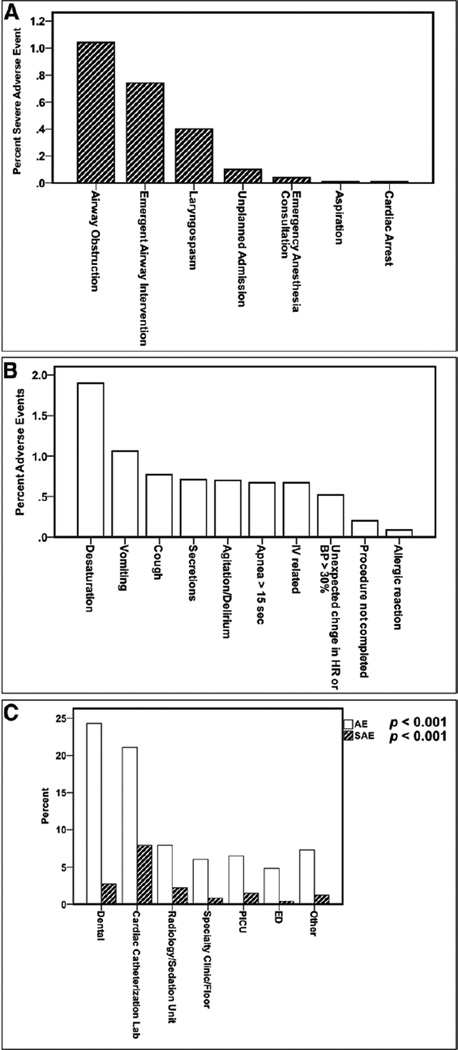
Of the 22,645 ketamine sedations, there were 1,643 sedations where at least one AE was reported (7.3%; 95% CI, 6.92–7.60%), with 2,202 total AEs reported. There were 401 sedations where at least one SAE occurred (1.77%; 95% CI, 1.60–1.94%), with 532 total SAEs reported. Figures 1, A and B show the percentages of SAEs and AEs most commonly associated with ketamine administration. AEs and SAEs varied considerably by the location of sedation (Fig. 1C) (p < 0.001 for both AEs and SAEs rates).
Figure 1.
A, The percentage of occurrence of individual severe adverse events (SAEs) and B, most frequently occurring adverse events (AEs) associated with ketamine sedation. No deaths occurred. C, The percentage of occurrence of any AEs or SAEs by location of sedation. Analysis of variance was performed resulting in a p < 0.001 for both AEs and SAEs. BP = blood pressure, ED = emergency department, HR = heart rate.
Cardiac Arrest Occurring During PS
Although no deaths were reported, cardiac arrest requiring cardiopulmonary resuscitation occurred in three patients. The first patient was 13 months old with a neurologic disorder and an ASA-PS II undergoing a radiology procedure in a radiology/sedation unit using ketamine and propofol. This child experienced laryngospasm requiring unplanned endotracheal intubation and ICU admission. The second patient was also 13 months old with an infection (site not specified) and an ASA-PS II undergoing a painful surgical procedure in a radiology/sedation unit with ketamine and propofol. This patient was reported to have coughing, laryngospasm, and an emergent anesthesiology consultation, resulting in endotracheal intubation and ICU admission. The third patient was 25 months old with a neurologic disorder and an ASA-PS I undergoing a radiology procedure in a radiology/sedation unit with ketamine and dexmedetomidine. This child experienced laryngospasm, but no further information was reported.
Aspiration Events During PS
The first patient was 3 years old with an ASA-PS III, a history of a transplant, and a gastrointestinal disorder and was undergoing a gastrointestinal procedure in a radiology/sedation unit using ketamine, propofol, and an anticholinergic. This child experienced an aspiration event accompanied by secretions, emergent endotracheal intubation, and an unplanned admission. The second patient was 7 years old with a gastrointestinal disorder and an ASA-PS II and was undergoing a gastrointestinal procedure in a radiology/sedation unit with ketamine and propofol. This patient was reported to have secretions, laryngospasm, and an aspiration event and required suctioning, repositioning, bag-mask ventilation, and emergency placement of an endotracheal tube. The third patient was 4 years old with burns and an ASA-PS II and was undergoing a painful procedure in a radiology/sedation unit with ketamine, propofol, and dexmedetomidine. This child experienced an aspiration event along with airway obstruction and coughing. Continuous positive airway pressure, jaw thrust/chin lift, bag-mask ventilation, suctioning, and repositioning were required interventions. All three patients were in compliance with ASA NPO guidelines.
AEs and SAEs by Age
There were more AEs (p < 0.001), but not SAEs (p = 0.997), in the age group of 0–3 months and in the age group over 13 years (eTable 4, Supplemental Digital Content 1, http://links.lww.com/PCC/A284).
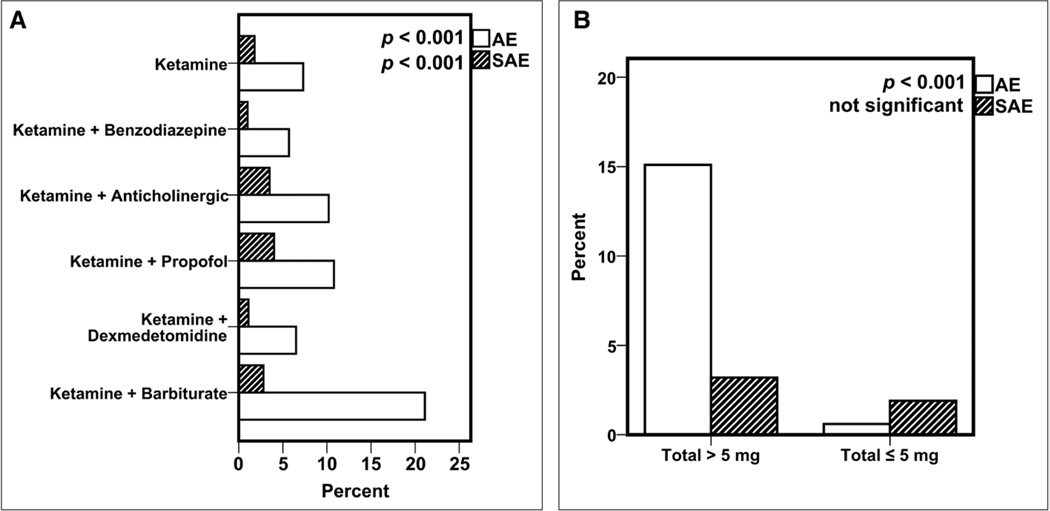
AEs and SAEs by Coadministration of Adjunctive Medications and Total Dose
The percentages of AEs and SAEs during sedations performed with ketamine alone were compared to the coadministration of ketamine with adjunctive medications (Fig. 2A). There was no significant difference in the report of agitation in the population of patients receiving benzodiazepine in conjunction with ketamine versus ketamine alone. The coadministration of benzodiazepine was associated with decreased odds of AEs and SAEs, whereas the coadministration of propofol, an anticholinergic, or a barbiturate was associated with increased AEs and SAEs (Fig. 2A). A total IV administered weight-adjusted dose of more than 5 mg/kg was associated with a statistically significant higher percentage of AEs and SAEs (Fig. 2B). A total IV administered weight-adjusted dose of more than 2.5 mg/kg was also associated with a significant increase in AEs (6.8% vs 4.9%; p = 0.008), but not SAEs (1.8% vs 1.7%; p = 0.962). However, we were not able to determine an optimal threshold above which AEs are more likely to occur. Because of the low number of SAEs, the study is not powered to detect differences in SAEs based on limited availability of dosing data. There was no difference in median total weight-adjusted dose of ketamine administered in patients who received benzodiazepine (1.40 mg/kg) compared with those patients who did not receive benzodiazepine (1.46 mg/kg). In addition, the proportion of patients who received more than 5 mg/kg of ketamine did not differ whether or not they received benzodiazepine (3.3% in both groups).
Figure 2.
A, The percentage of adverse events (AEs) and severe adverse events (SAEs) by coadministration of adjunctive medications. B, The percentage of AEs and SAEs for weight-adjusted total IV ketamine dose of greater than 5 mg/kg versus less than or equal to 5 mg/kg, regardless of coadministered medications. Data (in B) are from the 2007–2011 subcohort of patients for which dosing data are available.
Risk Factors for AEs
The final multiple variable logistic model for any AEs and SAEs is reported in Table 3. After adjusting for age, painful procedure, adjunctive medication coadministration, lower respiratory tract illness, and primary diagnosis, the location of sedation was still a significant predictor of AEs but not SAEs. Patients undergoing a painful procedure had significantly lower odds of SAEs than those not undergoing a painful procedure (odds ratio, 0.78; 95% CI, 0.62–0.97). Benzodiazepine administration as an adjunctive medication was associated with a decrease in the odds of SAEs (odds ratio, 0.42; 95% CI, 0.33–0.53); however, the use of propofol, anticholinergics, and barbiturates was associated with increased odds of SAEs (Table 3).
Table 3.
Multivariable Logistic Regression: Risk Factors for an Adverse Event and Severe Adverse Events
| Effect | OR Any AEs |
95% CI Any AEs |
OR Any SAEs |
95% CI Any SAEs |
|---|---|---|---|---|
| Age | ||||
| 0–3 mo | 1.01 | 0.68–1.52 | 1.23 | 0.51–2.92 |
| 4–12 mo | 0.79 | 0.63–0.99 | 1.05 | 0.65–1.70 |
| 1–13 yr | 0.86 | 0.75–1.00 | 0.34 | 0.8–1.84 |
| > 13 yr | Reference | Reference | ||
| American Society of Anesthesiologists- Physical Status |
||||
| ≥ III | 1.11 | 0.98–1.25 | 1.26 | 0.99–1.62 |
| < III | Reference | Reference | ||
| Location | ||||
| Cardiac catheterization laboratory | 1.19 | 0.49–2.89 | 1.61 | 0.36–7.14 |
| Dental suite | 3.75 | 1.74–8.06 | 1.39 | 0.18–10.48 |
| Emergency department | 0.71 | 0.57–0.88 | 0.42 | 0.21–0.84 |
| Other | 0.99 | 0.81–1.21 | 0.72 | 0.46–1.13 |
| PICU | 0.82 | 0.66–1.03 | 0.85 | 0.54–1.33 |
| Specialty floor/clinic | 1.13 | 0.91–1.41 | 0.64 | 0.37–1.12 |
| Radiology/sedation | Reference | Reference | ||
| Painful procedure: yes | 0.88 | 0.78–0.99 | 0.78 | 0.62–0.97 |
| Adjunctive medication | ||||
| Benzodiazepine | 0.61 | 0.54–0.68 | 0.42 | 0.33–0.53 |
| Anticholinergic | 1.67 | 1.48–1.89 | 2.92 | 2.35–3.63 |
| Propofol | 1.79 | 1.59–2.02 | 5.36 | 4.08–7.05 |
| Barbiturate | 3.34 | 1.83–6.12 | 2.03 | 0.48–8.58 |
| Primary diagnosis | ||||
| Cardiology | 2.38 | 1.64–3.46 | 1.41 | 0.60–3.31 |
| Hematology/oncology | 0.57 | 0.49–0.67 | 0.63 | 0.46–0.85 |
| Gastrointestinal | 1.40 | 1.18–1.65 | 1.10 | 0.79–1.53 |
| Lower respiratory illness | 1.67 | 1.23–2.27 | 1.53 | 0.86–2.71 |
AEs = adverse events, OR = odds ratio, SAEs = serious adverse events.
Boldface values denote statistical significance, p < 0.05.
DISCUSSION
This study is a description of prospectively collected data on a cohort of children undergoing PS using ketamine outside of the OR. The incidence of SAEs was low (1.77%) in this patient population receiving PS care from experienced sedation services within the PSRC.
In contrast to the PSRC propofol cohort (18), ketamine was used for painful procedures (ketamine 69.9% vs propofol 48.3%), the incision and drainage of an abscess (ketamine 15.2% vs propofol 4.9%), and orthopedic procedures (ketamine 12.4% vs propofol 2.9%) where the need for immobility is not required as in imaging studies. The performance of painful procedures has been shown to decrease AEs when propofol alone is administered as the sedative (18). However, in this study, the use of propofol with ketamine was associated with an increase in SAEs. We speculate that perhaps due to the analgesia provided by ketamine, the protective effect of a painful stimulus during PS is mitigated. Results of two emergency department (ED)-based, single-center studies did not show a difference in adverse respiratory events when comparing “ketofol” with propofol or ketamine alone (22–24). Given that serious adverse respiratory events are rare, we speculate that the studies comparing the combination of ketamine + propofol to either anesthetic agent alone may be underpowered to detect differences in serious adverse respiratory outcomes when fewer than 200 patients were enrolled.
In a meta-analysis of 8,282 children (32 ED studies) using ketamine for PS in the ED, Green et al (15) report risk factors associated with adverse respiratory events, emesis, and recovery agitation including high initial IV doses (≥ 2.5 mg/kg) or total dose (≥ 5 mg/kg), coadministered benzodiazepine or anticholinergic, and an age less than 2 years old or greater than 13 years old. Oropharyngeal procedures, such as dental and upper endoscopy procedures, have been associated with an increased incidence of laryngospasm (10, 25, 26); however, in the prospective subset of Green et al (16) meta-analysis, there were no significantly higher odds of laryngospasm for minor (i.e., no upper endoscopy or airway manipulation) oropharyngeal procedures. In contrast, we found a significant increase in SAEs, including airway obstruction, laryngospasm, and a need for emergent airway intervention, in our cohort of patients undergoing dental procedures. Because dental sedations comprised only 0.6% of the overall sedations performed using ketamine, the higher incidence of SAEs that occurred with dental sedations may be due to an under-reporting of dental sedation in the PSRC database. The overall difference in the incidence of AEs and SAEs between Green et al (16) meta-analysis (involving studies from ED) and the current study may be attributable to the less stimulating types of oropharyngeal procedures performed in a shorter amount of time in the ED on an emergency basis that likely differ from those performed electively, over a longer period of time, that contribute to the increased number of AEs.
Coadministration of anticholinergics has been advocated to mitigate the increased secretions and subsequent instigation of a cough, airway obstruction, and laryngospasm associated with ketamine (26–28). Despite the widespread practice of using anticholinergics with ketamine to decrease secretions, we have information from Green et al (10, 15, 16) meta-analyses, and now from our current study, that administration of adjunctive anticholinergic is associated with an increase in the odds of AEs and SAEs and sedation providers should consider forgoing such a practice. It is not possible from the PSRC database to determine whether the anticholinergic was administered therapeutically for secretions or prophylactically to prevent the development of excessive secretions.
Some randomized control studies and a meta-analysis have addressed the coadministration of benzodiazepines, principally midazolam, with ketamine (15, 29, 30). Several studies have shown that there is no clear benefit to coadministration with benzodiazepine to prevent or ameliorate recovery agitation associated with ketamine (10, 31). Kennedy and McAllister (32) have argued that if midazolam were administered before ketamine to decrease presedation agitation, the results of the above mentioned randomized control trials might have been different. In contrast with the earlier ED studies (30, 33), the current analysis indicates that the coadministration of benzodiazepine with ketamine for pediatric PS was associated with a significant decrease in the odds of having AEs or SAEs. It is not clear why coadministration of benzodiazepines are associated with decreased odds of having AEs and SAEs in the current study, and further research is required to confirm this finding. It is unclear whether benzodiazepine was administered prophylactically to prevent ketamine-related agitation or for emergence postsedation.
Ketamine is also associated with vomiting, especially in the early adolescent age group (31, 34, 35). The reported incidence of vomiting in children sedated with ketamine ranges from 8.4% to 30% (24, 35). We speculate that the occurrence of vomiting in the PSRC cohort is so much lower than that in earlier reports due to the adherence to the American Academy of Pediatrics NPO guidelines in our cohort; however, two ED PS studies found no association between preprocedural fasting times and vomiting (36, 37). The lower rates of vomiting and recovery agitation in our study could also be because these AEs occur in the postsedation period and are not captured in our data collection model.
There are several limitations to this study. Institutions voluntarily contributing to the PSRC are high-performance sedation services, making the generalizability of our data uncertain. This is an observational study, and as such, our subjects were not randomized, and data recorders were not blinded to drugs administered. We attempted to control for confounders using a logistic regression statistical model. Specific patient monitoring devices, such as pulse oximetry, capnography, and noninvasive blood pressure and heart rate monitoring, are recommended during sedation, but these are not mandated by the PSRC. Additionally, depth of sedation and vital sign data are not recorded in the database, limiting the unbiased reporting of AEs and SAEs as well as the adequacy of sedation depth. Although SAEs are clearly defined and agreed upon, there is still the potential for bias in the consistency of reporting AEs by sedation providers. Only limited pre-, intra-, and immediate postsedation information is available in the database, and as such there is no longer term neurologic follow-up data available for those patients who experienced SAEs. Dosing data were only available from September 2007 to November 2011.
CONCLUSIONS
This study describes a large cohort of ketamine PS in children performed outside of the OR within the PSRC. In accord with our hypothesis, the coadministration of anticholinergics, the presence of lower respiratory tract illness, and a higher ASA-PS greater than or equal to III were associated with increased AEs during ketamine sedation. Surprisingly the coadministration of benzodiazepines with ketamine decreased the odds of both AEs and SAEs. In addition, the coadministration of propofol and underlying cardiac/gastrointestinal disease were associated with increased odds of AEs during ketamine sedation.
Supplementary Material
Acknowledgments
We thank the Emory+Children’s Biostatistics Core for their help with statistical analysis.
Dr. Grunwell is supported by a T32GM095442.
Footnotes
Drs, Grunwell and Kamat conceived and developed the study and wrote the article. Drs. Travers and McCracken conducted statistical analyses and edited the article. Drs. Scherrer, Stormorken, Chumpitazi, Roback, and Stockwell edited the article. All authors read and approved the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Petrack EM, Marx CM, Wright MS. Intramuscular ketamine is superior to meperidine, promethazine, and chlorpromazine for pediatric emergency department sedation. Arch Pediatr Adolesc Med. 1996;150:676–681. doi: 10.1001/archpedi.1996.02170320022003. [DOI] [PubMed] [Google Scholar]
- 2.Peña BM, Krauss B. Adverse events of procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 1999;34:483–491. doi: 10.1016/s0196-0644(99)80050-x. [DOI] [PubMed] [Google Scholar]
- 3.Ip U, Saincher A. Safety of pediatric procedural sedation in a Canadian emergency department. CJEM. 2000;2:15–20. doi: 10.1017/s1481803500004346. [DOI] [PubMed] [Google Scholar]
- 4.Evered LM. Procedural sedation and analgesia for paediatric patients in the emergency department. Paediatr Child Health. 2003;8:503–507. doi: 10.1093/pch/8.8.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roback MG, Wathen JE, MacKenzie T, et al. A randomized, controlled trial of i.v. versus i.m. ketamine for sedation of pediatric patients receiving emergency department orthopedic procedures. Ann Emerg Med. 2006;48:605–612. doi: 10.1016/j.annemergmed.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Munro A, Machonochie I. Midazolam or ketamine for procedural sedation of children in the emergency department. Emerg Med J. 2007;24:579–580. doi: 10.1136/emj.2007.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacchetti A, Stander E, Ferguson N, et al. Pediatric Procedural Sedation in the Community Emergency Department: Results from the ProSCED registry. Pediatr Emerg Care. 2007;23:218–222. doi: 10.1097/PEC.0b013e31803e176c. [DOI] [PubMed] [Google Scholar]
- 8.Dallimore D, Herd DW, Short T, et al. Dosing ketamine for pediatric procedural sedation in the emergency department. Pediatr Emerg Care. 2008;24:529–533. doi: 10.1097/PEC.0b013e318180fdb5. [DOI] [PubMed] [Google Scholar]
- 9.Borland M, Esson A, Babl F, et al. Procedural sedation in children in the emergency department: A PREDICT study. Emerg Med Australas. 2009;21:71–79. doi: 10.1111/j.1742-6723.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 10.Green SM, Roback MG, Kennedy RM, et al. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57:449–461. doi: 10.1016/j.annemergmed.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Dachs RJ, Innes GM. Intravenous ketamine sedation of pediatric patients in the emergency department. Ann Emerg Med. 1997;29:146–150. doi: 10.1016/s0196-0644(97)70321-4. [DOI] [PubMed] [Google Scholar]
- 12.Green SM, Rothrock SG, Lynch EL, et al. Intramuscular ketamine for pediatric sedation in the emergency department: Safety profile in 1,022 cases. Ann Emerg Med. 1998;31:688–697. doi: 10.1016/s0196-0644(98)70226-4. [DOI] [PubMed] [Google Scholar]
- 13.Parker RI, Mahan RA, Giugliano D, et al. Efficacy and safety of intravenous midazolam and ketamine as sedation for therapeutic and diagnostic procedures in children. Pediatrics. 1997;99:427–431. doi: 10.1542/peds.99.3.427. [DOI] [PubMed] [Google Scholar]
- 14.Malviya S, Voepel-Lewis T, Tait AR. Adverse events and risk factors associated with the sedation of children by nonanesthesiologists. Anesth Analg. 1997;85:1207–1213. doi: 10.1097/00000539-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Green SM, Roback MG, Krauss B, et al. Emergency Department Ketamine Meta-Analysis Study Group. Predictors of airway and respiratory adverse events with ketamine sedation in the emergency department: An individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54:158–168. e151–e154. doi: 10.1016/j.annemergmed.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Green SM, Roback MG, Krauss B Emergency Department Ketamine Meta-Analysis Study Group. Laryngospasm during emergency department ketamine sedation: A case-control study. Pediatr Emerg Care. 2010;26:798–802. doi: 10.1097/PEC.0b013e3181fa8737. [DOI] [PubMed] [Google Scholar]
- 17.Melendez E, Bachur R. Serious adverse events during procedural sedation with ketamine. Pediatr Emerg Care. 2009;25:325–328. doi: 10.1097/PEC.0b013e3181a341e0. [DOI] [PubMed] [Google Scholar]
- 18.Kamat PP, McCracken CE, Gillespie SE, et al. Pediatric critical care physician-administered procedural sedation using propofol: A report from the Pediatric Sedation Research Consortium Database. Pediatr Crit Care Med. 2015;16:11–20. doi: 10.1097/PCC.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 19.Cravero JP, Beach ML, Blike GT, et al. Pediatric Sedation Research Consortium. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: A report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108:795–804. doi: 10.1213/ane.0b013e31818fc334. [DOI] [PubMed] [Google Scholar]
- 20.Cravero JP, Blike GT, Beach M, et al. Pediatric Sedation Research Consortium. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: Report from the Pediatric Sedation Research Consortium. Pediatrics. 2006;118:1087–1096. doi: 10.1542/peds.2006-0313. [DOI] [PubMed] [Google Scholar]
- 21.de Caen AR, Berg MD, Chameides L, et al. Part 12: Pediatric advanced life support: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S526–S542. doi: 10.1161/CIR.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David H, Shipp J. A randomized controlled trial of ketamine/propofol versus propofol alone for emergency department procedural sedation. Ann Emerg Med. 2011;57:435–441. doi: 10.1016/j.annemergmed.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Shah A, Mosdossy G, McLeod S, et al. A blinded, randomized controlled trial to evaluate ketamine/propofol versus ketamine alone for procedural sedation in children. Ann Emerg Med. 2011;57:425–433.e2. doi: 10.1016/j.annemergmed.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 24.Green SM, Andolfatto G, Krauss B. Ketofol for procedural sedation? Pro and con. Ann Emerg Med. 2011;57:444–448. doi: 10.1016/j.annemergmed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Green SM, Klooster M, Harris T, et al. Ketamine sedation for pediatric gastroenterology procedures. J Pediatr Gastroenterol Nutr. 2001;32:26–33. doi: 10.1097/00005176-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Green SM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation in children. Ann Emerg Med. 2004;44:460–471. doi: 10.1016/S0196064404006365. [DOI] [PubMed] [Google Scholar]
- 27.Reich DL, Silvay G. Ketamine: An update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36:186–197. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- 28.White PF, Way WL, Trevor AJ. Ketamine–its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–136. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Sherwin TS, Green SM, Khan A, et al. Does adjunctive midazolam reduce recovery agitation after ketamine sedation for pediatric procedures? A randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2000;35:229–238. doi: 10.1016/s0196-0644(00)70073-4. [DOI] [PubMed] [Google Scholar]
- 30.Wathen JE, Roback MG, Mackenzie T, et al. Does midazolam alter the clinical effects of intravenous ketamine sedation in children? A double-blind, randomized, controlled, emergency department trial. Ann Emerg Med. 2000;36:579–588. doi: 10.1067/mem.2000.111131. [DOI] [PubMed] [Google Scholar]
- 31.Green SM, Roback MG, Krauss B, et al. Emergency Department Ketamine Meta-Analysis Study Group. Predictors of emesis and recovery agitation with emergency department ketamine sedation: An individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54:171–180.e1. doi: 10.1016/j.annemergmed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy RM, McAllister JD. Midazolam with ketamine: Who benefits? Ann Emerg Med. 2000;35:297–299. [PubMed] [Google Scholar]
- 33.Roback MG, Wathen JE, Bajaj L, et al. Adverse events associated with procedural sedation and analgesia in a pediatric emergency department: A comparison of common parenteral drugs. Acad Emerg Med. 2005;12:508–513. doi: 10.1197/j.aem.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Langston WT, Wathen JE, Roback MG, et al. Effect of ondansetron on the incidence of vomiting associated with ketamine sedation in children: A double-blind, randomized, placebo-controlled trial. Ann Emerg Med. 2008;52:30–34. doi: 10.1016/j.annemergmed.2008.01.326. [DOI] [PubMed] [Google Scholar]
- 35.Green SM, Kuppermann N, Rothrock SG, et al. Predictors of adverse events with intramuscular ketamine sedation in children. Ann Emerg Med. 2000;35:35–42. doi: 10.1016/s0196-0644(00)70102-8. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal D, Manzi SF, Gupta R, et al. Preprocedural fasting state and adverse events in children undergoing procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 2003;42:636–646. doi: 10.1016/s0196-0644(03)00516-x. [DOI] [PubMed] [Google Scholar]
- 37.Roback MG, Bajaj L, Wathen JE, et al. Preprocedural fasting and adverse events in procedural sedation and analgesia in a pediatric emergency department: Are they related? Ann Emerg Med. 2004;44:454–459. doi: 10.1016/j.annemergmed.2004.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.