Abstract
Endothelial cell barrier function plays a prevalent regulatory mechanism for the integrity and homeostasis of blood vessels and modulates angiogenesis and immune responses. Cell adhesion molecules (CAMs) play a central role in the barrier function of endothelial cells. Although immunoglobulin containing and proline-rich receptor-1(IGPR-1) was recently identified as a novel CAM expressed in endothelial cells, the molecular mechanisms underlying the function of IGPR-1 in endothelial cells remain uncharacterized. In this report, we investigated the role of IGPR-1 in endothelial cell barrier function and the molecular mechanism of its activation in endothelial cells. We demonstrate that IGPR-1 is localized to endothelial adherens junctions, and through trans-homophilic dimerization regulates endothelial cell-cell adhesion and barrier function. Trans-homophilic dimerization of IGPR-1 stimulates phosphorylation of serine 220 (Ser220), which is required for IGPR-1 to regulate endothelial barrier function and angiogenesis. Moreover, IGPR-1 chimera, which mimics the trans-homophilic dimerization of IGPR-1, induced a sustained phosphorylation of Ser220 upon stimulation with a ligand. Coordinated dimerization of IGPR-1 and its homophilic interaction modulates its adhesive function and Ser220 phosphorylation. This adhesive function of IGPR-1 contributes to the barrier function of endothelial cells.
Graphical abstract
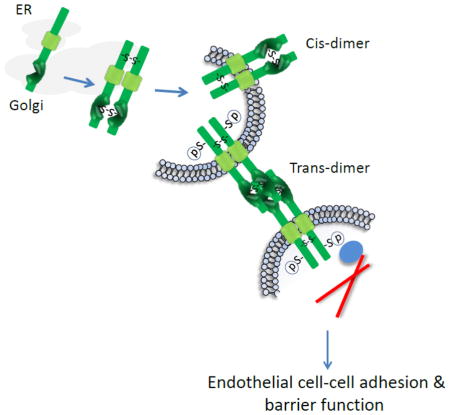
IGPR-1 is localized to endothelial adherens junctions and through trans-homophilic dimerization regulates endothelial cell-cell adhesion and barrier function. Trans-homophilic dimerization of IGPR-1 stimulates phosphorylation of serine 220 (Ser220), which is required for IGPR-1 to regulate endothelial barrier function and angiogenesis.
pSer220 likely recruits signaling proteins to IGPR-1 and links IGPR-1 to actin fibril assembly.
Introduction
Blood vessels are lined with a single layer of endothelial cells (ECs), which create a dynamic barrier between the blood and underlying tissue. The structural and functional integrity of ECs is essential for the physiological function of blood vessels, and their altered function plays a pivotal role in the pathogenesis of human diseases ranging from cancer to inflammation and diabetes and other human diseases [1–3]. Cell adhesion molecules (CAMs) are the key mediators of endothelial barrier function. CAMs mediate cell-cell, cell-matrix adhesion and transmit signals across the plasma membrane to process information from the extracellular environment involved in tissue morphogenesis, angiogenesis, and tumor progression [4, 5]. Cadherins, integrins, selectins and immunoglobulin- (Ig) like cell adhesion proteins (Ig-CAMs) are major CAMs present in the human genome [4, 6]. Ig-CAMs are cell surface glycoproteins with one or more Ig domains in the extracellular domain, a single transmembrane domain and a C-terminal intracellular domain. The Ig-containing extracellular domain of Ig-CAMs commonly mediates homophilic interaction by binding to the same structure on an opposing cell surface, or other cell surface receptors via heterophilic dimerization[7]. The C-terminal intracellular domains, on the other hand, interact with signaling proteins that regulate cell morphology and other cellular functions.
Ig containing and proline-rich receptor-1 (IGPR-1) is a newly identified Ig-CAM expressed by epithelial and endothelial cells. IGPR-1 is a transmembrane glycoprotein that consists of an extracellular domain with a single Ig domain, a single transmembrane domain, and a highly conserved intracellular domain. The C-terminal intracellular domain is highly enriched in proline followed by serine residues that have the potential to undergo phosphorylation. The intracellular domain of IGPR-1 interacts with multiple signaling proteins containing a Src homology 3 (SH3) domain, includingSPIN90/WISH to mediate the biological function of IGPR-1. IGPR-1 expression is highly conserved in higher mammals, but no obvious homolog of IGPR-1 is found in mouse or rat [8]. However,, transmembrane and immunoglobulin domain-containing 1 (TMIGD1), which is the second member of the IGPR-1 family of proteins, is expressed both in humans and rodents [9]. Ectopic expression of IGPR-1 in porcine aortic endothelial (PAE) cells increased cell aggregation and deletion of the extracellular domain of IGPR-1 abrogated its adhesive function, thereby demonstrating that IGPR-1 acts as a putative CAM in endothelial cells [8]. IGPR-1 regulates angiogenesis as capillary tube formation of endothelial cells was increased by its upregulation and decreased by its downregulation by siRNA [8]. In addition to its adhesive function, it was recently reported that IGPR-1 binds to HHLA2, a member of the B7 family of costimulatory molecules involved in the activation and downregulation of T lymphocytes [10, 11].
In this current study, we demonstrate that IGPR-1 is present in cis- and trans-dimeric forms, and through trans-homophilic dimerization regulates endothelial cell barrier function and adhesion. IGPR-1 is phosphorylated on multiple serine residues and its trans-homophilic dimerization stimulates Ser220 phosphorylation. The study thus identifies IGPR-1 as a novel and important player in endothelial cell adhesion and barrier function.
Materials and Methods
Cells, antibodies and other chemicals
Porcine aortic endothelial cells (PAE) were maintained in 10%FBS plus antibiotics in DMEM cell culture media. Human umbilical vein endothelial cells (HUVECs) were maintained in the complete endothelial cell growth medium (purchased from ENZO). HEK-293 (human embryonic kidney epithelial) cells were grown in 10%FBS in DMEM plus antibiotics. Rabbit polyclonal pan anti-IGPR-1 antibody was developed against peptides corresponding to the cytoplasmic domain of IGPR-1 as previously described [8]. Anti-phospho-serine 220 IGPR-1 (pSer220) antibody was developed using a peptide containing phospho-serine 220 and further purified by peptide affinity chromatography using phospho-serine220 containing peptide. The pSer220 antibody was used (1:10,000 dilution) in western blotting to detect Ser220 phosphorylation of IGPR-1 in the whole cell lysates derived from PAE cells or HEK-293 cells ectopically overexpressing IGPR-1. The same antibody (1:750 dilutions) was used to detect phosphorylated IGPR-1 in the whole cell lysates of HUVECs. The mouse monoclonal blocking IGPR-1 antibody was developed by injecting B16F cells ectopically expressing IGPR-1 into mice. Hybridomas were generated and three clones (1A12, 4F10 and 4D5) were isolated and further validated for their ability to block IGPR-1 activity. For blocking phosphorylation of Ser220, cells were treated with the blocking antibodies (1μg/ml) overnight. In some experiments, the blocking antibody was coupled to polystyrene beads (Sigma, cat # 796-33-5ml-F). Anti-FLAG antibody (cat#2368) and anti-c-Myc antibody (71D10, cat# 2278) were purchased from Cell Signaling.
Plasmids
Human IGPR-1 and ΔN-IGPR-1 (IGPR-1 without extracellular domain) were cloned into retroviral pMSCV vector as previously described [8]. Human IGPR-1 cDNA was used as a template to generate C-terminal tagged c-Myc and FLAG using standard PCR strategy. The following primers were used to generate the IGPR1-c-myc construct in pQCXIP-c-myc modified vector via Not1 and BamH1: Sense primer: 5′CTCGAGCGGCCGCAGTCTGTCAACTGGGAGGGGGA3′; Anti-sense primer: 5′GAGCTCGGATCCTCCTCTCCCACTTTGGGGAA3′. The following primers were used to generate IGPR1-FLAG tag construct in pQCXIP vector via NotI and BamHI. Sense primer: 5′CTCGAGCGGCCGCAGTCTGTCAACTGGGAGGGGGA3′ Anti-sense 5′GAGCTCGGATCCTACTTATCGTCGTCATCCTTGTAATCCTCCTCTCCC ACTTTGGGGAA3′. The following primers were used to generate the chimeric IGPR-1 (cIGPR-1) construct in pQCXIP-c-Myc vector.
CSF-1R-NotI-S: 5′GCACCGCGGCCGCACTTCCCCACCGAGGCCAT3′
CSF-1R/GPR1-AS: 5′ TCCTGGGAAGGGATGCGTGTGGGCTCCTGC3′
CSFR-1/GPR1-S: 5′ACGCATCCCTT CCAGGATTCCTCTTCGTGCTG3′
IGPR-1/BamHI-AS: 5′GAGCTCGGATCCTCCTCTCCCACTTTGGGGAA3′.
The C-terminal truncated IGPR-1 and serine mutant IGPR-1 were constructed using a similar strategy. All the constructs used in this study were sequenced to confirm their correct sequences. The following IGPR-1 siRNA oligos were used for silencing of IGPR-1 in human endothelial cells: IGPR-1-siRNA (#1): CAG CAA AGG GAC UCA GGU AUU, IGPR-1-siRNA (#2): AGG UAA CAG CCC AGG AAA UUU.
Retroviral virus production and Cell culture
PAE cells were grown in DMEM plus 10% FBS and antibiotics as described [12]. 293-GPG packaging cells were used to generate virus as described [12]. All the cells used in this study were free of contamination.
Recombinant GST-fusion IGPR-1 protein production and GST-pull down assay
To generate the glutathione S-transferase (GST) fusion extracellular domain of IGPR-1 (GST-E-IGPR-1), the extracellular domain of IGPR-1 was PCR amplified and cloned into pGEX-2T vector. The purified GST-fusion-IGPR-1 protein was subsequently used for GST pull-down assay, which was performed as described [13]. Other uses are described in the corresponding figure legends.
Disulfide bond dimerization of IGPR-1
PAE cells expressing IGPR-1, truncated IGPR-1, or control empty vector were lysed in the presence of the alkylating agent, iodoacetamide. Whole cell lysates were mixed with sample buffer (5X) with or without β-mercaptoethanol and resolved in SDS-PAGE followed by western blot analysis using anti-IGPR-1 antibody. In some experiments, the GST-extracellular domain of IGPR-1 (GST-E-IGPR-1) protein was produced in E- coli. The purified recombinant GST-E-IGPR-1 was similarly prepared in sample buffer with or without β-mercaptoethanol and resolved by SDS-PAGE.
Cell culture assays
To determine dimerization of IGPR-1, PAE expressing empty vector (pMSCV), IGPR1-myc or IGPR1-FLAG were cultured in 10 cm plates for 3 days until reaching close to 90–100% confluency. Cells were lysed in lysis buffer (10 mmol/L Tris-HCl, 10% glycerol, pH 7.4, 5 mmol/L EDTA, 50 mmol/L NaCl, 50 mmol/L NaF, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, 2 mmol/L Na3VO4, and 20 mg/mL aprotinin). Cell lysates derived from PAE cells expressing pMSCV and IGPR1-FLAG were subjected to immunoprecipitation with FLAG antibody for about 90 minutes, followed by a 60 minutes incubation with Protein G sepharose. Immunoprecipitated proteins were washed in lysis buffer (3X) then incubated with whole cell lysates of PAE cells expressing IGPR1-c-Myc. After 90 minutes, the co-immunoprecipitated proteins were washed in lysis buffer (3X) and resolved on 10% SDS-PAGE. The membrane was immunoblotted with rabbit anti-c-Myc antibody (71D10, cell signaling cat#2278). The same membrane was stripped and re-blotted with rabbit polyclonal anti-FLAG antibody. Co-culture assay was used to determine transdimerization of IGPR-1. In brief, PAE cells expressing empty vector (pMSCV), IGPR-1-myc, or IGPR-1-FLAG were co-cultured in 10cm plates at a ratio of 1:1 for 17hours at near 100% confluency in the following manner: pMSCV + IGPR1-myc, IGPR1-myc + IGPR1-FLAG, and pMSCV + IGPR1-FLAG. Cells were lysed and cell lysates were subjected to immunoprecipitation by using monoclonal anti-c-Myc antibody, 9E10 for 90 minutes, followed by protein G agarose incubation for an additional 60min. Immunoprecipitated proteins were washed in lysis buffer (3X) and resolved on 10% SDS-PAGE gel. The membrane was probed with rabbit polyclonal anti-FLAG antibody (cell signaling cat#2368). The membrane was then stripped and re-probed with rabbit monoclonal anti-c-Myc antibody. In some experiments, PAE cells expressing IGPR-1 or control cells were incubated with cross-linker BS3. Cells were lysed and whole cell lysates were resolved in reducing SDS-PAGE, followed by western blot analysis using anti-IGPR-1 antibody. Also, in some experiments, cells were incubated with 1% methycellulose overnight to prevent cell-cell interaction.
Immunofluorescent Microscopy
PAE cells expressing IGPR-1 were grown in chamber slides. Slides were fixed with freshly prepared 3% formaldehyde for 10 minutes at room temperature after washing once with TBS buffer. Cells were permeabilized with 0.5% Triton X-100 in 1x PBS for 10 min on ice. Slides were blocked with 1% BSA in TBS buffer followed by incubation with anti-IGPR-1 antibody as indicated in the figure legends or with phalloidin. Slides were then incubated with the mouse FITC-conjugated secondary antibody in 1% BSA with TBS for 1 hour. The secondary antibody solution was removed, and the slides were washed with TBS and mounted with mounting media with DAPI. A Nikon Deconvolution Wide-Field Epifluorescence System was used to take pictures (Imaging core facility, Boston University). For co-localization of IGPR-1 with VE-cadherin, PAE or HUVEC cells were plated on collagen coated cover slips for 48 hours at 37 °C with 5% CO2. Cells were washed twice with PBS and then fixed for 5 minutes in methanol at −20 °C. Fixed cells were washed twice with PBS and blocked with BSA (2% BSA in PBS) for 1 hour, then incubated with mouse anti-c-Myc (1:400) (Santa Cruz) and rabbit anti-VE cadherin (1:400) (Cell Signalling) for 90 minutes. Cells were washed with PBS (3X, 5minutes each) and then incubated for 1 hour with Alexa-488-coupled anti-mouse antibody (1:400) and Alexa-594-coupled anti-rabbit antibody (1:400) diluted in 2% BSA in PBS. After washing with PBS (3X, 5 minutes each) slides were mounted in Vectashield mounting medium with DAPI (H-1200, Burlingame, CA). For HUVECs, fixed HUVEC cells were incubated with rabbit anti-VE cadherin (1:400) (Cell Signalling) for 90 minutes washed with PBS (3X, 5 minutes each) and incubated (1 hour) with Alexa-488-coupled anti rabbit antibody (1:400) diluted in 2% BSA. HUVEC cells were again fixed in 4% paraformaldehyde, washed with PBS (2X) and blocked for 1 hour with 2%BSA in PBS. Cells were incubated with rabbit anti-IGPR-1 antibody (1:200) in 2%BSA in PBS for 90minutes, washed (3X,5 minutes each) and incubated with Alexa-594-coupled anti-rabbit immunoglobulin antibody in 2% BSA in PBS and mounted as done with PAE cells. Images were captured using the Leica SP5 confocal microscope.
Cell Permeability and TEER Assays
Cell permeability and TEER assays were performed as described [9]. Briefly, PAE cells expressing IGPR-1 or control vector were seeded on tissue culture polycarbonate membrane filters coated with collagen I (pore size, 3.0 mm) in 24-well Transwell plates at a seeding density of 5,000 cells/well. The complete DMEM was added to both the donor and the acceptor compartment and the cells were incubated at 37°C in a CO2 incubator for at least 4days or until a monolayer of cells was formed. Fluorescein isothiocyanate-dextran (70 KD,100 μL of 1X) was added to each insert and incubated at 37°C in a CO2 incubator for 60 minutes. After 60 minutes, inserts were removed, and solutions from the lower chambers were transferred into a 96-well plate and read at 485-nm excitation, 520-nm emission. For the trans-endothelial electrical resistance (TEER) assay, cells were similarly prepared, and TEER assay was performed with a Millicell ERS meter (Millipore, Bedford, MA). The TEER values were determined by measuring the potential difference between the two sides of the cell monolayer as recommended by the manufacturer.
Matrigel angiogenesis Assay
Equal numbers of PAE cells expressing IGPR-1 or other variants of IGPR-1 were plated on the Matrigel coated 24-well plates (1x103/well, triplicate wells/group) as described [13]. Cells were viewed under the microscope and pictures were taken after overnight incubation. Capillary tube formation of PAE cells was quantified using Angiogenesis Analyzer via ImageJ software (http://image.bio.methods.free.fr/ImageJ/?Angiogenesis-Analyzer-for-ImageJ.html).
MS analysis
IGPR-1 was immunoprecipitated with anti-IGPR-1 antibody from PAE cells ectopically expressing IGPR-1. The immunoprecipitated proteins were subjected to proteolytic digestion with trypsin on a ProGest device (Genomic Solutions). Samples were analyzed by nano–LC-MS/MS on a Thermo Fisher LTQ Orbitrap XL. Thirty microliters of hydrolysate was loaded onto a 5 mm × 75 mm inside diameter (ID) C12 (Jupiter Proteo, Phenomenex) vented column at a flow rate of 10 ml/min. Gradient elution was over a 15 cm × 75 mm ID C12 column at 300 nl/min. The mass spectrometer was operated in data-dependent mode, and the six most abundant ions were selected for MS/MS. The Orbitrap MS resolution was performed 60,000 full width at half maximum resolution. MS/MS data were searched using both MASCOT and Sequest algorithms. The search results were processed by Scaffold (http://www.proteomesoftware.com) and Proteome Discoverer (Thermo Fisher Scientific, version 1.3.0.339). Selected parameters for LTQ Orbitrap XL data require a minimum of two peptides matching per protein with minimum probabilities of 90% at the protein level and 50% at the corresponding peptide level.
Statistical analyses
Western blots were quantified using Image J software. Statistical analysis was performed by two-tailed t test. All the experiments presented in this manuscript were repeated at least three times or as indicated in the figure legends. In some experiments such as TEER and permeability assays, where multiple group comparisons were made, one-way ANOVA with Krusakal Wallis testswas performed.
Results
IGPR-1 is expressed in blood vessels and localized to adherens junctions of endothelial cells
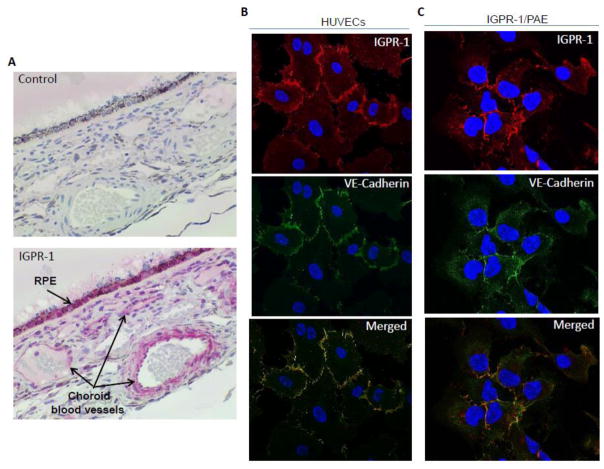
IGPR-1 is expressed in human blood vessels and regulates capillary tube formation of endothelial cells in cell culture [8]. Consistent with our previous observation, immunohistochemical staining of human ocular tissue showed that IGPR-1is highly expressed in choroidal blood vessels and retinal pigmented epithelial cells (Figure 1A). Adherens junctions mediate intercellular contacts of endothelial cells via CAMs on adjacent cells, by forming homophilic interactions with their extracellular domains. Vascular endothelial cadherin (VE-cadherin also known as cadherin-5) is a major endothelial CAM [14]. Considering the role of IGPR-1 in the regulation of cell adhesion, we examined co-localization of IGPR-1 with VE-cadherin by immunofluorescent staining of human primary umbilical vein endothelial cells (HUVECs) and porcine aortic endothelial (PAE) cells ectopically expressing IGPR-1 (IGPR-1/PAE). IGPR-1 was found to co-localize with VE-cadherin in both HUVECs and PAE cells (Figure 1B, 1C).Knockdown of IGPR-1 did not abolish the staining of VE-cadherin indicating that the anti-IGPR-1 antibody do not unexpectedly cross-react with VE-cadherin. Similarly, knockdown of VE-cadherin did not abolish the staining of IGPR-1(S. Figure 1A, 1C). Taken together, the data demonstrate that IGPR-1is expressed in human blood vessels and its expression is concentrated at the adherens junctions of endothelial cells.
Figure 1. IGPR-1 is expressed in human choroidal blood vessels and localized to adherens junctions of endothelial cells.
(A) Human ocular tissue was subjected to immunohistochemistry staining using anti-IGPR-1 antibody or control antibody. Expression of IGPR-1 in choroidal blood vessels and retinal pigmented epithelial cells (RPE) are shown. (B, C) PAE cells expressing IGPR-1 (IGPR-1/PAE) and human primary endothelial cells, HUVECs were prepared and subjected to immunofluorescence staining using anti-IGPR-1 and anti-VE-cadherin antibodies as described in the material and methods section. Slides were viewed under microscope and images were captured using the Leica SP5 confocal microscope.
IGPR-1 regulates endothelial cell barrier function
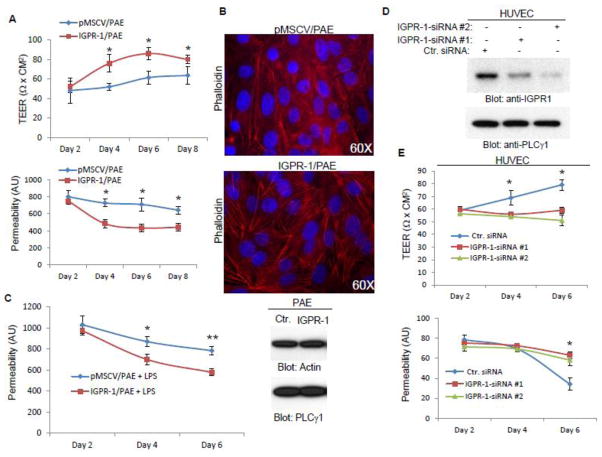
CAMs are enriched at sites of cell-cell contacts and support endothelial barrier function [15, 16]. Expression of IGPR-1 was also enriched at sites of cell-cell contacts (Figure 1B, 1C), suggesting a possible role for IGPR-1in the regulation of barrier function of endothelial cells. To investigate the role of IGPR-1 in endothelial barrier function, we initially measured transendothelial electrical resistance (TEER) of IGPR-1/PAE cells and the parental cells expressing empty vector. The TEER values were significantly increased in PAE cells expressing IGPR-1 (Figure 2A), demonstrating that IGPR-1 increases the transendothelial electrical resistance of endothelial cells. To further, corroborate the effect of IGPR-1 in endothelial cell barrier function, we measured the leakiness of PAE cells. Expression of IGPR-1 in PAE cells significantly reduced the permeability of fluorescently labeled dextran (Figure 2A). The effect of IGPR-1 in TEER of PAE cells significantly reduced when IGPR-1/PAE cells were pre-incubated with the recombinant GST-E-IGPR-1 (GST-E-IGPR-1), which is encompassed of the extracellular domain of IGPR-1 (S. Figure 2A).
Figure 2. IGPR-1 regulates endothelial cells barrier function.
(A) PAE cells expressing empty vector (pMSCV) or IGPR-1 were seeded in 24-well transwell (5000 cells/transwell, quadruple transwell/group). TEER and permeability of cells were measured every two days up to 8 days. The graphs are representative of two independent experiments of quadruple well/group. P < 0.05 for both TEER and permeability assay measurements at day 4, 6 and 8. (B) IGPR-1/PAE and pMSCV/PAE cells were stained for actin using phalloidin. Slides were viewed with a florescence microscope and pictures were taken (60X magnification). (C) IGPR-1/PAE and pMSCV/PAE cells were subjected to cell permeability assay as panel A with or without LPS stimulation. The graph is representative of two independent experiments of quadruple wells/group. *P= 0.003, **P= 0.012. (D) HUVECs were transfected with control siRNA or IGPR-1 siRNAs. The knock down of IGPR-1 by siRNA was confirmed by western blot analysis. (E) HUVECs transfected with control or two different IGPR-1 siRNA oligos were subjected to TEER and permeability assay as panel A. The graphs are representative of two independent experiments of quadruple wells/group. *P< 0.05. For the data presented in panels A, C and E, one-way ANOVA with Krusakal Wallis test was performed for multiple group comparison and unpaired two-tailed t-test analysis was performed for comparison of the TEER and permeability of IGPR-1 and control group at different time points.
Since in these experiments (Figure 2A), we measured TEER and cell permeability for several days after seeding them on the transwell, which may affect cell proliferation, we also seeded cells on the transwell that allows cells to become fully confluent and measured TEER and permeability after 24 hours. The results similarly showed that expression of IGPR-1 in PAE cells significantly increased TEER and reduced permeability (S. Figure 2A, 2B). Consistent with the effect of IGPR-1 in TEER increase, IGPR-1 also promoted F-actin redistribution in PAE cells (Figure 2A). Actin stress fibers in PAE cells expressing IGPR-1 were prominent at the cell-cell junctions, whereas in the parental PAE cells expressing empty vector actin stress fibers were sporadic, and less prominent at cell junctions (Figure 2B).
F-actin localization to cell-cell contact areas is thought to play a vital role in the strengthening of cell-cell adhesion [17, 18]. Various cytokines and inflammatory factors such as lipopolysaccharide (LPS) are known to increase permeability of endothelial cells [19, 20]. We therefore examined the effect of LPS on permeability of IGPR-1/PAE cells. The result demonstrated that PAE cells expressing IGPR-1 were less responsive to LPS-stimulated permeability compared to PAE cells expressing empty vector (Figure 2C).
To determine the role of endogenous IGPR-1 in endothelial barrier function, we silenced expression of IGPR-1 by siRNA in HUVECs and assessed TEER and transendothelial dextran permeability of HUVECs. Transfection of HUVECs with two different siRNAs significantly knocked down IGPR-1 expression (Figure 2C). Knockdown of IGPR-1 in HUVECs markedly reduced TEER and increased dextran permeability (Figure 2E). Additionally, knockdown of IGPR-1 inhibited capillary tube formation/in vitro angiogenesis (S. Figure 2C). Taken together, the data based on both the over-expression and knockdown of IGPR-1 demonstrate that IGPR-1 plays a critical role in the endothelial cell barrier function.
To link the adhesive function of IGPR-1 to its effect on the endothelial cell barrier function, we deleted the extracellular domain of IGPR-1 (herein called ΔN-IGPR-1), expressed it in PAE cells (S. Figure 3A, 3B), and then measured PAE cells permeability to dextran. Expression of ΔN-IGPR-1 in PAE cells, unlike IGPR-1, did not inhibit transendothelial permeability of PAE cells to dextran (S. Figure 3C). The data indicate that the extracellular domain of IGPR-1 is required for its ability to regulate endothelial cell barrier function.
IGPR-1 forms disulfide bond-linked dimerization
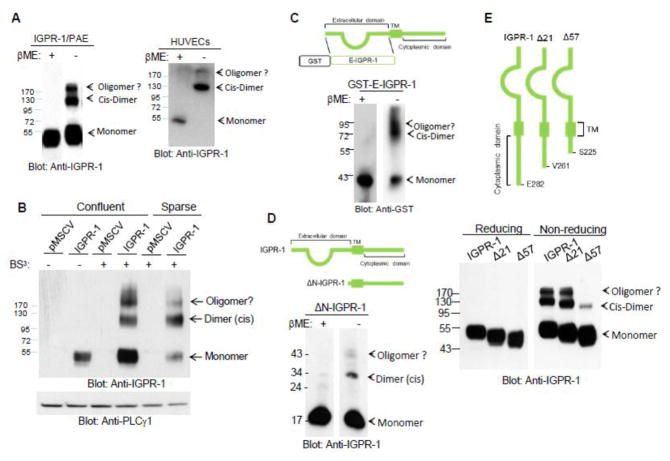
To gain further insight into the mechanism of the adhesive function of IGPR-1, we sought to examine dimerization of IGPR-1, a mechanism that is commonly utilized by CAMs to establish biological functions [14, 21]. Our initial observation in IGPR-1/PAE cells demonstrated that in non-reducing SDS-PAGE (without β-mercaptoethanol, β-ME) IGPR-1 is present with approximate molecular weights of 55kDa, 110kDa and 180kDa. However, in reducing SDS-PAGE (with β-ME), IGPR-1 was detected with an approximate molecular weight of 55kDa (Figure 3A). Additionally, cell lysates derived from HUVECs, in which IGPR-1is endogenously expressed, in reducing SDS-PAGE IGPR-1was detected as a 55kDa protein and in non-reducing SDS-PAGE it was detected as 110kDa and 180kDa forms (Figure 3A). The 55kDa IGPR-1 in the cell lysates of PAE and HUVECs in reducing SDS-PAGE corresponds to monomeric IGPR-1 (Figure 3A). The 110kDa and 180kDa IGPR-1 protein bands correspond to covalently linked dimeric and oligomeric IGPR-1, respectively. The observation indicates that IGPR-1 is present in covalent bond linked cis-dimers (i.e., parallel/lateral) in endothelial cells. Interestingly, the presence of 55kDa monomeric IGPR-1 was not detected in the cell lysates of HUVECs in non-reducing SDS-PAGE, indicating that IGPR-1 in non-ectopically expressing cells is mostly present in cis-dimeric and oligomeric forms and that the presence of monomeric IGPR-1 in PAE cells in non-reducing SDS-PAGE is likely due to the over-expression of IGPR-1 in PAE cells. Altogether, the observation indicates that IGPR-1 undergoes covalent cis-dimerization.
Figure 3. IGPR-1 forms disulfide bond linked dimerization.
(A) PAE cells expressing IGPR-1 (IGPR-1/PAE) or primary endothelial cells, HUVECs were lysed with lysis buffer containing 50mM iodoacetamide. Whole cell lysates were prepared in reducing loading sample buffer with β-mercaptoethanol (βME) (+) or in non-reducing loading buffer without βME (−). Cell lysates were resolved in 5% SDS-PAGE followed by western blot analysis using pan anti-IGPR-1 antibody. The size of protein markers is shown to the left of the blot. Arrows indicate the position of monomeric, dimeric and oligomeric IGPR-1. (B) Equal number of IGPR-1/PAE or pMSCV/PAE cells were seeded in either confluent (60cm plates) or sparse (100cm plates) conditions. Cells were incubated with cross-linker DS3 (+) or with control vehicle (−), lysed and resolved on reducing SDS-PAGE, followed by western blot analysis using pan anti-IGPR-1 antibody or anti-PLCγ1 antibody for loading control. (C) GST-E-IGPR-1 encompassing the extracellular domain of IGPR-1 was prepared in non-reducing or reducing conditions as panel A and blotted with pan anti-IGPR-1 antibody. (D) Cell lysates derived from PAE cells expressing extracellular deleted IGPR-1 (ΔN-IGPR-1) was prepared as shown Panel A, resolved on SDS-PAGE under reducing (+) or non-reducing (−) conditions as panel A, and blotted for IGPR-1. (E) PAE cells expressing wild type IGPR-1, C-terminal truncated IGPR-1 constructs, whereas the C-terminal of IGPR-1 was deleted by 21 (Δ21) or 57 (Δ57) amino acids, respectively. Whole cell lysates were resolved in non-reducing (−) or reducing (+) conditions as shown panel A and blotted for IGPR-1.
To examine the dimer forming potential of IGPR-1 further, we used homo-bifunctional bis (sulfosuccinimidyl)suberate (BS3), a membrane non-permeable cross-linker, and assessed the dimer formation of IGPR-1 in reducing SDS-PAGE. To distinguish the possible trans-dimerization (i.e., anti-parallel homophilic dimerization) of IGPR-1 from cis-dimerization, cells were seeded in both confluent and sparse conditions. The results demonstrated that in confluent cells treated with homobifunctional BS3 cross-linker, IGPR-1 was present in three major forms: monomer, dimer and oligomer (Figure 3B). However, in cells that were seeded in sparse conditions, IGPR-1 was present predominately in dimeric (110kDa) form (Figure 3B). Interestingly, the levels of both monomeric and oligomeric forms of IGPR-1 were decreased but not the dimeric IGPR-1 (110kDa) in non-reducing SDS-PAGE (Figure 3B), suggesting that the 110kDa form likely corresponds to cis-dimer IGPR-1.
Next, we investigated the contribution of the extracellular domain of IGPR-1 in covalent bond dimerization of IGPR-1. To this end, we generated a soluble GST-fusion recombinant IGPR-1 (GST-E-IGPR-1) encompassing only the extracellular immunoglobulin domain. We examined the dimer formation of the purified GST-E-IGPR-1 in non-reducing and reducing SDS-PAGE. Recombinant GST-E-IGPR-1 was detected in both monomer and dimer forms in non-reducing SDS-PAGE (Figure 3C), indicating that the extracellular domain of IGPR-1 is capable of forming covalent-linked dimer in E. coli.
To test whether the C-terminal cytoplasmic tail of IGPR-1 alone could also undergo covalent bond linked dimerization, we examined the ability of the extracellular domain deleted IGPR-1, ΔN-IGPR-1, to undergo dimerization. Curiously, ΔN-IGPR-1 also formed covalent dimers in non-reducing SDS-PAGE (Figure 3D). However, only a small fraction of ΔN-IGPR-1 formed dimers, indicating that the C-terminal tail does not significantly contribute to disulfide bond linked dimerization of IGPR-1. In an alternative approach, and also to alleviate the concern of a possible deleterious effect from deletion of the extracellular domain, we decided to delete the C-terminal tail of IGPR-1 by 57 and 21 amino acids. Deletion of 57 amino acids removed two cysteines (Cys241 and Cys247), which could mediate disulfide bond formation. Deletion of 57 amino acids significantly impaired dimer formation of IGPR-1, whereas deletion of 21 amino acids (which did not remove any cysteine residues) had no effect on the dimer formation of IGPR-1 (Figure 3E). Taken together, the data demonstrate that IGPR-1 is present in covalent linked cis-dimers and oligomers involving both extracellular and cytoplasmic domains.
IGPR-1 forms trans-homophilic dimerization
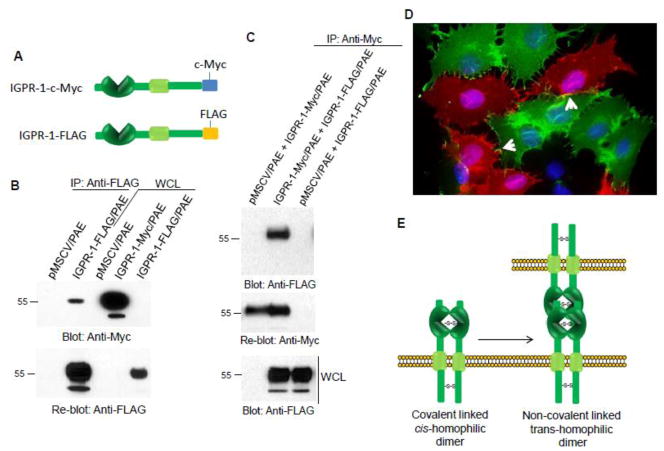
Next, we investigated whether IGPR-1 forms a trans-homophilic dimerization. To this end, we generated two PAE cell lines expressing either C-terminal c-Myc tagged IGPR-1 (IGPR-1-c-Myc) or C-terminal FLAG tagged IGPR-1(IGPR-1-FLAG) (Figure 4A). Immunofluorescence staining of PAE cells showed that both IGPR-1-c-Myc- and IGPR-1-FLAG constructs are expressed at similar levels at the cell surface (S. Figure 4). To demonstrate the homophilic dimerization of IGPR-1, the cell lysates of PAE cells expressing IGPR-1-FLAG were first immunoprecipitated with an anti-FLAG antibody, and then the purified immunoprecipitated proteins were incubated with the cell lysates derived from PAE cells expressing IGPR-1-c-Myc. The co-precipitated proteins were detected using western blot analysis. The result demonstrated that IGPR-1-FLAG formed dimers with IGPR-1-c-Myc (Figure 4B), supporting the hypothesis that IGPR-1 forms homodimer. To demonstrate specifically, whether IGPR-1 forms a trans-homophilic dimerization, PAE cells expressing IGPR-1-FLAG were co-cultured with PAE cells expressing IGPR-1-c-Myc in a 1:1 ratio. After 17 hours co-culture, cells were lysed, and the cell lysates were immunoprecipitated with c-Myc antibody and blotted with anti-FLAG antibody (Figure 4C). The data demonstrated that IGPR-1 monomers interact with each other in a homophilic trans-dimeric mechanism. Furthermore, staining of IGPR-1-FLAG and IGPR-1-c-Myc cells co-cultured together showed that IGPR-1-FLAG co-localizes with IGPR-1-c-Myc in the cell-cell contact regions (Figure 4D and S. Figure 5). Based on these observations, we propose that IGPR-1 is mostly present in a covalent-linked cis-homophilic dimer and that cis-homophilic dimeric IGPR-1 interacts with cis-homophilic IGPR-1dimers expressed on the opposing cells to form non-covalent trans-homophilic dimers, which mediate cell-cell interaction (Figure 4E).
Figure 4. IGPR-1 forms homophilic transdimerization complexes.
(A) Schematic of C-terminal tagged IGPR-1 (IGPR-1-FLAG) and c-Myc tagged IGPR-1 (IGPR-1-c-Myc). (B) PAE cells expressing IGPR-1-FLAG or empty vector (pMSCV) were lysed and whole cell lysates were subjected to immunoprecipitation using an anti-FLAG antibody. The immunoprecipitated proteins was extensively washed and then incubated with cell lysates derived from PAE cells expressing IGPR-1-c-Myc. After 90 minutes incubation, the co-immunoprecipitated protein complexes were washed (3X) and resolved in reducing SDS-PAGE and blotted for IGPR-1 using anti-c-Myc antibody. The same membrane was striped and re-blotted with anti-FLAG antibody. (C) PAE cells expressing IGPR-1-FLAG were co-cultured with PAE cells expressing empty vector or IGPR-1-c-Myc in an equal ratio. Cells were lysed and immunoprecipitated with anti-c-Myc antibody and subsequently blotted with anti-FLAG antibody. The same membrane was re-blotted with anti-c-Myc antibody. The whole cell lysates from the same groups were blotted with anti-FLAG antibody. (D) PAE cells expressing IGPR-1-FLAG were co-cultured with PAE cells expressing empty vector or IGPR-1-c-Myc in an equal ratio and after overnight cells were fixed and subjected to immunofluorescence staining using anti-Myc and anti-FLAG antibodies. (E) Based on the data presented in figure 3 and 4, we propose that IGPR-1 is mostly present at the cell surface as a cis-homodimer and forms trans-homophilic dimers by interacting with cis-homodimer IGPR-1 on the opposing cells.
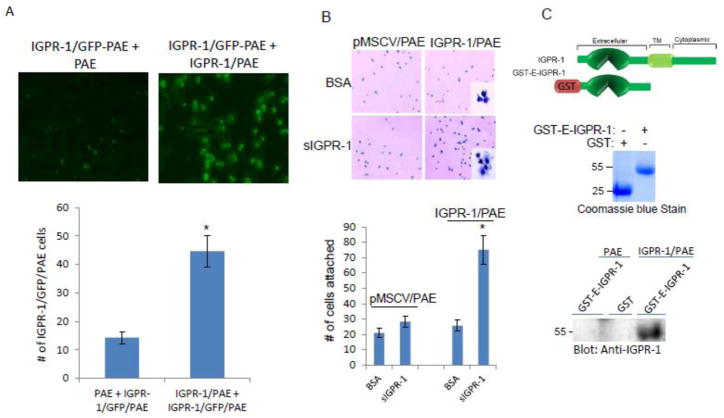
Figure 5. IGPR-1 mediates cell-cell adhesion.
(A) PAE cells expressing IGPR-1/GFP were added into the plates of fully confluent PAE cells either expressing empty vector or IGPR-1. After 15 minutes incubation, unattached IGPR-1/GFP-PAE cells were washed off the plates, the presence of attached cells was observed under a fluorescent microscope, and pictures (10X magnification) were taken. The graph is representative of three different fields. (B) PAE cells expressing either empty vector or IGPR-1 were seeded in the GST- extracellular domain of IGPR-1 (GST-E-IGPR-1) or BSA coated 24-well plates (quadruple wells/group). After 30 minutes incubation, the non-adhered cells were washed off the wells, cells were fixed and pictures were taken (10X magnification). The graph is presentative of quadruple wells/group. (C) GST fusion of extracellular domain (GST-E-IGPR-1) was used in GST pulldown assay to demonstrate the binding of GST-E-IGPR-1 to IGPR-1.
Trans-homophilic dimerization of IGPR-1 mediates cell-cell interaction
Next, we examined whether trans-homophilic dimerization of IGPR-1 mediates cell-cell interaction. To investigate the role of in cell-cell interaction, we generated green fluorescent protein (GFP) PAE (GFP-PAE) cells expressing IGPR-1 cell line (IGPR-1/GFP-PAE). IGPR-1/GFP-PAE cells were added into the plates of fully confluent PAE cells expressing either an empty vector or IGPR-1. After 15 minutes of incubation, unattached IGPR-1/GFP-PAE cells were washed off the plates and the presence of attached cells was observed under a fluorescent microscope. The result demonstrated that IGPR-1/GFP-PAE cells preferentially adhered to IGPR-1/PAE cells but not to pMSCV/PAE cells (Figure 5A), indicating that IGPR-1 mediates cell-cell adhesion through transdimerization.
In a complementary approach, we generated a soluble IGPR-1 encompassing the extracellular domain of IGPR-1 (sIGPR-1) and used it to coat cell culture plates. PAE cells expressing IGPR-1 were used to test whether they adhere to sIGPR-1 coated plates. The result showed that PAE cells expressing IGPR-1 adhered more strongly to sIGPR-1 coated plates than to the plates coated with BSA (Figure 5B). The adherence of PAE cells expressing empty vector (pMSCV) to the plates coated with sIGPR-1 and BSA were similar (Figure 5B). However, the adherence of PAE cells expressing pMSCV to sIGPR-1 coated plates was significantly lower than the adherence of PAE cells expressing IGPR-1 (Figure 5B). Of note, PAE cells expressing IGPR-1 displayed more spreading on the plates coated with sIGPR-1 compared to the plates coated with the control protein, BSA (Figure 5B). To demonstrate the physical interaction of sIGPR-1 with IGPR-1, a GST-fusion sIGPR-1 encompassing the extracellular domain of IGPR-1 (GST-E-IGPR-1) was generated (Figure 5C) and tested for its ability to interact with IGPR-1 in an in vitro GST-pull down assay. The GST-E-IGPR-1 strongly interacted with IGPR-1 in cell lysates derived from IGPR-1/PAE cells (Figure 5C). Taken together, the data further demonstrate that IGPR-1 mediates cell-cell interaction through trans-homophilic dimerization.
Trans-homophilic dimerization regulates IGPR-1 phosphorylation
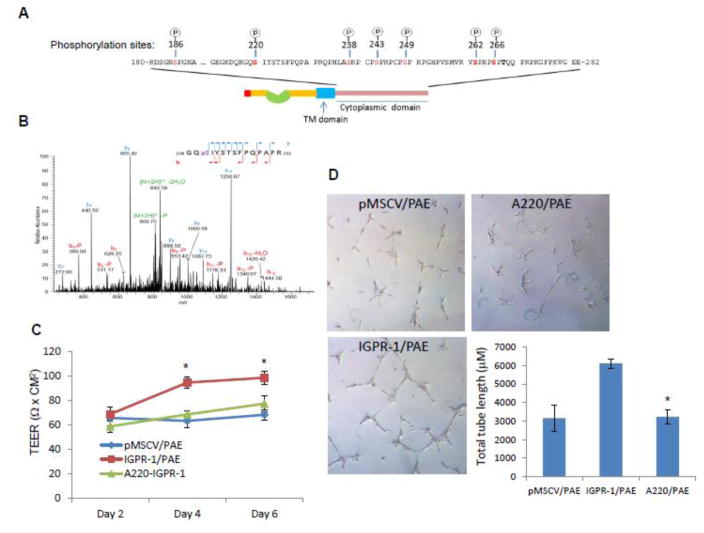
The cytoplasmic domain of IGPR-1 has 16 serine/threonine residues, accounting for 16% of the total amino acids in the cytoplasmic domain [8]. To identify potential serine/threonine phosphorylation sites on IGPR-1, we analyzed IGPR-1 immunoprecipitated from PAE cells expressing IGPR-1 by liquid chromatography–tandem mass spectrometry (LC-MS/MS). MS analysis identified seven phosphorylated serine residues on IGPR-1, including Ser186, Ser220, Ser238, Ser243, Ser249, Ser262 and Ser266 (Figure 6A, 6B, S. Figure 5A–E). To determine the potential role of serine phosphorylation in IGPR-1function, we mutated several of the serine phosphorylation sites, including Ser220 to the non-phosphorylatable residue, alanine (Ser220A).
Figure 6. IGPR-1 is phosphorylated at multiple serine sites.
(A) A schematic of serine phosphorylation sites on IGPR-1 is shown. (B) Shown is the LC-MS/MS spectrum of serine 220 on IGPR-1. (C) Equal number of PAE cells expressing pMSCV, IGPR-1 or serine mutant 220 IGPR-1 (A220-IGPR-1) were subjected to permeability assay as panel A. The graphs are representative of two independent experiments of quadruple wells/group. *P< 0.05. (D) pMSCV/PAE, IGPR-1/PAE and A220-IGPR-1/PAE cells were subjected to matrigel angiogenesis assay in triplicate of 24-well plates. Capillary tube formation of cells were viewed under microscope and pictures were taken after 12 hours. Capillary tube formation was quantified using the Image J/angiogenesis analyzer software. P<0.05.
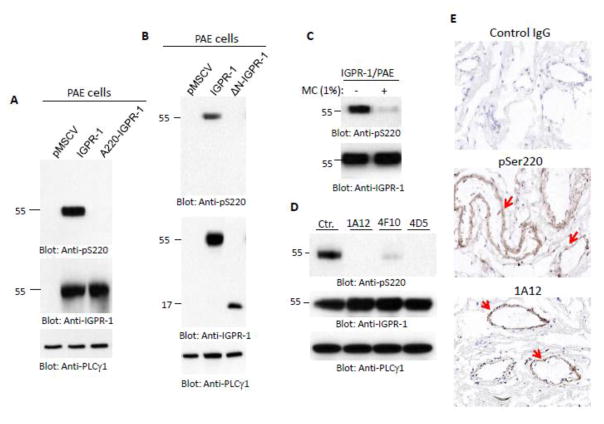
We observed that mutation of Ser220 significantly inhibited the ability of IGPR-1 to regulate the transendothelial electrical resistance (TEER) of PAE cells (Figure 6C). Further analysis showed that Ser220 mutation also inhibits the ability of IGPR-1 to stimulate angiogenesis/in vitro capillary tube formation (Figure 6D). Collectively, the data demonstrated that Ser220 phosphorylation plays an important role in mediating the biological function of IGPR-1. Considering the potential role of Ser220 phosphorylation in IGPR-1 function, we developed a rabbit polyclonal anti-phospho-Ser220 specific antibody (anti-pSer220) and used this antibody to demonstrate phosphorylation of Ser220 in PAE cells (Figure 7A). We validated specificity of the anti-pSer220 antibody in multiple ways. First, mutation of Ser220 abolished the immunoreactivity of the anti-pSer220 antibody with IGPR-1 (Figure 7A), indicating that the anti-pSer220 antibody specifically recognizes the phosphorylated Ser220 on IGPR-1. Second, pre-incubation of anti-pSer220 antibody with a pSer220 peptide inhibited the recognition of IGPR-1 by anti-pSer220 antibody (S. Figure 7A). Third, treatment of a whole cell lysate containing IGPR-1 with calf intestinal alkaline phosphatase (CIP) also inhibited the recognition of IGPR-1 by anti-pSer220 antibody (S. Figure 7B).
Figure 7. Blocking trans-homophilic dimerization of IGPR-1 inhibits IGPR-1 phosphorylation.
(A &B) Cell lysates derived from pMSCV/PAE, IGPR-1/PAE or ΔN-IGPR-1/PAE were subjected to western blot analysis and immunoblotted for total IGPR-1, pSer220 or loading control protein, PLCγ1. (C) Equal number of PAE cells expressing IGPR1 were plated in full growth medium or full growth medium plus 1% methycellulose (MC) for overnight. Cells were lysed and whole cell lysates were analyzed by western blot analysis and blotted for pSer220 and total IGPR-1. (D) PAE cells expressing IGPR-1 were incubated with full growth DMEM medium plus normal mouse IgG or with three different clones of monoclonal blocking anti-IGPR-1 antibody (1A12, 4F10 and 4D5) for overnight. Cells were lysed and whole cell lysates were blotted with anti-pS220 antibody and anti-IGPR-1 antibody. (E) 1A12 blocking anti-IGPR-1 antibody recognizes IGPR-1 in the blood vessels of frozen human colon tissue.
Having confirmed phosphorylation of IGPR-1 in PAE cells, we decided to examine phosphorylation of Ser220 in human umbilical vein endothelial cells (HUVECs) endogenously expressing IGPR-1. The result demonstrated that Ser220 was also phosphorylated on IGPR-1 in HUVECs (S. Figure 7C). Next, we investigated possible mechanisms involved in the regulation of Ser220 phosphorylation. Specifically, we examined the role of dimerization in the phosphorylation of Ser220. To this end, we first tested whether the presence of the extracellular domain is required for phosphorylation of Ser220. The results showed that deletion of the extracellular domain (ΔN-IGPR-1), which eliminated the trans-homophilic dimerization of IGPR-1 abrogated phosphorylation of Ser220.(Figure 7B), indicating that trans-homophilic dimerization of IGPR-1 regulates Ser220 phosphorylation (Figure 7B). Additionally, we interfered with the trans-homophilic dimerization of IGPR-1 by incubating the cells with methycellulose (MC) and examined phosphorylation of Ser220. We mixed PAE cells expressing IGPR-1 with 1% MC and seeded them in a cell culture plate. After overnight incubation, cells were lysed and phosphorylation of Ser220 was assessed. MC inhibits cell-cell contact by creating a physical barrier between cells [22, 23] and therefore reduces homophilic protein-protein interactions. MC significantly inhibited phosphorylation of Ser220 (Figure 7C), further indicating that trans-homophilic dimerization of IGPR-1is required for phosphorylation of Ser220.
To demonstrate the direct role of trans-homophilic dimerization in the regulation of Ser220 phosphorylation, we developed a mouse monoclonal blocking antibody against the extracellular domain of IGPR-1. We demonstrated that three different clones of this antibody were able to inhibit phosphorylation of IGPR-1 (Figure 7D). Moreover, treatment of IGPR-1/PAE cells with 1A12 blocking antibody coupled to a polystyrene (PS) resin also inhibited phosphorylation of Ser220 (S. Figure 7D), whereas the normal control antibody had no effect on the phosphorylation of Ser220 (S. Figure7D). Additionally, the IGPR-1 blocking antibody (clone 1A12) as well as -anti-pSer220 antibody specifically recognized IGPR-1 in the human blood vessels (Figure 7E). Taken together, the data demonstrate that trans-homophilic dimerization of IGPR-1 controls phosphorylation of Ser220.
To further illustrate the role of dimerization in the phosphorylation of Ser220, we generated a chimeric IGPR-1 by replacing the extracellular domain of IGPR-1 with human colony stimulating growth factor-1 receptor (CSF-1R) (herein referred to as cIGPR-1) (Figure 8A) and expressed it in HEK-293 cells. If trans-homophilic dimerization of IGPR-1was responsible for phosphorylation of Ser220, one would expect to observe an increase in the phosphorylation of Ser220 in response to CSF-1 stimulation. The data demonstrated that without CSF-1 stimulation, cIGPR-1 was partially phosphorylated at Ser220 and its phosphorylation was increased with CSF-1 stimulation in a time-dependent manner up to 60 minutes (Figure 8B). Additionally, longer stimulation of cIGPR-1 with CSF-1 showed that cIGPR-1 remained highly phosphorylated up to 4 days (96 hours) in a progressive manner (Figure 8B). The data indicated that CSF-1 mediated dimerization of cIGPR-1 stimulated phosphorylation of Ser220 and that CSF-1 stimulation of cIGPR-1 mimicked the trans-homophilic dimerization of IGPR-1. The data also revealed that trans-homophilic dimerization induced an unusually long-lasting phosphorylation of IGPR-1, which is strikingly different from growth factor induced phosphorylation of receptor tyrosine kinases (RTKs) that typically lasts only minutes [12, 24].
Figure 8. Chimeric IGPR-1 (cIGPR-1) is phosphorylated in a CSF-1 dependent manner.

(A) Schematic of cIGPR-1 is shown. (B) Serum-starved HEK-293 cells expressing cIGPR-1 were stimulated with CSF-1 (10ng/ml) for indicated times, cells were lysed, and whole cell lysates were immunoblotted for total IGPR-1, pSer220 or loading control β-actin. The graph is representative of two independent experiments. (C) Cells prepared as panel B, but they were stimulated with CSF-1 for extended period as indicated. Whole cell lysates were immunoblotted for total IGPR-1, pSer220 or loading control β-actin. The graph is representative of two independent experiments.
Discussion
Permeability of the endothelial barrier is maintained by inter-endothelial junctions, including tight junctions and adherens junctions and plays a prevalent regulatory mechanism in the physiological function of blood vessels and major human diseases such as cancer and inflammation [25]. In this study, we demonstrate that IGPR-1 is present at the endothelial adherens junctions and controls endothelial cell-cell adhesion and barrier function through trans-homophilic dimerization. The integrity of the endothelium and its barrier function is dependent on cell-cell adhesion [26, 27]. Vascular endothelial cadherin (VE-cadherin, also called cadherin-5), a prototypic of E-cadherin, is the only known CAM linked to endothelial cell-cell adhesion [15, 27]. Our findings that IGPR-1 is expressed in endothelial cells [8] and localized to adherens junctions underscores an important role for IGPR-1 in the function of endothelial cells. Adherens junction serves multiple functions, including regulation of cell-cell adhesion, control of the actin cytoskeleton fibril formation, intracellular signaling and transcriptional regulation [28], suggesting a critical role for IGPR-1 in the regulation of these key events. Furthermore, given the expression of IGPR-1 by other cell types, in addition to its role in the homophilic interaction of endothelial cells, IGPR-1 could also regulate the heterophilic interactions between endothelial cells and other cell types, including immune cells and cancer cells thus contributing to pathological conditions.
Both the extracellular and the cytoplasmic domains of IGPR-1 are essential for its function. Deletion of the extracellular domain abrogated the effect of IGPR-1 in the endothelial barrier function, indicating that engagement of the extracellular domain through trans-homophilic dimerization increases endothelial cell-cell adhesion and therefore reduces endothelial cell permeability. Moreover, PAE cells expressing IGPR-1 were resistant to LPS-mediated permeability, indicating that expression of IGPR-1 in endothelial cells could play a protective role during inflammatory conditions such as sepsis and edema. IGPR-1 is present in homophilic and oligomeric dimers and its dimerization is established through disulfide bonds involving both cytoplasmic and extracellular domains. Given that an alkylating agent, iodoacetamide, did not inhibit homophilic dimerization of IGPR-1, the data suggest that the dimerization of IGPR-1 is not an artifact of cell lysis but occurs in cells and that covalent bond linked dimerization is a primary mechanism of IGPR-1 dimerization. The observation that both cytoplasmic and extracellular domains are involved in the covalent bond linked dimerization, indicates that perhaps the cytoplasmic domain mediates the lateral cis-dimerization of IGPR-1, creating a more favorable conformational requirement for a trans-homophilic dimerization which is mediated through the extracellular domain. The extracellular domain could generate a sequential dimerization, involving cis- and trans-dimerization, which could in turn regulate IGPR-1 phosphorylation. In support of this hypothesis, blocking trans-homophilic dimerization of IGPR-1 either by methylcellulose or IGPR-1 blocking antibody inhibited phosphorylation of IGPR-1at Ser220. This type of dimerization has also been proposed for other CAMs, including E-cadherin [29, 30]. Growth factor receptors are also known for covalent bond cytoplasmic dimerization followed by ligand mediated dimerization at the extracellular domain. In favor of this model, chimeric IGPR-1 (cIGPR-1) showed a basal phosphorylation without CSF-1 stimulation, and upon stimulation with CSF-1, which mimics dimerization of IGPR-1, demonstrated increased and sustained phosphorylation of IGPR-1. We propose this higher level of dimerization for IGPR-1, which is established through cell-cell interaction, could coordinate IGPR-1 function. However, future studies, in particular, those resolving the crystal structure of IGPR-1 should shed light into the molecular regulation of IGPR-1dimerization.
Studies presented here indicate that through trans-dimerization, IGPR-1 undergoes phosphorylation at Ser220 to regulate endothelial barrier function. Furthermore, the demonstration that IGPR-1 modulates endothelial cell-cell adhesion and barrier function, suggest that IGPR-1 may play an important role in the pathological conditions such as diabetes, inflammation and tumor metastasis where endothelial cell-cell adhesion and barrier is altered. IGPR-1 is not expressed in rodents [8], making it difficult to examine the possible role of IGPR-1 in mouse model. However, data obtained from both over-expression and under-expression of IGPR-1 in cell culture system strongly suggest a critical role for IGPR-1 in angiogenesis and endothelial cell barrier function.
Supplementary Material
Summary.
IGPR-1 is localized to adherens junctions and through trans-dimerization undergoes phosphorylation at serine 220 to regulate endothelial barrier function and angiogenesis. The data suggest that IGPR-1 may play an important role in pathological conditions such as diabetes, inflammation and tumor metastasis, where endothelial cell-cell adhesion and barrier are altered.
Research Highlight.
Molecular mechanisms of endothelial cell-cell adhesion and barrier function are not fully understood. IGPR-1 is a novel cell adhesion molecule expressed in endothelial cells and is localized to endothelial adherens junctions. IGPR-1through trans-homophilic dimerization regulates endothelial cell-cell adhesion and barrier. Trans-homophilic dimerization of IGPR-1 stimulates phosphorylation of serine 220 (Ser220), which is required for IGPR-1 to stimulate endothelial barrier function and angiogenesis.
Acknowledgments
Funding: This work was supported in part through NIH grants R21CA191970 and R21CA193958 (to N.R.) and grants P41 RR010888/GM104603 and S10 RR020946, and NIH/National Heart, Lung, and Blood Institute contract N01 HHSN268201000031C (to C.E.C.).
Authors thank Nick Woolf and Bradley E. Pearson for their edits and comments on the manuscript. The authors also acknowledge Dr. Michael T Kirber (Boston University, Imaging Center) for his assistance with immunofluorescence microscopy.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Frontiers in oncology. 2013;3:211. doi: 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reymond N, d’Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nature reviews Cancer. 2013;13:858–70. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 3.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiological reviews. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 4.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nature reviews Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 5.Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, et al. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. The Journal of biological chemistry. 2003;278:19199–208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]
- 6.Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annual review of cell and developmental biology. 2008;24:309–42. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- 7.Barclay AN. Membrane proteins with immunoglobulin-like domains--a master superfamily of interaction molecules. Seminars in immunology. 2003;15:215–23. doi: 10.1016/s1044-5323(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 8.Rahimi N, Rezazadeh K, Mahoney JE, Hartsough E, Meyer RD. Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Molecular biology of the cell. 2012;23:1646–56. doi: 10.1091/mbc.E11-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arafa E, Bondzie PA, Rezazadeh K, Meyer RD, Hartsough E, Henderson JM, et al. TMIGD1 Is a Novel Adhesion Molecule That Protects Epithelial Cells from Oxidative Cell Injury. The American journal of pathology. 2015;185:2757–67. doi: 10.1016/j.ajpath.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, et al. B7-H5 costimulates human T cells via CD28H. Nature communications. 2013;4:2043. doi: 10.1038/ncomms3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janakiram M, Chinai JM, Fineberg S, Fiser A, Montagna C, Medavarapu R, et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:2359–66. doi: 10.1158/1078-0432.CCR-14-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. The Journal of biological chemistry. 2000;275:16986–92. doi: 10.1074/jbc.M000528200. [DOI] [PubMed] [Google Scholar]
- 13.Hartsough EJ, Meyer RD, Chitalia V, Jiang Y, Marquez VE, Zhdanova IV, et al. Lysine methylation promotes VEGFR-2 activation and angiogenesis. Science signaling. 2013;6:ra104. doi: 10.1126/scisignal.2004289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nature reviews Molecular cell biology. 2011;12:189–97. doi: 10.1038/nrm3068. [DOI] [PubMed] [Google Scholar]
- 15.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Developmental cell. 2013;26:441–54. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. Journal of cell science. 2008;121:2115–22. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP, et al. Actin at cell-cell junctions is composed of two dynamic and functional populations. Journal of cell science. 2005;118:5549–62. doi: 10.1242/jcs.02639. [DOI] [PubMed] [Google Scholar]
- 18.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, et al. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Molecular biology of the cell. 2002;13:1175–89. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leeuwenberg JF, Dentener MA, Buurman WA. Lipopolysaccharide LPS-mediated soluble TNF receptor release and TNF receptor expression by monocytes. Role of CD14, LPS binding protein, and bactericidal/permeability-increasing protein. J Immunol. 1994;152:5070–6. [PubMed] [Google Scholar]
- 20.Eutamene H, Theodorou V, Schmidlin F, Tondereau V, Garcia-Villar R, Salvador-Cartier C, et al. LPS-induced lung inflammation is linked to increased epithelial permeability: role of MLCK. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;25:789–96. doi: 10.1183/09031936.05.00064704. [DOI] [PubMed] [Google Scholar]
- 21.Gurevich VV, Gurevich EV. How and why do GPCRs dimerize? Trends in pharmacological sciences. 2008;29:234–40. doi: 10.1016/j.tips.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin J, Sherer NM, Heidecker G, Derse D, Mothes W. Assembly of the murine leukemia virus is directed towards sites of cell-cell contact. PLoS biology. 2009;7:e1000163. doi: 10.1371/journal.pbio.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YL, Lanni F, McNeil PL, Ware BR, Taylor DL. Mobility of cytoplasmic and membrane-associated actin in living cells. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:4660–4. doi: 10.1073/pnas.79.15.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer RD, Singh A, Majnoun F, Latz C, Lashkari K, Rahimi N. Substitution of C-terminus of VEGFR-2 with VEGFR-1 promotes VEGFR-1 activation and endothelial cell proliferation. Oncogene. 2004;23:5523–31. doi: 10.1038/sj.onc.1207712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annual review of physiology. 2010;72:463–93. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature medicine. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 27.Dejana E, Bazzoni G, Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue? Experimental cell research. 1999;252:13–9. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- 28.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochimica et biophysica acta. 2008;1778:660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Current biology : CB. 1997;7:308–15. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- 30.Murase S, Hirano S, Wang X, Kitagawa M, Natori M, Taketani S, et al. Lateral clustering of cadherin-4 without homophilic interaction: possible involvement in the concentration process at cell-cell adhesion sites as well as in the cell adhesion activity. Biochemical and biophysical research communications. 2000;276:1191–8. doi: 10.1006/bbrc.2000.3590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.